Abstract
Rectal cancer predominantly affects patients older than 70 years, with peak incidence at age 80 to 85 years. However, the standard treatment paradigm for rectal cancer oftentimes cannot be feasibly applied to these patients owing to frailty or comorbid conditions. There are currently little information and no treatment guidelines to help direct therapy for patients who are elderly and/or have significant comorbidities, because most are not included or specifically studied in clinical trials. More recently various alternative treatment options have been brought to light that may potentially be utilized in this group of patients. This critical review examines the available literature on alternative therapies for rectal cancer and proposes a treatment algorithm to help guide clinicians in treatment decision making for elderly and comorbid patients.
Introduction
Modern day treatment for locally advanced rectal cancer (LARC) (T3 or higher or lymph node positive) in the United States historically consists of neoadjuvant chemoradiation (CRT), followed by radical resection of the tumor and adjuvant chemotherapy. The current treatment paradigm has evolved remarkably from surgical management alone, which led to unacceptably high rates of local recurrences (1–3). Studies in the 1970s and 1980s investigated the role of preoperative or postoperative radiation therapy (RT) in the treatment of rectal cancer and found that inclusion of RT on average decreased the rates of local recurrence by 50% in the absence of total mesorectal excision (TME) (4–10). The addition of concomitant 5-fluorouracil (5-FU) with RT in the preoperative or postoperative setting further reduced the likelihood of recurrence and enhanced the tumoricidal effect (11–16). The seminal German Rectal Cancer Study Group trial addressed the question of preoperative versus postoperative CRT and found several improvements in clinical outcomes with preoperative CRT, including decreased rate of local recurrence, fewer acute and late toxicities, and higher rate of sphincter-preserving surgery in patients initially requiring abdominoperitoneal resection, which helped establish the commonly accepted standard of care of neoadjuvant CRT (17, 18).
Although these findings formed the basis of preoperative CRT as the standard of care for LARC, it is widely recognized that adherence to such a regimen is oftentimes impractical in elderly patients, who may have suboptimal functional status or comorbidities. Rectal cancer predominantly affects patients older than 70 years, with peak incidences in the 80- to 85-year-old age group (19). Yet patients in this age group were underrepresented in the majority of trials that shaped the current treatment paradigm for LARC. Understandably, these trials were designed to maximize treatment efficacy with aggressive therapy in patients with high performance status (PS), but PS may be compromised in the elderly or comorbid population. Thus, one purpose of this review is to underscore the dilemma in the management of LARC in the elderly population—a difficult balancing act between oncologic outcomes and treatment-associated morbidity and mortality. Although several geriatric assessment and prognostic tools can be helpful in predicting the tolerance of elderly patients receiving antineoplastic therapies, there is little information to guide adapted treatment for rectal cancer in this population (20–27). Therefore, it is the goal of this critical review to explore the various alternative therapeutic options for elderly patients regarding LARC management. We also believe that the evidence presented here potentially could be applied to other ill patients with significant comorbidities, and will henceforth refer to the population group as elderly/comorbid patients (ECPs). Finally, owing to the paucity of elderly data regarding alternative therapies for LARC, as well as the extensive extrapolation required to bridge the gap between available literature and applicability in the ECPs, we have divided this review into several sections to highlight the unmet needs of the ECP population with rectal cancer.
Assessment and Issues Relevant to ECPs
Understanding the unique needs of ECPs with rectal cancer is the first critical step for providing optimal care for these patients. One challenge of treating ECPs with LARC is the heterogeneity within this population, with wide spectrums of tolerability for and willingness to accept each given available treatment modality. We advocate for comprehensive pretreatment evaluations of ECPs with LARC to assess their fitness level to better guide treatment. The classification of patient fitness as described below will also form the basis of our proposed treatment algorithm. We also encourage treating physicians to respect the wishes and priorities of the ECPs when formulating treatment plans through the concept of shared decision making.
Comorbidity assessment in ECPs
Anticancer treatment is a double-edged sword: although it may provide cure or palliation, it may often cause a decline in the patient’s overall health or PS. Although otherwise healthy individuals with sufficient functional organ reserve can recover from the toxicities of various treatment modalities, those with pre-existing comorbidities might succumb to their adverse effects. Although comorbidities are more prevalent, on average, in older patients (19), chronological age is not an accurate predictor for treatment-related outcomes and toxicities on an individual basis.
Balducci and Extermann (20) proposed that categorizing elderly patients into 3 functional groups based on a comprehensive geriatric assessment (CGA) screening can better evaluate the balance between safety and effectiveness of treatment. These groups include fit patients who are functionally independent, who may receive the full treatment; frail patients who are candidates for only palliation; and intermediate patients in between the fit and frail groups, who may benefit from modified treatment with lower toxicity. Proposed factors for CGA include functional status, number and severity of comorbidities, socioeconomic conditions, cognitive function, emotional and mental health, medication reliance and requirement, nutritional status, presence of geriatric syndromes, and functional reserve of organs (eg, liver, kidney, bone marrow) (20, 28, 29).
The oncologic–multidimensional prognostic index is another prognostic tool that can accurately predict the 1-year mortality of older cancer patients to help guide treatment decisions. Of the 658 cancer patients aged ≥70 years prospectively enrolled, oncologic–multidimensional prognostic index scores were used to stratify the patients into low-, intermediate-, and high-risk patients, which translated into a significant divergence of 1-year mortality rate observed (2.1% vs 17.7% vs 80.8%, respectively), with high discriminatory power (21).
However, CGA is a time-consuming process, and much effort has been made to develop screening tests to identify frail patients who may benefit from full evaluation with CGA (30–32). Unfortunately, all of the proposed frailty screening methods lack either sensitivity or specificity, or both, to predict the outcomes of CGA, and the current recommendation is for all elderly cancer patients to undergo a full geriatric assessment (23).
A systemic review of studies that examined the outcomes of elderly surgical patients who underwent preoperative CGA assessment and patient-specific optimization substantiated a benefit in reducing postoperative adverse outcome (33). Studies also investigated assessment tools using various components of the CGA to predict tolerance and toxicity to chemotherapy for older cancer patients. A prospective study with 500 elderly patients with cancer generated a predictive model for risks of developing grade 3 to 5 toxicity using CGA variables, laboratory values, and tumor/treatment characteristics (26). Similarly, the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score was developed to predict the risk levels of hematologic and nonhematologic toxicities with chemotherapy in this older patient population (25).
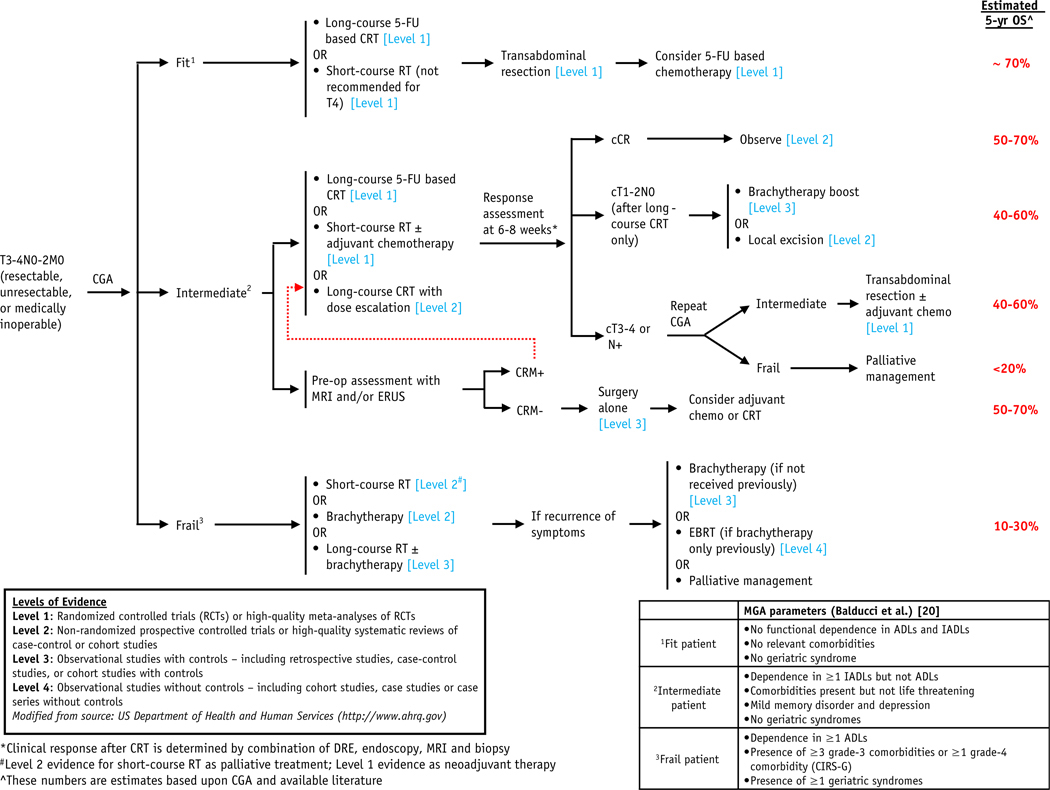
Geriatric assessment is currently still under-utilized, and the International Society of Geriatric Oncology task force recommends wider adoption by clinicians. Although there is increasing evidence of the predictive value of treatment-related risk with geriatric assessment, whether treatment adaptation guided by these predictions can improve outcome or quality of life is a question that remains to be answered. As such, we seek to better define the various treatment options to optimally manage ECPs with LARC in this review, especially for non-fit/frail patients. We proposed a classification of ECPs (fit, intermediate, and frail patients) largely based on Balducci and Extermann to help guide management; however, we acknowledge that a spectrum of patients is to be expected within each fitness class (Fig. 1, Table 1).
Fig. 1.
Proposed treatment algorithm for locally advanced rectal cancer in elderly/comorbid patients. Abbreviations: 5-FU = 5-fluorouracil; ADLs = activities of daily living; cCR = clinical complete response; CGA = comprehensive geriatric assessment; CIRS-G = Cumulative Illness Rating Scale–Geriatric; CRM = circumferential resection margins; CRT = chemoradiation; EBRT = external beam radiation therapy; DRE = digital rectal examination; ERUS = endorectal ultrasound; IADLs = instrumental activities of daily living; MGA = multidimensional geriatric assessment; RT = radiation therapy.
Table 1.
Elements of a comprehensive geriatric assessment from Balducci et al
| Parameter assessed | Elements of the assessment |
|---|---|
|
| |
| Functional status | Performance status ADLs IADLs |
| Comorbidity | No. of comorbid conditions Severity of comorbid conditions |
| Socioeconomic conditions | Living conditions Presence and adequacy of a caregiver |
| Cognitive function | Folstein’s mini-mental status |
| Emotional status | Geriatric depression scale |
| Pharmacy | No. of medications Appropriateness of medications Risk of drug interactions |
| Nutritional status | Mini-nutritional assessment Body mass index Albumin, hemoglobin, transferrin |
| Geriatric syndromes | Dementia Delirium Depression Falls Neglect and abuse Spontaneous bone fractures |
Abbreviations: ADLs = activities of daily living; IADLs = instrumental activities of daily living. See reference 20.
