Diabetes mellitus is one of the most common endocrine diseases in all populations and all age groups. It is a syndrome of disturbed intermediary metabolism caused by inadequate insulin secretion or impaired insulin action, or both. Diabetes is crudely grouped into the two types—insulin dependent diabetes mellitus and non-insulin dependent diabetes mellitus. Both types are associated with excessive morbidity and mortality. Relative mortality in people with insulin dependent diabetes is between 10 and 30 (equal to a 5-10 year reduction in life expectancy), depending on the age at diagnosis, current age, the duration of the disease, and the year of diagnosis.1 Although cohorts of patients with insulin dependent diabetes diagnosed after 1955 may have a better prognosis,1,2 this trend seems to be levelling off.3 Non-insulin dependent diabetes is associated with an overall age adjusted mortality that is about twice that of non-diabetic populations; life expectancy is reduced by 5 to 10 years in middle aged patients with this type of diabetes.4 There are no signs of a reduction in mortality from non-insulin dependent diabetes.
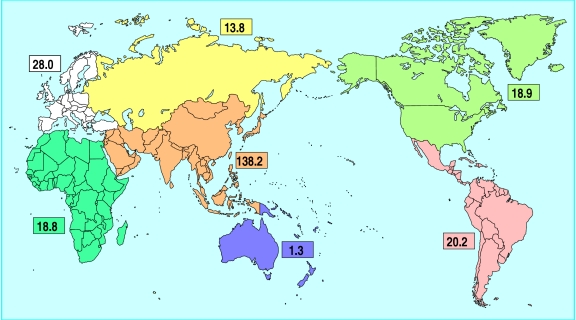
Diabetes has a serious economic impact. In 1992, the estimated cost of diabetes in the United States was between $85bn and $92bn, two thirds of which resulted from lost productivity because of admission to hospital and death.5 The worldwide prevalence of diabetes is expected to more than double between 1994 and 2010, to 239 million people (fig 1).6 Tight metabolic control, drug treatment of hypertension and hyperlipidaemia, and efforts directed at changes in lifestyle will therefore pose heavy financial burdens on global healthcare systems. Urgent political and organisational decisions need to be made now.
Figure 1.
Regional projections of the prevalence of diabetes (millions) in 2010. Reproduced with permission6
Methods
The references in this review were selected from reports on the epidemiology, aetiology, pathogenesis, and treatment of diabetes. Most were published in the past five years. The overall aim was to underline the increasing severity of diabetes worldwide. Being a clinical specialist in diabetes and a diabetes immunologist, I apologise for any selection bias resulting from this approach.
Recent advances
Diabetes is one of the most serious challenges to health care worldwide; according to recent projections, it will affect 239 million people by 2010—a doubling in prevalence since 1994
The incidence of insulin dependent diabetes is increasing in small children, who will be more prone to late complications of diabetes
Insulin dependent and non-insulin dependent diabetes are pathogenetically heterogeneous disorders caused by the interaction of several genetic and environmental factors
Although it is now shown that strict metabolic control prevents late microvascular complications, it increases the risk of hypoglycaemia, demands high motivation from patients, and is costly
All cardiovascular risk factors—not just hyperglycaemia—may need to be reduced if late macrovascular complications are to be prevented
Aetiology and pathogenesis
Insulin dependent diabetes
The aetiology and pathogenesis of insulin dependent diabetes mellitus are not fully understood. Patients with insulin dependent diabetes mellitus can be grouped according to their presenting characteristics as well as immune and genetic markers. Insulin dependent diabetes in early childhood is characterised by abrupt onset and by high frequencies of ketoacidosis and of insulin and IA2 autoantibodies. It is associated with the major histocompatibility complex heterozygosity, HLA DR3/4. Insulin dependent diabetes in older children and adolescents is associated with a high frequency of islet cell cytoplasmic autoantibodies, glutamic acid decarboxylase autoantibodies, and HLA DR3. Autoimmune diabetes of late onset is often be accompanied by typical immune markers. Whether these groupings represent separate diseases or simply reflect the rapidity with which β cell destruction takes place is unclear. Some lean people with insulin dependent diabetes lack the classic manifestations of autoimmune disease, and their diabetes may therefore have a different aetiology and pathogenesis. β cell destruction in immune mediated insulin dependent diabetes is caused by an inflammatory reaction in the islets of Langerhans, triggered by environmetal factors in genetically susceptible individuals (box).7
