Abstract
This study tested the hypothesis that ITRI Biofilm prevents adhesion of the chest cavity. Combined extracorporeal shock wave (ECSW) + bone marrow-derived autologous endothelial progenitor cell (EPC) therapy was superior to monotherapy for improving heart function (left ventricular ejection fraction [LVEF]) in minipigs with ischemic cardiomyopathy (IC) induced by an ameroid constrictor applied to the mid-left anterior descending artery. The minipigs (n = 30) were equally designed into group 1 (sham-operated control), group 2 (IC), group 3 (IC + EPCs/by directly implanted into the left ventricular [LV] myocardium; 3 [+]/3[–] ITRI Biofilm), group 4 (IC + ECSW; 3 [+]/[3] – ITRI Biofilm), and group 5 (IC + EPCs–ECSW; 3 [+]/[3] – ITRI Biofilm). EPC/ECSW therapy was administered by day 90, and the animals were euthanized, followed by heart harvesting by day 180. In vitro studies demonstrated that cell viability/angiogenesis/cell migratory abilities/mitochondrial concentrations were upregulated in EPCs treated with ECSW compared with those in EPCs only (all Ps < 0.001). The LVEF was highest in group 1/lowest in group 2/significantly higher in group 5 than in groups 3/4 (all Ps < 0.0001) by day 180, but there was no difference in groups 3/4. The adhesion score was remarkably lower in patients who received ITRI Biofilm treatment than in those who did not (all Ps <0.01). The protein expressions of oxidative stress (NOX-1/NOX-2/oxidized protein)/apoptotic (mitochondrial-Bax/caspase3/PARP)/fibrotic (TGF-β/Smad3)/DNA/mitochondria-damaged (γ-H2AX/cytosolic-cytochrome-C/p-DRP1), and heart failure/pressure-overload (BNP [brain natriuretic peptide]/β-MHC [beta myosin heavy chain]) biomarkers displayed a contradictory manner of LVEF among the groups (all Ps < 0.0001). The protein expression of endothelial biomarkers (CD31/vWF)/small-vessel density revealed a similar LVEF within the groups (all Ps < 0.0001). ITRI Biofilm treatment prevented chest cavity adhesion and was superior in restoring IC-related LV dysfunction when combined with EPC/ECSW therapy compared with EPC/ECSW therapy alone.
Keywords: ITRI Biofilm, ischemic cardiomyopathy, endothelial progenitor cells, extracorporeal shock wave, angiogenesis
Introduction
A few patients with left main or three-vessel diseases undergo coronary artery bypass surgery (CABG)1–3. Inevitably, many of these patients would receive a second open chest-wall surgery owing to restenosis of post-CABG coronary arteries (re-do CABG), valvular heart disease, or other disease entities, resulting in a high incidence of tissue–organ adhesion (ie, post-surgical adhesion)4–6. However, re-opening chest-wall surgical intervention (second cardio-thoracic surgery4–10) commonly causes higher morbidity and mortality7–10 and the unacceptably high cost of post-surgical intervention care. Moreover, there is no effective treatment for post-surgical adhesions. This highlights the need for developing a new methodology with safety and efficacy for surgeons and patients undergoing a second cardiovascular surgery, regardless of the causal etiology.
Currently, venous graft occlusion after CABG is considered ineffective. In addition, patients with vein graft failure frequently have myocardial ischemia and angina, which are commonly refractory to medication, resulting in hospital readmission for refractory angina and heart failure11–14. These medical issues are of utmost importance; therefore, there is a need to identify new therapeutic modalities that are safe and efficacious for these patients.
Angiogenesis/neovascularization is the key mechanism for restoring the blood flow in the ischemic zone to improve the ischemia-associated left ventricular (LV) dysfunction and clinical outcomes15–20. However, endogenous angiogenesis is inadequate, indicating that exogenous angiogenesis may be essential in patients with ischemic cardiomyopathy (IC). Endothelial progenitor cells (EPCs) play a critical role in endothelial cell repairmen and angiogenesis17,20–26. However, direct implantation of EPCs into the ischemic area of the LV myocardium is superior to venous or intracoronary arterial injection for long retention/tracking of EPCs in ischemic areas for angiogenesis 22 . Thus, we hypothesized that direct implantation of EPCs into the LV myocardium would be an innovative strategy for patients with total vein grafting occlusion, angina, and heart failure owing to coronary artery obstruction that is refractory to conventional medical treatment. However, the first CABG procedure caused tissue/heart adhesions that hindered the second successful opening chest-wall surgery for the implantation of EPCs, resulting in an increasingly impermissible high risk of mortality and morbidity in these patients.
Extracorporeal shock waves (ECSWs) have anti-inflammatory, angiogenic, and tissue regeneration capacities27,28. Our studies and previous other studies have further elucidated that ECSW therapy effectively improves ischemia-associated organ dysfunction, mainly through angiogenesis15,16,19,29–31. However, it remains unclear whether the combined EPCs and ECSW therapy is more effective than either therapy alone. The minipig IC model was used for the study because the systolic and diastolic blood pressure, heart rate, LV end-diastolic and LV systolic dimensions, LV ejection fraction, ratio of heart weight to body weight, and coronary artery anatomy were all similar between minipigs and humans.
The ITRI Biofilm (a bioresorbable porous film) developed by the Industrial Technology Research Institute of Taiwan is a Food and Drug Administration (FDA)-recognized implantable biomaterial that exhibits optimal compatibility and has undergone biocompatibility testing. Porous films are composed of biocompatible biodegradable materials with specially designed drug carrier characteristics that can deliver hydrophobic, hydrophilic, and protein-based drugs. They are applicable for guided tissue regeneration in wound care and anti-adhesive wound care; that is, the ingrowth of scar tissue and subsequent adhesion control. Thus, this study was designed to test whether the ITRI Biofilm material could inhibit the formation of adhesions resulting from surgical procedures in the chest cavity. The cardinal finding in this study was that this ITRI Biofilm treatment ensured that the chest cavity was far away from the adhesion, and combination therapy of EPCs and ECSW was better than individual therapies for restoring ischemia-induced LV dysfunction.
Materials and Methods
Creation of an Animal Model of LV IC and Application of ITRI Biofilm on the Heart Surface Area
The methodologies are described in our previous study 15 . Briefly, a male minipig (Taitung Animal Propagation Station, Livestock Research Institute, Taiwan), weighting 16–18 kg was anesthetized by intramuscular injections of ketamine (15 mg/kg) and maintained by inhalation of 1.5% isoflurane during the procedures. The minipig was placed in the supine position on a warming pad at 37°C followed by endotracheal intubation with positive-pressure ventilation (180 ml/min) with room air using a ventilator (Fig. 4).
