Abstract
Background
Thoracoscopy, which has an increasing role in the treatment of indexed neonatal surgical conditions, requires adequate training. To support this, the current study aimed to evaluate the feasibility and effectiveness of using live rabbit models in neonatal thoracoscopic skills training among paediatric surgeons.
Methods
Following didactic lectures and demonstrations, the participants were given hands-on opportunities to perform thoracoscopic procedures. The feasibility and effectiveness of using live rabbit models in neonatal thoracoscopic skills training among paediatric surgeons were evaluated with pre-/post-course procedural confidence scores and a questionnaire.
Results
This study included 13 paediatric surgeons—2 (15 %) males and 11 (85 %) females—who were evenly distributed. There were four basic surgical trainees, five higher surgical trainees and four fellows in paediatric surgery (mean surgical practice experience: 4.5 ± 3.7 years). Most had experience assisting paediatric (70 %) and neonatal (62 %) thoracoscopic surgery. Only 30 % had experience as the chief surgeon of paediatric thoracoscopic surgery, with none on neonates. Significant improvement was seen in procedural confidence as the assistant and chief surgeon of all procedures post-workshop. The surgeons rated the model positively.
Conclusion
The procedural confidence level of paediatric surgeons improved significantly after workshop participation. This realistic and easily reproducible model can help perfect thoracoscopic skills. Therefore, its integration into paediatric surgical training would promote surgical skill proficiency and could improve surgeons’ confidence in neonate operations.
Keywords: Thoracoscopic, Neonatal surgery, Surgical training, Animal surgery
1. Introduction
Thoracoscopy has an increasing role in the treatment of indexed neonatal surgical conditions (e.g. repair of oesophageal atresia, repair of congenital diaphragmatic hernia/eventration, etc.) in this era of minimally invasive surgery [1]. This technique requires different capabilities from those in open surgery; thus, surgeons must learn the necessary skills for its proper use. Individual centres and surgeons may have limited experience with these conditions and even less experience with the minimally invasive surgical approach as a result of the relatively lower occurrence of these congenital anomalies. Therefore, it is crucial to ensure that this relatively newer technique is part of paediatric surgical training.
Simulation-based training in both the simulation laboratory and the animal operating room has been a solution for training less commonly performed complex procedures. Animal models (e.g. rabbits) have been reported to provide realistic tissue handling as well as haptic feedback, which are irreplaceable by in vitro stimulators [2,3]. With the aim of enhancing neonatal thoracoscopic surgery training, we developed a training workshop with live rabbit models for paediatric surgeons who lacked neonatal thoracoscopic skills. The participants were provided with hands-on opportunities to perform thoracoscopic procedures. This study aimed to evaluate the feasibility and effectiveness of using live rabbit models in neonatal thoracoscopic skills training among paediatric surgeons.
2. Methods
2.1. Study design and participants
The rabbit model for training was approved and performed under the guidelines of care and use of experimental animals by the Committee on the Use of Live Animals in Teaching and Research, University of Hong Kong (CULATR number: 23–229). The study was also reviewed and approved by the hospital's institutional review board (reference number: UW 23–499). Surgeons of various training levels were invited to enrol in our neonatal thoracoscopic training course using a rabbit model. They received hands-on training on three thoracoscopic procedures on the rabbit model, which resembled the same pathologies in neonates. All participants enrolled in the training course were recruited into the study to evaluate the feasibility and effectiveness of the course using assessment instruments, as described below.
2.2. Course design
The training course was conducted for 3 h, during which the participants underwent intensive hands-on thoracoscopic skills training on three important neonatal pathologies: (1) thoracoscopic repair of trachea–oesophageal fistula and oesophageal atresia (TOF-OA); (2) thoracoscopic repair of congenital diaphragmatic hernia (CDH); and (3) thoracoscopic lung resection for congenital pulmonary airway malformation. The learning objectives included the following: (1) acquire knowledge and techniques in handling thoracoscopic instruments and a camera in neonates; (2) acquire knowledge and techniques in creating access to the thorax safely in neonates; (3) acquire knowledge and techniques in thoracoscopic suturing and knot-tying in neonates; (4) acquire knowledge and techniques in dissections and tissue handling during thoracoscopic surgery in neonates; (5) understand the perioperative considerations needed for thoracoscopic surgery in neonates; and (6) gain practical experience in thoracoscopic surgery on animal models.
The participants completed a pre-course procedural confidence survey on the three thoracoscopic procedures. They then received an introductory briefing from the course director on animal handling and important knowledge about thoracoscopic instrument handling, access to the thoracic cavity and key techniques of the three procedures to be covered. The participants were divided into groups comprised of 4–5 participants with variable levels of training. Each surgical table was coached by an experienced paediatric surgeon who had extensive thoracoscopic surgical experience in both neonates and older children. The skills for thoracoscopic repair of TOF-OA, CDH and lung resection were demonstrated by the instructor. The participants took turns as assistants and chief surgeons of the respective procedures. At the end of the course, the participants were given a questionnaire consisting of six statements about how realistic the model was and its usefulness for training.
