Abstract
Genetic studies in Borrelia burgdorferi have been hindered by the lack of a nonborrelial selectable marker. Currently, the only selectable marker is gyrBr, a mutated form of the chromosomal gyrB gene that encodes the B subunit of DNA gyrase and confers resistance to the antibiotic coumermycin A1. The utility of the coumermycin-resistant gyrBr gene for targeted gene disruption is limited by a high frequency of recombination with the endogenous gyrB gene. A kanamycin resistance gene (kan) was introduced into B. burgdorferi, and its use as a selectable marker was explored in an effort to improve the genetic manipulation of this pathogen. B. burgdorferi transformants with the kan gene expressed from its native promoter were susceptible to kanamycin. In striking contrast, transformants with the kan gene expressed from either the B. burgdorferi flaB or flgB promoter were resistant to high levels of kanamycin. The kanamycin resistance marker allows efficient direct selection of mutants in B. burgdorferi and hence is a significant improvement in the ability to construct isogenic mutant strains in this pathogen.
Borrelia burgdorferi, the spirochetal agent of Lyme disease (4), is maintained in nature by an infectious cycle involving tick vectors and small rodent hosts (13). The genome sequence of B. burgdorferi has provided a wealth of data about the genetic composition of this bacterium (7). However, relatively little is known about the function of most of the deduced proteins encoded by the genome. For example, 41% of the chromosomal open reading frames and 84% of plasmid open reading frames are either homologs to hypothetical proteins in other bacteria or have no match in databases (7). Although many B. burgdorferi gene products have been identified and characterized on the basis of antigenicity, abundance, membrane location, or pattern of synthesis, the functions of most of these proteins are unknown (5, 12, 20, 31, 32, 36). As a consequence, relatively little is known about the molecular mechanisms mediating B. burgdorferi variation and adaptation and the roles of these processes in the infectious cycle.
Identification of genes that encode virulence factors or participate in the transmission cycle of B. burgdorferi is hindered because this pathogen differs considerably from bacteria with well-developed genetic systems. These differences include an atypical outer membrane composition, unique genome structure with an undefined mechanism of replication, and complex, stringent in vitro growth requirements. One major factor that has impeded genetic studies in B. burgdorferi is the lack of an exogenous selectable marker. The only available selectable marker was derived by mutating the B. burgdorferi gene for the B subunit of DNA gyrase (gyrB), yielding a derivative gene (gyrBr) whose product confers resistance to the antibiotic coumermycin A1 (26). When the gyrBr gene is used for gene inactivation by allelic exchange or integration into the genome, the most common outcome (>99.5%) is recombination with the endogenous chromosomal gyrB gene rather than allelic exchange at the desired target locus (3, 21, 30, 33). Because of the possibility of unwanted recombination at a second genomic site and the occurrence of spontaneous drug resistance, very large numbers of coumermycin-resistant colonies must be screened by PCR to determine where recombination has occurred. This procedure is laborious, expensive, and time-consuming. Hence, development of an exogenous selectable marker would substantially improve the efficiency of targeted gene disruption in B. burgdorferi.
Our laboratory has previously generated B. burgdorferi transformants with an Escherichia coli plasmid integrated on the chromosome (30). Although this E. coli vector contained the intact kanamycin resistance gene (kan) from Tn903, these B. burgdorferi clones remained susceptible to kanamycin. In this paper, we report why B. burgdorferi transformants containing the native kan gene remain susceptible to kanamycin and describe how we have modified this gene to yield an efficient marker for targeted mutagenesis.
MATERIALS AND METHODS
Strains and culture conditions.
B. burgdorferi B31 (ATCC 35210), the prototype strain of B. burgdorferi sensu stricto, was originally isolated from a tick collected on Shelter Island, N.Y. (4). B. burgdorferi clones AB1 (30), KA1, KG1, and AB5 were derived from B31-A, a high passage, noninfectious clone of B31 (Table 1). Bacteria were grown in BSK-H (Sigma, St. Louis, Mo.) or BSK-II (1) supplemented with 6% rabbit serum at 34°C, unless otherwise indicated. Single colonies in solid medium were obtained as described previously (21).
TABLE 1.
B. burgdorferi strains used in this study
| Strain | Relevant integrated DNA | Drug resistance phenotypea | Kanamycin MIC (μg/ml) | Source or reference |
|---|---|---|---|---|
| B31-A | None/wild type | Cous Kans | ≤25 | This study |
| KS5 | kan gyrBr | Cour Kans | NDb | 30 |
| AB1 | kan gyrBr | Cour Kans | 50 | 30 |
| KA1 | PflaB-kan gyrBr | Cour Kanr | >1,600 | This study |
| KG1 | PflgB-kan gyrBr | Cour Kanr | >1,600 | This study |
| AB5 | PflaB-kan ΔoppAV | Cous Kanr | ND | This study |
Kans, inability to form colonies in the presence of 40 μg of kanamycin per ml; Kanr, ability to form colonies in the presence of 200 μg of kanamycin per ml; Cous, inability to form colonies in the presence of 0.5 μg of coumermycin per ml; Cour, ability to form colonies in the presence of 0.5 μg of coumermycin per ml.
ND, not determined.
Indirect immunofluorescence microscopy for B. burgdorferi FlaB in solid medium.
Twenty B. burgdorferi colonies were picked from an agarose plate and mixed in 100 μl of phosphate-buffered saline containing MgCl2. Ten-microliter aliquots were spotted on glass slides and were air dried, heat fixed, and acetone fixed for 10 min. Spirochetes were incubated with anti-flagellin monoclonal antibody H9724 (2) for 30 min followed by goat anti-mouse antibody coupled to fluorescein isothiocyanate (Kierkegaard & Perry Lab Inc., Gaithersburg, Md.). These preparations were examined by epifluorescence microscopy.
Construction of B. burgdorferi promoter-kan fusions.
