Abstract
Methods
30 male patients with primary inguinal hernias undergoing primary inguinal herniorrhaphy were prospectively recruited for ilioinguinal nerve resection and evaluation. Three samples of the resected ilioinguinal nerve (proximal, canal, and distal) were evaluated using Masson's trichrome stain to measure fascicle and total nerve cross-sectional area and detect changes in collagen.
Results
The fascicle cross-sectional area in the canal segment was significantly decreased compared to the proximal control with a large effect size observed (p = 0.016, η2 = 0.16). There was no significant difference in the nerve cross-sectional area between locations, but there was a moderate to large effect size observed between locations (p = 0.165, η2 = 0.105). There was no significant difference in collagen content nor effect size observed between locations (p = 0.99, η2 = 1.503 × 10−4). Interpretation. The decrease in the fascicle cross-sectional area within the inguinal canal further suggests that there is chronic pressure applied by hernia tissue consistent with axon degeneration. Collagen content is uniformly distributed along the length of the nerve. Further studies with larger samples are needed to confirm the observed effect of nerve location on the total nerve cross-sectional area and axon loss.
1. Introduction
Previous studies have reported apparent enlargement of the nerve distal to the external inguinal ring in as many as 63% primary inguinal hernia patients [1, 2] but have yet to investigate the pathophysiological change resulting in the size discrepancy. Compression neuropathy can result in fibrotic changes to the external epineurium and perineurium, thickening these layers and increasing the overall nerve diameter [3]. Many autopsy series have demonstrated that compression neuropathy commonly occurs at sites where nerves interact with fascia and bone [4, 5], characterized by perineural fibrosis and axon loss with varying degrees of Wallerian degeneration [6, 7]. In animal studies, this has been shown to be a dose-dependent response where increased pressure results in more damage over time [8]. This is thought to be an ischemic damage due to a loss of blood flow in the endoneurium and results in the degeneration of nerves without a significant inflammatory response [9]. Nerves displaying compression neuropathy in an inguinal hernia have been shown to demonstrate an increased diameter adjacent to the compression site, increased fascicle count, and increased myxoid content (edema) [1, 10, 11]. This is due to the nerve becoming constricted and eventually damaged due to nearby structures exerting increased pressure by the hernia sac over a prolonged period [1, 4, 5]. The purpose of this study is to investigate sections of the ilioinguinal nerve from primary inguinal hernia patients at several sites within the inguinal canal for evidence of collagen deposition, axon loss, and changes in the nerve cross-sectional area to further explain gross nerve size discrepancies.
2. Materials and Methods
Thirty patients undergoing primary inguinal herniorrhaphy were prospectively recruited and consented for ilioinguinal neurectomy and evaluation. These patients were not part of a previous primary inguinal hernia study. During primary inguinal hernia repair using the Lichtenstein tension-free mesh repair, the ilioinguinal nerve was visually inspected for size discrepancy at the external inguinal ring. Using the best intraoperative judgment, the surgeon resected ilioinguinal nerves which were grossly enlarged just beyond the external ring during this repair. Due to the ilioinguinal nerve's origin proximal to the inguinal canal, an uninvolved site of the ilioinguinal nerve was able to serve as a control to the involved sections of the nerve (Figure 1). Using the resected specimen, three segments of the nerve were selected: one 3 cm proximal to the internal inguinal ring, one in the inguinal canal within 1 cm of the external ring, and one just distal to the external inguinal ring (Figure 1). Specimens were fixed in formalin solution and sent for the permanent section. All measurements of the cross-sectional area were taken postfixation and may be smaller than in vivo due to shrinkage associated with formalin fixation. Histological characterization was conducted to quantify the total fascicle cross-sectional area, total nerve cross-sectional area, and collagen content. These measurements were pursued to identify suspected evidence of size discrepancy and collagen content that could be suggestive of compression neuropathy. To best accomplish this task, a trichrome stain was selected. Trichrome stains have a wide variety of applications in muscle fibers and other tissues and can be effectively applied to nerves to differentiate collagen fibers from other structures [12]. The collagen content of the nerves was examined due to its role in fibrosis and thus significance in determining presence of compression neuropathy [1, 7].
