Abstract
Background
Xylazine is a sedative found increasingly in the illicit fentanyl supply that can cause hypotension, bradycardia, necrosis and death. This pilot examined the real-world performance of BTNX xylazine test strips (XTS) in drug residue samples.
Methods
This study was nested within a drug checking service in Rhode Island. We tested unmeasured drug residue dissolved in 5 mL of distilled water using XTS and Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (LC-QTOF-MS). Analyses compared XTS and LC-QTOF-MS results to calculate XTS detection of xylazine in residue.
Results
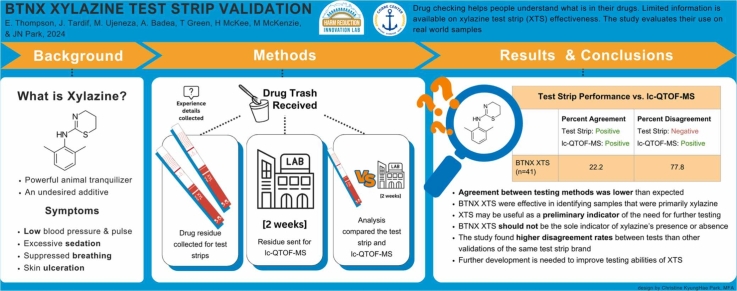
Among 41 residue samples, xylazine was detected in 11% by the XTS and 44 % by the laboratory. The LC-QTOF-MS detected xylazine in 18 samples: 4 major, 9 minor, 5 trace by volume relative to the whole sample. The XTS disagreed with the LC-QTOF-MS by indicating a negative result in 77.8 % (N=14) of the samples but never indicated a positive when the LC-QTOF-MS reported xylazine’s absence. The XTS correctly detected xylazine 22 % of the time, however, this increased to 100 % of the time if xylazine was a major active component.
Conclusions
In this study, the BTNX XTS often disagreed with LC-QTOF-MS by indicating a negative result, likely due to the dilution levels used and sample composition. The XTS may not be accurate in detecting residual amounts of xylazine, especially if xylazine is not a dominant component of the tested sample. Given the novelty of BTNX’s XTS products, we recommend XTS only be used in conjunction with other advanced drug checking modalities for residue testing.
Keywords: Harm reduction, Drug testing, Overdose, Opioids, Substance use
Graphical Abstract
Highlights
-
•
BTNX xylazine test strips effectively detect xylazine as a major sample component.
-
•
Overall detection using BTNX xylazine test strips was lower than expected.
-
•
BTNX xylazine test strips may be helpful as an indicator of need for further testing.
-
•
BTNX xylazine test strip results in street samples should be accepted with caution.
-
•
Further development is needed to improve testing abilities of xylazine test strips.
1. Introduction
People who use drugs (PWUD) rely on an unregulated, ever-changing illicit market, resulting in uncertainty of what they are using (Bhuiyan et al., 2023). The desired substance, if present, is often only a fraction of the total, with filler or undesired substances comprising the rest (Cruz et al., 2023; Żubryckaet al., 2022). Without knowing a substance's psychoactive components, PWUD are at increased risk for adverse health effects, including overdose.
Drug checking services (DCS) have existed since the 1960s, since becoming a key harm reduction strategy in many American and European countries (Renfroe, 1986; Barratt and Measham, 2022). Proponents believe understanding a substance’s contents empowers individuals to make informed decisions about when and how they use. Several technologies of varying complexity have been used in DCS including Fourier Transform Infrared Spectrometry, Quadruple Time of Flight Spectroscopy (LC-QTOF-MS), gas-chromatography, Raman spectroscopy, immunoassay test strips and reagents (Gozdzialski et al., 2023). While countless organizations distribute take-home tools such as fentanyl test strips (FTS), access to more advanced services is limited (Park et al., 2022). In 2022, only sixteen programs responded to a survey aiming to monitor advanced DCS implementation across the Americas (Park et al., 2023).