Model of pathogenesis of insulin dependent diabetes
Environmental factors (such as viruses, chemicals, and nutrition) trigger the process by causing restricted and focal damage to β cells; this leads to the release of β cell antigens that were either modified or have previously been “hidden” from recognition by the immune system. These antigens are taken up by resident macrophages or dendritic cells, or both, or later by antigen presenting cells recruited to the islets in which focal β cell damage has taken place. After processing and presentation of β cell antigen, and provision of appropriate soluble and surface membrane second signals, specifically reactive T helper cells will be activated to transcribe cytokine genes. The synthesised and released cytokines recruit both antigen specific and non-specific mononuclear cells to build up the insulitis infiltrate and activate endothelial cells. Recruited macrophages are stimulated by interferon γ to produce interleukin 1 and tumour necrosis factor α, which in synergy with interferon γ lead to β cell destruction via the induction of toxic nitric oxide radicals and pathways that activate apoptosis (programmed cell death) specific to β cells.7
Aetiology
Interesting contributions to our understanding of the aetiology of insulin dependent diabetes mellitus have recently been made. Although the incidence is highest in schoolchildren and adolescents, it is now clear that in its classic form insulin dependent diabetes may develop at any age.8 Between 1960 and 1989, steady temporary increases were reported in North America, northern Europe, Japan, and New Zealand.9 Recent disturbing data from Finland and the United Kingdom show that the growing incidence is mainly explained by an increase in the frequency of onset in children under 5 years.10,11 This finding points to changes in major environmental aetiological factors early in life, such as viral infections and nutritional factors.
Viral infections
Interestingly, mothers whose children became diabetic before age 15 had increased titres of low avidity antibody to enteroviruses such as the Coxsackie B group (indicating recent viral infection) compared with mothers whose children were not diabetic by age 15.12 Immune cross reactivity to similar sequences in the Coxsackie B virus and the putative β cell autoantigen, glutamic acid decarboxylase, has been found, suggesting that molecular mimicry may be the underlying mechanism.13
Nutritional factors
Early exposure to cows’ milk proteins is associated with an increased risk of childhood diabetes; it shows an odds ratio of 1.57 (95% confidence interval 1.19 to 2.07).14 Molecular mimicry between bovine serum albumin and ICA 69, another putative β cell autoantigen, has been suggested.15 However, use of bovine serum albumin to induce tolerance in animal models of spontaneous insulin dependent diabetes mellitus failed to show protection against diabetes despite tolerance to the bovine serum albumin.16 Intriguingly, formula milks based on cows’ milk and on hydrolysed casein contain bovine insulin. Children fed cows’ milk formula had higher titres of IgG antibodies to bovine insulin that cross reacted with human insulin, and the presence of these autoantibodies correlated with the presence of autoantibodies to human insulin.17 These findings may explain the high incidence of insulin autoantibodies in young children and raise the possibility that sensitisation to bovine insulin could prime immune system autoaggression towards β cells in people who are genetically predisposed.17 Finally, excess interuterine growth has been reported as an independent risk factor for insulin dependent diabetes mellitus, but the mechanism remains to be explained.18
Special problems in young children
The increased incidence of insulin dependent diabetes mellitus in very young children is worrying. This is because of the special problems of managing diabetes in this group. These problems include more frequent ketoacidois, severe hypoglycaemia, and more frequent admission to hospital,19,20 together with the well known correlation between early onset insulin dependent diabetes mellitus and increased morbidity and mortality from late diabetic complications.