Figure 1.

Illustration of the location of the sample site along the ilioinguinal nerve. Proximal control sample (nerve not in contact with hernia tissue) in orange. Inguinal canal segment proximal to external ring in green. Distal segment found distal to the external inguinal ring in pink.
After formalin fixation, the segments were paraffin-embedded, oriented, cross-sectioned, and stained with modified Masson's trichrome (collagen green, myelin red, and nuclei dark red-brown) [13]. The stained sections were digitized (Aperio AT2, Leica Biosystems, USA) and deidentified. The samples were then analyzed by a blinded reviewer using Fiji ImageJ (Version 1.52n, NIH, USA). The nerve cross-sectional area (mm2) was measured by manually drawing the region of interest (ROI) around the entire nerve (Figure 2). An additional set of ROIs were drawn around the individual nerve fascicles (within the nerve sections, excluding the perineurium). The multiple ROIs selecting the nerve fascicles were then summed to determine the total cross-sectional area covered by nerve fascicle. Using a filter customized to isolate the green wavelength dominant in Masson's trichrome stain, color deconvolution in Fiji was applied to separate the image into the primary green, blue, and red components [5, 6]. To quantify collagen content, the total cross-sectional area (mm2) covered by the green component was measured against fascicle ROI total cross-sectional area. The proximal section of the ilioinguinal nerve, unaffected by hernia tissue, was used as the control segment from which to compare the inguinal canal segment and the distal segment. This study has undergone continuous review and approval by our Institutional Review Board.
Figure 2.
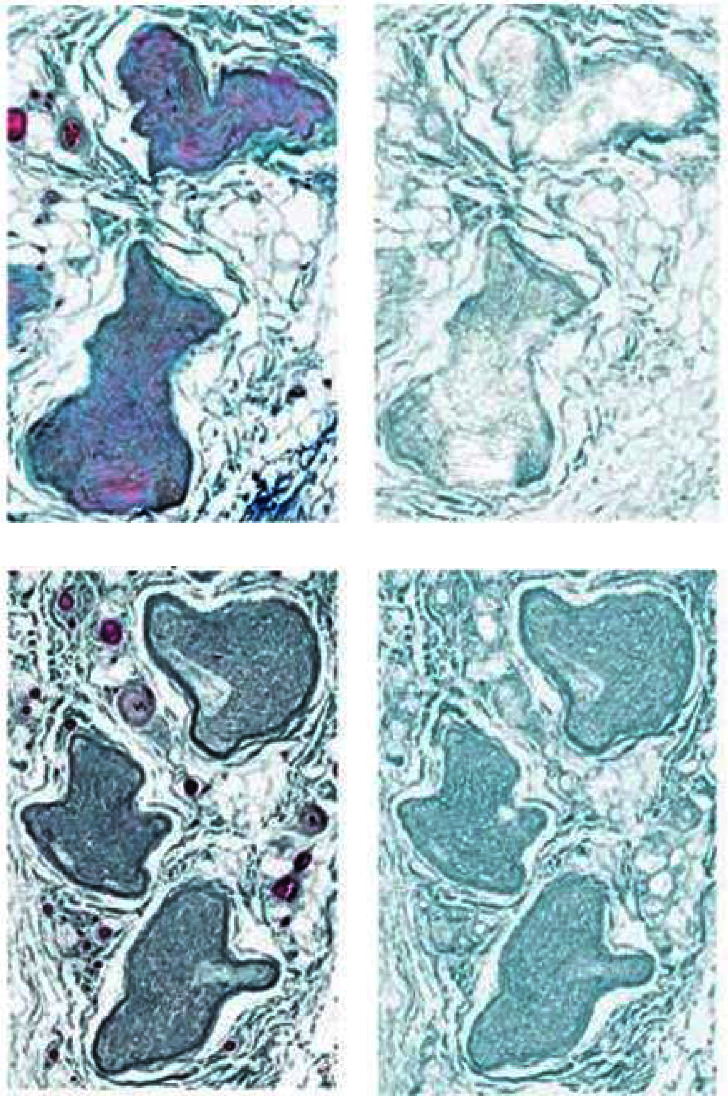
Representative protected and affected ilioinguinal nerve samples stained with trichrome (a, c), next to the resultant color deconvoluted image containing only the green component (b, d), showing increased collagen content in the inguinal canal sample. (a) Proximal sample. (b) Proximal green component. (c) Inguinal sample. (d) Inguinal green component.