DCS and drug surveillance have confirmed the ever-changing nature of an unregulated drug market (Bhuiyan et al., 2023). Drug supply trends have transitioned several times in the past 20 years. Opioid overprescribing, illegal marketing practices, and reformulation of Oxycontin contributed to a substantial increase in opioid use, addiction, and heroin availability in the 2000s (Evans et al., 2018). By 2016, fentanyl overtook heroin as the predominantly available opioid, which stands to this day (Althoff et al., 2020; United Nations Office on Drugs and Crime & Division for Treaty Affairs, 2022). Xylazine, an alpha-2 adrenergic substance, was detected in the US drug supply intermittently from 2006 to 2018. Since 2018, xylazine has become regularly present, having increased by 1238 % as of 2021 (Alexander et al., 2022; Gupta et al., 2023; Quijano et al., 2023). Despite its prevalence, research suggests xylazine is not desired by PWUD (Spadaro et al., 2023) and is linked to severe health implications (Ball et al., 2022, Ruiz-Colón et al., 2014).
In the 1960s, xylazine was evaluated for treatment of hypertension, but never received FDA approval due to excessive sedation including reduced blood pressure and pulse (Greene and Thurmon, 1988). Today, xylazine is only approved as a large animal tranquilizer in Europe and the Americas. In 2013, Ruiz-Colón et al. reviewed 43 cases of human xylazine ingestion. They identified xylazine’s toxidrome: sedation, bradycardia, hypotension, hyperglycemia, miosis, and respiratory depression (Ruiz-Colón et al., 2014). PWUD report deep sedation lasting between 8 and 72 h after using xylazine (Ball et al., 2022, Ruiz-Colón et al., 2014). Beyond the symptoms of acute poisoning, PWUD report withdrawal, skin ulcerations, soft tissue necrosis, and infection (Bishnoi, 2023; Haymann, 2022). Lesions associated with xylazine consumption often occur on extremities regardless of administration route. The wounds may resist healing which can result in amputation (Bishnoi, 2023; Haymann, 2022). The mechanism of action and toxic threshold for health complications are unclear.
As of November 2022, xylazine was detected in confiscated drugs in 48 US states (Drug Enforcement Administration, 2022). It was increasingly seen in overdose deaths from Connecticut, Vermont, and Maryland between January 2021 to June 2022, ultimately reaching over 20 % prevalence (Kariisa et al., 2023). One surveillance project found that, despite xylazine being detected in 41.6 % of samples tested, it was never expected by the sample submitter (Collins et al., 2023). Xylazine is almost exclusively found in drug samples also containing fentanyl, suggesting that countries where fentanyl is present may be at risk of xylazine in the future (Friedman et al., 2022, Collins et al., 2023).
PWUD have expressed interest in xylazine test strips (XTS) to identify its presence (Reed et al., 2022). Early in 2023, the first XTS became available to the public market. While manufacturing companies typically report specificity and detection limits from laboratory-based testing, there are no regulatory standards to guide them. Additionally, some manufacturers only include instructions for testing urine samples or omit reporting interferent substances. Two studies recently examined the detection limit of the BTNX XTS, which the manufacturer reported as 1000 μg/mL (Sisco et al., 2023; Krotulski et al., 2023). Lack of regulation and a volatile illicit drug market indicate the importance of evaluating XTS’ accuracy and effectiveness in real-world settings. Accordingly, our aim was to explore the performance of BTNX XTS for testing drug refuse residue at a community-based DCS.
2. Materials and methods
A pilot DCS was hosted by a community drop-in center serving PWUD in Rhode Island. The research and drop-in center teams collaborated closely during protocol development and result dissemination. This study was approved by the Institutional Review Board (IRB) of Rhode Island Hospital. Further details about the methodology are provided elsewhere (Cepeda et al., 2023).
A peer outreach specialist employed by the research team known to the center's community was vital to building trust between the community and research team. They promoted DCS utilization by informing clients about submission criteria and $5 compensation per sample between operating hours.