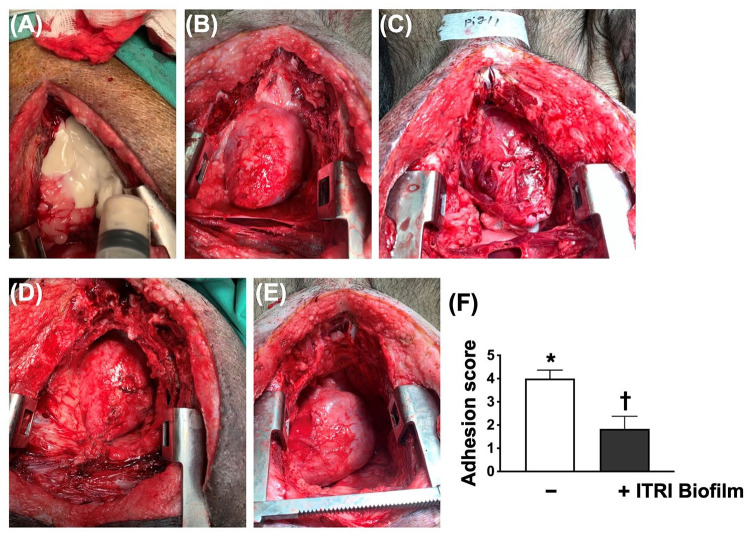
Figure 4.
Morphological features of adhesion and the adhesive score by day 60 after ischemic induction. (A) The application of ITRI Biofilm (white color) to the heart surface just after opening the chest wall. (B and C) The anatomical features of the two animals without ITRI Biofilm just after opening the chest wall. Remarkably, adhesive appearance was noted in these two animals. (D and E) The anatomical features of the two animals received ITRI Biofilm just after opening the chest wall. The adhesive appearance was remarkably attenuated in these two animals compared with (B) and (C) (without ITRI Biofilm treatment). (F) Adhesion score, * vs †, P < 0.0001 (n = 9 for each group).
Under sterile conditions, the heart was exposed through a mid-thoracotomy. After gentle removal of the pericardium, an ameroid constrictor was applied to the mid-left anterior descending artery (LAD) just distal to the first diagonal branch.
After applying ameroid constrictors to the LAD, the ITRI Biofilm (which was a gift for us from the Industrial Technology Research Institute of Taiwan) was immediately patched/covered on the surface of the whole heart.
The animals were then categorized into group 1 (sham-operated control [SC], just by opening the skin and muscle of chest wall, followed by closing the skin and muscle layers), group 2 (IC, including six animals without ITRI Biofilm use during the first opening chest-wall procedure), group 3 (IC + autologous EPCs [1.0 × 107 cells] implanted into LV ischemic area 3 months after ameroid constrictor over LAD induced IC, included three animals with and three animals without ITRI Biofilm use during the first opening chest-wall procedure), group 4 (IC + ECSW 0.12 mJ/mm2 at total 800 impulses/time, first applied to LV surface at four points during the second time of opening the chest wall, followed by the same dosage undergoing echo guide 1 week after the second opening chest-wall procedure; three animals with and three animals without receiving ITRI Biofilm treatment during the first opening chest-wall procedure) and group 5 (combined EPC + ECSW, included three animals with and three animals without ITRI Biofilm use during the first opening chest-wall procedure), respectively. Accordingly, nine animals with and nine animals without ITRI Biofilm treatment were included.
Functional Assessment of Left Ventricular Ejection Fraction by Echocardiography
The procedure and protocol have been described in our previous reports15,16,19. Briefly, transthoracic echocardiographic examination was conducted before ischemic myocardial induction and on days 90 and 180 after the procedure using an iE33 (Philips Medical System, Bothell, WA, USA) with S5 transducers. Recordings were stored for offline two-dimensional image analysis using computer software (Q-lab v9.0; Philips Medical Systems). The left ventricular ejection fraction (LVEF) was calculated as follows: LVEF (%) = [(LVEDd3–LVESd3)/LVEDd3] × 100. All measurements were performed by an animal cardiologist blinded to the treatment and non-treatment groups.
Application of ECSW to LV Myocardium of Minipigs
The procedure and protocol were based on those described in our previous reports15,16,19. By day 90 after the procedure, each minipig in groups 4 and 5 was anesthetized again without intubation. ECSW therapy was applied to these animals on days 90 and 97 after myocardial ischemia induction using a shockwave generator system (Storz Duolith, SD1 STORZ MEDICAL AG, Switzerland). Briefly, ECSW was applied once to each of the four ischemic zones at an intensity of 200 impulses at 0.12 mJ/mm2/zone at a total of 800 impulses/time after echocardiographic localization of the four ischemic zones of the LV (middle anterior, middle anteroseptal, middle inferior, and middle inferolateral segments) using the parasternal axis approach.
Methodology for Assessing the Adhesive Scores Over the Chest Cavity
The procedure and protocol for the assessment of the adhesive scores over the chest cavity were based on previous reports 32 . Surgical intervention was performed by a senior cardiovascular surgeon (CVS) who had performed CABG for >10 years. Meanwhile, another CVS who has conducted CABG for 25 years and has a license for heart transplantation was blinded to the study design and the treatment strategy to carefully assess the adhesive scores over the chest cavity using the following list (1), (2), and (3) protocols for identification of the variables and then summarized them on a computer for data analysis for objective observational purposes.
First, we carefully dissected and isolated retrosternal and intrapericardial adhesions. The adhesions were categorized into three groups:
- (1) Using cardiac regions directionally divided into A–D
- A) Behind the heart
- B) Left side of the heart
- C) Right side of the heart
- D) Front side of the heart and the area between the pericardium and sternum at the surgical site
- E) In front of the heart, the pericardium and sternum are located before the opening
- (2) Scoring for all regional assessments
- 0 = No sticking
- 1 = Evacuated adhesion that could be easily dissected by hand
- 2 = Uncharacteristic, can be easily dissected by hand
- 3 = Frequent, requiring sharp dissection, but was easy to dissect
- 4 = Rich, required sharp and appropriate dissection
- 5 = Rich, requires careful and clear dissection and is difficult to dissect.
