Abstract
Purpose of Review
Cannabis may have beneficial anti-inflammatory effects in people with HIV (PWH); however, given this population’s high burden of persisting neurocognitive impairment (NCI), clinicians are concerned they may be particularly vulnerable to the deleterious effects of cannabis on cognition. Here, we present a systematic scoping review of clinical and preclinical studies evaluating the effects of cannabinoid exposure on cognition in HIV.
Recent Findings
Results revealed little evidence to support a harmful impact of cannabis use on cognition in HIV, with few eligible preclinical data existing. Furthermore, the beneficial/harmful effects of cannabis use observed on cognition were function-dependent and confounded by several factors (e.g., age, frequency of use).
Summary
Results are discussed alongside potential mechanisms of cannabis effects on cognition in HIV (e.g., anti-inflammatory), and considerations are outlined for screening PWH that may benefit from cannabis interventions. We further highlight the value of accelerating research discoveries in this area by utilizing translatable cross-species tasks to facilitate comparisons across human and animal work.
Keywords: NeuroHIV, Cannabinoids, Cognition, HIV-associated neurocognitive disorders
Introduction
Approximately 38.4 million people live with human immunodeficiency virus (HIV) worldwide (UNAIDS, 2021). While advances in combination-antiretroviral therapy (cART) have reduced HIV progression, secondary effects of long-term infection arise, including self-reported [1–3] and clinically diagnosed neurocognitive impairment [NCI; 4–6]. NCI is estimated to affect 40–45% of people with HIV (PWH), typically impacting verbal fluency/language skills, attention/working memory, executive function, learning and memory, information-processing speed, and motor skills [7–11]. Treatment development is complicated by several factors. For example, while affected cognitive domains vary across PWH, HIV-associated NCI is diagnosed based on the degree of impairment, ranging from mild (i.e., asymptomatic neurocognitive impairment) to moderate (i.e., mild neurocognitive impairment) to severe (i.e., HIV-associated dementia) [12]. This means individuals often share the same categorical diagnoses, yet experience deficits in different functional domains. This approach is problematic given that distinct “cognitive trajectories” of HIV-associated-NCI are observed, suggesting discrete underlying network aberrations across PWH [13•, 14, 15].
Moreover, worse NCI is observed in PWH who reported current [16–19] or past [20–22] substance use, necessitating considerations of the exacerbating effects of drug exposure. Cannabis, however, has anti-inflammatory and neuroprotective properties [23] which may combat the neuroinflammation and associated neurodegeneration thought to partly mediate HIV-associated NCI. Importantly, cannabis use (CU) is reported by PWH at two-to-three times the rate of the general population [24], driven in large part by self-medication (e.g., anxiety and pain management) [25, 26], but its interactions with HIV infection are not well understood.
Particularly, the interactive effects of CU and HIV infection on domain-specific cognition are required to facilitate development of targeted therapeutics for HIV-associated NCI.
Cannabis and Interactions with the Endocannabinoid System
Cannabis acts primarily via the endocannabinoid (eCB) system, which comprises the G-protein coupled cannabinoid-1 and cannabinoid-2 receptors (CB1R and CB2R), and their endogenous ligands (i.e., endocannabinoids), N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) [27]. AEA and 2-AG are retrograde signaling molecules that are synthesized on-demand following neuronal depolarization and decrease further neurotransmitter release by activating presynaptic eCB receptors [28].
CB1Rs are one of the most abundantly expressed receptors in the central nervous system (CNS), primarily expressed on neurons [29]. CB2Rs, while less abundant in the CNS, are expressed at higher levels on immune-activated glial cells and astrocytes and are largely upregulated under pathological conditions, e.g., neuroinflammation [30]. The eCB system mediates widespread physiological and behavioral processes—including cognition [31]—and has therapeutic potential for HIV-associated neuropathology, e.g., blocking neuronal excitotoxicity (CB1R) [32, 33] and reducing neuroinflammation via attenuation of microglia activation (CB2R) [34]. Interestingly, AEA and related eCB ligands are decreased in PWH, suggesting dysregulation of the eCB system (although this result may be confounded by heavier drug use in the HIV cohort) [35]. The eCB system should, therefore, be further explored for potential biomarkers of HIV-related states, such as HIV-associated NCI.
Over 100 phytocannabinoids have been identified in the cannabis plant, each of which may contribute to its effects on cognition. Of these, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are the best characterized. THC, the primary psychoactive constituent, is a partial agonist at both eCB receptors [36], although its cognitive effects are largely CB1R-mediated [37, 38]. The pharmacology of CBD includes antagonist/inverse-agonist activity at eCB receptors [36, 39], and agonist properties at several other non-eCB receptors [40–42]. Interestingly, THC and CBD can produce differential, or even opposing effects on cognition and associated brain activity [43–47]; thus, the THC:CBD ratio influences the pharmacological properties of cannabis, and likely its effects on cognition.
Essential to determining causal mechanisms of CU on HIV-associated NCI, including the role of the eCB system, is the use of neuroHIV animal models. NeuroHIV animal models enable well-controlled studies of cannabis and/or cannabinoid effects on HIV-associated NCI by minimizing confounds inherent to clinical HIV research (e.g., clinical demographics), as well as those relating to CU (e.g., inter-individual variation in dosing, THC/CBD content). Below we provide a brief overview of current animal models and their benefits and limitations in determining the effects of CU on cognition in HIV.
Animal Models of HIV
Several transgenic (tg) animal models exist that incorporate HIV proteins into the host genome. For example, the glycoprotein (gp)120 tg mouse expresses the envelope protein gp120 in brain astrocytes and develops a similar neuropathology to that observed in humans with acquired immunodeficiency syndrome (AIDS) [48]. However, since gp120 is expressed constitutively from birth, generalizability to PWH is limited, apart from those born with HIV. Meanwhile, the inducible Tat (iTat) mouse expresses the HIV regulatory protein Tat only following additional treatment (doxycycline), thereby providing temporal control over viral exposure [49]. While the gp120 and iTat lines are useful in parsing the effects of individual HIV proteins on behavioral outcomes, they do not fully recapitulate the human disease, which entails exposure to several interacting viral proteins. The HIV-1tg rat partly addresses this limitation by constitutively expressing most of the HIV genome (excluding gag and pol) [50]. However, this model, too, is limited as it does not reproduce a key feature of viral pathogenesis—the replication and transmission of the infection to other host cells.
Other animal models recreate aspects of viral replication and propagation. For example, humanized mouse models comprising immunodeficient mice reconstituted with HIV-infected human cells enable the study of HIV transmission and chronic infection [51]. However, these mice have numerous drawbacks that limit their use in behavioral assessments (e.g., longevity, cost, transmissibility concerns). A new approach utilizes EcoHIV, an injectable chimeric HIV virus in which the gp120 gene has been replaced with ecotropic gp80 (from murine leukemia virus), thus enabling viral entry into cells of healthy mice [52] and rats [53]. EcoHIV is sexually transmissible, virally suppressible with cART, and not transmissible to humans, making this model an ideal approach for recreating HIV in rodents.
Finally, simian immunodeficiency virus (SIV) and feline immunodeficiency virus (FIV)—lentiviruses found in non-human primates and felines, respectively [54, 55]—recreate aspects of HIV, though their species-specificity limits their translatability to the human disease. To address this, chimeric viruses (“SHIVs”) in which one or more HIV genes are inserted into the SIV viral genome have been developed [56]; however, given the complexity of utilizing larger animals in research, rodent models are far more commonly used to study neuroHIV.
Objective
Here, we performed a systematic scoping review of the clinical (i.e., human) and preclinical (i.e., animal model) studies assessing the impact of cannabis exposure or manipulations of the eCB system on cognitive functioning in HIV. The aims of this review are to (1) summarize the existing literature on the effects of cannabinoids on individual cognitive domains; (2) highlight modulating factors of cannabinoid effects on cognition in HIV; and (3) discuss the use of cannabinoids, and alternative approaches in treating HIV-associated NCI.
Methods
Information Sources and Search Strategy
A systematic scoping review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [204]. Reports were identified by searching PubMed, EMBASE, PsycINFO, and Web of Science for articles published through May 2023. Studies with available data on aspects of cognition, cannabis or the eCB system, and HIV or non-human animal models of HIV. The full search strategies used for each database are provided in Supplemental File 1.
Eligibility Criteria and Study Selection
Figure 1 summarizes the systematic scoping review results and applied inclusion/exclusion criteria used for study selection. Eligible studies required all three major components relevant to our review: (1) a study population of PWH or an appropriate model of HIV; (2) assessment of cognition; and (3) self-report of CU data (human studies), administration of cannabis, and measurement/manipulation of the eCB system. Only studies published or translated into the English language were included.
Fig. 1.
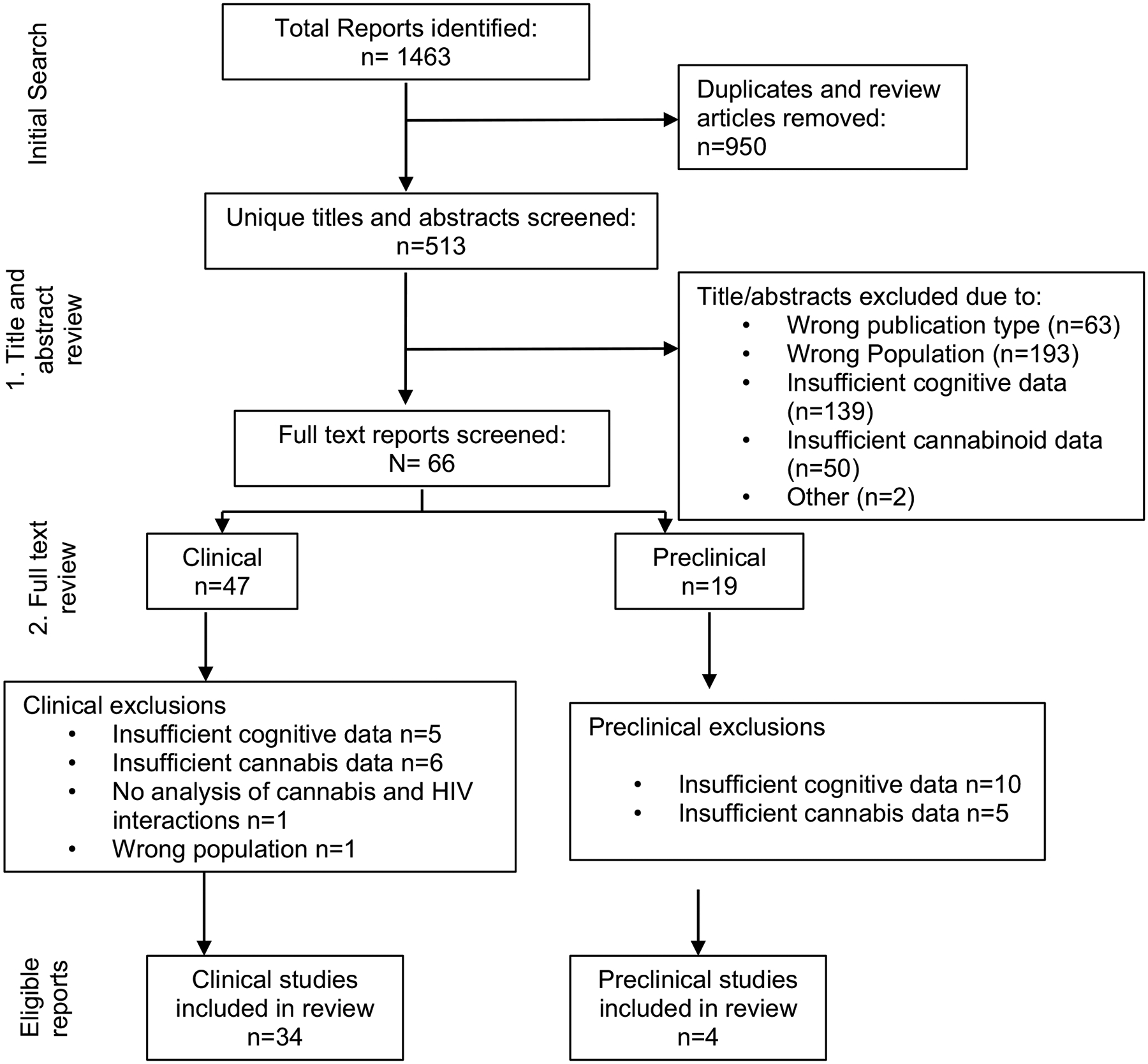
Flowchart of systematic scoping review results and inclusion/exclusion criteria
Data Collection
Database search results were uploaded into EndNote to delete duplicated results. Unique articles were then uploaded to the systematic review software Rayyan QCRI. First and secondary assessments were performed by four reviewers, SA, BH, BR, and AHM, following a previously published systematic scoping review protocol [205, 206]. First and secondary assessments required reviews of the tile and abstract, and full text review, respectively, based on the inclusion and exclusion criteria. Articles were approved by at least two reviewers to be considered eligible. Data was extracted from eligible reports using a standardized form, including: first author, publication year, gender distribution, sample-size, diagnoses/disease model, cognitive assessments, and CU criteria or relevant eCB measurement/manipulation method. Conflicts were resolved via discussion between the reviewers.
Results
Table 1 summarizes eligible preclinical findings, including sample size, animal model, drug-administration, and cognitive task. Table 2 includes eligible clinical findings, including participant demographics, CU criteria, and cognitive tasks. It should be noted that articles may appear in several sections if they assessed multiple domains and may also reappear in beneficial, adverse, or null categories if reported findings were dependent on a varying factor (i.e., dose).
Table 1.
Data extraction from studies that examined the effects of cannabinoid exposure on cognition in the context of HIV in non-human animals
| Author/year | HIV animal model and subject characteristics | Cannabinoid measure/manipulation | Cognitive assessments | Cognitive domains | Main findings |
|---|---|---|---|---|---|
| Jacobs et al. 2019 | Mouse—iTat (50% female) 7/group: Tat + /Tat − |
Measured CB1R expression | Go/no-go task | Inhibitory-control | Female iTat + mice demonstrated less inhibitory-control compared to male iTat + mice and had higher CB1R expression in mPFC relative to all other groups; Higher infralimbic mPFC CB1R expression correlated with less inhibitory-control |
| League et al. 2021 | Mouse—iTat (100% female) | MAG-L inhibition via MJN110 | Discrimination and reversal-learning task | Learning and cognitive flexibility | Initial and reversal-learning was quicker in Tat + mice than in Tat − mice; MJN 110 slowed reversal-learning to Tat- levels |
| 12/group: Tat +/Tat− | |||||
| Wang et al. 2022 | Rat-hippocampal gpl20 injection (100% male) 8–9/group: control, sham, gpl20 |
eCB agonist WIN-55,212–2 (3 mg/kg) and/or CB2R antagonist AM630 (1.5 mg/kg) prior to gpl20 | Morris water maze | Learning and memory | Prophylactic WIN-55,212–2 treatment attenuated Gpl20-induced spatial memory deficits; WIN-55,212–2 effects were blocked by CB2R antagonist treatment, which also prevented hippocampal gpl20-induced inflammation and apoptosis |
| Winsauer et al. 2011 | Rhesus monkeys (100% males) | Acute THC (0.032–0.32 mg/kg; IM); | Repeated acquisition task | Learning/working memory | Prior to SIV acute THC decreased response-rates without affecting %errors; tolerance developed to chronic THC irrespective of SIV. Chronic THC did not affect viral load but reduced neuropathology, opportunistic infections and inflammation in SIV + animals, and decreased hippocampal CB1R and CB2R expression across all animals (SIV + / −) |
| 3–4/group: SIV + / − ; THC + / − | Chronic 28-day THC (2×/day; 0.32 mg/kg; IM) |
Table 2.