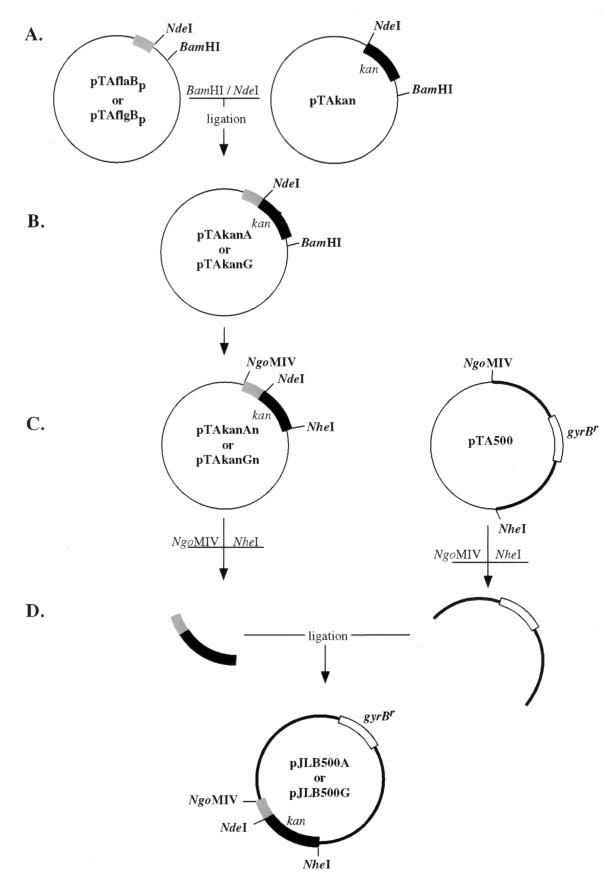
Oligonucleotides used for gene cloning and DNA probe synthesis are listed in Table 2. The source of the Tn903 kanamycin resistance gene (kan) was the plasmid vector pOK12 (34). To replace the Tn903 kan promoter with B. burgdorferi promoters (Fig. 1), the promoterless Tn903 kan gene and B. burgdorferi promoters were amplified with primers that introduced a NdeI restriction enzyme site (the 3′ end of the promoter fragment and the 5′ end of the kan gene) and were separately cloned into pCR2.1 (Invitrogen, San Diego, Calif.). PCR amplifications were performed with a DNA Thermal Cycler (Perkin-Elmer, Norwalk, Conn.) for 25 cycles at the following conditions: 94°C for 1 min, 50°C for 30 s, and 68°C for either 1 or 2 min, for the promoter region and kan gene, respectively. A 916-bp fragment extending from the ATG translational start codon of the kan gene to 106 bp downstream of the stop codon was amplified with oligonucleotides 1 and 2 (pTAkan) (Fig. 1A). Oligonucleotides 5 and 6 were used to amplify a region beginning at the flgB ATG start codon and extending 410 bp upstream (pTAflgBp) (Fig. 1A). Oligonucleotides 3 and 4 were used to amplify an analogous 354-bp region upstream of the flaB gene (pTAflaBp) (Fig. 1A).
TABLE 2.
Oligonucleotides (5′→3′) used in this study
| Oligonucleotide | Sequencea | Application |
|---|---|---|
| 1. kan 5′ + NdeI | CATATGAGCCATATTCAACGGGAAACG | 5′ kan fusion with Borrelia promoter |
| 2. pOK.3 | GATCGCCCTTCCCAACAGTTGC | 3′ end of kan gene 3. |
| 3. flaB 5′ prom + NotI | TGTCTGTCGCCTCTTGCGGCCGCCGGAGGAG | 5′ end of flaB promoter |
| 4. flaB 3′ prom + NdeI | GATTGATAATCATATGTCATTCCTCCATG | flaB promoter 3′ fusion with kan |
| 5. flgB 5′ prom | TAATACCCGAGCTTCAAGGAAG | 5′ end of flgB promoter |
| 6. flgB 3′ prom + NdeI | CTTTCAAAATCATTCATATGGAAACCTCCCTC | flgB promoter 3′ fusion with kan |
| 7. flaB 5′ prom + NgoMIV | GCCGGCTGTCTGTCGCCTCTTGTGGCTTCCGG | pJLB 500A |
| 8. flgB 5′ prom + NgoMIV | GCCGGCTAATACCCGAGCTTCAAGGAG | pJLB 500G |
| 9. pOK.7 + NheI | GGCGAATGAGCTAGCGCCGTCCC | pJLB 500A and -G |
| 10. pOK.8 + NheI | GGGACGGCGCTAGCTCATTCGCC | pJLB 500A and -G |
| 11. pOK.9 + NgoMIV | GCCGGCGACAGTTTTATTGTTCATGATG | pJLB 500A and -G |
| 12. pOK.6 | ATGCAAAAGCACCACTGGCAGC | Sequencing promoter fusions |
| 13. FL-6 | TTCAGGGTCTCAAGCGTCTTGGACT | flaB gene-specific probe |
| 14. FL-7 | GCATTTTCAATTTTAGCAAGTGATG | flaB gene-specific probe |
| 15. oppA.76 | CGTGCTCAGCAATGTCTAAAC | 5′ ΔoppAV |
| 16. oppA.83 | CATGTTTCAGGCTCAATTTCTAC | 3′ ΔoppAV |
| 17. pOK12-Rev2 | TGTGGAATTGTGAGCGGATAAC | Screen for oppAV integrants |
| 18. oppA.73 | GCGTCTTTTTAAGCCTTTTGCC | Screen for oppAV integrants |
Restriction sites in boldface.
FIG. 1.
Construction of pJLB500A and pJLB500G plasmids. (A) PCR fragments of B. burgdorferi promoters and the kan gene were cloned into pCR2.1. (B) The kan gene was excised and ligated into plasmids containing B. burgdorferi promoters. The resulting plasmids contained PflaB-kan or PflgB-kan fusions (pTAkanA and pTAkanG, respectively). (C) pTAkanAn and pTAkanGn plasmids were constructed by amplifying PflaB-kan and PflgB-kan from pTAkanA and pTAkanG, respectively, with primers that contained NgoMIV and NheI restriction sites. The PCR product amplified with oligonucleotides 10 and 11 from pBLS500 was cloned into pCR2.1 (pTA500). (D) PflaB-kan and pTA500 or PflgB-kan and pTA500 were digested, purified, and ligated to create pJLB500A or pJLB500G, respectively. The shaded box represents either the B. burgdorferi flaB or flgB promoter region. The black box and open box represent the kan and gyrBr genes, respectively. The thick line represents pOK12 sequence. Plasmids are not drawn to scale.