2.3. Animal care and surgical procedures
Three New Zealand white rabbits were utilised in this animal-teaching workshop. The thoracic wall was first shaved and disinfected. All surgical procedures were carried out under aseptic conditions and general anaesthesia. Each rabbit was intubated and ventilated after the commencement of general anaesthesia. Continuous intraoperative monitoring of vital signs and recordings, including oxygen saturation, blood pressure, respiration rate and heart rate, were carried out throughout the operations to ensure well-balanced anaesthesia care. The parameters of any other signs (e.g. pupil size, toe reflex) that might be used for anaesthetic depth assessment were also monitored to avoid unnecessary suffering and pain in the animals.
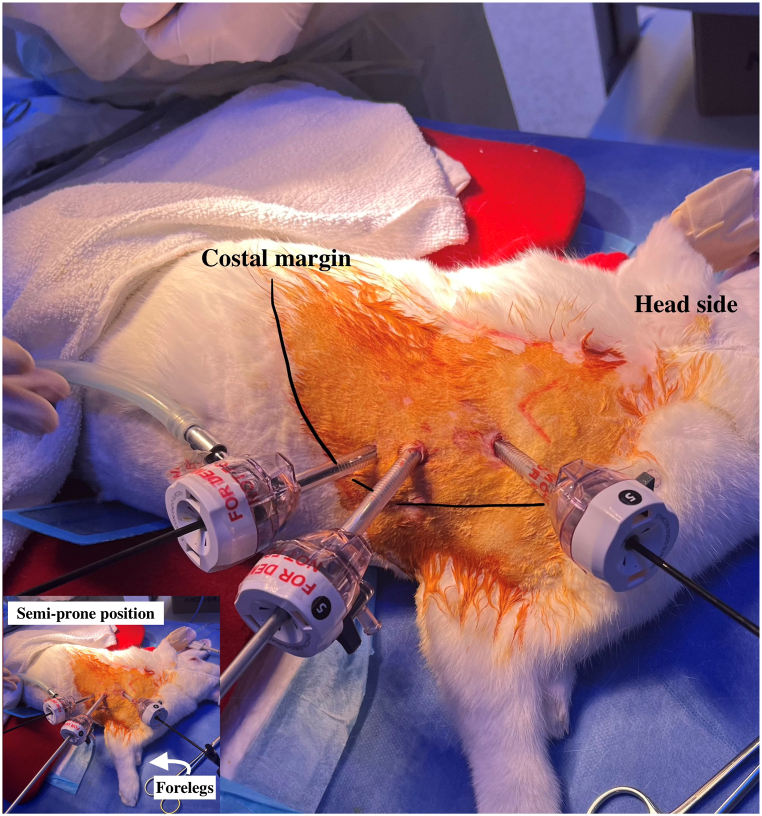
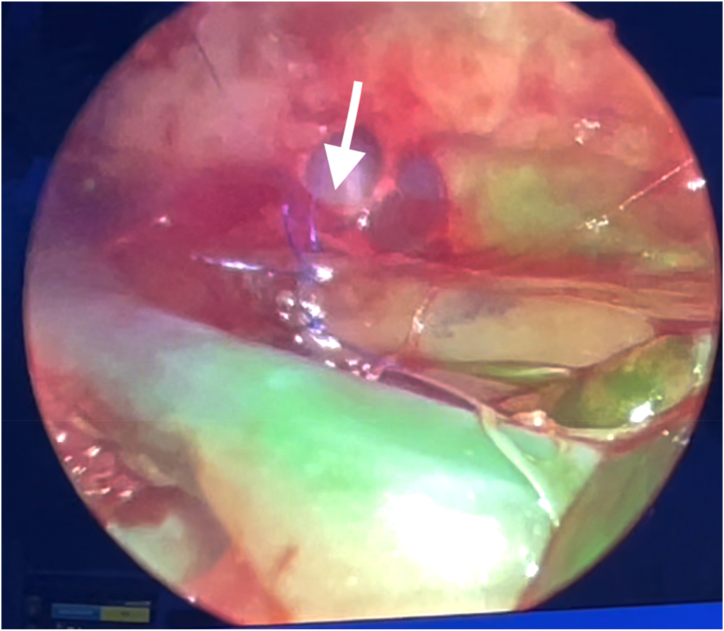
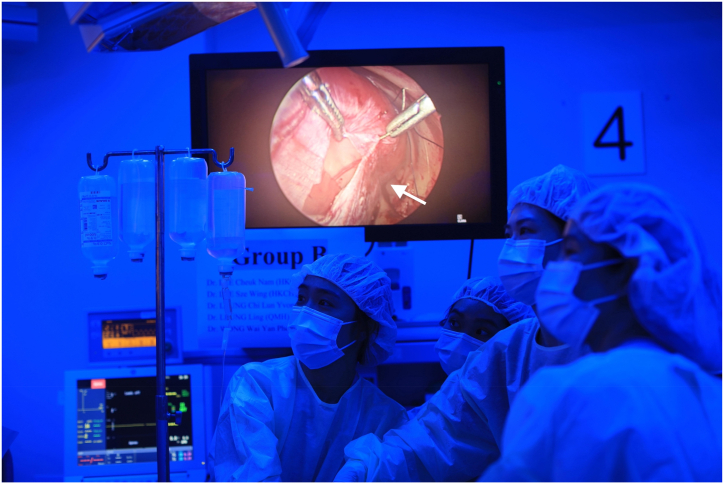
After the establishment of general anaesthesia and skin disinfection, the rabbits were placed in a right lateral position [Fig. 1]. After three 5-mm trocar insertions, pneumothorax was established with the instillation of CO2 at 4 mm Hg, with a flow rate of 1 L/min. The procedures began with TOF-OA repair. The oesophagus was identified and dissected with 3-mm thoracoscopic instruments. It was then transected and re-anastomosed with 5-0 monofilament absorbable sutures using intracorporeal knot-tying, simulating the repair of oesophageal atresia. The vascular status of the anastomosis was then assessed by indocyanine green fluorescence angiography [Fig. 2]. Following that, the participants performed wedge resection of the lung with the aid of energy devices, simulating lung resection for congenital pulmonary airway malformation. Finally, a defect was created thoracoscopically over the diaphragm. The participants were required to reduce the abdominal content back to the abdominal cavity, and repair of congenital diaphragmatic hernia was performed using non-absorbable sutures [Fig. 3].
Fig. 1.
Trocar placement in the rabbit model.
Fig. 2.
Assessment of oesophageal anasotmosis (arrowed) with indocyanine green fluorescence angiography. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Repair of congenital diaphragmatic hernia (arrowed) in rabbit model.
All animals were kept alive throughout the procedure under satisfactory general anaesthesia. At the end of the workshop, the animals were sacrificed with an overdose of anaesthesia.
2.4. Assessment instruments
2.4.1. Procedural confidence survey
Participants were asked to self-rate their pre-course and post-course procedural confidence for the three thoracoscopic procedures taught on a five-point rating scale. This scoring system has been reported in the literature, and scores ranged from 1 = very little confidence to 5 = a lot of confidence [4].
2.5. Survey of the model and course