Shared decision making
Given the complexity in optimally treating ECPs with LARC, to have the clinicians involve the patients directly in the treatment decision making is of paramount importance (34). Patients and physicians oftentimes have different preferences in the treatment options for rectal cancer. Significantly more patients with LARC are willing to trade survival or disease control for quality of life than clinicians, who tend to favor more aggressive treatments (35, 36). In reality, however, treating physicians often overlook the priorities and wishes of their patients. A systemic review demonstrated low rates of shared decision making in consultations across all specialties (37). Additionally, a patient value study from The Netherlands showed that in a mere 18% of rectal cancer consultations did physicians explicitly consider the patient’s values or treatment preferences. If patients’ values were considered, the study showed increased patient-perceived involvement in the treatment decision process (38). Another study from the same Dutch group observed that only 3% of rectal and breast cancer consultations conducted discussion on treatment decision with the patients (39).
Current evidence suggests that shared decision making is an under-utilized concept. Therefore, it is important to emphasize the need for frank discussion of various treatment options for LARC, with the associated risks and benefits, to allow the ECPs to make informed treatment decisions.
Data on LARC Management With Background from Non-ECPs and Supporting Data Specific to ECPs
As mentioned earlier, treatment outcome data from various treatment modalities for LARC are lacking in the ECP population. In this section, we aim to discuss specific management strategies that stem from studies involving younger cohorts, with supporting data on treatment tolerability and efficacy in the ECPs. The available elderly data in this section help shape our understanding on how we may apply the following treatment regimens in the ECPs.
Surgery alone
Radical surgery remains the cornerstone for curative therapy in LARC (T3–4, N0–2, M0). The advent of TME has significantly improved local control with surgery alone and is the standard surgery for rectal cancer (40). However, an epidemiologic study showed that less-radical surgeries were performed for patients older than 70 years, likely attributable to known elevated risk of postoperative complications in the elderly, as well as patient or clinician preference (41). Nevertheless, for ECPs who are surgically fit, TME with curative intent alone is a viable option.
Trimodality therapy for LARC aims to optimize local and distant control of disease. However, treatment mortality and morbidity were shown to be higher in CRT compared with surgery alone in elderly patients (42, 43). Interestingly, studies have implicated that TME alone may result in excellent outcomes in select patients with LARC. Frasson et al (44) reported a 5-year local recurrence rate of 9.5% from an institutional cohort of 152 patients (mean age, 70 years) with cT2N+ or cT3N0/N+ rectal cancer who underwent TME alone without neoadjuvant or adjuvant therapies. Furthermore, patients preoperatively staged with free margin >2 mm from mesorectal fascia using MRI or endorectal ultrasound had a 5-year local recurrence rate of 5.4%, compared with 19.4% in those with threatened circumferential resection margins (CRM) (44). Indeed, CRM remains to be a significant prognostic factor for local recurrence, distant metastases, and overall survival in rectal cancer, and hence the need for neoadjuvant therapy for tumors involving the CRM (45, 46). A United Kingdom single-institutional study also demonstrated remarkable local control in patients with TME alone without residual disease, reporting an overall local recurrence rate (mean follow-up of 8.7 years) of 7.5% in Dukes C (lymph node-positive) patients (47). The local control rates from these retrospective data are remarkably comparable to those from large randomized trials using neoadjuvant radiation therapy or CRT, suggesting feasibility of TME alone in LARC patients with uninvolved CRM.
In contrast to the younger cohort, older patients who underwent TME surgery did not seem to have improved overall survival (OS) compared with non-TME surgery, owing to increased perioperative mortality, yet cancer-specific survival remained excellent (19, 42, 48, 49). Analyses from the Dutch TME trial and Comprehensive Cancer Centre registry showed that 1-month and 6-month postoperative mortality in patients aged ≥75 years were 4.5% to 7.8% and 14% to 16%, respectively, compared with 0.8% to 2.5% and 3.3% to 3.9% in those younger than 75 years (48). Furthermore, a systemic review of 28 studies with a total of 34,194 patients who underwent colorectal surgeries concluded that incidence of postoperative morbidity and mortality increased progressively with advanced age. When comparing patients with age groups of <65 years, 65 to 74 years, 75 to 84 years, and 85+ years, the median postoperative mortality rates reported were 3.0%, 6.4%, 8.6%, and 19.4%, respectively. Incidence of postoperative morbidities from anesthesia, such as respiratory complications, cardiovascular complications, cerebrovascular accidents, thromboembolism, and memory decline, all significantly increased with age (50, 51). Although not yet widely adopted, preoperative CGA assessment and appropriate interventions based on each patient’s pre-existing comorbidities may improve postoperative outcomes in elderly patients (33).
Despite potentially increasing the morbidity and mortality of surgery for ECPs, open surgery remains the standard surgical technique for TME. Laparoscopic surgery for rectal cancer is gradually gaining awareness, given its better tolerability and faster recovery demonstrated in colectomy. A laparoscopic approach for TME surgery was recently shown in the COLOR II trial in patients with mean age of 66 years to have decreased blood loss, sooner return of bowel function, and shorter hospital stay compared with open surgery, without compromising locoregional control or survival for rectal cancer >2 mm from endopelvic fascia (52, 53). A single-institution, prospective, randomized trial demonstrated decreased surgical morbidity rate in elderly (age ≥70 years) patients undergoing laparoscopic surgery for colorectal cancer compared with open surgery (20.2% vs 37.5%, P = .01) (54). Furthermore, a case-matched control study showed that, despite octagenarians (age ≥80 years) having expected higher American Society of Anesthesia score than middle-aged controls (age 60–69 years), there were no significant differences in both morbidity incidence or 5-year cancer-specific survival in both cohorts (55). Although data suggest feasibility and utility of laparoscopic surgery in elderly patients, future randomized studies in ECPs with LARC comparing laparoscopic versus open surgery with specific attention to the preoperative CRM status are warranted, to better select patients for the optimal surgical technique.
In the meantime, thorough preoperative workup, CGA assessment, and frank discussion with patients regarding pros and cons of radical surgery are recommended. Surgery alone remains an appropriate option for those who are low risk for surgery with favorable CRM status. However, for patients who are fit or adequate surgical candidates, implying potential longevity, it would be reasonable to consider (neo)adjuvant therapies as well. Ultimately, discussion with the fit patients must make clear that reducing treatment morbidity by omitting preoperative RT or CRT may come with the potential cost of higher risk of local recurrence, especially if CRM are positive. For intermediate patients, surgery alone is therefore a reasonable option to provide curative therapy without adding treatment toxicity from (neo)adjuvant therapies.
CRT in elderly patients
Although CRT for rectal cancer is most known for its use in the neoadjuvant setting, many of the ECP population may not be surgical candidates. Given the aforementioned possible risks of TME surgery in the ECP population, nonoperative management (NOM) with CRT is a suitable alternative in ECPs with significant comorbidities who may not tolerate surgery, whereas preoperative CRT may be considered in fit patients.
Concurrent CRT has been shown in non-ECPs to have greater therapeutic effect compared with radiation or chemotherapy alone and is the current standard for neoadjuvant or adjuvant therapy for rectal cancer. Although the pathologic complete response (pCR) rates in these studies are in the range of 10% to 15%, this evidence substantiates the tumoricidal effect of CRT as a feasible nonoperative treatment option for ECPs (11–13).
Extrapolation of the results of several retrospective studies on CRT for elderly patients suggests that both definitive and neoadjuvant CRT are effective in ECPs. A French study that aimed at analyzing the treatment efficacy for rectal cancer in elder patients found that patients with age ≥85 years were treated preferentially with CRT and nonsurgical management, which demonstrated a 5-year OS rate of 45% and disease-free survival (DFS) rate of 65%. Although the OS in this elderly cohort was inferior (owing to complications and comorbidities) to that of the younger patients enrolled in large randomized studies, the DFS was, in fact, similar or improved (56). In terms of the biological effect of treatment, Choi et al (57) described a single-institution retrospective experience, which demonstrated that of the 160 patients treated with neoadjuvant CRT, older patients (age ≥70 years) exhibit no differences in tolerance to CRT, pCR rate, and treatment-related complications compared with younger patients (age ˂70 years). Likewise, a small series of 36 patients with age ≥70 years with rectal cancer were categorized as “fit” or “vulnerable” according to the number and severity of comorbidities, and all patients regardless of fitness were able to tolerate the full course of concurrent CRT (50.4 Gy with bolus or continuous infusion 5-FU), with similar rates of pathologic down-staging and without differences in toxicity (58). Finally, a Surveillance, Epidemiology, and End Results program analysis of 4121 elderly patients aged >75 years with LARC showed that the 5-year cancer-specific survival was best for those who underwent neoadjuvant CRT (70.4%), followed by adjuvant RT (60.4%), surgery alone (52.1%), and RT alone (27.7%) (59). Although these retrospective data are limited by the inherent bias of patient selection and inclusion of patients for whom CRT was successfully administered, they suggest that CRT remains to be an effective therapy in elderly patients who can tolerate treatment, and this likelihood of tolerance should be assessed by CGA.
Neoadjuvant CRT, however, can potentially cause greater toxicity in ECPs compared with their younger counterparts. In an unplanned subset analysis of the ACCORD12/PRODIGE2 phase 3 trial, in elderly patients (age ≥70 years) compared with those with aged <70 years, preoperative CRT led to more severe grade 3 or 4 toxicities (25.6% vs 15.8%) and more permanent stomas (33.3% vs 22.8%). The lower rate of stoma reversal in older patients was due, in part, to higher rates of anastomic fistula compared with younger patients (38.9% vs 10.9% of those who develop complications) (60). These outcomes indicate that ECPs may have lower tolerance for preoperative CRT, and thus careful selection of patients for neoadjuvant CRT and individualized tailoring of therapy are warranted to ensure treatment safety.
Concomitant CRT is effective in ECPs. Although the standard preoperative CRT consisting of RT doses of 45 to 50.4 Gy and concurrent 5-FU—based chemotherapy achieves rather low rates of pCR as mentioned previously, it forms the basis of various treatment combinations as discussed in later sections [see “CRT and observation (nonoperative management),” “CRT with dose escalation,” and “CRT with local excision”].
Brachytherapy
The 2 most common forms of brachytherapy for treatment of rectal cancer are Papillon contact X-ray 50-kV brachytherapy (CXB; also known as endocavitary brachytherapy) and high-dose-rate endorectal 192Ir brachytherapy (HDREBT). The advantage of brachytherapy is that large doses of radiation can be delivered locally for excellent tumor control, while minimizing radiation-induced side effects to adjacent organs. For early-stage disease, brachytherapy is a feasible alternative to local excision (LE). For advanced disease, brachytherapy alone can achieve high rates of local control and/or pCR and is an alternative for ECPs who cannot tolerate CRT or surgery. Brachytherapy can also be used to salvage local recurrences after NOM or in the setting of reirradiation, as detailed below.