The kan gene from pTAkan was digested with the restriction enzymes BamHI and NdeI, was separated by agarose gel electrophoresis, and was purified with a QIAEX II gel extraction kit (QIAGEN, Chatsworth, Calif.). pTAflaBp and pTAflgBp vectors were then digested with BamHI and NdeI, and the gel-purified kan gene was ligated into both pTAflaBp and pTAflgBp constructs to make pTAkanA (PflaB-kan) and pTAkanG (PflgB-kan), respectively (Fig. 1B).
Construction of pJLB500A and pJLB500G.
To replace the Tn903 kan promoter and gene in pBLS500 with the B. burgdorferi promoter-kan fusion, PCR primers that introduced restriction enzyme sites were used to separately amplify pBLS500 without the kan gene and the promoter-kan fusions (Fig. 1C). Oligonucleotide 10, containing a NgoMIV restriction enzyme site, was located 31 bp upstream of the translational start codon of the kan gene and in the reverse orientation. Oligonucleotide 11, containing a NheI restriction enzyme site, was located 75 bp downstream of the translational stop codon (TAA) of the kan gene and in the forward orientation. By using oligonucleotides 10 and 11 and pBLS500 as a template, an amplified PCR product of the expected size of 3.3 kb was obtained and cloned into pCR 2.1 (pTA500) (Fig. 1C). 5′ oligonucleotides 7 and 8 had NgoMIV restriction sites incorporated into them and were used to amplify the PflaB-kan and PflgB-kan fusions, respectively, in conjunction with 3′ oligonucleotide 9, which included an NheI restriction enzyme site (Fig. 1B), and PCR fragments were cloned into pCR2.1 (Fig. 1C).
The amplified vector and B. burgdorferi promoter-kan fusions were excised from pCR2.1 with the restriction enzymes NgoMIV and NheI, were separated by agarose gel electrophoresis, and were purified with a QIAEX II gel purification kit (QIAGEN). The purified pBLS500 fragment was ligated with either the PflaB-kan or PflgB-kan fragments to yield pJLB500A and pJLB500G, respectively (Fig. 1D). The ligation junctions from all the clones were verified by sequencing with a 373A automated DNA sequencer (Applied Biosystems Inc., Foster City, Calif.).
Construction of pJLBA.V and pJLBG.V.
A 5′- and 3′-truncated oppAV gene was cloned into a derivative of pJLB500 lacking the gyrBr marker (pJLB12A and pJLB12G) and was tested for targeted integration at the oppAV locus. pJLB12A and pJLB12G were constructed in the same manner as pJLB500A and pJLB500G, respectively, except pOK12 (34) instead of pBLS500 was amplified with oligonucleotides 10 and 11 (Fig. 1). Amplified PCR products were cloned into pCR2.1 (Invitrogen), were digested with NheI and NgoMIV, and were ligated with either PflaB-kan (pJLB12A) or PflgB-kan (pJLB12G). Oligonucleotide 15, located 370 bp 5′ of the stop codon, and oligonucleotide 16, located 60 bp 3′ of the start codon, were used to amplify a 1,164-bp internal fragment of oppAV. The ΔoppAV PCR fragment was cloned into pCR2.1 and sequenced. The ΔoppAV PCR fragment was digested from pCR2.1 with BamHI and PstI and was ligated into pJLB12A or pJLB12G to make pJLBA.V and pJLBG.V, respectively.
Transformation of B. burgdorferi.
Twenty-four micrograms of pJLB500A and pJLB500G plasmid DNA was used to transform B. burgdorferi B31-A by electroporation as previously described (21, 25). Twenty-four hours after electroporation, the transformation was diluted 1:10 in BSK-II containing 100 μg of kanamycin per ml and was incubated at 35°C. Six days after kanamycin selection in liquid medium, the culture was plated in solid BSK with 200 μg of kanamycin per ml.
Twenty-four micrograms of pJLBA.V and pJLBG.V plasmid DNA was used to transform B. burgdorferi B31-A by electroporation as described above. Twenty-four hours after electroporation, the transformations were plated directly in solid BSK containing 200 μg of kanamycin per ml. Kanr B. burgdorferi colonies were screened as previously described (21, 30, 33).
Southern blot analysis.
Total genomic DNA was isolated from B. burgdorferi clones B31-A, AB1, KA1, KG1, and AB5 as previously described (22), was digested with restriction enzyme XbaI, and was separated on a 0.8% agarose gel by field inversion electrophoresis for 24 h at 7 V cm−1 with program 3 of a PPI-200 programmable power inverter (MJ Research, Watertown, Mass.). DNA was bidirectionally transferred to Biotrans nylon membranes (ICN Pharmaceuticals Inc., Irvine, Calif.), was prehybridized, and was hybridized with radioactive probes in a solution containing 6X SSC (where 20X SSC is 3 M sodium chloride plus 0.3 M sodium citrate), 0.1% sodium dodecyl sulfate (SDS), 0.5% nonfat dried milk, and 0.1 mM sodium pyrophosphate at 55°C in a hybridization oven (Bellco, Vineland, N.J.) (22). Membranes were probed with PCR-generated fragments of the kan (oligonucleotides 1 and 2) and gyrB genes as previously described (30, 33). Probe fragments were radiolabelled with [α-32P]dATP (DuPont, NEN Research Products, Boston, Mass.) by random priming (Life Technologies, Gaithersburg, Md.). Blots were washed in 0.2X SSC and 0.1% SDS at 55°C and were visualized by autoradiography.
MIC of kanamycin.