A questionnaire consisting of six statements about how realistic the model was and its usefulness for training was given to the participants at the end of the workshop [2]. This questionnaire included a five-point ordinal scale (1 = not at all, 2 = a little, 3 = enough, 4 = a lot and 5 = completely). These statements included the following: (1) similarities between animal model and newborn, (2) similarities between rabbit thoracic dimension and newborn thorax, (3) degree of technical difficulty compared with real scenario, (4) training capacities of the model to improve thoracoscopic skills, (5) training capacities of the model to facilitate management of neonatal thoracic surgical complications and (6) degree of usefulness of this model in a training programme in neonatal thoracoscopy surgery.
2.6. Outcome measures
The primary outcome of this study was the participants' procedural confidence scores before and after the course. The scores were analysed and compared with the background of the participants to identify any factors that could have affected the scores. Participants’ satisfaction with the model and course for neonatal thoracoscopic skill training was analysed as the secondary outcome.
2.7. Statistical methods
We analysed and compared all the data statistically using the Statistical Package for the Social Sciences® (SPSS®; IBM®, USA), version 26, and Excel. Graphs were constructed with Prism 10 (GraphPad, USA). Descriptive statistics are given as the number of units (n) and the percentage (%). The data are expressed as mean plus standard deviation (SD). We analysed the continuous variables using the Student's t-test and analysis of variance (ANOVA), as appropriate. We performed a paired samples t-test to compare the procedural confidence scores before and after the workshop. The Pearson correlation test was used to explore the impact of training experience on the procedural confidence scores, and we considered a p-value of <0.05 statistically significant.
3. Results
3.1. Demographics and experience of participants
A total of 13 paediatric surgeons participated in the workshop. Complete evaluation data were collected and used for the statistical analysis. Of the 13 participants, 2 (15 %) were male and 11 (85 %) were female, with a mean age of 29.7 ± 4 years. The surgeons were evenly distributed among the three training levels (four basic surgical trainees, five higher surgical trainees and four fellows in paediatric surgery), with a mean surgical practice experience of 4.5 ± 3.7 years. Most had experience assisting paediatric (70 %, n = 9) and neonatal (62 %, n = 8) thoracoscopic surgery. However, few (30 %, n = 4) had experience as the chief surgeon of paediatric thoracoscopic surgery, and none had such experience with neonates. Among the four surgeons with experience as the chief surgeon of a paediatric thoracoscopic surgery, one had operated on two children, one had operated on three children and two had operated on five children.