Advanced tumors with high T stage or nodal involvement often require multimodality therapy. However, for ECPs with poor performance status who cannot tolerate chemotherapy or a full course of external beam radiation therapy (EBRT), treatment or palliation with brachytherapy may be an option, as extrapolated from surgical evidence derived from non-ECPs. Vuong et al (61) at McGill University were one of the first groups to investigate the efficacy of neoadjuvant brachytherapy for LARC. Using HDREBT to 26 Gy delivered over 4 consecutive days, Vuong et al treated 49 patients with T2 to early T4 operable tumors, followed by surgical resection after 4 to 8 weeks, and pathology revealed that 32% of patients had pCR and another 36% had only microscopic residual disease at the primary tumor site. The authors’ follow-up study, which examined 100 patients with mostly T3 tumor using the same brachytherapy regimen, resulted in 29% of patients with ypT0N0–2 and 37% with micro-foci residual disease in surgical specimens. All of the patients developed acute proctitis, but 99% of them had grade 2 proctitis (62). Preoperative brachytherapy alone for LARC may be an excellent neoadjuvant option for fit patients. More importantly, the above evidence suggests that high doses of radiation delivered locally with brachytherapy, although they may not be curative owing to involvement of lymphatic spread or extent of primary disease, can still achieve high rates of local control at the site of primary disease for LARC. This notion is supported by retrospective data from investigations of the role of brachytherapy for advanced rectal cancer for which the patients were inoperable owing to old age with poor performance status, advanced disease, or presence of metastasis. With a median age of 82 years, these studies demonstrated high rates of local tumor control (local tumor response in 85% of patients, with complete response in approximately 60%) in elderly patients who were unfit for surgery or were treated with palliative intent. Definitive treatments were delivered via HDREBT up to 36 Gy in 6 fractions or as a boost of 12 Gy after conventional CRT, and the median survivals of those treated with radical intent were 18.5 to 25 months (63, 64). Late toxicities with rectal ulcers, strictures, and fistula were reported in 8% of patients (64). Altogether, evidence suggests that frail patients with LARC who cannot tolerate surgery or CRT may benefit from brachytherapy alone for local control and symptom palliation, with acceptable toxicity.
Brachytherapy is also a nonsurgical alternative for salvaging residual disease after NOM. Sun Myint et al (65) reported a series of 83 patients (median age, 72 years) with cT2–3 rectal cancer who had residual disease (≤3 cm) after CRT/EBRT and subsequently received salvage treatment with CXB. A total CXB dose of 90 Gy was delivered in 3 fractions over 4 weeks, and clinical complete response (cCR) was achieved in 53 (63.8%) patients. Several NOM studies [see “CRT and observation (nonoperative management),” below] also successfully used brachytherapy for salvage of disease progression after initial cCR (66–68). These results demonstrate that brachytherapy is a feasible alternative to surgical management in ECPs after appropriate down-staging of disease with CRT or EBRT.
Brachytherapy may also be a preferred mode of treatment for ECPs with rectal cancer who previously received pelvic irradiation. Additional EBRT is not recommended for patients who have had pelvic irradiation because of increased risk of long-term complications in adjacent organs or tissues. As such, reirradiation using HDREBT is dosimetrically more conformal to spare previously irradiated tissues while allowing dose escalation to the tumor. Currently, available data on reirradiation with brachytherapy are limited. Chuong et al (69) reported a retrospective analysis of a small group of patients (n = 10) with median age of 74 years receiving endorectal brachytherapy after previous radiation, with 3 patients achieving at least near-complete pathologic response and none with grade ≥3 acute toxicity. Future studies investigating brachytherapy for rectal cancer in the setting of reirradiation are highly encouraged.
Brachytherapy alone can be used in frail patients with LARC for both radical and palliative treatments. It is also a viable nonsurgical option for salvage therapy. Although additional data are needed, definitive or preoperative brachytherapy are likely feasible in the setting of reirradiation in ECPs.
Extrapolation From Non-ECP Studies for Clinical Application in ECPs
A major obstacle in optimizing LARC management in ECPs is the lack of high-level evidence supporting the use of various alternative therapies in this patient cohort. Ironically, these less-invasive treatment modalities are well suited to ECPs and are intended to minimize treatment toxicity while maintaining good oncologic outcomes. In this section we endeavor to explore the various treatment regimens studied in younger/healthier cohorts and extrapolate their clinical utility to ECPs.
CRT and observation (nonoperative management)
The knowledge that CRT can achieve pCR has prompted several investigations to query whether patients with LARC can be treated with up-front CRT, with surgery reserved only for salvage therapy, also known as nonoperative management (NOM). Nonoperative management aims to avoid or delay the complications and morbidity of radical surgery for treatment of rectal cancer. Although the majority of the NOM studies enrolled or analyzed patients in the younger cohort (median age of late 50s to mid-60s), the results are very encouraging for its application in ECPs with rectal cancer (Table 2), especially for those who wish to avoid perioperative surgical complications or who are at high risk for surgery (66–68, 70–78). Moreover, a decision-analytic model from the United Kingdom using Markov chain simulation found an absolute survival benefit in fit (10.1%) and comorbid (13.5%) 80-year-old patients at 1-year after treatment when NOM was implemented instead of radical surgery, underscoring a compelling adverse effect of surgical risks in ECPs (79).
Table 2.
Chemoradiation and observation (nonoperative management)
| Authors (reference) | N | Age (y) | f/u (mo) | Stage | RT | Chemotherapy | cCR, n (%) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Habr-Gama et al (2004) (66) | 265 | Mean 54.8 (range, 25–92) | Mean 50.5 | Stage I-III, resectable | 50.4 Gy @ 180 cGy/fx | 5-FU and folinic acid | 71 (26.8) |
| Dalton et al (70) | 49 | Median 64 | Mean 25.5 | T3–4N0–2 | 45 Gy in 25 fx | Capecitabine | 6 (12.2) |
| Maas et al (71) | 192 | Mean 65 | Mean 25 | Stage I-III | 50.4 Gy @ 180 cGy/fx | Capecitabine | 21 (10.9) |
| Smith et al (72) | N/A | Median 70 | Median 28 | T2–4N0–2 | 50.4 Gy (median dose) | 5-FU or capecitabine | 32 |
| Habr-Gama et al (2013)(73) | 70 | Mean 60.2 | Median 56 | T2–4N0–2 | 54 Gy @ 180 cGy/fx | 5-FU and folinic acid | 47 (68) |
| Appelt et al (74) | 51 | Median 67 | Median 23.9 | T2–3N0–1 | 60 Gy in 30 fx + 5 Gy brachytherapy boost | Tegafur-uracil | 40 (78.4) |
| Araujo et al (75) | N/A | Median 63.6 | Median 47.7 | LARC | 45–50.4 Gy | 5-FU and leucovorin | 42 |
| Renehan et al (68) | N/A | Median 66.9 | Median 33 | Stage I-III | 45 Gy | Capecitabine | 129 |
| Response assessment (timing*) | Adjuvant chemotherapy after cCR, n (%) | LF after cCR, n (%) | DF after cCR, n (%) | Salvage after LF, n (%) | Survival (cCR cohort) (%) |
|---|---|---|---|---|---|
|
| |||||
| Proctoscopy, rebiopsy, DRE, CT A/P, chest X-ray (8 wk) | None | 2 (2.8) | 3 (4.2) | 2 (100) | 10-y OS, 97.7; 10-y DFS, 84 |
| MRI, EUA, rebiopsy, PET/CT (6–8 wk) | None | 0 | 0 | N/A | N/A |
| MRI, DRE, endoscopy, rebiopsy (6–8 wk) | 17 (81) | 1 (4.8) | 0 | 1 (100) | 2-y OS, 100; 2-yDFS, 89 |
| DRE, endoscopy, selective biopsy (4–10 wk) | 17 (53) | 6 (18.8) | 3 (9.4) | 6 (100) | 2-y OS, 97; 2-y DFS, 88 |
| DRE, proctoscopy, CEA, MRI, and/or | None | 12 (25.5) | 3 (6.4) | 11 (91.7) | 3-y OS, 94; 3-y DFS, 75 |
| PET/CT (10 wk) | |||||
| DRE, endoscopy, rebiopsy, MRI pelvis | None | 9 (22.5) | 3 (7.5) | 9 (100) | N/A |
| (6 wk) | |||||
| MRI, DRE, endoscopy (not stated) | 2 (4.8) | 8 (19.0) | 7 (16.7) | 4(50) | 5-y OS, 71.6; 5-y |
| DFS, 60.9 | |||||
| MRI pelvis, DRE, endoscopy (≥8 wk) | 8(6) | 44 (34.1) | 7 (5.4) | 37 (84.1) | 3-y OS, 96; 3-y DFS, 88 |
Abbreviations: 5-FU = 5-fluorouracil; cCR = clinical complete response; CEA = carcinoembiyonic antigen; CT A/P = computed tomography abdomen and pelvis; DF = distant failure; DFS = disease-free survival; DRE = digital rectal examination; EUA = examination under anesthesia; f/u = follow-up; fx = fractions; LARC = locally advanced rectal cancer; LF = local failure; OS = overall survival; N/A = not available; PET = positron emission tomography.
Timing of clinical response assessment after completion of chemoradiation.
In the setting of NOM, Habr-Gama et al (56) spearheaded this initiative and published their results on 265 patients with stage I-III potentially resectable adenocarcinoma of the distal rectum treated with neoadjuvant 5-FU, leucovorin, and 50.4 Gy of radiation concurrently. Patients were re-evaluated with endoscopic examination after 8 weeks from completion of CRT; those with gross residual disease or ulcer underwent immediate radical surgery, whereas those with cCR underwent strict observation. Seventy-one patients (26.8%) achieved cCR with CRT alone, with an overall recurrence rate of 7.0% (n = 5) at a mean follow-up period of 57.3 months. Of the 5 recurrences, 2 patients with local recurrence were salvaged with brachytherapy and LE. Interestingly, 8% of patients with incomplete responses who under-went surgery had pCRs. When comparing the observation cohort with cCR versus those with pCR after surgical resection, the 5-year overall survival was 100% and 88%, and DFS was 92% and 83%, respectively, with patients in the observation arm having similar, if not better, outcomes (66). Although the mean age of this cohort was only 54.8 years, these favorable results speak to the possibility of avoiding surgery in ECPs achieving cCR after CRT. Several studies, thereafter, reported similarly favorable outcomes of NOM, further providing credence for NOM as an alternative in ECPs (68, 71, 72).
Although NOM is an attractive alternative to surgical resection in the ECPs, careful selection of those who are likely to have true cCR is critical (76). Moreover, there is a considerable risk for patients with cCR to still harbor microscopic disease in the rectal wall and/or mesorectum and therefore remain at risk for developing local recurrence. An important question, consequently, is whether local recurrences after NOM can be effectively salvaged without compromising oncologic outcomes. Although most of the NOM studies demonstrate that most local recurrences were salvaged, albeit with limited follow-up data, this specific question was investigated only recently in a study by Habr-Gama et al (67). The study enrolled a total of 183 patients (mean age, 58 years) with T2–4N0–2 locally advanced distal rectal cancer who were treated with CRT (50.4–54 Gy RT with 5-FU-based chemotherapy), and 90 patients (49%) achieved cCR after 8 weeks from treatment completion. Of the cCR patients, 28 (31%) experienced local recurrences, with the majority of the recurrences developing within 12 months, and 26 patients underwent salvage therapy (mostly with TME surgery), with an overall salvage rate of 93%. The 5-year cancer-specific survival and DFS of the entire cCR cohort was 91% and 68%, respectively, and the 5-year local recurrence-free survival was 94% after salvage. For the 26 patients who were salvaged after local recurrence, the 3-year cancer-specific survival and DFS were 88% and 78%, respectively (67). This study demonstrated that salvage after NOM is effective without jeopardizing patient outcomes and can be applied to ECPs.