Non-insulin dependent diabetes
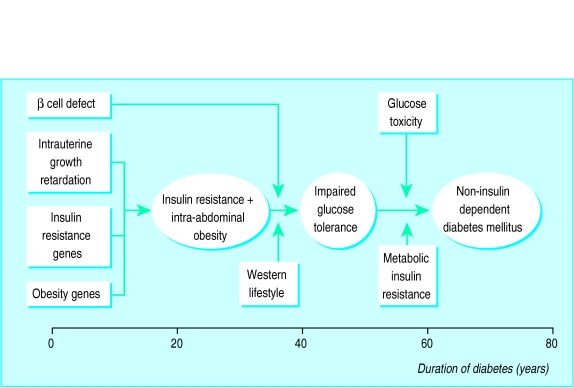
This type of diabetes is caused by relative insulin deficiency because of impaired insulin action combined with the inability of the β cell to compensate for the reduction in insulin sensitivity. Which of these effects is the primary one remains unknown. Figure 2 shows a model of the aetiology and pathogenesis of non-insulin dependent diabetes mellitus.
Figure 2.
Aetiology and pathogenesis of the development of obesity and non-insulin dependent diabetes mellitus
Fetal malnutrition
Fetal malnutrition may contribute to the later development of non-insulin dependent diabetes mellitus by impairing early fetal growth and reducing the β cell mass. This might limit β cell function in adult life and hamper the ability of the β cell to compensate for insulin resistance caused by obesity, pregnancy, or drugs such as glucocorticoids.21 Support for this view has been published recently. Low birth weight in adult first degree relatives of patients with non-insulin dependent diabetes was associated with impaired β cell function,22 and monozygotic twins who became concordant for non-insulin dependent diabetes after 60 years of follow up were growth retarded at birth compared with discordant twins.23 Furthermore, low birth weight has been found to predispose to obesity and hypertension, suggesting that intrauterine growth retardation is a risk factor for several features of the so called metabolic syndromes.24 These data show that increased focus on early fetal growth and nutrition may prevent non-insulin dependent diabetes mellitus in the coming generation.
Genetics of diabetes
Two different methodological approaches have recently been taken to clarify the genetic bases of insulin dependent and non-insulin dependent diabetes. The random marker approach uses information from the human genome project on the location of so called chromosomal “tags” spread all over the human genome, and the segregation of these tags with diabetes in genetically informative families. This method identifies chromosomal regions that may contain genes predisposing to disease; subsequent analysis is necessary to identify the structural genes of pathogenetic relevance. A complementary method is the candidate gene approach. Here genes encoding for proteins believed to have a role in the pathogenesis of a disease (based on a formulated pathogenetic model) are searched for variations associated with the disease in case-control studies or linked to the disease in family studies, or both.
Insulin dependent diabetes mellitus
In insulin dependent diabetes mellitus the random marker approach has identified 18 chromosomal regions that show evidence of linkage to insulin dependent diabetes mellitus.25 Formal linkage has been shown only for the major histocompatibility complex class II region, a variable number of tandem repeats 5′ to the insulin gene and the CTLA-4 gene, coding for the ligand B7, an important accessory molecule for T cell activation.
Studies comparing different racial groups have shown evidence of appreciable genetic heterogeneity between ethnic groups. Much work is needed before the complex genetic susceptibility of insulin dependent diabetes mellitus is understood and the functional role of the products of these genes in pathogenesis is clarified.26
Non-insulin dependent diabetes
More than 250 candidate genes have been tested for association with or linkage to non-insulin dependent diabetes mellitus, but none has shown consistent results in different study populations.27 Most success for the candidate gene approach has been provided in the study of maturity onset diabetes in young people, an autosomal dominant form of early onset non-insulin dependent diabetes mellitus. Although maturity onset diabetes in young people may seem to be a homogeneous phenotype, four genetically and pathogenetically distinct forms have recently been described. The first form is caused by a mutation in the transcription factor hepatic nuclear factor 4α,28 the second results from different mutations in the glucokinase gene affecting β cell glucose sensing,29 the third is caused by mutations in the gene encoding for hepatic nuclear factor 1α,30 and the fourth results from mutation of the insulin promoter factor-1. The precise role of the hepatic nuclear factor 4α and 1α mutations is not known, but it is suspected that mutations in these transcription factors affect the β cell response to glucose.31
These genes account for a small percentage of non-insulin dependent diabetes and underline the genetic complexity behind apparently homogeneous diabetic phenotypes. However, these discoveries may lead to new drugs that modify the β cell response to glucose, and to the development of pharmacological principles for treating common non-insulin dependent diabetes.