Data extraction from clinical studies examining associations between CU and HIV status in humans
| Author/year | Sample characteristics (% male) | Cannabis use criteria | Cognitive assessments | Cognitive domains | Main findings |
|---|---|---|---|---|---|
| Attonito et al. 2014 | 370 PWH: > 50% CU + (49% M) | no. of days of CU in the past 3 months | AVLT, The Color Trails Test 2; Short Category Test | Executive function, attention, information-processing, memory | Higher number of CU days correlated with worse psychomotor speed and attention (p = 0.051) |
| Bedi et al. 2010 | 7 PWH: 100% current and chronic CU (≥ 2 × /week) (100% M) | 16-day dronabinol (5–10 mg) + 16-day placebo (4×/day) | DSS, RAT, DAT, immediate and delayed DRT, 10-min rapid information-processing task | Attention, information-processing, verbal memory | Dronabinol was associated with worse processing-speed, latency (after 9–16 day treatment), and more false alarms (days 1–8), but better acquisition compared to placebo; No effect on information-processing or recall |
| Byrd et al. 2011 | 1284 PWH: 924 CU + (67% M) | Interview-Substance Abuse Module for frequent use (≥5×) | WAIS-III DS, WAIS-III SS, TMA, SMT, FMT, WCST, TMB, COWAT, CF, WAIS-III LNS, PASAT, GPT | Global cognition [executive function, attention, information processing, learning, memory, verbal-fluency, motor]; individual subdomain scores also reported | Lifetime “dosage” of cannabis was weakly associated with better verbal-fluency (p<0.1) |
| Chang et al. 2006 | PWH: 21 C − (81% M) 21 C + (86% M) PWoH: 30 C − (80% M) 24 C + (83% M) |
C + : regular CU or history of chronic CU C − : < 1 joint per month or no use |
Timed gait, RAVLT, GPT, Stroop, New adult reading test revised, TMA, TMB, SDM, CalCAP, Tests for working memory, and visual discrimination and response inhibition (degraded words with distracters, response reversal/visual scanning, and form discrimination tasks) | Executive function, information processing, verbal memory, motor function | CU, irrespective of HIV diagnosis, was associated with better executive function, information-processing and motor skills, but no effects on any cognitive function after age was included as a covariate |
| Christopher-Hayes et al. 2021 | PWH: 22 C − (82% M) 18 C + (78% M) PWoH: 20 C − (55% M) 21 C + (57% M) |
≥ 4 × CU/month; no other illicit substance use; no CU on test day | GPT, HVLT-R, Phonemic and semantic verbal-fluency, WAIS-III DS, WAIS-III SS, TMA, Stroop, TMB | Executive function, attention, information-processing, learning, memory, verbal-fluency | No effects or interaction between cannabis and HIV on any cognitive functions; main effect of HIV on reaction time in visuospatial task (PWH slower than PWoH) |
| Cristiani et al. 2004 | PWH: 46 asympt. C − 79 asympt. C + 29 sympt. C − 55 sympt. C + PWoH: 24 C − 49 C + No gender data |
Past 12 month self-report; ≥ 1 × CU/week | WAIS-R, Selective Reminding Test, Wechsler Memory Scale-Revised, Verbal Concept Attainment Test, WCST, Verbal and figural fluency, TMA, TMB, GPT, PASAT | Global cognition [executive function, attention, information-processing, learning, memory, language, motor] | Cannabis related to worse overall impairment; driven by interaction effect on delayed memory; CU worsened delayed memory, particularly for those with more severe HIV disease) |
| Crook et al. 2021 | PWH: 91 past CUD (79% M) 47 past CUD + (69% M) |
Past CUD measured by DSM-IV interview; excluded current CUD | WAIS-III LNS, PASAT, TMA, WAIS-III DS, WAIS-III SS, TMB, WCST, COWAT, HVLT, BVMT, GPT | Executive function, informa-tion-processing, attention, learning, memory, verbal-fluency, motor | CUD + associated with better processing-speed, visual learning, memory, delayed recall, and dominant hand motor ability compared to CUD− |
| Dastgheyb et al. 2021 | 929 PWH 717 PWoH 100% F |
Self-reported CU | HVLT-R, LNS, TMA, TMB, Stroop, SDMT, COWAT; Animal fluency; GPT | Neuropsychological “profiles” created using the following domains: executive function, attention, learning, memory, language, motor | Current CU associated with lower likelihood of impairment “profile” consisting of learning, information-processing and executive function in women with HIV |
| Flannery et al. 2021 | PWH: 27 C − (67% M) 32 C + (91% M) PWoH: 22 C − (50% M) 28 C + (57% M) |
≥ 1 × CU/week for 3 months; ≥ 20 × in the past year | EAT (go/no-go motor inhibition paradigm) during MRI; Cognitive Failures Questionnaire | Executive function | Greater lifetime CU associated with worse inhibition-related neural activity in PWoH but not PWH |
| Flannery et al. 2022 | PWH: 28 C − (67% M) 32 C + (91% M) PWoH: 22 C − (50% M) 24 C + (57% M) |
≥ 1 × CU/week for 3 months; ≥ 20 × in the past year | WAIS-IV, WCST, IGT, HVLT-R, BVMT, GPT, MST | Executive function, attention, information-processing, learning, memory, and motor | CU was associated with increased resting state functional connectivity in regions associated with executive function in PWH and PWoH, but no effect of CU on cognitive functions |
| Flannery et al. 2022 | PWH: 28 C − (67% M) 32 C + (91% M) PWoH: 22 C − (50% M) 24 C + (57% M) |
≥ 1 × CU/week for 3 months; ≥ 20 × in the past year | EAT (go/no-go motor inhibition paradigm) during MRI | Executive function | CU was associated with better executive function in PWH (error-awareness) relative to PWH who do not use cannabis |
| Gomez et al. 2017 | 138 neurocognitively normal (NN) PWH/C − 67 NN PWH/C + 69 NCI PWH/C − 14 NCI PWH/C + (84–90% M/group) |
Patient chart review for the past cannabis abuse | Game of Dice Task (GDT), SDM, TMT-2/4, GPT, HVLT, WCST | Executive function | CU predicted worse executive function (GDT net score) |
| Gonzalez et al. 2011 | PWH: 25 C − 17C + PWoH: 21 C − 23 C + Mostly men no gender data; polysubstance users |
DSM-IV + Kreek-McHugh-Schluger-Kellog scale; lifetime history of cannabis dependence | Rotary pursuit task (RPT), Star mirror tracing task (SMT), weather prediction task (WPT) | Procedural learning of motor skills | History of cannabis dependence was not associated with procedural learning of motor skills; Motor skills performance on the RPT and SMT adversely affected among PWH with history of polysubstance dependence |
| Haney et al. 2005 | 15 PWH/C + with muscle loss (80% M) 15 PWH/C + no muscle loss (100% M) 100% current CU + ≥ 2×/ week for 4 weeks |
Acute dronabinol (0, 10, 20, 30 mg) or cannabis (0.0, 1.8%, 2.8%, 3.9% THC) | DSS, RAT, DAT, Immediate and a delayed DRT | Attention, learning, memory | CU associated with no effects on performance in either group compared to placebo; 20-mg dronabinol associated with worse memory and attention in clinical muscle loss group; 30-mg dronabinol was associated with worse memory in normal muscle mass group |
| Haney et al. 2007 | 10 PWH/C + (90% M): 100% current cannabis users (≥ 2 ×/week) for the past 4 weeks |
Chronic (4 day; QUID) dronabinol (0, 5, 10 mg) or cannabis (THC 0%, 2%, 3.9%) | DSS, RAT, DAT, immediate and a delayed DRT, 50-item visual analogue scale | Attention, information-processing, learning, memory | Cannabis or dronabinol was associated with no effects on cognitive performance compared to placebo |
| Heaton et al. 2023 | 402 PWH 76% M |
Past or present CUD | DVT, WAIS-III DS, WAIS-III SS, TMA, SMT, FMT, HCT, WCST, TMB, COWAT, CF, WAIS-R DS, WAIS-III LNS, PASAT, GPT | Global cognition [executive function, attention, information-processing, learning, verbal-fluency] | History of CUD was associated with worse neurocognitive decline (decrease from baseline after 12 years) |
| Kallianpur et al. 2020 | PWH (85% M): 4 C − 25 recent C + 23 remote C + PWoH (73%M): 11 C − 9 recent C + 35 remote C + |
Self-report of CU; recent use (use in the last 12 months), remote use (> 12 months ago); also reported 12-month CU frequency |
CalCAP, RAVLT, RCF Copy and Recall, TMA, TMB, WAIS-R, WAIS-III LNS, GPT, Verbal-fluency test, Animal and Boston Naming tests, Delis-Kaplan Executive Function System, Stroop, Timed gait | Executive function, information-processing, learning, memory | Occasional CU was associated with better executive function in PWH compared to non-use; duration of CU positively correlated with psychomotor speed and executive function in PWH but not PWoH; recent CU was associated with worse psychomotor speed compared to remote CU in PWoH |
| Lorkiewicz et al. 2018 | 215 PWH 65% M |
Lifetime CU history; current use: no. of days of use in the past 20 days); lifetime CU (no. of years use;≥3×/week) |
Montreal Cognitive Assessment (memory and attention) | Attention, memory | Current CU associated with no effects on cognitive function; no significant association between lifetime CU and cognitive function |
| Meade et al. 2018 | PWH: 29 C − (72% M) 20 C + (75% M) PWoH: 19 C + (68% M) 25 C − (68% M) |
≥ 4 days of CU in the past month and ≥ 1 year of regular CU | Stroop, Counting stroop (during fMRI) | Executive function | CU was associated with no effects on cognitive interference, but an HIV X cannabis interaction effect on fMRI activity in task-dependent brain regions. PWH/C + had the greatest activity in these regions |
| Murdoch et al. 2023 | PWH: 33 C + 12 C − /cocaine + 22 C + /cocaine + 43 C − /cocaine − (58–91% M/group) |
Lifetime history of regular use and ≥ 12 days of use in the past 90 days | PASAT, WAIS-IV DS, WAIS-IV LNS, TMA, TMB, WAIS-IV coding, Stroop, HVLT-R, CF, GPT | Global cognition [executive function, speed of information-processing, attention, learning, memory, verbal-fluency, motor function] | CU was associated with no effect on global score |
| Naveed et al. 2022 | 581 PWH-159 use cannabis 79% M |
Self-reported CU | TMA, TMB, WCST, WAIS-III DS, WAIS-III SS WAIS-III LNS, PASAT, BVMT-R, HVLT-R, COWAT, GPT | global cognition [executive function, speed of information-processing, attention and working memory, learning, memory, verbal-fluency, motor function] | Lifetime CU associated with worse neurocognitive decline |
| Okafor et al. 2019 | PWH: 498 C − 290 C + PWoH: 755 C − 377 C + 100% M |
Self-reported CU in the past 6 months; CU years = total days used during study; categorized “monthly,” “weekly,” “daily” | TMA, TMB, SDM | Executive functioning, attention, information-processing, psychomotor speed | Daily and monthly CU associated with worse processing-speed compared to non-users in PWH; no association between cumulative cannabis years and cognitive function in PWH men; each additional 5 years of CU was associated with worse in processing-speed in men without HIV |
| Rogers et al. 2023 | PWH (82–92% M): 187 Meth − /C − 68 Meth − /C + 82 PWH/Meth + /C − 135 Meth +/C + |
Timeline follow back and DSM-IV Interview for CUD | DVT, WAIS-III DS, WAIS-III SS, TMA, SMT, FMT, HCT, WCST, TMB, COWAT, CF, WAIS-R DS, WAIS-III LNS, PASAT, GPT | Global cognition [executive function, information-processing speed, learning, memory, verbal-fluency, motor]; individual subdomains also reported | Meth + /C + performed better than Meth + /C− in executive function, learning, memory, working memory and better than Meth − /C − on verbal-fluency, but worse than Meth − /C − in learning and memory. Meth − /C + performed better than Meth − /C − on executive function, learning, memory and working memory |
| Saloner et al. 2019 | All participants 50–64 years 734 PWH 123 PWoH PWH: 84% M PWoH: no gender distribution |
Lifetime CUD | CF, Letter fluency, PASAT, WAIS-III LNS, WAIS-III SS, WAIS-III DS, WMS-III spatial span, TMA, Stroop, WCST, TMB, Halstead Category Test, HVMT-R, BVMT-R, SMT, FMT, GPT | Global cognition, [executive function, attention, information-processing, learning, verbal-fluency, motor] | Higher rates of lifetime CUD among “super ager” PWH (participants with-above average GDS cognitive performance relative to their age) compared to cognitively impaired individuals PWH and cognitively normal PWH |
| Schantell et al. 2022 | 33 PWoH/C − (48 %M) 32 PWoH/C + (59% M) 17 PWH/C − (47% M) 18 PWH/C + (72% M) |
≥ 2 ×CU/week for 6 months; other substance use< 1 ×/month | Eriksen flanker task during magnetoencephalography | Executive function | PWH/C − had larger flanker interference effect than PWoH/C + and PWoH/C −; PWoH/C + had smaller flanker interference effect relative to PWH/C + |
| Schouten et al. 2016 | 74 PWoH 103 PWH 100% M |
Daily to monthly self-reported CU | CF, LF, TMB, WCST, Stroop, TMA, DS, SS, PASAT, LNS, RAVLT, Visual reproduction test, GPT | Global cognition [executive function, attention, information-processing, memory, fluency, and motor] | Cognitive impairment detected in 17% of men with HIV; CU was associated with worse cognitive performance among men with HIV |
| Skalski et al. 2018 | PWH (67–75%): 42 C − 12EC + 15LC + EC + = early CU onset LC + = late CU onset (before/after age 18 respectively) |
C + : ≥ 10 days/month ≥ 1 year or ≥ 3 ×/week of binging or problematic regular use | HVLT-R, BVMT-R, TMA, TMB, GPT, COWAT, PASAT | Attention, information-processing, learning, memory, verbal-fluency, psychomotor ability | EC + more likely to have worse learning and memory compared to C − (learning, memory) but more likely to have better attention/working memory. No differences between LC + and C − |
| Thames et al. 2017 | PWH (83% M): 24 PWH/C − 24 PWH/C+ PWoH (52% M): 16 PWoH/C − 13 PWoH/C+ |
Average CU amount (in grams) smoked/day times no. of days/week of CU in the past month | WTAR, TMA, Stroop, WAIS-IV LNS, WAIS-IV DS, WAIS-IV SS, COWAT, BVMT-R, HVLT-R, TMB | Global cognition, [executive function, information-processing, attention, learning, memory, verbal-fluency]; individual subdomains also reported | Higher levels of CU associated with worse global cognition in PWoH, no effect in PWH, driven by processing-speed and memory; simple effects: low CU (<1.43 g/week) was associated with worse global cognition in PWH vs. PWoH; no between group effects observed when CU > 1.43 g/week |
| Thames et al. 2016 | PWH: 14 C − (85% M) 30 light C +(95% M) 31 heavyC + (54% M) PWoH: 32 C − (45% M) 12 light C + (75% M) 10 heavy C + (100% M) |
Light C + :2–14 ×/week> 12 months; Heavy C +: 18–90 ×/week > 12 months |
WTAR, TMA, Stroop, WAIS − IV LNS, WAIS-IV DS, WAIS-IS SS, COWAT, BVMT-R, HVLT-R TMB | Executive function, attention, information-processing, learning, memory, verbal-fluency | Moderate to heavyC + associated with worse global cognition, processing-speed, learning/memory and executive function compared to LightC + and C − ; PWH/ HeavyC + performed worse in learning and memory compared to all groups, PWH/LightC + performed better in verbal-fluency than PWoH/LightC + |
| Wang et al. 2020 | PWH: 23 C − (96% M) 21 C + (95% M) PWoH: 24 C − (88% M) 22 C + (86% M) |
Chronic CU (> 3 ×/week for > 2 years) | RAVLT, RCFT, D-KEFS, Stroop, Trail-making (number-letter switching); WAIS-IV, Fluency test, COWAT, GPT | Executive function, attention, information-processing, learning, memory, design and verbal-fluency, motor | Nonsignificant trend indicating PWH/C + performed better than PWH/C- in executive function and verbal-fluency, executive function, and information processing |
| Watson et al. 2020 | PWH: 573 C −(80% M) 106 C + (92% M) PWoH: 229 C − (60% M) 44 C + (79% M) |
C + : history of CUD and/or CU in the past year | COWAT, CF, WCST, TMA, TMB, Stroop, WAIS-III DS, WAIS-III SS, WAIS-III LNS, HVLT-R, BVMT-R, PASAT, GPT | Executive function, attention, information processing, learning, verbal-fluency, motor | PWH/C + associated with better performance in verbal-fluency, learning; no effect of cannabis in PWoH on any cognitive domain |
| Watson et al. 2021 | PWH (82–95% M): 105 C− 62 moderate C + 31 dailyC + PWoH: 65 C −(68% M) |
Moderate C + : 3 days of CU/week to 3 × of use within the past 6 months | HVLT-R, BVMT-R, COWAT, VF, TMA, TMB, WCST, HCT, PASAT, GPT, WAIS-III DS, WAIS-III SS, WAIS-III LNS, Stroop | Executive function, attention, information-processing, learning, verbal-fluency, motor | PWH/Daily C + had better global cognition compared to PWH/moderate C + and PWH/C−(ns); Similar trend in verbal-fluency, attention/working memory, processing-speed, learning and motor skills |