One hundred microliters of BSK-II medium was added to each well of a 96-well tissue culture plate (Corning, Corning, N.Y.). One hundred microliters of a solution containing 6.4 mg of kanamycin per ml was added to the first row of wells, and twofold dilutions were performed up to the last row of wells, to which no kanamycin was added. Log-phase cultures of clones B31-A, AB1, KA1, and KG1 were diluted to 105 bacteria/ml, and 100 μl of the suspension was added to duplicate wells. The concentration of kanamycin tested spanned from 25 to 1,600 μg/ml. Each plate was covered with Breathe-Easy, a gas-permeable sealing membrane for microtiter plates (Diversified Biotech, Boston, Mass.), and was incubated at 35°C in 1% CO2. Growth was monitored by color change of the medium. As previously demonstrated, the change of the pH indicator in the medium from pink to yellow accurately reflects bacterial growth (23).
Northern blot analysis of kan transcripts.
Total RNA was isolated from exponentially growing cultures of B. burgdorferi clones B31-A, AB1, KA1, and KG1 by using the Ultraspec RNA isolation system (Biotecx, Houston, Tex.) (3, 33). RNA was denatured with glyoxal and dimethyl sulfoxide and was electrophoresed in a 1% agarose gel in 10 mM sodium phosphate buffer, pH 7.0 (24). The RNA was transferred to a nylon membrane (Micron Separations Inc., Westboro, Mass.), cross-linked with ultraviolet light, and air dried. Prehybridization and hybridization were conducted at 55°C in 1% bovine serum albumin, 7% SDS, 0.5 M sodium phosphate (pH 7.0), and 1 mM EDTA in rotating bottles in a hybridization oven (Bellco). Membranes were probed with PCR-generated fragments of the kan (described above) and flaB (oligonucleotides 13 and 14) genes, radiolabelled as described above. Blots were washed in 0.2X SSC and 0.1% SDS at 55°C and were visualized by autoradiography.
Cloning and sequencing of the promoter-kan fusions from B. burgdorferi clones KA1 and KG1.
Oligonucleotides 2 and 12 were used to amplify the regions that flank the B. burgdorferi promoter-kan fusion genes from clones KA1 and KG1. The resulting PCR fragments were cloned into pCR2.1 (Invitrogen), were purified by using the QIAprep Spin Miniprep Kit (QIAGEN), and were sequenced.
Rescue of integrated plasmids from B. burgdorferi.
Approximately 420 ng of KA1 and 190 ng of KG1 total genomic DNA were each digested with BamHI, followed by phenol-chloroform extraction and ethanol precipitation (30). Digested DNA was ligated with 400 U of T4 DNA ligase. One-sixth of the ligation mixture was transformed into competent E. coli DH5α cells (Life Technologies), which were then plated on medium containing 40 μg of kanamycin per ml. DNA from five clones from each transformation was purified with the QIAprep Spin Miniprep Kit (QIAGEN), was digested with BamHI, was separated by agarose gel electrophoresis, and was visualized by ethidium bromide staining.
RESULTS
Kanamycin resistance phenotype of B. burgdorferi clone AB1.
B. burgdorferi clones KS5 and AB1 (Table 1) contain the plasmid pBLS500 (pOK12 vector with the B. burgdorferi gyrBr gene) integrated at the gyrB chromosomal locus. Clone AB1 also contains a copy of the oppAV gene at this site (30). B. burgdorferi with the kan gene from Tn903 does not form colonies in the presence of 40 μg of kanamycin per ml of medium (30). We first determined if the lack of kanamycin resistance of B. burgdorferi clones KS5 and AB1 was due to inadequate expression of the kan gene or to the inability of the kan gene product to confer kanamycin resistance.
Comparison of the MIC of kanamycin for growth in liquid culture demonstrated that clone AB1 was resistant to a twofold higher concentration of kanamycin than the wild-type clone B31-A from which it was derived (MIC of 50 μg/ml versus ≤25 μg/ml, respectively) (Table 1). Taken together, these results indicated that the kan gene product conferred a low level of kanamycin resistance. Next, Northern blot analysis was used to detect kan expression in clone AB1. A discrete transcript was not observed after a 2-week exposure, but a faint smear of approximately the correct size was present (data not shown).
The lack of an abundant kan transcript in clone AB1 suggested that a selectable, kanamycin-resistant phenotype could be generated if the level of kan expression was increased. The flaB and flgB genes of B. burgdorferi encode components of the periplasmic flagella, a major and defining structural feature of spirochetes. Previous Northern blot results indicated that an abundant and invariant amount of flaB transcript was made under various culture conditions (3, 31, 33). The borrelial flaB and flgB promoters have also been shown to be actively transcribed in E. coli (9, 27). Taken together, these observations suggested that replacing the Tn903 promoter with the flaB or flgB promoter could increase the level of kan expression in B. burgdorferi.
Presence of flagella in B. burgdorferi grown on solid medium.
We first confirmed that flagellar gene promoters (such as flaB and flgB) would be utilized by B. burgdorferi forming colonies in solid media, since this is where the selection for kanamycin resistance would be imposed. B. burgdorferi colonies were excised from solid media and spread on a glass microscope slide, and indirect immunofluorescence was performed with a monoclonal antibody directed against flagellin, the product of the flaB gene. Since flgB encodes part of the flagellar rod and is the first gene in a 21-kb motility operon, the presence of intact flagella implies that FlgB protein is also synthesized. Fluorescent, spiral-shaped spirochetes were detected in all colonies, demonstrating that flagella were present and that the flaB and flgB promoters were active in B. burgdorferi colonies on plates (data not shown).
Construction of pJLB500A and pJLB500G plasmids.
To increase kan gene expression, the flaB and flgB promoters (Fig. 1A and B) were fused to the translational start site of the kan gene to create PflaB-kan or PflgB-kan, respectively (Fig. 2). PflaB-kan or PflgB-kan fusions were integrated into a plasmid lacking a kanamycin resistance gene and were assayed for their ability to confer kanamycin resistance in E. coli. Both constructs yielded kanamycin-resistant colonies (data not shown), indicating that the borrelial promoter-kan fusions were functional in E. coli. To test if the PflaB-kan or PflgB-kan fusions were functional in B. burgdorferi, the kan gene from pBLS500 was replaced with the new promoter-kan constructs to produce pJLB500A and pJLB500G, respectively (Fig. 1).
FIG. 2.