3.2. Procedural confidence
All participants showed a low level of procedural confidence at the beginning of the workshop. The mean procedural confidence scores in being the assistant of TOF repair, CDH repair and lung resection were 2.8 ± 1.4, 2.6 ± 1.4 and 2.7 ± 1.4 out of 5, respectively. By contrast, the mean procedural confidence scores in being the chief surgeon of TOF repair, CDH repair and lung resection were 1.2 ± 0.4, 1.4 ± 0.7 and 1.2 ± 0.4 out of 5, respectively [Table 1]. The level of training had a significant correlation with the level of pre-course procedural confidence of being the assistant and chief surgeon of all three procedures. The pre-course procedural confidence levels were significantly lower in the participants with a more junior level of training across all three procedures [Table 2, Table 3(supplementary table)].
Table 1.
The Procedural confidence score of participants.
| Procedure | Pre-course | Post-course | p |
|---|---|---|---|
| TOF – Assistant | 2.8 ± 1.4 | 3.8 ± 1.2 | <0.0001* |
| TOF – Chief | 1.2 ± 0.4 | 2.4 ± 1.3 | 0.005* |
| CDH – Assistant | 2.6 ± 1.4 | 3.8 ± 1.2 | <0.0001* |
| CDH – Chief | 1.4 ± 0.7 | 2.5 ± 1.2 | 0.006* |
| Lung resection – Assistant | 2.7 ± 1.4 | 3.9 ± 1.2 | <0.0001* |
| Lung resection – Chief | 1.2 ± 0.4 | 2.7 ± 1.3 | 0.043* |
TOF: Repair of Tracheo-oesophgeal fistula and oesophageal atresia.
CDH: Repair of congenital diaphragmatic hernia.
Table 2.
The pre-course procedural confidence score according to level of training status.
| Procedure | Level of training | Procedural Confidence | p |
|---|---|---|---|
| TOF – Assistant | BST | 1 | <0.0001* |
| HST | 3.2 ± 0.4 | ||
| Fellow | 4 ± 0.8 | ||
| TOF – Chief | BST | 1 | 0.004* |
| HST | 1 | ||
| Fellow | 1.8 ± 0.5 | ||
| CDH – Assistant | BST | 1 | <0.0001* |
| HST | 2.8 ± 0.4 | ||
| Fellow | 4 ± 1.2 | ||
| CDH – Chief | BST | 1 | <0.0001* |
| HST | 1 | ||
| Fellow | 2.3 ± 0.5 | ||
| Lung resection – Assistant | BST | 1 | 0.003* |
| HST | 3.2 ± 0.4 | ||
| Fellow | 3.75 ± 1.5 | ||
| Lung resection – Chief | BST | 1 | 0.004* |
| HST | 1 | ||
| Fellow | 1.8 ± 0.5 |
TOF: Repair of Tracheo-oesophgeal fistula and oesophageal atresia.
CDH: Repair of congenital diaphragmatic hernia.
BST: Basic surgical trainee.
HST: Higher surgical trainee.
Table 3.
Correlation of Training years and Pre-course Procedural confidence score.
| Pearson correlation coefficient (r)a | p | |
|---|---|---|
| TOF – Assistant | 0.812** | 0.001 |
| TOF – Chief | 0.833** | <0.0001 |
| CDH – Assistant | 0.750** | 0.003 |
| CDH – Chief | 0.767** | 0.002 |
| Lung resection – Assistant | 0.625* | 0.022 |
| Lung resection – Chief | 0.833** | <0.0001 |
TOF: Repair of Tracheo-oesophgeal fistula and oesophageal atresia.
CDH: Repair of congenital diaphragmatic hernia.
2-tailed Pearson correlation with number of training years.
