Abstract
Clinical risk scores based on traditional risk factors of atherosclerosis correlate imprecisely to an individual’s complex pathophysiological predisposition to atherosclerosis and provide limited accuracy for predicting major adverse cardiovascular events (MACE). Over the past two decades, computed tomography scanners and techniques for coronary computed tomography angiography (CCTA) analysis have substantially improved, enabling more precise atherosclerotic plaque quantification and characterization. The accuracy of CCTA for quantifying stenosis and atherosclerosis has been validated in numerous multicentre studies and has shown consistent incremental prognostic value for MACE over the clinical risk spectrum in different populations. Serial CCTA studies have advanced our understanding of vascular biology and atherosclerotic disease progression. The direct disease visualization of CCTA has the potential to be used synergistically with indirect markers of risk to significantly improve prevention of MACE, pending large-scale randomized evaluation.
Keywords: Coronary artery disease, Atherosclerotic cardiovascular disease, Coronary computed tomography angiography, Major adverse cardiovascular events, Prevention
Graphical Abstract
Graphical Abstract.
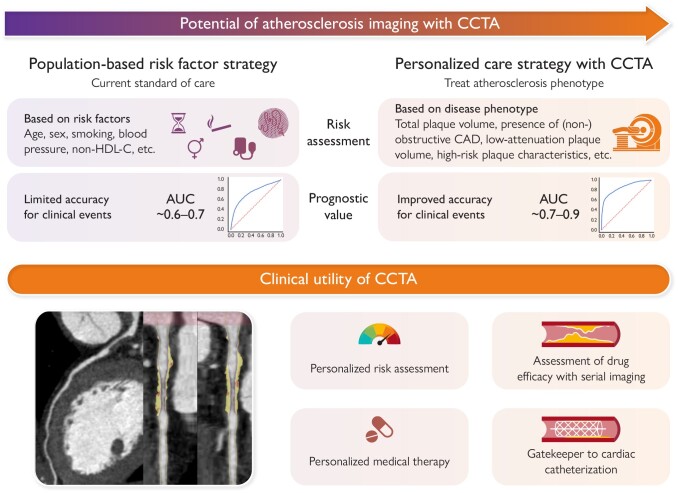
Coronary computed tomography angiography (CCTA) can change the population risk-based approach to a personalized care approach and provides important clinical utility. Coronary computed tomography angiography allows for precise atherosclerotic plaque quantification and characterization, and CCTA studies have advanced our understanding of vascular biology that holds potential to change our population risk-based approach to a personalized care approach. Coronary computed tomography angiography also provides clinical utility for assessment of drug efficacy and as a gatekeeper to cardiac catheterization lab. AUC, area under the curve; CAD, coronary artery disease; CCTA, coronary computed tomography angiography; HDL-C, high-density lipoprotein cholesterol.
Background
The identification of patients at risk of cardiovascular events from atherosclerotic coronary artery disease (CAD) poses a major challenge in current prevention. Risk algorithms utilized in clinical practice, including the Systemic Coronary Risk Evaluation 2 (SCORE2) system and the Second Manifestations of ARTerial disease 2 (SMART2), Pooled Cohort Equations, and the Framingham Risk Score (FRS),1–5 are based on traditional cardiovascular risk factors and, while effective at a population level, precision for predicting events at an individual level is limited.6–8
Limitations to current approaches in cardiovascular risk assessment: measurement of upstream and downstream indirect markers of heart disease rather than heart disease itself
Risk factors underpinning current clinical algorithms, including standard modifiable components of smoking, hypertension, diabetes, and hypercholesterolaemia, as well as age and sex, have been identified in large population studies. While these factors are associated with atherosclerosis progression/instability, they do not precisely integrate the dynamic complexity of pathophysiological processes that contribute to the development and progression of CAD. As a result, they cannot account for the heterogeneity in inter-individual atherogenic vulnerability or resilience. This is illustrated by poor discrimination and calibration in several validation studies.8,9 Indeed, many individuals develop atherosclerotic CAD, plaque rupture, and myocardial infarction without a single standard modifiable risk factor (SMuRF) meeting threshold for ‘action’.10–14
The fact that most asymptomatic patients remain unidentified before their myocardial infarction, and that more than two of three culprit plaques implicated in events are non-obstructive (<50% stenosis),15,16 highlights the need for reliable measures of atherosclerotic CAD itself. The inability to detect high-risk atherosclerosis is highlighted further by the many patients that experience out-of-hospital sudden cardiac death as their first and final cardiovascular event.17
The high event rates in symptomatic and secondary prevention patients can likely also be attributed to the absence of a reliable measure of atherosclerotic CAD itself—particularly of angiographically non-obstructive disease not detected by functional stress imaging. In symptomatic patients, non-invasive functional testing strategies, such as nuclear myocardial perfusion imaging using single-photon emission computed tomography (SPECT) or positron emission tomography (PET) are the gold standard for the non-invasive detection of myocardial ischaemia as an indirect marker for epicardial coronary stenosis and thus, need for revascularization. However, the majority of events occurs in patients with normal stress testing, highlighting the importance of detecting non-obstructive CAD for more effective prevention of major adverse cardiovascular events (MACE).16
The limitations of traditional risk estimation paradigms are an even more urgent problem considering the now globally rising deaths from cardiovascular diseases18 and the opportunity to stem this tide with novel preventive treatments to target different components of the atherosclerotic process, such as with monoclonal antibody PCSK9 inhibitors and inclisiran,19 icosapent ethyl,20 sodium-glucose co-transporter 2 (SGLT2) inhibitors,21–23 glucagon-like peptide-1 (GLP1) receptor agonists,24–26 bempedoic acid,27 low-dose rivaroxaban,28 and colchicine,29 amongst others. Due to the high costs associated with these medications and issues of polypharmacy as well as adherence, methods to distribute them to high-risk patients who are likely to derive the most benefit are needed.30
This review evaluates (i) the current use of coronary computed tomography angiography (CCTA) in clinical practice, (ii) its role in quantifying and characterizing CAD as the primary determinant for future MACE in symptomatic patients, (iii) its role in asymptomatic individuals or populations, and (iv) future directions for clinical trials investigating the role of CCTA in a personalized approach to prevent atherosclerotic cardiovascular disease (ASCVD).
Non-invasive imaging for coronary artery disease: coronary computed tomography angiography
Technological advancements enabling detailed coronary artery disease evaluation
Over the last 20 years, computed tomography (CT) scanners and techniques for CCTA have substantially improved, enabling more precise atherosclerotic plaque quantification and characterization. The first CT scanners used in large-scale CCTA trials were single-source 64-row detector scanners that had a isotropic spatial resolution of 0.625 mm and temporal resolution of 175 ms.31 The current third-generation, dual-source 2 × 192-row detector CT scanners can achieve a maximum spatial resolution of 0.24 mm and a temporal resolution up to 66 ms,31 while prospective triggering has drastically reduced the average radiation dose to below 3 mSv, equivalent to the amount of annual exposure due to background radiation for an individual living at sea level.32 Using a scanner with ≥320-row detector enables full-heart imaging within a single gantry rotation, and further reduces radiation dose (average ∼1 mSv) and eliminates step artefacts.32,33 In comparison to CCTA, median exposures are 13 mSv for SPECT,34 4 mSv for PET,34 and 3 mSv for diagnostic cardiac catheterization.35 Most recently, novel photon-counting CT scanners have been introduced into clinical practice.36 These CT scanners measure the energy of individually counted X-ray photons resulting in a higher contrast-to-noise ratio, providing the ability to further reduce radiation exposure while improving spatial resolution and spectral imaging capabilities.
Diagnostic accuracy of coronary computed tomography angiography for coronary atherosclerosis
The accuracy of CCTA for characterizing coronary atherosclerosis compared to intracoronary imaging has been validated in numerous studies. To detect any coronary plaque, compared to intravascular ultrasound (IVUS), a meta-analysis of 1360 patients from 42 studies showed a sensitivity and specificity of 93% and 92%, respectively, resulting in an area under the curve (AUC) of 0.97.37 A more recent study using 181 lesions from 151 patients reported an overestimation of lesion severity by CCTA compared to IVUS.38 Other studies reported differing degrees of over- and underestimation of lesion severity, most likely due to differences in lesion characteristics, lesion severity, and CCTA quality.39–42
Several studies also compared CCTA accuracy for luminal morphology using optical coherence tomography (OCT), showing good correlation despite the lower spatial resolution of CCTA.43–45 Findings with near-infrared spectroscopy (NIRS) have also been compared with plaque morphology observed with CCTA. The accuracy of CCTA-determined high-risk plaque for detecting NIRS lipid-rich plaque in 133 plaques from 47 patients was 94%, with a sensitivity of 93% and specificity of 94% (AUC 0.97).46
Advantages and limitations of non-invasive coronary computed tomography angiography: comparison to invasive approaches
Coronary computed tomography angiography has several advantages over traditional invasive methods to visualize atherosclerosis (Table 1). First, CCTA enables ‘whole-heart’ coronary imaging, whereas invasive methods such as IVUS only allow imaging of a maximum of two thirds of the major epicardial vessels, and typically are performed on a single vessel. Second, CCTA allows for comprehensive characterization of CAD, offering the advantages of multiple invasive imaging modalities in a single non-invasive imaging examination, and additionally provides information on valvular and structural heart disease. As examples, IVUS is mainly used to estimate plaque burden, OCT is employed to determine vascular morphology and NIRS evaluates lipid-rich plaque. Finally, the major advantage of CCTA is that it is a safe procedure with negligible complication rates and low radiation burden, thereby allowing for serial measurement in a clinical setting, which is not feasible with traditional invasive procedures. Resulting from this non-invasive approach, CCTA costs are low (∼€280) and now on par with routine blood tests and considerably less expensive than nuclear stress testing. The introduction of fractional flow reserve from CCTA (FFRCT) has further extended the utility of CCTA to non-invasive assessment of coronary flow.50–52
Table 1.
Advantages and disadvantages of coronary computed tomography angiography over traditional invasive approaches for atherosclerosis characterization
| Measurement | CCTA | IVUS | OCT | NIRS |
|---|---|---|---|---|
| Requires invasive ICA | No | Yes | Yes | Yes |
| Whole-heart CAD assessment | +++ | + | + | − |
| Plaque volume/burden | +++ | +++ | + | − |
| Positive remodelling | +++ | +++ | + | − |
| Plaque composition | ++ | ++ | +++ | + |
| Calcium identification | +++ | +++ | +++ | − |
| Calcium quantification | ++ | + | +++ | − |
| Lipid core | ++ | + | ++ | +++ |
| Thin-cap fibroatheroma | − | − | +++ | − |
| Plaque rupture | − | + | +++ | − |
| Intraluminal thrombus | + | ++ | +++ | − |
| PCI guidance | + | +++ | +++ | − |
| Interobserver variability | ++ | ++ | + | + |
| Procedure costs (€) | ∼280 | ∼1000 + ICA costs47 | ∼600 + ICA costs48 | ∼1000 + ICA costs49 |
| Radiation dosea (mSv) | 1–3 | − | − | − |
| Spatial resolution (mm) | 0.6 | 0.15–020 | 0.012–0.015 | 0.1 |
aRadiation doses for IVUS, OCT, and NIRS are limited to the doses used during coronary angiography and are not increased by the invasive imaging modalities.
CAD, coronary artery disease; CCTA, coronary CT angiography; ICA, invasive coronary angiography; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; NIRS, near-infrared spectroscopy.