Three dependent variables (nerve cross-sectional area, fascicle total cross-sectional area, and collagen total cross-sectional area) were measured across the three sites (proximal, canal, and distal.) Statistical analysis was performed using JASP computer software (Version 0.18.3, JASP Team 2024.) Outliers were identified based on the interquartile range for each dependent variable and removed prior to analysis such that acceptable levels of skewness and kurtosis [14] could be achieved. Q-Q plots were also examined after the removal of outliers for each dependent variable at each location to identify potential deviations from normality. It was determined that all dependent variables at all locations met the assumption of normality once outliers were removed from the data. Parametric methods were utilized for all analyses.
Given that three separate ANOVAs will be conducted to address the research questions, there is the possibility for an inflated family-wise error rate. To avoid this, sequential Holm–Sidak adjustment [15] was implemented for the p values from each omnibus ANOVA. In this process, p values resulting from the analysis are ordered from small-to-large and then adjusted based on the sequential Holm–Sidak formula. This method has the benefit of limiting family-wise type-I error while maintaining higher statistical power than the commonly utilized Bonferroni procedure [15]. Both adjusted and unadjusted p values are to be reported. The overall type-I error rate was set to 5% for all analyses (α=0.05).
To further assess whether assumptions were met for ANOVA, the dependent variables across sites were examined for sphericity. Results of the analyses suggested that sphericity could not be assumed for the collagen outcome (p=0.034), so a Greenhouse–Geisser correction was used when analyzing that dependent variable. Sphericity could be assumed for all other dependent variables.
3. Results
A total of thirty patients underwent repair and ilioinguinal nerve resection. Patient characteristics are listed in Table 1. Of these resected nerves, the data from four patients were incomplete, resulting in twenty-six complete sample sets. To determine if differences existed for the fascicle cross-sectional area depending on proximal, canal, or distal location, a repeated measures ANOVA was conducted. Repeated measures analysis was used due to all measures coming from the same participants, and the assumption of sphericity was met according to Mauchly's test of sphericity (p=0.271). Results of the analysis did not reveal a statistically significant difference for the fascicle cross-sectional area between all three locations (F(2, 46)=4.46, Holm − Sidak p=0.050, η2=0.16). However, the effect size observed would be considered large according to Cohen's rules of thumb [16]. The effect size suggests that 16% of the variance in the fascicle cross-sectional area could be explained by which location was measured. A larger sample would be needed to confirm the observed effect, and the result was very close to statistical significance even after adjusting for multiple testing. Given that a meaningful effect was found, Holm post hoc comparisons were conducted to see where potential differences could be found. A statistically significant, moderate-to-large difference was found between canal and proximal locations (p=0.016, d=−0.67 [95% CI : −1.29, −0.05]). The effect size for this difference suggests that the proximal fascicle cross-sectional area was over 0.67 standard deviations higher than the canal fascicle cross-sectional area. Although nonsignificant, a moderate effect size difference was found between canal and distal locations (p=0.107, d=−0.46 [95% CI : −1.05, 0.136]). Distal and proximal locations only showed a small effect size difference that was not statistically significant (p=0.35, d=−0.22[95% CI : −0.79, 0.357]) (Figure 3).