- (3) Using an anatomical profile plane rating scale
- 0 = No adhesion, retained the anatomical plane
- 1 = The stickiness was thin and could be dissected by hand and the anatomy could be preserved
- 2 = Moderate adhesion could be dissected and the anatomical plane was preserved
- 3 = The adhesion is thick and could not be dissected and the anatomical plane disappeared
Western Blot Analysis
This procedure was based on previous reports15,16,19. Equal amounts (50 μg) of protein extract were loaded and separated by SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) using a 12% acrylamide gradient. After electrophoresis, the separated proteins were transferred onto a polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences). Nonspecific sites were blocked by incubating the membrane in a blocking buffer (5% nonfat dry milk in T-TBS [TBS containing 0.05% Tween 20]) overnight. Membranes were incubated with the indicated primary antibodies.
We used six animals per group for each experiment, indicating that six sets of samples were prepared for western blot analysis. In each set of samples, we utilized the antibody against β-actin for loading control and normalization. Therefore, the image of actin for normalization should be the same if the western blotting images for different proteins were derived from the same set of samples.
The Time Points of Ischemia Induction, Treatment, Second Surgical Intervention, and Echocardiographic Evaluation and the End of the Study Period
A stylized figure that outlines the overall study timeline with all relevant time points and interventions is provided in Supplementary Figure 3 for readers to easily and quickly realize the complexity of the cohort subgroups.
Data Analysis
Quantitative data were expressed as mean ± SD. Data analysis was performed using analysis of variance (ANOVA), followed by a Bonferroni multiple comparison post hoc test. Statistical analyses were performed using SAS statistical software for Windows version 8.2 (SAS Institute, Cary, NC, USA). Statistical significance was set at P < 0.05 significant.
Results
Impact of ECSW Therapy on Enhancing the EPC Angiogenesis and Cell Viability
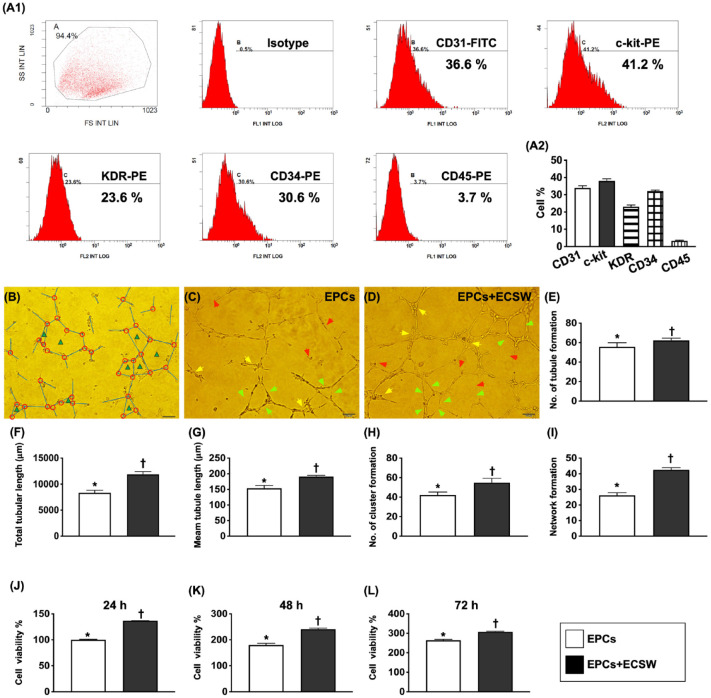
To elucidate whether ECSW would offer additional benefit for enhancing the angiogenesis, the bone marrow-derived endothelial progenitor cells (BMDEPCs) were pretreated by ECSW (0.12 mJ/mm2 with a total of 180 shots) before incubating for Matrigel assay (Fig. 1). The angiogenic capacity of the EPCs was significantly lower than in the EPC + ECSW group.
Figure 1.
Impact of ECSW therapy on enhancing the pig bone marrow-derived EPCs angiogenesis and cell viability. (A1) Flow cytometry analysis to identify endothelial progenitor cells (EPCs) and hematopoietic stem cells, including CD31+, c-Kit+, KDR+, CD34+, and CD45+ cells. (A2) Percentage expression in different stem cells. The most common cell types were c-Kit+ and CD31+ cells, followed by CD34+ and KDR+ cells. (B) Illustrating how to identify angiogenesis parameters, the number of tubules (blue color line), total tubular length (expressed as the length of the tubule, unit = μM), mean tubular length (expressed as mean length of the tubule, unit = μM), cluster formation (red color circle), and network formation (green color triangle), respectively. (C and D) Morphological features (200×) of the Matrigel assay to elucidate angiogenesis in EPCs (D) and EPCs + ECSW (E). The assessed parameters of angiogenesis included (1) tubular formation (red arrows), (2) cluster formation (yellow arrows), and (3) network formation (green). The scale bar in the right lower corner represents 50 µm. E) Analytical result of the number of tubules, * vs †, P < 0.001. (F) Analytical result of total tubular length, * vs †, P < 0.001. G) Analytical result of mean tubular length, * vs †, P < 0.001. (H) Analytical result of cluster formation, * vs †, P < 0.001. (I) Analytical result of network formation, * vs †, P < 0.001. (J) Cell viability at 24 h, * vs †, P < 0.0001. (K) Cell viability at 48 h, * vs †, P < 0.0001. L) Cell viability at 72 h, * vs †, P < 0.0001. n = 6 for each group. EPCs, endothelial progenitor cells; ECSW, extracorporeal shock wave.
In addition, the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay showed that cell viability at the time points of 24, 48, and 72 h was significantly higher in the EPC + ECSW group than in the EPCs alone, indicating that ECSW application to EPCs upregulated angiogenesis and cell proliferation.
Impact of ECSW Therapy on Enhancing the Mitochondrial Cytochrome C in Mitochondria and Migratory Ability of EPCs
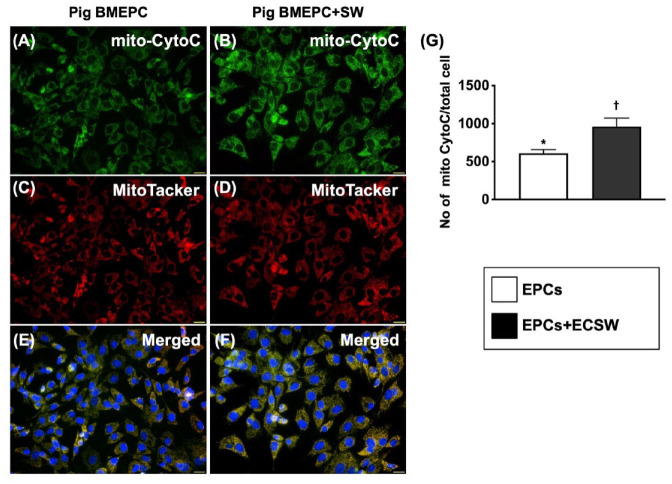
The number of mitochondrial cytochrome C in mitochondria (mit) and the relative mitDNA were significantly higher in the EPCs + ECSW group than in the EPC-only group (Fig. 2). In addition, this parameter was significantly increased in the EPC + ECSW group compared with the EPC-only group when examining EPC migratory capacity (Supplementary Figure 1). These data implied that ECSW treatment enhanced cell proliferation and mitochondrial duplication, resulting in increased ATP (adenosine triphosphate)/energy for EPC migration.
