Abstract
Tremelimumab plus durvalumab (Dur/Tre) is the first-line treatment for advanced hepatocellular carcinoma (HCC) worldwide. The present report describes the case of a 68-year-old man diagnosed with advanced HCC and a bile duct tumor thrombus (BDTT) who achieved a complete response to Dur/Tre therapy. The BDTT progressed to the bifurcation of the left and right hepatic ducts. Over time, both the tumors and BDTT progressively decreased in size, and a complete response was confirmed using the Response Evaluation Criteria in Solid Tumors (version 1.1.) 6 months after treatment administration. Subsequently, immune-related adverse events, including type 1 diabetes mellitus and diabetic ketoacidosis, emerged, leading to treatment discontinuation. The patient was undergoing outpatient follow-up in a drug-free state with no signs of recurrence 290 days after the initial administration of Dur/Tre. Although long-term and meticulous observations are required, the present findings could influence the choice of systemic chemotherapy for advanced HCC.
Keywords: HCC, Dur/Tre, BDTT, CR, case report
Introduction
Primary liver cancer ranks as the sixth most commonly diagnosed cancer and the third leading cause of cancer-related deaths worldwide (1). Hepatocellular carcinoma (HCC) accounts for approximately 80% of primary liver cancers (1). In recent years, there have been remarkable advancements in systemic chemotherapy for HCC. Specifically, the use of immune checkpoint inhibitors (ICIs) in combination therapy has emerged as a pivotal component of systemic chemotherapy, revolutionizing the treatment landscape for HCC. In the IMbrave150 trial, the combination of atezolizumab plus bevacizumab (Ate/Bev), incorporating anti-programmed death ligand 1 (PD-L1) and anti-vascular endothelial growth factor (VEGF), demonstrated superior survival outcomes compared to sorafenib, with a median survival time of 19.2 months, according to the updated analysis (2,3). Similarly, tremelimumab plus durvalumab (Dur/Tre), which targets both anti-cytotoxic T lymphocyte-associated antigen 4 and anti-PD-L1, demonstrated superior outcomes compared to sorafenib, showcasing a median survival time of 16.4 months in the HIMALAYA trial (4). Accordingly, both regimens have been recommended as first-line treatments for advanced HCC in the Barcelona Clinic Liver Cancer guidelines (5). In clinical practice, the optimal choice for first-line treatment and the most effective sequence for multidisciplinary management of unresectable HCC remains unclear.
HCC with bile duct tumor thrombus (BDTT) is a rare occurrence, with reported incidences ranging from 0.4 to 12.9% in previous reports (6,7). The survival outcomes of patients with HCC with BDTT after curative resection were significantly inferior to those of patients without BDTT (6). Owing to the rarity of HCC with BDTT, evidence regarding the therapeutic efficacy of systemic chemotherapy for this condition remains insufficient.
Here, we report a case of advanced HCC with BDTT extending to the common bile duct, which shows a significant response to Dur/Tre treatment.
Case report
A 68-year-old Japanese man sought medical attention from his family physician due to abdominal pain. He had no family or past medical history, including viral infections such as hepatitis B or C. Blood tests revealed elevated serum bilirubin levels (total bilirubin, 3.7 mg/dl; direct bilirubin, 2.3 mg/dl) and C-reactive protein levels (5.6 mg/dl). Abdominal computed tomography (CT) revealed two intrahepatic tumors in the right lobe and an intraductal mass in the right hepatic duct, suggestive of hilar cholangiocarcinoma or liver metastasis. After receiving antibiotic treatment for cholangitis, the patient was referred to our hospital for diagnosis and treatment.
