Abstract
Genetic and non-genetic factors contribute to obsessive-compulsive disorder (OCD), with strong evidence of familial clustering. Genomic studies in psychiatry have used the concepts of families that are “simplex” (one affected) versus “multiplex” (multiple affected). Our study compares demographic and clinical data from OCD probands in simplex and multiplex families to uncover potential differences. We analyzed 994 OCD probands (501 multiplex, 493 simplex) from the Brazilian Research Consortium on Obsessive-Compulsive Spectrum Disorders (C-TOC). Clinicians administered the Structured Clinical Interview for DSM-IV (SCID-IV) to diagnose, Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) to assess severity, and Dimensional Yale–Brown Obsessive-Compulsive Scale (DY-BOCS) to assess symptom dimensionality. Demographics, clinical history, and family data were collected. Compared to simplex probands, multiplex probands had earlier onset, higher sexual/religious and hoarding dimensions severity, increased comorbidity with other obsessive-compulsive-related disorders (OCRD), and higher family history of psychiatric disorders. These comparisons provide the first insights into demographic and clinical differences between Latin American simplex and multiplex families with OCD. Distinct clinical patterns may suggest diverse genetic and environmental influences. Further research is needed to clarify these differences, which have implications for symptom monitoring and management.
Keywords: Obsessive-compulsive disorder, Family history, Clinical differences, Latin America
1. Introduction
Obsessive-compulsive disorder (OCD) is a chronic neuropsychiatric condition characterized by the presence of obsessions (intrusive and recurrent thoughts, impulses, or mental images) and compulsions (repetitive and persistent behaviors or mental acts performed in response to obsessions) (American Psychiatric Association et al., 2013; Stein et al., 2019). OCD is a heterogeneous disorder (Mataix-Cols et al., 2013; Miguel et al., 2005) that affects approximately 1 to 3 % of the global population (Leckman et al., 2010) and typically emerges during childhood or adolescence (Miguel et al., 2008; Ruscio et al., 2010).
Most individuals with OCD experience at least one co-existing psychiatric disorder, such as mood, anxiety, and impulsive disorders (Mahjani et al., 2022; Ruscio et al., 2010). OCD is also associated with a high risk of developing chronic tic disorders, including Tourette Syndrome (TS) (Brander et al., 2021; Browne et al., 2015). Additionally, OCD is frequently linked to other obsessive-compulsive related disorders (OCRD), such as body dysmorphic disorder (BDD), trichotillomania (TTM), and skin-picking (American Psychiatric Association et al., 2013). Although the hallmarks of OCD are obsessions and compulsions, individual patients differ with regard to specific OCD symptom dimensions (Rosario-Campos et al., 2006), age of symptom onset, comorbidity profile, and family history of OCD (Brakoulias et al., 2017; Mattina and Steiner, 2016).
Genetic and non-genetic factors have been implicated in OCD (Fernandez et al., 2018; Hirschtritt et al., 2017; Mahjani et al., 2020; Stein et al., 2019), and more work is needed to provide a comprehensive picture of its risk factors. Twin studies first demonstrated a significant familial and genetic component to OCD, with ~40 % heritability (Browne et al., 2014; Taylor, 2011a). Recent work by Mahjani et al. with 822,843 individuals from a national Swedish epidemiological sample supports this, providing heritability estimates of 32–35 % and evidence for significant maternal effects in OCD risk (Mahjani et al., 2020). Studies have estimated single-nucleotide polymorphism (SNP) based heritability at 25–43 %, with potentially higher estimates for childhood-onset OCD. Common and rare genetic variants are associated with risk for OCD (International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS), 2018; Mahjani et al., 2020). Nevertheless, a recent genome-wide association study (GWAS) with 14, 140 OCD cases led to just a single genome-wide significant common variant finding (Strom et al., 2021), and there are only two sufficiently powered studies using whole-exome sequencing (WES) to detect rare variants in OCD (Cappi et al., 2020; Halvorsen et al., 2021), suggesting CHD8 as a risk gene for the disorder. These studies indicate that increasing sample size will lead to the identification of additional OCD risk loci and genes, as in other psychiatric disorders (Demontis et al., 2019; Fu et al., 2022; Grove et al., 2019; Satterstrom et al., 2020; Wray et al., 2018).
In psychiatry, families with multiple affected subjects are called “multigenerational families” (Mataix-Cols et al., 2013; Mathews et al., 2007). In the psychiatric genomics field, multigenerational families are also called “multiplex,” while families with only one affected are called “simplex” (Constantino et al., 2010; Gerdts et al., 2013; Hoffmann et al., 2014; Itsara et al., 2010; Klei et al., 2012; Leppa et al., 2016; Ronemus et al., 2014; Sanders, 2013; Sebat et al., 2007; Virkud et al., 2009; Wroten et al., 2023). In OCD, Cappi et al. performed WES in simplex families and observed that rates of de novo likely protein-truncating variation (PTV) (i.e., creation or loss of a stop codon, disruption of a canonical splice site, or a frameshift indel) are significantly elevated in OCD simplex trios compared to controls (Cappi et al., 2020). Wang et al. observed an overrepresentation of de novo damaging variants in simplex, but not multiplex, families of TS (Wang et al., 2018), though the sample size was limited. Similarly, different genetic mechanisms are involved in childhood attention deficit hyperactivity disorder (ADHD) (individuals diagnosed with ADHD in childhood) and late-diagnosed ADHD (individuals diagnosed with their first ADHD diagnosis as adults). Childhood ADHD showed a greater overlap with hyperactivity and autism, as well as a higher burden of rare variants, while late-diagnosed ADHD, which exhibited overlap with depression, did not show an increased burden of rare variants (Rajagopal et al., 2022). Although these studies show that genetic risk mechanisms may differ between simplex and multiplex families, no genomic studies have examined whether demographic and clinical variables differ between probands of these families.
Up to now, there have been two published reviews and meta-analyses concerning the genetic epidemiology of OCD. The initial review from 2001 encompassed 4 family studies on OCD, yet none originated from Latin America (Hettema et al., 2001). A more recent review and meta-analysis analyzed 24 family studies on OCD and chose to focus on 14 of them (Blanco-Vieira et al., 2023). These studies were pre-dominantly conducted in the United States and Nordic countries, with no studies focused on the Latin American demographic. Furthermore, they did not estimate the prevalence of OCD symptom dimensions in probands within simplex or multiplex families, nor did they characterize the comorbidity profiles.
A greater understanding of how family history impacts the clinical presentation of OCD may shed light on the etiology of OCD, including risk factors and different heritability patterns (Berends et al., 2019). Characterizing families and investigating family history is foundational for differentiating between diagnoses, assessing genetic risks, and guiding genetic testing, healthcare, and patient support. Beyond its informational value, the collection of family history also facilitates the examination of family dynamics, support systems, risk perceptions, and direct experiences with the disorder (Guttmacher et al., 2004; Walter and Emery, 2005; Slomp et al., 2018). This study aims to analyze the phenotypic and clinical differences between simplex and multiplex probands with OCD from a large Brazilian sample. We hypothesize that multiplex probands exhibit a distinct pattern of OCD phenotypes compared to simplex probands.
2. Methods
2.1. Ethics
This study is part of The Brazilian Research Consortium on Obsessive-Compulsive Spectrum Disorders (C-TOC) and was approved by the local ethics committee of each participating site. All participants gave written informed consent. Participants were interviewed in person by a trained psychologist or psychiatrist for 3 to 6 h. All clinicians were trained for reliability in the use of the assessment instruments. Recruitment information is in Fig. 1, and more detailed information about the Consortium and procedures regarding recruitment, instruments, and methods can be found in Miguel et al. (2008).
Fig. 1. Recruitment Flowchart.
