Shareable abstract
The Lung Flute ECO, a self-powered, low-cost oscillatory positive expiratory pressure device, assisted people with presumptive tuberculosis to produce an adequate sputum volume for diagnostic testing and was well tolerated https://bit.ly/47sDq8W
To the Editor:
Tuberculosis (TB) mainly affects people in resource-limited settings, and in 2022 an estimated 3.1 million of the total 10.6 million people with TB were not diagnosed or linked to treatment for the disease [1]. While multiple nonsputum diagnostics are in development and may help close this gap in care, most TB diagnostic testing is currently still based on sputum, and many people with presumptive TB have difficulties producing adequate sputum for diagnostic testing [2–4]. In high-resource settings, sputum induction with nebulised hypertonic saline is often routinely used to assist people to produce sputum for diagnostic testing for TB [5]. However, sputum induction is an invasive and resource-intensive technique, and it has not been adopted for routine use in many resource-limited settings.
The Lung Flute (Medical Acoustics, Santa Monica, CA, USA) is an oscillatory positive expiratory pressure (OPEP) device that has been evaluated to assist people to produce sputum for therapeutic and diagnostic applications [6–8]. The Lung Flute ECO (Acoustic Innovations, Tokyo, Japan) was developed in 2018 as a low-cost (<USD 1) alternative to the Lung Flute. Both devices are ∼30 cm long, thin rectangular tubes with a plastic reed inside; the tube of the Lung Flute ECO is made of paper, while the tube of the original Lung Flute is plastic. The user blows into the end where the reed is attached, which produces sound waves (16–25 Hz). The device operates on the principle that these sound waves induce vibrations in the lungs that reduce mucus viscosity and loosen mucus from the walls of the lung, enabling the user to produce sputum more readily. As a low-cost, self-powered, highly portable device, the Lung Flute ECO has potential value at point-of-need for applications requiring the collection of sputum.
We aimed to assess whether use of the Lung Flute ECO assists in sputum collection and is tolerable among people to be evaluated for TB, as compared to video instruction without device assistance, in this, the first phase of a two-phase trial of this device. This randomised crossover trial consisted of two intervention periods separated by a washout period of ≥2 h and at most 2 weeks. Each participant received both interventions, following 1:1 randomisation either to sequence 1 (A–B) or sequence 2 (B–A). In intervention A, the comparator, participants received 3 min of video instruction [9] prior to attempting to collect sputum. In intervention B, participants used the Lung Flute ECO, following video instruction on how to use the device, prior to attempting to collect sputum. Sequences were assigned in randomly permuted blocks of 50, stratified by site, by a person not involved in the interventions using a random sequence generator software (https://www.studyrandomizer.com) and sealed in sequentially numbered opaque envelopes.
People presenting at eight health facilities in Cameroon were systematically screened and were considered eligible if they were aged ≥15 years, reported at least one TB symptom and were able to provide informed consent. People were excluded if currently on TB treatment or contraindicated for sputum induction.
This study was approved by the institutional review boards of the Cameroon Baptist Convention Health Board (IRB2022–02), ITM Antwerp (1546/21) and the University Hospital of Antwerp (2022–3088). Participants aged >20 years provided written informed consent; participants aged 15–20 years provided assent and a parent or guardian provided written informed consent.
The primary outcome was the proportion of people who produced >1 mL of sputum, as recommended for diagnostic testing with the Xpert MTB/RIF Ultra assay [10]. Sputum collected in a 50-mL conical tube was photographed in a custom-built box to standardise the angle, lighting and distance for the photo, which was subsequently read by a blinded external reader with >7 years’ expertise in TB laboratory methods. Secondary outcomes included frequency, severity and time to resolution of new symptoms and symptom aggravation related to sputum collection attempts [11] and device tolerability as reported by participants. From sample size calculations, 938 participants (469 per sequence) were required to provide 80% power to detect an absolute 5% difference in the proportion of people who produced >1 mL sputum, with an estimated standard deviation of paired differences of 0.54, as obtained from a feasibility study including 237 participants (unpublished data). To account for screen failures and early exclusions, the enrolment target was 1250.