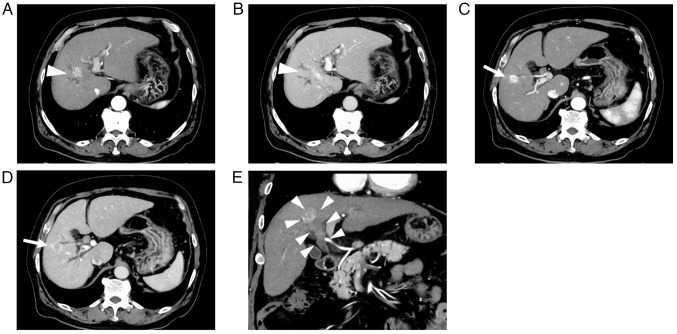
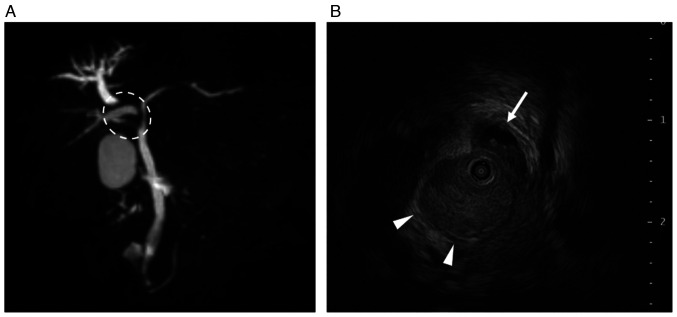
Abdominal contrast-enhanced CT (CECT) revealed two intrahepatic tumors, one measuring 20.0 mm in segment 8 with BDTT and the other measuring 18.8 mm in segment 5. These tumors exhibited enhancement during the arterial phase and washout during the late phase. Moreover, no extrahepatic metastases were detected (Fig. 1). Magnetic resonance cholangiopancreatography revealed the dilated peripheral intrahepatic bile ducts in the right lobe (Fig. 2A). Intraductal ultrasonography revealed that the BDTT had progressed to the bifurcation of the left and right hepatic ducts and the right hepatic duct was obstructed by the BDTT (Fig. 2B). Subsequently, endoscopic retrograde cholangiopancreatography was performed, and intraductal stents were inserted to prevent jaundice progression. Thereafter, the serum bilirubin levels decreased to normal levels (total bilirubin, 1.3 mg/dl; direct bilirubin, 0.3 mg/dl). Despite brush cytology of the bile duct showing malignant components, BDTT biopsy did not confirm the presence of HCC. Furthermore, the serum α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) levels were 44 ng/ml and 57 mAU/ml, respectively, while carcinoembryonic antigen and carbohydrate antigen 19–9 levels were within normal ranges. Liver biopsy was not performed owing to the risk of complications and delay in treatment initiation. Accordingly, we diagnosed the tumors as HCC. The Child-Pugh score was 6 (class A), and the albumin-bilirubin score was −1.64 (modified albumin-bilirubin grade 2b) (8). The indocyanine green retention rate at 15 min was 16.8%. Given that right hepatectomy with bile duct reconstruction is necessary for curative surgery, we regarded these tumors as unresectable HCC due to impaired liver function and planned to administer systemic chemotherapy. We opted for Dur/Tre treatment as the first-line approach because Ate/Bev seemed unsuitable, given the biliary bleeding after the biopsy.
Figure 1.
Abdominal CT before tremelimumab plus durvalumab treatment. The tumor in segment 8 (white arrowheads) was shown in the axial CT images during the (A) arterial phase and (B) late phase. The tumor in segment 5 (white arrows) was shown in the axial CT images during the (C) arterial phase and (D) late phase. (E) The bile duct tumor thrombus originated from the tumor in segment 8 and progressed to the bifurcation of the left and right hepatic ducts according to the coronal CT image. CT, computed tomography.
Figure 2.
MRCP and IDUS before tremelimumab plus durvalumab treatment. (A) MRCP showed the stenosis of proximal hepatic ducts (white dotted circle) and dilated peripheral intrahepatic bile ducts in the right lobe. (B) IDUS showed that the bile duct tumor thrombus progressed to the bifurcation of the left and right hepatic ducts. The left hepatic duct is indicated by a white arrow, and the right hepatic duct is indicated by white arrowheads. IDUS, intraductal ultrasonography; MRCP, magnetic resonance cholangiopancreatography.