Figure 2.
Impact of ECSW therapy on enhancing cytochrome C expression in mitochondria of minipig bone marrow-derived nuEPCs. (A and B) Immunofluorescent (IF) microscopic findings (400x) for identifying the expression of mitochondrial cytochrome C in mitochondria (green color). (C and D) MitoTracker stain (400×) for identifying endogenous mitochondria (red color). (E and F) Merged picture (400×) of (A)–(D). The pink-yellow color indicated the endogenous mitochondrial cytochrome C. The blue color in (E) and (F) indicated the DAPI stain (400×) for identifying the nuclei of EPCs in two groups, respectively. Scale bars in right lower corner represent 20 µm. (E) Mitochondrial cytochrome C expression in endogenous mitochondria/high-power field in the cells, * vs †, P < 0.001. BMDEPCs = bone marrow-derived endothelial progenitor cells.
Time Courses of LVEF and Left Ventricular Fractional Shortening Evaluated by Transthoracic Echocardiography
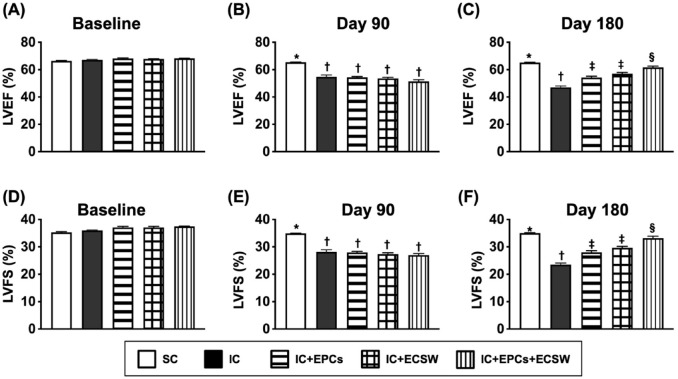
As expected, baseline LVEF did not differ among the five groups (Fig. 3). However, LVEF and left ventricular fractional shortening (LVFS) were significantly higher in group 1 than in groups 2–5 by day 90 after LV ischemia induction. Meanwhile, there was no difference among the latter four groups. On the other hand, these two parameters were highest in group 1, lowest in group 2, and significantly higher in group 1 than in groups 3 and 4 by day 180 after LV ischemia induction; however, they were similar between groups 3 and 4, suggesting that the combined EPCs and ECSW therapy was superior to either one in preserving heart function.
Figure 3.
Assessment of time courses of LVEF and LVFS by transthoracic echocardiography. (A) LVEF at baseline, P > 0.5. (B) LVEF by day 90 after LV ischemia induction, * vs †, P < 0.0001. (C) LVEF by day 180 after LV ischemia induction, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (D) LVFS at baseline, P > 0.5. (E) LVFS by day 90 after LV ischemia induction, * vs †, P < 0.0001. (F) LVFS by day 180 after LV ischemia induction, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. All statistical analyses were performed using one-way ANOVA, followed by Bonferroni multiple comparison post-hoc tests (n = 6 for each group). Symbols (*, †, ‡, and §) indicate significance (at 0.05 level). SC, sham-operated control; IC, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; LVFS, left ventricular fractional shortening.
Anatomical Features and Adhesive Severity of the Heart and Chest Cavity by Day 60 After the Second Opening Chest-Wall Procedure
After opening the chest wall, the degree of adhesion of the chest cavity and heart was remarkably serious in the case without ITRI Biofilm (Fig. 4). Thus, the adhesion score was significantly higher in animals without ITRI Biofilms than in those with ITRI Biofilms, indicating that ITRI Biofilm treatment prevented chest cavity adhesion after the second surgical intervention.
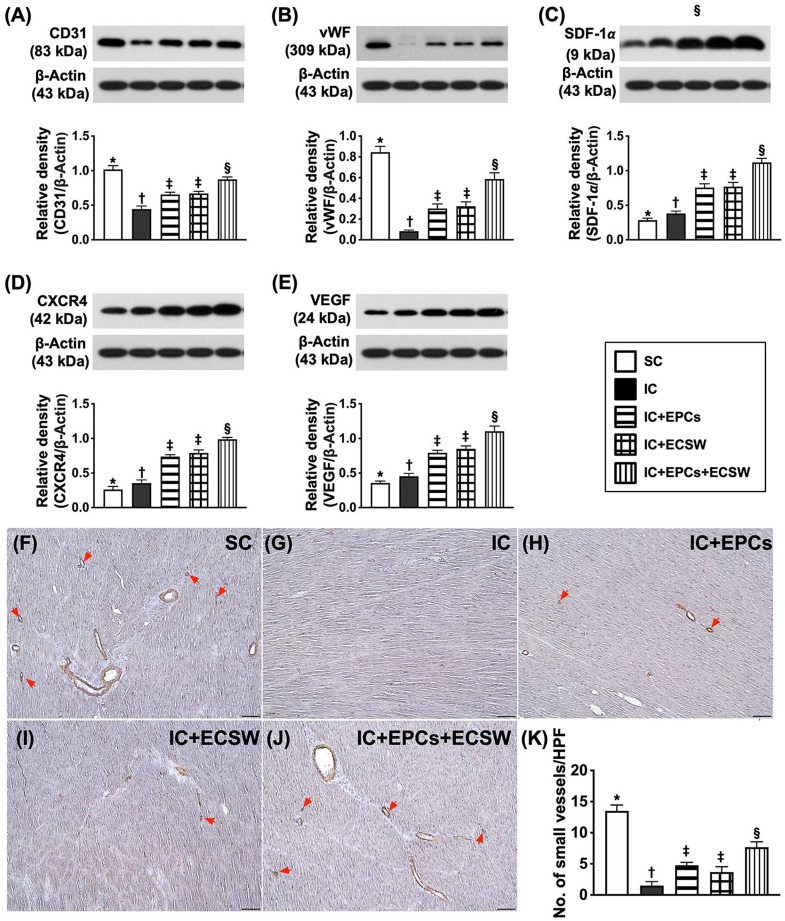
Protein Expressions of Angiogenesis Factors of LV Myocardium by Day 60 After Ischemic Induction
The protein expression of CD31 and vWF (von Willebrand factor) (two indicators of angiogenesis/integrity of endothelial cells) was the highest in group 1, lowest in group 2, and significantly higher in group 5 than in groups 3 and 4; however, there was no difference between groups 3 and 4 (Fig. 5). In addition, the protein expressions of stromal cell-derived factor (SDF)-1α, CXCR4, and VEGF (vascular endothelial growth factor) (three indicators of angiogenesis) were significantly and progressively increased from groups 1 to 5. Furthermore, the number of small vessels (defined as diameter ≤ 25 μM)15,16,19 (an indicator of the cellular level of angiogenesis) displayed an identical pattern of CD31.