Analyses of the primary outcome were conducted for participants who were randomised, attempted to produce sputum twice and had complete data available. Analyses of the secondary outcomes were conducted for all participants who were randomised (intention-to-treat). The analysis of the primary outcome was based on the subject-specific logistic regression model described by Mainland and Gart, which takes period of intervention into account [12, 13]. Analyses were conducted with R version 4.2.2 (www.r-project.org).
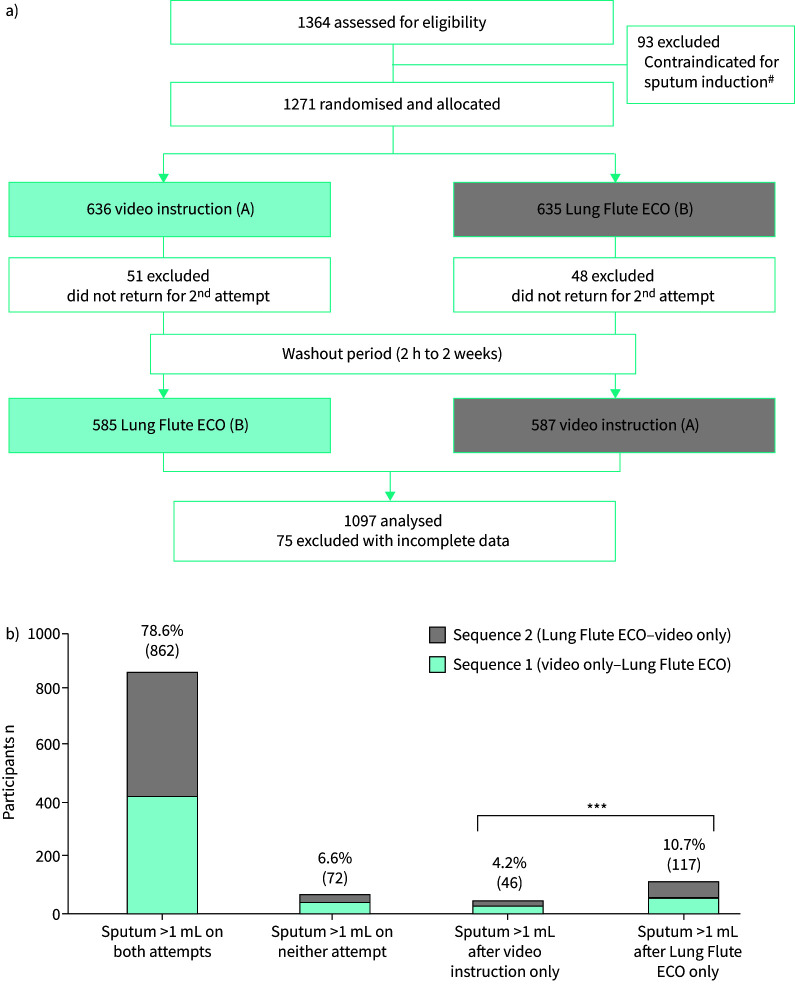
Results for 1097 people enrolled from 8 March to 31 May 2022 were included in the analysis of the primary outcome (figure 1a). Participant characteristics were similar by sequence; the median age was 40 years (interquartile range 29–55 years); 53% were female; 24% were known HIV-positive; 11% were hospitalised; 12% had any history of TB treatment; 80% participated in both interventions on the same day. At baseline, participants reported having any cough (80%), fever (57%), night sweats (32%) and/or weight loss (63%).
FIGURE 1.
a) Study flow, with two-period crossover design. In sequence A–B, participants first received video instruction only (intervention A), followed by use of the Lung Flute ECO (intervention B). In sequence B–A, participants received the two interventions in the opposite order. b) Proportions of participants for primary outcome of whether participant produced >1 mL of sputum, by sequence, for each combination of outcomes on the two sputum collection attempts. #: reasons for exclusion: vomiting (n=36), haemoptysis (n=18), asthma with recent acute exacerbation (n=15), nausea grade 3 or 4 (n=12), dyspnoea grade 3 or 4 (n=12), other (n=27); some reasons overlap. ***: p<0.001.