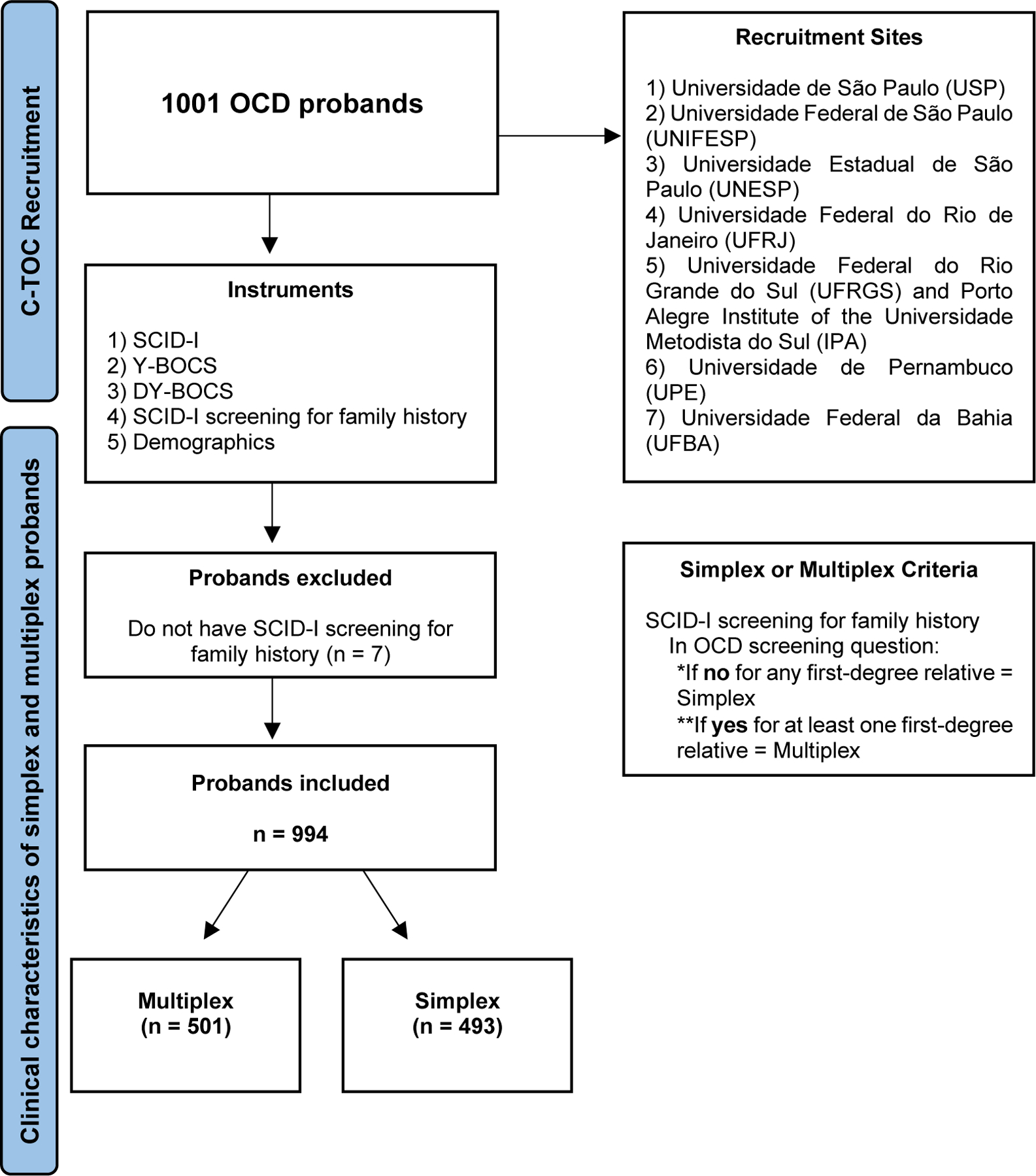
Illustrates the participant recruitment flow. A total of 994 probands with OCD were selected across seven recruitment sites, with inclusion criteria requiring a primary diagnosis of OCD, to be in treatment at one of the centers, and the ability to engage in the research protocol. Following the research protocol, involving interviews with five instruments (SCID-I, Y-BOCS, DY-BOCS, SCID-I screening, and Demographics), participants were categorized into simplex (n = 493) or multiplex (n = 501) families.
2.2. Participants
Nine hundred ninety-four probands with a primary OCD diagnosis were recruited between 2006 and 2015 by C-TOC, a cross-sectional study conducted by seven university sites specialized in OCD in Brazil. We consider “simplex” the families where only the proband has OCD (n = 493), and “multiplex” the families where the proband has OCD and at least one first-degree relative (FDR) has OCD or obsessive-compulsive symptoms (OCS) (n = 501). We included OCS in the multiplex group since well-controlled family studies support a shared genetic component between OCD and OCS (do Rosario-Campos et al., 2005; Hanna et al., 2005; Mathews et al., 2007; Nestadt et al., 2000; Taylor, 2011b). Additionally, Bralten et al. have demonstrated a genetic overlap between OCD and OCS using polygenic risk scores from OCD GWAS data (Bralten et al., 2020).
2.3. Clinical measures
For this study, we compared groups for the presence of current and past psychiatric diagnoses using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID-IV) – Axis I Disorders (Schizophrenia; Mood Disorders; Substances Use Disorders; Anxiety Disorders; Eating Disorders; and Impulse Control Disorders).
We aggregated the psychiatric comorbidities into a numerical variable referred to as the “number of psychiatric comorbidities”. To classify OCRD, we created a group that includes BDD, skin-picking, and TTM. Consistent with DSM-V, this classification is supported by evidence that these disorders share a common genetic basis (American Psychiatric Association et al., 2013; Mathews et al., 2008; Phillips et al., 2010).
We considered the age of onset as the age of the first OCD symptom regardless of distress, and the OCD duration was calculated by subtracting the age of onset from the age at assessment. Due to their higher negative correlation (Pearson correlation: R = −0.31, p < 2.2e-16), and considering the bias of duration of illness variable, which can vary depending on the proband’s age, we opted to use the age of onset variable in the statistical model.
The severity of OCD symptoms was measured by the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989); Y-BOCS scores range from 0 to 40, with higher scores corresponding to higher severity. To assess the severity of specific OCD symptom dimensions (obsessions and compulsions related to aggression, injury, violence, and natural disasters; obsessions and compulsions related to sexual, moral, and religion; obsessions and compulsions related to symmetry/just-right and compulsions to count, order and arrange; obsessions to contamination and cleaning compulsions; obsessions and compulsions related to hoarding and miscellaneous obsessions and compulsions related to somatic concerns, superstitions and other symptoms), we used the Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS) (Rosario-Campos et al., 2006). The score of each DY-BOCS dimension ranges from 0 to 15, and the global score ranges from 0 to 30.
We investigated the psychiatric diagnoses in family members using the screening session of the SCID-IV. Additionally, the rating clinicians assessed whether any first or second-degree relatives had received a diagnosis of OCD from a qualified mental health clinician.
Fig. 1. Recruitment flowchart.
2.4. Statistical analysis
Normality assumptions for the continuous variables were checked using the Shapiro–Wilk test. Logistic regression models, adjusted for age and sex, were used to compare simplex and multiplex probands in sociodemographic and clinical variables. Statistically significant variables in those models were selected as predictor variables in a multiple logistic regression model, adjusted for age and sex. In all logistic regression models, the response variable was the proband group (simplex vs. multiplex), with the reference level being the multiplex group. Odds ratios (ORs) for predictor variables were obtained by exponentiating their respective coefficients estimated in the models. 95 % confidence intervals for those ORs were estimated using the profile likelihood method.
3. Results
3.1. Sample demographics
The sample comprised 994 adults with OCD (493 simplex, 501 multiplex), with a mean age of 34.8 years (34.8 years for simplex and 34.9 years for multiplex) at the time of assessment. Most probands were female (55 % simplex, 58.9 % multiplex). There were no statistically significant differences in age and sex between the groups.