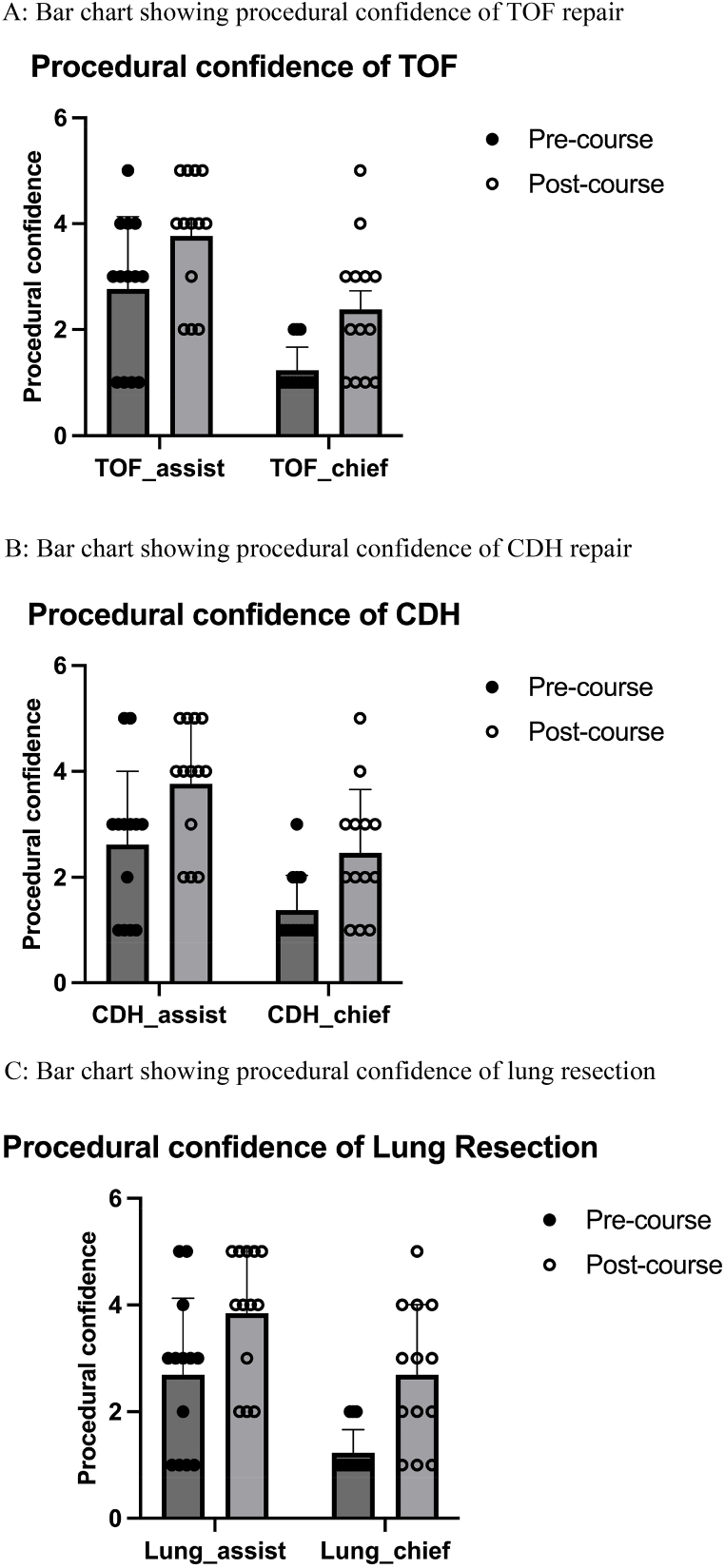
There was significant improvement of procedural confidence in being the assistant and chief of all three procedures after the workshop (assistant, TOF repair: 2.8 ± 1.4 vs. 3.8 ± 1.2, p < 0.0001; chief, TOF repair: 1.2 ± 0.4 vs. 2.4 ± 1.3, p = 0.005; assistant, lung resection: 2.7 ± 1.4 vs. 3.9 ± 1.2, p < 0.0001; chief, lung resection: 1.2 ± 0.4 vs. 2.7 ± 1.3, p = 0.043; assistant, CDH repair: 2.6 ± 1.4 vs. 3.8 ± 1.2, p < 0.0001; chief, CDH repair: 1.4 ± 0.7 vs. 2.5 ± 1.2, p = 0.006) [Fig. 4 and Table 1]. However, there was no significant correlation between the degree of procedural confidence improvement and the surgeons’ experience [Table 4(supplementary table)].
Fig. 4.
(A): Bar chart showing procedural confidence of TOF repair. (B): Bar chart showing procedural confidence of CDH repair. (C): Bar chart showing procedural confidence of lung resection.
Table 4.
Correlation of Training years and Procedural confidence score difference.
| Pearson correlation coefficient (r)a | p | |
|---|---|---|
| TOF – Assistant | 0.219 | 0.358 |
| TOF - Chief | 0.272 | 0.368 |
| CDH - Assistant | −0.196 | 0.522 |
| CDH - Chief | 0.241 | 0.427 |
| Lung resection - Assistant | −0.128 | 0.676 |
| Lung resection - Chief | 0.450 | 0.123 |
TOF: Repair of Tracheo-oesophgeal fistula and oesophageal atresia.
CDH: Repair of congenital diaphragmatic hernia.
2-tailed Pearson correlation with number of training years.
3.3. Evaluation of the model
Table 5 shows the mean ratings of the participants’ impressions of the rabbit model. The participants rated the model positively, highlighting the similarities between the model and newborn pathologies and its usefulness for neonatal thoracoscopic skills training. The highest mean score was for the question about the usefulness of this model for neonatal thoracoscopic surgery training and the capacity of the model to improve thoracoscopic skills. The statistical analysis revealed no significant difference across the scores from the three levels of training (p > 0.1), except for the usefulness of this model for improvement of thoracoscopic skills, indicating a general agreement on all questions. In particular, this model was perceived to be more useful in improving thoracoscopic skills by more experienced participants (BST 4 vs. HST 4.4 ± 0.5 vs. Fellow 5, p = 0.007).