| Watson et al. 2023 | PWH (81–92% M): 191 C− 83 Occasional C + 23 Frequent C + |
Occasional: 1 ×/week to< 1 ×/month Frequent: 2–7 days/week |
HVLT-R, BVMT-R, COWAT, VF, TMA, TMB, WCST, HCT, PASAT, GPT, WAIS-III DS, WAIS-III SS, WAIS-III LNS, Stroop | Executive function, attention, information-processing, learning, verbal-fluency, motor | Occasional, but not frequent, CU was associated with better global cognition; driven by better performance in attention (significant), verbal-fluency (ns), and learning (ns). Recent THC use was associated with worse global cognition, driven by worse memory |
AVLT, auditory verbal learning test; WAIS-III DS, Wechsler Adult Intelligence Scale Third Edition-III digit symbol; WAIS-III SS, WAIS-III symbol search; TMA, Trail Making Test Part A; SMT, Story Memory Test; FMT, Figure Memory Test; WCST, Wisconsin Card Sorting Task; TMB, Trail Making Test Part B; COWAT , Controlled Oral Word Association Test; CF, category fluency; WAIS-III LNS, WAIS-III letter-number sequencing; PASAT, paced auditory serial addition task; GPT, Grooved Pegboard Test; RAVLT, Rey Auditory Verbal Learning Test; EAT, error-awareness task; Stroop, Stroop Color Interference Test; SDM, symbol digit modalities; CalCAP, California Computerized Assessment Package; HVLT-R, Hopkins Verbal Learning Test-Revised; WAIS-R, Wechsler Adult Intelligence Scale-Revised; BVMT-R, Brief Visuospatial Memory Test-Revised; MST, Mirror Star Tracing Test; TMT-2, TMT-4, Trail Making Test 2 and 4; HCT, Halstead Category Test; WTAR , Wechsler Test of Adult Reading; RCFT, Rey-Osterreith Complex Figure Test; DSS, 3-min digit–symbol substitution task; RAT , 3-min repeated acquisition task; DAT, 10-min divided attention task; DRT, immediate and delayed digit-recall task; D-KEFS, Delis-Kaplan Executive Function System; SUD, substance use disorder; QUID, four times daily
Cognitive Domains
Global Cognition
As discussed, HIV-associated NCI affects various cognitive domains and is diagnosed based on overall NCI. Notably, several studies reported global NCI calculated from a comprehensive battery, such as the Global Deficit Score (GDS) [57, 58]. The GDS averages performance across tasks spanning the seven domains commonly affected in HIV, weighted such that no single exceptionally high-test score disproportionately influences the resultant value. While less useful for determining domain-specific CU/HIV interactions on cognition, this approach enables direct comparison across studies on global functioning. Our review identified 16 clinical studies examining associations between CU and global deficits among PWH. Of these, five studies found adverse effects [57, 59–62], six studies found beneficial effects [15, 58, 63–66], and the remaining studies reported null effects [62, 66–71].
Two studies reported adverse effects of CU on GDS in PWH enrolled in the NIH CHARTER initiative, a 12-year prospective observational study. One reported neurocognitive decline was unrelated to disease or treatment characteristics, possibly because most were virally suppressed on cART at follow-up assessment; however, lifetime (i.e., not current) history of CU disorder (CUD) predicted worsened global neurocognitive change overtime [57]. The other reported that lifetime CU was associated with worse GDS in PWH [59], suggesting CUD diagnoses was not necessary for the adverse CU effects observed in the previous study. Two additional studies described similar results utilizing composite scores other than the GDS. Cristiani et al. [60] reported that frequent CU was associated with greater NCI, an effect more pronounced in cognitively symptomatic versus asymptomatic PWH; thus, disease state may influence the impact of CU on global cognition in PWH (although the negative impact of CU reported above were observed in cohorts that were virally suppressed). Similarly, CU was strongly associated with global impairment in a male cohort of PWH, but not people without HIV (PWoH) [61]. In contrast, in a female-only cohort of PWH, a specific cognitive impairment profile (deficits in executive function, learning, and processing-speed) was less likely in cannabis-using versus cannabis-abstinent PWH [15]; however, other impairment profiles incorporating these domains (e.g., the learning and memory profile) were not associated with CU. This divergence in cannabis effects on select cognitive impairment profiles emphasizes the importance of interpreting global deficit scores with caution. The protective effect of CU identified by this latter study may therefore contrast with the adverse effects reported above because of differences in definition of global deficit scores, and/or differences in gender distributions within the sample population.
Two studies suggested that dosage modulates the impact of CU on cognition in PWH. For example, PWH who engaged in moderate-to-heavy CU demonstrated greater NCI compared to those that reported light-to-no-use, underscoring the detrimental effects of heavier consumption patterns [62]. However, a subsequent study reported global NCI in non-using PWH versus PWoH, but no group differences when reported CU exceeded 1.43 g/week [66], suggesting a minimum threshold of exposure was required to mitigate observed NCI in PWH. Unreported frequency of CU patterns likely accounted for discrepant findings since this factor appears to modulate the beneficial effects of CU on GDS (see below).
The remaining studies identified indicated that CU may be beneficial for global cognition. Cannabis exposure in PWH was associated with better global cognition, an effect that did not vary by age [58], and a follow-up reported similar trends in daily users versus occasional or non-users [64], together suggesting more frequent use as beneficial. CU may be particularly favorable for global cognition in older PWH. For example, higher rates of lifetime CUD were observed in “super ager” PWH (i.e., above average performers on the GDS relative to their age) compared to cognitively normal and impaired PWH [63]. Surprisingly, these data were from a subset of participants in the CHARTER study, yet are at odds with the negative effects described by both studies described above [57, 59]. This divergence may be related to age of the samples since beneficial effects were observed in a sample that was over a decade older (~ 55 years old) [63]. CU frequency may mediate the beneficial CU effects in older PWH, as occasional (≤ 1 × /week), but not frequent, use was associated with better global cognition in older PWH [65]. Furthermore, positive THC urine toxicology at cognitive assessment was linked to worse NCI, suggesting that time since last use also impacts CU effects on cognition in aging PWH. Together, these studies suggest that frequent-previous (i.e., CUD) [63], but not -current [65], CU benefits aging PWH, in addition to current moderate use. Frequency of CU and age may interact to differentially impact global NCI, warranting further investigation.
Overall, similar numbers of studies reported adverse effects of CU on global cognition in PWH as beneficial effects. Potential moderating factors included age, frequency of use, and disease severity, with a potential interaction between age and frequency of use. It is important to note that while these studies reported composite/summary measures of cognition, observed effects could have been driven by specific cognitive domains. Reporting NCI in this manner therefore complicates identification of those domains most affected by CU, as well as developing targeted treatments. As such, the differential effects of cannabis on specific cognitive domains are reviewed below.
Executive Function
Our review yielded 21 clinical studies examining the effects of CU on executive function (EF) in PWH. These studies tested various aspects of EF, including problem solving, decision-making, cognitive-control, and inhibition. Five studies found beneficial effects [69–73], two reported adverse effects [62, 74], and the remaining studies reported null effects [58, 60, 62, 64, 66, 68, 75–83].
As with GDS, frequency of use may moderate cannabis’ beneficial effects on EF. For example, PWH who reported frequent/occasional lifetime CU had better EF compared with non-users, with duration of CU correlating positively with task performance [71]. Chronicity of use was also a factor in Wang et al. [70], in which a non-significant trend indicated that PWH who engaged in regular ongoing CU (≥ 3 × /week for ≥ 2 years) outperformed cannabis-abstinent PWH on tasks of EF. Furthermore, lifetime CUD history in PWH was associated with better EF performance, and also appeared to mitigate methamphetamine (METH)-induced EF impairments [69]; together suggesting chronic use may benefit EF, as well as prevent the deleterious effects of other substances on this domain. However, a different study suggested that chronic moderate-to-heavy (≥ 18 × /week), but not light-to-moderate (≤ 14 × /week), CU was associated with worse EF regardless of HIV status [62]. Therefore, while chronic CU appears to benefit EF in PWH, exceptionally high frequencies of use may be detrimental.
Beneficial effects of CU were also observed on neurological correlates of cognitive/inhibitory-control and related cognitive performance in PWH. Two studies utilized fMRI in combination with the error-awareness task (EAT), a modified go/no-go paradigm (with elements of the Stroop test) that required participants to (1) respond and inhibit responding to target versus non-target stimuli; and (2) report when they made an error. The first study found that lifetime CU was associated with reduced task-related brain activity in regions implicated in inhibitory-control in PWoH, but not PWH; however, no group differences were detected in task performance (80). The follow-up study reported impaired error-awareness and heightened rsFC in associated regions in cannabis-abstinent, but not cannabis-using, PWH [73]. Interestingly, an interactive effect of HIV and CU on cognition was observed such that PWH who used cannabis exhibited comparable error-awareness to that of controls. Finally, cannabis-using PWH exhibited similar magnetoencephalographic activity in task-relevant brain regions as PWoH during performance of the Eriksen flanker task, although this activity was increased in cannabis-abstinent PWH; neither CU nor HIV status was associated with altered inhibitory-control, though CU nonsignificantly increased processing-speed in this EF-based task, selectively in PWH [72].
In contrast to the beneficial effects listed above, current CU predicted impaired risk-based decision-making in cognitively impaired and unimpaired PWH as measured by a mock gambling task (Game of Dice Task; GDT), irrespective of group differences in performance [74]. Interestingly, reported CU was higher in neurocognitively normal PWH compared to those diagnosed with HIV-associated NCI. However, as explained, HIV-associated NCI diagnoses are based on global impairment. It should be noted that the GDT taps into risk-based decision-making, a faucet of executive function, deficits of which have been observed in PWH [84] and cannabis-users [85]; this specific aspect of executive function may therefore be differentially affected by CU.
Our review also identified two preclinical studies assessing the relationship between eCB signaling and HIV in the context of EF. One study assessed behavioral inhibition in iTat mice using a go/no-go task, after which medial PFC (mPFC) CB1R expression was quantified and correlated with performance [86]. Inhibitory-control deficits were reported in female Tat+ mice, though post hoc analyses revealed that this group only differed from male Tat+ mice, and only arithmetically (p > 0.1) from Tat− females. These data therefore do not describe a conclusive deficit relative to normal animals, though the study was likely insufficiently powered (3–4 animals/group). Importantly, CB1R expression was increased in the infralimbic (IL)-mPFC of Tat+ females relative to all other groups. IL-mPFC CB1R expression in turn negatively correlated with overall task performance across all mice (likely driven by Tat+ females), accounting for 30% of the total variance in inhibitory-control. Assuming a genuine effect of Tat expression on behavioral inhibition (sex-dependent or otherwise), these findings link subregion-specific increases in cortical eCB signaling to poor cognitive-control in the presence of Tat.
The other preclinical study assessed the effect of increased eCB signaling on reversal-learning in female iTat mice by inhibiting the degradative enzyme for 2-AG, monoacylglycerol lipase (MAGL) [87]. After learning the initial reward-predictive (target) stimulus in a spatial discrimination task, mice were treated with the MAGL inhibitor MJN110 prior to (and throughout) reversal training, in which the designations of the target and non-target stimuli were switched. Consistent with previous findings in Tat+ mice [88] and HIV transgenic rats [89], Tat+ mice acquired the original discrimination in fewer trials than controls, indicative of faster learning. Reversal-learning was also faster in Tat+ mice, but was reduced to control levels by MAGL inhibition. However, no genotypic differences were evident in either treatment group, calling into question the functional significance of both the effect of genotype itself as well as its mitigation by MJN110 treatment. MAGL inhibition also increased 2-AG expression in Tat+ and Tat− mice, and PFC AEA in Tat+ mice, though eCB levels did not correlate with EF. Interestingly, response latency correlated with arachidonic acid (2-AG metabolite) and AEA levels in Tat+ and MJN110-treated mice, respectively. While not directly related to EF per se, the relation of 2-AG metabolism to response-speed in Tat+ mice is compelling given the reduction in Tat+ reversal-learning and concomitant increase in 2-AG following MAGL inhibition. This study therefore provides preliminary evidence that manipulating eCB metabolism may produce functionally relevant, differential effects on both regional eCB levels and cognition in the context of HIV. Furthermore, the link between eCB metabolism and response latency may be relevant to the effects of CU on information-processing in PWH (see “Attention/Information-processing”). Extrapolation of these findings to the clinic remains difficult, however. For example, while the behavioral effects of MAGL inhibition were interpreted as mitigating Tat-induced neurocognitive alterations, the fact that this normalization was achieved by impairing reversal-learning hardly recommends MAGL inhibition as a treatment for PWH. Additionally, lack of reversal-learning impairment in the iTat mouse indicates this line may not be the best model of HIV-associated EF deficits.
Overall, clinical studies indicate that there may be a beneficial effect of CU on EF in PWH. Furthermore, adverse consequences of CU on EF in PWH were either specific to heavier use patterns [62], or associated with deficits in a subdomain of EF (i.e., risky decision-making) [74]. Although few conclusive preclinical studies were identified, they suggested that expression of specific components of the eCB system (CB1R and 2-AG metabolites) may correlate with reversal-learning and cognitive-control in the context of HIV; therefore, cannabis may exert its effects on EF in PWH via eCB mechanisms.
Attention/Information-Processing
Our review identified 20 clinical studies examining associations between CU (or dronabinol exposure) and attention, information-processing, and/or processing-speed in PWH. Three studies found beneficial effects [65, 77, 83], five studies found adverse effects [62, 75, 78, 90, 91], two studies reported nonsignificant beneficial trends [64, 70], and the remaining studies reported no associations [58, 60, 62, 66, 68, 69, 71, 76, 81, 92, 93].