Most probands in both simplex and multiplex groups were categorized as white (80.9 % simplex, 87.8 % multiplex). However, the proportion of white and black probands was different between the groups (p < 0.01). The mean years of education were significantly higher in multiplex probands compared to simplex probands, respectively 15.2 and 13.9 (p < 0.01) (Table 1).
Table 1:
Sociodemographic characteristics and clinical features of probands
| Total (n = 994) | Simplex (n = 493) | Multiplex (n = 501) | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Sociodemographic | ||||||
|
| ||||||
| Age at assessment [mean years (SD)] | 34.8 (13) | 34.8 (13.3) | 34.9 (12.7) | 1.00 | (0.99 – 1.01) | 0.927 |
| Sex (Women) [n (%)] | 566 (56.9%) | 271 (55%) | 295 (58.9%) | 1.18 | (0.91 – 1.52) | 0.214 |
| Race (Black) [n (%)] | 153 (15.4%) | 93 (19.1%) | 60 (12.2%) | 0.59 | (0.41 – 0.84) | <0.01 |
| Years of Education [mean (SD)] | 14.5 (4.9) | 13.9 (4.6) | 15.2 (5.2) | 1.06 | (1.03 – 1.09) | <0.01 |
|
| ||||||
| Clinical Features | ||||||
|
| ||||||
| Age of OCD Onset [mean years (SD)] | 12.6 (7.5) | 13.7 (7.8) | 11.4 (6.6) | 0.95 | (0.93 – 0.97) | <0.01 |
| OCD duration [mean years (SD)] | 22.3 (13.6) | 21 (13.6) | 23.6 (12.7) | 1.05 | (1.03 – 1.07) | <0.01 |
| Y-BOCS Total Score [mean (SD)] | 25.5 (7.5) | 25.4 (7.5) | 25.7 (7.5) | 1.00 | (0.99 – 1.02) | 0.634 |
| DY-BOCS Global Score [mean (SD)] | 21.1 (6.3) | 20.9 (6.3) | 21.4 (6.2) | 1.01 | (0.99 – 1.03) | 0.327 |
| DY-BOCS Aggressive Score [mean (SD)] | 5.3 (4.9) | 5.1 (5) | 5.4 (4.9) | 1.01 | (0.99 – 1.04) | 0.393 |
| DY-BOCS Sexual/Religious Score [mean (SD)] | 4.3 (4.9) | 3.9 (4.8) | 4.7 (5) | 1.03 | (1.01 – 1.06) | <0.01 |
| DY-BOCS Symmetry Score [mean (SD)] | 7.3 (4.6) | 7.1 (4.7) | 7.5 (4.6) | 1.02 | (0.99 – 1.04) | 0.271 |
| DY-BOCS Contamination Score [mean (SD)] | 6.2 (5.1) | 6 (5.2) | 6.4 (5.1) | 1.01 | (0.99 – 1.04) | 0.359 |
| DY-BOCS Hoarding Score [mean (SD)] | 3.1 (4.1) | 2.7 (3.8) | 3.6 (4.3) | 1.05 | (1.02 – 1.09) | <0.01 |
| DY-BOCS Miscellaneous Score [mean (SD)] | 7.5 (4.7) | 7.5 (4.7) | 7.6 (4.7) | 1.00 | (0.97 – 1.03) | 0.906 |
| Number of comorbidities [mean (SD)] | 3.2 (2.6) | 3.1 (2.5) | 3.4 (2.6) | 1.05 | (1 – 1.1) | 0.058 |
| Major Depression Disorder (MDD) comorbidity [n (%)] | 669 (67.3%) | 318 (64.5%) | 351 (70.1%) | 1.29 | (0.99 – 1.68) | 0.065 |
| Generalized Anxiety Disorder (GAD) comorbidity [n (%)] | 333 (33.5%) | 151 (30.7%) | 182 (36.6%) | 1.30 | (0.99 – 1.69) | 0.055 |
| Social Phobia comorbidity [n (%)] | 316 (31.8%) | 156 (31.6%) | 160 (31.9%) | 1.02 | (0.78 – 1.34) | 0.858 |
| Specific Phobia comorbidity [n (%)] | 306 (30.8%) | 158 (32.2%) | 148 (29.6%) | 0.87 | (0.66 – 1.14) | 0.320 |
| Tics Disorder or TS comorbidity [n (%)] | 186 (18.7%) | 90 (18.3%) | 96 (19.2%) | 1.08 | (0.78 – 1.49) | 0.645 |
| Post Traumatic Stress Disorder (PTSD) comorbidity [n (%)] | 98 (9.8%) | 55 (11.4%) | 43 (8.9%) | 0.74 | (0.48 – 1.13) | 0.161 |
| OCRD comorbidity [n (%)] | 261 (26.2%) | 113 (22.9%) | 148 (29.5%) | 1.40 | (1.05 – 1.87) | 0.023 |
| Family history of psychiatric disorders (excluding OCD) [n (%)] | 863 (86.8%) | 412 (83.6%) | 451 (90%) | 1.75 | (1.20 – 2.57) | <0.01 |
SD: Standard deviation; OR: Odds Ratio / 95% CI: 95% Confidence Interval; p-values calculated using logistic regression
3.2. Simplex versus multiplex analysis
Compared to simplex probands, multiplex probands exhibited an earlier age of onset (mean: 13.7 years for simplex, 11.4 years for multiplex; p < 0.01). The Y-BOCS total score and DY-BOCS global score were not significantly different between the groups. However, multiplex probands had higher severity in the DY-BOCS dimensions of Sexual/Religious (mean: 3.9 for simplex, 4.7 for multiplex; p < 0.01) and Hoarding (mean: 2.7 for simplex, 3.6 for multiplex; p < 0.01). No significant differences were observed between the two groups regarding other DY-BOCS dimensions or Y-BOCS scores (Table 1).
The variable “number of psychiatric comorbidities”, which determines the total number of psychiatric comorbidities presented by the proband at the time of the interview, was not significantly different between the groups. The most common comorbid disorders in the whole sample were MDD, with frequencies of 64.5 % for simplex and 70.1 % for multiplex probands (p = 0.065), followed by GAD, with frequencies of 30.7 % for simplex and 36.6 % for multiplex probands (p = 0.055). However, multiplex probands had a higher frequency of OCRD comorbidity compared to simplex probands, respectively 29.5 % and 22.9 %, (p = 0.023) (Table 1). Furthermore, a higher prevalence of family history of psychiatric disorders, other than OCD, was found in the multiplex group (90 %) compared to the simplex group (83.6 %) (p < 0.01) (Table 1).
Finally, we performed a multiple logistic regression model using the statistically significant variables (p < 0.05) in the previous model, along with age and sex, as independent predictor variables. The following characteristics remained associated with multiplex probands: higher proportion of self-reported white race (OR: 1.71, p < 0.01), higher years of education (OR: 1.06, p < 0.01), earlier age of onset (OR: 0.96, p = 0), higher DY-BOCS Sexual/Religious severity score (OR: 1.03, p = 0.011), higher DY-BOCS Hoarding severity score (OR: 1.05, p < 0.01), increased OCRD comorbidities (OR: 1.41, p = 0.018) and higher prevalence family history of psychiatric disorders (OR: 1.77, p < 0.01). Self-reported black race was more prevalent in simplex probands (OR: 0.59, p < 0.01) (Table 2).