Altogether, the various studies on NOM have validated, albeit in younger patients, its feasibility and effectiveness as an alternative treatment option to standard of care for ECPs, with emphasis on minimizing treatment-related mortality and morbidity, which are particularly vital considerations when treating intermediate or frail patients.
CRT with dose escalation
While achieving cCR or pCR with CRT alone for LARCis the ideal outcome, the majority of patients, unfortunately, would be expected to have an incomplete clinical response (80). One contributing factor is the limited radiation dose with pelvic RT that can be safely delivered to the rectum and pelvis to avoid bowel toxicity. Patients with incomplete response after CRT can benefit from TME surgery as per standard of care. However, for ECPs not amenable to surgery, radiation boost with brachytherapy and/or EBRT can be used to increase local control of the disease to avoid surgery.
Numerous studies have evaluated the radiation dose—response effect on rectal cancer (Table 3) (74, 81–88). A higher radiation dose of 45 Gy has been identified as an independent factor for rectal tumor achieving pCR (84). Several phase 2 dose escalation studies have shown that pCR rates with doses from 46 to 50.4 Gy are significantly higher than that with <40 Gy (85–87). Moreover, modeling of radiation dose—response effect demonstrated that radiation dose of 92 Gy (equivalent dose in 2 Gy per fraction) is required to achieve pCR, whereas 72.1 Gy is required for major response, in 50% of patients (88).
Table 3.
Chemoradiation with dose escalation
| Authors (reference) | Accrual year | N | Median age (range) (y) | Median f/u (mo) | Stage | RT | Chemo | pCR (%) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Jakobsen et al (2006) (81) | N/A | 50 | 61 | N/A | T3N0–2 | 60 Gy @ 1.8–2 Gy/fx + 5 Gy ERB boost | Tegafur-uracil + leucovorin | 27 |
| Jakobsen et al (2012) (82) | 2005–2010 | 120 | 64 (38–78) | N/A | T3–4, resectable | 50.4 Gy in 28 fx + 10 Gy in 2 fx ERB boost | Tegafur-uracil + leucovorin | 18 |
| Appelt et al (74) | 2009–2013 | 51 | 67 | 23.9 | T2–3N0–1 | 60 Gy in 30 fx + 5 Gy ERB boost | Tegafur-uracil | cCR 78.4 |
Abbreviations: ERB = endorectal brachytherapy; pCR = pathologic complete response. Other abbreviations as in Table 2.
Jakobsen et al (81) published one of the first studies that increased the radiation dose in concurrent CRT even higher, with dose escalation of the EBRT boost plus a brachytherapy boost. By treating the pelvis to 48.6 Gy and boost to gross disease to 60 Gy with concurrent tegafur-uracil, followed by a single 5-Gy endorectal brachytherapy boost to tumor bed, 27% of the 48 patients with T3 rectal cancer had pCR, and another 27% had only microscopic tumor left after resection. The median age of the study cohort was 61 years, and only 6% of the patients had grade 3 nonhematologic toxicities. The same group later conducted a randomized trial comparing the standard CRT regimen of 50.4 Gy in 28 fractions with or without endorectal boost with brachytherapy (10 Gy in 2 fractions). Although the overall pCR rate was equivalent at 18% between the 2 arms, dose escalation with brachytherapy resulted in a significant increase in the rate of major tumor response (44%) compared with CRT alone (28%), including both complete response and residual microscopic disease. Toxicity rates between the 2 arms were shown to be similar (82). Outcomes and tolerability reported from these studies support the use of dose escalation and may be extrapolated to ECPs.
Using a similar dose-escalated regimen as Jakobsen et al, a Dutch study treated 51 patients with low-lying resectable rectal cancer (T2-T3, N0-N1) with 50 Gy to the pelvis and 60 Gy to the gross tumor using intensity modulated RT with concomitant boost, followed by a 5-Gy endorectal high-dose-rate brachytherapy boost with concurrent tegafur-uracil. Forty patients (78%) were found to have cCR and subsequently underwent observation, and the local recurrence rate at 1 year was 15.5% for the observation group. Interestingly, the cCR rate in this study seems to be higher than those of other NOM studies, potentially as a consequence of dose escalation, but it may also be due to a higher proportion of smaller tumors. The major late toxicity noted in this study was rectal bleeding, which occurred in roughly 80% of patients, although most cases were mild (<10% with grade 3 bleeding) (74). Although enrolled patients had a median age of 67 years, this regimen provides an opportunity to increase the rate of cCR and reduce the potential need for surgery in ECPs.
Dose escalation can lead to more tumor cell killing and greater tumor regression; but more importantly, to achieve an optimal balance between tumor control and treatment toxicity is crucial. Brachytherapy seems to be a feasible modality for delivering higher doses of radiation to improve local control while minimizing toxicity. The absence of increase in toxicity with dose escalation in the aforementioned studies is promising and supports this alternative option for ECPs. However, optimization of dose and fractionation of brachytherapy or EBRT boost done specifically in the elderly cohort may further refine this treatment concept to ECPs (similar to the HERBERT study discussed below in “EBRT with brachytherapy”).
CRT with local excision
Local excision is an acceptable alternative to TME surgery for early node-negative rectal cancer, such as select T1N0 tumors (size <3 cm, well to moderately differentiated, involving <30% of rectal circumference, and no lympho-vascular space invasion), because the survival outcome with minimally invasive surgery is similar to that of radical resection, albeit with higher rates of local recurrence (89–91). The benefit of LE, namely transanal excision and transanal endoscopic microsurgery, include organ preservation, lower rates of surgical morbidity, and improved quality of life. However, LE is not sufficient for T2 tumors or T1 cancers with high-risk features, owing to higher risk of local recurrence, higher risk of lymph node dissemination, and inferior survival outcome (92–94). As such, TME surgery is the standard of care for T2 rectal cancer, whereas T3/T4 cancers often require the addition of neoadjuvant or adjuvant therapy. However, TME is associated with higher risk of long-term complications and postoperative mortality, especially in older patients (49). Alternatively, several retrospective and prospective studies have shown that neoadjuvant CRT before LE for T2 to T3 (mostly node-negative) rectal cancer can achieve similar local control compared with TME and therefore is a feasible treatment option for ECPs with these tumors to minimize surgical complications (Table 4) (95–101).
Table 4.
Chemoradiation with local excision
| Authors or study (reference) | Accrual year | N | Age (y)* | Median f/u (mo) | Stage | RT |
|---|---|---|---|---|---|---|
|
| ||||||
| Kim et al (95) | 1994–2000 | 26 | Mean 63 (44–90) | 19 | T2–3N0–1 | 45 Gy in 25 fx |
| Bonnen et al (96) | 1990–2002 | 26 | 60 | 46 | T3N0–1 | 45 Gy in 25 |
| Lezoche et al (97) | 1997–2004 | 50 | 66 (58–70) | 9.6 y | cT2N0 | fx ± boost to 52.5 Gy 50.4 Gy in 28 fx |
| Nair et al (98) | 1994–2006 | 44 | 69 (43–89) | 64 | T2–3N0–1 | 50.4 Gy in 28 fx |
| ACOSOG Z6041 (99) | 2006–2009 | 79 | 62 (30–83) | 56 | T2N0 | 54 Gy @ 180 cGy/fx |
| CARTS (100) | 2010–2012 | 55 | 64 (39–82) | 17 | cT1–3N0 | 50 Gy in 25 fx; 50.4 Gy in 28 fx |
| Chemo | LE criteria† | LR, n (%) | DM, n (%) | OS (%) | DFS (%) |
|---|---|---|---|---|---|
|
| |||||
| 5-FU | ypT0 | 1 (3.8) | 0 | N/A | N/A |
| 5-FU | N/A | 2 (7.7) | 3 (11.5) | N/A | 5-y = 80 |
| 5-FU | ypT0–2 | 4 (8) | 2 (4) | OS = 72; CSS = 89 (at end of f/u) |
N/A |
| 5-FU | N/A | 4 (9.1) | 5 (11.4) | 5-y = 81–84 | N/A |
| Cape-ox | ypT0–2 | 3 (3.8) | 5 (6.3) | N/A | 3-y = 88.2 |
| Capecitabine | ypT0–1 | 4 (7.3) | 1 (1.8) | N/A | N/A |
Abbreviations: Cape-ox = capecitabine/oxaliplatin; CR = complete response; CSS = cancer-specific survival; DM = distant metastases; LE = local excision; LR = local recurrence. Other abbreviations as in Table 2.
Age expressed as median unless otherwise noted. Values in parentheses are range.
Acceptable pathologic staging after neoadjuvant chemoradiation and local excision without recommendation for radical surgery.
A single-institution prospective study in Italy compared LE with endoluminal locoregional resection versus standard TME surgery for select T2 rectal cancers (grade 1–2, tumor size <3 cm, and within 6 cm of the anal verge) after neoadjuvant CRT. One hundred patients with median age of 66 years were randomized to receive the 2 surgical approaches, and after a median follow-up of 9.6 years the local recurrence rates of the endoluminal locoregional resection (8%) and TME (6%) arms were similar (97). Additionally, the American College of Surgeons Oncology Group published results of their multi-institutional phase 2 trial, demonstrating the efficacy of CRT followed by LE in clinically T2 rectal cancer (ACOGSOG Z6041). With 79 eligible patients (cT2N0, size <4 cm, involving <40% circumference, and located <8 cm from anal verge), 72 patients (median age, 62 years) underwent RT to 50.4 to 54 Gy with concomitant capecitabine and oxaliplatin, and the 3-year DFS for the per-protocol group was 86.9%. Of note, 3 patients who underwent LE were found to have ypT3 tumor (excluded from per-protocol analysis), and 2 of the patients underwent salvage TME (99).
In some studies, this multimodality treatment was also used in cT3 rectal cancer. In the CARTS prospective multicenter study, 47 patients (median age, 64 years) with cT1–3 distal rectal cancer underwent CRT (50 Gy or 50.4 Gy with capecitabine) followed by LE. Significant down-staging of the tumor was observed, and those with ypT2 or higher after LE were offered salvage TME. After a median follow-up of 17 months, none of the ypT0 patients had recurrence, and only 1 patient with ypT1 developed local recurrence, with organ preservation in half of patients who would have required TME surgery (100).