Table 5.
Evaluation of model.
| Statement | Score |
p | |||
|---|---|---|---|---|---|
| Overall | BST | HST | Fellow | ||
| The rabbit model of the pathologies resembles the pathologies in neonates | 4.1 ± 0.3 | 4 | 4 | 4.25 ± 0.5 | 0.354 |
| The rabbit thoracic dimension resembles that of a neonate | 4.4 ± 0.5 | 4 | 4.4 ± 0.5 | 4.75 ± 0.5 | 0.102 |
| The technical difficult is comparable to real scenario | 4.2 ± 0.7 | 4 | 4 ± 1 | 4.5 ± 0.6 | 0.523 |
| The model can improve thoracoscopic skills | 4.5 ± 0.5 | 4 | 4.4 ± 0.5 | 5 | 0.007* |
| The model can facilitate management of neonatal thoracic surgical complications | 4.2 ± 0.4 | 4 | 4.2 ± 0.4 | 4.5 ± 0.6 | 0.289 |
| The training program is useful in training neonatal thoracoscopic surgery | 4.5 ± 0.5 | 4.25 ± 0.5 | 4.4 ± 0.5 | 4.75 ± 0.5 | 0.408 |
BST: basic surgical trainee; HST: higher surgical trainee.
4. Discussion
In this era of minimally invasive surgery, there has been a paradigm shift towards thoracoscopic surgery in the diagnosis and treatment of thoracic conditions, leading to better postoperative outcomes with less pain and better quality of life compared to traditional open thoracotomy [5]. Thoracoscopy also plays an increasing role in the treatment of indexed neonatal surgical conditions (e.g. repair of oesophageal atresia, repair of congenital diaphragmatic hernia/eventration, etc.) [1]. Therefore, adequate training is important. Given the lower occurrence of these conditions in neonatal populations, individual centres and surgeons may have limited experience with these conditions and even less experience with the minimally invasive surgical approach.
Simulation-based training in both the simulation laboratory and the animal operating room should ideally precede the human operating room. Importantly, simulation-based training has been shown to shorten learning curves, enhance technical skills and improve proficiency in minimally invasive surgical skills, which are transferrable to the operating theatre [6,7]. Various training models for surgical skill training have been reported, and the choice of a suitable model hinges on the consideration of availability, similarity to human tissues, reliability and cost [8,9]. A systematic review by Yokoyama et al. (2019) revealed limitations in the use of simulation-based training using an in vitro model compared to animal models [10]. After the settlement of cost and ethical concerns, live animal models could offer better opportunities for demonstrating and practicing surgical skills and might simulate human tissue better in comparison with other models [11].
Rabbit models have been reported to provide realistic tissue handling as well as haptic feedback, which are irreplaceable by in vitro stimulators [2,3]. Our team developed this training workshop with live rabbit models for paediatric surgeons who were not acquainted with neonatal thoracoscopic skills. With this practical session, participants gained invaluable experience in performing thoracoscopic procedures on rabbits, which have a body size like that of human neonates. To the best of our knowledge, we are the first to report on the experience of neonatal thoracoscopic surgical skills training of TOF-OA repair, CDH repair and lung resection with a rabbit model. Furthermore, our current research is the first study of its kind to use multidimensional assessment tools to evaluate the feasibility and effectiveness of this novel neonatal thoracoscopic surgical skill training.
The success of this training course was evidenced by the significant improvement in procedural confidence in being the assistant and chief surgeon of all three procedures after the course. Furthermore, the participants rated the model positively, highlighting the similarities between the model and newborn pathologies and its usefulness for neonatal thoracoscopic skills training. The generalisability of our data to paediatric surgeons of various experience was strengthened by the fact that the participants in this cohort were evenly distributed among the three training levels.
Our data also revealed a general lack of confidence in and opportunities to perform the three essential thoracoscopic procedures. Although a majority of the surgeons had experience in assisting paediatric (70 %) and neonatal (62 %) thoracoscopic surgery, the pre-course procedural confidence scores of being an assistant were low (less than three out of five) across the three procedures. Moreover, only 30 % had experience as the chief surgeon of a paediatric thoracoscopic surgery, and none were performed on neonates. Understandably, the participants generally lacked confidence in being the chief surgeon for thoracoscopic surgery in neonates, which highlights the need for this surgical skill course.