Table 2:
Multiple Logistic Regression Model
| OR | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.00 | 0.99 – 1.01 | 0.902 |
| Sex (Women) | 1.17 | 0.91 – 1.51 | 0.213 |
| Race (White) | 1.71 | 1.2 – 2.44 | < 0.01 |
| Years of education | 1.06 | 1.03 – 1.08 | < 0.01 |
| Age of onset (in years) | 0.96 | 0.94 – 0.97 | 0 |
| DY-BOCS Sexual/Religious Score | 1.03 | 1.01 – 1.06 | 0.011 |
| DY-BOCS Hoarding Score | 1.05 | 1.02 – 1.09 | < 0.01 |
| OCRD Comorbidity (Presence) | 1.41 | 1.06 – 1.88 | 0.018 |
| Family history of psychiatric disorders (excluding OCD) | 1.77 | 1.22 – 2.6 | < 0.01 |
OR: Odds Ratio / 95% CI: 95% Confidence Interval
4. Discussion
This study investigated demographic and clinical variables associated with simplex (n = 493) and multiplex (n = 501) families of 994 Brazilian probands with OCD. Our findings suggest that OCD probands from multiplex families demonstrate several distinguishing characteristics compared to simplex OCD probands. These include an earlier onset of OCD symptoms, higher severity in the sexual/religious and hoarding symptom dimensions, a higher prevalence of OCRD, and a higher occurrence of psychiatric disorders in their family history.
To our knowledge, this is the first study to explore and compare demographic and clinical characteristics of simplex and multiplex probands with OCD. In our study, the overall sample primarily comprised of women and individuals of white race with similar educational backgrounds, primarily at the high school level. The C-TOC collected data from seven sites located in three regions of Brazil (South, Southeast, and Northeast). Additionally, the study conducted by Miguel et al., using the same sample, revealed that the Northeast region had the lowest proportion of white patients, while the South and Southeast regions had a higher concentration of white patients (Miguel et al., 2008). Nevertheless, it is noteworthy that simplex families exhibited a higher representation of black probands and displayed a statistically significant difference in educational attainment. However, the specific interplay of these characteristics in the context of OCD remains largely uninvestigated.
OCD probands from multiplex families experienced their first symptoms at an earlier age than those from simplex families. These findings are in line with previous studies that have likewise observed associations between first-degree relatives affected by OCD and earlier age of OCD onset in the proband (Geller et al., 1998; Mataix-Cols et al., 2013; Nestadt et al., 2000).
Our analysis demonstrates a similar number of comorbidities for probands from simplex and multiplex families. However, our findings indicate that OCRD, particularly BDD, TTM, and skin-picking, were more prevalent in multiplex probands. Previous studies have established that OCD often co-occurs with related disorders (Bienvenu et al., 2000; Cullen et al., 2007; Frías et al., 2015; LaSalle et al., 2004; Strom et al., 2021; Tükel et al., 2002), however, our research is pioneering in showing that this comorbidity is more common in multiplex families than in simplex families. Consistent with our results, early onset (i.e., before 10 years of age) has been associated with a specific profile regarding concurrent psychiatric conditions, including tic disorders, TMM, and BDD (Hemmings et al., 2004; de Mathis et al., 2009).
Our findings suggest that multiplex probands are more likely to have a family history of other psychiatric disorders than simplex probands. Further studies are needed to better understand the relationship between simplex and multiplex OCD families and the presence of psychiatric comorbidities.
Our analysis did not find any evidence of differences between simplex and multiplex probands concerning the severity of OCD symptoms. However, the severity of sexual/religious and hoarding symptoms was higher in subjects from multiplex families. In line with this, consistent evidence from multiple genetic methodologies indicates that symptom dimensions may have distinct genetic architectures. For instance, investigations within multiplex families have demonstrated increased concordance among family members for the aggressive/sexual/religious, symmetry/ordering, and hoarding dimensions (Brakoulias et al., 2016; Leckman et al., 2003). In addition, family and twin studies have yielded divergent findings concerning the heritability of specific dimensions of OCD symptoms (Alemany-Navarro et al., 2020; Alsobrook et al., 1999; Brakoulias et al., 2016; Hasler et al., 2007; Leckman et al., 2003). Finally, genotyping 399 individuals with OCD, Alemany-Navarro et al. identified distinct biological pathways associated with the aggressive, ordering, sexual/religious, and hoarding dimensions, implying specific genetic mechanisms for each symptom dimension, with hoarding standing out (Alemany-Navarro et al., 2020).
This study addressed a critical gap in the OCD literature by extending a well-established family study methodology to populations previously overlooked in genetic studies. Zhang et al. found that high-income countries produce 88 % of psychiatric research, with the largest shares from the US (32.68 %), UK (8.59 %), Germany (6.77 %), Australia (5.87 %), and Canada (4.9 %). Middle-income countries contribute 12 %, while less than 1 % comes from low-income countries (Zhang et al., 2017). Challenges to the generalizability of psychological research persist due to an overreliance on Western, Educated, Industrialized, Rich, and Democratic (WEIRD) samples, despite efforts to enhance inclusive collaboration and address cultural diversity, authorship diversity, and reproducibility issues (Towards a Global Psychological Science, 2022). Therefore, this manuscript represents an initial step toward broader genetic research into OCD across diverse populations. Furthermore, despite the substantial progress in psychiatric genomics through GWAS studies, there remains a significant imbalance in subject representation, with approximately 78 % of participants having European ancestry, while only 1.3 % of GWAS samples originate from Latin America (Riehm et al., 2023; Sirugo et al., 2019; Cavazos and Witte, 2021). Recent studies show that including non-European subjects increases the number of risk genomic associations and contributes to the mapping of new risk genomic regions (Riehm et al., 2023). This has raised concerns, not only from an ethical perspective in medicine but also regarding scientific implications, as GWAS study findings may not be replicable in diverse populations. Furthermore, Latin America stands out for its population diversity, characterized by variations in allelic frequencies due to historical migration patterns, with Brazil being the most populous nation in the region (Ruiz-Linares et al., 2014; Fonseca et al., 2021). Thus, increasing studies characterizing the samples from these countries is of fundamental importance.
The primary limitation of our study is the indirect method used to obtain the psychiatric family history, rather than directly interviewing the family members themselves. We recognize that direct interviews are considered the gold standard for collecting clinical data in family studies. Nonetheless, prior research has not found statistically significant differences in the clinical data collected from relatives through direct versus indirect interviews (Rougemont-Buecking et al., 2008; Vandeleur et al., 2015). Additionally, it has been noted that individuals with a disorder tend to report familial occurrences of the same disorder (Vandeleur et al., 2015), which suggests direct interviews might yield more precise data. Despite this, we are confident that our method for collecting clinical data from relatives serves as a reasonably accurate approximation of the gold standard. Another limitation is related to the variability in the expression of comorbidities during the lifetime of OCD, which can change the classification of the types of families proposed in this study. In this sense, Yaryura-Tobias et al. (2000) reported a preferential temporal sequence of comorbid conditions in OCD over a person’s lifespan. According to those authors, it may be likely for an anxiety disorder, a mood disorder, an eating disorder, or a tic disorder to manifest first in patients who later develop a comorbid diagnosis. Furthermore, the periods when these symptoms begin may influence the clinical profile and progression of each disorder (Yaryura-Tobias et al., 2000). Despite the clinical importance of this topic, few studies have adopted a developmental perspective to investigate how the onset age of comorbid disorders affects the course and severity of OCD. A study using the same database as ours reported that OCD is a heterogeneous disorder and that the presence of specific comorbid diagnoses predating the onset of OCD may affect its clinical manifestation (de Mathis et al., 2013).
In conclusion, these results underscore the necessity for more exhaustive genetic and phenotypic investigations to elucidate the origins of this clinical diversity in OCD. Moreover, our findings suggest that the type of family pedigree, whether simplex or multiplex, plays a crucial role in the phenotypic expression of OCD, and despite its significance, this factor is often omitted in genetic studies of OCD.
Acknowledgments
We would like to thank the subjects who participated in this study and all members of The Brazilian Research Consortium of Obsessive-Compulsive Disorder (C-TOC).