Figure 5.
Protein expression of angiogenesis factors in LV myocardium by day 60 after ischemic induction. (A) Protein expression of CD31, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (B) Protein expression of von Willebrand factor (vWF), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (C) Protein expression of stromal cell-derived factor (SDF)-1α, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (D) Protein expression of CXCR4, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (E) Protein expression of vascular endothelial growth factor (VEGF), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (F–J) Illustrating the microscopic finding (200×) of alpha-smooth muscle actin (α-SMA) (gray color). (K) The number of small vessels (diameter ≤ 25 μM), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6 for each group). Symbols (*, †, ‡, and §) indicate significance (at 0.05 level). SC = sham-operated control; IC = ischemic cardiomyopathy.
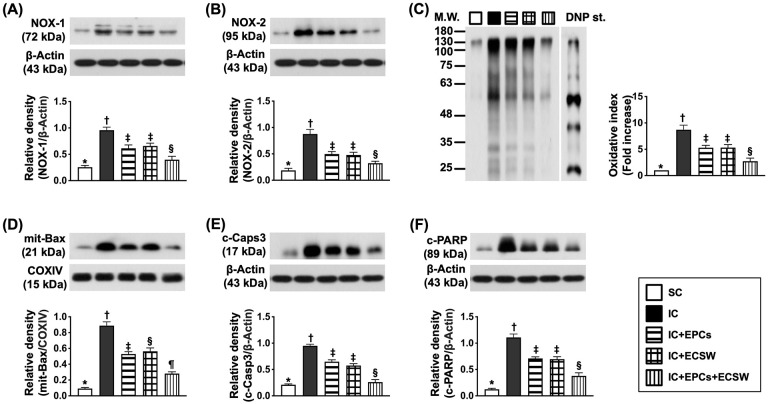
Protein Expressions of Apoptotic and Fibrotic Biomarkers of LV Myocardium by Day 60 After Ischemic Induction
The protein expression of NOX-1, NOX-2, and oxidized proteins (three indicators of oxidative stress) and the protein expression of mitochondrial Bax, cleaved caspase 3, and cleaved PARP (three indices of apoptosis) were the lowest in group 1, highest in group 2, and significantly higher in groups 3 and 4 than in group 5, but there was no difference between groups 3 and 4 (Fig. 6).
Figure 6.
Protein expression of oxidative stress, apoptotic, and fibrotic biomarkers in LV myocardium by day 60 after ischemic induction. (A) Protein expression of NOX-1, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (B) Protein expression of NOX-2, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (C) Oxidized protein expression, * vs other groups with different symbols (†, ‡, and §), P < 0.0001 (Note: the left and right lanes shown on the upper panel represent protein molecular weight marker and control oxidized molecular protein standard, respectively). M.W. = molecular weight; DNP = 1–3 dinitrophenylhydrazone. (D) Protein expression of mitochondrial (mit)-Bax, * vs other groups with different symbols (†, ‡, §, and ¶), P < 0.0001. (E) Protein expression of cleaved caspase 3 (c-Casp3), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (F) Protein expression of cleaved poly(ADP-ribose) polymerase (c-PARP), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6 for each group). Symbols (*, †, ‡, §, and ¶) indicate significance (at 0.05 level).
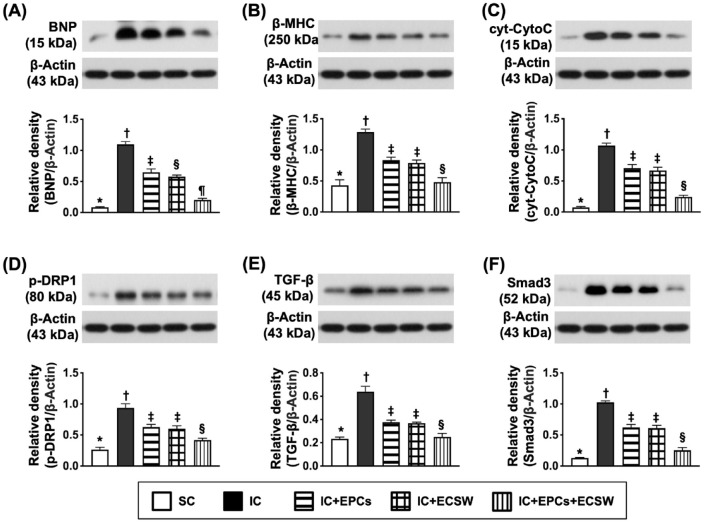
Protein Expression of Heart Failure, Mitochondrial-Damaged and Fibrotic Markers of LV Myocardium by Day 60 After Ischemic Induction
The protein expressions of BNP (brain natriuretic peptide) and β-MHC (beta myosin heavy chain) (two indices of pressure overload/heart failure) were lowest in group 1, highest in group 2, and significantly lower in group 5 than in groups 3 and 4 (Fig. 7). In addition, the protein expression levels of cytosolic cytochrome C and DRP1 (two indicators of mitochondrial damage) displayed similar BNP patterns between the two groups. Moreover, the protein expressions of transforming growth factor (TGF)-β and Smad3 (two indices of fibrosis) showed a similar pattern of heart failure markers among the groups.
Figure 7.
Protein expressions of heart failure, mitochondrial-damaged-, and fibrotic markers of LV myocardium by day 60 after ischemic induction. (A) Protein expression of brain natriuretic peptide (BNP), * vs other groups with different symbols (†, ‡, §, and ¶), P < 0.0001. (B) Protein expression of beta myosin heavy chain (β-MHC), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (C) Protein expression of cytosolic cytochrome C (cyt-CytoC), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (D) Protein expression of phosphorylated dynamin-related protein 1 (p-DRP1), * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (E) Protein expression of transforming growth factor (TGF)-β, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. (F) Protein expression of Smad3, * vs other groups with different symbols (†, ‡, and §), P < 0.0001. All statistical analyses were performed by one-way ANOVA, followed by Bonferroni multiple comparison post hoc test (n = 6 for each group). Symbols (*, †, ‡, §, and ¶) indicate significance (at the 0.05 level).