Studies for LE after CRT for T2-T3 rectal cancer show promising results. In the era of increasing interest in NOM or less-invasive surgeries, adopting this regimen in the ECP population would be a reasonable consideration for those who would benefit from the addition of LE, albeit with certain challenges. Currently it is unknown whether patients with cCR after CRT would benefit from additional surgery with LE. Furthermore, if surgical pathology after LE demonstrates ypT2–3 or nodal involvement, the utility of LE may be present but requires additional data. Hypothetically, patients with residual ypT1 disease may benefit the most from LE after CRT. However, determining response or degree of residual disease after CRT may be challenging, given the limitations in staging techniques such as endorectal ultrasound, MRI, CT, or positron emission tomography (102–107). Indeed, this notion is exemplified by the results of the GRECCAR-2 prospectively randomized phase 3 study, in which 148 patients (median age, 61–64 years) with T2–3N0–1 rectal cancer with good response (residual tumor ≤2 cm) after CRT (50 Gy 3-dimensional conformal pelvic RT in 2-Gy fractions with concurrent capecitabine and oxaliplatin) were randomized to receive either LE or TME. Clinical response of CRT was assessed by pelvic MRI 6 to 8 weeks afterward. Of the 73 patients in the LE arm, 34 patients had unfavorable pathological response after LE (ypT2–3 or R1 resection), for which TME was recommended, and 26 of those patients received TME. Although the study hypothesized lower morbidity and side effects with LE, the intention-to-treat analysis showed no differences in all primary outcomes (death, tumor recurrence, morbidity, and side effects), presumably owing to a significant number of patients in the LE arm receiving TME. Furthermore, of the patients in the study who were considered as clinically inadequate responders after CRT and who were nonrandomly assigned to receive TME, 39% had good pathologic responses (ypT0–1) (108). These results underscore the importance of optimizing restaging assessment and patient selection for optimal use of LE after neoadjuvant therapy. Furthermore, the results suggested that few positive lymph nodes (8%) occurred in such small irradiated tumors, indicating that completion TME could be limited to less than 10% of patients who had been down-staged to ypT2N1 and ypT3. This notion will need additional prospective evaluation.
Again, the above studies were conducted primarily in non-ECPs, but given the tolerability of the therapy and the potential to avoid TME, LE for ECPs could be a reasonable alternative when appropriately or sufficiently down-staged by CRT.
Short-course RT
Short-course RT (SCRT) before radical surgery for rectal cancer, similar to the effect of preoperative CRT, improves local control compared with surgery alone (Table 5) (109–112). Randomized trials also showed that rectal cancer patients receiving preoperative SCRT also have better local control than with postoperative CRT (113, 114). Preoperative SCRT has been shown to achieve locoregional control and OS comparable to that with standard conventional preoperative CRT. Both the Polish and the Trans Tasman Radiation Oncology Group 01.04 trials showed that SCRT (5 Gy × 5 delivered in 1 week) followed by immediate surgery can achieve outcomes similar to those with long-course CRT (50.4 Gy in 28 fractions with concomitant 5-FU) followed by surgery 4 to 6 weeks later (115, 116).
Table 5.
Short-course radiation therapy
| Study (reference) | Accrual year | N | Age (y)* | Median f/u | Stage | Study arms |
|---|---|---|---|---|---|---|
|
| ||||||
| Stockholm I (109) | 1980–1987 | 424 | Mean 69 | 107 mo | Any stage resectable |
Arm 1: 5 × 5 Gy with immediate surgery |
| 425 | Mean 67 | Arm 2: surgery alone | ||||
| Stockholm II (110) | 1987–1993 | 272 | 66 (30–80) | 8.8 y | Any stage resectable | Arm 1: 5 × 5 Gy with immediate surgery |
| 285 | Arm 2: surgery alone | |||||
| Swedish trial (111) | 1987–1990 | 585 | 69 (50–78) | 13 y | T1–3 | Arm 1: 5 × 5 Gy with immediate surgery |
| 583 | Arm 2: surgery alone | |||||
| Dutch TME trial (112) | 1996–1999 | 924 | 65 (26–88) | 12 y | Any stage resectable |
Arm 1: 5 × 5 Gy with immediate surgery |
| 937 | 66 (23–92) | Arm 2: selective postoperative RT for positive margins, 50.4 Gy in 28 fx | ||||
| Uppsala (113) | 1980–1985 | 235 | N/A | >5 y | Dukes A-C | Arm 1: 5 × 5.1 Gy with immediate surgery |
| 236 | Arm 2: selective postoperative RT for Dukes B/C, 60 Gy in 30 fx |
|||||
| MRC CR07 (114) | 1998–2005 | 674 | 65 (38–87) | 4 y | Stage I-III | Arm 1: 5 × 5 Gy with immediate surgery |
| 676 | 65 (36–87) | Arm 2: selective postoperative CRT for positive margin, 45 Gy in 25 fx + 5-FU | ||||
| Polish trial (115) | 1999–2002 | 155 | Mean 60 (30–75) | 48 mo | T3–4, resectable | Arm 1: 5 × 5 Gy with immediate surgery |
| 157 | Mean 59 (34–73) | Arm 2: 50.4 Gy in 28 fx and 5-FU/leucovorin followed by surgery at 4–6 wk | ||||
| TROG 01.04 (116) | 2001–2006 | 163 | 63 (26–80) | 5.9 y | cT3N0–2 | Arm 1: 5 × 5 Gy with immediate surgery |
| 163 | 64 (29–82) | Arm 2: 50.4 Gy in 28 fx and 5-FU followed by surgery at 4–6 wk | ||||
| Stockholm III (3-arm) (117–119) | 1998–2013 | 129 | 67 (62–74) | 5.2 y | Any stage resectable |
Arm 1: 5 × 5 Gy with immediate surgery |
| 128 | 67 (62–75) | Arm 2: 5 × 5 Gy with surgery at 4–8 wk | ||||
| 128 | 66 (61–73) | Arm 3: 25 × 2 Gy with surgery at 4–8 wk | ||||
| Stockholm III (2-arm) (117–119) | 228 | 67 (61–74) | Arm 1: 5 × 5 Gy with immediate surgery | |||
| 227 | 67 (61–74) | Arm 2: 5 × 5 Gy with surgery at 4–8 wk | ||||
| Bujko et al. (120) | 2008–2014 | 261 | 60 (54–66) | 35 mo | cT3–4 | Arm 1: 5 × 5 Gy followed by FOLFOX |
| 254 | 60 (56–65) | Arm 2: 50.4 Gy in 28 fx with concurrent 5-FU/leucovorin | ||||
| pCR (%) | Acute toxicity (%) | Late toxicity (%) | Local control | OS (%) |
|---|---|---|---|---|
|
| ||||
| N/A | 26 s 19 (P < .01) | 86 vs 72 P < .01) | 30 vs 31 (NS) | |
| N/A | 41 vs 28 (P < .01) | 88 vs 75 (P < .001) | 39 vs 36 (P = .2) | |
| N/A | 32 vs 19 (P < .01) | 56 vs 49 (P = .01) | 91 vs 74 (P < .001) | 38 vs 30 (P = .008) |
| N/A | 95 vs 89 (P < .0001) | 48 vs 49 (P = .86) | ||
| N/A | 33 vs 18 (sepsis, P < .01) | 19.4 vs 18.2 (NS) | 87 vs 78 (P = .02) | No difference |
| N/A | 35 vs 22 (nonhealing perineum) | 95.6 vs 89.4 (P < .0001) | 5 yr OS: 70.3 vs 67.9 (P = .4) 5 yr DFS: 73.6 vs 66.7 (P = .013) |
|
| 0.7 | 3.2 vs 18.2 (grade ≥3, P < .001) |
10.1 vs 7.1 (grade ≥3, P = .36) | 91 vs 85.8 (P = .17) | 67.2 vs 66.2 (P = .96) |
| 15.2 | ||||
| 1.2 | N/A | 5.8 vs 8.2 (grade ≥3, P = .53) | 92.5 vs 95.6 (P = .24) | 74 vs 70 (P = .62) |
| 14.7 | ||||
| N/A | 46.6 vs 40 vs 32 (P = .164) | 50 vs 38 vs 39 (P = .075) | 97.7 vs 96.9 vs 94.5 (P = .48) | 73 vs 76 vs 78 (P = .61) |
| N/A | ||||
| N/A | ||||
| 1.7† | N/A | 53 vs 41 (P = .001)‡ | 97.8 vs 97.2 (P = .58)‡ | N/A |
| 11.8† | ||||
| 16 | 75 vs 83 (all events, P = .006) | 20 vs 21 (NS) | 3-y: 78 vs 79 | 3-y: 73 vs 65 (P = .046) |
| 12 | ||||
Age expressed as median unless otherwise noted. Values in parentheses are range.
Pooled analysis of both 2-arm and 3-arm randomization of Stockholm III trial (20).
Pooled analysis of both 2-arm and 3-arm randomization of Stockholm III trial.
Neoadjuvant SCRT can be more convenient and with a higher compliance rate for ECPs who are surgical candidates. However, the Dutch TME subgroup analysis showed that although patients older than 75 years responded well to preoperative SCRT in terms of oncologic outcomes, it is also associated with significantly higher treatment-related mortality at 6 months compared with surgery alone (48). Another Dutch study of patients older than 75 years with T2–3N0–2M0 rectal cancer diagnosed between 2002 and 2004 reported a significant increase in postoperative complications in patients receiving preoperative RT (short or standard course) compared with those who underwent surgery alone (58% vs 42%), especially wound and deep infections (43). Although meta-analysis of trials comparing preoperative SCRT with standard CRT demonstrated large differences in rates of acute toxicity (which is largely due to patients undergoing SCRT receiving immediate surgery and bypassing the manifestation of acute radiation toxicity), rates of late toxicity between the 2 neoadjuvant regimens were similar (121). As such, patient selection for SCRT followed by surgery should be as rigorous as that for standard of care with preoperative CRT and TME surgery.
The Stockholm III trial results have broadened our knowledge of SCRT. In this multicenter, randomized, phase 3 noninferiority trial, patients with stage I-III resectable rectal cancer were randomized to 3 arms: (1) SCRT (5 Gy × 5) with immediate surgery; (2) SCRT with surgery 4 to 8 weeks thereafter; and (3) long-course RT (2 Gy × 25) with surgery after 4 to 8 weeks. After a median follow-up of 5.2 years, very few local recurrences occurred, without significant differences among the 3 arms. No difference in distant metastases was observed as well. However, when comparing SCRT with or without delay to surgery, patients who underwent delayed surgery experienced significantly lower rates of postoperative complications than those with immediate surgery (41% vs 53%). Although timing of surgery after SCRT did not affect local control of tumor, patients who underwent delayed surgery had higher rates of tumor down-staging and pCR (11.8% vs 1.7%), which was not unexpected given that there was more time for tumor regression to occur (117–119). This 1-week regimen could be applied to ECPs for purposes of convenience without apparent compromise in outcomes.
Although the Stockholm III trial demonstrated that SCRT with delayed surgery is a good alternative to conventional therapy, because it reduces treatment time while preserving effectiveness of tumor control, it also raises interesting questions that may be pertinent for ECPs who may not be able to tolerate surgery.
Is SCRT alone an effective treatment?
According to Stockholm III tumor regression data, the pCR rate 4 to 8 weeks after SCRT appears comparable to historic data with conventional CRT. Thus, it may be potentially beneficial to utilize SCRT in lieu of conventional CRT in NOM or a dose-escalation approach (see above, “CRT and observation” and “CRT with dose escalation”).