Funding
This work was supported by the Sao Paulo Research Foundation (FAPESP) [Grant 2019/23513–0] and was supported in part by funding from the National Institute of Mental Health [Grant 1K99MH128540–01A1 and Grant U01MH125062]. The contents of this publication do not necessarily reflect the views or policies of the NIH. The mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Footnotes
CRediT authorship contribution statement
Monicke O Lima: Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Formal analysis, Writing – review & editing. Leonardo C Saraiva: Formal analysis, Writing – review & editing. Vanessa R Ramos: Investigation, Data curation. Melaine C Oliveira: Formal analysis. Daniel L C Costa: Writing – review & editing. Thomas V Fernandez: Writing – review & editing, Supervision. James J Crowley: Writing – review & editing. Eric A Storch: Writing – review & editing, Supervision. Roseli G Shavitt: Writing – review & editing, Supervision. Euripedes C Miguel: Writing – review & editing, Supervision. Carolina Cappi: Writing – review & editing, Supervision, Conceptualization, Methodology, Data curation, Writing – original draft, Visualization, Formal analysis.
Declaration of Competing Interest
Dr. Shavitt reports receiving research funding to her institution from the Brazilian National Council for Scientific and Technological Development (CNPq) and the Sao Paulo Research Foundation (FAPESP).
Dr. Storch reports receiving research funding to his institution from the Ream Foundation, International OCD Foundation, and NIH. He was a consultant for Brainsway and Biohaven Pharmaceuticals (past 12 months). He owns stock less than $5000 in NView (for distribution of the Y-BOCS and CY-BOCS). He receives book royalties from Elsevier, Wiley, Oxford, American Psychological Association, Guildford, Springer, Routledge, and Jessica Kingsley.
Dr. Fernandez reports receiving research funding to his institution from the National Institute of Mental Health (NIMH) and from the New Venture Fund.
References
- Alemany-Navarro M, Cruz R, Real E, Segalàs C, Bertolín S, Rabionet R, Carracedo Á, Menchón JM, Alonso P, 2020. Looking into the genetic bases of OCD dimensions: a pilot genome-wide association study. Transl. Psychiatry 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsobrook JP II, Leckman JF, Goodman WK, Rasmussen SA, Pauls DL, 1999. Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am. J. Med. Genet. 88, 669–675. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, D.S., Association, A.P., Others, 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric association. Washington, DC. [Google Scholar]
- Berends D, Dissanayake C, Lawson LP, 2019. Differences in cognition and behaviour in multiplex and simplex autism: does prior experience raising a child with autism matter? J. Autism Dev. Disord. 49, 3401–3411. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Samuels JF, Riddle MA, Hoehn-Saric R, Liang KY, Cullen BA, Grados MA, Nestadt G, 2000. The relationship of obsessive-compulsive disorder to possible spectrum disorders: results from a family study. Biol. Psychiatry 48, 287–293. [DOI] [PubMed] [Google Scholar]
- Blanco-Vieira T, Radua J, Marcelino L, Bloch M, Mataix-Cols D, do Rosário MC, 2023. The genetic epidemiology of obsessive-compulsive disorder: a systematic review and meta-analysis. Transl. Psychiatry 13 (1), 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakoulias V, Starcevic V, Belloch A, Brown C, Ferrao YA, Fontenelle LF, Lochner C, Marazziti D, Matsunaga H, Miguel EC, Reddy YCJ, do Rosario MC, Shavitt RG, Shyam Sundar A, Stein DJ, Torres AR, Viswasam K, 2017. Comorbidity, age of onset and suicidality in obsessive-compulsive disorder (OCD): an international collaboration. Compr. Psychiatry 76, 79–86. [DOI] [PubMed] [Google Scholar]
- Brakoulias V, Starcevic V, Martin A, Berle D, Milicevic D, Viswasam K, 2016. The familiality of specific symptoms of obsessive-compulsive disorder. Psychiatry Res. 239, 315–319. [DOI] [PubMed] [Google Scholar]
- Bralten J, Widomska J, Witte WD, Yu D, Mathews CA, Scharf JM, Buitelaar J, Crosbie J, Schachar R, Arnold P, Lemire M, Burton CL, Franke B, Poelmans G, 2020. Shared genetic etiology between obsessive-compulsive disorder, obsessive-compulsive symptoms in the population, and insulin signaling. Transl. Psychiatry 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brander G, Kuja-Halkola R, Rosenqvist MA, Rück C, Serlachius E, Fernández de la Cruz L, Lichtenstein P, Crowley JJ, Larsson H, Mataix-Cols D, 2021. A population-based family clustering study of tic-related obsessive-compulsive disorder. Mol. Psychiatry 26, 1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HA, Gair SL, Scharf JM, Grice DE, 2014. Genetics of obsessive-compulsive disorder and related disorders. Psychiatr. Clin. North Am. 37, 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne HA, Hansen SN, Buxbaum JD, Gair SL, Nissen JB, Nikolajsen KH, Schendel DE, Reichenberg A, Parner ET, Grice DE, 2015. Familial clustering of tic disorders and obsessive-compulsive disorder. JAMA Psychiatry 72, 359–366. [DOI] [PubMed] [Google Scholar]
- Cappi C, Oliphant ME, Péter Z, Zai G, Conceição do Rosário M, Sullivan CAW, Gupta AR, Hoffman EJ, Virdee M, Olfson E, Abdallah SB, Willsey AJ, Shavitt RG, Miguel EC, Kennedy JL, Richter MA, Fernandez TV, 2020. De Novo damaging DNA coding mutations are associated with obsessive-compulsive disorder and overlap with Tourette’s disorder and autism. Biol. Psychiatry 87, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazos TB, Witte JS, 2021. Inclusion of variants discovered from diverse populations improves polygenic risk score transferability. HGG Adv. 2 10.1016/j.xhgg.2020.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P, 2010. Sibling recurrence and the genetic epidemiology of autism. Am. J. Psychiatry 167, 1349–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B, Brown CH, Riddle MA, Grados M, Bienvenu OJ, Hoehn-Saric R, Shugart YY, Liang K-Y, Samuels J, Nestadt G, 2007. Factor analysis of the Yale-Brown Obsessive Compulsive Scale in a family study of obsessive-compulsive disorder. Depress. Anxiety 24, 130–138. [DOI] [PubMed] [Google Scholar]
- de Mathis MA, Diniz JB, Hounie AG, Shavitt RG, Fossaluza V, Ferrão Y, Leckman JF, de Bragança Pereira C, do Rosario MC, Miguel EC, 2013. Trajectory in obsessive-compulsive disorder comorbidities. Eur. Neuropsychopharmacol. 23, 594–601. [DOI] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, Baldursson G, Belliveau R, Bybjerg-Grauholm J, Bækvad-Hansen M, Cerrato F, Chambert K, Churchhouse C, Dumont A, Eriksson N, Gandal M, Goldstein JI, Grasby KL, Grove J, Gudmundsson OO, Hansen CS, Hauberg ME, Hollegaard MV, Howrigan DP, Huang H, Maller JB, Martin AR, Martin NG, Moran J, Pallesen J, Palmer DS, Pedersen CB, Pedersen MG, Poterba T, Poulsen JB, Ripke S, Robinson EB, Satterstrom FK, Stefansson H, Stevens C, Turley P, Walters GB, Won H, Wright MJ,, ADHD Working Group of the Psychiatric Genomics Consortium (PGC), Early Lifecourse & Genetic Epidemiology (EAGLE) Consortium, 23andMe Research Team, Andreassen OA, Asherson P, Burton CL, Boomsma DI, Cormand B, Dalsgaard S, Franke B, Gelernter J, Geschwind D, Hakonarson H, Haavik J, Kranzler HR, Kuntsi J, Langley K, Lesch K-P, Middeldorp C, Reif A, Rohde LA, Roussos P, Schachar R, Sklar P, Sonuga-Barke EJS, Sullivan PF, Thapar A, Tung JY, Waldman ID, Medland SE, Stefansson K, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Daly MJ, Faraone SV, Børglum AD, Neale BM, 2019. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 51, 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Rosario-Campos MC, Leckman JF, Curi M, Quatrano S, Katsovitch L, Miguel EC, Pauls DL, 2005. A family study of early-onset obsessive-compulsive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 136B, 92–97. [DOI] [PubMed] [Google Scholar]