During the pilot, we explored the performance of XTS (BTNX Inc., Ontario, Canada, lot DOA2212085, expiration 12/12/2024), the same brand of FTS commonly distributed to PWUD (Park et al., 2021). Availability of the XTS was limited due to their recent development and high demand in communities where xylazine was present. The team was able to obtain and use 41 BTNX XTS from April-May 2023.
An AB Sciex X500R SCIEX LC-QTOF-MS (Framingham, MA) housed at Rhode Island Hospital’s toxicology laboratory was used for untargeted qualitative confirmatory testing which served as the gold standard when calculating XTS agreement rates. The laboratory established its concentration detection limits for xylazine to be between 1 and 10 ng/mL, depending on solvents and sample composition. Limits were established by adapting the dilute-and-shoot method (Thoren et al., 2016, Collins et al., 2023). While the laboratory could not provide a complete picture of sample contents (i.e., absolute volumes, inactive components), they were able to derive relative amounts of the psychoactive drug component. The strongest signal psychoactive component and anything ≥30 % of its strength were considered major, anything 10 %-29 % of the strongest component was minor and anything <10 % was considered trace.
All parties participating in the studies design agreed that these cutoffs appropriately reflected what is considered significant in harm reduction messaging. Additionally, they aligned with reporting methods previously used in a state-wide drug surveillance project (Collins et al., 2023).
2.1. Testing procedures
Center clients submitted dry drug refuse with visible drug residue (i.e. empty bags, intact pipes, and once-used cookers) procured from Providence County. Sharps (i.e. needles, broken glass) were excluded for technician safety. Due to existing drug criminalization laws in the state of Rhode Island, larger quantities of drugs were not accepted.
After initial confirmation that a sample was eligible, the research assistant collected details about the sample’s origin, suspected contents, visual appearance, and physical characteristics. These details were recorded in the StreetCheck Web Application, a tool developed to standardize data collection and results communication for DCS.
No manufacturer-recommended procedures for testing drug residue with XTS existed at the time so we diluted one portion of each residue sample in 5 mL of water to align with a similar FTS residue testing protocol (Green et al., 2022). The XTS was dipped 1 cm into the resulting solution for 10–15 s then placed horizontally for 5-min before reading.
One line at the control point indicated a positive test, two indicated a negative test, and any other result would have been deemed invalid. Each result was interpreted by the research assistant and photographed for verification by a second team member to ensure accurate interpretation. XTS results were not shared with participants as this testing protocol was not yet validated.
The research assistant packaged the remaining undissolved sample after onsite testing was complete in a labeled disposable centrifuge tube and ziplock bag for confirmatory testing via LC-QTOF-MS. Refuse remaining after extracting the sample was properly disposed of. Samples were transported to the Rhode Island Hospital Toxicology Lab weekly and results were reported within 30 days via a shared spreadsheet. The lab was blinded to the research team's results. The technicians interpretation of LC-QTOF-MS results were verified by the director (AB) before being reported to donors using the Streetcheck application.
2.2. Analysis
The data were merged using a common sample ID and cleaned for analysis. Specifically, agreement rates between XTS and LC-QTOF-MS results were calculated using Stata/MP Version 16 (StataCorp, TX). We first ran the calculations using the full dataset. Given that lidocaine is a known confounder (Krotulski et al., 2023) and trace xylazine may be below the reported detection limit, the calculations were repeated excluding samples with each compound. We explored the potential contribution of the relative amount of xylazine detected on the XTS’ performance.
3. Results
Of the 41 samples included, most contained fentanyl (84 %) and/or cocaine (74 %) per LC-QTOF-MS results. Only 2 % (n=1) of submitters reported suspecting their drugs contained xylazine. The LC-QTOF-MS detected xylazine in 43.9 % of tested drug samples (n=18) of which 5 had trace amounts, 9 had minor, and the remaining 4 had major. After excluding trace amounts of xylazine, its presence fell to 31.7 % (n=13).