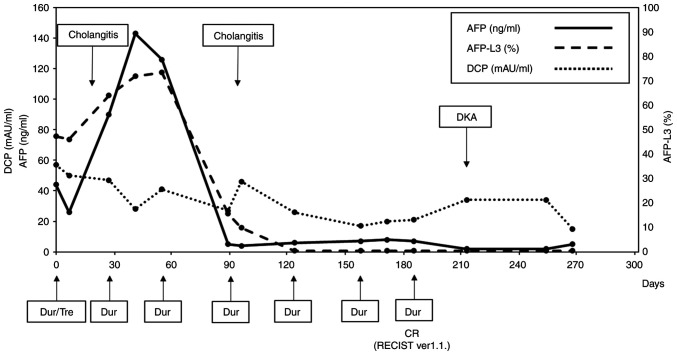
After treatment with tremelimumab (300 mg) and durvalumab (1500 mg), durvalumab monotherapy was administered every 4 weeks. Although serum AFP levels increased from 44 ng/ml (before treatment) to 143 ng/ml 1 month after treatment administration, the response was evaluated as a stable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) (9) guidelines. Thereafter, the serum AFP levels drastically decreased and normalized after three courses of durvalumab monotherapy. The AFP L3 isoform followed a similar course and normalized. The DCP levels also gradually normalized after treatment. The clinical course and transition of tumor marker levels are shown in Fig. 3. During treatment, the patient was hospitalized twice for acute cholangitis treatment, during which intraductal stents were replaced. Cholangiography, conducted 106 days after initiating Dur/Tre treatment, showed improvement in bile duct obstruction. Abdominal CECT showed continued shrinkage of the tumors and BDTT, confirming complete response (CR) after six courses of durvalumab monotherapy according to RECIST (version 1.1). The patient presented to our emergency unit with a 4-day history of appetite loss, vomiting, and impaired consciousness 215 days after the first Dur/Tre treatment. Blood tests revealed hyperglycemia (987 mg/dl), severe metabolic acidosis (pH: 6.88), and renal failure (blood urea nitrogen, 71.5 mg/dl; serum creatinine, 3.05 mg/dl). The patient was diagnosed with diabetic ketoacidosis (DKA) induced by an ICI and was treated with fluid resuscitation and intravenous insulin administration in the intensive care unit. The patient was discharged home with insulin injection therapy for ICI-induced type 1 diabetes mellitus (T1DM) and was followed up without any systemic chemotherapy for HCC.
Figure 3.
Tumor marker levels and clinical events during Dur/Tre treatment. Dur/Tre, tremelimumab plus durvalumab; Dur, durvalumab; CR, complete response; RECIST, Response Evaluation Criteria in Solid Tumors; AFP, α-fetoprotein; AFP-L3, α-fetoprotein L3 isoform; DCP, des-γ-carboxy prothrombin; DKA, diabetic ketoacidosis.
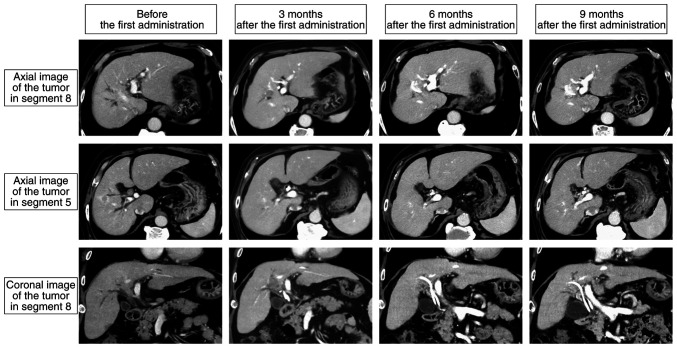
The patient is currently being followed up as an outpatient in a drug-free state 290 days after the initial administration of Dur/Tre without any sign of recurrence and other new adverse events (Fig. 4).
Figure 4.
Computed tomography findings during tremelimumab plus durvalumab treatment. Two intrahepatic tumors and a bile duct tumor thrombus shrank and completely disappeared 6 months after the first treatment administration. At 9 months after the first administration, there were no signs of recurrence.