- Fernandez TV, Leckman JF, Pittenger C, 2018. Genetic susceptibility in obsessive-compulsive disorder. Handb. Clin. Neurol. 148, 767–781. [DOI] [PubMed] [Google Scholar]
- Fonseca L, Sena BF, Crossley N, Lopez-Jaramillo C, Koenen K, Freimer NB, Bressan RA, Belangero SI, Santoro ML, Gadelha A, 2021. Diversity matters: opportunities in the study of the genetics of psychotic disorders in low- and middle-income countries in Latin America. Braz. J. Psychiatry 43, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías Á, Palma C, Farriols N, González L, 2015. Comorbidity between obsessive-compulsive disorder and body dysmorphic disorder: prevalence, explanatory theories, and clinical characterization. Neuropsychiatr. Dis. Treat. 11, 2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu JM, Satterstrom FK, Peng M, Brand H, Collins RL, Dong S, Wamsley B, Klei L, Wang L, Hao SP, Stevens CR, Cusick C, Babadi M, Banks E, Collins B, Dodge S, Gabriel SB, Gauthier L, Lee SK, Liang L, Ljungdahl A, Mahjani B, Sloofman L, Smirnov AN, Barbosa M, Betancur C, Brusco A, Chung BHY, Cook EH, Cuccaro ML, Domenici E, Ferrero GB, Gargus JJ, Herman GE, Hertz-Picciotto I, Maciel P, Manoach DS, Passos-Bueno MR, Persico AM, Renieri A, Sutcliffe JS, Tassone F, Trabetti E, Campos G, Cardaropoli S, Carli D, Chan MCY, Fallerini C, Giorgio E, Girardi AC, Hansen-Kiss E, Lee SL, Lintas C, Ludena Y, Nguyen R, Pavinato L, Pericak-Vance M, Pessah IN, Schmidt RJ, Smith M, Costa CIS, Trajkova S, Wang JYT, Yu MHC,, Autism Sequencing Consortium (ASC), Broad Institute Center for Common Disease Genomics (Broad-CCDG), iPSYCH-BROAD Consortium, Cutler DJ, De Rubeis S, Buxbaum JD, Daly MJ, Devlin B, Roeder K, Sanders SJ, Talkowski ME, 2022. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet 54, 1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Jones J, Shapiro S, Schwartz S, Park KS, 1998. Obsessive-compulsive disorder in children and adolescents: a review. Harv. Rev. Psychiatry 5, 260–273. [DOI] [PubMed] [Google Scholar]
- Gerdts JA, Bernier R, Dawson G, Estes A, 2013. The broader autism phenotype in simplex and multiplex families. J. Autism Dev. Disord. 43, 1597–1605. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS, 1989. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch. Gen. Psychiatry 46, 1006–1011. [DOI] [PubMed] [Google Scholar]
- Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, Pallesen J, Agerbo E, Andreassen OA, Anney R, Awashti S, Belliveau R, Bettella F, Buxbaum JD, Bybjerg-Grauholm J, Bækvad-Hansen M, Cerrato F, Chambert K, Christensen JH, Churchhouse C, Dellenvall K, Demontis D, De Rubeis S, Devlin B, Djurovic S, Dumont AL, Goldstein JI, Hansen CS, Hauberg ME, Hollegaard MV, Hope S, Howrigan DP, Huang H, Hultman CM, Klei L, Maller J, Martin J, Martin AR, Moran JL, Nyegaard M, Nærland T, Palmer DS, Palotie A, Pedersen CB, Pedersen MG, dPoterba T, Poulsen JB, Pourcain BS, Qvist P, Rehnström K, Reichenberg A, Reichert J, Robinson EB, Roeder K, Roussos P, Saemundsen E, Sandin S, Satterstrom FK, Davey Smith G, Stefansson H, Steinberg S, Stevens CR, Sullivan PF, Turley P, Walters GB, Xu X,, Autism Spectrum Disorder Working Group of the Psychiatric Genomics Consortium, BUPGEN, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, 23andMe Research Team, Stefansson K, Geschwind DH, Nordentoft M, Hougaard DM, Werge T, Mors O, Mortensen PB, Neale BM, Daly MJ, Børglum AD, 2019. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 51, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmacher AE, Collins FS, Carmona RH, 2004. The family history–more important than ever. N. Engl. J. Med. 351, 2333–2336. [DOI] [PubMed] [Google Scholar]
- Halvorsen M, Samuels J, Wang Y, Greenberg BD, Fyer AJ, McCracken JT, Geller DA, Knowles JA, Zoghbi AW, Pottinger TD, Grados MA, Riddle MA, Bienvenu OJ, Nestadt PS, Krasnow J, Goes FS, Maher B, Nestadt G, Goldstein DB, 2021. Exome sequencing in obsessive-compulsive disorder reveals a burden of rare damaging coding variants. Nat. Neurosci. 24, 1071–1076. [DOI] [PubMed] [Google Scholar]
- Hanna GL, Fischer DJ, Chadha KR, Himle JA, Van Etten M, 2005. Familial and sporadic subtypes of early-onset obsessive-compulsive disorder. Biol. Psychiatry 57, 895–900. [DOI] [PubMed] [Google Scholar]
- Hasler G, Pinto A, Greenberg BD, Samuels J, Fyer AJ, Pauls D, Knowles JA, McCracken JT, Piacentini J, Riddle MA, Rauch SL, Rasmussen SA, Willour VL, Grados MA, Cullen B, Bienvenu OJ, Shugart Y-Y, Liang K-Y, Hoehn-Saric R, Wang Y, Ronquillo J, Nestadt G, Murphy DL, OCD Collaborative Genetics Study, 2007. Familiality of factor analysis-derived YBOCS dimensions in OCD-affected sibling pairs from the OCD Collaborative Genetics Study. Biol. Psychiatry 61, 617–625. [DOI] [PubMed] [Google Scholar]
- Hemmings SMJ, Kinnear CJ, Lochner C, Niehaus DJH, Knowles JA, Moolman-Smook JC, Corfield VA, Stein DJ, 2004. Early- versus late-onset obsessive-compulsive disorder: investigating genetic and clinical correlates. Psychiatry Res. 128 (2), 175–182. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS, 2001. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry 158 (10), 1568–1578. [DOI] [PubMed] [Google Scholar]
- Hirschtritt ME, Bloch MH, Mathews CA, 2017. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA 317, 1358–1367. [DOI] [PubMed] [Google Scholar]
- Hoffmann TJ, Windham GC, Anderson M, Croen LA, Grether JK, Risch N, 2014. Evidence of reproductive stoppage in families with autism spectrum disorder: a large, population-based cohort study. JAMA Psychiatry 71, 943–951. [DOI] [PubMed] [Google Scholar]