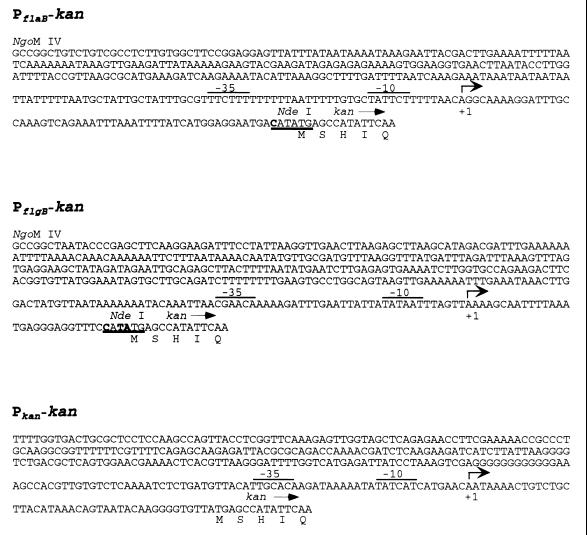
Sequence of the promoter regions from flaB and flgB of B. burgdorferi and the kan gene of Tn903. The NgoMIV site was added to the 5′ end of the flaB and flgB promoters for cloning. The underlined sequence denotes the NdeI fusion site between the promoters and the kan gene. The nucleotides shown in boldface within the underlined sequence differ from the wild-type sequence. The transcription initiation sites are indicated by arrows, and the proposed −10 and −35 sequences of the promoters are overlined (8, 9, 11, 14, 15). The first five amino acids of the kan product are shown below their corresponding codons.
Transformation of B. burgdorferi with pJLB500A and pJLB500G.
The pBLS500 vector contains one copy of the gyrBr gene, which can mediate direct integration at the gyrB locus on the B. burgdorferi chromosome (30). Similarly, pJLB500A or pJLB500G can integrate at the gyrB locus, permitting stable maintenance of these DNAs. By so doing, the B. burgdorferi promoter-kan constructs could be tested for their ability to confer kanamycin resistance. B. burgdorferi clone B31-A was transformed by electroporation with pJLB500A and pJLB500G, and transformants were selected in liquid BSK medium containing 100 μg of kanamycin per ml. Four days after electroporation, no viable spirochetes were visible by dark-field microscopy from a control transformation conducted without transforming DNA. In contrast, viable spirochetes were visible at this time in the culture transformed with plasmid DNA. The transformed culture containing spirochetes was plated in solid medium containing 200 μg of kanamycin per ml 6 days after transformation, and the resultant Kanr colonies were screened with primers specific for either PflaB-kan or PflgB-kan. PCR-positive colonies were obtained for each promoter construct. B. burgdorferi clones KA1 (PflaB-kan) and KG1 (PflgB-kan) (Table 1) were excised from agarose plates for further characterization.
DNA analysis of KA1 and KG1.
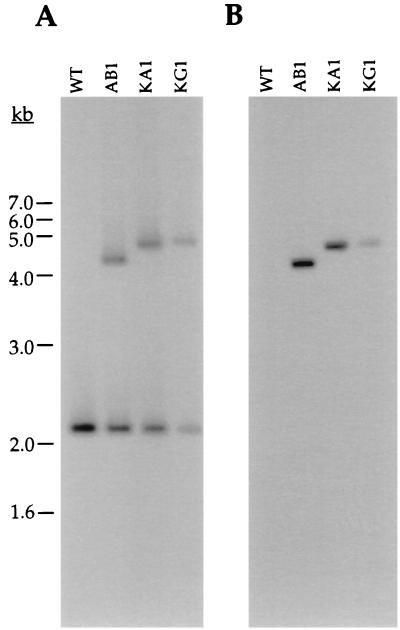
Southern blot analysis confirmed that plasmid integration had occurred at the chromosomal gyrB locus in Kanr transformants KA1 and KG1. Southern blots containing XbaI-digested genomic DNA from wild-type B31-A and clones AB1, KA1, and KG1 were hybridized with gyrB- or kan-specific probes. As expected, the gyrB probe hybridized to a single 2.1-kb fragment in B31-A (Fig. 3A). The gyrB probe hybridized to two fragments of approximately 2.1 kb and 4.5 or 4.9 kb (Fig. 3A) in clones AB1, KA1, and KG1. This result is consistent with integration of each plasmid at the chromosomal gyrB locus and duplication of the gyrB gene. The approximately 400-bp size difference in the larger fragment from clones KA1 and KG1 relative to clone AB1 is caused by the insertion of B. burgdorferi promoter sequences in these plasmids (Fig. 3A). The kan probe also hybridized with the 4.5- or 4.9-kb fragments in clones AB1, KA1, and KG1, but not to any fragment in B31-A (Fig. 3B).
FIG. 3.
Southern blot analysis of DNA from various B. burgdorferi clones. Total genomic DNA from clones B31-A (WT), AB1, KA1, and KG1 digested with XbaI and hybridized with either a gyrB (A) or a kan (B) probe (strain source of DNA indicated at the top of each lane). The size standards are indicated on the left.
To confirm that the promoter-kan fusions were not altered in B. burgdorferi, the promoter regions from clones KA1 and KG1 were amplified by PCR, were sequenced, and were found to be identical to the original constructs. E. coli plasmids tested to date do not autonomously replicate in B. burgdorferi. To test the possibility that the deficiency in this case results from the loss or alteration of essential replication genes in B. burgdorferi, integrated plasmid DNA was rescued from clones KA1 and KG1 by digesting total genomic DNA with BamHI (which cuts at both ends of the integrated plasmid, once in the B. burgdorferi gyrB gene and once in the plasmid multiple cloning site) followed by ligation and transformation back into E. coli. Kanr E. coli were recovered, and all clones contained a plasmid of the predicted size (data not shown). These and previous results (30) indicated that the pOK12 sequences required for plasmid replication and maintenance in E. coli were not sufficient to confer autonomous replication in B. burgdorferi, but they can be introduced into, stably maintained in, and later recovered from spirochetes.
Kanr phenotype of clones KA1 and KG1.