Histopathological Finding of LV Myocardium and Protein Level of Fibrosis by Day 60 After Ischemic Induction
Masson’s trichrome stain demonstrated that the fibrotic area and the cellular level of γ-H2AX (an indicator of DANA-damaged biomarker in LV myocardium) were highest in group 2, lowest in group 1, and significantly lower in group 5 than in groups 3 and 4 (Supplementary Figure 2).
Discussion
Our previous animal models and clinical studies demonstrated that ECSW therapy remarkably improved ischemia-related organ dysfunction and outcomes15,16,19,29–31,33–35. Consistently, our clinical trials have previously demonstrated that intracoronary administration of EPCs effectively improves LVEF, and short-term and long-term outcomes. The results of these animal studies and clinical trials15,16,19,29–31,33–35 highlight that EPCs and ECSW may be new tools for the treatment of ischemic heart disease, especially in patients with diffuse CAD (defined as end-stage CAD) who are non-candidates for either catheter-based coronary artery intervention or CABG. The essential finding of this study is that LV function was significantly preserved in IC animals treated with EPCs or ECSW. Importantly, EPC therapy was comparable with ECSW therapy in improving ischemia-related LV dysfunction. Thus, our findings are consistent with those of previous studies15,16,19,29–31,33–35. The most novel finding in this study was that the combined EPC and ECSW treatment was superior to ECSW alone in improving ischemia-related LV dysfunction. Therefore, our findings extend those of the previous studies15,16,19,29–31,33–35.
An increase in LV fibrosis is a strong indicator of increasing fibroblast number and enhanced cardiomyocyte death, resulting in LV mass reduction and LV chamber dilatation as a consequence of increasing LV remodeling and heart failure. Thus, LV fibrosis and remodeling are strong predictors of unfavorable outcomes18,19,36–38. In this study, we observed that the fibrotic area and DNA-damaged markers were remarkably increased in IC animals compared with SC animals when looking at the histopathological findings. In addition, the protein levels of fibrosis biomarkers (TGF-β and Smad3) were consistently expressed as the pathological fining of fibrosis among the groups. These findings strengthen previous research18,19,36–38, and could explain (at least in part) why LVEF was reduced in the IC group compared with the SC group. Of particular importance was that these parameters were substantially reversed in IC animals treated by EPCs or ECSW and further substantially reversed in those of IC animals treated by a combination of EPCs and ECSW, highlighting that this combination regimen offered an additional benefit in the setting of IC.
BNP and β-MHC are two well-known heart failure/pressure-overload biomarkers 39 . A cardinal finding in this study was that these two aforementioned biomarkers and cardiomyocyte size increased in IC animals compared with those in SC animals. These findings were in agreement with those of Masson’s trichrome staining (the fibrotic area). In addition, the protein levels of apoptosis and mitochondrial damage markers were markedly upregulated in the LV myocardium of the IS group compared with those in the SC group. These aforementioned molecular–cellular perturbations could partially explain why heart function was reduced in IC animals compared with SC animals. The distinctive findings in this study were that these molecular–cellular perturbations were substantially suppressed by EPCs or ECSW treatment, and more substantially suppressed by the combined EPCs and ECSW treatment. This explains why LVEF was preserved in IC animals after EPC–EPCs–ECSW treatment.
Readers would be keen to explore the underlying mechanisms involved in improving LVEF. Our previous studies15–17,19–21,29–31,33,34 demonstrate that EPCs and ECSW treatments improve heart function mainly through the upregulation of angiogenesis, cell proliferation, migratory capacity, anti-inflammation, and the preservation of mitochondrial integrity. Our in vitro studies demonstrated that ECSW-treated EPCs augmented angiogenesis, cell viability, EPC migratory ability, and mitochondrial duplication. Of fundamental importance was that our in vivo studies showed that the angiogenesis biomarkers were markedly upregulated using EPC–ECSW treatment, suggesting that our in vitro studies (in addition to supporting the in vivo study) could explain why LV function was preserved in IC animals after receiving—the EPCs–ECSW treatment.
The second cardio-thoracic surgery4–10 inevitably causes unacceptably high morbidity and mortality7–10 and an extremely high cost of post-surgical intervention care. An intriguing finding in this study was that the adhesions in the chest cavity and myocardium were substantially reduced in these animals compared with those without ITRI Biofilm. This finding highlights that innovative management should be encouraged for patients who receive second chest-wall opening surgery, regardless of the disease entity.
Study Limitation
Our study has several limitations. First, the direct implantation of EPCs into the LV myocardium is less practical than intracoronary administration. Second, the animal model was an IC rather acute myocardial infarction (AMI) setting. Therefore, we do not know whether this treatment strategy can be extrapolated to patients with AMI.
Third, the sample size of this study was relatively small, which may have distorted the results of the statistical analysis. Thus, we could not completely exclude statistical bias in this study. Fourth, our study did not include a positive-control cohort that might affect the true impact of ITRI Biofilms on the prevention of adhesion. Finally, while interpreting our findings, the authors should be cautious about the following limitations of our scoring specific for adhesions: (1) not all adhesions will be clinically significant and (2) the process of opening the chest would disrupt the anatomy of the adhesions, resulting in impairment of one’s ability to gauge the severity.
In conclusion, the results of this study demonstrate that EPCs–ECSW therapy effectively preserved IC-related LV dysfunction, predominantly via angiogenesis. The innovative results of this study encourage us to apply this modality in clinical practice for patients with IC with severe diffuse disease17,21 when they undergoing CABG without unethical issues.
Supplemental Material
Supplemental material, sj-jpg-1-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-2-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-3-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-4-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Footnotes
Authors’ Contributions: Hon-Kan Yip and Jun Guo designed and directed the project; Jiunn-Jye Sheu, Yi-Ting Wang, and Yi-Ling Chen performed the experiments; Jui-Ning Yeh analyzed spectra; Pei-Hsun Sung and John Y. Chiang performed the calculations and aided in interpreting the results.
Availability of Data and Material: All data generated and/or analyzed during this study are included in this published article.