- International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS), 2018. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol. Psychiatry 23, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itsara A, Wu H, Smith JD, Nickerson DA, Romieu I, London SJ, Eichler EE, 2010. De novo rates and selection of large copy number variation. Genome Res. 20, 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, Willsey AJ, Moreno-De-Luca D, Yu TW, Fombonne E, Geschwind D, Grice DE, Ledbetter DH, Lord C, Mane SM, Martin CL, Martin DM, Morrow EM, Walsh CA, Melhem NM, Chaste P, Sutcliffe JS, State MW, Cook EH Jr, Roeder K, Devlin B, 2012. Common genetic variants, acting additively, are a major source of risk for autism. Mol. Autism 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle VH, Cromer KR, Nelson KN, Kazuba D, Justement L, Murphy DL, 2004. Diagnostic interview assessed neuropsychiatric disorder comorbidity in 334 individuals with obsessive-compulsive disorder. Depress. Anxiety 19, 163–173. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Denys D, Simpson HB, Mataix-Cols D, Hollander E, Saxena S, Miguel EC, Rauch SL, Goodman WK, Phillips KA, Stein DJ, 2010. Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress. Anxiety 27, 507–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Pauls DL, Zhang H, Rosario-Campos MC, Katsovich L, Kidd KK, Pakstis AJ, Alsobrook JP, Robertson MM, McMahon WM, Walkup JT, van de Wetering BJM, King RA, Cohen DJ, Tourette Syndrome Assocation International Consortium for Genetics, 2003. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 116B, 60–68. [DOI] [PubMed] [Google Scholar]
- Leppa VM, Kravitz SN, Martin CL, Andrieux J, Le Caignec C, Martin-Coignard D, DyBuncio C, Sanders SJ, Lowe JK, Cantor RM, Geschwind DH, 2016. Rare inherited and De Novo CNVs reveal complex contributions to ASD risk in multiplex families. Am. J. Hum. Genet. 99, 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjani B, Klei L, Hultman CM, Larsson H, Devlin B, Buxbaum JD, Sandin S, Grice DE, 2020. Maternal effects as causes of risk for obsessive-compulsive disorder. Biol. Psychiatry 87, 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjani B, Klei L, Mattheisen M, Halvorsen MW, Reichenberg A, Roeder K, Pedersen NL, Boberg J, de Schipper E, Bulik CM, Landén M, Fundín B, Mataix-Cols D, Sandin S, Hultman CM, Crowley JJ, Buxbaum JD, Rück C, Devlin B, Grice DE, 2022. The genetic architecture of obsessive-compulsive disorder: contribution of liability to OCD from alleles across the frequency spectrum. Am. J. Psychiatry 179, 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols D, Boman M, Monzani B, Rück C, Serlachius E, Långström N, Lichtenstein P, 2013. Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA Psychiatry 70, 709–717. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Greenwood T, Wessel J, Azzam A, Garrido H, Chavira DA, Chandavarkar U, Bagnarello M, Stein M, Schork NJ, 2008. Evidence for a heritable unidimensional symptom factor underlying obsessionality. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 676–685. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Nievergelt CM, Azzam A, Garrido H, Chavira DA, Wessel J, Bagnarello M, Reus VI, Schork NJ, 2007. Heritability and clinical features of multigenerational families with obsessive-compulsive disorder and hoarding. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 174–182. [DOI] [PubMed] [Google Scholar]
- Mathis MA, Diniz JB, Shavitt RG, Torres AR, Ferrão YA, Fossaluza V, Pereira C, Miguel E, do Rosario MC, 2009. Early onset obsessive-compulsive disorder with and without tics. CNS Spectr. 14 (7), 362–370. [DOI] [PubMed] [Google Scholar]
- Mattina GF, Steiner M, 2016. The need for inclusion of sex and age of onset variables in genetic association studies of obsessive-compulsive disorder: overview. Prog. Neuropsychopharmacol. Biol. Psychiatry 67, 107–116. [DOI] [PubMed] [Google Scholar]
- Miguel EC, Ferrão YA, Rosário MC, Mathis MA, Torres AR, Fontenelle LF, Hounie AG, Shavitt RG, Cordioli AV, Gonzalez CH, Petribú K, Diniz JB, Malavazzi DM, Torresan RC, Raffin AL, Meyer E, Braga DT, Borcato S, Valério C, Gropo LN, Prado HS, Perin EA, Santos SI, Copque H, Borges MC, Lopes AP, Silva ED, Brazilian Research Consortium on Obsessive-Compulsive Spectrum Disorders, 2008. The Brazilian research consortium on obsessive-compulsive spectrum disorders: recruitment, assessment instruments, methods for the development of multicenter ollaborative studies and preliminary results. Braz. J. Psychiatry 30, 185–196. [DOI] [PubMed] [Google Scholar]
- Miguel EC, Leckman JF, Rauch S, do Rosario-Campos MC, Hounie AG, Mercadante MT, Chacon P, Pauls DL, 2005. Obsessive-compulsive disorder phenotypes: implications for genetic studies. Mol. Psychiatry 10, 258–275. [DOI] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R, 2000. A family study of obsessive-compulsive disorder. Arch. Gen. Psychiatry 57, 358–363. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Stein DJ, Rauch SL, Hollander E, Fallon BA, Barsky A, Fineberg N, Mataix-Cols D, Ferrão YA, Saxena S, Wilhelm S, Kelly MM, Clark LA, Pinto A, Bienvenu OJ, Farrow J, Leckman J, 2010. Should an obsessive-compulsive spectrum grouping of disorders be included in DSM-V? Depress. Anxiety 27, 528–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal VM, Duan J, Vilar-Ribó L, Grove J, Zayats T, Ramos-Quiroga JA, Satterstrom FK, Artigas MS, Bybjerg-Grauholm J, Bækvad-Hansen M, Als TD, Rosengren A, Daly MJ, Neale BM, Nordentoft M, Werge T, Mors O, Hougaard DM, Mortensen PB, Ribasés M, Børglum AD, Demontis D, 2022. Differences in the genetic architecture of common and rare variants in childhood, persistent and late-diagnosed attention-deficit hyperactivity disorder. Nat. Genet. 54, 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehm KE, Keyes KM, Susser ES, 2023. Social determinants of health and selection bias in genome-wide association studies. World Psychiatry 22, 160–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronemus M, Iossifov I, Levy D, Wigler M, 2014. The role of de novo mutations in the genetics of autism spectrum disorders. Nat. Rev. Genet. 15, 133–141. [DOI] [PubMed] [Google Scholar]
- Rosario-Campos MC, Miguel EC, Quatrano S, Chacon P, Ferrao Y, Findley D, Katsovich L, Scahill L, King RA, Woody SR, Tolin D, Hollander E, Kano Y, Leckman JF, 2006. The Dimensional Yale-Brown Obsessive-Compulsive Scale (DY-BOCS): an instrument for assessing obsessive-compulsive symptom dimensions. Mol. Psychiatry 11, 495–504. [DOI] [PubMed] [Google Scholar]
- Rougemont-Buecking A, Rothen S, Jeanprêtre N, Lustenberger Y, Vandeleur CL, Ferrero F, Preisig M, 2008. Inter-informant agreement on diagnoses and prevalence estimates of anxiety disorders: direct interview versus family history method. Psychiatry Res. 157, 211–223. [DOI] [PubMed] [Google Scholar]