Could chemotherapy be integrated with SCRT?
In the Stockholm III trial in which patients did not receive concomitant chemotherapy with RT, and most did not receive adjuvant chemotherapy, the majority of disease recurrences were distant metastases. For patients planned for delayed surgery after SCRT, this waiting period provided an opportunity for neoadjuvant chemotherapy to be given to improve distant control of disease. Similarly, ECPs not fit for surgery may potentially benefit from chemotherapy after SCRT. In a phase 3 Polish trial, patients with fixed cT3 or cT4 rectal cancer were randomized to either: (1) SCRT (5 Gy × 5) followed by 5-FU—based chemotherapy; or (2) 5-FU—based concurrent CRT to 50.4 Gy in 28 fractions. Interestingly, SCRT with sequential chemotherapy compared with concurrent CRT demonstrated a similar R0 resection rate (77% vs 71%), pCR rate (16% vs 12%), and incidence of local failure (22% vs 21%) and distant metastases (30% vs 27%) but exhibited improved 3-year OS (73% vs 65%) and decreased rates of acute toxicity (75% vs 83%) (120). Although the primary endpoint of the study was rate of R0 resection, the overall findings can arguably be extrapolated to support the use of SCRT with sequential chemotherapy (preoperatively or definitively) as an alternative to standard CRT for ECPs.
Adjuvant local excision after SCRT
Despite that minimally invasive surgeries are potentially acceptable alternatives to radical surgeries in select cases (see above, “CRT with local excision”), current available literature does not recommend the use of LE after SCRT. A recent pilot study evaluating the efficacy of SCRT followed by LE 4 to 10 weeks after for T1-T2 rectal cancer revealed surprisingly high rates of major postoperative complications, including rectal suture dehiscence and enterocutaneous fistula, which prompted early closure of the trial (122). Although reasons are unclear, preoperative standard CRT seems to be associated with lower rates of major surgical complications after LE, without significant adverse effect on quality of life (99, 100). As such, LE should only be performed adjuvantly after standard long-course RT or CRT. For patients who received SCRT in the preoperative setting with the plan to undergo surgery, TME is recommended.
Palliation
Finally, given the convenience of the fractionation, SCRT alone can also be used in the palliative setting for symptomatic control. In a recently published phase 2 study with 18 patients with obstructive rectal cancer not amenable to curative treatment, 38.9% and 50% of patients had complete and partial symptomatic resolution after 4 weeks of completing the 5 Gy × 5 regimen, respectively. The study reported that the need for colostomy for obstructive symptoms was avoided in most patients, with 3-year colostomy-free survival of 47.6% and overall survival at 39.8% (123). Providing good local tumor responses for symptom palliation, the 5 Gy × 5 regimen is an excellent option for frail patients with advanced disease.
Adjuvant chemotherapy
The clinical benefit of adjuvant chemotherapy after definitive treatment with neoadjuvant CRT followed by surgery for LARC stage II-III has long been a controversial subject, given the lack of support with strong evidence. This is reflected in the large differences among guidelines throughout the world, ranging from National Comprehensive Cancer Network guidelines recommending adjuvant chemotherapy in stage II/III rectal cancer after receiving neoadjuvant CRT and surgery to the Dutch and Norwegian guidelines that do not recommend postoperative chemotherapy in patients who have received preoperative CRT (124). Results from large phase 3 randomized trials investigating the utility of adjuvant chemotherapy after preoperative RT (with or without preoperative chemotherapy) do not show survival benefits (125–128). In the largest of such studies to date, the European Organization for Research and Treatment of Cancer 22921 trial randomized 1011 patients with clinical T3–4 resectable rectal cancer to adjuvant chemotherapy or surveillance after preoperative RT (with or without concomitant chemotherapy) and surgery, but failed to show survival advantages in the adjuvant chemotherapy cohort compared with the surveillance cohort after a median follow-up of 10.4 years (10-year OS 51.8% vs 48.4%, P = .32; 10-year DFS 47.0% vs 43.7%, P = .29) (125). One argument for the lack of survival benefits in these trials was that the sample sizes were not large enough, but 2 independent meta-analyses of the randomized trials also failed to show significant benefit (129, 130).
Because of the limited benefit, if any, of adjuvant chemotherapy in LARC, its risks and benefits of application in ECPs need to be carefully weighed. Quality of life for patients receiving adjuvant 5-FU chemotherapy significantly deteriorated during the length of the treatment in a prospective study (131). Postoperative chemotherapy can also cause significant late adverse effects that can have a negative impact on quality of life after treatment, as described in the quality-of-life evaluation of patients treated in the European Organization for Research and Treatment of Cancer 22921 trial (132). Furthermore, a Surveillance, Epidemiology, and End Results program analysis by Lund et al (133) reported no benefit of postoperative 5-FU or capecitabine in patients with age ≥75 years in terms of reduction of all-cause or cancer-specific mortality. In line with the minimally invasive approach in NOM, such as the Habr-Gama et al series, most patients in the NOM studies did not receive adjuvant chemotherapy, although may be largely reflected by institutional practices (Table 2). However, the incremental benefit of chemotherapy after NOM remains unclear; whether further chemotherapy after CRT could prolong the disease-free interval or improve the rate of pCR are questions that remain unanswered (134). Although adjuvant chemotherapy can be offered to fit patients with frank discussion of the risks and benefits, it may be avoided in most ECPs, unless there are poor prognostic features.
Data Specific to ECPs: EBRT With Brachytherapy
As previous sections have illustrated, evidence regarding the use of most alternative therapies for ECPs with rectal cancer is lacking, but clinical application of the aforementioned treatment modalities can be extrapolated to ECPs from non-ECP studies in these disciplines. However, the combination modality using EBRT alone with brachytherapy, as discussed below, was evaluated almost exclusively in ECPs on the basis of the available literature. This is not surprising, because this regimen is only suitable for frail patients with significant comorbidities who are unfit for surgery and cannot tolerate chemotherapy owing to poor performance status.
Recent efforts in formulating alternative treatment options have shown that standard RT is feasible for elderly patients with T2 to T4 rectal cancer. In the absence of chemotherapy, locoregional control of tumor, as well as control of disseminated disease, would be compromised. Therefore, achieving comparable local control would require higher doses of radiation than the standard 45 to 50.4 Gy of EBRT. Several studies, mostly retrospective, have shown that dose escalation beyond the standard pelvic radiation with EBRT is viable using brachytherapy for attaining reasonable rates of local control (see also above, “CRT with dose escalation”) (135–140).
A Washington University experience of treating 199 patients, with a substantial number of elderly patients (median age, 71 years), with most receiving EBRT (mean 45 Gy in 25 fractions) followed by endocavitary brachytherapy (most received 60 Gy to the mucosal surface in 2 fractions 2 weeks apart) without chemotherapy, showed a local control rate of 85% for cT2 and 56% for cT3 tumors after a median follow-up of 70 months. Of the patients treated, 54 had prior endoscopic removal of macroscopic disease; 71% of patients were treated with RT alone, whereas some underwent salvage surgery afterward (135). Similarly, the French experience at Lyon Sud reported a primary local tumor control rate of 60% to 95% for T2-T3 rectal cancer in elderly patients with median age of 77 years treated with a combination of EBRT and endocavitary brachytherapy alone; approximately 27% of the patients treated were medically inoperable (136–139). Although these data are only retrospective, they provide a glimpse of a potential treatment regimen for frail patients for whom therapeutic options are otherwise limited.
The dose relationship between treatment efficacy and toxicity for HDREBT in ECPs has not been studied until recently. A Dutch group published the results of its phase 1 HERBERT study designed to identify the maximum tolerated dose, which is defined as the dose level below the dose at which 3 patients experienced grade ≥3 proctitis at <6 weeks after HDREBT. Thirty-eight patients with cT2–4N0–1M0–1 rectal cancer with a median age of 83 years were enrolled in this dose-escalation trial, and HDREBT dosed at 7 Gy per fraction per week for 3 weeks after EBRT (13 × 3 Gy) was concluded to be the maximum tolerated dose. Response occurred in 87.9% of patients eligible for response evaluation, and 60.6% of patients achieved cCR. The 2-year local progression-free survival and OS were 42% and 63%, respectively, and those with cCR showed significant improvement in local progression-free survival (60%) and trending improvement in OS (80%). However, because of high rates of severe late toxicity occurring in 10 of 32 evaluable patients with grade ≥3 toxicity (rectal bleeding, proctitis, and ulceration), the authors recommended further evaluation of risks and benefits to optimize this treatment regimen (140). At this time, if considering HDREBT boost after EBRT, clinicians would need to discuss with the patients regarding the benefit of improving tumor control weighing against the risk of late toxicity. Alternatively, endocavitary brachytherapy can be used for smaller tumors to minimize irradiated tissue to limit toxicity.
Both endocavitary brachytherapy and HDREBT can feasibly boost the radiation dose delivered to the gross disease after EBRT in frail patients, to increase tumor response. However, because of concomitant comorbidities, these patients may also experience greater a degree of adverse effects. Nevertheless, these treatment regimens may be effective for ECPs who are unable to tolerate surgery.
Conclusions
Clinicians are often faced with difficult decisions when treating LARC in ECPs, because many ECPs have medical contraindications to various aspects of the standard trimodality therapy. Optimization of the balance between oncologic outcomes and treatment morbidity/mortality in these patients is a challenge yet to be resolved. It is unlikely that large randomized studies evaluating various treatment modalities in ECPs will be conducted. Therefore, we have critically reviewed the literature on alternative therapeutic strategies that can be applied to ECPs and extrapolated pertinent findings to provide a basic treatment algorithm, presented in Figure 1. On the basis of the available evidence, we recommended the following treatment approach, depending on patient status. For fit patients without medical contraindications to therapy, the standard long-course CRT, or short-course RT with or without adjuvant chemotherapy, with standard TME will likely achieve optimal oncologic outcomes. Alternatively, for fit or intermediate patients with the goal of less-invasive therapy, NOM after CRT in the setting of cCR or minimally invasive surgery (ie, local excision) after appropriate down-staging with CRT are acceptable options as well. Select patients with negative CRM preoperatively may opt for TME alone without neoadjuvant therapies. For intermediate patients who are medically inoperable, long-course CRT followed by NOM or dose escalation with brachytherapy boost can attain good local control of the tumor. For frail patients who cannot tolerate surgery or chemotherapy, EBRT with brachytherapy boost is an alternative option. In the palliative setting, frail patients may still benefit from brachytherapy or SCRT alone for symptomatic control. Finally, clinicians should embrace the concept of shared decision making to devise treatment plans that are considerate of the patient’s values and priorities. Although these recommendations are not meant to serve as definitive guidelines, we hope to present an array of treatment options to help clinicians formulate treatment decisions for ECPs with rectal cancer.
Footnotes
An online CME test for this article can be taken at https://academy.astro.org.
Conflict of interest: none.