Ethical Approval: All animal experimental protocols and procedures were approved by the Institute of Animal Care and Use Committee at Kaohsiung Chang Gung Memorial Hospital (Affidavit of Approval of Animal Use Protocol No. 2018092003) and performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article, and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a project grant from Chang Gung Memorial Hospital, Chang Gung University [CMRPG8I0241].
ORCID iDs: Hon-Kan Yip  https://orcid.org/0000-0002-6305-5717
https://orcid.org/0000-0002-6305-5717
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Athappan G, Patvardhan E, Tuzcu ME, Ellis S, Whitlow P, Kapadia SR. Left main coronary artery stenosis: a meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc Interv. 2013;6(12):1219–30. [DOI] [PubMed] [Google Scholar]
- 2. Palmerini T, Serruys P, Kappetein AP, Genereux P, Riva DD, Reggiani LB, Christiansen EH, Holm NR, Thuesen L, Makikallio T, Morice MC, et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J. 2017;190:54–63. [DOI] [PubMed] [Google Scholar]
- 3. Nerlekar N, Ha FJ, Verma KP, Bennett MR, Cameron JD, Meredith IT, Brown AJ. Percutaneous coronary intervention using drug-eluting stents versus coronary artery bypass grafting for unprotected left main coronary artery stenosis. Circ Cardiovasc Interv. 2016;9(12):e004729. [DOI] [PubMed] [Google Scholar]
- 4. Launcelott S, Ouzounian M, Buth KJ, Légaré JF. Predicting in-hospital mortality after redo cardiac operations: development of a preoperative scorecard. Ann Thorac Surg. 2012;94(3):778–84. [DOI] [PubMed] [Google Scholar]
- 5. LaPar DJ, Ailawadi G, Harris DA, Hajzus VA, Lau CL, Kern JA, Kron IL. A protocol-driven approach to cardiac reoperation reduces mortality and cardiac injury at the time of resternotomy. Ann Thorac Surg. 2013;96(3):865–70; discussion 870. [DOI] [PubMed] [Google Scholar]
- 6. Imran Hamid U, Digney R, Soo L, Leung S, Graham AN. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg. 2015;47(5):819–23. [DOI] [PubMed] [Google Scholar]
- 7. Goodwin AT, Ooi A, Kitcat J, Nashef SA. Outcomes in emergency redo cardiac surgery: cost, benefit and risk assessment. Interact Cardiovasc Thorac Surg. 2003;2(3):227–30. [DOI] [PubMed] [Google Scholar]
- 8. Kaneko T, Loberman D, Gosev I, Rassam F, McGurk S, Leacche M, Cohn L. Reoperative aortic valve replacement in the octogenarians-minimally invasive technique in the era of transcatheter valve replacement. J Thorac Cardiovasc Surg. 2014;147(1):155–62. [DOI] [PubMed] [Google Scholar]
- 9. Kirmani BH, Brazier A, Sriskandarajah S, Azzam R, Keenan DJ. A meta-analysis of computerized tomography scan for reducing complications following repeat sternotomy for cardiac surgery. Interact Cardiovasc Thorac Surg. 2016;22(4):472–79. [DOI] [PubMed] [Google Scholar]
- 10. Ejiofor JI, Ramirez-Del Val F, Nohria A, Norman A, McGurk S, Aranki SF, Shekar P, Cohn LH, Kaneko T. The risk of reoperative cardiac surgery in radiation-induced valvular disease. J Thorac Cardiovasc Surg. 2017;154(6):1883–95. [DOI] [PubMed] [Google Scholar]
- 11. Gegouskov V, Tochtermann U, Badowski-Zyla D, Thomas G, Hagl S, Osswald B. Long-term results after coronary artery reconstructive surgery. Thorac Cardiovasc Surg. 2007;55(5):293–97. [DOI] [PubMed] [Google Scholar]
- 12. Lytle BW, Loop FD, Taylor PC, Goormastic M, Stewart RW, Novoa R, McCarthy P, Cosgrove DM. The effect of coronary reoperation on the survival of patients with stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg. 1993;105(4):605–12; discussion 612–14. [PubMed] [Google Scholar]
- 13. Hess CN, Lopes RD, Gibson CM, Hager R, Wojdyla DM, Englum BR, Mack MJ, Califf RM, Kouchoukos NT, Peterson ED, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation. 2014;130(17):1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harskamp RE, Lopes RD, Baisden CE, de Winter RJ, Alexander JH. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg. 2013;257(5):824–33. [DOI] [PubMed] [Google Scholar]
- 15. Fu M, Sun CK, Lin YC, Wang CJ, Wu CJ, Ko SF, Chua S, Sheu JJ, Chiang CH, Shao PL, Leu S, et al. Extracorporeal shock wave therapy reverses ischemia-related left ventricular dysfunction and remodeling: molecular-cellular and functional assessment. PLoS ONE. 2011;6(9):e24342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sheu JJ, Lee FY, Yuen CM, Chen YL, Huang TH, Chua S, Chen YL, Chen CH, Chai HT, Sung PH, Chang HW, et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69–83. [DOI] [PubMed] [Google Scholar]
- 17. Lee FY, Chen YL, Sung PH, Ma MC, Pei SN, Wu CJ, Yang CH, Fu M, Ko SF, Leu S, Yip HK. Intracoronary transfusion of circulation-derived CD34+ cells improves left ventricular function in patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention. Crit Care Med. 2015;43(10):2117–32. [DOI] [PubMed] [Google Scholar]
- 18. Chen YL, Sun CK, Tsai TH, Chang LT, Leu S, Zhen YY, Sheu JJ, Chua S, Yeh KH, Lu HI, Chang HW, et al. Adipose-derived mesenchymal stem cells embedded in platelet-rich fibrin scaffolds promote angiogenesis, preserve heart function, and reduce left ventricular remodeling in rat acute myocardial infarction. Am J Transl Res. 2015;7(5):781–803. [PMC free article] [PubMed] [Google Scholar]
- 19. Sheu JJ, Ali HEE, Cheng BC, Chiang HJ, Sung PH, Chen KH, Yang CC, Chen YT, Chiang JY, Lin PY, Chua S, et al. Extracorporeal shock wave treatment attenuated left ventricular dysfunction and remodeling in mini-pig with cardiorenal syndrome. Oncotarget. 2017;8(33):54747–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang TH, Sun CK, Chen YL, Sung PH, Chu CH, Lee MS, Lin YP, Yip HK, Lee FY. Correlation between therapeutic efficacy of CD34(+) cell treatment and directed in vivo angiogenesis in patients with end-stage diffuse coronary artery disease. Stem Cells Int. 2018;2018:9591421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sung PH, Lee FY, Tong MS, Chiang JY, Pei SN, Ma MC, Li YC, Chen YL, Wu CJ, Sheu JJ, Lee MS, et al. The five-year clinical and angiographic follow-up outcomes of intracoronary transfusion of circulation-derived CD34+ cells for patients with end-stage diffuse coronary artery disease unsuitable for coronary intervention-phase I clinical trial. Crit Care Med. 2018;46(5):e411–18. [DOI] [PubMed] [Google Scholar]