When comparing BTNX XTS to the LC-QTOF-MS, the XTS correctly identified xylazine in 22.2 % of the samples and correctly indicated its absence in 100 %. After removing samples that contained lidocaine, a known confounding substance, and samples with trace amounts of xylazine, the agreement between the instruments rose slightly to 25.0 % for xylazine-positive samples and fell to 75.0 % for xylazine-negative ones. However, if xylazine was a major component of the sample, the agreement rose to 100 % (Table 1).
Table 1.
Performance of BTNX xylazine test strips compared to laboratory testing using LC-QTOF-MS.
| BTNX XTS |
Positive XTS and LC-QTOF-MS (agreement)a |
Negative XTS and Positive LC-QTOF-MS (disagreement) |
||
|---|---|---|---|---|
| n | % | n | % | |
| Any xylazine i.e., major, minor, trace (n=41)b | 4 | 22.2 | 14 | 77.8 |
| Major xylazine only (n=4) | 4 | 100.0 | 0 | 0.0 |
| Minor xylazine only (n=9) | 0 | 0.0 | 9 | 100.0 |
| Major or minor xylazine (excluding trace xylazine and lidocaine) |
3 | 25.0 | 9 | 75.0 |
ability to correctly identify xylazine.
33 of the samples were submitted as fentanyl or fentanyl with another substance, 4 were reported to contain methamphetamines, 2 were reported to be adderall, 2 were reported to be cocaine, 2 were reported to be benzodiazepines, 1 was reported to be xylazine, and 1 was unknown at the time of submission.
4. Discussion
This study examined the performance of BTNX XTS for testing drug residue. The detection of xylazine’s presence was lower than expected although increased if xylazine was the sample major psychoactive component. Our findings suggest further research and field testing are needed to develop rapid XTS and procedures for residue testing in point-of-care DCS.
Few studies have evaluated the performance of XTS in the community. One small laboratory study on samples from Philadelphia found no false-negatives compared to confirmatory results (i.e., a sensitivity of 100 %). Notably, the Philadelphia study tested samples rather than residue using a 1 mg:1 mL dilution factor (Krotulski et al., 2023), compared to our procedure of dissolving an unquantified amount of residue in 5 mL of water. A second study using 100 residue samples from Maryland and Nevada assessed crossreactivity of 77 different compounds found only lidocaine produced false-positives (Sisco et al., 2023). Each study tested different XTS batch lots which may have contributed to result discrepancies; quality and accuracy of lateral flow strips can vary between lots (Hayden et al., 2014).
This study's limitations include its small sample size, limited collection radius and timeframe, and non-random collection procedure. Tested samples primarily contained fentanyl or other opioids; further research should explore the performance of testing non-opioids. Additionally, no validated procedure existed for testing drug residue at the time of the study. While collecting larger amounts of drugs to test would likely increase generalizability and confidence in findings, non-laboratory DCS fall into a legal gray area due to the Controlled Substances Act and resultantly often rely on drug refuse residue. Finally, while quantification was outside the scope of this study, it would provide stronger evidence of whether XTS perform in accordance with their stated detection limit in a community setting and should be considered for future studies. Given these uncertainties, our findings, while initial, caution against reliance upon the XTS alone for xylazine detection in drug residue and, if used, should be accompanied by a thorough discussion of the test’s limitations when interpreting the results or coupled with laboratory testing.
Given the low community awareness of xylazine and its severe health effects, XTS may be a useful preliminary indicator for further testing using more advanced, comprehensive technologies. Future research is needed to examine and replicate these findings using other, real-world drug and residue samples.
Funding
This study was funded by the NIGMS Center of Biomedical Research Excellence (COBRE) on Opioids and Overdose (P20GM125507). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of our funders.
CRediT authorship contribution statement
Traci C. Green: Writing – review & editing, Conceptualization. Merci Ujeneza: Writing – review & editing, Investigation. Adina Badea: Writing – review & editing, Conceptualization. Ju Nyeong Park: Methodology, Funding acquisition, Formal analysis, Conceptualization. Jessica Tardif: Writing – review & editing, Investigation. Erin Thompson: Writing – original draft, Project administration, Methodology, Investigation, Formal analysis. Haley McKee: Writing – review & editing. Michelle McKenzie: Writing – review & editing.