Wild-type B31-A and transformants AB1, KA1, and KG1 were each inoculated into liquid medium containing varying concentrations of kanamycin to determine the MIC of the drug for these strains. Clones KA1 and KG1 grew in the presence of 1.6 mg of kanamycin per ml, the highest antibiotic concentration tested (Table 1), whereas wild-type clone B31-A and the integrant AB1 were inhibited by 25 and 50 μg of kanamycin per ml, respectively (Table 1). There was no difference between the growth rate of clones KA1 and KG1 in kanamycin and the growth rate of control cultures without antibiotic (data not shown). Identical MICs were obtained for clones KA1 and KG1 grown without kanamycin prior to the assay (data not shown). Hence, the Kanr marker is relatively stable during six to seven doublings in the absence of antibiotic selection. Moreover, no induction with antibiotic is required for high-level resistance to kanamycin.
Expression of the kan gene in B. burgdorferi.
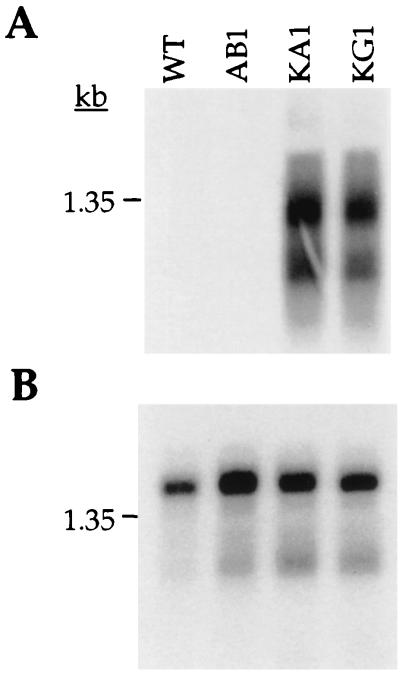
The increased level of kanamycin resistance of clones KA1 and KG1 relative to clone AB1 suggested that the kan gene was expressed at a higher level in B. burgdorferi when fused to the flaB- and flgB-promoters. To test this interpretation, total RNA was extracted from B. burgdorferi clones B31-A, AB1, KA1, and KG1, and a Northern blot was hybridized with a probe from the kan gene. Clones KA1 and KG1 each contained an abundant kan transcript, whereas none was detected in clones B31-A and AB1 after a comparable exposure (Fig. 4A). Two hybridizing bands were observed with clones KA1 and KG1. The smaller band of approximately 1 kb is the predicted size of the kan gene transcript. The larger band of approximately 1.4 kb could represent transcriptional read-through (Fig. 4A). Hybridization of the same Northern blot with a probe to the flaB gene indicated roughly equivalent amounts and integrity of RNA in all samples (Fig. 4B).
FIG. 4.
Northern blot analysis of kan transcripts from B. burgdorferi clones. A blot containing total RNA from B. burgdorferi clones B31-A (WT), AB1, KA1, and KG1 was hybridized with a radioactive kan probe and exposed for 8 h at −80°C (A). After decay, these blots were hybridized with an internal flagellin probe to assure equivalent loading of RNA in each lane (B). An RNA size marker (in kilobases) is indicated to the left of each panel.
Inactivation of the oppAV gene.
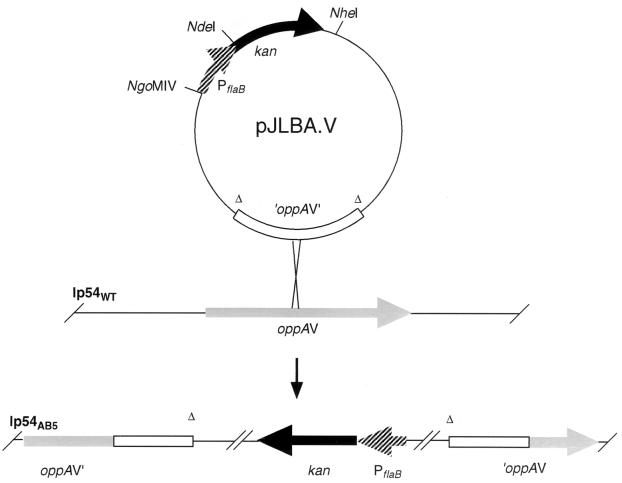
Integration of circular plasmids by homologous recombination can be used as a method for site-directed gene disruption. This is accomplished by cloning an internal fragment of the targeted gene lacking the 5′ and 3′ ends of the open reading frame. To determine the usefulness of plasmids containing the Kanr marker for gene inactivation, a truncated fragment of the B. burgdorferi oppAV gene was cloned into plasmids pJLBA.V and pJLBG.V, containing PflaB-kan or PflgB-kan, respectively (Fig. 5). Plasmid integration will result in disruption of the oppAV gene, as diagrammed in Fig. 5. oppAV encodes a homolog of the substrate binding component of oligopeptide permease and is located on a linear plasmid (lp54) (3, 7), which also represents a distinct genomic component in which to attempt plasmid integration. One hundred twenty-five Kanr colonies were recovered after electroporation of B. burgdorferi with pJLBA.V and pJLBG.V; two Kanr colonies were obtained in the control transformation without plasmid. Eighty-seven of 93 Kanr colonies (93%) screened by PCR (oligonucleotides 1 and 9) contained the kan gene (data not shown). Additional PCR analyses indicated that 84 of these transformants were plasmid integrants at the oppAV locus, and three were at the flaB promoter (data not shown). The six Kanr colonies negative by PCR for the kan gene probably represent background resistance mutants. Southern blot, PCR, and sequence analyses of a kan-positive clone, AB5, were consistent with inactivation of the oppAV gene by plasmid integration (data not shown). The in vitro growth rates of clones AB5 and B31-A in BSK-II were indistinguishable (data not shown). The lack of an impaired growth phenotype may reflect functional redundancy among the OppA proteins.
FIG. 5.
Formation of B. burgdorferi clone AB5. The top of the figure shows the plasmid pJLBA.V and integration at the oppAV locus in the 54-kb linear plasmid (lp54). The lower part of the figure represents the oppAV locus of the B. burgdorferi clone AB5 with an integrated copy of pJLBA.V.