Table 1.
Patient characteristics.
| N ∗ | Mean | Std. dev | Range | |
|---|---|---|---|---|
| Age | 29 | 57 | 14.3 | 27–87 |
| BMI | 29 | 28.3 | 4.1 | 21–38 |
| Right side | Left side | |||
| Hernia laterality | 29 | 13 | 16 | |
| Yes | No | |||
| Incarcerated? | 29 | 21 | 8 | |
| <1.5 cm | <3 cm | >3 cm | ||
| Hernia type-direct | 23 | 13 | 9 | 1 |
| Hernia type-indirect | 6 | 4 | 2 |
∗ Of the original thirty patients, one patient's dataset was incomplete.
Figure 3.
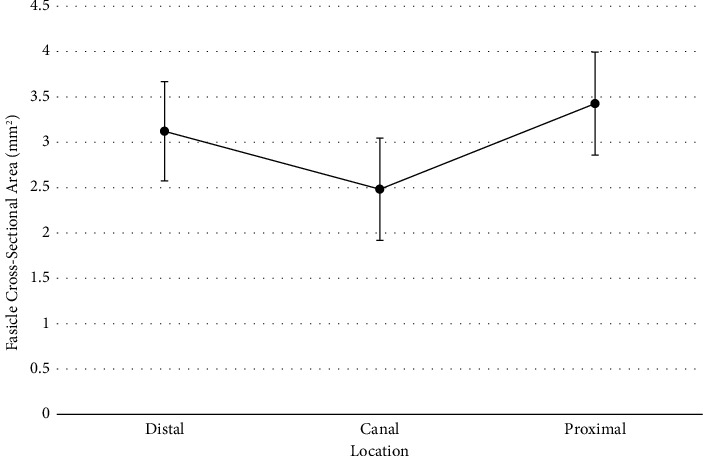
Fascicle area means for each location with 95% confidence intervals.
To determine if differences existed for the nerve cross-sectional area depending on proximal, canal, or distal location, a repeated measures ANOVA was conducted. Repeated measures analysis was used due to all measures coming from the same participants, and the assumption of sphericity was met according to Mauchly's test of sphericity (p=0.539). Results of the analysis did not reveal a statistically significant difference for the nerve cross-sectional area between the locations (F(2, 44)=2.59, Holm − Sidak p=0.165, η2=0.105). However, the effect size observed would be considered moderate to large according to Cohen's rules of thumb [16]. The effect size suggests that almost 11% of the variance in the nerve cross-sectional area could be explained by which location was measured. A larger sample would be needed to confirm the observed effect. Given that a meaningful effect was found, Holm post hoc comparisons were conducted to see where potential differences could be found. Although no statistically significant differences were found, a small-to-moderate effect size difference was found between canal and distal (p=0.19, d=0.42 [95% CI : −0.21, 1.05]), and a moderate effect size difference was found between canal and proximal (p=0.109, d=0.53 [95% CI : −0.11, 1.17]). Average nerve cross-sectional areas were 0.42 standard deviations apart for canal and distal locations and 0.53 standard deviations apart for canal and proximal locations (Figure 4).
Figure 4.
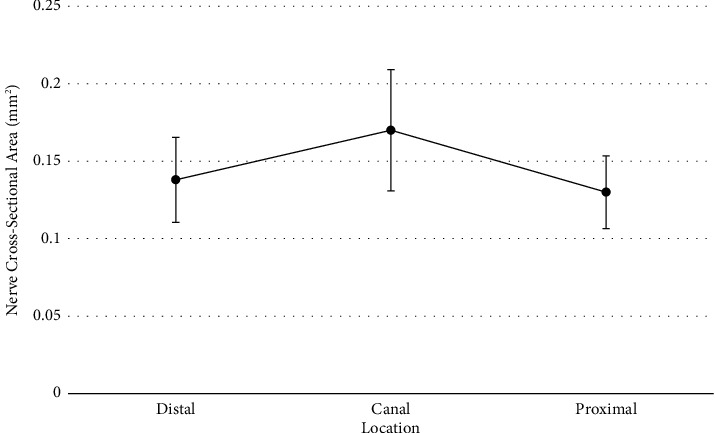
Nerve cross-sectional area means for each location with 95% confidence intervals.