Dronabinol is a synthetic partial agonist of eCB receptors, like THC, and is used medicinally to enhance food intake and promote weight gain in AIDS. Three studies assessed the clinical efficacy of dronabinol on clinical outcomes and information-processing and/or attention in PWH who regularly smoked cannabis. In one study, acutely administered mid-to-high doses of dronabinol (20–30 mg), but not lower dronabinol doses or low-potency (0–3.9% THC) smoked cannabis, impaired divided attention and psychomotor speed in PWH [90]. A follow-up study reported that neither chronic administration (4 × /day for 4 days) of lower-dosed dronabinol (5–10 mg) nor low-potency cannabis (4 × /day for 4 days) affected attention and psychomotor speed in PWH [92], suggesting chronicity and dosage contributed to the formerly observed adverse effects of dronabinol. The null effect of low-potency cannabis on cognition in both studies should be cautioned; however, given these THC% do not reflect those found in modern markets [94, 95]. Nonetheless, when comparing longer-term administration, dronabinol (10 mg; 4 × /day) worsened processing-speed and attention after 9–16 treatment days [91]. The repeated findings that dronabinol, but not cannabis, impaired performance suggests dosing differences or the interaction of phytocannabinoid exposure (in cannabis but not dronabinol) could have contributed to their differential effects.
Cannabis-induced alterations were also observed in studies which utilized a composite score for information-processing. In one, moderate-to-heavy CU was associated with worse processing-speed compared to light-use and non-use cohorts, irrespective of HIV status [62], suggesting heavy-, but not light-, CU harms this function. In contrast, two studies reported a nonsignificant trend indicating that PWH who used cannabis performed better than non-users on information-processing and processing-speed [64, 70]. These results are seemingly contradictory; however, CU criteria varied substantially across studies. Particularly, negative effects were specific to heavier CU (up to 90 × /week) [62] whereas beneficial effects were reported in studies that defined CU as “moderate” and “daily” [64]or “chronic active-use” (see Table 2 for CU definitions) [70]; thus, frequency of CU likely contributed to discrepant findings. Consistency in definitions of reported use would prove useful for collating findings in the future.
CU was also associated with worse performance in PWH using specific processing-speed outcome measures. For example, greater number of cannabis-using days were associated with worse performance in the Color Trails Test 2 (CMT2) in PWH [75]. Similarly, both daily and monthly CU in PWH was associated with worse performance in the Trail Making Test A (TMTA) compared to nonusers [78]. Importantly, the adverse effect of CU on processing-speed was observed both cross-sectionally [75] and longitudinally across 17 years [78]. However, the Okafor et al. [78] report demonstrated that each additional 5 years of CU significantly worsened processing-speed in PWoH, but not PWH, which may indicate that PWH are less sensitive to the effects of prolonged CU exposure. These findings are consistent with interactive effects observed between CU and HIV wherein recent/current CU was associated with worse processing-speed in PWoH, but not PWH [66]. In fact, PWH with a previous CUD diagnoses have performed better on the TMTA compared to those with no CUD history [77], supporting that a prolonged history of regular CU may instead confer a beneficial effect on this domain in PWH.
Age may also moderate CU effects on attention and information-processing. Few studies reported specific positive benefits; however, a recent study observed that in older PWH (mean = 56.2 years), occasional CU (< 1 × /week) was associated with better attention, compared to non-users [65]. The average age of CU onset within this study was 19.4 years [65], again indicating chronic CU may be particularly beneficial to EF in PWH. Similarly, in a younger cohort (mean = 36 years), PWH who initiated regular CU early in life (< 18 years old) were less likely to demonstrate impaired working-memory/attention compared to non-users [83]. However, these data are at odds with existing literature linking earlier age of CU onset with poorer cognitive function in healthy populations and PWH [83, 96, 97]. A final study linked CU with better processing-speed irrespective of HIV status, though after accounting for age, those effects were no longer significant [81]; once again highlighting that age may be an important moderating factor.
Overall, several studies associated CU or dronabinol administration with adverse effects on attention and information-processing in PWH, with processing-speed the most affected function. Although most studies reported null effects, broadly, the composite scores used to assess this domain make it difficult to ascertain the effects of CU on specific types of attention (i.e., sustained versus focused). Duration and dosage of CU were important moderating factors of processing-speed, with heavy, but not necessarily chronic, CU negatively impacting this function. Frequency of use may also be a moderating factor; however, inconsistent definitions for moderate, heavy, and light CU hinder comparison across studies. Interestingly, some studies indicated that CU impairs attention in PWoH, but not PWH; therefore, research should determine whether PWH are indeed less sensitive to CU effects on attention and potential mechanisms underlying such effects. Importantly, and consistent with the reviewed literature, specific populations may uniquely benefit from CU such as aging PWH.
Learning and Memory
Our review identified 20 clinical studies that examined CU effects on learning and memory in PWH. Five studies reported adverse effects [60, 62, 83, 90, 98], three found beneficial effects [58, 69, 77], two studies reported nonsignificant beneficial trend effects [64, 65], and the remaining reported null effects [68, 70, 71, 75, 76, 79, 81, 91–93].
Age of CU onset appeared as a moderating factor in a study that reported PWH who initiated CU at an early age (< 18 years old) were eight times more likely to demonstrate learning impairment and four times more likely to demonstrate memory impairment compared to nonusers or later onset users [83]. These data contrast with the beneficial effects of early onset CU on attention (see above), suggesting this factor may differentially modulate CU impact on cognition dependent on functional domain. Thus, in the context of learning and memory functions, initiating CU during adolescence could have deleterious effects.
Disease state may have an important impact on the effects of CU on memory. For example, current CU was associated with poorer global cognition in PWH, an effect more pronounced in symptomatic versus asymptomatic PWH and driven by delayed memory impairments [60]. In another study, administration of 20-mg dronabinol to PWH with clinical muscle loss, but not those without muscle loss, decreased memory task performance [90]. Thus, PWH with advanced disease state may be particularly impacted by adverse CU effects on memory. Outside of health-related factors, recency of use may also modulate CU impact on memory; for example, a positive THC urine toxicology was associated with poorer global NCI, driven by poor memory/delayed-recall, despite reported CU not being associated with such impairments [65].
A study by Woods and colleagues [98] assessed a sub-type of memory, event-based prospective memory (PM), in a young-adult cohorts of PWH with and without comorbid substance use disorder (SUD). Here, reported SUD in PWH was associated with lower PM accuracy, which was driven by participants with CUD. These data contrast with a study in an older cohort that reported PWH with a past CUD diagnosis (i.e., not current) demonstrated better learning and memory compared to PWH with no history of CUD [77]. Similarly, and in an older cohort, lifetime history of cannabis dependence was associated with better learning and memory in PWH, and also appeared protective against the negative effects of lifetime METH dependence on cognitive performance in this domain (like the findings described in the EF section) [69]. Together, these studies suggest age may modulate the impact of CUD on memory in PWH, with adverse and beneficial effects observed in younger and older adults, respectively. However, beneficial effects were reported using composite domain scores in previous users, as opposed to the adverse effects that were reported in PM specifically in current users. Thus, task differences and recency of use may have also influenced discrepant findings.
The remaining studies suggest the beneficial effects of CU on this domain appear more specific to learning rather than memory tasks. For example, cannabis exposure (prior CUD or recent use) in PWH was linked to better learning performance [58], and two studies reported nonsignificant trend effects of CU in PWH to be associated with improved learning functions [64, 65]. Instead, when a composite learning and memory score was reported, moderate-heavy CU (18–90 uses/week) was associated with impaired performance in PWH [62]. Thus, the latter study’s discrepant finding could be due to tasks utilized or specific to the heavy CU reported in that cohort; however, this latter factor was difficult to interpret given the varied CU criteria used across all studies (refer to Table 2).
Our review also identified two preclinical studies assessing the effects of cannabinoid compounds on learning and/or memory. The first reported the individual and combined effects of THC (0.032–0.32 mg/kg) and SIV infection on repeated-acquisition performance [99]. Higher doses of acute THC decreased response-rate—but not accuracy—prior to SIV infection, both when learning new stimulus–response patterns and repeating old ones. This latter effect on learned behavior was also detected following SIV inoculation, with behavioral tolerance observed following chronic administration. Therefore, neither acute nor chronic THC differentially affected behavior in SIV+ versus SIV− animals, and consistent with previous findings [100–102], higher doses of THC eliminated responding, severely limiting the assay’s ability to detect explicit cognitive effects. Thus, THC-induced response-rate reductions were more likely indicative of the gross sedative-like effects of THC observed at such doses [103], rather than to alterations in information-processing. Importantly, the tendency for THC to more potently affect response-rate than accuracy in repeated-acquisition paradigms was also observed in the clinic, as was similar inter-individual variability [104], highlighting the cross-species predictive validity, and thus utility of repeated-acquisition paradigms. Nonetheless, these data suggest neither increased susceptibility to, nor benefit from, the effects of THC on learning and memory in HIV.
The other preclinical study identified by our search assessed spatial learning and memory. Male Sprague–Dawley rats were treated with a subchronic (3-day) course of the potent and non-specific cannabinoid receptor agonist Win55,212–2 prior to intra-hippocampal injection of the HIV envelope glycoprotein gp120 [105]. Rats were then assessed daily for 6 days in the Morris water maze, in which rodents learn to use spatial cues to navigate to an escape platform [106]. Gp120 treatment slowed escape latency, increased swimming distance, and decreased time spent in the target quadrant, suggestive of spatial learning impairment. This deficit was prevented by prophylactic (i.e., pre-gp120) Win55,212–2 in a CB2R-dependent manner, although the contribution of CB1R signaling was not assessed.
Overall, clinical studies indicate that CU variably affects learning and memory in PWH. Early age (< 18) of onset and more severe HIV disease state were associated with worse learning and memory, indicating that these factors may be critical in determining functional outcome for these domains. Differential effects of CU were also observed within the context of polysubstance use in which age and recency of use appeared to moderate the beneficial/adverse observed outcomes. Furthermore, limited data suggested memory functions may be more adversely affected in PWH than learning functions, which instead may be improved by cannabis exposure. The two animal studies identified were well-controlled in moderating factors and suggested null or beneficial effects of cannabinoid exposure on this cognitive domain. However, both preclinical studies were performed in males only, limiting generalizability of findings across sex.
Language/Verbal-Fluency
Our review identified 12 clinical studies that examined associations between CU and language and/or verbal-fluency domains in PWH. Four studies reported beneficial effects [58, 62, 68, 69], three studies reported beneficial trends [64, 65, 70], and eight studies reported null effects [60, 62, 64–66, 76, 77, 83].
Overall, CU tends to be associated with improved verbal-fluency in PWH. Watson et al. [58] reported that cannabis exposure, defined as a history of CUD or recent use, associated with better verbal-fluency in PWH. While such CU criteria cannot distinguish between current, or former chronic (i.e., CUD) CU, cannabis-exposed PWH reported an estimated 1724 g lifetime used suggesting they represented a sample with high exposure. These data somewhat align with a cross-sectional study that reported higher lifetime “dosage” of cannabis was correlated with improved verbal-fluency [68]. Here, “dosage” was achieved by multiplying standard cannabis units (undefined) used per day by the number of days used across periods of consistent CU. Similarly, PWH who reported chronic current CU (≥ 3 × /week for ≥ 2 years) performed slightly, but nonsignificantly, better on verbal-fluency tasks than those who reported little-to-use [70]. Together, these data suggest high levels of CU in PWH, whether former or current, may benefit verbal-fluency.
Studies with defined variations in frequency of CU patterns also report beneficial effects, though frequency effects were not consistent across studies. For example, Watson et al. [64] reported a nonsignificant trend for better verbal-fluency in PWH who used cannabis daily compared to those who used occasionally (> 1 × /month, < 3 × /week) and compared to non-users, suggesting more frequent CU modulated beneficial CU effects. In contrast, better verbal-fluency was observed in PWH engaged in occasional (2–14 × /week), but not more frequent (18–90 × /week) CU, compared to PWoH within the corresponding CU category [62]. However, CU definitions between studies were drastically different, and discrepant findings may be accounted for by the magnitude of CU, such that daily [64], but not necessarily excessive dosing of [62] CU may confer beneficial effects. In an older cohort, nonsignificant beneficial trend effects of occasional (< weekly), but not frequent (> weekly) CU was reported [65]; thus, as described in other sections, less frequent use in aging PWH may be beneficial for cognition.
A final study reported PWH with no lifetime CUD or METH-use disorder (CUD − /MUD −) performed worse than CUD + /MUD + , though no differences were found between other groups (CUD − /MUD + or CUD + /MUD −) [69]. Thus, the beneficial impact of CU on verbal-fluency only appeared in a cohort with concomitant MUD.
Overall, CU appeared to benefit language/verbal-fluency processes in PWH, and no data supported adverse effects of CU on this cognitive domain. Studies reported inconsistent CU frequency effects on language and verbal-fluency; however, it is difficult to draw coherent conclusions given the variable CU criteria across studies and insignificant trend effects. Finally, it is important to recognize that language and verbal-fluency domains cannot be measured in animals and therefore represent a domain whereby mechanistic animal literature will be inherently lacking.
Motor Skills
Our review identified 12 clinical studies that examined the association between CU and motor skills in PWH. One study reported beneficial effects [77], and one study reported adverse effects [107]. An additional study reported a nonsignificant association between daily CU and better motor task performance among PWH compared to abstinent PWH or PWH who moderately used (≤ 3 days/week) [64], suggesting more frequent use mediated this beneficial effect. Somewhat similarly, a history of CUD, indicative of prior frequent use, was associated with improved motor skill function in PWH, compared to PWH without prior CUD diagnoses [77]. However, a cannabis dependence history and HIV infection were associated with poorer motor function on procedural motor skill learning tasks among individuals with a history of polysubstance dependence [107]; thus, CU may interact with other substance use to impair motor function. The remaining studies reported null associations [58, 60, 68–70, 76, 79, 81, 83]. In all, there is a paucity of literature on the effects of cannabis on motor functioning in PWH, and more research is needed to guide interpretations.
Discussion
The Effects of Cannabis on HIV-Associated NCI Are Likely Function-Dependent
This review synthesized published data on the effects of CU and/or cannabinoid exposure on HIV-relevant cognitive domains. Results suggested that heavy or recent CU may have deleterious effects on global cognition in PWH, particularly in aging populations, though light-to-moderate use may confer beneficial effects. Otherwise, the effects of CU on cognition in PWH appeared function-dependent, and modulated by several factors (see Table 3 for a summary). For example, no studies reported adverse associations between CU and language/verbal-fluency in PWH, and similarly there is little support for adverse effects on motor functioning. CU tended to be associated with improved EF in PWH, particularly cognitive-control, and preliminary preclinical data supported that eCB ligands (i.e., 2-AG via MAGL inhibition) [87] and receptors (i.e., CB1) [86] relate to EF in HIV, suggesting that CU effects in PWH are likely mediated through eCB mechanisms. Furthermore, CU tended to be associated with adverse effects on attention/information-processing, driven by poor processing-speed, and in learning/memory domains, driven by memory impairment. Overall, the number of reported adverse effects were largely outnumbered by beneficial or null findings, providing insufficient support for the detrimental impact of CU on cognition in PWH.
Table 3.