DISCUSSION
Integration of pBLS500 into the B. burgdorferi chromosome provides a means to stably maintain heterologous DNA and to assay for the expression of foreign genes (30). We exploited this approach to develop the first exogenous antibiotic resistance marker that can be used to select B. burgdorferi mutants. The advantage of this exogenous kan marker relative to gyrBr stems from the elimination of B. burgdorferi transformants that arise by unwanted recombination at the gyrB locus rather than at the desired targeted site. We are currently exploiting this approach to test other antibiotic resistance genes for their usefulness as additional selectable markers in B. burgdorferi.
The main concern in using a B. burgdorferi promoter-kan fusion for genetic manipulation was whether an abundant transcript would be produced under the desired culture conditions. Synthesis of the FlaB protein has been documented under various B. burgdorferi growth conditions. For example, a flagellum-specific monoclonal antibody detected the FlaB protein on spirochetes in liquid culture (2), in colonies on plates (as shown in this paper), and in the tick midgut (T. G. Schwan, personal communication). These observations and molecular genetic data (8) indicate that flagellar genes are constitutively expressed in B. burgdorferi. This is different than what has been described in E. coli and Salmonella, where the flagellar genes are under strict regulation (16). Interestingly, both borrelial promoters are recognized by the E. coli transcriptional machinery, but the converse is not true for the native kan promoter in B. burgdorferi. A comparison of the kan, flaB, and flgB promoters does not yield an obvious explanation for this result (Fig. 2) (8, 9, 14, 15).
A similar approach of fusing a native promoter with an antibiotic resistance marker was used to develop a puromycin (pac) selectable marker for genetic studies in Methanococcus voltae, a methanococcal archaebacterium (10). Novel genetic strategies were necessary because, similar to borreliae, the archaebacterial transcriptional machinery does not recognize eubacterial promoter and terminator sequences. The methanococcal transcriptional signals from the pac cassette were also fused with the Tn903 neomycin/kanamycin resistance gene to generate a second selectable marker (35). The availability of an antibiotic resistance marker represented a major breakthrough in the genetic analysis of methanococci (17, 35).
In theory, integration of the Kanr marker could occur at any site in the genome with sufficient homology to plasmid-borne sequences. pBLS500 and pJLB500 integrated at the gyrB locus, near the chromosomal origin of replication (6, 7, 18, 19), because the gyrBr gene was also present on these plasmids. Integration of the E. coli plasmid pOK12 near the origin of replication does not appear to alter DNA replication (30). However, preliminary results suggest that not all heterologous sequences can be tolerated at this site (J. L. Bono, unpublished data). In addition to targeted gene disruption, plasmid integration at other loci should permit introduction of foreign DNA that is incompatible with the chromosomal origin.
Promoter-reporter fusion constructs can now be integrated at a targeted locus in B. burgdorferi and tested under different conditions that mimic the tick or mammalian environment. A transient assay for B. burgdorferi promoters has been developed that utilizes borrelial sequences linked to a chloramphenicol acetyltransferase (cat) gene (27, 29). Stable cat integrants were not previously obtained, and direct selection for chloramphenicol resistance may not be possible in B. burgdorferi due to a competing esterase activity in the BSK growth medium (28). Since the Kanr marker confers resistance in B. burgdorferi to greater than 1.6 mg of kanamycin per ml, its activity is apparently unaffected by medium components. Stable promoter-cat fusion constructs should be possible if plasmid integration is selected with the Kanr marker.
Shuttle vectors are another important genetic tool that is not available for B. burgdorferi analyses. pJLB500 will enhance the ability to construct such a vector. Although much of this plasmid has a guanine-plus-cytosine content that is significantly higher than that of the B. burgdorferi genome (approximately 50 versus 30%, respectively) (7; J. L. Bono, unpublished data), it is tolerated and stably replicated on the chromosome and lp54. This result indicates that if the appropriate B. burgdorferi plasmid replication genes were present, pJLB500-derived plasmids should be able to replicate autonomously. B. burgdorferi naturally contains many plasmids, and various cloning strategies can be used with pJLB500 to identify the factors necessary for plasmid replication in B. burgdorferi.
Previously, we tried to inactivate the oppAV gene through targeted integration of a derivative of pBLS500 that contained a 5′- and 3′-truncated fragment of oppAV, with gyrBr as the selectable marker. No integrants were detected by PCR screening of 788 Cour B. burgdorferi colonies from four separate transformations (J. L. Bono, unpublished data). As described in this paper, we repeated this experiment with the same truncated fragment of oppAV cloned into a derivative of pJLB500 lacking the gyrBr gene, but containing PflaB-kan or PflgB-kan as the selectable marker. Eighty-four of the 93 Kanr colonies were positive for plasmid integration at the oppAV locus. Although the actual transformation frequency of B. burgdorferi B31-A was not enhanced with kan (5.5 × 10−7) compared to gyrBr (2.91 × 10−6), the large number of irrelevant transformants resulting from recombination with the chromosomal gyrB locus was eliminated, thus facilitating recovery of the desired integrant. Taken together, our data clearly demonstrate the enhanced utility of the kan gene to generate targeted gene disruptions in noninfectious B. burgdorferi relative to what is now possible with the gyrBr marker. To date, we have not isolated Kanr transformants from infectious B. burgdorferi, presumably due to the approximately 100-fold lower transformation frequency of infectious relative to noninfectious B. burgdorferi (P. A. Rosa, unpublished data). When a method becomes available to transform infectious B. burgdorferi, the Kanr marker should be a useful tool for these spirochetes as well. This efficient method of targeted mutagenesis will facilitate ongoing investigations of the roles of specific B. burgdorferi gene products in the biological processes underlying the infectious cycle and pathogenesis of Lyme disease.
ACKNOWLEDGMENTS
We thank Martine Bos, James M. Musser, Paul Policastro, Tom Schwan, and Philip Stewart for helpful comments on the manuscript, Tom Schwan for help with the indirect immunofluorescence microscopy for FlaB, Nyles Charon for discussions about flagellar gene expression, Gary Hettrick and Robert Evans for graphic support, and Kelly Matteson for secretarial assistance.