- Ruiz-Linares A, Adhikari K, Acuña-Alonzo V, Quinto-Sanchez M, Jaramillo C, Arias W, Fuentes M, Pizarro M, Everardo P, de Avila F, Gómez-Valdés J, León-Mimila P, Hunemeier T, Ramallo V, Silva de Cerqueira CC, Burley M-W, Konca E, de Oliveira MZ, Veronez MR, Rubio-Codina M, Attanasio O, Gibbon S, Ray N, Gallo C, Poletti G, Rosique J, Schuler-Faccini L, Salzano FM, Bortolini M-C, Canizales-Quinteros S, Rothhammer F, Bedoya G, Balding D, Gonzalez-José R, 2014. Admixture in Latin America: geographic structure, phenotypic diversity and self-perception of ancestry based on 7,342 individuals. PLOS Genet. 10, e1004572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC, 2010. The epidemiology of obsessive-compulsive disorder in the national comorbidity survey replication. Mol. Psychiatry 15, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S, Volkmar FR, 2013. Multiplex-simplex comparisons. Encyclopedia of Autism Spectrum Disorders. Springer New York. New York, NY: 1960–1960. [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An J-Y, Peng M, Collins R, Grove J, Klei L, Stevens C, Reichert J, Mulhern MS, Artomov M, Gerges S, Sheppard B, Xu X, Bhaduri A, Norman U, Brand H, Schwartz G, Nguyen R, Guerrero EE, Dias C,, Autism Sequencing Consortium, iPSYCH-Broad Consortium, Betancur C, Cook EH, Gallagher L, Gill M, Sutcliffe JS, Thurm A, Zwick ME, Børglum AD, State MW, Cicek AE, Talkowski ME, Cutler DJ, Devlin B, Sanders SJ, Roeder K, Daly MJ, Buxbaum JD, 2020. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180, 568–584 e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, Leotta A, Pai D, Zhang R, Lee Y-H, Hicks J, Spence SJ, Lee AT, Puura K, Lehtimäki T, Ledbetter D, Gregersen PK, Bregman J, Sutcliffe JS, Jobanputra V, Chung W, Warburton D, King M-C, Skuse D, Geschwind DH, Gilliam TC, Ye K, Wigler M, 2007. Strong association of de novo copy number mutations with autism. Science 316, 445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirugo G, Williams SM, Tishkoff SA, 2019. The missing diversity in human genetic studies. Cell 177, 1080. [DOI] [PubMed] [Google Scholar]
- Slomp C, Morris E, Inglis A, Lehman A, Austin J, 2018. Patient outcomes of genetic counseling: assessing the impact of different approaches to family history collection. Clin. Genet. 93, 830–836. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, van den Heuvel OA, Simpson HB, 2019. Obsessive-compulsive disorder. Nat. Rev. Dis. Primers 5, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom NI, Grove J, Meier SM, Bækvad-Hansen M, Becker Nissen J, Damm Als T, Halvorsen M, Nordentoft M, Mortensen PB, Hougaard DM, Werge T, Mors O, Børglum AD, Crowley JJ, Bybjerg-Grauholm J, Mattheisen M, 2021. Polygenic heterogeneity across obsessive-compulsive disorder subgroups defined by a comorbid diagnosis. Front. Genet. 12, 711624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S, 2011a. Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin. Psychol. Rev. 31, 1361–1372. [DOI] [PubMed] [Google Scholar]
- Taylor S, 2011b. Early versus late onset obsessive-compulsive disorder: evidence for distinct subtypes. Clin. Psychol. Rev. 31, 1083–1100. [DOI] [PubMed] [Google Scholar]
- Towards a global psychological science. Nat Rev Psychol 1 (7), 2022. 10.1038/s44159-022-00087-3, 369. [DOI] [Google Scholar]
- Tükel R, Polat A, Ozdemir O, Aksüt D, Türksoy N, 2002. Comorbid conditions in obsessive-compulsive disorder. Compr. Psychiatry 43, 204–209. [DOI] [PubMed] [Google Scholar]
- Vandeleur CL, Rothen S, Lustenberger Y, Glaus J, Castelao E, Preisig M, 2015. Inter-informant agreement and prevalence estimates for mood syndromes: direct interview vs. family history method. J. Affect. Disord. 171, 120–127. [DOI] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN, 2009. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter FM, Emery J, 2005. Coming down the line’– patients understanding of their family history of common chronic disease. Ann. Fam. Med. 3, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Mandell JD, Kumar Y, Sun N, Morris MT, Arbelaez J, Nasello C, Dong S, Duhn C, Zhao X, Yang Z, Padmanabhuni SS, Yu D, King RA, Dietrich A, Khalifa N, Dahl N, Huang AY, Neale BM, Coppola G, Mathews CA, Scharf JM,, Tourette International Collaborative Genetics Study (TIC Genetics), Tourette Syndrome Genetics Southern and Eastern Europe Initiative (TSGENESEE), Tourette Association of America International Consortium for Genetics (TAAICG), Fernandez TV, Buxbaum JD, De Rubeis S, Grice DE, Xing J, Heiman GA, Tischfield JA, Paschou P, Willsey AJ, State MW, 2018. De Novo sequence and copy number variants are strongly associated with Tourette disorder and implicate cell polarity in pathogenesis. Cell Rep. 24, 3441–3454 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, Adams MJ, Agerbo E, Air TM, Andlauer TMF, Bacanu S-A, Bækvad-Hansen M, Beekman AFT, Bigdeli TB, Binder EB, Blackwood DRH, Bryois J, Buttenschøn HN, Bybjerg-Grauholm J, Cai N, Castelao E, Christensen JH, Clarke T-K, Coleman JIR, Colodro-Conde L, Couvy-Duchesne B, Craddock N, Crawford GE, Crowley CA, Dashti HS, Davies G, Deary IJ, Degenhardt F, Derks EM, Direk N, Dolan CV, Dunn EC, Eley TC, Eriksson N, Escott-Price V, Kiadeh FHF, Finucane HK, Forstner AJ, Frank J, Gaspar HA, Gill M, Giusti-Rodríguez P, Goes FS, Gordon SD, Grove J, Hall LS, Hannon E, Hansen CS, Hansen TF, Herms S, Hickie IB, Hoffmann P, Homuth G, Horn C, Hottenga J-J, Hougaard DM, Hu M, Hyde CL, Ising M, Jansen R, Jin F, Jorgenson E, Knowles JA, Kohane IS, Kraft J, Kretzschmar WW, Krogh J, Kutalik Z, Lane JM, Li Y, Li Y, Lind PA, Liu X, Lu L, MacIntyre DJ, MacKinnon DF, Maier RM, Maier W, Marchini J, Mbarek H, McGrath P, McGuffin P, Medland SE, Mehta D, Middeldorp CM, Mihailov E, Milaneschi Y, Milani L, Mill J, Mondimore FM, Montgomery GW, Mostafavi S, Mullins N, Nauck M, Ng B, Nivard MG, Nyholt DR, O’Reilly PF, Oskarsson H, Owen MJ, Painter JN, Pedersen CB, Pedersen MG, Peterson RE, Pettersson E, Peyrot WJ, Pistis G, Posthuma D, Purcell SM, Quiroz JA, Qvist P, Rice JP, Riley BP, Rivera M, Saeed Mirza S, Saxena R, Schoevers R, Schulte EC, Shen L, Shi J, Shyn SI, Sigurdsson E, Sinnamon GBC, Smit JH, Smith DJ, Stefansson H, Steinberg S, Stockmeier CA, Streit F, Strohmaier J, Tansey KE, Teismann H, Teumer A, Thompson W, Thomson PA, Thorgeirsson TE, Tian C, Traylor M, Treutlein J, Trubetskoy V, Uitterlinden AG, Umbricht D, Van der Auwera S, van Hemert AM, Viktorin A, Visscher PM, Wang Y, Webb BT, Weinsheimer SM, Wellmann J, Willemsen G, Witt SH, Wu Y, Xi HS, Yang J, Zhang F, eQTLGen, 23andMe, Arolt V, Baune BT, Berger K, Boomsma DI, Cichon S, Dannlowski U, de Geus ECJ, DePaulo JR, Domenici E, Domschke K, Esko T, Grabe HJ, Hamilton SP, Hayward C, Heath AC, Hinds DA, Kendler KS, Kloiber S, Lewis G, Li QS, Lucae S, Madden PFA, Magnusson PK, Martin NG, McIntosh AM, Metspalu A, Mors O, Mortensen PB, Müller-Myhsok B, Nordentoft M, Nöthen MM, O’Donovan MC, Paciga SA, Pedersen NL, Penninx BWJH, Perlis RH, Porteous DJ, Potash JB, Preisig M, Rietschel M, Schaefer C, Schulze TG, Smoller JW, Stefansson K, Tiemeier H, Uher R, Völzke H, Weissman MM, Werge T, Winslow AR, Lewis CM, Levinson DF, Breen G, Børglum AD, Sullivan PF, Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium, 2018. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroten M, Yoon S, Andrews P, Yamrom B, Ronemus M, Buja A, Krieger AM, Levy D, Ye K, Wigler M, Iossifov I, 2023. Sharing parental genomes by siblings concordant or discordant for autism. Cell Genom. 3, 100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaryura-Tobias JA, Grunes MS, Todaro J, McKay D, Neziroglu FA, Stockman R, 2000. Nosological insertion of axis I disorders in the etiology of obsessive-compulsive disorder. J. Anxiety Disord. 14, 19–30. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen X, Gao X, Yang H, Zhen Z, Li Q, Lin Y, Zhao X, 2017. Worldwide research productivity in the field of psychiatry. Int. J. Ment. Health Syst. 11 (1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]