Importantly, CCTA is not without limitations compared to its invasive analogues: it possesses lower spatial and temporal resolution than invasive procedures. This precludes assessment of important atherosclerotic features, such as thin-cap fibroatheroma or macrophage infiltration of plaque. Given the recent introduction of photon-counting CCTA as well as deep learning reconstruction techniques that improve spatial resolution, the near-term future will ideally advance CCTA’s further contribution to the study of atherosclerosis.53 Additionally, CCTA is complicated in patients with a fast heart rate or arrhythmias and in patients with a low estimated glomerular filtration rate.
The role of coronary computed tomography angiography in patients with symptoms or proven coronary artery disease
Coronary computed tomography angiography-defined atherosclerosis for prediction of major adverse cardiovascular events in symptomatic patients
The burden of CCTA-defined extent of CAD is a strong predictor for MACE in patients with symptomatic or proven CAD, with consistent incremental prognostic value over the clinical risk spectrum as demonstrated in several subpopulations.54–64
A large body of studies has shown that the degree or extent of obstructive CAD (defined as either ≥50% or ≥70%) has important prognostic implications in patients undergoing clinically indicated CCTA.65–68 Compared with a normal CCTA, the multivariable adjusted hazard ratios (HR) for myocardial infarction or death were 2.2, 2.9, 3.5, and 4.7 for the presence of angiographically non-obstructive, one-vessel obstructive, two-vessel obstructive, and three-vessel or left main obstructive CAD in Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter (CONFIRM).65 In 17 793 patients from this registry, the two visually assessed CCTA parameters ‘number of proximal segments with mixed or calcified plaques’, and ‘the number of proximal segments with a stenosis ≥50%’ provided independent discriminatory value for death at 2.3-year beyond 3 commonly used clinical risk scores for future MACE, including the FRS.69
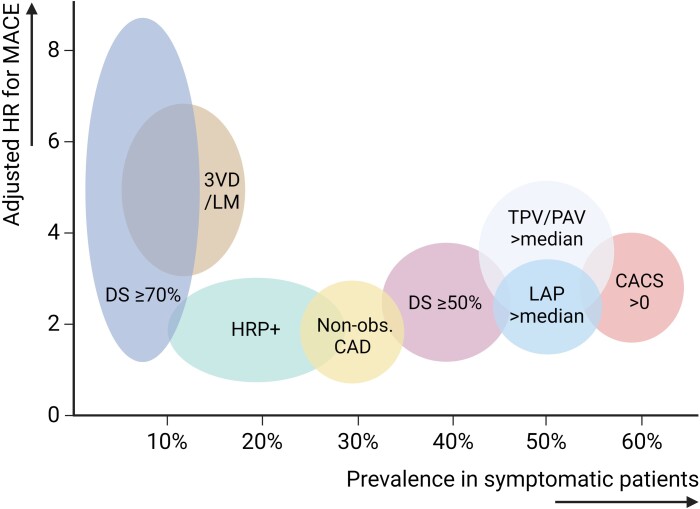
Coronary atherosclerotic burden and stenosis severity are correlated, but atherosclerosis is the primary disease process while stenosis is a sequela of atherosclerosis in some patients; and, in the CONFIRM risk score, diffuseness of atherosclerosis dominated risk prediction over the severity of stenosis. The risk of MACE for non-obstructive CAD in >4 segments was equivalent to the risk of MACE for angiographically obstructive CAD.70 Similarly, increased risk for myocardial infarction or death was observed with every increasing diseased segment in patients without any obstructive stenosis.71 Nevertheless, as stenosis and atherosclerosis are strongly correlated in the majority of patients, studies incorporating the degree and extent of obstructive disease have shown similar predictive values for obstructive stenosis and the extent of non-obstructive CAD as indicators of total atherosclerosis burden (Figure 1).
Figure 1.
Prevalence and hazard of coronary computed tomography angiography characteristics in symptomatic patients. Estimates of the relative hazards and prevalence of different CCTA characteristics, derived from different large studies in symptomatic patients.62–64,71–73 3VD, three-vessel disease; CACS, coronary artery calcium score; CCTA, coronary computed tomography angiography; DS, diameter stenosis; HR, hazard ratio; HRP+, presence of high-risk plaque; LAP, low-attenuation plaque; LM, left main disease; MACE, major adverse cardiovascular event; non-obs. CAD, non-obstructive coronary artery disease; PAV, percent atheroma volume; TPV, total plaque volume
Subsequent studies have investigated the role of coronary plaque characterization, including compositional assessment, vascular morphology, or positive remodelling of the artery, which has also been reported to yield prognostic utility in symptomatic patients or those suspected of CAD (Figure 1; Table 2). In the Incident Coronary Syndromes Identified by Computed Tomography (ICONIC), Scottish Computed Tomography of the Heart (SCOT-HEART), and Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trials, plaque composition or plaque burden, next to the degree of coronary stenosis, was the strongest predictor of future acute coronary syndromes (ACS).62–64,71,72 These studies need to be interpreted in light of the degree of adjustment for overall CAD burden, which remains a stronger predictor than detailed morphological and compositional plaque characterization (Figure 1). In ICONIC, 234 patients who suffered core-lab verified ACS after baseline CCTA were matched to 234 patients not experiencing ACS after baseline CCTA, based on age, sex, risk factors, and stenosis severity (normal, non-obstructive, one-vessel, two-vessel, and three-vessel/left main obstructive CAD).71 In this cohort, total plaque volume was similar between cases and controls (289.7 mm3 ± 308.4 vs. 267.2 mm3 ± 285.7, P = .32), whilst the presence of severe stenosis (≥70%), the volume of low-density non-calcified plaque, and presence of high-risk plaque was higher in ACS cases. Low-density non-calcified, high-risk plaque, and severe stenosis had comparable HRs for ACS on a per-patient level (low-density non-calcified plaque: HR 1.44, high-risk plaque: HR 1.59, severe stenosis: HR 1.53). Additionally, diffuseness of disease, presence of high-risk plaque, and volume of fibro-fatty plaque was higher in culprit ACS cases. In analysis from the multicentre PROMISE trial in 4451 patients, the presence of obstructive CAD defined as a >70% stenosis or >50% left main stenosis was associated with an approximately nine-fold increased risk of MACE, defined as myocardial infarction, unstable angina, or death.72 Independent of luminal stenosis and ASCVD risk factors, but unadjusted for total plaque burden, which was not analysed in this study, high-risk plaque was associated with an additional modest increase in MACE risk [adjusted HR (aHR) 1.72, 95% confidence interval (CI) 1.13–2.62], predominantly in those without obstructive CAD.72 Furthermore, Williams et al.64 demonstrated the prognostic value of low-attenuation plaque independently of plaque burden, coronary artery calcium score (CACS) and presence of obstructive CAD in 1769 patients with stable chest pain undergoing CCTA in the SCOT-HEART trial. Low-attenuation plaque burden (<30 HU) was associated with an aHR of 1.60 per doubling (95% CI 1.10–2.34; P = .014) for myocardial infarction. In the same model, CACS was also associated with myocardial infarction [aHR 1.13 per doubling (95% CI 1.01–1.27); P = .041]. Also using external validation in SCOT-HEART, Lin et al.63 found that a total plaque volume above 238.5 mm3, as defined by deep learning plaque analysis, was associated with a more than five-fold increased risk of myocardial infarction [aHR 5.36 (95% CI 1.70–16.86); P = .0042]. Similarly, stratification based on total plaque volume also demonstrated a prognostic benefit beyond traditional CACS and clinical risk factors in a long-term 10-year outcomes study in 536 patients (Figure 2).62 Nevertheless, the highest plaque stage provided a similar HR to the presence of obstructive stenosis. Most recently, a post hoc analysis of the CORE320 study found no additional prognostic benefit of plaque burden quantification beyond CACS (AUC 0.64 for CAD staging vs. AUC 0.65 for CACS).73
Table 2.
Studies evaluating independent prognostic value of detailed plaque characterization in symptomatic patients
| Study | Design | Type of plaque quantification | Outcomes |
|---|---|---|---|
| Chang et al. (ICONIC)71 | Case-control study, 234 patients with ACS propensity matched (based on clinical risk factors and CAD extent) to 234 patients without ACS | Quantitative evaluation of the total coronary tree. Low-density non-calcified plaque was defined by <30 HU. | Low-density non-calcified plaque (HR 1.44), high-risk plaque (HR 1.59), and severe stenosis (HR 1.53) were associated with a higher risk of ACS. |
| Ferencik et al. (PROMISE)72 | RCT, 4451 symptomatic patients undergoing CCTA, followed for 25 months for death, MI, or unstable angina (n = 31) | Significant stenosis (>70% or >50% in left main) and high-risk plaque (positive remodelling, low computed tomographic attenuation, or napkin-ring sign) was visually assessed | Significant stenosis was associated with a 9-fold increase in MACE. Adjusted for significant stenosis and ASCVD risk score, patient-level presence of HRP was additionally associated with a modest risk increase [aHR 1.72 (95% CI 1.13–2.62)]. |
| Williams et al. (SCOT-HEART)64 | RCT, 1769 patients with stable chest pain undergoing CCTA, followed for 4.7 years for MI (n = 41) | Quantitative semi-automated evaluation of plaque burden and low-attenuation plaque | Adjusted for plaque burden, non-calcified and calcified plaque burden, CACS, the presence of visually obstructive CAD, and cardiovascular risk score, low-attenuation plaque was independently associated with MI [aHR 1.60 per doubling (95% CI 1.10–2.34); P = .014]. In the same model, CACS was also associated with MI [aHR 1.13 per doubling (95% CI 1.01–1.27); P = .041]. |
| Lin et al. (SCOT-HEART)63 | Multicentre observational study, external prognostic validation in SCOT-HEART, 1611 patients followed for 4.7 years for MI (n = 41) | Deep learning plaque analysis | Adjusted for stenosis and clinical risk factors, a total plaque volume of 238.5 mm3 or higher was associated with an increased risk of myocardial infarction (aHR, 5.36, [95%CI 1.70–16.86]; P = .0042). |
| Nurmohamed et al.62 | Observational cohort study, 536 patients suspected of CAD, followed for 10 years for non-fatal MI, non-fatal stroke, revascularization and all-cause mortality (n = 116) | AI-guided plaque and stenosis quantification | Adjusted for clinical risk factors, stage 3 plaque based on PAV was associated with the composite outcome [aHR 3.57 (95% CI 2.12–6.00); P < .001], while obstructive stenosis >70% was associated with an aHR of 4.66 (95% CI 2.73–7.94). |
| Oeing et al. (CORE320)73 | Observational cohort study, 372 symptomatic patients, followed for 4.9 years for cardiac death, MI, cardiac hospitalization, and late revascularization (n = 97) | Semi-automated plaque quantification | Atheroma burden, CAD-RADS, high-risk plaque provided no additional prognostic value beyond CACS (AUC 0.65 for CACS vs. 0.64 for CAD stage). |
ACS, acute coronary syndrome; AI-QCT, Atherosclerosis Imaging-Quantitative Computed Tomography; CAD, coronary artery disease; CI, confidence interval; HU, Hounsfield units; HR, hazard ratio; MI, myocardial infarction; ICONIC, Incident Coronary Syndromes Identified by Computed Tomography; PAV, percent atheroma volume; PROMISE, Prospective Multicenter Imaging Study for Evaluation of Chest Pain; RCT, randomized clinical trial; SCOT-HEART, Scottish Computed Tomography of the Heart.