Summary of function-dependent effects of cannabis use in people with HIV
| Functional domain | Beneficial CU effects (no. of reports) | Adverse CU effects (no. of reports) | Null findings (no. of reports) | Moderating factors | Conclusions from synthesized data |
|---|---|---|---|---|---|
| Global cognition | 6ǂ | 5 ~ | 7 | Frequency of CU Age& Disease severity |
Inconsistent CU frequency effects, though heavy use appears detrimental CU may benefit aging PWH, particularly frequent previous use or current moderate use CU may be harmful in advanced disease stages |
| Executive functioning | 5ǂ | 2 ~ | 15 | Chronicity of CU& Frequency of CU |
Chronic CU appears beneficial, but not when used at exceptionally high frequencies CU appears to have adverse consequences on risk-based decision-making |
| Attention and information-processing | 5ǂǂ | 5 | 11 | Dosage Frequency of CU Chronicity of CU Age |
CU appears to have adverse effects, particularly on processing-speed Dronabinol at high, but not low doses, appears adverse Heavy, but not necessarily chronic, CU appears detrimental, with some beneficial effects observed with moderate CU CU may be beneficial for older PWH |
| Learning and memory | 5ǂǂ | 5 ~ | 11 | Age of CU onset Age Disease severity |
CU may benefit learning functions, and hinder memory functions Early age of CU onset appears detrimental CU may be beneficial for older PWH, while having adverse effects in younger PWH CU may be harmful in advanced disease stages |
| Language and verbal fluency | 7ǂǂǂ | 0 | 8 | Frequency of CU | CU appears beneficial No data to support adverse effects Inconsistent frequency of use effects |
| Motor skills | 2ǂ | 1 | 9 | Little data to support beneficial or harmful effects of CU |
CU, cannabis use
indicates no. of beneficial trend effects included in total reports count
indicates no. of adverse effects selective to current heavy-use patterns (i.e., dose-dependency within the same study)
moderated by frequency of CU
Discrepant results between studies are likely be attributed to sparsely defined CU criteria. For example, many studies reported minimum CU criteria (e.g., > 3 × /week) and/or stratified CU by frequency groups (e.g., “occasional,” “moderate”), while few studies reported ranges of CU frequency (e.g., 6–37 days over the past 6 months) [64]. Only one study clearly accounted for independent daily instances of CU [66]. Additionally, age of CU onset appeared to have distinct effects on individual cognitive domains in PWH, however, was seldom reported.
Time of study may also account for inconsistencies reported across clinical data. As reviewed, cannabis dosing may alter its impact on cognition, and THC potency has steadily increased over the last decade [108]. Furthermore, given increased access to cART therapies, more recent publications likely preclude participants with low nadir immunosuppression, a factor strongly associated with NCI in PWH [7, 8, 69, 109–113]. The legal status of cannabis at the time of the study completion may have also impacted participant self-report of cannabis or changed the proportion of cannabis-using PWH, including reasons for use (i.e., recreational versus medicinal), and the potential impact of using drugs gained illicitly versus legally.
Demographic and clinical differences between study participants further complicated the synthesis of results. Most clinical studies reported in this review consisted of largely male populations (see Table 2); though the sole study in which females were exclusively assessed [15] agreed with the group of literature suggesting a beneficial impact of CU on EF. Clinical studies also largely consisted of virally managed participants, limiting interpretations of CU-induced effects on cognition in advanced HIV stages. Future work must study more diverse populations to draw conclusions regarding potential sex- or gender- or clinical-based differences on observed effects.
Interpretation of clinical findings reviewed above would greatly benefit from animal studies that can precisely control for confounding conditions. Given the numerous existing cognitive tasks that are consistent across species [114, 115], future research utilizing such tasks should be prioritized.
Importance of Utilizing Cross-Species Paradigms
To date, only one preclinical study has directly assessed the effect of a phytocannabinoid (THC) on cognition in HIV [99]; the remaining studies provide insight into how the eCB system interacts with HIV-related factors to regulate cognition [86, 87, 105]. While the reviewed preclinical literature describes limited differential impact of cannabinergic/eCB manipulations on HIV models versus controls, limitations of these studies exist, particularly those pertaining to the domain-specificity and/or clinical translatability of their cognitive tasks. For example, the finding that prophylactic Win55,212–2 prevented gp120-induced deficits in the Morris water maze (MWM) [105] is difficult to translate to the clinic. For one, there is no analogous human task. Additionally, central to the MWM is the element of stress [116, 117]; therefore, it is difficult to determine non-specific actions of Win55,212–2 on MWM performance given: (1) reported elevated cortices-terone levels in naïve gp120 transgenic mice [118] and (2) the eCB system’s role in regulating the stress response [119]. Nonetheless, between-sessions, learning occurs in the MWM, and therefore these findings merit confirmation using less aversive, and more translatable, assays (e.g., radial arm maze).
Many translational limitations of ethologically based assays like the MWM may be mitigated by operant conditioning paradigms, which enable assessment of several cognitive processes in a consistent manner across species [114]. For example, the cross-species go/no-go paradigm utilized to correlate PFC CB1R expression with behavioral inhibition in iTat mice represents a relative strength of this study [86]. Therefore, while lack of statistical power hindered interpretability of their findings (see above), sufficient sample sizes may identify a cognitive-control deficit in iTat mice that is directly relevant to the clinic, although translatability may yet be improved by utilizing the cross-species continuous performance task (CPT gold standard clinical assessment of vigilance and cognitive-control) [120].
Operant paradigms also enable large numbers of trials per session (≥ 100). A major limitation of League and colleagues’ study of the effects of Tat and MAGL inhibition on reversal-learning [87] was the lack of trials assessed due to task design. This dearth of trials precluded more detailed analyses that may have shed light on the specific processes impacted by Tat expression and MAGL inhibition, as afforded by operant paradigms. Increased trial numbers also permit the calculation of decision-making metrics (e.g., win-stay, lose-shift) [121] and thereby detection of more subtle differences in reversal-learning than could be gleaned from overall accuracy.
Operant-based cross-species reversal-learning tasks represent an opportunity to determine the effects of CU in PWH in a manner that would enable a subsequent preclinical mechanistic study. While reversal-learning paradigms (e.g., the probabilistic reversal-learning task; PRLT) consistently detect deficits in both neuropsychiatric populations [122–125] and relevant animal models [126–129], PWH have yet to be assessed in such a translational paradigm. Cognitive flexibility deficits are instead more commonly assessed using the Wisconsin Card Sorting Test in PWH, in which participants sort a deck of cards according to a rule that changes after every ten consecutive correct responses [130–132]; however, HIVtg rats detected rule shifts at the same rate as controls in the PRLT [89]. This divergence in preclinical and clinical findings demonstrates the need for consistent assessment of a given cognitive function (e.g., cognitive flexibility) across species. Importantly, the wide variety of cross-species translatable operant tasks means that this strategy may also be applied to the study of many other cognitive domains in addition to cognitive flexibility (e.g., vigilance, effort- and risk-based decision-making) [114].
Putative Mechanisms of HIV-Associated NCI and the Interactive Effects of Cannabis: Insights from Clinical Data
Structurally, reduced cortical thickness and subcortical volumes, and the presence of white matter hyperintensitites (found in aging and Alzheimer’s disease), correlate with greater NCI in PWH [133–141]. However, data on the interactive effects of CU and HIV on brain structure are few and inconclusive. Larger caudate and cerebral white matter volumes have been linked to recent CU in PWH but not people without HIV (PWoH) [71]; however, duration of use negatively correlated with cortical volumes suggesting prolonged CU may exacerbate brain volumetric loss despite possible short-term benefits. Other studies reported interactive effects of CU and HIV on brain structure that were unrelated to cognition, or consistent structural changes across cannabis-using PWH and PWoH (null interactions) [66, 70].
Functional (f)MRI revealed increased brain activation in cognitively normal PWH relative to PWoH during decision-making [142] and memory task performance [143, 144], suggesting that hyperactivation in PWH may be compensatory, enabling comparable performance. Indeed, longitudinal assessments detected similar attentional ability and concomitant brain activity in PWH and PWoH at baseline, but an HIV-specific increase in brain activation a year later, with no change in performance [145]. In contrast, cognitively normal PWH demonstrated decreased task-dependent brain activation during an attention task, but enhanced activity in adjacent and contralateral regions [146]. Thus, elevated brain activity may compensate for reduced network efficacy in PWH prior to detectable NCI. Meanwhile, studies in cognitively impaired PWH instead report reduced activation of task-dependent networks [147, 148], suggesting prolonged compensatory overactivation may result in NCI.
Interestingly, CU may normalize aberrant brain activity in PWH during cognitive performance. For example, CU in PWH was associated with normalization of elevated parietal-occipital gamma [76] and spontaneous frontocortical alpha activity [72] to control levels during visuospatial-processing, but performance behavior was not associated with, or directly compared to such changes. Instead, reduced theta oscillations have been linked to visuospatial-processing deficits in PWH, but chronic CU did not alter these findings [76, 149]. Meanwhile, HIV and CU were independently associated with abnormal insular-sensori-motor resting-state functional connectivity (rsFC), which in turn correlated with visuospatial-processing deficits; neither cannabis-using PWH nor cannabis-abstinent controls demonstrated these neurophysiological or cognitive deficits, supporting a beneficial effect of CU on these measures in PWH but a detrimental effect in PWoH [150]. However, CU did not affect salience-default mode network rsFC, which was elevated in PWH and correlated with deficient error-awareness in the same task [73]. Therefore, the beneficial effects of CU task-related brain activity in PWH appear dependent upon specific outcome measures (i.e., function-dependent).
Finally, additional studies reported null interactions between CU and HIV status on brain function [80, 151], or reported additive effects that do not relate to cognitive performance [71, 82]. Taken together, CU may partially remediate aberrant brain activity in PWH, though such effects were not always associated with cognition or relevant analyses were not conducted. Further studies are therefore required to determine the interactive effects of HIV and CU on co-occurring brain function and cognitive performance.
In addition to brain volume and activity, blood- and CSF-based biomarkers have been correlated to cognitive dysfunction in PWH. HIV-associated NCI is not always associated with viral loads [57, 152, 153], indicating that factors beyond the virus alone interact to contribute to the severity of NCI. Similarly, current immunosuppression indicators (i.e., CD4 + cell count) are not typically associated with cognition in PWH; however, lower nadir immunosuppression is linked to poorer cognitive outcomes [7, 8, 69, 109–113], suggesting delayed treatment as a significant risk factor for HIV-associated NCI. Low nadir immunosuppression has also been linked to cortical thinning and concomitance NCI in PWH [111]. Nonetheless, reported CU in PWH does not appear to be associated with changes to immune-related biomarkers in PWH [35, 83, 154, 155]. Furthermore, multiple studies observed no changes to viral-load or immune-related biomarkers in PWH following a 21-day low-dose (3.95% THC cigarette 3 × /day) cannabis intervention [156, 157]. Few studies have reported links between suppressed viral loads and better immune function in cannabis-using PWH; however, these effects were observed alongside NCI [62], or cognition was not measured [158]. Hence, there is little evidence to support direct viral/immunological mechanisms underlying the effects of CU on HIV-associated NCI.
In contrast, CNS proinflammatory markers tend to be linked to cognition in HIV [64, 159, 160]. Current and recent CU is consistently linked with reduced inflammation in PWH [64, 71, 154, 158, 161], including reductions in monocyte chemoattractant protein-1 (MCP-1), which mediates CNS leukocyte perturbation [162], and is associated with cognitive deficits arising from HIV [163], aging, and Alzheimer’s populations [164, 165]. Specifically, lower MCP-1 and interferon gamma-induced protein (IP-10) levels were observed in cannabis-using PWH, which correlated with better learning task performance [64]. CU may therefore mitigate HIV-associated NCI through anti-inflammatory mechanisms, though more studies that directly relate such biomarkers to NCI are required to confirm this effect.
CU may also impact HIV-associated NCI through its interactions with the blood–brain barrier (BBB). Reduced BBB integrity is observed in PWH, which may facilitate entry of HIV-infected monocytes into the CNS [166, 167]. While BBB dysfunction has yet to be studied in the context of HIV-associated NCI, it has been associated with neuronal damage in PWH [168], indicating its potential to harm cognition. A recent study linked frequent CU to less BBB dysregulation in PWH [169]; however, explicit investigation of the interactions between CU, HIV, and the BBB on cognition is lacking and warranted.
Other risk factors for HIV-associated NCI include individual genetics [170], coinfections [171], and comorbid psychiatric [172], vascular [173–176], metabolic [74], and substance-use disorders (16, 17, 19, 21, 22), which are difficult to control for when studying human populations. Animal models, meanwhile, enable precise mechanistic study of cannabinoid effects on HIV-associated NCI by minimizing these confounds inherent to clinical HIV research, as well as those relating to CU (e.g., inter-individual variation in dosing, THC/CBD content). These models also permit determination of directionality of cannabis and HIV effects, thus elucidating causal mechanisms. Below, we provide a brief overview of current animal models as well as their benefits and limitations in determining the effects of CU on cognition in HIV.
Putative Mechanisms of HIV-Associated NCI and the Interactive Effects of Cannabis: Insights from Preclinical Models
Cannabis may alter HIV-associated NCI by activating the eCB system to promote neurogenesis, and attenuate neuroinflammation and neurodegeneration associated with HIV viral exposure. Particularly, CB2R activation has attenuated gp120-induced neurodegeneration in vivo and in vitro across studies [177–180]. Interestingly, in vitro Tat-induced neurodegeneration was reduced by AEA, 2-AG, and WIN 55,212–2, which was prevented by CB1R, but not CB2R antagonism [32, 181]. These studies suggest the discrete HIV viral proteins Tat and gp120 have independent mechanisms (CB1R and CB2R, respectively) through which they produce neurodegeneration. Inhibition of AEA’s degradative enzyme, fatty acid amide hydrolase (FAAH), is also protective against neurodegeneration and inflammatory responses induced by gp120 or Tat exposure [182–184]. Since proinflammatory markers are linked to NCI in PWH [64, 159, 160], and are reduced in cannabis-using PWH [64, 71, 154, 158, 161], the FAAH-induced reductions of inflammation may describe the mechanisms by which cannabis affects cognition in PWH. It should be noted that AEA is only one of several fatty acids degraded by FAAH [185, 186]; therefore, those molecules (e.g., OEA) and their respective receptor systems (e.g., PPARa receptors) may also mediate the neuroprotective effects of FAAH inhibition on HIV-induced neurodegeneration and inflammation.
The eCB system may alleviate neuroinflammation and neurodegeneration induced by HIV-proteins through its ability to alter neurotransmission. Indeed, HIV-relevant proteins dysregulate neurotransmission, and the eCB system has a prominent role in regulating such processes. For example, Tat-induced attenuation of PFC inhibitory transmission was blocked by a CB1R in vitro [187]. In a follow-up study utilizing PFC slices from iTat mice, Tat-induction sex-dependently altered GABAergic activity and FAAH inhibition (i.e., a general increase in eCB signaling) suppressed GABAergic activity regardless of sex or Tat-expression [188], suggesting sex impacts the role of the eCB in modulating Tat effects.
Interestingly, application of Tat to rat hippocampal cultures reduced the magnitude of CB1R-mediated presynaptic inhibition of glutamatergic, but not GABAergic, neurotransmission [189]. The effects of Tat on neurotransmission may therefore also be dependent upon brain region (i.e., hippocampus versus PFC) and/or excitatory versus inhibitory signaling. Given the apparent sex-, region-, and neurotransmitter-specificity of Tat and/or eCB effects on neurotransmission, it is likely that any specific cognitive/behavioral outcomes of these mechanisms would be similarly context-dependent. For example, the sex-dependent effect of Tat expression on PFC eCB signaling [188] may partially account for the findings of poorer inhibitory-control in female versus male Tat + mice, especially given that females had higher CB1R expression, which also correlated with behavioral inhibition [86].