REFERENCES
- 1.Barbour A G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour A G, Hayes S F, Heiland R A, Schrumpf M E, Tessier S L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986;52:549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bono J L, Tilly K, Stevenson B, Hogan D, Rosa P. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology. 1998;144:1033–1044. doi: 10.1099/00221287-144-4-1033. [DOI] [PubMed] [Google Scholar]
- 4.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 5.Carroll J A, Garon C F, Schwan T G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casjens S, Huang W M. Linear chromosomal physical and genetic map of Borrelia burgdorferi, the Lyme disease agent. Mol Microbiol. 1993;8:967–980. doi: 10.1111/j.1365-2958.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 7.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 8.Ge Y, Old I G, Saint Girons I, Charon N W. The flgK motility operon of Borrelia burgdorferi is initiated by a sigma 70-like promoter. Microbiology. 1997;143:1681–1690. doi: 10.1099/00221287-143-5-1681. [DOI] [PubMed] [Google Scholar]
- 9.Ge Y, Old I G, Saint Girons I, Charon N W. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus sigma 70 promoter. J Bacteriol. 1997;179:2289–2299. doi: 10.1128/jb.179.7.2289-2299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gernhardt P, Possot O, Foglino M, Sibold L, Klein A. Construction of an integration vector for use in the archaebacterium Methanococcus voltae and expression of a eubacterial resistance gene. Mol Gen Genet. 1990;221:273–279. doi: 10.1007/BF00261731. [DOI] [PubMed] [Google Scholar]
- 11.Grindley N D, Joyce C M. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc Natl Acad Sci USA. 1980;77:7176–7180. doi: 10.1073/pnas.77.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indest K J, Ramamoorthy R, Sole M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane R S, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 14.Lee K Y, Hopkins J D, Syvanen M. Direct involvement of IS26 in an antibiotic resistance operon. J Bacteriol. 1990;172:3229–3236. doi: 10.1128/jb.172.6.3229-3236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee K Y, Hopkins J D, Syvanen M. Evolved neomycin phosphotransferase from an isolate of Klebsiella pneumoniae. Mol Microbiol. 1991;5:2039–2046. doi: 10.1111/j.1365-2958.1991.tb00826.x. [DOI] [PubMed] [Google Scholar]
- 16.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 17.Metcalf W W. Genetic analysis in the domain Archae. In: Smith M C, Sockett R E, editors. Methods in microbiology: genetic methods for diverse prokaryotes. London, England: Academic Press; 1999. pp. 277–326. [Google Scholar]
- 18.Old I G, MacDougall J, Saint Girons I, Davidson B E. Mapping of genes on the linear chromosome of the bacterium Borrelia burgdorferi: possible locations for its origin of replication. FEMS Microbiol Lett. 1992;99:245–250. doi: 10.1016/0378-1097(92)90034-l. [DOI] [PubMed] [Google Scholar]
- 19.Picardeau M, Lobry J R, Hinnebusch B J. Physical mapping of an origin of bidirectional replication at the centre of the Borrelia burgdorferi linear chromosome. Mol Microbiol. 1999;32:437–445. doi: 10.1046/j.1365-2958.1999.01368.x. [DOI] [PubMed] [Google Scholar]
- 20.Porcella S F, Popova T G, Akins D R, Li M, Radolf J D, Norgard M V. Borrelia burgdorferi supercoiled plasmids encode multi-copy tandem open reading frames and a lipoprotein gene family. J Bacteriol. 1996;178:3293–3307. doi: 10.1128/jb.178.11.3293-3307.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa P, Samuels D S, Hogan D, Stevenson B, Casjens S, Tilly K. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J Bacteriol. 1996;178:5946–5953. doi: 10.1128/jb.178.20.5946-5953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosa P A, Schwan T G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989;160:1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- 23.Sadziene A, Thompson P A, Barbour A G. In vitro inhibition of Borrelia burgdorferi growth by antibodies. J Infect Dis. 1993;167:165–172. doi: 10.1093/infdis/167.1.165. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 7.40–7.42. [Google Scholar]
- 25.Samuels D S, Mach K E, Garon C F. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. J Bacteriol. 1994;176:6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels D S, Marconi R T, Huang W M, Garon C F. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J Bacteriol. 1994;176:3072–3075. doi: 10.1128/jb.176.10.3072-3075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sohaskey C D, Arnold C, Barbour A G. Analysis of promoters in Borrelia burgdorferi by use of a transiently expressed reporter gene. J Bacteriol. 1997;179:6837–6842. doi: 10.1128/jb.179.21.6837-6842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sohaskey C D, Barbour A G. Esterases in serum-containing growth media counteract chloramphenicol acetyltransferase activity in vitro. Antimicrob Agents Chemother. 1999;43:655–660. doi: 10.1128/aac.43.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sohaskey C D, Zückert W R, Barbour A G. The extended promoters for two outer membrane lipoprotein genes of Borrelia spp. uniquely include a T-rich region. Mol Microbiol. 1999;33:41–51. doi: 10.1046/j.1365-2958.1999.01443.x. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson B, Bono J, Elias L A, Tilly K, Rosa P. Transformation of the Lyme disease spirochete Borrelia burgdorferi with heterologous DNA. J Bacteriol. 1998;180:4850–4855. doi: 10.1128/jb.180.18.4850-4855.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson B, Bono J L, Schwan T G, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in tick-bite-infected mammals, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 34.Vieira J, Messing J. New pUC-derived cloning vectors with different selectable markers and DNA replication origins. Gene. 1991;100:189–194. doi: 10.1016/0378-1119(91)90365-i. [DOI] [PubMed] [Google Scholar]
- 35.Whitman W B, Tumbula D L, Yu J-P, Kim W. Development of genetic approaches for the methane-producing archaebacterium Methanococcus maripaludis. Biofactors. 1997;6:37–46. doi: 10.1002/biof.5520060105. [DOI] [PubMed] [Google Scholar]
- 36.Zückert W R, Meyer J, Barbour A G. Comparative analysis and immunological characterization of the Borrelia Bdr protein family. Infect Immun. 1999;67:3257–3266. doi: 10.1128/iai.67.7.3257-3266.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]