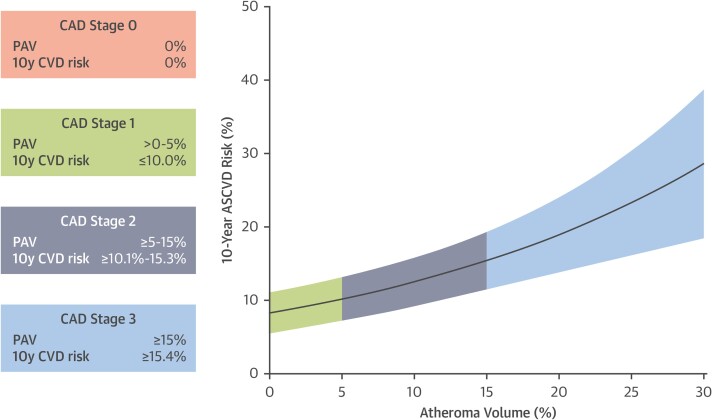
Figure 2.
Relationship between coronary computed tomography angiography-derived plaque burden and 10-year risk for cardiovascular events. Ten-year risk of cardiovascular events according to different plaque stages based on percent atheroma volume. The risk for cardiovascular events increases with increasing plaque volume. ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CVD, cardiovascular disease; CCTA, coronary computed tomography angiography; PAV, percent atheroma volume. Adapted from Nurmohamed et al.62
Dynamic changes in coronary computed tomography angiography-defined atherosclerosis over time
Several studies have investigated the changes in CCTA-defined atherosclerosis over time, of which the PARADIGM (Progression of Atherosclerotic Plaque Determined by Computed Tomographic Angiography Imaging) has been the largest study to date.74–76 The PARADIGM registry included over 1000 patients with serial CCTA ≥2 years apart upon which atherosclerotic evaluation was performed. In PARADIGM, adjusted for baseline PAV, annualized increase in PAV was independently associated with MACE with a 23% increased risk per standard deviation increase during 8 years of follow-up, consistent in multiple subpopulations.76,77 For plaque progression, a clinically relevant threshold was defined by an increase of plaque volume associated with MACE, that is, annualized 1.0% increase in PAV.76 Specifically, patients experiencing MACE during follow-up previously had a three-fold higher progression rate compared with patients not experiencing MACE: 0.93% (IQR 0.34–1.96) vs. 0.32% (IQR 0.02–0.90; P < .001).76 Plaque progression evaluation using serial IVUS has yielded comparable results. Among combined data of six clinical trials utilizing serial IVUS, patients with MACE had an annual increase in PAV of 0.95% vs. 0.46% in patients without events (P < .001).78
Serial coronary computed tomography angiography studies investigating the impact of medical therapy
Numerous studies have evaluated the impact of statin therapy on plaque burden and plaque composition.79 The PARADIGM registry involving 1255 patients who underwent serial CCTA, showed statin therapy to accelerate calcifications of non-calcified plaques, thus conferring plaque stabilization.77 Progression of calcified plaque was not associated with MACE, while in contrast rapid progression of non-calcified lesions was reflective of unstable CAD and associated with future adverse outcomes.76 The greatest reduction in non-calcified plaque volume was observed in a small but prospective randomized trial in HIV patients who were randomized to 20 mg atorvastatin vs. placebo. Non-calcified plaque volume decreased by 19% in the statin arm and increased by 20% during 1-year follow-up in the placebo arm. In addition, in comparison to the placebo group, patients with statin therapy had a lower total plaque burden, low-attenuation plaque volume and experienced a reduction in the number of plaques with positive remodelling after treatment.80 In line with this smaller study, a recent prespecified mechanistic substudy in 804 HIV-positive patients from the REPRIEVE trial found that pitavastatin significantly lowered non-calcified plaque volume compared with placebo (−1.7 mm3 vs. 2.6 mm3; P = .044).81 In parallel, the GLAGOV, PACMAN-AMI and HUYGENS studies have shown that, compared to placebo, PCSK9 inhibition reduced percent atheroma volume from IVUS by 1%, reduced lipid core burden and increased fibrous cap thickness.82–84 Similar results were found in a study using serial CCTA imaging.85 Both icosapent ethyl and colchicine have also shown to result in reductions in low-density or non-calcified plaque using serial CCTA (Table 3).86–89
Table 3.
Studies investigating effect of medication on coronary computed tomography angiography-defined atherosclerosis progression
| Intervention | Main inclusion criterion | Sample size | Duration of follow-up | Effect on plaque morphology |
|---|---|---|---|---|
| Statins90 | Suspected or known coronary artery disease | 857 patients with 2458 coronary lesions | 3.6 years |
|
| Atorvastatin80 | HIV | 19 patients receiving atorvastatin 21 patients receiving placebo |
12 months |
|
| Pitavastatin81 | HIV | 402 patients receiving pitavastatin 402 patients receiving placebo |
2 years |
|
| PCSK9 inhibition85 | Presence of vulnerable plaque | 98 patients with 136 vulnerable plaques (lesions with HU < 50) | 6 months |
|
| Icosapent ethyl86,87,89 | CAD and elevated triglyceride levels | 31 patients receiving icosapent ethyl 37 receiving placebo |
18 months |
|
| Colchicine88 | Recent ACS <1 month | 40 patients receiving colchicine 40 controls |
13 months |
|
| Diet intervention91 | Non-obstructive CAD (<70%) | 45 in diet intervention 44 controls |
15 months |
|
ACS, acute coronary syndrome; CAD, coronary artery disease; HIV, human immunodeficiency virus; PCSK9, proprotein convertase subtilisin-kexin type 9.
The hypothesis that plaque transformation from non-calcified to calcified is a risk-lowering phenomenon is supported by three observations. First, high-density calcium is associated with lower risk for future coronary syndrome as compared with non-calcified plaque (Figure 3).92 Second, statins (as well as other medications and lifestyle interventions) provoke a more rapid transformation of low-density non-calcified plaque to high-density calcium. Third, the higher the density of calcified plaque at baseline, the lower the plaque progression rates of these lesions.90 The notion that statins increase coronary calcium explains why serial CACS is not useful in patients receiving preventive medical therapy, and suggests that serial CCTA-guided atherosclerosis evaluation can enhance our understanding of the dynamic nature of CAD that predisposes an individual to plaque vulnerability.
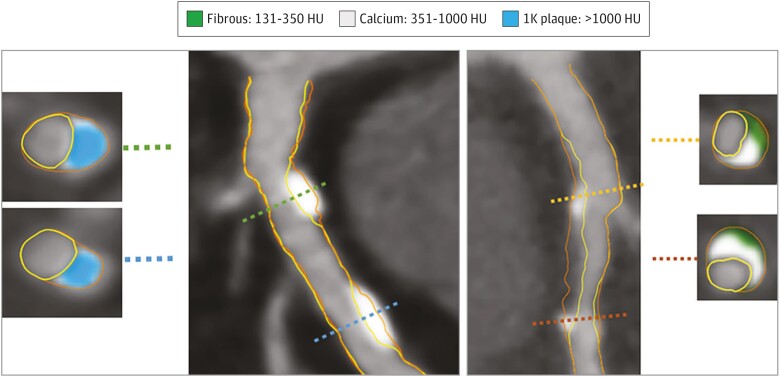
Figure 3.
Example of 1K plaque. The artery segment in the left panel (A) shows two lesions composed of 1K plaque without non-calcified plaque. Cross-sectional examples are shown with 1K plaque. The artery segment in (B) shows calcifications between 351 and 1000 HU intermingled in non-calcified plaque. Two cross-sections show 351 to 1000 HU calcium together with fibrous plaque tissue. HU, Hounsfield units. Adapted from van Rosendael et al.92
Treating atherosclerosis, not stenosis or ischaemia, improves patient outcomes
To date, only one large-scale randomized controlled trial has demonstrated the use of non-invasive CAD imaging to guide therapy in a manner that reduces MACE. The SCOT-HEART trial randomized patients with stable chest pain to standard care vs. CCTA-guided care, which resulted in a 40% reduction in death from CHD or myocardial infarction at 5-year follow-up.93 Importantly, nearly 50% of events occurred in patients with non-obstructive stenosis which would not have been detected by ischaemia imaging modalities. The direct visualization of atherosclerosis by CCTA compared with standard care led to higher rates of statin and aspirin prescription, and more appropriate prescriptions targeting patient with actual disease, which can explain the benefit observed in SCOT-HEART.94,95 The results from SCOT-HEART96 are consistent with findings from prior studies—including COURAGE,97 BARI 2D,98 ORBITA,99 and ISCHEMIA100—wherein a stenosis- or ischaemia-guided approach did not improve prognosis, highlighting the inadequacy of emphasizing these downstream sequelae of atherosclerosis over the disease itself.
Role of coronary computed tomography angiography as a ‘gatekeeper’ to downstream unnecessary procedures
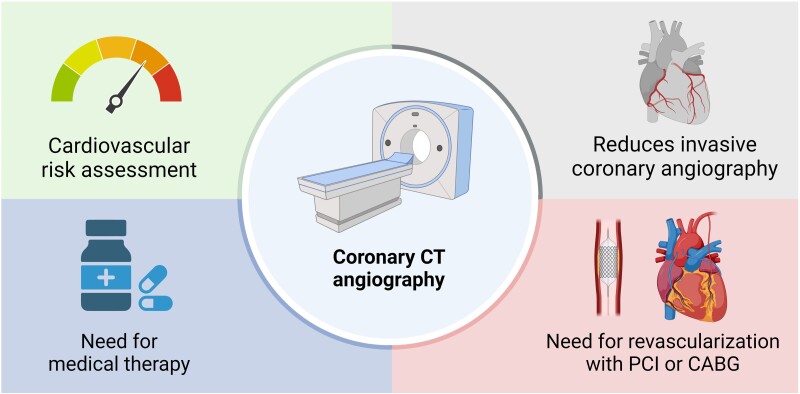
An important aim of non-invasive diagnostic testing suspected CAD is to identify those with ischaemia who might benefit from coronary revascularization (Figure 4). While ischaemia testing has held a historically prominent position in the diagnostic work-up of symptomatic suspected CAD, its benefit has been proven for symptom relief but not risk reduction. Despite its widespread use, ischaemia testing has limited diagnostic performance. Amongst 398 978 patients undergoing elective invasive angiography, only 37.6% of patients had obstructive CAD.101 Notably, 83.9% of patients underwent previous non-invasive imaging, mainly functional imaging by stress testing.102,103 This ischaemia-driven referral was driven, in part, by results of the FAME trials showing ischaemia-guided coronary intervention to confer symptomatic benefit.104,105 However, in the study by Patel et al.,101 the yield of obstructive CAD on invasive coronary angiography (ICA) was low, with nearly two of three patients referred for ICA identified as having no actionable CAD. This raises significant concerns about the ability of current testing methods to correctly identify patients referred for ICA.
Figure 4.
