Abstract
A 54-year-old woman with a diagnosis of Erdheim-Chester disease under therapy with dabrafenib presents with clinical signs of heart failure. After discontinuing the offending medication and initiating guideline-directed medical therapy for heart failure with reduced ejection fraction, the clinical picture improved.
Key Words: aortitis, cardiorenal syndrome, dabrafenib, Erdheim-Chester disease, heart failure with reduced ejection fraction
Graphical Abstract
History of Presentation
A 54-year-old woman with a diagnosis of Erdheim-Chester disease (ECD), recently started on dabrafenib, arrived at the emergency department endorsing dyspnea. The patient presented with proptosis, pulmonary rales at bases, jugular ingurgitation, and dysarthria. Imaging (Figures 1 and 2B, Video 1) revealed cardiomegaly, widened mediastinum, pulmonary congestion, and left ventricular ejection fraction (LVEF) of 25% to 30%. The patient’s laboratory results on arrival are shown in Table 1.
Learning Objectives
-
•
To understand that Erdheim-Chester disease is driven by BRAF and MEK mutations.
-
•
To highlight that BRAF and MEK inhibition has been linked to a reduction in LVEF.
-
•
To acknowledge that BRAF inhibitor cardiotoxicity and treatment remain incompletely understood.
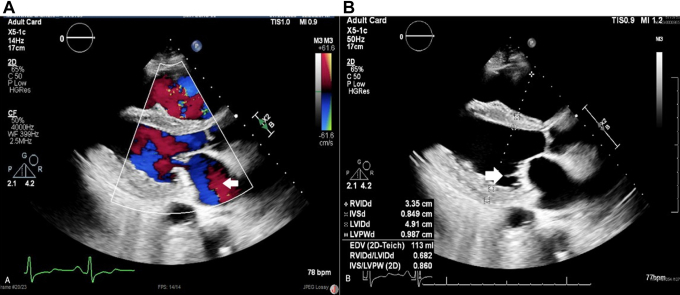
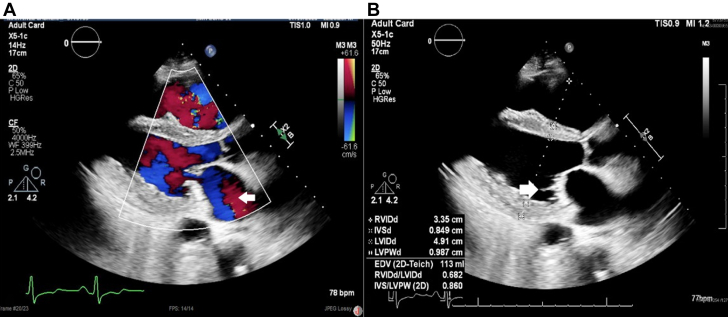
Figure 1.
Transthoracic Echocardiogram Parasternal View
(A) Mitral regurgitation (open arrow). (B) Lambl excrescences in the posterior mitral leaflet (open arrow).
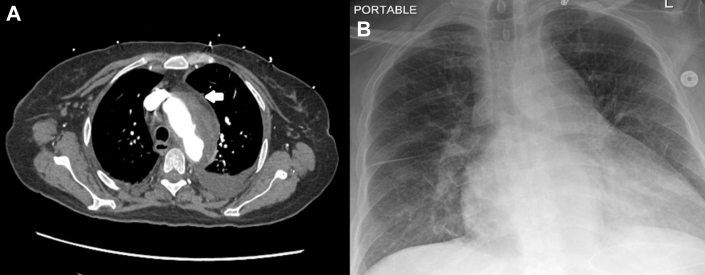
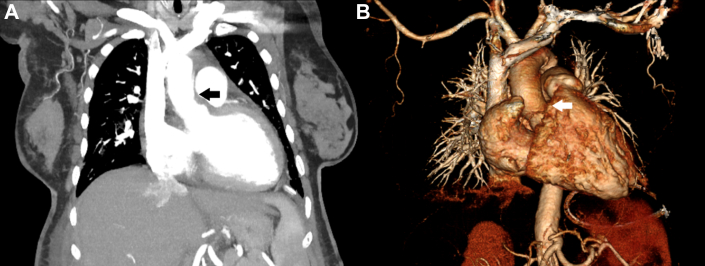
Figure 2.