References
- 1.McDermott FT, Hughes ES, Pihl E, et al. Local recurrence after potentially curative resection for rectal cancer in a series of 1008 patients. Br J Surg 1985;72:34–37. [DOI] [PubMed] [Google Scholar]
- 2.Pilipshen SJ, Heilweil M, Quan SH, et al. Patterns of pelvic recurrence following definitive resections of rectal cancer. Cancer 1984; 53:1354–1362. [DOI] [PubMed] [Google Scholar]
- 3.McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis 1995; 10:126–132. [DOI] [PubMed] [Google Scholar]
- 4.Gerard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg 1988;208:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cedermark B, Dahlberg M, Glimelius B, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980–987. [DOI] [PubMed] [Google Scholar]
- 6.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638–646. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21–29. [DOI] [PubMed] [Google Scholar]
- 8.Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Medical Research Council Rectal Cancer Working Party. Lancet 1996;348:1605–1610. [PubMed] [Google Scholar]
- 9.Balslev I, Pedersen M, Teglbjaerg PS, et al. Postoperative radiotherapy in Dukes’ B and C carcinoma of the rectum and rectosigmoid. A randomized multicenter study. Cancer 1986;58:22–28. [DOI] [PubMed] [Google Scholar]
- 10.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–1304. [DOI] [PubMed] [Google Scholar]
- 11.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006;24:4620–4625. [DOI] [PubMed] [Google Scholar]
- 12.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 13.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results—EORTC 22921. J Clin Oncol 2005;23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 14.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000;92:388–396. [DOI] [PubMed] [Google Scholar]
- 15.Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med 1985;312:1465–1472. [DOI] [PubMed] [Google Scholar]
- 16.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991; 324:709–715. [DOI] [PubMed] [Google Scholar]
- 17.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 18.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 19.Rutten HJ, den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9:494–501. [DOI] [PubMed] [Google Scholar]
- 20.Balducci L, Extermann M. Management of cancer in the older person: A practical approach. Oncologist 2000;5:224–237. [DOI] [PubMed] [Google Scholar]
- 21.Brunello A, Fontana A, Zafferri V, et al. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J Cancer Res Clin Oncol 2016;142:1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giantin V, Falci C, De Luca E, et al. Performance of the Multidimensional Geriatric Assessment and Multidimensional Prognostic Index in predicting negative outcomes in older adults with cancer. Eur J Cancer Care (Engl) 2016; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol 2012;13:e437–e444. [DOI] [PubMed] [Google Scholar]
- 24.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 2013;31:3877–3882. [DOI] [PubMed] [Google Scholar]
- 25.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 26.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hathout L, Maloney-Patel N, Malhotra U, et al. Management of locally advanced rectal cancer in the elderly: A critical review and algorithm. J Gastrointest Oncol 2017; Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241–252. [DOI] [PubMed] [Google Scholar]
- 29.Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maas HA, Janssen-Heijnen ML, Olde Rikkert MG, et al. Comprehensive geriatric assessment and its clinical impact in oncology. Eur J Cancer 2007;43:2161–2169. [DOI] [PubMed] [Google Scholar]
- 31.Luciani A, Ascione G, Bertuzzi C, et al. Detecting disabilities in older patients with cancer: Comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol 2010;28:2046–2050. [DOI] [PubMed] [Google Scholar]
- 32.Kellen E, Bulens P, Deckx L, et al. Identifying an accurate pre-screening tool in geriatric oncology. Crit Rev Oncol Hematol 2010; 75:243–248. [DOI] [PubMed] [Google Scholar]
- 33.Partridge JS, Harari D, Martin FC, et al. The impact of pre-operative comprehensive geriatric assessment on postoperative outcomes in older patients undergoing scheduled surgery: A systematic review. Anaesthesia 2014;69 Suppl 1:8–16. [DOI] [PubMed] [Google Scholar]
- 34.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: Really putting patients at the centre of healthcare. BMJ 2012;344:e256. [DOI] [PubMed] [Google Scholar]
- 35.Solomon MJ, Pager CK, Keshava A, et al. What do patients want? Patient preferences and surrogate decision making in the treatment of colorectal cancer. Dis Colon Rectum 2003;46:1351–1357. [DOI] [PubMed] [Google Scholar]
- 36.Harrison JD, Solomon MJ, Young JM, et al. Patient and physician preferences for surgical and adjuvant treatment options for rectal cancer. Arch Surg 2008;143:389–394. [DOI] [PubMed] [Google Scholar]
- 37.Couet N, Desroches S, Robitaille H, et al. Assessments of the extent to which health-care providers involve patients in decision making: A systematic review of studies using the OPTION instrument. Health Expect 2015;18:542–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunneman M, Marijnen CA, Baas-Thijssen MC, et al. Considering patient values and treatment preferences enhances patient involvement in rectal cancer treatment decision making. Radiother Oncol 2015;117:338–342. [DOI] [PubMed] [Google Scholar]
- 39.Kunneman M, Engelhardt EG, Ten Hove FL, et al. Deciding about (neo-)adjuvant rectal and breast cancer treatment: Missed opportunities for shared decision making. Acta Oncol 2016;55: 134–139. [DOI] [PubMed] [Google Scholar]
- 40.Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; 133:894–899. [DOI] [PubMed] [Google Scholar]
- 41.Chang GJ, Skibber JM, Feig BW, et al. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: A population-based study. Eur J Cancer 2006;42:3015–3021. [DOI] [PubMed] [Google Scholar]
- 43.Maas HA, Lemmens VE, Nijhuis PH, et al. Benefits and drawbacks of short-course preoperative radiotherapy in rectal cancer patients aged 75 years and older. Eur J Surg Oncol 2013;39:1087–1093. [DOI] [PubMed] [Google Scholar]
- 44.Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer 2011;117:3118–3125. [DOI] [PubMed] [Google Scholar]
- 45.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008; 26:303–312. [DOI] [PubMed] [Google Scholar]
- 46.Bernstein TE, Endreseth BH, Romundstad P, et al. Circumferential resection margin as a prognostic factor in rectal cancer. Br J Surg 2009;96:1348–1357. [DOI] [PubMed] [Google Scholar]
- 47.Cecil TD, Sexton R, Moran BJ, et al. Total mesorectal excision results in low local recurrence rates in lymph node-positive rectal cancer. Dis Colon Rectum 2004;47:1145–1149. discussion 1149–1150. [DOI] [PubMed] [Google Scholar]
- 48.Rutten H, den Dulk M, Lemmens V, et al. Survival of elderly rectal cancer patients not improved: Analysis of population based data on the impact of TME surgery. Eur J Cancer 2007;43:2295–2300. [DOI] [PubMed] [Google Scholar]
- 49.Paun BC, Cassie S, MacLean AR, et al. Postoperative complications following surgery for rectal cancer. Ann Surg 2010;251:807–818. [DOI] [PubMed] [Google Scholar]
- 50.Surgery for colorectal cancer in elderly patients: A systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–974. [PubMed] [Google Scholar]
- 51.Patel D, Lunn AD, Smith AD, et al. Cognitive decline in the elderly after surgery and anaesthesia: Results from the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort. Anaesthesia 2016;71:1144–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210–218. [DOI] [PubMed] [Google Scholar]
- 53.Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372:1324–1332. [DOI] [PubMed] [Google Scholar]
- 54.Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300. [DOI] [PubMed] [Google Scholar]
- 55.Otsuka K, Kimura T, Hakozaki M, et al. Comparative benefits of laparoscopic surgery for colorectal cancer in octogenarians: A case-matched comparison of short- and long-term outcomes with middle-aged patients. Surg Today 2017;47:587–594. [DOI] [PubMed] [Google Scholar]
- 56.Moureau-Zabotto L, Resbeut M, Gal J, et al. Management and clinical outcome of rectal cancer in patients ≥ 80 years treated in southern France (PACA region) between 2006 and 2008. J Surg Oncol 2013;108:450–456. [DOI] [PubMed] [Google Scholar]
- 57.Choi Y, Kim JH, Kim JW, et al. Preoperative chemoradiotherapy for elderly patients with locally advanced rectal cancer-a real-world outcome study. Jpn J Clin Oncol 2016;46:1108–1117. [DOI] [PubMed] [Google Scholar]
- 58.Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–3923. [PubMed] [Google Scholar]
- 59.Wan JF, Zhu J, Li GC, et al. Implications for determining the optimal treatment for locally advanced rectal cancer in elderly patients aged 75 years and older. Oncotarget 2015;6:30377–30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Francois E, Azria D, Gourgou-Bourgade S, et al. Results in the elderly with locally advanced rectal cancer from the ACCOR12/-PRODIGE 2 phase III trial: Tolerance and efficacy. Radiother Oncol 2014;110:144–149. [DOI] [PubMed] [Google Scholar]
- 61.Vuong T, Belliveau PJ, Michel RP, et al. Conformal preoperative endorectal brachytherapy treatment for locally advanced rectal cancer: Early results of a phase I/II study. Dis Colon Rectum 2002; 45:1486–1493. discussion 1493–1495. [DOI] [PubMed] [Google Scholar]
- 62.Vuong T, Devic S, Podgorsak E. High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:701–705. [DOI] [PubMed] [Google Scholar]
- 63.Hoskin PJ, de Canha SM, Bownes P, et al. High dose rate after-loading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Radiother Oncol 2004;73:195–198. [DOI] [PubMed] [Google Scholar]
- 64.Corner C, Bryant L, Chapman C, et al. High-dose-rate after-loading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Brachytherapy 2010;9:66–70. [DOI] [PubMed] [Google Scholar]
- 65.Sun Myint A, Smith FM, Gollins S, et al. Dose escalation using contact X-ray brachytherapy after external beam radiotherapy as a non-surgical treatment option for rectal cancer: Outcomes from a single-centre experience. Int J Radiat Oncol Biol Phys 2018;100: 565–573. [DOI] [PubMed] [Google Scholar]
- 66.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: Long-term results. Ann Surg 2004;240:711–717. discussion 717–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014; 88:822–828. [DOI] [PubMed] [Google Scholar]
- 68.Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): A propensity-score matched cohort analysis. Lancet Oncol 2016;17:174–183. [DOI] [PubMed] [Google Scholar]
- 69.Chuong MD, Fernandez DC, Shridhar R, et al. High-dose-rate endorectal brachytherapy for locally advanced rectal cancer in previously irradiated patients. Brachytherapy 2013;12:457–462. [DOI] [PubMed] [Google Scholar]
- 70.Dalton RS, Velineni R, Osborne ME, et al. A single-centre experience of chemoradiotherapy for rectal cancer: Is there potential for nonoperative management? Colorectal Dis 2012;14:567–571. [DOI] [PubMed] [Google Scholar]
- 71.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633–4640. [DOI] [PubMed] [Google Scholar]
- 72.Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256:965–972. [DOI] [PubMed] [Google Scholar]
- 73.Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: Are we getting closer to anal cancer management? Dis Colon Rectum 2013;56:1109–1117. [DOI] [PubMed] [Google Scholar]
- 74.Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: A prospective observational study. Lancet Oncol 2015;16:919–927. [DOI] [PubMed] [Google Scholar]
- 75.Araujo RO, Valadao M, Borges D, et al. Nonoperative management of rectal cancer after chemoradiation opposed to resection after complete clinical response. A comparative study. Eur J Surg Oncol 2015;41:1456–1463. [DOI] [PubMed] [Google Scholar]