Multidimensional role of coronary computed tomography (CT) in coronary artery disease. Coronary CT angiography can refine risk stratification, determine need for medical therapy, can reduce unnecessary invasive coronary angiography and determined the need for revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)
A growing body of evidence indicates that, at present, patients referred for ICA are lower risk and possess lower prevalence of ischaemia and ischaemic burden, which is also observed in numerous studies showing the proportion of patients with ischaemia has decreased considerably over the past years.106,107 This trend towards lower disease prevalence results in a reduction in specificity and positive predictive value creating more false-positive findings. The high negative predictive value of CCTA renders it an ideal tool for excluding obstructive CAD (i.e. ≥50% or ≥70% diameter stenosis) with near to absolute certainty in low-to-intermediate risk populations.108 As such, CCTA can serve as an effective gatekeeper for avoidance of ICA in low-to-intermediate populations with mild or no CAD. In this regard, the CONSERVE trial investigated normalcy rates, number of invasive procedures and outcomes in patients in whom decision to proceed to ICA was informed by CCTA compared to a direct referral strategy.42 The authors observed that ICA was effectively avoided in 77% of patients by CCTA, with a concomitant cost reduction of 57%. ICA avoidance was safe, with MACE (4.6% in both arms) similar between groups, whereas the ICA normalcy rates were significantly lower in the CCTA arm (25% vs. 61%, P < .001).42 Similar findings were seen in the DISCHARGE trial, where no difference in MACE was observed between a CCTA and ICA strategy (2.1% vs. 3.0%; P = .10).109 Importantly, frequency of procedure-related complications was a three-fold lower in patients undergoing CCTA vs. ICA (0.5% vs. 1.9%; odds ratio 0.26; 95% CI 0.13–0.55).96,110
The potential role of coronary computed tomography angiography in asymptomatic patients
Relationship between cardiovascular risk factors and coronary computed tomography angiography-defined atherosclerosis in asymptomatic individuals
Elevated serum lipid levels (low-density lipoprotein cholesterol, lipoprotein(a), triglycerides), inflammatory proteins (high-sensitivity C-reactive protein), glucose/glycated haemoglobin, and blood pressure are traditional CAD risk factors that increase the likelihood of atherosclerosis in the coronary arteries, and hence are important targets for ASCVD risk-lowering therapies. On a population level, significant associations have been observed between risk factors, atherosclerosis, and MACE.111–113 Coronary atherosclerosis is more prevalent in the presence of risk factors, but the association between risk factors and atherosclerosis demonstrates imprecision and high variability at the individual level. Table 4 shows the risk ratios for the presence of any coronary atherosclerosis by CCTA and obstructive stenosis in a cohort of 2359 asymptomatic individuals from the Miami Heart study,114 ranging from one- to four-fold increases for obesity, hypercholesterolaemia, diabetes, current smoking, family history with premature CAD, and hypertension. On an individual patient level, clinical risk estimates have proven to be a poor predictor of the actual burden of coronary atherosclerosis. As an example, among 10 000 patients without prior CAD from the CONFIRM registry, although not all asymptomatic, only slightly more atherosclerosis was present in patients with diabetes compared to those without diabetes, when matched for age, sex, and the other classic cardiovascular risk factors: normal, non-obstructive, and obstructive CCTA was observed in 28% vs. 36% (P < .001), 35% vs. 37% (P = .04), and 37% vs. 27% (P < .001), respectively.57 Moreover, CCTA has revealed that the notion that diabetes is equivalent to established atherosclerosis is inaccurate, given the ∼30% of patients with diabetes who have no identifiable atherosclerosis.115–117 An overview of atherosclerosis severity per specific risk group is provided in Table 5. Overall, a normal CCTA was observed in 28% to 63% of patients, while obstructive CAD was present in 5% to 26% of patients, depending on the cohort.
Table 4.
Associations between risk factors and coronary computed tomography angiography-defined atherosclerosis in asymptomatic individuals (Miami Heart Study)
| Presence of atherosclerotic plaque (univariate OR with 95% CI) | ≥50 stenosis (univariate OR with 95% CI) | |
|---|---|---|
| Obesity | 2.59 (2.08–3.24) | 3.79 (2.10–6.82) |
| Hypercholesterolaemia | 2.48 (2.09–2.95) | 2.40 (1.59–3.62) |
| Diabetes | 2.28 (1.67–3.11) | 3.92 (2.57–5.97) |
| Current smoking | 2.38 (1.43–3.96) | 2.72 (1.36–5.43) |
| Family history with premature CAD | 1.10 (0.80–1.52) | 1.08 (0.55–2.09) |
| Hypertension | 2.24 (1.90–2.64) | 2.90 (1.93–4.36) |
Data from the Miami Heart Study.114
CAD, coronary artery disease; CCTA, coronary CT angiography; OR, odds ratio; CI, confidence interval.
Table 5.
Coronary atherosclerosis presence by risk factor in individuals without prior coronary artery disease
| Risk factor | n | Symptoms | Age (years) | Normal CCTA (%) | Non-obstructive CAD (%) | Obstructive CAD (%) |
|---|---|---|---|---|---|---|
| Familial hypercholesterolaemia118 | 50 | Asymptomatic | 48 | 50 | 22 | 26 |
| Hypertension119 | 1434 | 38% asymptomatic | 57 | 44 | 38 | 18 |
| Diabetes57 | 3370 | 30% asymptomatic | 61 | 28 | 35 | 37 |
| Metabolic syndrome120 | 690 | 39% asymptomatic | 58 | 53 | 32 | 15 |
| Asymptomatic US population114 | 2359 | Asymptomatic | 53 | 51 | 43 | 7 |
| Asymptomatic US population, ASCVD score 7.5%–20%114 | 219 | Asymptomatic | – | 25 | 61 | 14 |
| Asymptomatic South Korean population, Lp(a) quartile 1121 | 1804 | Asymptomatic | 53 | 66 | 29 | 5 |
| Asymptomatic South Korean population, Lp(a) quartile 4121 | 1798 | Asymptomatic | 55 | 63 | 30 | 7 |
ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CCTA, coronary CT angiography; Lp(a), lipoprotein(a).
Coronary computed tomography angiography-defined atherosclerosis for prediction of major adverse cardiovascular events in asymptomatic individuals
Since CCTA is not routinely recommended in cardiovascular risk screening in asymptomatic patients,3,4 there are relatively few large studies evaluating the value of CCTA for cardiovascular risk stratification. In two studies from CONFIRM with 2 and 6 years of follow-up, restricted to asymptomatic patients without known CAD, CCTA did not add any relevant incremental prognostic value beyond CACS when added to a model with clinical risk factors.56,122 In a more recent study from the Copenhagen General Population Study, the prognostic value of CCTA was investigated in 9533 asymptomatic patients with a mean age of 60 years.123 In this population study, subclinical atherosclerosis was found in 61% of men and 36% of women. After adjustment for sex, age, and cardiovascular risk factors, the presence of subclinical obstructive atherosclerosis was associated with a more than 8-fold up to 12-fold increased risk of myocardial infarction, depending on the extent of the CAD. Furthermore, presence of a high-risk plaque feature (spotty calcification, napkin-ring sign, or non-calcified plaque) was associated with a three-fold increased risk of myocardial infarction. Although CACS >300 was also associated with a seven-fold risk increase of myocardial infarction, the study did not investigate the incremental prognostic value of CCTA beyond clinical risk factors or CACS.
While CACS has shown incremental value and might be considered in asymptomatic individuals around risk thresholds, it remains unknown whether CCTA can further improve risk stratification in asymptomatic patients, considering the limited amount of data available.3,4 The fact that relative reductions in events in the SCOT-HEART trial were similar in those with non-cardiac chest pain, suggests a directionally similar effect, but further studies are highly needed.93
Coronary computed tomography angiography-based atherosclerosis assessment in apparently healthy individuals to guide preventive therapy
To date, results of randomized studies evaluating the use of CT in asymptomatic, apparently healthy individuals have been less robust. The St. Francis Heart Study randomized 1005 asymptomatic subjects with CACS >80th percentile for age and sex to atorvastatin vs. placebo.124 At 4.3 years, no differences were seen in patients for CACS progression, with treatment failing to reduce MACE. Notably, patients with CACS >400 did experience a reduction in events (8.7% vs. 15.0%, P = .046).124 While this was not prespecified, it nevertheless raises the possibility of benefit of atherosclerosis screening by CT if proper identification of high-risk individuals can be achieved.
In 2022, the results of DANCAVAS were reported for men 65–74 years of age who underwent non-contrast CT for CACS, ankle-brachial indices and cholesterol and diabetes serum biomarker diagnosis.125 In this population-based screening study, a total of 46 611 men underwent randomization. For a primary endpoint of all-cause mortality at 5 years, the study was not statistically significant but directionally suggested possible benefit of screening with CT (HR 0.95, 95% CI 0.90–1.00, P = .06). In a prespecified subgroup analysis, potential benefits were seen in those of younger age (65–69 years), with an 11% lower rate of death (P = .007) and a 7% lower rate of a primary composite endpoint of death, stroke, or myocardial infarction (P = .016).
That both the St. Francis Heart Study and the DANCAVAS trials used CACS over CCTA precludes assessment of the value of non-calcified plaque for better identification and image-guided treatment. To date, only one study has been reported to evaluate the use of CCTA of screening—the FACTOR-64 study.126 In this study of 900 patients randomized to CCTA or no CCTA, patients with diabetes were recommended for treatment of cholesterol and diabetes and lifestyle based upon CCTA findings of stenosis and without atherosclerosis evaluation. The endpoint—all-cause mortality, non-fatal myocardial infarction, or hospitalization for unstable angina—did not differ between the CCTA and no CCTA arms. Whether latest generation quantitative atherosclerosis evaluation tools may have influenced these findings requires future study.
Future directions
Atherosclerosis evaluation by coronary computed tomography angiography encourages a shift in the preventive care paradigm from population-based to personalized
CCTA has the potential to individualize risk assessment by allowing for direct visualization of atherosclerosis in a non-invasive manner. It offers the ability to quantify plaque burden and assess plaque morphology, as well as the ability to define high-risk measures of plaque and vascular morphology. Diameter stenosis, plaque burden, and specific plaque phenotypes, such as non-calcified and, in particular, low-attenuation plaque, are considered some of the most potent markers for future MACE and carry the potential to be the standard of care for risk assessment. Temporal changes in plaque burden and morphology further refine risk stratification, offering the impetus to achieve personalized medicine.
Nevertheless, current risk prediction tools are traditionally based upon traditional cardiac risk factors such as hypertension, diabetes, hypercholesterolaemia, age, sex, and a few tools also incorporate C-reactive protein and lipoprotein(a).1–5 These risk factors of atherosclerosis reflect population-level factors associated with CAD that correlate imprecisely to any given individual’s disease. Although these risk tools are accessible, they fail to accurately predict future adverse events in asymptomatic individuals with diabetes and symptomatic patients, cannot discriminate individuals with vs. without high-risk CAD, and may give false security to those with no risk factors but significant CAD (Figure 5).
Figure 5.
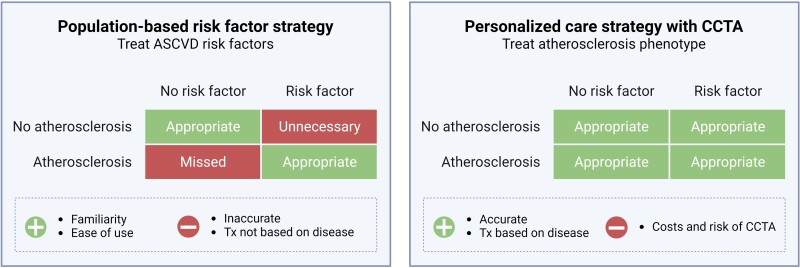
Comparison of population risk-based prevention with personalized prevention strategies using coronary computed tomography angiography. Differences in a population-based approach based on risk factors (e.g. commonly used ASCVD risk scores) and a personalized care strategy based on the actual disease phenotype observed with CCTA. In a population-based risk factor strategy, patients are treated based on the presence of risk factors. If the presence of risk factors aligns with the presence of atherosclerosis, patients are treated appropriately. In a population-based risk factor strategy, patients without risk factors but with presence of atherosclerosis are missed, while patients with risk factors but without atherosclerosis (above a certain age threshold) are unnecessarily treated. In a personalized care strategy with CCTA, patients can receive appropriate therapy based on their individual atherosclerosis phenotype. ASCVD, atherosclerotic cardiovascular disease; CCTA, coronary computed tomography angiography; Tx, treatment
Numerous studies have shown a CACS =0 to be a potent negative risk marker in general populations, one that downgrades individual risk and identifies individuals with a negligible risk of future MACE outcome.127–133 Yet, contemporary research has demonstrated the inadequacy of CAC scoring for identifying the totality of at-risk individuals. Coronary artery calcium score data from population-based studies have revealed up to 1 in 4 younger individuals who will suffer MACE will have a CACS =0 at the time of imaging,16,134 emphasizing the urgency of improved approaches to pinpoint at-risk individuals. That CACS has been prognostically useful in large-scale outcomes studies is unsurprising, as many who have CAC also possess higher-risk non-calcified plaques. However, as adverse atherosclerotic plaque morphology is defined by lower-density non-calcified plaques, CCTA may represent an improved approach to evaluate individuals in a personalized fashion for total plaque burden, as well as makeup of plaque morphology, which has been shown to be a better guide for risk stratification.62
Visualization of atherosclerosis as the primary heart disease process holds the potential to shift our current preventive care paradigm emphasis on population-based risk factors to individualized disease burden and type in a manner that may guide therapeutic decision making of medical therapy and lifestyle interventions (Figure 4). Coronary atherosclerosis is a single trackable metric that represents an individual’s lifelong exposure to all known and unknown risk factors. In this manner, clinical evaluation of atherosclerosis may both allow for deferral of initiating lifelong therapy or escalation of medical therapy based upon disease or changes in disease, thereby increasing the prognostic benefit of our therapeutics.