Computerized Tomography and Chest X Ray
(A) Tissue deposition in the aortic arch (open arrow) and luminal irregularity. (B) Widened mediastinum and cardiomegaly.
Table 1.
Laboratory Values at Arrival
| Glucose, mg/dL | 95 |
| Sodium, mmol/L | 133 |
| Potassium, mmol/L | 4.8 |
| Chloride, mmol/L | 98 |
| CO2, mmol/L | 23 |
| BUN, mmol/L | 34 |
| Creatinine, mg/dL | 1.6 |
| Troponin I, ng/mL | <20 |
| NT-proBNP, pg/mL | 9,200 |
BUN = blood urea nitrogen; CO2 = carbon dioxide; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Past Medical History
The patient was recently diagnosed with ECD, a very rare non-Langerhans histiocytosis, with around 1,000 cases reported.1 ECD is a type of histiocytosis characterized by multisystem infiltration, creating a wide range of pathologic findings. Deposited histiocytes can induce local inflammation, eventually resulting in tissue damage. Characteristic imaging signs include coated aorta (Figure 2A), hairy kidney, and multiple bony lesions. The diagnosis is established by taking a biopsy of the affected tissue and running an analysis of the genetic mutations affecting the mitogen-activated protein kinase (MAPK) pathway.1, 2, 3 It is common for the disease to infiltrate the aorta and heart valves, leading to heart failure.1 The patient had baseline dyspnea NYHA functional class I, which worsened to NYHA functional class IV before hospitalization. Unfortunately, the patient did not have a baseline cardiovascular evaluation, and she only followed up with her oncologist, who made the diagnosis of ECD.
Differential Diagnosis
The patient arrived with dyspnea and had clinical signs of heart failure. The initial electrocardiogram showed no evidence of infarction, troponin I was not elevated, and angiogram performed during admission revealed no signs of obstruction, which made acute coronary syndrome less likely. The patient had recently started treatment with dabrafenib for ECD. Our thought then shifted toward pharmacologic cardiotoxicity associated with the use of BRAF inhibitors; therefore, we decided to stop the medication and begin guideline-directed medical therapy (GDMT) for heart failure with reduced ejection fraction (HFrEF). Imaging studies allowed us to observe the soft tissue deposition, which is characteristic of ECD in distinct tissues, including the aorta, the left renal artery, the retro-orbital muscles, the cerebellum, the spleen, and the mitral valve. At the time, the patient already had a diagnosis of ECD, made through the findings of a skin biopsy 4 months prior to the hospitalization. She was immediately started on dabrafenib by her oncologist. Although in the differential diagnosis the activity of ECD on the heart could still be responsible for the heart failure, it was less likely because dabrafenib was treating the disease and is known to impair the left ventricular function negatively. Therefore, given the strong possibility of the diagnosis being pharmacologic cardiotoxicity, we decided to stabilize the patient, control the volume status, and recover the ejection fraction before discussing initiating another treatment for ECD.4
Investigations
The patient underwent a multi-image assessment to characterize the activity of the disease. Transthoracic echocardiography revealed a severely reduced LVEF of 25% to 30% and restrictive physiology. The right ventricle was dilated with compromised systolic function and estimated severe pulmonary hypertension with proper ventricular systolic pressure of 60 to 70 mm Hg. The aortic valve presented trace regurgitation, and the mitral valve had tethered leaflets concerning Lambl excrescences with moderate mitral regurgitation (Figure 1, Video 2).
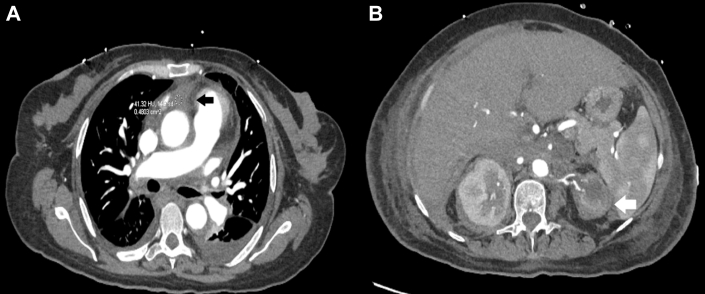
Computed tomography revealed soft tissue deposition in the aortic root, spleen, and left aortic arch, suggestive of aortitis, giving the characteristic ECD sign of a coated aorta (Figure 2A). An atrophic left-sided kidney was identified, and Doppler ultrasound confirmed unilateral left renal artery stenosis (Figures 3 and 4). Diffuse luminal irregularities of the aortic arch and descending thoracic aorta were identified, consistent with histiocyte deposition rather than atherosclerosis due to the characteristics of the density variations and Hounsfield units. The commonly involved layers include the adventitial and periadventitial periaortic spaces (Figures 2A and 4A).5
Figure 3.