Discussion
We present a rare case demonstrating a significant response to Dur/Tre treatment in HCC with BDTT. Although serum AFP levels increased after the first administration of Dur/Tre, the tumor size remained unchanged at the first CECT evaluation. Subsequently, serum AFP levels drastically decreased to normal levels. Intrahepatic tumors and BDTT shrank following the tumor markers and ultimately became undetectable. To the best of our knowledge, this is the first HCC case with BDTT to achieve ‘clinical CR’ (10) using Dur/Tre without sequential local therapy. Despite Dur/Tre exhibiting an objective response of 20.1% and a progressive disease rate of 39.9%, respectively, which may appear inferior to those reported for Ate/Bev (objective response rate: 27.3%, progressive disease rate: 19.6%) (2,4), Dur/Tre demonstrated superiority in overall survival's long-tail effect owing to the synergistic action of anti-CTLA4 and anti-PD-L1 agents (11). Moreover, Dur/Tre is preferable for patients at risk of bleeding or proteinuria, conditions for which bevacizumab, a VEGF inhibitor, might be contraindicated (12). In this specific case, owing to the bleeding risk from the BDTT post-biopsy, Dur/Tre emerged as an ideal first-line option over Ate/Bev.
ICIs play an important role in enhancing the cancer immunity cycle (13), leading to various immune-related adverse events (irAEs). In the HIMALAYA trial, grade 3 or 4 irAEs occurred in 12.6% of the patients (4), but the incidence of ICI-induced T1DM in patients who received Dur/Tre for HCC remains unknown. The occurrence of ICI-induced T1DM is exceptionally rare, with a reported frequency of approximately 1% after administration of ICIs (14). In a clinical trial of lung cancer using tremelimumab, durvalumab, or both, T1DM was observed in a few patients (15). Although the mechanism underlying ICI-induced T1DM is not yet fully understood, it may involve unintended immune responses against pancreatic islets. ICI-induced T1DM occurs several weeks to months after the initiation of ICI, and the onset is exceptionally rapid due to DKA; therefore, careful glucose level monitoring becomes mandatory (14). In this case, it might be difficult to re-introduce Dur/Tre or change the regimen in the event tumor progression emerges. Long-time follow-up for other potential irAEs is also required.
Bile duct invasion is associated with malignant features such as portal vein invasion, intrahepatic metastasis, poor tumor differentiation, and gross classification (6,7). Yeh et al (16) demonstrated that silencing of the microRNA-200 family was related to BDTT via ZEB1-mediated epithelial-to-mesenchymal transition. Recently, Xu et al (17) demonstrated that tumor-initiating cells expressing high BMI1 induced trans-intrahepatic biliary epithelial migration via secreting lysosomal cathepsin B. Evidence regarding the pathophysiology of HCC with BDTT is currently limited, especially regarding the efficacy of systemic chemotherapy, including ICIs. Among a cohort of 10 patients with HCC and BDTT treated with sorafenib, the objective response and disease control rates were 20.0 and 70.0%, respectively. The overall survival and time to progression of patients with HCC and BDTT were comparable to those of patients without BDTT (18). Another cohort study of patients with HCC ineligible for the REFLECT trial demonstrated more promising outcomes with lenvatinib treatment, revealing an 85.7% objective response rate and a 100% disease control rate for patients with BDTT (19). In the IMbrave150 trial, patients with high-risk statuses, including tumor invasion of the main trunk of the portal vein, tumor occupancy of ≥50%, and bile duct invasion, were treated with Ate/Bev. They had an overall survival of 7.6 months and an objective response rate of 25.0% (3). Although there have been reports of HCC cases with portal vein tumor thrombus achieving CR with Ate/Bev (20,21), reports regarding CR to HCC with BDTT are lacking. Robust analyses with larger sample sizes are needed to elucidate the biological features and identify suitable treatment strategies for HCC with BDTT.