Among people who produced sputum on only one attempt, a sputum volume of >1 mL was obtained from more participants after use of the Lung Flute ECO versus after video instruction only (117 (10.7%) out of 1097 versus 46 (4.2%) out of 1097; OR 2.6, 95% CI 1.9–3.7; p<0.001) (figure 1b). Among the 212 people who reported only one symptom, >1 mL sputum was collected from 13.2% (28 out of 212) of participants after use of the Lung Flute ECO alone as compared to 4.7% after video instruction alone (OR 3.0, 95% 1.4–6.5; p=0.006); overall 74.1% (157 out of 212) produced >1 mL sputum by both methods.
Satisfaction with use of the Lung Flute ECO was generally high: 99% of participants (1165 out of 1198) said they understood the instructions, 97% reported hearing the reed flutter, and 90% found it comfortable to use. Overall, 7.1% (85 out of 1198) of participants reported new or aggravated symptoms after use of the Lung Flute ECO versus 3.8% (45 out of 1200) after video instruction alone. The most common symptoms reported following use of the Lung Flute ECO were chest tightness (1.8%), headache (1.3%), dizziness (1.8%) and sore throat (1.3%). All new events were grade 1 and all aggravation events increased from grade from 1 to 2; all resolved on their own within 30 min.
Here we found that use of the Lung Flute ECO device assisted in collection of sputum with volume >1 mL among people to be evaluated for TB. Of the people who did not produce >1 mL sputum after video instruction alone, 62% (117 out of 189) were able to produce >1 mL sputum after use of the Lung Flute ECO. This finding is consistent with increased sputum volume reported after use of OPEP devices for therapeutic purposes among people with chronic conditions [14]. A strength of this study is the crossover design, where each participant served as their own control [15].
While participants in this study were randomised to the order of interventions, and the primary outcome of sputum volume was read by a blinded reader, neither the participants nor the healthcare providers were blinded to the type of intervention administered.
In conclusion, use of the Lung Flute ECO was well tolerated and assisted people to be evaluated for TB to produce an adequate volume of sputum (>1 mL) for TB diagnostic testing. These initial results support further investigation of the performance of the Lung Flute ECO to assist in sputum collection and TB detection in different and larger populations.
Acknowledgements
We thank the study participants and their families for their participation in this study. We also thank the National TB Program and all the members of the teams at the study sites for their contributions to this study.
Provenance: Submitted article, peer reviewed.
Lung Flute ECO Trial Consortium: Michelle Barbara Ngono (Meskine Baptist Hospital, Maroua, Cameroon); Guy Zero Molesa (Mboppi Baptist Hospital, Douala, Cameroon); Jeanne Rachel Leslie Ngo Mode (Hopital Notre Dame des Apotres de Djamboutou, Garoua, Cameroon); Eldred Mabughe Chongwain (Nkwen Baptist Hospital, Bamenda, Cameroon); Nina Lubeka (Bonaberi Baptist Hospital, Bonaberi, Cameroon); Hibbert Cyrille Houpa (CMA Founangue, Maroua, Cameroon); Adamou Dodo Balkissou (Garoua Regional Hospital, Garoua, Cameroon); Kwemu Njakoi Clinton (Baptist Hospital Mutengene, Mutengene, Cameroon); Elie Olivier Habaga (CSPP Djarengol Kodeck); Angela Neh, Fuh Boris Nforbi, Frinwie Mary-Carmel Ndifor, Claudia Asanji, Pascale Sandrine Bih, Nguifu Kelly Ngwafung and Miranda Ngumbusi Tumanjong (Center for Health Promotion and Research); Liliane Keugni and Hamada Beloko (Tuberculosis Reference Laboratory Douala, Douala, Cameroon); Daniel Tollo Tollo, Theo Mpaba Minkat and Henri Manga (National TB Program).
This study is registered at https://pactr.samrc.ac.za/ with identifier number PACTR202301719591790. All data used for the analysis are available in the Dryad Data Repository (https://doi.org/10.5061/dryad.b8gtht7m7) .
Conflict of interest: All authors have nothing to disclose.
Support statement: This work was funded by the Global Health Innovative Technology Fund (grant number G2021-114). Funding information for this article has been deposited with the Crossref Funder Registry.