To determine if differences existed for the collagen cross-sectional area depending on proximal, canal, or distal location, a repeated measures ANOVA was conducted. Repeated measures analysis was used due to all measures coming from the same participants, but the assumption of sphericity was not met according to Mauchly's test of sphericity (p=0.034). The ANOVA results were adjusted using a Greenhouse–Geisser correction. Results of the analysis did not reveal a statistically significant difference for the collagen cross-sectional area between the locations (F(1.58, 36.37)=0.003, Holm − Sidak p=0.99, η2 < 0.01). No post hoc comparisons were conducted since no overall mean differences existed (Figure 5). This suggests that collagen content appears to be uniform at each segment of the nerve. Statistics are summarized in Table 2.
Figure 5.
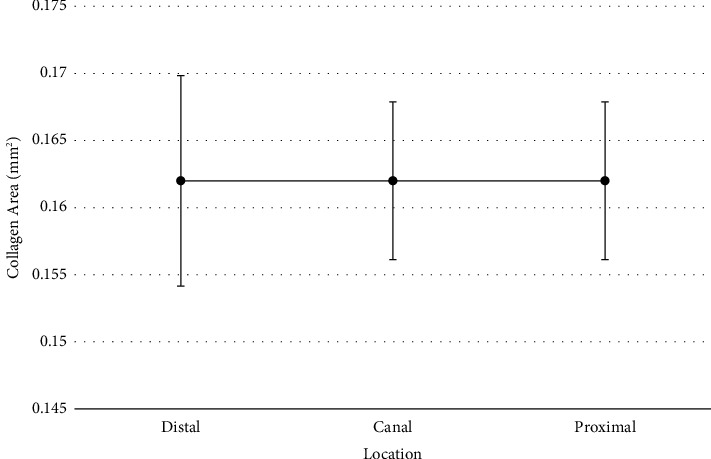
Collagen area means for each location with 95% confidence intervals.
Table 2.
Measurements of the total nerve cross-sectional area, total fascicle cross-sectional area, and total collagen cross-sectional area at the three sample sites.
| Sample location | N ∗ | Proximal (control) | Canal | Distal | Canal vs. proximal | Canal vs. distal | Proximal vs. distal |
|---|---|---|---|---|---|---|---|
| Mean (mm2) | Mean (mm2) | Mean (mm2) | p | p | p | ||
| Total nerve cross-sectional area | 23 | 0.130 | 0.170 | 0.138 | 0.109 | 0.190 | 0.655 |
| Total fascicle cross-sectional area | 24 | 3.426 | 2.482 | 3.121 | 0.016 | 0.107 | 0.350 |
| Total collagen cross-sectional area | 24 | 0.162 | 0.162 | 0.162 | 1 | 1 | 1 |
∗ N is calculated with outliers removed; see Materials and Methods section. Numbers in bold indicate significance.
4. Discussion
Prior research on size disparity of nerves in the inguinal canal has demonstrated significant correlation with preoperative hernia pain. This current study demonstrated a decrease in the fascicle cross-sectional area in the canal region of the ilioinguinal nerve compared to the proximal control which suggests compression damage to the nerve from the hernia. This could be indicative of axon loss in the canal region of the ilioinguinal nerve. Additionally, this study found similar cross-sectional areas between the proximal and distal regions of the nerve suggesting that damage is occurring within the canal, and the nerve may be narrowing in the canal region rather than enlarging at the distal region. Apparent decrease in the size of the nerve in the inguinal canal correlates with preoperative pain “most of the time” on questionnaire series. Additionally, it correlates with heavy fibrosis of the external inguinal ring as noted during surgery [10]. In another study, decreased canal segment diameter visually noted during surgery correlates with four of eight pain measures on Carolina's Comfort Scale for preoperative pain [2].