Perspectives for future clinical trials
Over the last 20 years, CCTA has witnessed important advances in its technology, with improved spatial and temporal resolution, larger volume coverage, and lower radiation exposure and contrast requirements. In conjunction with continued automation in quantitative CCTA analysis with AI-supported algorithms,62–64,135–141 allowing for reliable and reproducible identification of coronary plaque volume and potential high-risk plaque, atherosclerosis assessment by CCTA has the potential to further improve cardiovascular risk stratification. However, large-scale prospective studies comparing atherosclerosis assessment by CCTA to the clinical standard of care are highly needed before widespread implementation in clinical practice. Furthermore, the direct visualization of coronary atherosclerosis by CCTA allows for the evaluation CAD burden and morphology and, given its non-invasive nature, allows for serial assessment to identify temporal changes in an individual’s disease process.79 Quantitative CCTA evaluation of atherosclerosis135–141—coupled with the near universal availability of CT scanners—may provide a useful tool to evaluate the effectiveness of medical therapy and lifestyle interventions over time integrating large-scale randomized controlled trial data with an ‘n of 1’ approach that is dictated by actual CAD compared to optimization of imprecisely associated risk factors within a single individual (Table 5). In the meantime, assessment of both stenosis and a visual assessment of plaque burden such as the segment involvement score, as advocated by CAD-RADS 2.0,142 could significantly improve ASCVD risk stratification in current clinical practice.
To date, most CCTA studies have been performed in symptomatic patients with stable chest pain. Given its safety and ease of performance, use of CCTA has been advocated by some to serve as a ‘mammogram of the heart,’ i.e. to leverage its use in screening large populations for early identification, risk stratification, and treatment. While no study to date has been performed to directly addressing the screening indication of CCTA, in aggregate, the previously performed large-scale randomized trials offer hypothesis-generating results that image-guided screening by CCTA may serve as an effective tool to identify asymptomatic individuals at risk of MACE and to guide judicious use of preventive therapies. These studies also highlight limitations that must be considered for expansion of CCTA use in screening populations:
Tiered treatment commensurate to individualized disease burden. In the prior studies, there has been ambiguity in treatment recommendations based upon CT for atherosclerosis or stenosis. Notably, these trials were performed at a time when effective medical prevention of CAD primarily consisted of statins, with no access to contemporary agents such as PCSK9i monoclonals, inclisiran, icosapent ethyl, bempedoic acid, low-dose rivaroxaban, GLP1 receptor agonists, GLP1/GIP agonists, SGLT2 inhibitors, SGLT1/2 inhibitors, and upcoming therapies targeting inflammation and lipoprotein(a). A contemporary assessment of CT-guided vs. non-CT-guided treatment leveraging the entirety of contemporary medical therapy is needed.
Appropriate clinical endpoints. Prior studies have either evaluated endpoints of all-cause mortality (with its intrinsic competing risks from non-cardiovascular deaths that cannot be influenced by treating CAD) or ‘soft’ endpoints comprising revascularization.
Appropriate CAD measurements. To date, only FACTOR-64 has evaluated the effectiveness of CCTA for screening but was performed at a time when CCTA was primarily used for stenosis severity evaluation. No study to date has leveraged total atherosclerotic plaque burden and type to guide therapy.
Adequate sample size and follow-up. Previous studies have been underpowered to observe realistic clinical differences between image-based and non-image-based arms. To address this, future trials would be ideally event-driven along with minimal treatment durations. These primary endpoints should also emphasize important patient-centric clinical outcomes that are of uniform importance to patients and will require very large sample sizes and adequate follow-up time to achieve. The SCOT-HEART2 trial (ClinicalTrials.gov: NCT03920176), the DANE-HEART trial (ClinicalTrials.gov: NCT05677386), and the TRANSFORM trial (ClinicalTrials.gov: NCT06112418) will each have an estimated sample size of 6000 to 7500 patients with a follow-up duration of 4–5 years, which optimizes the chance of demonstrating clinically significant benefit.
Conclusions
Over two decades, CCTA has advanced our understanding of coronary biology in a manner that can now effectively pinpoint risk and guide therapy in a personalized fashion (Graphical Abstract). Adoption of this precision heart care approach may be beneficial, but large-scale trials will be required to demonstrate the benefit with clinical outcomes. Given that the most successful preventive care paradigms have relied upon advanced imaging for direct visualization—including mammograms, colonoscopies, and lung CT for early identification and treatment of breast, colon, and lung cancer, respectively—it is conceivable that emulation of these approaches for CAD prevention over sole use of indirect risk factors may hold the potential to improve patient outcomes by combining large-scale RCT evidence with clinic-based ‘n of 1’ approaches to personalized care.
Acknowledgments
Figures 1, 4, and 5 were created with BioRender.com.
Contributor Information
Nick S Nurmohamed, Department of Cardiology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Vascular Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands; Division of Cardiology, The George Washington University School of Medicine, Washington, DC, United States.
Alexander R van Rosendael, Department of Cardiology, Leiden University Medical Center, Leiden, The Netherlands.
Ibrahim Danad, Department of Cardiology, University Medical Center Utrecht, Utrecht, The Netherlands; Department of Cardiology, Radboud University Medical Center, Nijmegen, The Netherlands.
Quyen Ngo-Metzger, Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, CA, United States.
Pam R Taub, Section of Cardiology, Department of Medicine, University of California, San Diego, CA, United States.
Kausik K Ray, Department of Primary Care and Public Health, Imperial College London, London, United Kingdom.
Gemma Figtree, Faculty of Medicine and Health, University of Sydney, Australia, St Leonards, Australia.
Marc P Bonaca, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States.
Judith Hsia, Department of Medicine, University of Colorado School of Medicine, Aurora, CO, United States.
Fatima Rodriguez, Department of Medicine, Stanford University School of Medicine, Stanford, CA, United States.
Alexander T Sandhu, Department of Medicine, Stanford University School of Medicine, Stanford, CA, United States.
Koen Nieman, Department of Medicine, Stanford University School of Medicine, Stanford, CA, United States.
James P Earls, Cleerly, Inc., Denver, CO, United States; Department of Radiology, The George Washington University School of Medicine, Washington, DC, United States.
Udo Hoffmann, Cleerly, Inc., Denver, CO, United States.
Jeroen J Bax, Department of Cardiology, Leiden University Medical Center, Leiden, The Netherlands.
James K Min, Cleerly, Inc., Denver, CO, United States.
David J Maron, Department of Medicine, Stanford University School of Medicine, Stanford, CA, United States.
Deepak L Bhatt, Mount Sinai Fuster Heart Hospital, Icahn School of Medicine at Mount Sinai, 1 Gustave Levy Place, Box 1030, New York, NY 10029, United States.
Supplementary Data
Supplementary data are not available at European Heart Journal online.
Declarations
Disclosure of Interest
N.S.N. is co-founder of Lipid Tools. Q.N.-M. is a consultant for Cleerly. P.R.T. receives consulting fees from Amgen, Boehringer Ingelheim, Cleerly, Esperion, Lilly, Merck, and Novo Nordisk. K.K.R. reports unrestricted research grants (last 3 years) to Imperial College London, Amgen, Sanofi, Regeneron, Daiichi Sankyo, and Ultragenix; Consulting fees from Novartis, Daiichi Sankyo, Kowa, Esperion, Novo Nordisk, MSD, Lilly, Silence Therapeutics, AZ, New Amsterdam Pharma, Bayer, Beren Therapeutics, CLEERLY, EMENDOBIO, SCRIBE, CRISPR, VAXXINITY, Amarin, Regeneron, Ultragenix, Cargene, Resverlogix for serving as a member of the SC or EC of clinical trials and roles as PI, NLI. Attending advisory boards, providing advice on data its interpretation and future lines of research; lecture fees from Novartis, BI, AZ, Novo Nordisk, Viatris, Amarin, Biologix Pharma, Sanofi, Amgen, Esperion, Daiichi Sankyo, Macleod Pharma for CME and non-CME symposia at international meetings; stock options from New Amsterdam Pharma and PEMI31 and serving as EAS President (unpaid). G.F. acknowledges support from the National Health and Medical Research Council (Australia) and New South Wales Health, and consulting fees/advisory board for Abbott, Amgen, Astra Zeneca, Cleerly, Novartis, and equity in Prokardia and Kardiomics. M.P.B. and J.H. receive salary support from CPC, a non-profit academic research organization affiliated with the University of Colorado, that receives or has received research grant/consulting funding between February 2021 and July 2023 from the following organizations: Abbott Laboratories, Adamis Pharmaceuticals Corporation, Agios Pharmaceuticals, Inc., Alexion Pharma, Alnylam Pharmaceuticals, Inc., Amgen Inc., Angionetics, Inc., ARCA Biopharma, Inc., Array BioPharma, Inc., AstraZeneca and Affiliates, Atentiv LLC, Audentes Therapeutics, Inc., Bayer and Affiliates, Beth Israel Deaconess Medical Center, Better Therapeutics, Inc., BIDMC, Boston Clinical Research Institute, Bristol-Meyers Squibb Company, Cambrian Biopharma, Inc., Cardiol Therapeutics Inc., CellResearch Corp., Cleerly Inc., Cook Medical Incorporated, Cook Regentec, Covance, CSL Behring LLC, Eidos Therapeutics, Inc., EP Trading Co. Ltd., EPG Communication Holdings Ltd., Epizon Pharma, Inc., Esperion Therapeutics, Inc., Everly Well, Inc., Exicon Consulting Pvt. Ltd., Faraday Pharmaceuticals, Inc., Foresee Pharmaceuticals Co. Ltd., Fortress Biotech, Inc., HDL Therapeutics Inc., HeartFlow Inc., Hummingbird Bioscience, Insmed Inc., Ionis Pharmaceuticals, IQVIA Inc., JanOne Biotech Holdings Inc., Janssen and Affiliates, Kaneka, Kowa Research Institute, Inc., Kyushu University, Lexicon Pharmaceuticals, Inc., LSG Kyushu University, Medimmune Ltd., Medpace, Merck & Affiliates, Novartis Pharmaceuticals Corp., Novate Medical, Ltd., Novo Nordisk, Inc., Pan Industry Group, Pfizer Inc., PhaseBio Pharmaceuticals, Inc., PPD Development, LP, Prairie Education and Research Cooperative, Prothena Biosciences Limited, Regeneron Pharmaceuticals, Inc., Regio Biosciences, Inc., Rexgenero, Sanifit Therapeutics S.A., Sanofi-Aventis Groupe, Silence Therapeutics PLC, Smith & Nephew plc, Stealth BioTherapeutics Inc., State of Colorado CCPD Grant, The Brigham & Women's Hospital, Inc., The Feinstein Institutes for Medical Research, Thrombosis Research Institute, University of Colorado, University of Pittsburgh, VarmX, Virta Health Corporation, WCT Atlas, Worldwide Clinical Trials Inc., WraSer, LLC, and Yale Cardiovascular Research Group. J.H. also reports owning AstraZeneca stock. M.P.B. receives support from the AHA SFRN under award numbers 18SFRN3390085 (BWH-DH SFRN Center) and 18SFRN33960262 (BWH-DH Clinical Project). M.P.B. also reports stock in Medtronic and Pfizer. F.R. disclosures consulting for NovoNordisk, Novartis, HealthPals, Movano Health, Edwards, Inclusive Health, and Esperion. A.S. disclosures consulting for Lexicon Pharmaceuticals and Reprieve Cardiovascular. K.N. acknowledges support from the NIH (NIH R01- HL141712; NIH R01—HL146754), and reports unrestricted institutional research support from Siemens Healthineers, Bayer, HeartFlow Inc., Novartis unrelated to this work, consulting for Siemens Medical Solutions USA, Cleerly, Artrya, and Novartis and equity in Lumen Therapeutics. Drs. Earls, Hoffmann and Min are employees of and hold equity in Cleerly Inc. D.J.M. discloses support from NIH (1R01HL149888–01A1) and consulting for HeartFlow, Carta. D.L.B. discloses the following relationships—Advisory Board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; Board of Directors: American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); Consultant: Broadview Ventures, Hims, SFJ, Youngene; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo. The other authors report no relevant disclosures.