Computerized Tomography With Contrast
(A) Soft tissue deposition on the aortic root (solid arrow). (B) Left atrophic kidney (open arrow) and multiple splenic hypodensities.
Figure 4.
Computerized Tomography With Contrast and Orthogonal 3D Reconstruction
(A) Aortic root compression due to tissue deposition (solid arrow). Luminal irregularity of the aorta. (B) Aortic root compression (open arrow).
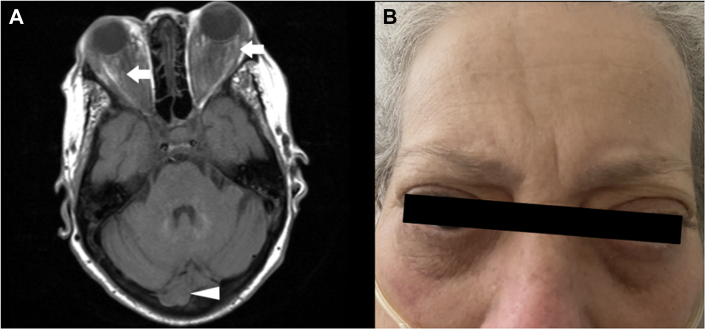
Magnetic resonance imaging revealed soft tissue deposition in the retro-orbital tissue and cerebellar involvement of the right hemisphere (Figures 4B and 5A).
Figure 5.
Magnetic Resonance Imaging and Physical Findings
(A) Retro-orbital (open arrows) and cerebellar (open arrowhead) tissue deposition (open arrows). (B) Bilateral proptosis.
Management
Dabrafenib was stopped due to HFrEF. The patient improved with GDMT, which consisted of carvedilol 6.25 mg twice a day, dapagliflozin 10 mg every 24 hours, sacubitril-valsartan 49/51 mg twice a day, and spironolactone 25 mg every 24 hours. Transthoracic echocardiography showed LVEF of 30% to 35% (Video 3). The patient experienced acute renal failure due to cardiorenal syndrome and pneumonia with sepsis, both treated successfully with diuretic and antibiotic therapy, respectively. The patient presented hemodynamic instability during admission and was strictly supervised in the intensive care unit. Because of the patient’s ECD diagnosis, we considered it overly invasive to perform an endomyocardial biopsy; we thought it would not add significant information that could modify our therapeutic approach and could lead to complications. We did not order cardiac magnetic resonance because we had the computed tomography scan findings. We focused on treating heart failure and did not fully characterize disease activity during admission. We decided to order it during the follow-up instead.
The patient had aortitis due to ECD, and dabrafenib was put on hold due to heart failure. Our priority was to stabilize the patient’s heart failure before discussing potential therapeutic strategies. The patient was discharged due to the resolution of the mentioned conditions and the stability of heart function in the absence of symptoms.