Recommendations for Treating HIV-Associated NCI with Cannabis/Cannabinoids
This review has highlighted that CU may be detrimental or beneficial for HIV-associated NCI depending on the measured function. These results can be used as a starting guide for the individualized treatment of HIV-associated NCI with cannabis/cannabinoids. For example, medicinal cannabis may be particularly beneficial for PWH demonstrating EF or verbal-fluency impairments, but should be avoided in populations with impairments specific to information-processing and memory. In addition to the potential benefits for HIV-associated NCI, CU in PWH has been linked to lower inflammatory markers [59, 91–94], increased appetite [90, 92], and stronger BBB integrity [169]. In all, the number of beneficial or null effects of CU on cognition reviewed suggests cannabis and medications can be prescribed to PWH while posing little threat to cognitive function.
Despite this medicinal potential, certain phytocannabinoids, particularly THC, produce adverse side effects such as altered mobility, thermodysregulation, and anxiogenic responses [190–192]. The potential therapeutic effects of cannabis would also occur alongside its psychoactive properties, which could hinder daily functional ability (i.e., capacity to operate motor vehicles). Such adverse reactions can be avoided by using compounds known to elevate endogenous eCB tone by inhibiting eCB enzymatic degradation [193]. Both eCBs AEA and 2-AG are created on demand, and following eCB receptor binding, are rapidly degraded by their respective enzymes, FAAH (for AEA) and MAGL (for 2-AG). Thus, such enzyme inhibitors enhance/prolong naturally occurring eCB activity rather than artificially stimulating the eCB system via exogenous cannabinoid exposure (i.e., cannabis), produce fewer adverse side effects, and are devoid of psychoactive properties. FAAH inhibitors are especially known for being well tolerated in healthy populations [194, 195] and in clinical trials [196, 197], and also increase other fatty acid signaling molecules known to have anti-inflammatory and neuroprotective effects (i.e., OEA and PEA). Therefore, FAAH inhibition may possess greater clinical potential for HIV-associated NCI.
Considering all articles discussed, few linked low-dose CU to cognitive dysfunction in PWH. Low potency cannabis is well tolerated in PWH [90, 92], but is not easily accessible in today’s legal/illegal markets [94]. Ensuring prescribers and PWH which have access to low-dose medicinal cannabis for the potential treatment of HIV-associated NCI, or other HIV-related symptoms, should therefore be prioritized. Providing access to such low-dose cannabis may also reduce the number of PWH using high-dose, and likely harmful amounts of cannabis for self-medication.
In further relevance to the clinic, it will be important to consider interactions between CU and cART. Little data exists on the effects of CU on cART efficacy; however, phytocannabinoids and HIV medications share common metabolic pathways, thus concomitant use could lead to altered drug bioavailability and possible toxicity [198]. CU has also been linked to poorer cART adherence [199–203]; therefore, monitoring medication use patterns in PWH prescribed cannabis is of particular importance.
Recommendations for Future Research
Our findings can be used as means to design future research to disentangle the impact of CU effects on HIV-associated NCI. Our summarized function-dependent findings highlight the importance of moving away from global cognitive scores to better define relationships between CU and HIV on cognition. Additionally, research is needed to better understand the specific effects of CU on motor functioning in PWH since the study of this domain is underrepresented in the literature, despite being integral to performance of cognitive tasks. We further recommend designing studies with explicit CU criteria, considering factors that impacted the beneficial/adverse effects on discrete cognitive domains in PWH reported in this review (e.g., dosage of CU on EF, disease state on CU-induced memory impairment). Such factors must also be clearly defined in future work, and preferably relative to previous published definitions to enhance the interpretation of study results and enable comparisons across studies.
Further investigation of low-dosed CU on HIV-associated NCI is particularly warranted given the majority of accessible cannabis contains high THC levels known to produce strong psychoactive and potentially adverse side effects. Additionally, given the adverse health consequences associated with cannabis smoke, future work should explore the impact of alternative CU methods (e.g., orally dosed) or compounds (eCB enzyme inhibitors) on cognition in the context of HIV. Since preclinical evidence support the neuroprotective effects of FAAH and MAGL inhibitors, and these compounds are tolerated in the clinic, their impact on HIV-associated NCI in human and animal studies is particularly warranted.
Undoubtedly, the use of cross-species cognitive tasks will permit the collection of translatable data on the effects of CU on cognition in PWH. Several cognitively based tasks can be completed in humans and animals, including the PRLT which measures reinforcement learning and cognitive flexibility. Additionally, the cross-species Iowa Gambling Task, a measure of risk-based decision-making, could be utilized to further explore the adverse effects of CU on risk-based decision-making in PWH. Clinical studies should therefore utilize cross-species tasks to allow for future mechanistic determination of any effects observed while preclinical studies must similarly use such tasks to ensure data collected is translatable.
Future preclinical work will be essential for defining the impact of confounding factors since these can more easily be controlled for in animal studies. Furthermore, as highlighted by our review, specific exploration of phytocannabinoid impact on cognitive function in animal models of HIV is lacking and warranted. Particularly, future preclinical work should aim to increase face-validity by utilizing comparable cannabis administration methods used in humans (i.e., vapor or oral exposure) which would also expose the animal to the entirety of phytocannabinoids present in cannabis, rather than the administration of an individual component (i.e., CBD).
Conclusions
This review revealed little support for the detrimental effects of CU on cognitive function in PWH. Further reviewed studies suggested beneficial/adverse effects of CU on cognition is function-dependent in the context of HIV, and modulated by several confounding factors. The scarcity of preclinical support for the interactions of cannabinoids and HIV on cognition is concerning since animal models of neuroHIV can help disentangle such effects in well-controlled experiments that allow for mechanistic explorations.
Supplementary Material
Funding
SMA supported by 2R25MH081482-16 and BMH supported by T32MH018399.
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- AEA
N-Arachidonoylethanolamine (anandamide)
- AIDS
Acquired immunodeficiency syndrome
- BBB
Blood-brain barrier
- cART
Combination antiretroviral therapy
- CMT2
Color Trails Test 2
- CPT
Continuous performance task
- CUD
Cannabis use disorder
- CB1R
Cannabinoid-1 receptor
- CB2R
Cannabinoid-2 receptor
- CBD
Cannabidiol
- CNS
Central nervous system
- CU
Cannabis use
- EAT
Error-awareness task
- eCB
Endocannabinoid
- EF
Executive function
- FAAH
Fatty acid amide hydrolase
- FIV
Feline immunodeficiency virus
- fMRI
Functional magnetic resonance imaging
- GDS
Global deficit score
- IL
Infralimbic
- IP-10
Interferon gamma-induced protein (IP-10)
- MAGL
Monoacylglycerol lipase
- MCP-1
Monocyte chemoattractant protein-1
- METH
Methamphetamine
- mPFC
Medial prefrontal cortex
- MRI
Magnetic resonance imaging
- MWM
Morris water maze
- NCI
Neurocognitive impairment
- PFC
Prefrontal cortex
- PL
Procedural learning
- PM
Prospective memory
- PRLT
Probabilistic reversal learning task
- PWH
People with HIV
- PWoH
People with out HIV
- RPT
Rotary pursuit task
- rsFC
Resting-state functional connectivity
- SIV
Simian immunodeficiency virus
- SMT
Star mirror tracing task
- SUD
Substance use disorder
- THC
Δ9-Tetrahydrocannabinol (THC)
- TMTA
Trail Making Test A
- tg
Transgenic
- WPT
Weather prediction task
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11904-024-00698-w.
Competing Interests The authors declare no competing interests.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: an examination of subjective appraisal versus objective performance. Clin Neuropsychol. 2011;25(2):224–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knippels HM, Goodkin K, Weiss JJ, Wilkie FL, Antoni MH. The importance of cognitive self-report in early HIV-1 infection: validation of a cognitive functional status subscale. AIDS. 2002;16(2):259–67. [DOI] [PubMed] [Google Scholar]
- 3.Yoo-Jeong M, Anderson A, Rahman AF, Baumann M, McB-room J, Waldrop-Valverde D. Associations of mood on objective and subjective cognitive complaints in persons living with HIV/AIDS. J HIV/AIDS. 2018;4(1). 10.16966/2380-5536.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao A, Yamanouchi J, Takenaka K, Takada K. The Iowa Gambling Task on HIV-infected subjects. J Infect Chemother. 2020;26(3):240–4. [DOI] [PubMed] [Google Scholar]
- 5.Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13(2):306–16. [DOI] [PubMed] [Google Scholar]
- 6.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Lam MN, Stefaniak M, et al. Verbal and spatial working memory performance among HIV-infected adults. J Int Neuropsychol Soc. 2002;8(4):532–8. [DOI] [PubMed] [Google Scholar]
- 7.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95(19):e2610–21. [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Hou J, Su B, Jiang T, Guo C, Wang W, et al. The prevalence of Frascati-criteria-based HIV-associated neurocognitive disorder (HAND) in HIV-infected adults: a systematic review and meta-analysis. Front Neurol. 2020;11:581346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korten V, Ay U, Hari E, Tigen Tukenmez E, Gencer S, Akca Kalem S, et al. Prevalence of HIV-associated neurocognitive disorder (HAND) in Turkey and assessment of Addenbrooke’s Cognitive Examination Revised (ACE-R) test as a screening tool. HIV Med. 2021;22(1):60–6. [DOI] [PubMed] [Google Scholar]
- 11.Flatt A, Gentry T, Kellett-Wright J, Eaton P, Joseph M, Urasa S, et al. Prevalence and 1-year incidence of HIV-associated neurocognitive disorder (HAND) in adults aged >/=50 years attending standard HIV clinical care in Kilimanjaro Tanzania. Int Psychogeriatr. 2023;35(7):339–50. [DOI] [PubMed] [Google Scholar]
- 12.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•.Dastgheyb RM, Sacktor N, Franklin D, Letendre S, Marcotte T, Heaton R, et al. Cognitive trajectory phenotypes in human immunodeficiency virus-infected patients. J Acquir Immune Defic Syndr. 2019;82(1):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]; (This work showed that a specific cognitive impairment profile (deficits in executive function, learning, and processing-speed) was less likely in cannabis-using versus cannabis-abstinent PWH. However, other impairment profiles incorporating these domains (e.g., the learning and memory profile) were not associated with CU. These data highlight the necessity of testing discrete cognitive domain functions in PWH, and the importance of interpreting global cognition scores with caution).
- 14.Rubin LH, Saylor D, Nakigozi G, Nakasujja N, Robertson K, Kisakye A, et al. Heterogeneity in neurocognitive change trajectories among people with HIV starting antiretroviral therapy in Rakai Uganda. J Neurovirol. 2019;25(6):800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dastgheyb RM, Buchholz AS, Fitzgerald KC, Xu Y, Williams DW, Springer G, et al. Patterns and predictors of cognitive function among virally suppressed women with HIV. Front Neurol. 2021;12:604984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J Int Neuropsychol Soc. 2004;10(1):1–14. [DOI] [PubMed] [Google Scholar]
- 17.Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, et al. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the digit symbol test: differential deficits in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31(8):1315–24. [DOI] [PubMed] [Google Scholar]
- 19.Schulte T, Mueller-Oehring EM, Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Differential effect of HIV infection and alcoholism on conflict processing, attentional allocation, and perceptual load: evidence from a Stroop Match-to-Sample task. Biol Psychiatry. 2005;57(1):67–75. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez Salgado D, Rodriguez Alvarez M, Seoane PG. Neuropsychological impairment among asymptomatic HIV-positive former intravenous drug users. Cogn Behav Neurol. 2006;19(2):95–104. [DOI] [PubMed] [Google Scholar]
- 21.Wakim KM, Freedman EG, Molloy CJ, Vieyto N, Cao Z, Foxe JJ. Assessing combinatorial effects of HIV infection and former cocaine dependence on cognitive control processes: a high-density electrical mapping study of response inhibition. Neuropharmacology. 2021;195:108636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakim KM, Freedman EG, Tivarus ME, Heinecke A, Foxe JJ. Assessing combinatorial effects of HIV infection and former cocaine dependence on cognitive control processes: a functional neuroimaging study of response inhibition. Neuropharmacology. 2022;203:108815. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Mahato DK, Kamle M, Borah R, Sharma B, Pandhi S, et al. Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: an overview. Phytother Res. 2021;35(11):6010–29. [DOI] [PubMed] [Google Scholar]
- 24.Shiau S, Arpadi SM, Yin MT, Martins SS. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addict Behav. 2017;68:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costiniuk CT, Saneei Z, Salahuddin S, Cox J, Routy JP, Rueda S, et al. Cannabis consumption in people living with HIV: reasons for use, secondary effects, and opportunities for health education. Cannabis Cannabinoid Res. 2019;4(3):204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris GE, Dupuis L, Mugford GJ, Johnston L, Haase D, Page G, et al. Patterns and correlates of cannabis use among individuals with HIV/AIDS in Maritime Canada. Can J Infect Dis Med Microbiol. 2014;25(1):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. [DOI] [PubMed] [Google Scholar]
- 28.Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–80. [DOI] [PubMed] [Google Scholar]
- 29.Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–4. [DOI] [PubMed] [Google Scholar]
- 30.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58(9):1017–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu C, Hermes DJ, Nwanguma B, Jacobs IR, Mackie K, Mukhopadhyay S, et al. Endocannabinoids exert CB(1) receptor-mediated neuroprotective effects in models of neuronal damage induced by HIV-1 Tat protein. Mol Cell Neurosci. 2017;83:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302(5642):84–8. [DOI] [PubMed] [Google Scholar]
- 34.Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J Neuroinflammation. 2005;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray CH, Javanbakht M, Cho GD, Gorbach PM, Fulcher JA, Cooper ZD. Changes in immune-related biomarkers and endocannabinoids as a function of frequency of cannabis use in people living with and without HIV. Cannabis and cannabinoid research. Advance online publication. 2023. 10.1089/can.2022.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wise LE, Thorpe AJ, Lichtman AH. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology. 2009;34(9):2072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nidadavolu P, Bilkei-Gorzo A, Kramer M, Schurmann B, Palmisano M, Beins EC, et al. Efficacy of Delta(9) -Tetrahydrocannabinol (THC) alone or in combination with a 1:1 ratio of cannabidiol (CBD) in reversing the spatial learning deficits in old mice. Front Aging Neurosci. 2021;13:718850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Aguirre C, Carmona-Cruz F, Velasco AL, Velasco F, Aguado-Carrillo G, Cuellar-Herrera M, et al. Cannabidiol acts at 5-HT(1A) receptors in the human brain: relevance for treating temporal lobe epilepsy. Front Behav Neurosci. 2020;14:611278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etemad L, Karimi G, Alavi MS, Roohbakhsh A. Pharmacological effects of cannabidiol by transient receptor potential channels. Life Sci. 2022;300:120582. [DOI] [PubMed] [Google Scholar]
- 42.Morales P, Reggio PH. An Update on Non-CB(1), Non-CB(2) Cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. 2017;2(1):265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, et al. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13(4):421–32. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, et al. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of delta9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. [DOI] [PubMed] [Google Scholar]