Data Availability
No data were generated or analysed for or in support of this article.
Funding
Dutch Heart Foundation (grant number 03-007-2023-0068) and the European Atherosclerosis Society (2023) to N.S.N.
References
- 1. D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 2. Deanfield J, Sattar N, Simpson I, Wood D, Bradbury K, Fox K, et al. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart 2014;100:ii1–67. 10.1136/heartjnl-2014-305693 [DOI] [PubMed] [Google Scholar]
- 3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Visseren FLJ, MacH F, Smulders YM, Carballo D, Koskinas KC, Bäck MMM, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 5. Hageman SHJ, McKay AJ, Ueda P, Gunn LH, Jernberg T, Hagström E, et al. Estimation of recurrent atherosclerotic cardiovascular event risk in patients with established cardiovascular disease: the updated SMART2 algorithm. Eur Heart J 2022;43:1715–27. 10.1093/eurheartj/ehac056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaasenbrood L, Boekholdt SM, Van Der GY, Ray KK, Peters RJG, Kastelein JJP, et al. Distribution of estimated 10-year risk of recurrent vascular events and residual risk in a secondary prevention population. Circulation 2016;134:1419–29. 10.1161/CIRCULATIONAHA.116.021314 [DOI] [PubMed] [Google Scholar]
- 7. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US preventive services task force recommendation statement. JAMA 2018;320:272–80. 10.1001/jama.2018.8359 [DOI] [PubMed] [Google Scholar]
- 8. DeFilippis AP, Young R, Carrubba CJ, McEvoy JW, Budoff MJ, Blumenthal RS, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015;162:266–75. 10.7326/M14-1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA 2007;297:611–9. 10.1001/jama.297.6.611 [DOI] [PubMed] [Google Scholar]
- 10. Vernon ST, Coffey S, Bhindi R, Soo Hoo SY, Nelson GI, Ward MR, et al. Increasing proportion of ST elevation myocardial infarction patients with coronary atherosclerosis poorly explained by standard modifiable risk factors. Eur J Prev Cardiol 2017;24:1824–30. 10.1177/2047487317720287 [DOI] [PubMed] [Google Scholar]
- 11. Figtree GA, Vernon ST, Hadziosmanovic N, Sundström J, Alfredsson J, Arnott C, et al. Mortality in STEMI patients without standard modifiable risk factors: a sex-disaggregated analysis of SWEDEHEART registry data. Lancet 2021;397:1085–94. 10.1016/S0140-6736(21)00272-5 [DOI] [PubMed] [Google Scholar]
- 12. Figtree GA, Vernon ST, Harmer JA, Gray MP, Arnott C, Bachour E, et al. Clinical pathway for coronary atherosclerosis in patients without conventional modifiable risk factors: JACC State-of-the-Art Review. J Am Coll Cardiol 2023;82:1343–59. 10.1016/j.jacc.2023.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kelly C, Lan NSR, Phan J, Hng C, Matthews A, Rankin JM, et al. Characteristics and outcomes of young patients with ST-elevation myocardial infarction without standard modifiable risk factors. Am J Cardiol 2023;202:81–9. 10.1016/j.amjcard.2023.06.045 [DOI] [PubMed] [Google Scholar]
- 14. Kong G, Chin YH, Chong B, Goh RSJ, Lim OZH, Ng CH, et al. Higher mortality in acute coronary syndrome patients without standard modifiable risk factors: results from a global meta-analysis of 1,285,722 patients. Int J Cardiol 2023;371:432–40. 10.1016/j.ijcard.2022.09.062 [DOI] [PubMed] [Google Scholar]
- 15. Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–71. 10.1161/01.CIR.92.3.657 [DOI] [PubMed] [Google Scholar]
- 16. Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136:1993–2005. 10.1161/CIRCULATIONAHA.117.030578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cavallari I, Bhatt DL, Steg PG, Leiter LA, McGuire DK, Mosenzon O, et al. Causes and risk factors for death in diabetes: a competing-risk analysis from the SAVOR-TIMI 53 trial. J Am Coll Cardiol 2021;77:1837–40. 10.1016/j.jacc.2021.02.030 [DOI] [PubMed] [Google Scholar]
- 18. World Heart Federation . Deaths from cardiovascular disease surged 60% globally over the last 30 years: Report. 2023. https://world-heart-federation.org/news/deaths-from-cardiovascular-disease-surged-60-globally-over-the-last-30-years-report/ (13 July 2023).
- 19. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med 2020;382:1507–19. 10.1056/NEJMoa1912387 [DOI] [PubMed] [Google Scholar]
- 20. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 21. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 22. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–28. 10.1056/NEJMoa2030183 [DOI] [PubMed] [Google Scholar]
- 23. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–39. 10.1056/NEJMoa2030186 [DOI] [PubMed] [Google Scholar]
- 24. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 25. Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2019;381:841–51. 10.1056/NEJMoa1901118 [DOI] [PubMed] [Google Scholar]
- 26. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023;389:2221–32. 10.1056/NEJMoa2307563 [DOI] [PubMed] [Google Scholar]
- 27. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med 2023;388:1353–64. 10.1056/NEJMoa2215024 [DOI] [PubMed] [Google Scholar]
- 28. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–30. 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- 29. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020;383:1838–47. 10.1056/NEJMoa2021372 [DOI] [PubMed] [Google Scholar]
- 30. Varghese MS, Liu CL, Kazi DS. The price of progress: cost, access, and adoption of novel cardiovascular drugs in clinical practice. Curr Cardiol Rep 2021;23:163. 10.1007/s11886-021-01598-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tridandapani S, Banait-Deshmane S, Aziz MU, Bhatti P, Singh SP. Coronary computed tomographic angiography: a review of the techniques, protocols, pitfalls, and radiation dose. J Med Imaging Radiat Sci 2021;52:S1–11. 10.1016/j.jmir.2021.08.014 [DOI] [PubMed] [Google Scholar]
- 32. Seppelt D, Kolb C, Kühn JP, Speiser U, Radosa CG, Hoberück S, et al. Comparison of sequential and high-pitch-spiral coronary CT-angiography: image quality and radiation exposure. Int J Cardiovasc Imaging 2019;35:1379–86. 10.1007/s10554-019-01568-y [DOI] [PubMed] [Google Scholar]
- 33. Finck T, Klambauer K, Hendrich E, Will A, Martinoff S, Hadamitzky M. Radiation dose and image quality of a high-pitch prospective spiral first approach in coronary computed tomography angiography (CCTA). J Cardiovasc Dev Dis 2021;8:119. 10.3390/jcdd8100119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Desiderio MC, Lundbye JB, Baker WL, Farrell MB, Jerome SD, Heller G V. Current status of patient radiation exposure of cardiac positron emission tomography and single-photon emission computed tomographic myocardial perfusion imaging. Circ Cardiovasc Imaging 2018;11:e007565. 10.1161/CIRCIMAGING.118.007565 [DOI] [PubMed] [Google Scholar]
- 35. Stocker TJ, Abdel-Wahab M, Möllmann H, Deseive S, Massberg S, Hausleiter J. Radiation dose in diagnostic cardiac catheterization: results from the PROTECTION VII study. JACC Cardiovasc Interv 2021;14:1958–60. 10.1016/j.jcin.2021.07.023 [DOI] [PubMed] [Google Scholar]
- 36. Willemink MJ, Persson M, Pourmorteza A, Pelc NJ, Fleischmann D. Photon-counting CT: technical principles and clinical prospects. Radiology 2018;289:293–312. 10.1148/radiol.2018172656 [DOI] [PubMed] [Google Scholar]
- 37. Fischer C, Hulten E, Belur P, Smith R, Voros S, Villines TC. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: a meta-analysis. J Cardiovasc Comput Tomogr 2013;7:256–66. 10.1016/j.jcct.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 38. Doh J-H, Koo B-K, Nam C-W, Kim J-H, Min JK, Nakazato R, et al. Diagnostic value of coronary CT angiography in comparison with invasive coronary angiography and intravascular ultrasound in patients with intermediate coronary artery stenosis: results from the prospective multicentre FIGURE-OUT (Functional Imaging criteria for GUiding REview of invasive coronary angiOgraphy, intravascular Ultrasound, and coronary computed Tomographic angiography) study. Eur Hear J Cardiovasc Imaging 2014;15:870–7. 10.1093/ehjci/jeu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kristensen TS, Engstrøm T, Kelbæk H, Von Der RP, Nielsen MB, Kofoed KF. Correlation between coronary computed tomographic angiography and fractional flow reserve. Int J Cardiol 2010;144:200–5. 10.1016/j.ijcard.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 40. Papadopoulou SL, Neefjes LA, Schaap M, Li HL, Capuano E, van der Giessen AG, et al. Detection and quantification of coronary atherosclerotic plaque by 64-slice multidetector CT: a systematic head-to-head comparison with intravascular ultrasound. Atherosclerosis 2011;219:163–70. 10.1016/j.atherosclerosis.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 41. Voros S, Rinehart S, Qian Z, Vazquez G, Anderson H, Murrieta L, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions. JACC Cardiovasc Interv 2011;4:198–208. 10.1016/j.jcin.2010.10.008 [DOI] [PubMed] [Google Scholar]
- 42. Chang HJ, Lin FY, Gebow D, An HY, Andreini D, Bathina R, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging 2019;12:1303–12. 10.1016/j.jcmg.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 43. van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, Wijeratne R, Jinnouchi H, Finn A, et al. What atherosclerosis findings can CT see in sudden coronary death: plaque rupture versus plaque erosion. J Cardiovasc Comput Tomogr 2020;14:214–8. 10.1016/j.jcct.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 44. Wang ZQ, Zhang HX, Wu W, Yuan YS, Dou YN, Yin D, et al. Combined coronary CT angiography with plain scan for diagnosis of ruptured plaque: comparison with optical coherence tomography. Int J Cardiovasc Imaging 2021;37:3073–80. 10.1007/s10554-021-02253-9 [DOI] [PubMed] [Google Scholar]
- 45. Sugiura J, Watanabe M, Nobuta S, Okamura A, Kyodo A, Nakamura T, et al. Prediction of optical coherence tomography-detected calcified nodules using coronary computed tomography angiography. Sci Rep 2022;12:22296. 10.1038/s41598-022-26599-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Omori H, Matsuo H, Fujimoto S, Sobue Y, Nozaki Y, Nakazawa G, et al. Determination of lipid-rich plaques by artificial intelligence-enabled quantitative computed tomography using near-infrared spectroscopy as reference. Atherosclerosis 2023;386:117363. 10.1016/j.atherosclerosis.2023.117363 [DOI] [PubMed] [Google Scholar]
- 47. Zhou J, Liew D, Duffy SJ, Shaw J, Walton A, Chan W, et al. Intravascular ultrasound versus angiography-guided drug-eluting stent implantation: a health economic analysis. Circ Cardiovasc Qual Outcomes 2021;14:e006789. 10.1161/CIRCOUTCOMES.120.006789 [DOI] [PubMed] [Google Scholar]