- 76.Habr-Gama A, Perez RO, Wynn G, et al. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 2010;53:1692–1698. [DOI] [PubMed] [Google Scholar]
- 77.Hughes R, Harrison M, Glynne-Jones R. Could a wait and see policy be justified in T3/4 rectal cancers after chemo-radiotherapy? Acta Oncol 2010;49:378–381. [DOI] [PubMed] [Google Scholar]
- 78.Lim L, Chao M, Shapiro J, et al. Long-term outcomes of patients with localized rectal cancer treated with chemoradiation or radiotherapy alone because of medical inoperability or patient refusal. Dis Colon Rectum 2007;50:2032–2039. [DOI] [PubMed] [Google Scholar]
- 79.Smith FM, Rao C, Oliva Perez R, et al. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: Results of a decision-analytic model. Dis Colon Rectum 2015;58: 159–171. [DOI] [PubMed] [Google Scholar]
- 80.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 81.Jakobsen A, Mortensen JP, Bisgaard C, et al. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys 2006;64:461–465. [DOI] [PubMed] [Google Scholar]
- 82.Jakobsen A, Ploen J, Vuong T, et al. Dose-effect relationship in chemoradiotherapy for locally advanced rectal cancer: A randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys 2012;84:949–954. [DOI] [PubMed] [Google Scholar]
- 83.Sun Myint A, Mukhopadhyay T, Ramani VS, et al. Can increasing the dose of radiation by HDR brachytherapy boost following pre operative chemoradiotherapy for advanced rectal cancer improve surgical outcomes? Colorectal Dis 2010;12 Suppl 2:30–36. [DOI] [PubMed] [Google Scholar]
- 84.Sanghera P, Wong DW, McConkey CC, et al. Chemoradiotherapy for rectal cancer: An updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) 2008;20:176–183. [DOI] [PubMed] [Google Scholar]
- 85.Chan AK, Wong AO, Langevin J, et al. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: A phase II dose escalation study. Int J Radiat Oncol Biol Phys 2000;48:843–856. [DOI] [PubMed] [Google Scholar]
- 86.Valentini V, Coco C, Cellini N, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: Acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys 2001;51:371–383. [DOI] [PubMed] [Google Scholar]
- 87.Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: Effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys 2006;64:709–716. [DOI] [PubMed] [Google Scholar]
- 88.Appelt AL, Pløen J, Vogelius IR, et al. Radiation dose-response model for locally advanced rectal cancer after preoperative chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013;85:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willett CG, Compton CC, Shellito PC, et al. Selection factors for local excision or abdominoperineal resection of early stage rectal cancer. Cancer 1994;73:2716–2720. [DOI] [PubMed] [Google Scholar]
- 90.Sengupta S, Tjandra JJ. Local excision of rectal cancer: What is the evidence? Dis Colon Rectum 2001;44:1345–1361. [DOI] [PubMed] [Google Scholar]
- 91.Lu JY, Lin GL, Qiu HZ, et al. Comparison of transanal endoscopic microsurgery and total mesorectal excision in the treatment of T1 rectal cancer: A meta-analysis. PLoS One 2015;10:e0141427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mellgren A, Sirivongs P, Rothenberger DA, et al. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum 2000;43: 1064–1071. discussion 1071–1074. [DOI] [PubMed] [Google Scholar]
- 93.You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: A nationwide cohort study from the National Cancer Database. Ann Surg 2007;245:726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stitzenberg KB, Sanoff HK, Penn DC, et al. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol 2013; 31:4276–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim CJ, Yeatman TJ, Coppola D, et al. Local excision of T2 and T3 rectal cancers after downstaging chemoradiation. Ann Surg 2001; 234:352–358. discussion 358–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys 2004;60:1098–1105. [DOI] [PubMed] [Google Scholar]
- 97.Lezoche E, Baldarelli M, Lezoche G, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012;99:1211–1218. [DOI] [PubMed] [Google Scholar]
- 98.Nair RM, Siegel EM, Chen DT, et al. Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg 2008;12:1797–1805. discussion 1805–1806. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): Results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verseveld M, de Graaf EJ, Verhoef C, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 2015;102:853–860. [DOI] [PubMed] [Google Scholar]
- 101.Borschitz T, Wachtlin D, Möhler M, et al. Neoadjuvant chemoradiation and local excision for T2–3 rectal cancer. Ann Surg Oncol 2008;15:712–720. [DOI] [PubMed] [Google Scholar]
- 102.Garcia-Aguilar J, Pollack J, Lee SH, et al. Accuracy of endorectal ultrasonography in preoperative staging of rectal tumors. Dis Colon Rectum 2002;45:10–15. [DOI] [PubMed] [Google Scholar]
- 103.Landmann RG, Wong WD, Hoepfl J, et al. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum 2007;50:1520–1525. [DOI] [PubMed] [Google Scholar]
- 104.Hanly AM, Ryan EM, Rogers AC, et al. Multicenter Evaluation of Rectal cancer ReImaging pOst Neoadjuvant (MERRION) Therapy. Ann Surg 2014;259:723–727. [DOI] [PubMed] [Google Scholar]
- 105.Zhao RS, Wang H, Zhou ZY, et al. Restaging of locally advanced rectal cancer with magnetic resonance imaging and endoluminal ultrasound after preoperative chemoradiotherapy: A systemic review and meta-analysis. Dis Colon Rectum 2014;57:388–395. [DOI] [PubMed] [Google Scholar]
- 106.Joye I, Deroose CM, Vandecaveye V, et al. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: A systematic review. Radiother Oncol 2014;113:158–165. [DOI] [PubMed] [Google Scholar]
- 107.Guillem JG, Ruby JA, Leibold T, et al. Neither FDG-PET nor CT can distinguish between a pathological complete response and an incomplete response after neoadjuvant chemoradiation in locally advanced rectal cancer: A prospective study. Ann Surg 2013;258:289–295. [DOI] [PubMed] [Google Scholar]
- 108.Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): A prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017;390:469–479. [DOI] [PubMed] [Google Scholar]
- 109.Cedermark B, Johansson H, Rutqvist LE, et al. The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma. A prospective randomized trial. Stockholm Colorectal Cancer Study Group. Cancer 1995;75:2269–2275. [DOI] [PubMed] [Google Scholar]
- 110.Martling A, Holm T, Johansson H, et al. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: Long-term follow-up of a population-based study. Cancer 2001;92:896–902. [DOI] [PubMed] [Google Scholar]
- 111.Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644–5650. [DOI] [PubMed] [Google Scholar]
- 112.van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–582. [DOI] [PubMed] [Google Scholar]
- 113.Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: Final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum 1993;36:564–572. [DOI] [PubMed] [Google Scholar]
- 114.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009;373:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215–1223. [DOI] [PubMed] [Google Scholar]
- 116.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012; 30:3827–3833. [DOI] [PubMed] [Google Scholar]
- 117.Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg 2010;97:580–587. [DOI] [PubMed] [Google Scholar]
- 118.Pettersson D, Lörinc E, Holm T, et al. Tumour regression in the randomized Stockholm III trial of radiotherapy regimens for rectal cancer. Br J Surg 2015;102:972–978, discussion 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): A multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18: 336–346. [DOI] [PubMed] [Google Scholar]
- 120.Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: Results of a randomized phase III study. Ann Oncol 2016;27:834–842. [DOI] [PubMed] [Google Scholar]
- 121.Chen C, Sun P, Rong J, et al. Short course radiation in the treatment of localized rectal cancer: A systematic review and meta-analysis. Sci Rep 2015;5:10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arezzo A, Arolfo S, Allaix ME, et al. Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for t1-t2 n0 extraperitoneal rectal cancer. Int J Radiat Oncol Biol Phys 2015;92:299–306. [DOI] [PubMed] [Google Scholar]
- 123.Picardi V, Deodato F, Guido A, et al. Palliative short-course radiation therapy in rectal cancer: A phase 2 study. Int J Radiat Oncol Biol Phys 2016;95:1184–1190. [DOI] [PubMed] [Google Scholar]
- 124.Poulsen LO, Qvortrup C, Pfeiffer P, et al. Review on adjuvant chemotherapy for rectal cancer - why do treatment guidelines differ so much? Acta Oncol 2015;54:437–446. [DOI] [PubMed] [Google Scholar]
- 125.Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184–190. [DOI] [PubMed] [Google Scholar]
- 126.Sainato A, Cernusco Luna Nunzia V, Valentini V, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol 2014;113:223–229. [DOI] [PubMed] [Google Scholar]
- 127.Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: A Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696–701. [DOI] [PubMed] [Google Scholar]
- 128.Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007;370:2020–2029. [DOI] [PubMed] [Google Scholar]
- 129.Bujko K, Glimelius B, Valentini V, et al. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo) therapy: A meta-analysis of randomized trials comparing surgery ± a fluoropyrimidine and surgery + a fluoropyrimidine ± oxaliplatin. Eur J Surg Oncol 2015;41:713–723. [DOI] [PubMed] [Google Scholar]
- 130.Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200–207. [DOI] [PubMed] [Google Scholar]
- 131.Chau I, Norman AR, Cunningham D, et al. Longitudinal quality of life and quality adjusted survival in a randomised controlled trial comparing six months of bolus fluorouracil/leucovorin vs. twelve weeks of protracted venous infusion fluorouracil as adjuvant chemotherapy for colorectal cancer. Eur J Cancer 2005;41:1551–1559. [DOI] [PubMed] [Google Scholar]
- 132.Tiv M, Puyraveau M, Mineur L, et al. Long-term quality of life in patients with rectal cancer treated with preoperative (chemo)-radiotherapy within a randomized trial. Cancer Radiother 2010;14:530–534. [DOI] [PubMed] [Google Scholar]
- 133.Lund JL, Sturmer T, Sanoff HK. Comparative effectiveness of postoperative chemotherapy among older patients with non-metastatic rectal cancer treated with preoperative chemoradiotherapy. J Geriatr Oncol 2016;7:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol 2015;16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: Results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys 2001;51:363–370. [DOI] [PubMed] [Google Scholar]
- 136.Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys 2002;54:142–149. [DOI] [PubMed] [Google Scholar]
- 137.Gerard JP, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol 2003;4:158–166. [DOI] [PubMed] [Google Scholar]
- 138.Gerard JP, Chapet O, Ortholan C, et al. French experience with contact X-ray endocavitary radiation for early rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:661–673. [DOI] [PubMed] [Google Scholar]
- 139.Gerard JP, Frin AC, Doyen J, et al. Organ preservation in rectal adenocarcinoma (T1) T2-T3 Nx M0. Historical overview of the Lyon Sud - nice experience using contact x-ray brachytherapy and external beam radiotherapy for 120 patients. Acta Oncol 2015;54:545–551. [DOI] [PubMed] [Google Scholar]
- 140.Rijkmans EC, Cats A, Nout RA, et al. Endorectal brachytherapy boost after external beam radiation therapy in elderly or medically inoperable patients with rectal cancer: Primary outcomes of the phase 1 HERBERT study. Int J Radiat Oncol Biol Phys 2017;98:908–917. [DOI] [PubMed] [Google Scholar]