- 46.Curran T, Devillez H, YorkWilliams SL, Bidwell LC. Acute effects of naturalistic THC vs. CBD use on recognition memory: a preliminary study. J Cannabis Res. 2020;2(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woelfl T, Rohleder C, Mueller JK, Lange B, Reuter A, Schmidt AM, et al. Effects of cannabidiol and delta-9-tetrahydrocannabinol on emotion, cognition, and attention: a double-blind, placebo-controlled, randomized experimental trial in healthy volunteers. Front Psychiatry. 2020;11:576877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–93. [DOI] [PubMed] [Google Scholar]
- 49.Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162(5):1693–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr, Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001;98(16):9271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denton PW, Garcia JV. Humanized mouse models of HIV infection. AIDS Rev. 2011;13(3):135–48. [PMC free article] [PubMed] [Google Scholar]
- 52.Potash MJ, Chao W, Bentsman G, Paris N, Saini M, Nitkiewicz J, et al. A mouse model for study of systemic HIV-1 infection, antiviral immune responses, and neuroinvasiveness. Proc Natl Acad Sci U S A. 2005;102(10):3760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, McLaurin KA, Mactutus CF, Booze RM. A rat model of ecohiv brain infection. J visualized experiments. 2021;(167). 10.3791/62137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mallard J, Williams K. An SIV macaque model of SIV and HAND: the need for adjunctive therapies in HIV that target activated monocytes and macrophages. J Neurovirol. 2018;24(2):213–9. [DOI] [PubMed] [Google Scholar]
- 55.Miller C, Abdo Z, Ericsson A, Elder J, VandeWoude S. Applications of the FIV model to study HIV pathogenesis. Viruses. 2018;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Prete GQ, Eilers B, Moldt B, Keele BF, Estes JD, Rodriguez A, Sampias M, Oswald K, Fast R, Trubey CM, Chertova E, Smedley J, LaBranche CC, Montefiori DC, Burton DR, Shaw GM, Markowitz M, Piatak M, KewalRamani VN Jr, Bieniasz PD, Hatziioannou T. Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins. Cell host & microbe, 2014;16(3):412–418. 10.1016/j.chom.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heaton RK, Ellis RJ, Tang B, Marra CM, Rubin LH, Clifford DB, et al. Twelve-year neurocognitive decline in HIV is associated with comorbidities, not age: a CHARTER study. Brain. 2023;146(3):1121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson CW, Paolillo EW, Morgan EE, Umlauf A, Sundermann EE, Ellis RJ, et al. Cannabis exposure is associated with a lower likelihood of neurocognitive impairment in people living with HIV. J Acquir Immune Defic Syndr. 2020;83(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Naveed Z, Fox HS, Wichman CS, May P, Arcari CM, Meza J, et al. An assessment of factors associated with neurocognitive decline in people living with HIV. Int J STD AIDS. 2022;33(1):38–47. [DOI] [PubMed] [Google Scholar]
- 60.Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. J Neuropsychiatry Clin Neurosci. 2004;16(3):330–5. [DOI] [PubMed] [Google Scholar]
- 61.Schouten J, Su T, Wit FW, Kootstra NA, Caan MW, Geurtsen GJ, et al. Determinants of reduced cognitive performance in HIV-1-infected middle-aged men on combination antiretroviral therapy. AIDS. 2016;30(7):1027–38. [DOI] [PubMed] [Google Scholar]
- 62.Thames AD, Mahmood Z, Burggren AC, Karimian A, Kuhn TP. Combined effects of HIV and marijuana use on neurocognitive functioning and immune status. AIDS Care. 2016;28(5):628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saloner R, Campbell LM, Serrano V, Montoya JL, Pasipanodya E, Paolillo EW, et al. Neurocognitive superaging in older adults living with HIV: demographic, neuromedical and everyday functioning correlates. J Int Neuropsychol Soc. 2019;25(5):507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson CW, Campbell LM, Sun-Suslow N, Hong S, Umlauf A, Ellis RJ, et al. Daily cannabis use is associated with lower CNS inflammation in people with HIV. J Int Neuropsychol Soc. 2021;27(6):661–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson CW, Sundermann E, Helm J, Paolillo EW, Hong S, Ellis RJ, Letendre S, Marcotte TD, Heaton RK, Morgan EE, Grant I. A longitudinal study of cannabis use and risk for cognitive and functional decline among older adults with HIV. AIDS and behavior. 2023;27(10):3401–3413. 10.1007/s10461-023-04056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thames AD, Kuhn TP, Williamson TJ, Jones JD, Mahmood Z, Hammond A. Marijuana effects on changes in brain structure and cognitive function among HIV+ and HIV− adults. Drug Alcohol Depend. 2017;170:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murdoch DM, Barfield R, Chan C, Towe SL, Bell RP, Volkheimer A, et al. Neuroimaging and immunological features of neurocognitive function related to substance use in people with HIV. J Neurovirol. 2023;29(1):78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rogers JM, Iudicello JE, Marcondes MCG, Morgan EE, Cherner M, Ellis RJ, Letendre SL, Heaton RK, Grant I. The combined effects of cannabis, methamphetamine, and HIV on neurocognition. Viruses. 2023;15(3):674. 10.3390/v15030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang HA, Liang HJ, Ernst TM, Oishi K, Chang L. Microstructural brain abnormalities in HIV+ individuals with or without chronic marijuana use. J Neuroinflammation. 2020;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kallianpur KJ, Birn R, Ndhlovu LC, Souza SA, Mitchell B, Paul R, et al. Impact of cannabis use on brain structure and function in suppressed HIV infection. J Behav Brain Sci. 2020;10(8):344–70. [PMC free article] [PubMed] [Google Scholar]
- 72.Schantell M, Springer SD, Arif Y, Sandal ME, Willett MP, Johnson HJ, et al. Regular cannabis use modulates the impact of HIV on the neural dynamics serving cognitive control. J Psychopharmacol. 2022;36(12):1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Flannery JS, Riedel MC, Hill-Bowen LD, Poudel R, Bottenhorn KL, Salo T, et al. Altered large-scale brain network interactions associated with HIV infection and error processing. Netw Neurosci. 2022;6(3):791–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez D, Power C, Gill MJ, Fujiwara E. Determinants of risk-taking in HIV-associated neurocognitive disorders. Neuropsychology. 2017;31(7):798–810. [DOI] [PubMed] [Google Scholar]
- 75.Attonito JM, Devieux JG, Lerner BD, Hospital MM, Rosenberg R. Exploring substance use and HIV treatment factors associated with neurocognitive impairment among people living with HIV/AIDS. Front Public Health. 2014;2:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christopher-Hayes NJ, Lew BJ, Wiesman AI, Schantell M, O’Neill J, May PE, et al. Cannabis use impacts pre-stimulus neural activity in the visual cortices of people with HIV. Hum Brain Mapp. 2021;42(16):5446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crook CL, Savin MJ, Byrd D, Summers AC, Guzman VA, Morris EP, et al. The neurocognitive effects of a past cannabis use disorder in a diverse sample of people living with HIV. AIDS Care. 2021;33(11):1482–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okafor CN, Plankey MW, Li M, Chen X, Surkan PJ, Shoptaw S, et al. Association of marijuana use with changes in cognitive processing speed and flexibility for 17 years in HIV-seropositive and HIV-seronegative men. Subst Use Misuse. 2019;54(4):525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flannery JS, Riedel MC, Salo T, Hill-Bowen LD, Poudel R, Adams AR, et al. Interactive effects of HIV infection and cannabis use on insula subregion functional connectivity. J Neuroimmune Pharmacol. 2022;17(1–2):289–304. [DOI] [PubMed] [Google Scholar]
- 80.Flannery JS, Riedel MC, Salo T, Poudel R, Laird AR, Gonzalez R, et al. HIV infection is linked with reduced error-related default mode network suppression and poorer medication management abilities. Prog Neuropsychopharmacol Biol Psychiatry. 2021;111:110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Meade CS, Bell RP, Towe SL, Chen NK, Hobkirk AL, Huettel SA. Synergistic effects of marijuana abuse and HIV infection on neural activation during a cognitive interference task. Addict Biol. 2019;24(6):1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skalski LM, Towe SL, Sikkema KJ, Meade CS. Memory impairment in HIV-infected individuals with early and late initiation of regular marijuana use. AIDS Behav. 2018;22(5):1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iudicello JE, Woods SP, Cattie JE, Doyle K, Grant I, Group HIVNRP. Risky decision-making in HIV-associated neurocognitive disorders (HAND). Clin Neuropsychol. 2013;27(2):256–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, et al. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. J Math Psychol. 2010;54(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobs IR, Xu C, Hermes DJ, League AF, Xu C, Nath B, et al. Inhibitory control deficits associated with upregulation of CB(1) R in the HIV-1 Tat transgenic mouse model of HAND. J Neuroimmune Pharmacol. 2019;14(4):661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.League AF, Gorman BL, Hermes DJ, Johnson CT, Jacobs IR, Yadav-Samudrala BJ, et al. Monoacylglycerol lipase inhibitor MJN110 reduces neuronal hyperexcitability, restores dendritic arborization complexity, and regulates reward-related behavior in presence of HIV-1 Tat. Front Neurol. 2021;12:651272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kesby JP, Fields JA, Chang A, Coban H, Achim CL, Semenova S, et al. Effects of HIV-1 TAT protein and methamphetamine exposure on visual discrimination and executive function in mice. Behav Brain Res. 2018;349:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roberts BZ, He YV, Chatha M, Minassian A, Grant I, Young JW. HIV Transgenic rats demonstrate superior task acquisition and intact reversal learning in the within-session probabilistic reversal learning task. Cogn Affect Behav Neurosci. 2021;21(6):1207–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181(1):170–8. [DOI] [PubMed] [Google Scholar]
- 91.Bedi G, Foltin RW, Gunderson EW, Rabkin J, Hart CL, Comer SD, et al. Efficacy and tolerability of high-dose dronabinol maintenance in HIV-positive marijuana smokers: a controlled laboratory study. Psychopharmacology. 2010;212(4):675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, et al. Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr. 2007;45(5):545–54. [DOI] [PubMed] [Google Scholar]
- 93.Lorkiewicz SA, Ventura AS, Heeren TC, Winter MR, Walley AY, Sullivan M, et al. Lifetime marijuana and alcohol use, and cognitive dysfunction in people with human immunodeficiency virus infection. Subst Abus. 2018;39(1):116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cash MC, Cunnane K, Fan C, Romero-Sandoval EA. Mapping cannabis potency in medical and recreational programs in the United States. PLoS ONE. 2020;15(3):e0230167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pennypacker SD, Cunnane K, Cash MC, Romero-Sandoval EA. Potency and therapeutic THC and CBD ratios: U.S. cannabis markets overshoot. Front Pharmacol. 2022;13:921493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23(2):117–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woods SP, Doyle KL, Morgan EE, Naar-King S, Outlaw AY, Nichols SL, et al. Task importance affects event-based prospective memory performance in adults with HIV-associated neurocognitive disorders and HIV-infected young adults with problematic substance use. J Int Neuropsychol Soc. 2014;20(6):652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winsauer PJ, Molina PE, Amedee AM, Filipeanu CM, McGoey RR, Troxclair DA, et al. Tolerance to chronic delta-9-tetrahydrocannabinol (Delta(9)-THC) in rhesus macaques infected with simian immunodeficiency virus. Exp Clin Psychopharmacol. 2011;19(2):154–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans EB, Wenger GR. Effects of drugs of abuse on acquisition of behavioral chains in squirrel monkeys. Psychopharmacology. 1992;107(1):55–60. [DOI] [PubMed] [Google Scholar]
- 101.Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W Jr, et al. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J Pharmacol Exp Ther. 1988;245(1):178–86. [PubMed] [Google Scholar]
- 102.Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10(5):497–511. [DOI] [PubMed] [Google Scholar]
- 103.Beardsley PM, Scimeca JA, Martin BR. Studies on the agonistic activity of delta 9–11-tetrahydrocannabinol in mice, dogs and rhesus monkeys and its interactions with delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1987;241(2):521–6. [PubMed] [Google Scholar]
- 104.Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects of delta(9)-tetrahydrocannabinol on repeated acquisition and performance of response sequences and on self-reports in humans. Behav Pharmacol. 1994;5(1):71–8. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Zeng Y, Zhou Y, Yu J, Liang M, Qin L, et al. Win 55,212–2 improves neural injury induced by HIV-1 glycoprotein 120 in rats by exciting CB2R. Brain Res Bull. 2022;182:67–79. [DOI] [PubMed] [Google Scholar]
- 106.Morris R Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. [DOI] [PubMed] [Google Scholar]
- 107.Gonzalez R, Schuster RM, Vassileva J, Martin EM. Impact of HIV and a history of marijuana dependence on procedural learning among individuals with a history of substance dependence. J Clin Exp Neuropsychol. 2011;33(7):735–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A comprehensive review of cannabis potency in the United States in the last decade. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6(6):603–6. [DOI] [PubMed] [Google Scholar]
- 109.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12(6):333–8. [DOI] [PubMed] [Google Scholar]
- 111.Hassanzadeh-Behbahani S, Shattuck KF, Bronshteyn M, Dawson M, Diaz M, Kumar P, et al. Low CD4 nadir linked to widespread cortical thinning in adults living with HIV. Neuroimage Clin. 2020;25:102155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12(5):387–91. [DOI] [PubMed] [Google Scholar]
- 113.Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retrovir. 2008;24(10):1301–7. [DOI] [PubMed] [Google Scholar]
- 114.Young JW. Development of cross-species translational paradigms for psychiatric research in the Research Domain Criteria era. Neurosci Biobehav Rev. 2023;148:105119. [DOI] [PubMed] [Google Scholar]
- 115.Roberts BZ, Young JW. Translational cognitive systems: focus on attention. Emerg Top Life Sci. 2022;6(5):529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, et al. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82(5–6):306–19. [DOI] [PubMed] [Google Scholar]
- 117.Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198(1):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raber J, Toggas SM, Lee S, Bloom FE, Epstein CJ, Mucke L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: evidence for involvement of NMDA receptors and nitric oxide synthase. Virology. 1996;226(2):362–73. [DOI] [PubMed] [Google Scholar]
- 119.Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81(2):331–42. [DOI] [PubMed] [Google Scholar]
- 120.Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS ONE. 2009;4(1):e4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, et al. Serotonin modulates sesitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35(6):1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Culbreth AJ, Gold JM, Cools R, Barch DM. Impaired activation in cognitive control regions predicts reversal learning in schizophrenia. Schizophr Bull. 2016;42(2):484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reddy LF, Waltz JA, Green MF, Wynn JK, Horan WP. Probabilistic reversal learning in schizophrenia: stability of deficits and potential causal mechanisms. Schizophr Bull. 2016;42(4):942–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Linke J, King AV, Rietschel M, Strohmaier J, Hennerici M, Gass A, et al. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169(3):316–25. [DOI] [PubMed] [Google Scholar]
- 125.Mukherjee D, Filipowicz ALS, Vo K, Satterthwaite TD, Kable JW. Reward and punishment reversal-learning in major depressive disorder. J Abnorm Psychol. 2020;129(8):810–23. [DOI] [PubMed] [Google Scholar]