Linkage studies in non-insulin dependent diabetes are hampered by a general lack of parents of patients with diabetes of adult onset. Very large numbers of affected sibling pairs are needed for linkage studies, and this calls for international collaboration.
The glucose hypothesis
Insulin dependent diabetes
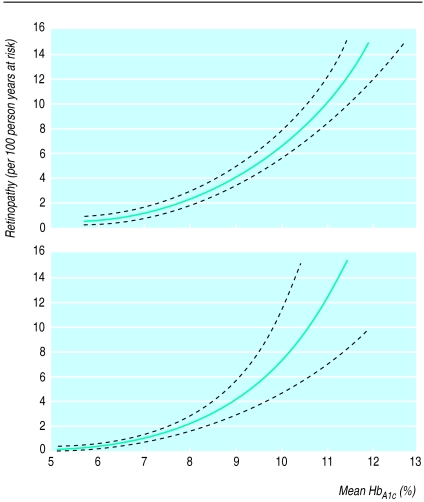
For many years specialists in diabetes have quarrelled about the importance of glycaemic control in preventing the late complications of diabetes. The diabetes control and complications trial, which showed that improved glycaemic control reduced microvascular complications in insulin dependent diabetes mellitus, ended the debate.32 Subsequent analyses showed that there was no glycaemic threshold for the development of microvascular complications (fig 3).33,34 Although improved glycaemic control was associated with a twofold to threefold increase in severe hypoglycaemia,32,35 the finding that repeated hypoglycaemia did not affect neuropsychological performance or lead to serious accidents was reassuring.36
Figure 3.
Glycated haemoglobin in relation to the risk of retinopathy in conventionally treated (top) and intensively treated (bottom) patients with insulin dependent diabetes. Reproduced with permission33
It is noteworthy, however, that the diabetes control and complications trial excluded children aged less than 13 and adults older than 39; patients with advanced retinopathy, nephropathy, and neuropathy; misusers of alcohol and drugs; patients with major psychological disturbances such as psychoses and abnormal eating habits; and patients with epilepsy, frequent ketoacidosis, and frequent hypoglycaemic incidents. Furthermore, the study failed to show a statistically significant reduction in macrovascular events. The challenge now is to implement the recommendation of the trial group—to intensify treatment in as many patients as possible as early as possible and as safely as possible. The most difficult decision for specialists in diabetes may be to know when they should not recommend intensive treatment.
Non-insulin dependent diabetes
There are fewer studies on people with non-insulin dependent diabetes. The Kumamoto study, which used a similar design to the diabetes control and complications trial, but with far fewer patients, also showed that improved metabolic control reduced microvascular complications in Japanese patients with non-insulin dependent diabetes mellitus.37 Studies designed to detect differences in cardiovascular events in patients with optimal glycaemic control are clearly needed.
Selecting treatment
Results from the United Kingdom prospective diabetes study are awaited and will have a major impact in selecting treatment for patients with non-insulin dependent diabetes. Meanwhile, recent data point to the advantage of using metformin in obese patients, either alone or with insulin.38–41 In short term studies, metformin was as effective as sulphonylurea or insulin, or both, in improving glycaemic control, and seemed to cause less weight gain when used alone or with insulin. Whether reducing cardiovascular morbidity and mortality will require intervention against risk factors other than glycaemia (high blood pressure, raised lipid concentrations, smoking) also remains to be determined. Finally, we still do not know whether early treatment with angiotensin converting enzyme inhibition in non-insulin dependent diabetic patients with microalbuminuria and normal blood pressure will not only preserve kidney function but also improve the long term prognosis.42
Conclusion
Urgent action is needed to plan for the demands that patients with diabetes will place on healthcare systems and the global economy. Resources will be needed to manage the projected global explosion in the prevalence of diabetes (particularly in developing countries), the increase in early onset insulin dependent diabetes mellitus, and the improved treatment for preventing late complications of diabetes.
Acknowledgments
I thank Professor Henning Beck-Nielsen, University of Odense, for supplying figure 2 and Anne Brock Rasmussen for preparing the manuscript.
Footnotes
Funding: No additional funding.