- 48. Ma T, Zhou B, Hsiai TK, Shung KK. A review of intravascular ultrasound-based multimodal intravascular imaging: the synergistic approach to characterizing vulnerable plaques. Ultrason Imaging 2016;38:314–31. 10.1177/0161734615604829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alkhalil M. Novel applications for invasive and non-invasive tools in the era of contemporary percutaneous coronary revascularisation. Curr Cardiol Rev 2021;18:e190122191004. 10.2174/1573403X17666210202102549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Curzen NP, Nolan J, Zaman AG, Nørgaard BL, Rajani R. Does the routine availability of CT–derived FFR influence management of patients with stable chest pain compared to CT angiography alone?: The FFRCT RIPCORD study. JACC Cardiovasc Imaging 2016;9:1188–94. 10.1016/j.jcmg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 51. Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, Van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 2012;308:1237–45. 10.1001/2012.jama.11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen PA, et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol 2019;73:161–73. 10.1016/j.jacc.2018.10.056 [DOI] [PubMed] [Google Scholar]
- 53. Sun Z, Ng CKC. Finetuned super-resolution generative adversarial network (artificial intelligence) model for calcium deblooming in coronary computed tomography angiography. J Pers Med 2022;12:1354. 10.3390/jpm12091354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–60. 10.1016/j.jacc.2011.02.074 [DOI] [PubMed] [Google Scholar]
- 55. Villines TC, Hulten EA, Shaw LJ, Goyal M, Dunning A, Achenbach S, et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) Registry. J Am Coll Cardiol 2011;58:2533–40. 10.1016/j.jacc.2011.10.851 [DOI] [PubMed] [Google Scholar]
- 56. Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, et al. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM registry (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry). Circulation 2012;126:304–13. 10.1161/CIRCULATIONAHA.111.081380 [DOI] [PubMed] [Google Scholar]
- 57. Rana JS, Dunning A, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, et al. Differences in prevalence, extent, severity, and prognosis of coronary artery disease among patients with and without diabetes undergoing coronary computed tomography angiography: results from 10,110 individuals from the CONFIRM (COronary CT Angiography Evaluation For Clinical Outcomes): an InteRnational Multicenter Registry. Diabetes Care 2012;35:1787–94. 10.2337/dc11-2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dwivedi G, Cocker M, Yam Y, Achenbach S, Al-Mallah M, Berman DS, et al. Predictive value of cardiac computed tomography and the impact of renal function on all cause mortality (from coronary computed tomography angiography evaluation for clinical outcomes). Am J Cardiol 2013;111:1563–9. 10.1016/j.amjcard.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 59. Otaki Y, Gransar H, Berman DS, Cheng VY, Dey D, Lin FY, et al. Impact of family history of coronary artery disease in young individuals (from the CONFIRM registry). Am J Cardiol 2013;111:1081–6. 10.1016/j.amjcard.2012.12.042 [DOI] [PubMed] [Google Scholar]
- 60. Arsanjani R, Berman DS, Gransar H, Cheng VY, Dunning A, Lin FY, et al. Left ventricular function and volume with coronary CT angiography improves risk stratification and identification of patients at risk for incident mortality: results from 7758 patients in the prospective multinational CONFIRM observational cohort study. Radiology 2014;273:70–7. 10.1148/radiol.14122816 [DOI] [PubMed] [Google Scholar]
- 61. Opolski MP, Hartaigh BO, Berman DS, Budoff MJ, Achenbach S, Al-Mallah M, et al. Current trends in patients with chronic total occlusions undergoing coronary CT angiography. Heart 2015;101:1212–8. 10.1136/heartjnl-2014-306616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nurmohamed NS, Bom MJ, Jukema RA, de Groot RJ, Driessen RS, van Diemen PA, et al. AI-guided quantitative plaque staging predicts long-term cardiovascular outcomes in patients at risk for atherosclerotic CVD. JACC Cardiovasc Imaging 2024;17:269–80. 10.1016/j.jcmg.2023.05.020 [DOI] [PubMed] [Google Scholar]
- 63. Lin A, Manral N, McElhinney P, Killekar A, Matsumoto H, Kwiecinski J, et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: an international multicentre study. Lancet Digit Heal 2022;4:e256–65. 10.1016/S2589-7500(22)00022-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish Computed Tomography of the HEART). Circulation 2020;141:1452–62. 10.1161/CIRCULATIONAHA.119.044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schulman-Marcus J, Hartaigh BÓ, Gransar H, Lin F, Valenti V, Cho I, et al. Sex-Specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events the CONFIRM long-term registry. JACC Cardiovasc Imaging 2016;9:364–72. 10.1016/j.jcmg.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–32. 10.1161/CIRCULATIONAHA.116.024360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;78:e187–285. 10.1016/j.jacc.2021.07.053 [DOI] [PubMed] [Google Scholar]
- 68. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407–77. 10.1093/eurheartj/ehz425 [DOI] [PubMed] [Google Scholar]
- 69. Hadamitzky M, Achenbach S, Al-Mallah M, Berman D, Budoff M, Cademartiri F, et al. Optimized prognostic score for coronary computed tomographic angiography: results from the CONFIRM registry (COronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry). J Am Coll Cardiol 2013;62:468–76. 10.1016/j.jacc.2013.04.064 [DOI] [PubMed] [Google Scholar]
- 70. Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. 10.1161/CIRCIMAGING.113.001047 [DOI] [PubMed] [Google Scholar]
- 71. Chang H-J, Lin FY, Lee S-E, Andreini D, Bax J, Cademartiri F, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol 2018;71:2511–22. 10.1016/j.jacc.2018.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ferencik M, Mayrhofer T, Bittner DO, Emami H, Puchner SB, Lu MT, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol 2018;3:144–52. 10.1001/jamacardio.2017.4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oeing CU, Matheson MB, Ostovaneh MR, Rochitte CE, Chen MY, Pieske B, et al. Coronary artery disease grading by cardiac CT for predicting outcome in patients with stable angina. J Cardiovasc Comput Tomogr 2023;17:310–7. 10.1016/j.jcct.2023.07.004 [DOI] [PubMed] [Google Scholar]
- 74. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol 2015;66:337–46. 10.1016/j.jacc.2015.05.069 [DOI] [PubMed] [Google Scholar]
- 75. Lee SE, Sung JM, Rizvi A, Lin FY, Kumar A, Hadamitzky M, et al. Quantification of coronary atherosclerosis in the assessment of coronary artery disease. Circ Cardiovasc Imaging 2018;11:e007562. 10.1161/CIRCIMAGING.117.007562 [DOI] [PubMed] [Google Scholar]
- 76. van Rosendael AR, Lin FY, van den Hoogen IJ, Ma X, Gianni U, Al Hussein Alawamlh O, et al. Progression of whole-heart atherosclerosis by coronary CT and major adverse cardiovascular events. J Cardiovasc Comput Tomogr 2021;15:322–30. 10.1016/j.jcct.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 77. Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 2018;11:1475–84. 10.1016/j.jcmg.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 78. Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 2010;55:2399–407. 10.1016/j.jacc.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 79. Figtree GA, Adamson PD, Antoniades C, Blumenthal RS, Blaha M, Budoff M, et al. Noninvasive plaque imaging to accelerate coronary artery disease drug development. Circulation 2022;146:1712–27. 10.1161/CIRCULATIONAHA.122.060308 [DOI] [PubMed] [Google Scholar]
- 80. Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch K, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2015;2:e52–e63. 10.1016/S2352-3018(14)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu MT, Ribaudo H, Foldyna B, Zanni MV, Mayrhofer T, Karady J, et al. REPRIEVE Trial Writing Group. Effects of pitavastatin on coronary artery disease and inflammatory biomarkers in HIV: mechanistic substudy of the REPRIEVE randomized clinical trial. JAMA Cardiol 2024;21:e235661. 10.1001/jamacardio.2023.5661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJP, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA 2016;316:2373–84. 10.1001/jama.2016.16951 [DOI] [PubMed] [Google Scholar]
- 83. Räber L, Ueki Y, Otsuka T, Losdat S, Häner JD, Lonborg J, et al. Effect of alirocumab added to high-intensity statin therapy on coronary atherosclerosis in patients with acute myocardial infarction: the PACMAN-AMI randomized clinical trial. JAMA 2022;327:1771–81. 10.1001/jama.2022.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nicholls SJ, Kataoka Y, Nissen SE, Prati F, Windecker S, Puri R, et al. Effect of evolocumab on coronary plaque phenotype and burden in statin-treated patients following myocardial infarction. JACC Cardiovasc Imaging 2022;15:1308–21. 10.1016/j.jcmg.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 85. Hirai K, Imamura S, Hirai A, Ookawara S, Morishita Y. Effect of evolocumab on vulnerable coronary plaques: a serial coronary computed tomography angiography study. J Clin Med 2020;9:3338. 10.3390/jcm9103338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 2020;41:3925–32. 10.1093/eurheartj/ehaa652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Rabbat MG, Lakshmanan S, Benjamin MM, Doros G, Kinninger A, Budoff MJ, et al. Benefit of icosapent ethyl on coronary physiology assessed by computed tomography angiography fractional flow reserve: EVAPORATE-FFRCT. Eur Heart J Cardiovasc Imaging 2023;24:866–73. 10.1093/ehjci/jead063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vaidya K, Arnott C, Martínez GJ, Ng B, McCormack S, Sullivan DR, et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC Cardiovasc Imaging 2018;11:305–16. 10.1016/j.jcmg.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 89. Buckler AJ, Doros G, Kinninger A, Lakshmanan S, Le VT, Libby P, et al. Quantitative imaging biomarkers of coronary plaque morphology: insights from EVAPORATE. Front Cardiovasc Med 2023;10:1204071. 10.3389/fcvm.2023.1204071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Van Rosendael AR, Van Den Hoogen IJ, Gianni U, Ma X, Tantawy SW, Bax AM, et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol 2021;6:1257–66. 10.1001/jamacardio.2021.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Henzel J, Kępka C, Kruk M, Makarewicz-Wujec M, Wardziak Ł, Trochimiuk P, et al. High-Risk coronary plaque regression after intensive lifestyle intervention in nonobstructive coronary disease: a randomized study. JACC Cardiovasc Imaging 2021;14:1192–202. 10.1016/j.jcmg.2020.10.019 [DOI] [PubMed] [Google Scholar]
- 92. van Rosendael AR, Narula J, Lin FY, van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, et al. Association of high-density calcified 1 K plaque with risk of acute coronary syndrome. JAMA Cardiol 2020;5:282–90. 10.1001/jamacardio.2019.5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924–33. 10.1056/NEJMoa1805971 [DOI] [PubMed] [Google Scholar]
- 94. Adamson PD, Williams MC, Dweck MR, Mills NL, Boon NA, Daghem M, et al. Guiding therapy by coronary CT angiography improves outcomes in patients with stable chest pain. J Am Coll Cardiol 2019;74:2058–70. 10.1016/j.jacc.2019.07.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Blankstein R, Bittencourt MS, Bhatt DL. Coronary CTA in the evaluation of stable chest pain: clear benefits, but not for all. J Am Coll Cardiol 2017;69:1771–3. 10.1016/j.jacc.2017.02.011 [DOI] [PubMed] [Google Scholar]