Discussion
ECD treatment aims to inhibit the MAPK pathway; medicines approved to provide this effect include BRAF and MEK inhibitors, which have been demonstrated to slow the disease process and even regression.1, 2, 3
Few prospective therapeutic studies for ECD have been reported. Treatment regimens have been mixed. However, in recent years, identifying the BRAFV600 mutation and the activity of the MAPK pathway played an essential part in the pathologic process. Cohen Aubart et al2 reported the outcomes of 54 patients with ECD treated with BRAF inhibitors (vemurafenib, dabrafenib), showing a 91% response rate in patients with the BRAFV600 mutation. The disease showed regression in vascular, central nervous system, and retroperitoneal tissues and significantly lessened symptoms.1,2 Diamond et al3 conducted a trial with MEK1/2 inhibitors, where 72% of patients presented a complete response and 17% presented a partial response.3 Some authors, such as Goyal et al,6 recommend empirical therapy with either MEK inhibitors, BRAF inhibitors, or both for BRAF-V600–mutated ECD.6 The duration of the regimen and the disease behavior after completing the medication cycles remain unknown.1,2
BRAF and MEK inhibitors have been identified as cardiotoxic; however, the mechanism of injury, reversibility, and response to treatment have not yet been entirely determined.7 The mechanism of injury seems to be secondary to the fact that inhibition of BRAF and MEK negatively interferes with cardiovascular MAPK signaling, which generates oxidative stress, myocyte apoptosis, and angiogenesis impairment. The cardiotoxicity may increase morbidity, requiring a temporary or permanent therapy termination.4 The recent start on dabrafenib, the NYHA functional class progression, signs of pulmonary congestion, and decreased ejection fraction were noted in imaging studies, allowing us to conclude signs of cardiotoxicity in the patient. We did not conduct a biopsy for reasons that were mentioned earlier. The clinical picture improved after prompt initiation of GDMT and discontinuation of dabrafenib. Waliany et al8 found an increased OR of heart failure (OR: 2.24; P = 0.01) relative to other targeted therapies in a median time to onset of 116 days. Higher odds of prolonged QT were also reported (relative risk (RR): 2.69; P = 0.01).8 Mincu et al4 found similar results, with an increased risk for a high-grade decrease in LVEF (RR: 2.79; P = 0.005) and development of arterial hypertension (RR: 1.54; P = 0.005) when using BRAF+MEK inhibitors vs BRAF monotherapy.4,8
Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers represent a cornerstone in the prevention and treatment of cardiovascular sequelae of a multitude of conditions, including the cardiotoxicity induced by chemotherapy.9 Similarly, spironolactone and beta blockers have demonstrated a cardioprotective effect in patients receiving anthracyclines, attenuating the LVEF decrease.9 Fadol10 mentions a similar resolution, which is currently the standard treatment at the MD Anderson Cancer Center for patients with anthracycline-induced cardiotoxicity. Angiotensin-converting enzyme inhibitors and beta blockers have shown improvement in left ventricular function.10 However, the need for more evidence and information regarding the toxicity mechanism of BRAF inhibitors increased the difficulty of our decision-making. It oriented us toward an empirical decision based on the current knowledge of the disease and the pharmacologic properties of dabrafenib.
Currently, the treatment for HFrEF consists of 4 groups of drugs: sodium glucose co-transporter 2 inhibitors, renin-angiotensin-aldosterone system antagonists, beta blockers, and aldosterone antagonists, because they have improved mortality outcomes.11
The current proposed ECD treatment involves inhibiting the MAPK pathway, which has been demonstrated to be potentially cardiotoxic, setting us in a dilemma where no evidence suggests that heart failure could be reversible with these agents.8 Adverse effects on the heart can occur even when treating a patient with the best available agent at 75 mg twice a day. We took new actions to counter the cardiotoxicity associated with BRAF inhibitors. Although the ideal treatment for ECD is unknown, given the consequences of current therapies, we found that discontinuing the offending agent and initiating GDMT for heart failure resulted in a favorable clinical outcome. The possibility of continuing GDMT simultaneously to prevent or lessen heart failure has yet to be reported.12 Glen et al12 proposed a pathway in which patients with an LVEF more than 40% should permanently suspend all MEK inhibitors and temporarily suspend BRAF inhibitors while initiating therapy for heart failure and undergoing a cardio-oncologic evaluation to plan as a team the best step forward. We agree with the proposed pathway by Glen et al,12 and that is exactly how we managed the patient. However, this therapeutic decision has very few precedents. Therefore, even if we can have an empirical consensus, more data are necessary to increase the prognostic value of the therapeutic interventions. We hope to expand the barriers to cardiologic and oncologic knowledge by reporting this case, our intervention, and the eventual outcome during follow-up. Reporting this case can clear the path to prospective studies in which the cardiotoxicity of these therapeutic agents is treated with GDMT. The results of these studies can provide data regarding the effectiveness and prognosis of GDMT for HFrEF in the context of BRAF inhibitor employment.