Conflict of interest: None.
References
- 1.Green A, Borch-Johnsen K, Andersen PK, Hougaard P, Keiding N, Kreiner N, et al. Relative mortality of type 1 (insulin-dependent) diabetes in Denmark: 1933-1981. Diabetologia. 1985;28:339–342. doi: 10.1007/BF00283140. [DOI] [PubMed] [Google Scholar]
- 2.Joner G, Patrick S. The mortality of children with type 1 (insulin-dependent) diabetes mellitus in Norway, 1973-1988. Diabetologia. 1991;34:29–32. doi: 10.1007/BF00404021. [DOI] [PubMed] [Google Scholar]
- 3.Portuese E, Orchard T. Diabetes in America. Bethesda, MD: National Institutes of Health; 1995. Mortality in insulin-dependent diabetes; pp. 221–232. . (NIH publication No 95-1468.) [Google Scholar]
- 4.Geiss LS, Herman WH, Smith PJ. Diabetes in America. Bethesda, MD: National Institutes of Health; 1995. Mortality in non-insulin-dependent diabetes; pp. 233–255. . (NIH publication No 95-1468.) [Google Scholar]
- 5.Javitt JC, Chiang Y. Diabetes in America. Bethesda, MD: National Institutes of Health; 1995. Economic impact of diabetes; pp. 601–611. . (NIH publication No 95-1468.) [Google Scholar]
- 6.McCarty D, Zimmet P. Diabetes 1994 to 2010: global estimates and projections. Leverkusen: Bayer AG; 1994. pp. 1–46. [Google Scholar]
- 7.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39:1005–1029. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 8.M¢lbak AG, Marner B, Borch-Johnsen K, Nerup J. Incidence of insulin-dependent diabetes mellitus in age groups over 30 years in Denmark. Diabetic Med. 1994;11:650–665. doi: 10.1111/j.1464-5491.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 9.Karvonen M, Tuomilehto J, Libman I, LaPorte R. A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:883–892. doi: 10.1007/BF02374468. [DOI] [PubMed] [Google Scholar]
- 10.Tuomilehto J, Virtala E, Karvonen M. Increase in incidence of insulin-dependent diabetes mellitus among chrildren in Finland. Int J Epidemiol. 1995;24:984–992. doi: 10.1093/ije/24.5.984. [DOI] [PubMed] [Google Scholar]
- 11.Gardner SG, Bingley PJ, Sawtell PA, Weeks S, Gale EAM the Bart’s-Oxford Study Group. Rising incidence of insulin dependent diabetes in children aged under 5 years in the Oxford region: time trend analysis. BMJ. 1997;315:713–717. doi: 10.1136/bmj.315.7110.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlquist G, Ivarsson S, Lindberg B, Forsgren M. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM: a population-based case-control study. Diabetes. 1995;44:408–413. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 13.Tian J, Lehmann PV, Kaufmann DL. T cell cross-reactivity between Coxsackie virus and glutamate decarboxylase is associated with a murine diabetes susceptibility allele. J Exp Med. 1994;180:1979–1984. doi: 10.1084/jem.180.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstein H. Cow’s milk exposure and type 1 diabetes. Diabetes Care. 1994;17:13–19. doi: 10.2337/diacare.17.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Pietropaolo M, Castano L, Babu S, Buelow R, Kuo YLS, Martin S, et al. Islet cell autoantigen 69-kD (ICA69): molecular cloning and characterization of a novel diabetes-associated autoantigen. J Clin Invest. 1993;92:359–371. doi: 10.1172/JCI116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen JS, Karlsen AE, Markholst H, Worsaae H, Dyrberg T, Michelsen B. Neonatal tolerization with glutamic acid decarboxylase but not with bovine serum albumin delays the onset of diabetes in NOD mice. Diabetes. 1994;43:1478–1484. doi: 10.2337/diab.43.12.1478. [DOI] [PubMed] [Google Scholar]
- 17.Vaarala O, Paronen J, Otonkoski T, Åkerblom HK. Cow milk feeding induces antibodies to insulin in children—a link between cow milk and insulin-dependent diabetes mellitus? Scand J Immunol. 1998;47:131–135. doi: 10.1046/j.1365-3083.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 18.Dahlquist G, Bennich SS, Källén B. Intrauterine growth pattern and risk of childhood onset insulin dependent (type 1): population based case-control study. BMJ. 1996;313:1174–1177. doi: 10.1136/bmj.313.7066.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinkney JH, Bingley PJ, Sawtell PA, Dunger DB, Gale EAM. Presentation and progress of childhood diabetes mellitus: a prospective population-based study. Diabetologia. 1994;37:70–74. doi: 10.1007/BF00428780. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen HB, Hougaard P. Comparison of metabolic control in a cross-sectional study of 2,873 children and adolescents with IDDM from 18 countries. Diabetes Care. 1997;20:714–720. doi: 10.2337/diacare.20.5.714. [DOI] [PubMed] [Google Scholar]