- 126.Amitai N, Young JW, Higa K, Sharp RF, Geyer MA, Powell SB. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cogn Affect Behav Neurosci. 2014;14(1):388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Milienne-Petiot M, Kesby JP, Graves M, van Enkhuizen J, Semenova S, Minassian A, et al. The effects of reduced dopamine transporter function and chronic lithium on motivation, probabilistic learning, and neurochemistry in mice: modeling bipolar mania. Neuropharmacology. 2017;113(Pt A):260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roberts BZ, Young JW, He YV, Cope ZA, Shilling PD, Feifel D. Oxytocin improves probabilistic reversal learning but not effortful motivation in Brown Norway rats. Neuropharmacology. 2019;150:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tranter MM, Aggarwal S, Young JW, Dillon DG, Barnes SA. Reinforcement learning deficits exhibited by postnatal PCP-treated rats enable deep neural network classification. Neuropsychopharmacology. 2023;48(9):1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moradi AR, Miraghaei MA, Parhon H, Jabbari H, Jobson L. Posttraumatic stress disorder, depression, executive functioning, and autobiographical remembering in individuals with HIV and in carers of those with HIV in Iran. AIDS Care. 2013;25(3):281–8. [DOI] [PubMed] [Google Scholar]
- 131.Chang L, Lim A, Lau E, Alicata D. Chronic tobacco-smoking on psychopathological symptoms, impulsivity and cognitive deficits in HIV-infected individuals. J Neuroimmune Pharmacol. 2017;12(3):389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kanmogne GD, Fonsah JY, Tang B, Doh RF, Kengne AM, Umlauf A, et al. Effects of HIV on executive function and verbal fluency in Cameroon. Sci Rep. 2018;8(1):17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanford R, Fellows LK, Ances BM, Collins DL. Association of brain structure changes and cognitive function with combination antiretroviral therapy in HIV-positive individuals. JAMA Neurol. 2018;75(1):72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alakkas A, Ellis RJ, Watson CW, Umlauf A, Heaton RK, Letendre S, et al. White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol. 2019;25(1):32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Patel SH, Kolson DL, Glosser G, Matozzo I, Ge Y, Babb JS, et al. Correlation between percentage of brain parenchymal volume and neurocognitive performance in HIV-infected patients. AJNR Am J Neuroradiol. 2002;23(4):543–9. [PMC free article] [PubMed] [Google Scholar]
- 136.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol. 2010;16(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thames AD, Foley JM, Wright MJ, Panos SE, Ettenhofer M, Ramezani A, et al. Basal ganglia structures differentially contribute to verbal fluency: evidence from human immunodeficiency virus (HIV)-infected adults. Neuropsychologia. 2012;50(3):390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bonnet F, Amieva H, Marquant F, Bernard C, Bruyand M, Dauchy FA, et al. Cognitive disorders in HIV-infected patients: are they HIV-related? AIDS. 2013;27(3):391–400. [DOI] [PubMed] [Google Scholar]
- 139.Kuper M, Rabe K, Esser S, Gizewski ER, Husstedt IW, Maschke M, et al. Structural gray and white matter changes in patients with HIV. J Neurol. 2011;258(6):1066–75. [DOI] [PubMed] [Google Scholar]
- 140.Watson C, Busovaca E, Foley JM, Allen IE, Schwarz CG, Jahanshad N, et al. White matter hyperintensities correlate to cognition and fiber tract integrity in older adults with HIV. J Neurovirol. 2017;23(3):422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su T, Wit FW, Caan MW, Schouten J, Prins M, Geurtsen GJ, et al. White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS. 2016;30(15):2329–39. [DOI] [PubMed] [Google Scholar]
- 142.Connolly CG, Bischoff-Grethe A, Jordan SJ, Woods SP, Ellis RJ, Paulus MP, et al. Altered functional response to risky choice in HIV infection. PLoS ONE. 2014;9(10):e111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–7. [DOI] [PubMed] [Google Scholar]
- 144.Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59(9):1343–9. [DOI] [PubMed] [Google Scholar]
- 145.Ernst T, Yakupov R, Nakama H, Crocket G, Cole M, Watters M, et al. Declined neural efficiency in cognitively stable human immunodeficiency virus patients. Ann Neurol. 2009;65(3):316–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang L, Tomasi D, Yakupov R, Lozar C, Arnold S, Caparelli E, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56(2):259–72. [DOI] [PubMed] [Google Scholar]
- 147.Melrose RJ, Tinaz S, Castelo JM, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188(2):337–47. [DOI] [PubMed] [Google Scholar]
- 148.Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wiesman AI, O’Neill J, Mills MS, Robertson KR, Fox HS, Swindells S, et al. Aberrant occipital dynamics differentiate HIV-infected patients with and without cognitive impairment. Brain. 2018;141(6):1678–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hall SA, Lalee Z, Bell RP, Towe SL, Meade CS. Synergistic effects of HIV and marijuana use on functional brain network organization. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naive HIV patients. Neuroimage. 2002;17(3):1638–48. [DOI] [PubMed] [Google Scholar]
- 152.Vitiello B, Goodkin K, Ashtana D, Shapshak P, Atkinson JH, Heseltine PN, et al. HIV-1 RNA concentration and cognitive performance in a cohort of HIV-positive people. AIDS. 2007;21(11):1415–22. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y, Qiao L, Ding W, Wei F, Zhao Q, Wang X, et al. An initial screening for HIV-associated neurocognitive disorders of HIV-1 infected patients in China. J Neurovirol. 2012;18(2):120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ellis RJ, Peterson SN, Li Y, Schrier R, Iudicello J, Letendre S, Morgan E, Tang B, Grant I, Cherner M. Recent cannabis use in HIV is associated with reduced inflammatory markers in CSF and blood. Neurology(R) neuroimmunology & neuroinflammation, 2020;7(5):e809. 10.1212/NXI.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Marcellin F, Lions C, Rosenthal E, Roux P, Sogni P, Wittkop L, et al. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort). Drug Alcohol Rev. 2017;36(2):227–38. [DOI] [PubMed] [Google Scholar]
- 156.Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial. Ann Intern Med. 2003;139(4):258–66. [DOI] [PubMed] [Google Scholar]
- 157.Bredt BM, Higuera-Alhino D, Shade SB, Hebert SJ, McCune JM, Abrams DI. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J Clin Pharmacol. 2002;42(S1):82S–S89. [DOI] [PubMed] [Google Scholar]
- 158.Manuzak JA, Gott TM, Kirkwood JS, Coronado E, Hensley-McBain T, Miller C, et al. Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin Infect Dis. 2018;66(12):1872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Burlacu R, Umlauf A, Marcotte TD, Soontornniyomkij B, Diaconu CC, Bulacu-Talnariu A, et al. Plasma CXCL10 correlates with HAND in HIV-infected women. J Neurovirol. 2020;26(1):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Portilla I, Reus S, Leon R, van-der Hofstadt C, Sanchez J, Lopez N, et al. Neurocognitive impairment in well-controlled HIV-infected patients: a cross-sectional study. AIDS Res Hum Retrovir. 2019;35(7):634–41. [DOI] [PubMed] [Google Scholar]
- 161.Yin L, Dinasarapu AR, Borkar SA, Chang KF, De Paris K, Kim-Chang JJ, et al. Anti-inflammatory effects of recreational marijuana in virally suppressed youth with HIV-1 are reversed by use of tobacco products in combination with marijuana. Retrovirology. 2022;19(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anderson AM, Jang JH, Easley KA, Fuchs D, Gisslen M, Zetterberg H, et al. Cognitive and neuronal link with inflammation: a longitudinal study in people with and without HIV infection. J Acquir Immune Defic Syndr. 2020;85(5):617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sanchez-Sanchez JL, Giudici KV, Guyonnet S, Delrieu J, Li Y, Bateman RJ, et al. Plasma MCP-1 and changes on cognitive function in community-dwelling older adults. Alzheimers Res Ther. 2022;14(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci Rep. 2018;8(1):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol. 2012;91(3):401–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Caligaris G, Trunfio M, Ghisetti V, Cusato J, Nigra M, Atzori C, Imperiale D, Bonora S, Di Perri G, Calcagno A. Blood-brain barrier impairment in patients living with HIV: Predictors and Associated Biomarkers. Diagnostics (Basel). 2021;11(5):867. 10.3390/diagnostics11050867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Calcagno A, Atzori C, Romito A, Vai D, Audagnotto S, Stella ML, et al. Blood brain barrier impairment is associated with cerebrospinal fluid markers of neuronal damage in HIV-positive patients. J Neurovirol. 2016;22(1):88–92. [DOI] [PubMed] [Google Scholar]
- 169.Ellis RJ, Peterson S, Cherner M, Morgan E, Schrier R, Tang B, et al. Beneficial effects of cannabis on blood-brain barrier function in human immunodeficiency virus. Clin Infect Dis. 2021;73(1):124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Olivier IS, Cacabelos R, Naidoo, V. Risk factors and pathogenesis of HIV-associated neurocognitive disorder: The Role of Host Genetics. Int J Mol Sci 2018;19(11):3594. 10.3390/ijms19113594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hinkin CH, Castellon SA, Levine AJ, Barclay TR, Singer EJ. Neurocognition in individuals co-infected with HIV and hepatitis C. J Addict Dis. 2008;27(2):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Namagga JK, Rukundo GZ, Niyonzima V, Voss J. Depression and HIV associated neurocognitive disorders among HIV infected adults in rural southwestern Uganda: a cross-sectional quantitative study. BMC Psychiatry. 2021;21(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gutierrez J, Porras TN, Yoo-Jeong M, Khasiyev F, Igwe KC, Laing KK, et al. Cerebrovascular contributions to neurocognitive disorders in people living with HIV. J Acquir Immune Defic Syndr. 2021;88(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wright EJ, Grund B, Robertson K, Brew BJ, Roediger M, Bain MP, et al. Cardiovascular risk factors associated with lower baseline cognitive performance in HIV-positive persons. Neurology. 2010;75(10):864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Becker JT, Kingsley L, Mullen J, Cohen B, Martin E, Miller EN, et al. Vascular risk factors, HIV serostatus, and cognitive dysfunction in gay and bisexual men. Neurology. 2009;73(16):1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Foley J, Ettenhofer M, Wright MJ, Siddiqi I, Choi M, Thames AD, et al. Neurocognitive functioning in HIV-1 infection: effects of cerebrovascular risk factors and age. Clin Neuropsychol. 2010;24(2):265–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Zvonok A, et al. The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis. Br J Pharmacol. 2014;171(2):468–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kim HJ, Shin AH, Thayer SA. Activation of cannabinoid type 2 receptors inhibits HIV-1 envelope glycoprotein gp120-induced synapse loss. Mol Pharmacol. 2011;80(3):357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hu S, Sheng WS, Rock RB. CB2 receptor agonists protect human dopaminergic neurons against damage from HIV-1 gp120. PLoS ONE. 2013;8(10):e77577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang X, Thayer SA. Monoacylglycerol lipase inhibitor JZL184 prevents HIV-1 gp120-induced synapse loss by altering endocannabinoid signaling. Neuropharmacology. 2018;128:269–81. 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Esposito G, Ligresti A, Izzo AA, Bisogno T, Ruvo M, Di Rosa M, et al. The endocannabinoid system protects rat glioma cells against HIV-1 Tat protein-induced cytotoxicity Mechanism and regulation. J Biol Chem. 2002;277(52):50348–54. [DOI] [PubMed] [Google Scholar]
- 182.Avraham HK, Jiang S, Fu Y, Rockenstein E, Makriyannis A, Wood J, et al. Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme. Br J Pharmacol. 2015;172(19):4603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hermes DJ, Yadav-Samudrala BJ, Xu C, Paniccia JE, Meeker RB, Armstrong ML, et al. GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration. Exp Neurol. 2021;341:113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hermes DJ, Xu C, Poklis JL, Niphakis MJ, Cravatt BF, Mackie K, et al. Neuroprotective effects of fatty acid amide hydrolase catabolic enzyme inhibition in a HIV-1 Tat model of neuroAIDS. Neuropharmacology. 2018;141:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bisogno T, De Petrocellis L, Di Marzo V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates Possible therapeutic implications. Curr Pharm Des. 2002;8(7):533–47. [DOI] [PubMed] [Google Scholar]
- 186.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7. [DOI] [PubMed] [Google Scholar]
- 187.Xu C, Hermes DJ, Mackie K, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Cannabinoids occlude the HIV-1 Tat-induced decrease in GABAergic neurotransmission in prefrontal cortex slices. J Neuroimmune Pharmacol. 2016;11(2):316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xu C, Yadav-Samudrala BJ, Xu C, Nath B, Mistry T, Jiang W, Niphakis MJ, Cravatt BF, Mukhopadhyay S, Lichtman AH, Ignatowska-Jankowska BM, Fitting S. Inhibitory neurotransmission is sex-dependently affected by tat expression in transgenic mice and suppressed by the fatty acid amide hydrolase enzyme inhibitor pf3845 via cannabinoid type-1 receptor mechanisms. Cells, 2022;11(5):857. 10.3390/cells11050857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wu MM, Thayer SA. HIV tat protein selectively impairs cb1 receptor-mediated presynaptic inhibition at excitatory but not inhibitory synapses. eNeuro, 2020;7(3). 10.1523/ENEURO.0119-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247(3):1046–51. [PubMed] [Google Scholar]
- 191.Moreira FA, Grieb M, Lutz B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract Res Clin Endocrinol Metab. 2009;23(1):133–44. [DOI] [PubMed] [Google Scholar]
- 192.Wang XF, Galaj E, Bi GH, Zhang C, He Y, Zhan J, et al. Different receptor mechanisms underlying phytocannabinoid-versus synthetic cannabinoid-induced tetrad effects: opposite roles of CB(1) /CB(2) versus GPR55 receptors. Br J Pharmacol. 2020;177(8):1865–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.van Egmond N, Straub VM, van der Stelt M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu Rev Pharmacol Toxicol. 2021;61:441–63. [DOI] [PubMed] [Google Scholar]
- 194.Li GL, Winter H, Arends R, Jay GW, Le V, Young T, et al. Assessment of the pharmacology and tolerability of PF-04457845, an irreversible inhibitor of fatty acid amide hydrolase-1, in healthy subjects. Br J Clin Pharmacol. 2012;73(5):706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mayo LM, Asratian A, Linde J, Morena M, Haataja R, Hammar V, et al. Elevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trial. Biol Psychiatry. 2020;87(6):538–47. [DOI] [PubMed] [Google Scholar]
- 196.Huggins JP, Smart TS, Langman S, Taylor L, Young T. An efficient randomised, placebo-controlled clinical trial with the irreversible fatty acid amide hydrolase-1 inhibitor PF-04457845, which modulates endocannabinoids but fails to induce effective analgesia in patients with pain due to osteoarthritis of the knee. Pain. 2012;153(9):1837–46. [DOI] [PubMed] [Google Scholar]
- 197.D’Souza DC, Cortes-Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trial. Lancet Psychiatry. 2019;6(1):35–45. [DOI] [PubMed] [Google Scholar]
- 198.Desai N, Burns L, Gong Y, Zhi K, Kumar A, Summers N, et al. An update on drug-drug interactions between antiretroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol. 2020;16(11):1005–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–80. [DOI] [PubMed] [Google Scholar]
- 200.Hicks PL, Mulvey KP, Chander G, Fleishman JA, Josephs JS, Korthuis PT, et al. The impact of illicit drug use and substance abuse treatment on adherence to HAART. AIDS Care. 2007;19(9):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bonn-Miller MO, Oser ML, Bucossi MM, Trafton JA. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med. 2014;37(1):1–10. [DOI] [PubMed] [Google Scholar]
- 202.Zhang Y, Wilson TE, Adedimeji A, Merenstein D, Milam J, Cohen J, et al. The impact of substance use on adherence to antiretroviral therapy among HIV-infected women in the United States. AIDS Behav. 2018;22(3):896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Manuzak JA, Granche J, Tassiopoulos K, Rower JE, Knox JR, Williams DW, et al. Cannabis use is associated with decreased antiretroviral therapy adherence among older adults with HIV. Open Forum Infect Dis. 2023;10(1):ofac699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jordan Walter T, Pocuca N, Young JW, Geyer MA, Minassian A, Perry W. The relationship between cannabis use and cognition in people with bipolar disorder: a systematic scoping review. Psychiatry Res. 2021;297:113695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pocuca N, Walter TJ, Minassian A, Young JW, Geyer MA, Perry W. The effects of cannabis use on cognitive function in healthy aging: a systematic scoping review. Arch Clin Neuropsychol. 2021;36(5):673–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