- 22. Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackie AR, Losordo DW. CD34-positive stem cells: in the treatment of heart and vascular disease in human beings. Tex Heart Inst J. 2011;38(5):474–85. [PMC free article] [PubMed] [Google Scholar]
- 24. Henry TD, Schaer GL, Traverse JH, Povsic TJ, Davidson C, Lee JS, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, et al. Autologous CD34(+) cell therapy for refractory angina: 2-year outcomes from the ACT34-CMI study. Cell Transplant. 2016;25(9):1701–11. [DOI] [PubMed] [Google Scholar]
- 25. Mathiyalagan P, Liang Y, Kim D, Misener S, Thorne T, Kamide CE, Klyachko E, Losordo DW, Hajjar RJ, Sahoo S. Angiogenic mechanisms of human CD34(+) stem cell exosomes in the repair of ischemic hindlimb. Circ Res. 2017;120(9):1466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry TD, Losordo DW, Traverse JH, Schatz RA, Jolicoeur EM, Schaer GL, Clare R, Chiswell K, White CJ, Fortuin FD, Kereiakes DJ, et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: a patient-level pooled analysis of randomized double-blinded trials. Eur Heart J. 2018;39(23):2208–16. [DOI] [PubMed] [Google Scholar]
- 27. Ma HZ, Zeng BF, Li XL. Upregulation of VEGF in subchondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcif Tissue Int. 2007;81(2):124–31. [DOI] [PubMed] [Google Scholar]
- 28. Wang CJ, Wang FS, Yang KD, Weng LH, Hsu CC, Huang CS, Yang LC. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003;21(6):984–89. [DOI] [PubMed] [Google Scholar]
- 29. Yuen CM, Chung SY, Tsai TH, Sung PH, Huang TH, Chen YL, Chen YL, Chai HT, Zhen YY, Chang MW, Wang CJ, et al. Extracorporeal shock wave effectively attenuates brain infarct volume and improves neurological function in rat after acute ischemic stroke. Am J Transl Res. 2015;7(6):976–94. [PMC free article] [PubMed] [Google Scholar]
- 30. Chai HT, Chen KH, Wallace CG, Chen CH, Sung PH, Chen YL, Yuen CM, Shao PL, Sun CK, Chang HW, Wang CJ, et al. Extracorporeal shock wave therapy effectively protects brain against chronic cerebral hypo-perfusion-induced neuropathological changes. Am J Transl Res. 2017;9(11):5074–93. [PMC free article] [PubMed] [Google Scholar]
- 31. Yin TC, Wu RW, Sheu JJ, Sung PH, Chen KH, Chiang JY, Hsueh SK, Chung WJ, Lin PY, Hsu SL, Chen CC, et al. Combined therapy with extracorporeal shock wave and adipose-derived mesenchymal stem cells remarkably improved acute ischemia-reperfusion injury of quadriceps muscle. Oxid Med Cell Longev. 2018;2018:6012636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iliopoulos J, Cornwall GB, Evans RO, Manganas C, Thomas KA, Newman DC, Walsh WR. Evaluation of a bioabsorable polylactide film in a large animal model for the reduction of retrosternal adhesions. J Surg Res. 2004;118(2):144–53. [DOI] [PubMed] [Google Scholar]
- 33. Sung PH, Fu M, Chiang HJ, Huang CR, Chu CH, Lee MS, Yip HK. Reduced effects of cardiac extracorporeal shock wave therapy on angiogenesis and myocardial function recovery in patients with end-stage coronary artery and renal diseases. Biomed J. 2021;44(6, Suppl 2):S201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sung PH, Li YC, Lee MS, Hsiao HY, Ma MC, Pei SN, Chiang HJ, Lee FY, Yip HK. Intracoronary injection of autologous CD34+ cells improves one-year left ventricular systolic function in patients with diffuse coronary artery disease and preserved cardiac performance-a randomized, open-label, controlled phase II clinical trial. J Clin Med. 2020;9(4):1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sung PH, Chiang HJ, Li YC, Chiang JY, Chu CH, Shao PL, Lee FY, Lee MS, Yip HK. Baseline factors identified for the prediction of good responders in patients with end-stage diffuse coronary artery disease undergoing intracoronary CD34+ cell therapy. Stem Cell Res Ther. 2020;11(1):324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang J, Yang F, Wan K, Mui D, Han Y, Chen Y. Left ventricular midwall fibrosis as a predictor of sudden cardiac death in non-ischaemic dilated cardiomyopathy: a meta-analysis. ESC Heart Fail. 2020;7(5):2184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu HY, Yang ZG, Zhang Y, Peng WL, Xia CC, Li ZL, He Y, Xu R, Rao L, Peng Y, Li YM, et al. Prognostic value of heart failure in hemodialysis-dependent end-stage renal disease patients with myocardial fibrosis quantification by extracellular volume on cardiac magnetic resonance imaging. BMC Cardiovasc Disord. 2020;20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Pezel T, Viallon M, Croisille P, Sebbag L, Bochaton T, Garot J, Lima JAC, Mewton N. Imaging interstitial fibrosis, left ventricular remodeling, and function in stage A and B heart failure. JACC Cardiovasc Imaging. 2021;14(5):1038–52. [DOI] [PubMed] [Google Scholar]
- 39. Chen YL, Chung SY, Chai HT, Chen CH, Liu CF, Chen YL, Huang TH, Zhen YY, Sung PH, Sun CK, Chua S, et al. Early administration of carvedilol protected against doxorubicin-induced cardiomyopathy. J Pharmacol Exp Ther. 2015;355(3):516–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-2-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-3-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation
Supplemental material, sj-jpg-4-cll-10.1177_09636897241253144 for ITRI Biofilm Prevented Thoracic Adhesion in Pigs That Received Myocardial Ischemic Induction Treated by Myocardial Implantation of EPCs and ECSW Treatment by Jiunn-Jye Sheu, Jui-Ning Yeh, Pei-Hsun Sung, John Y. Chiang, Yi-Ling Chen, Yi-Ting Wang, Hon-Kan Yip and Jun Guo in Cell Transplantation