- 21.Hales CN, Barker DJP. Type 2 (non-insulin dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 22.Cook JTE, Levy JC, Page RCL, Shaw AG, Hattersley AT, Turner RC. Association of low birth weight with beta cell function in the adult first degree relatives of non-insulin dependent diabetic subjects. BMJ. 1993;306:302–306. doi: 10.1136/bmj.306.6873.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulsen P, Vaag AA, Kyvik KO, M¢ller Jensen D, Beck-Nielsen H. Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia. 1997;40:439–446. doi: 10.1007/s001250050698. [DOI] [PubMed] [Google Scholar]
- 24.Leon DA, Koupilova I, Lithell HO, Berglund L, Mohsen R, Vågerö D, et al. Failure to realise growth potential in utero and adult obesity in relation to blood pressure in 50 year old Swedish men. BMJ. 1996;312:401–406. doi: 10.1136/bmj.312.7028.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies JL, Kawaguchi Y, Bennett ST, Copeman JB, Cordell HJ, Pritchard LE, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371:130–136. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- 26.Owerbach D, Gabbay KH. The search for IDDM susceptibility genes. Diabetes. 1996;45:544–551. doi: 10.2337/diab.45.5.544. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Schork NJ. Genetic analysis of NIDDM. Diabetes. 1996;45:1–14. doi: 10.2337/diab.45.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, et al. Mutations in the hepatocyte nuclear factor-4alfa gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 29.Vionnet N, Stoffel M, Takeda J, Yasuda K, Bell G, Zouali M, et al. Nonsense mutation in the glucokinase gene causes early-onset non-insulin-dependent diabetes mellitus. Nature. 1992;356:721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- 30.Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, et al. Mutations in the hepatocyte nuclear factor-1alfa gene in maturity-onset diabetes of the young (MODY3) Nature. 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]
- 31.Hansen T, Eiberg H, Rouard M, Vaxillaire M, Möller AM, Rasmussen SK, et al. Novel MODY3 mutations in the hepatocyte nuclear factor-1alfa gene. Diabetes. 1997;46:726–730. doi: 10.2337/diab.46.4.726. [DOI] [PubMed] [Google Scholar]
- 32.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 34.Diabetes Control and Complications Trial Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the diabetes control and complications trial. Diabetes. 1996;45:1289–1298. [PubMed] [Google Scholar]
- 35.Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 36.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes therapy on neuropsychological function on adults in the diabetes control and complications trial. Ann Intern Med. 1996;124:379–388. doi: 10.7326/0003-4819-124-4-199602150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 38.United Kingdom Prospective Diabetes Study Group. United Kingdom prospective diabetes study (UKPDS) 13: relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83–88. [PMC free article] [PubMed] [Google Scholar]
- 39.Defronzo RA, Goodman AM Multicenter Metformin Study Group. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–549. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 40.Yki-Järvinen H, Nikkilä K, Ryysy L, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin regimens in NIDDM: metformin prevents insulin-induced weight gain [abstract] Diabetologia. 1996;39:A33. [Google Scholar]
- 41.Bloomgarden ZT. American Diabetes Association scientific sessions, 1995. Non-insulin-dependent diabetes mellitus. Diabetes Care. 1995;18:1215–1219. doi: 10.2337/diacare.18.8.1215. [DOI] [PubMed] [Google Scholar]
- 42.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118:577–581. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]