Ethics approval: This study was approved by the institutional review board of the Cameroon Baptist Convention Health Board (IRB2022-02), the institutional review board of the ITM Antwerp (1546/21) and the University Hospital of Antwerp (2022-3088).
Contributor Information
Collaborators: Michelle Barbara Ngono, Guy Zero Molesa, Jeanne Rachel Leslie Ngo Mode, Eldred Mabughe Chongwain, Nina Lubeka, Houpa Hibbert Cyrille, Balkissou Nyako Wadjore, Kwemu Njakoi Clinton, Olivier Elie Habaga, Angela Neh, Fuh Boris Nforbi, Frinwie Mary-Carmel Ndifor, Claudia Asanji, Pascale Sandrine, Nguifu Ngwafung Kelly, Miranda Ngumbusi Tumanjong, Liliane Keugni, Hamada Beloko, Tollo Tollo Daniel, Theo Mpaba Minkat, and Henri Manga
References
- 1.World Health Organization (WHO) . Global Tuberculosis Report 2023. Geneva, WHO, 2023. www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023. [Google Scholar]
- 2.Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, et al. . Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? EBioMedicine 2022; 78: 103939. doi: 10.1016/j.ebiom.2022.103939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) . WHO Consolidated Guidelines on Tuberculosis. Module 3: Diagnosis – Rapid Diagnostics for Tuberculosis Detection 2021 Update. 2021. www.who.int/publications/i/item/9789240029415.
- 4.Datta S, Shah L, Gilman RH, et al. . Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. Lancet Glob Health 2017; 5: e760–e771. doi: 10.1016/S2214-109X(17)30201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewinsohn DM, Leonard MK, LoBue PA, et al. . Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin Infect Dis 2017; 64: e1–e33. doi: 10.1093/cid/ciw694 [DOI] [PubMed] [Google Scholar]
- 6.Sakashita K, Fujita A, Takamori M, et al. . Efficiency of the Lung Flute for sputum induction in patients with presumed pulmonary tuberculosis. Clin Respir J 2018; 12: 1503–1509. doi: 10.1111/crj.12697 [DOI] [PubMed] [Google Scholar]
- 7.Su J, Anjuman N, Guarnera MA, et al. . Analysis of Lung Flute-collected sputum for lung cancer diagnosis. Biomark Insights 2015; 10: 55–61. doi: 10.4137/BMI.S26883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization (WHO) . Compendium of Innovative Health Technologies for Low-Resource Settings 2011–2014: Assistive Devices, eHealth Solutions, Medical Devices, Other Technologies, Technologies for Outbreaks. 2015. https://apps.who.int/iris/handle/10665/202537.
- 9.IRD Global . “Good Sputum, Better Diagnosis” – (English) Instructional Video for Sputum Submission. 2013. www.youtube.com/watch?v=92dT_1kbbek. Date last accessed: 8 March 2023.
- 10.World Health Organization (WHO) . GLI Training Package: Diagnostic Network Strengthening and Xpert MTB/RIF (Ultra) Implementation. Part 2: Module 2. 2021. https://stoptb.org/wg/gli/TrainingPackage_XPERT_MTB_RIF_Ultra.asp.
- 11.National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services . Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Corrected version 2.1. 2017. https://rsc.niaid.nih.gov/clinical-research-sites/daids-adverse-event-grading-tables. Date last accessed: 12 August 2021.
- 12.Jones B, Kenward MG. Design and Analysis of Cross-Over Trials. 3rd edn. Boca Raton, CRC Press, 2015. [Google Scholar]
- 13.Gart JJ. An exact test for comparing matched proportions in crossover designs. Biometrika 1969; 56: 75–80. doi: 10.1093/biomet/56.1.75 [DOI] [Google Scholar]
- 14.Lee A, Burge A, Holland AE. Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev 2015; 5: CD011699. DOI: 10.1002/14651858.CD011699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dwan K, Li T, Altman DG, et al. . CONSORT 2010 statement: extension to randomised crossover trials. BMJ 2019; 366: l4378. doi: 10.1136/bmj.l4378 [DOI] [PMC free article] [PubMed] [Google Scholar]