It was originally hypothesized for this study that collagen was depositing in the nerve, accounting for nerve enlargement, but this study did not find unequal collagen deposition in the different locations (Table 2). However, in yet another study of nerve, decreased canal diameter correlated with increased pain as well as increased evidence of myxoid material which also correlated with pain [1]. The reduced fascicle area within the canal region of the nerve could be indicative of axon loss. Demyelination and axonal degradation have been found in chronic compression studies of rat sciatic and sural nerves. Increased pressure on the nerve can create ischemic changes that result in nerve fiber degeneration [5] which is consistent with the findings of this study and further suggestive of compression neuropathy. Masson's trichrome stain has limited ability to study axon loss, further research with Toluidine blue could be considered to better visualize demyelination and the severity of axon loss within nerves and conclude the state of nerve injury. These studies suggest that the hernia itself is causing nerve compression neuropathy and intervention prior to pain may prevent the progression of compression neuropathy. It is well established that the degree of preoperative pain when in higher levels correlates with chronic postoperative pain [17], so prevention of preoperative pain is a worthy goal. It is also a mystery as to why so many postoperative hernia patients have pain by whatever approach the repair is done. The key may lie in compression neuropathy occurrence.
Currently, there is an acceptable “watch and wait” approach to inguinal hernia repair. Patients are routinely told that it is advisable to delay treatment related to their hernia until it is bothering them more due to increased pain. This is based on the prospective data demonstrating that the yearly risk of acute strangulation of inguinal hernias is about 1–3% [18]. However, if the hernia sac is exhibiting a chronic pressure on the ilioinguinal nerve, it is likely contributing to the degeneration of nerve fibers. This challenges the current “watch and wait” approach to avoid further nerve damage.
4.1. Limitations
This study does not have large numbers and is a single-surgeon series. The moderate-to-large effect sizes seen in the analyses of total nerve and fascicle cross-sectional areas indicate that there may be a variance in cross-sectional areas by location, and larger studies would help investigate this relationship further. Tissue shrinkage commonly known to occur with formalin fixation may have made previously visible size discrepancies less noticeable and may have affected the specimens microscopically and not uniformly. The measurements are performed by one researcher without intraobserver repeatability testing. Also, to study the ilioinguinal nerve, it must be sacrificed during herniorrhaphy which is not advised as a standard practice. If compression neuropathy is to be established as a real entity in patients with primary inguinal hernias, larger funded studies will need to be performed which include stains that can demonstrate axon loss and Wallerian degeneration.
5. Conclusion
In patients with a primary inguinal hernia who present with a visible size discrepancy between the ilioinguinal nerve in the inguinal canal and the same nerve at the external inguinal ring, the smaller canal segment may be a result of compression neuropathy. This is supported by the findings of the decreased cross-sectional area of the nerve fascicle in the inguinal canal compared to the proximal nerve. Collagen deposition is uniformly distributed, so the size difference in the canal segment fascicle may be due to axon loss which is common in compression neuropathy.
Acknowledgments
Dr. Donald Born funded this project through discretionary research funds awarded by the Stanford University School of Medicine, Department of Neurosurgery, as part of his employment with the university. The authors acknowledge Trey DeJong Ph.D. with Washington State University's Center for Interdisciplinary Statistical Education and Research (CISER) for statistical analysis.
Data Availability
The data that support the findings of this study are available from the corresponding author under reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest as outlined by the International Committee of Medical Journal Editors.