Follow-Up
The patient will continue the GDMT and will follow-up with the oncologist. The continuation of directed therapy for ECD will be imperative, and the balance between therapeutic options to avoid cardiotoxicity and treat the condition is the main objective.
Conclusions
Clear guidelines are needed to aid in decision-making. Standard therapy for ECD-induced HFrEF was given, and GDMT led to improved clinical outcomes. Thus, this case represents an unexplored area of cardiology and oncology.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Arrival Transthoracic Echocardiogram
Apical 4-chamber view with observed left ventricular ejection fraction of 25% to 30%.
Arrival Transthoracic Echocardiogram
Long parasternal view with Lambl excrescences appreciated.
Post-Treatment Transthoracic Echocardiogram
Apical 4-chamber view showing improved left ventricular ejection fraction of 30% to 35%.
Post-Treatment Transthoracic Echocardiogram
Long parasternal view with Lambl excrescences appreciated.
References
- 1.Starkebaum G., Hendrie P. Erdheim–Chester disease. Best Pract Res Clin Rheumatol. 2020;34(4) doi: 10.1016/j.berh.2020.101510. [DOI] [PubMed] [Google Scholar]
- 2.Cohen Aubart F., Emile J.-F., Carrat F., et al. Targeted therapies in 54 patients with Erdheim-Chester disease, including follow-up after interruption (The love study) Blood. 2017;130(11):1377–1380. doi: 10.1182/blood-2017-03-771873. [DOI] [PubMed] [Google Scholar]
- 3.Diamond E.L., Durham B.H., Ulaner G.A., et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567(7749):521–524. doi: 10.1038/s41586-019-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mincu R.I., Mahabadi A.A., Michel L., et al. Cardiovascular adverse events associated with BRAF and MEK inhibitors. JAMA Network Open. 2019;2(8):e198890. doi: 10.1001/jamanetworkopen.2019.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haroche J., Cohen-Aubart F., Amoura Z. Erdheim-Chester disease. Blood. 2020;135(16):1311–1318. doi: 10.1182/blood.2019002766. [DOI] [PubMed] [Google Scholar]
- 6.Goyal G., Heaney M.L., Collin M., et al. Erdheim-Chester disease: consensus recommendations for evaluation, diagnosis, and treatment in the molecular era. Blood. 2020;135(22):1929–1945. doi: 10.1182/blood.2019003507. [DOI] [PubMed] [Google Scholar]
- 7.Jain D., Aronow W. Cardiotoxicity of cancer chemotherapy in clinical practice. Hosp Pract (1995) 2018;47(1):6–15. doi: 10.1080/21548331.2018.1530831. [DOI] [PubMed] [Google Scholar]
- 8.Waliany S., Zhu H., Wakelee H., et al. Pharmacovigilance analysis of cardiac toxicities associated with targeted therapies for metastatic NSCLC. J Thorac Oncol. 2021;16(12):2029–2039. doi: 10.1016/j.jtho.2021.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Trapani D., Zagami P., Nicolò E., Pravettoni G., Curigliano G. Management of cardiac toxicity induced by chemotherapy. J Clin Med. 2020;9(9):2885. doi: 10.3390/jcm9092885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadol A. Management of chemotherapy-induced left ventricular dysfunction and heart failure in patients with cancer while undergoing cancer treatment: The MD Anderson Practice. Front Cardiovasc Med. 2018;5:24. doi: 10.3389/fcvm.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidenreich P., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Glen C., Tan Y.Y., Waterston A., et al. Mechanistic and clinical overview cardiovascular toxicity of BRAF and MEK inhibitors. J Am Coll Cardiol CardioOnc. 2022;4(1):1–18. doi: 10.1016/j.jaccao.2022.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arrival Transthoracic Echocardiogram
Apical 4-chamber view with observed left ventricular ejection fraction of 25% to 30%.
Arrival Transthoracic Echocardiogram
Long parasternal view with Lambl excrescences appreciated.
Post-Treatment Transthoracic Echocardiogram
Apical 4-chamber view showing improved left ventricular ejection fraction of 30% to 35%.
Post-Treatment Transthoracic Echocardiogram
Long parasternal view with Lambl excrescences appreciated.