A successful animal training model lies not only in the model itself but also in the delivery of the course content by instructors. Peyton's four-step teaching approach for the acquisition of procedural skills (i.e. demonstration, deconstruction, comprehension and performance) was employed in the workshop [12]. It has been demonstrated to be superior to the traditional ‘see one-do one’ approach, especially in small group settings with few students per teacher (our workshop had four to five participants per teacher) [13]. The participants were encouraged to comment on other members in the same group and seek methods to improve their skills to learn through interaction and co-operation with other members via social learning theory [14]. We believe that this training course was equally useful to both novice and experienced learners. Our data demonstrated that participants at various training levels perceived similar benefits from the course, as evidenced by similarities in the improvement of procedural confidence after the course. However, this model was perceived to be more useful in improving thoracoscopic skills by more experienced participants, possibly due to better appreciation of the live tissue model simulation given their better foundations in surgical skills. Furthermore, it could also reflect that the difficulty of skills involved in the model could be considered as too advanced for less experienced participants. Although rabbits slightly differ from humans in terms of thoracic anatomy (e.g. a rabbit's heart is more centrally, anteriorly and cephalically located, rabbits have four lobes over the right lung and three lobes over the left lung, etc.), most participants in this study, under the guidance of mentors experienced with animal surgeries, rated the model positively, highlighting the similarities between the model and newborn pathologies.
One of the shortcomings of this study was the lack of other objective measures to evaluate the effectiveness of the course. To achieve a more comprehensive assessment of the participants’ performance, future studies should include other objective assessment tools for differences in procedural knowledge, independence and dexterity after the course to overcome the potential limitations of procedural confidence self-rating (especially by novices). Future studies would also be warranted by repeating with experts solely to enhance the assessment of validity of the training model. Notably, there have been ongoing debates on the ethical concerns of using animals for single-use lab training. Donated bodies had been advocated as the model of choice for anatomical education, research, and clinical training [15]; and that the utilization of active willed-body donation programs, positively corresponds with the development of beneficial donor-based surgical simulation programs [16]. However, their use is limited by the potential ethical concerns and high cost incurred with procurement of human body parts for educational purpose; as well as the challenges of acquiring donated bodies given their scarcity [[17], [18], [19]]. Adult cadaveric model had been reported useful for video-assisted thoracoscopic lobectomy simulation and training, yet challenges existed in simulating on newborn bodies, in particular scarcity of body donation for deceased children [19,20]. Although attempts have been made to replace live animals in laparoscopic training with donated bodies or high-fidelity synthetic models, the realism of the tissues and haptic feedback of the material are by no means comparable to that of live animals.
5. Conclusion
The procedural confidence levels of paediatric surgeons in this study improved significantly after participation in our workshop. This realistic and easily reproducible model can help perfect thoracoscopic skills using a realistic recreation of various neonatal pathologies. Its integration into paediatric surgical training would promote surgical skill proficiency and could improve surgeons’ confidence before they operate on neonates.
Ethical approval
The study is reported in accordance with ARRIVE guidelines. The rabbit model for training was approved and performed under the guidelines of care and use of experimental animals by the Committee on the Use of Live Animals in Teaching and Research, University of Hong Kong (CULATR number: 23–229). The study was also reviewed and approved by the hospital's institutional review board (reference number: UW 23–499).
Financial disclosures and conflicts of interest
Adrian Chi Heng FUNG, Patrick Ho Yu CHUNG, Ivy Hau Yee CHAN, Eugene Chin Tung LAU, Jana Yim Hung WO, Kenneth Kak Yuen WONG declared no conflict of interest.
Financial support statement