In conclusion, we reported a rare case of HCC with BDTT exhibiting a significant response to Dur/Tre treatment, accompanied by severe irAEs in the form of ICI-induced T1DM. Although long-term and further careful observations are required, the present findings may have a substantial impact on the selection of systemic chemotherapy for advanced HCC.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitor
- Ate/Bev
atezolizumab plus bevacizumab
- PD-L1
programmed cell death-ligand 1
- VEGF
vascular endothelial growth factor
- Dur/Tre
tremelimumab plus durvalumab
- BDTT
bile duct tumor thrombus
- CT
computed tomography
- CECT
contrast-enhanced CT
- AFP
α-fetoprotein
- DCP
des-γ-carboxy prothrombin
- RECIST
Response Evaluation Criteria in Solid Tumors
- CR
complete response
- DKA
diabetic ketoacidosis
- T1DM
type 1 diabetes mellitus
- irAE
immune-related adverse events
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
NI and SK conceptualized the case report. NI wrote the manuscript and performed additional data analysis. SK, MK, HG, KF, TU, TY, KA, HY, HT and TF were involved in the treatment and follow-up in this case. MK, HG, KF, TU, TY, KA, HY and HT critically revised the manuscript and provided valuable feedback. TF provided supervision and approved the final manuscript for publication. NI and SK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent for publication of individual data and any accompanying images was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 3.Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni EN, et al. Tremelimumab plus durvalumab in unresected hepatocellular carcinoma. N Eng J Med Evid. 2022;1:EVIDoa2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 5.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado A, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al. BCLB strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikenaga N, Chijiwa K, Otani K, Ohuchida J, Uchiyama S, Kondo K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492–497. doi: 10.1007/s11605-008-0751-0. [DOI] [PubMed] [Google Scholar]
- 7.Wu JY, Sun JX, Wu JY, Huang XX, Bai YN, Wei YG, Zhang ZB, Zhou JY, Cheng SQ, Yan ML. Impact of bile duct tumor thrombus on the long-term surgical outcomes of hepatocellular carcinoma patients: A propensity score matching analysis. Ann Surg Oncol. 2022;29:949–958. doi: 10.1245/s10434-021-10799-0. [DOI] [PubMed] [Google Scholar]
- 8.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6:325–36. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenheauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1.) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 10.Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, Takita M, Hagiwara S, Minami Y, Ida H, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: A multicenter proof-of-concept study. Liver Cancer. 2023;12:321–338. doi: 10.1159/000529574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DWM, Lim HY, Yau T, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M. Current therapeutic strategies for hepatocellular carcinoma in Japan. Liver Cancer. 2023;12:497–509. doi: 10.1159/000534304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers. 2020;12:1089. doi: 10.3390/cancers12051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho YK, Jung CH. Immune-checkpoint inhibitors-induced type 1 diabetes mellitus: From its molecular mechanisms to clinical practice. Diabetes Metab J. 2023;47:757–766. doi: 10.4093/dmj.2023.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G, Hussein M, Lim FL, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: The phase III POSEIDON study. J Clin Oncol. 2023;41:1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeh TS, Wang F, Chen TC, Yeh CN, Yu MC, Jan YY, Chen MF. Expression profile of microRNA-200 family in hepatocellular carcinoma with bile duct tumor thrombus. Ann Surg. 2014;259:346–354. doi: 10.1097/SLA.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 17.Xu LB, Qin YF, Su L, Huang C, Xu Q, Zhang R, Shi XD, Sun R, Chen J, Song Z, et al. Cathepsin-facilitated invasion of BMI1-high hepatocellular carcinoma cells drives bile duct tumor thrombi formation. Nat Commun. 2023;14:7033. doi: 10.1038/s41467-023-42930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka T, Kuzuya T, Ishigami M, Ito T, Ishizu Y, Honda T, Ishikawa T, Fujishiro M. Efficacy and safety of sorafenib in unresectable hepatocellular carcinoma with bile duct invasion. Oncology. 2020;98:621–629. doi: 10.1159/000507051. [DOI] [PubMed] [Google Scholar]
- 19.Sho T, Suda G, Ogawa K, Shigesawa T, Suzuki K, Nakamura A, Ohara M, Umemura M, Kawagishi N, Natsuizaka M, et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol Res. 2020;50:966–977. doi: 10.1111/hepr.13511. [DOI] [PubMed] [Google Scholar]
- 20.Komatsu S, Fujishima Y, Kido M, Kuramitsu K, Goto T, Yanagimoto H, Toyama H, Fukumoto T. Significant response to atezolizumab plus bevacizumab treatment in unresectable hepatocellular carcinoma with portal vein tumor thrombus: A case report. BMC Gastroenterol. 2021;21:470. doi: 10.1186/s12876-021-02053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurisaki K, Soyama A, Hara T, Matsushima H, Imamura H, Tanaka T, Adachi T, Ito S, Kanetaka K, Hidaka M, et al. Pathologic complete response after chemotherapy with atezolizumab plus bevacizumab for hepatocellular carcinoma with tumor thrombus in the main portal trunk. Dig Surg. 2023;40:84–89. doi: 10.1159/000529405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.