References
- 1.Wright R., Born D. E., D’Souza N., Hurd L., Gill R., Wright D. Why do inguinal hernia patients have pain? histology points to compression neuropathy. The American Journal of Surgery . 2017;213(5):975–982. doi: 10.1016/j.amjsurg.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Wright R., Born D. E., D’Souza N., Hurd L., Gill R., Wright D. Pain and compression neuropathy in primary inguinal hernia. Hernia . 2017;21(5):715–722. doi: 10.1007/s10029-017-1641-8. [DOI] [PubMed] [Google Scholar]
- 3.Mackinnon S. E. Pathophysiology of nerve compression. Hand Clinics . 2002;18(2):231–241. doi: 10.1016/s0749-0712(01)00012-9. [DOI] [PubMed] [Google Scholar]
- 4.Amato G., Ober E., Romano G., et al. Nerve degeneration in inguinal hernia specimens. Hernia . 2011;15(1):53–58. doi: 10.1007/s10029-010-0735-3. [DOI] [PubMed] [Google Scholar]
- 5.Amato G., Romano G., Salamone G., et al. Damage to the vascular structures in inguinal hernia specimens. Hernia . 2012;16(1):63–67. doi: 10.1007/s10029-011-0847-4. [DOI] [PubMed] [Google Scholar]
- 6.Rempel D. M., Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. Journal of Electromyography and Kinesiology . 2004;14(1):71–75. doi: 10.1016/j.jelekin.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Mackinnon S. E., Dellon A. L., Hudson A. R., Hunter D. A. Chronic human nerve compression- A histological assessment. Neuropathology and Applied Neurobiology . 1986;12(6):547–565. doi: 10.1111/j.1365-2990.1986.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Thatte M. R., Mansukhani K. A. Compressive neuropathy in the upper limb. Indian Journal of Plastic Surgery . 2011;44(2):283–297. doi: 10.4103/0970-0358.85350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundborg G., Myers R., Powell H. Nerve compression injury and increased endoneurial fluid pressure: a miniature compartment syndrome. Journal of Neurology, Neurosurgery and Psychiatry . 1983;46(12):1119–1124. doi: 10.1136/jnnp.46.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wright R., Salisbury T., Landes J. Groin anatomy, preoperative pain, and compression neuropathy in primary inguinal hernia: what really matters. The American Journal of Surgery . 2019;217(5):873–877. doi: 10.1016/j.amjsurg.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Alant J., Kemp S., Webb A., Midha R. Traumatic neuroma in continuity injury model in rodents: a preliminary report. Evidence-Based Spine-Care Journal . 2010;1(02):52–55. doi: 10.1055/s0028-1100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carriel V., Garzón I., Alaminos M., Cornelissen M. Histological assessment in peripheral nerve tissue engineering. Neural Regeneration Research . 2014;9(18):1657–1660. doi: 10.4103/16735374.141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamble N., Shukla D., Bhat D. Peripheral nerve injuries: electrophysiology for the neurosurgeon. Neurology India . 2019;67(6):1419–1422. doi: 10.4103/0028-3886.273626. [DOI] [PubMed] [Google Scholar]
- 14.George D., Mallery P. IBM SPSS Statistics 23 Step by Step: A Simple Guide and Reference . New York, NY, USA: Routledge; 2016. [Google Scholar]
- 15.Abdi H. Holm’s sequential bonferroni procedure. Encyclopedia of Research Design . 2010;1(8):1–8. [Google Scholar]
- 16.Cohen J. Statistical Power Analysis for the Behavioral Sciences . Cambridge, MA, USA: Academic Press; 2013. [Google Scholar]
- 17.Reinpold W. M. J., Nehls J., Eggert A. Nerve management and chronic pain after open inguinal hernia repair. Annals of Surgery . 2011;254(1):163–168. doi: 10.1097/sla.0b013e31821d4a2d. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos N. C., Dawson J., Jarvis M., Hobsley M. Risk of strangulation in groin hernias. British Journal of Surgery . 2005;78(10):1171–1173. doi: 10.1002/bjs.1800781007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author under reasonable request.