The researchers did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study is confidential and not deposited into a publicly available repository. However, they would be available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Adrian Chi Heng Fung: Writing – review & editing, Writing – original draft, Validation, Formal analysis, Data curation, Conceptualization. Patrick Ho Yu Chung: Writing – review & editing, Methodology, Data curation, Conceptualization. Ivy Hau Yee Chan: Validation, Supervision, Conceptualization. Eugene Chin Tung Lau: Validation, Supervision, Conceptualization. Jana Yim Hung Wo: Methodology, Investigation, Data curation, Conceptualization. Kenneth Kak Yuen Wong: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31498.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bawazir O.A. Thoracoscopy in pediatrics: surgical perspectives. Ann. Thorac. Med. 2019;14(4):239–247. doi: 10.4103/atm.ATM_114_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usón-Casaús J., Pérez-Merino E.M., Rivera-Barreno R., Rodríguez-Alarcón C.A., Sánchez-Margallo F.M. Evaluation of a Bochdalek diaphragmatic hernia rabbit model for pediatric thoracoscopic training. J. Laparoendosc. Adv. Surg. Tech. 2014;24(4):280–285. doi: 10.1089/lap.2013.0358. [DOI] [PubMed] [Google Scholar]
- 3.Marecos M.C., Torres R.A., Bailez M.M., Vagni R.L., Klappenbach R.F. Pediatric thoracoscopic training in an experimental pleural empyema rabbit model. J. Laparoendosc. Adv. Surg. Tech. 2006;16(4):397–399. doi: 10.1089/lap.2006.16.397. [DOI] [PubMed] [Google Scholar]
- 4.Bowyer M.W., Andreatta P.B., Armstrong J.H., Remick K.N., Elster E.A. A novel paradigm for surgical skills training and assessment of Competency. JAMA Surg. 2021;156(12):1103–1109. doi: 10.1001/jamasurg.2021.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow S.C., Ng C.S. Recent developments in video-assisted thoracoscopic surgery for pulmonary nodule management. J. Thorac. Dis. 2016;8(Suppl 6):S509–S516. doi: 10.21037/jtd.2016.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehabadi M., Fernando B., Berlingieri P. The use of simulation in the acquisition of laparoscopic suturing skills. Int. J. Surg. 2014;12(4):258–268. doi: 10.1016/j.ijsu.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Shetty S., Zevin B., Grantcharov T.P., Roberts K.E., Duffy A.J. Perceptions, training experiences, and preferences of surgical residents toward laparoscopic simulation training: a resident survey. J. Surg. Educ. 2014;71(5):727–733. doi: 10.1016/j.jsurg.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Bauer F., Rommel N., Kreutzer K., Weitz J., Wagenpfeil S., Gulati A., et al. A novel approach to teaching surgical skills to medical students using an ex vivo animal training model. J. Surg. Educ. 2014;71(4):459–465. doi: 10.1016/j.jsurg.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Lipman J.M., Marderstein E.L., Zeinali F., Phitayakorn R., Ponsky J.L., Delaney C.P. Objective evaluation of the performance of surgical trainees on a porcine model of open colectomy. Br. J. Surg. 2010;97(3):391–395. doi: 10.1002/bjs.6864. [DOI] [PubMed] [Google Scholar]
- 10.Yokoyama S., Mizunuma K., Kurashima Y., Watanabe Y., Mizota T., Poudel S., et al. Evaluation methods and impact of simulation-based training in pediatric surgery: a systematic review. Pediatr. Surg. Int. 2019;35(10):1085–1094. doi: 10.1007/s00383-019-04539-5. [DOI] [PubMed] [Google Scholar]
- 11.Qayumi A.K., Cheifetz R.E., Forward A.D., Baird R.M., Litherland H.K., Koetting S.E. Teaching and evaluation of basic surgical techniques: the University of British Columbia experience. J. Invest. Surg. 1999;12(6):341–350. [PubMed] [Google Scholar]
- 12.Peyton J.W.R. 1998. Teaching and Learning in Medical Practice: Manticore Europe. [Google Scholar]
- 13.Giacomino K., Caliesch R., Sattelmayer K.M. The effectiveness of the Peyton's 4-step teaching approach on skill acquisition of procedures in health professions education: a systematic review and meta-analysis with integrated meta-regression. PeerJ. 2020;8 doi: 10.7717/peerj.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vygotsky L.S., Kozulin A. MIT Press; 2012. Thought and Language, Revised and Expanded Edition. [Google Scholar]
- 15.Zdilla M.J., Balta J.Y. Human body donation and surgical training: a narrative review with global perspectives. Anat. Sci. Int. 2023;98(1):1–11. doi: 10.1007/s12565-022-00689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bagian L.K., Wyatt T.B., Mosley C.F., Balta J.Y. Investigating the status of whole-body donation across the United States of America. Anat. Sci. Educ. 2024;17(3):646–659. doi: 10.1002/ase.2387. [DOI] [PubMed] [Google Scholar]
- 17.Champney T.H., Hildebrandt S., Gareth Jones D., Winkelmann A. Bodies R us: ethical views on the commercialization of the dead in medical education and research. Anat. Sci. Educ. 2019;12(3):317–325. doi: 10.1002/ase.1809. [DOI] [PubMed] [Google Scholar]
- 18.Champney T.H. The business of bodies: ethical perspectives on for-profit body donation companies. Clin. Anat. 2016;29(1):25–29. doi: 10.1002/ca.22643. [DOI] [PubMed] [Google Scholar]
- 19.Brenner E., Bleys R.L.A.W., de Caro R., Catereniuc I., Chirculescu A.R.M., Destrieux C., et al. The legal and ethical framework governing body donation in Europe - 2nd update on current practice. Ann. Anat. 2024;252 doi: 10.1016/j.aanat.2023.152195. [DOI] [PubMed] [Google Scholar]
- 20.Dell'Amore A., Boscolo-Berto R., Schiavon M., Pangoni A., Porzionato A., Macchi V., et al. Human corpse model for video-assisted thoracoscopic lobectomy simulation and training. Interact. Cardiovasc. Thorac. Surg. 2020;31(5):632–637. doi: 10.1093/icvts/ivaa169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study is confidential and not deposited into a publicly available repository. However, they would be available from the corresponding author on reasonable request.