- 96. Newby D, Williams M, Hunter A, Pawade T, Shah A, Flapan A, et al. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. 10.1016/S0140-6736(15)60291-4 [DOI] [PubMed] [Google Scholar]
- 97. Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med 2007;356:1503–16. 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 98. Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. 10.1016/S0140-6736(17)32714-9 [DOI] [PubMed] [Google Scholar]
- 100. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395–407. 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. 10.1056/NEJMoa0907272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol 2012;60:e44–164. 10.1016/j.jacc.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 103. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, et al. 2013 ESC guidelines on the management of stable coronary artery disease. Eur Heart J 2013;34:2949–3003. 10.1093/eurheartj/eht296 [DOI] [PubMed] [Google Scholar]
- 104. Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, et al. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med 2018;379:250–9. 10.1056/NEJMoa1803538 [DOI] [PubMed] [Google Scholar]
- 105. Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 106. Rozanski A, Gransar H, Hayes SW, Min J, Friedman JD, Thomson LEJ, et al. Temporal trends in the frequency of inducible myocardial ischemia during cardiac stress testing: 1991 to 2009. J Am Coll Cardiol 2013;61:1054–65. 10.1016/j.jacc.2012.11.056 [DOI] [PubMed] [Google Scholar]
- 107. Al Badarin FJ, Chan PS, Spertus JA, Thompson RC, Patel KK, Kennedy KF, et al. Temporal trends in test utilization and prevalence of ischaemia with positron emission tomography myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging 2020;21:318–25. 10.1093/ehjci/jez159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Knuuti J, Ballo H, Juarez-Orozco LE, Saraste A, Kolh P, Rutjes AWS, et al. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: a meta-analysis focused on post-test disease probability. Eur Heart J 2018;39:3322–30. 10.1093/eurheartj/ehy267 [DOI] [PubMed] [Google Scholar]
- 109. Maurovich-Horvat P, Bosserdt M, Kofoed KF, Rieckmann N, Benedek T, Donnelly P, et al. CT or invasive coronary angiography in stable chest pain. N Engl J Med 2022;386:1591–602. 10.1056/NEJMoa2200963 [DOI] [PubMed] [Google Scholar]
- 110. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al-Khalidi HR, Cavanaugh B, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kannel WB. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979;241:2035–8. 10.1001/jama.1979.03290450033020 [DOI] [PubMed] [Google Scholar]
- 112. Stokes J, Kannel WB, Wolf PA, D’Agostino RB, Adrienne Cupples L. Blood pressure as a risk factor for cardiovascular disease the Framingham study-30 years of follow-up. Hypertension 1989;13:I13–8. 10.1161/01.HYP.13.5_Suppl.I13 [DOI] [PubMed] [Google Scholar]
- 113. Castelli WP, Anderson K, Wilson PWF, Levy D. Lipids and risk of coronary heart disease the Framingham study. Ann Epidemiol 1992;2:23–8. 10.1016/1047-2797(92)90033-M [DOI] [PubMed] [Google Scholar]
- 114. Nasir K, Cainzos-Achirica M, Valero-Elizondo J, Ali SS, Havistin R, Lakshman S, et al. Coronary atherosclerosis in an asymptomatic U.S. population: Miami Heart Study at Baptist Health South Florida. JACC Cardiovasc Imaging 2022;15:1604–18. 10.1016/j.jcmg.2022.03.010 [DOI] [PubMed] [Google Scholar]
- 115. Blanke P, Naoum C, Ahmadi A, Cheruvu C, Soon J, Arepalli C, et al. Long-term prognostic utility of coronary CT angiography in stable patients with diabetes mellitus. JACC Cardiovasc Imaging 2016;9:1280–8. 10.1016/j.jcmg.2015.12.027 [DOI] [PubMed] [Google Scholar]
- 116. Dimitriu-Leen AC, Scholte AJHA, Van Rosendael AR, Van Den Hoogen IJ, Kharagjitsingh AV, Wolterbeek R, et al. Value of coronary computed tomography angiography in tailoring aspirin therapy for primary prevention of atherosclerotic events in patients at high risk with diabetes mellitus. Am J Cardiol 2016;117:887–93. 10.1016/j.amjcard.2015.12.023 [DOI] [PubMed] [Google Scholar]
- 117. Kishi S, Magalhães TA, Cerci RJ, Matheson MB, Vavere A, Tanami Y, et al. Total coronary atherosclerotic plaque burden assessment by CT angiography for detecting obstructive coronary artery disease associated with myocardial perfusion abnormalities. J Cardiovasc Comput Tomogr 2016;10:121–7. 10.1016/j.jcct.2016.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Viladés Medel D, Leta Petracca R, Carreras Costa F, Cardona Olle M, Barros Membrilla A, Hidalgo Perez JA, et al. Coronary computed tomographic angiographic findings in asymptomatic patients with heterozygous familial hypercholesterolemia and null allele low-density lipoprotein receptor mutations. Am J Cardiol 2013;111:955–61. 10.1016/j.amjcard.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 119. Nakanishi R, Baskaran L, Gransar H, Budoff MJ, Achenbach S, Al-Mallah M, et al. Relationship of hypertension to coronary atherosclerosis and cardiac events in patients with coronary computed tomographic angiography. Hypertension 2017;70:293–9. 10.1161/HYPERTENSIONAHA.117.09402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ahmadi A, Leipsic J, Feuchtner G, Gransar H, Kalra D, Heo R, et al. Is metabolic syndrome predictive of prevalence, extent, and risk of coronary artery disease beyond its components? Results from the multinational coronary CT angiography evaluation for clinical outcome: an international multicenter registry (confirm). PLoS One 2015;10:e0118998. 10.1371/journal.pone.0118998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee H, Park KS, Jeon YJ, Park EJ, Park S, Ann SH, et al. Lipoprotein(a) and subclinical coronary atherosclerosis in asymptomatic individuals. Atherosclerosis 2022;349:190–5. 10.1016/j.atherosclerosis.2021.09.027 [DOI] [PubMed] [Google Scholar]
- 122. Cho I, Al’Aref SJ, Berger A, Hartaigh BÓ, Gransar H, Valenti V, et al. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: a 6-year follow-up from the prospective multicentre international CONFIRM study. Eur Heart J 2018;39:934–41. 10.1093/eurheartj/ehx774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Fuchs A, Kühl JT, Sigvardsen PE, Afzal S, Knudsen AD, Møller MB, et al. Subclinical coronary atherosclerosis and risk for myocardial infarction in a Danish cohort. Ann Intern Med 2023;176:433–42. 10.7326/M22-3027 [DOI] [PubMed] [Google Scholar]
- 124. Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis heart study randomized clinical trial. J Am Coll Cardiol 2005;46:166–72. 10.1016/j.jacc.2005.02.089 [DOI] [PubMed] [Google Scholar]
- 125. Lindholt JS, Søgaard R, Rasmussen LM, Mejldal A, Lambrechtsen J, Steffensen FH, et al. Five-year outcomes of the Danish Cardiovascular Screening (DANCAVAS) trial. N Engl J Med 2022;387:1385–94. 10.1056/NEJMoa2208681 [DOI] [PubMed] [Google Scholar]
- 126. Muhlestein JB, Lappé DL, Lima JAC, Rosen BD, May HT, Knight S, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014;312:2234–43. 10.1001/jama.2014.15825 [DOI] [PubMed] [Google Scholar]
- 127. Valenti V, Hartaigh BÓ, Heo R, Cho I, Schulman-Marcus J, Gransar H, et al. A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. JACC Cardiovasc Imaging 2015;8:900–9. 10.1016/j.jcmg.2015.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease : the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–58. 10.1161/CIRCULATIONAHA.115.018524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sandesara PB, Mehta A, O’Neal WT, Kelli HM, Sathiyakumar V, Martin SS, et al. Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2020;292:224–9. 10.1016/j.atherosclerosis.2019.09.014 [DOI] [PubMed] [Google Scholar]
- 130. Van Der Aalst CM, Denissen SJAM, Vonder M, Gratama JWC, Adriaansen HJ, Kuijpers D, et al. Screening for cardiovascular disease risk using traditional risk factor assessment or coronary artery calcium scoring: the ROBINSCA trial. Eur Heart J Cardiovasc Imaging 2020;21:1216–24. 10.1093/ehjci/jeaa168 [DOI] [PubMed] [Google Scholar]
- 131. Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, et al. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol 2018;72:3233–42. 10.1016/j.jacc.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Miedema MD, Duprez DA, Misialek JR, Blaha MJ, Nasir K, Silverman MG, et al. Use of coronary artery calcium testing to guide aspirin utilization for primary prevention: estimates from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes 2014;7:453–60. 10.1161/CIRCOUTCOMES.113.000690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Indraratna P, Naoum C, Ben ZS, Gransar H, Blanke P, Sellers S, et al. Aspirin and statin therapy for nonobstructive coronary artery disease: five-year outcomes from the CONFIRM registry. Radiol Cardiothorac Imaging 2022;4:e210225. 10.1148/ryct.210225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Hussain A, Ballantyne CM, Nambi V. Zero coronary artery calcium score: desirable, but enough? Circulation 2020;142:917–9. 10.1161/CIRCULATIONAHA.119.045026 [DOI] [PubMed] [Google Scholar]
- 135. Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr 2022;16:124–37. 10.1016/j.jcct.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 136. Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J 2012;33:1007–16. 10.1093/eurheartj/ehr465 [DOI] [PubMed] [Google Scholar]
- 137. Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology 2010;257:516–22. 10.1148/radiol.10100681 [DOI] [PubMed] [Google Scholar]
- 138. Fujimoto S, Kondo T, Kodama T, Fujisawa Y, Groarke J, Kumamaru KK, et al. A novel method for non-invasive plaque morphology analysis by coronary computed tomography angiography. Int J Cardiovasc Imaging 2014;30:1373–82. 10.1007/s10554-014-0461-5 [DOI] [PubMed] [Google Scholar]
- 139. Sheahan M, Ma X, Paik D, Obuchowski NA, St Pierre S, Newman WP, et al. Atherosclerotic plaque tissue: noninvasive quantitative assessment of characteristics with software-aided measurements from conventional CT angiography. Radiology 2018;286:622–31. 10.1148/radiol.2017170127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Choi AD, Marques H, Kumar V, Griffin WF, Rahban H, Karlsberg RP, et al. CT evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): a multi-center, international study. J Cardiovasc Comput Tomogr 2021;15:470–6. 10.1016/j.jcct.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 141. Tzimas G, Gulsin GS, Everett RJ, Akodad M, Meier D, Sewnarain K, et al. Age- and sex-specific nomographic CT quantitative plaque data from a large international cohort. JACC Cardiovasc Imaging 2024;17:165–75. 10.1016/j.jcmg.2023.05.011 [DOI] [PubMed] [Google Scholar]
- 142. Cury RC, Blankstein R, Leipsic J, Abbara S, Achenbach S, Berman D, et al. CAD-RADSTM 2.0–2022 coronary artery disease—reporting and data system an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2022;16:536–57. 10.1016/j.jcct.2022.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this article.