Declaration of Competing Interest
No conflict declared.
Acknowledgments
We are grateful to Project Weber/RENEW and study participants without whom the work would not be possible. This study was funded by the NIGMS Center of Biomedical Research Excellence (COBRE) on Opioids and Overdose (P20GM125507). TCG is funded by NIDA (5UG3DA056881). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of our funders.
Disclosures
BTNX Inc. provided the xylazine test strips to the research team but did not have a role in the study design, conduct or interpretation.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dadr.2024.100241.
Contributor Information
Erin Thompson, Email: ethompson1@lifespan.org.
Adina Badea, Email: Abadea@lifespan.org.
Traci C. Green, Email: tracigreen@brandeis.edu.
Haley McKee, Email: Hmckee@lifespan.org.
Michelle McKenzie, Email: mmckenzie@lifespan.org.
Ju Nyeong Park, Email: ju_park@brown.edu.
Appendix A. Supplementary material
Supplementary material
References
- Alexander R.S., Canver B.R., Sue K.L., Morford K.L. Xylazine and overdoses: trends, concerns, and recommendations. Am. J. Public Health. 2022;112(8):1212–1216. doi: 10.2105/AJPH.2022.306881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff K.N., Leifheit K.M., Park J.N., Chandran A., Sherman S.G. Opioid-related overdose mortality in the era of fentanyl: monitoring a shifting epidemic by person, place, and time. Drug Alcohol Depend. 2020;216 doi: 10.1016/j.drugalcdep.2020.108321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball N.S., Knable B.M., Relich T.A., Smathers A.N., Gionfriddo M.R., Nemecek B.D., Montepara C.A., Guarascio A.J., Covvey J.R., Zimmerman D.E. Vol. 60. Taylor and Francis Ltd; 2022. Xylazine poisoning: a systematic review; pp. 892–901. (Clinical Toxicology). [DOI] [PubMed] [Google Scholar]
- Barratt M.J., Measham F. What is drug checking, anyway? Drugs Habits Soc. Policy. 2022;23(3):176–187. doi: 10.1108/dhs-01-2022-0007. [DOI] [Google Scholar]
- Bhuiyan I., Tobias S., Ti L. Responding to changes in the unregulated drug supply: the need for a dynamic approach to drug checking technologies. Am. J. Drug Alcohol Abuse. 2023 doi: 10.1080/00952990.2023.2226312. [DOI] [PubMed] [Google Scholar]
- Bishnoi Anuradha, Singh Vaneet, Khanna Urmi, Vinay Keshavamurthy. Skin ulcerations caused by xylazine: A lesser-known entity. J. Am. Acad. Dermatol. 2023;89:e99–e102. doi: 10.1016/j.jaad.2023.04.009. [DOI] [PubMed] [Google Scholar]
- Cepeda J.A., Thompson E., Ujeneza M., Tardif J., Walsh T., Morales A., Rosen J.G., Green T.C., Park J.N. Costing analysis of a point-of-care drug checking program in Rhode Island. Drug Alcohol Depend. 2023;253 doi: 10.1016/j.drugalcdep.2023.111028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A.B., Wightman R.S., Macon E.C., Guan Y., Shihipar A., Krieger M., Elmaleh R., Smith M.C., Morales A., Badea A. Comprehensive testing and rapid dissemination of local drug supply surveillance data in Rhode Island. Int. J. Drug Policy. 2023;118 doi: 10.1016/j.drugpo.2023.104118. [DOI] [PubMed] [Google Scholar]
- Cruz S.L., Bencomo-Cruz M., Medina-Mora M.E., Vázquez-Quiroz F., Fleiz-Bautista C. First drug-checking study at an electronic festival and fentanyl detection in the central region of Mexico. Harm Reduct. J. 2023;20(1) doi: 10.1186/s12954-023-00905-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2022. DEA Reports Widespread Threat of Fentanyl Mixed with Xylazine. DEA Public Safety Alert. 〈https://www.dea.gov/alert/dea-reports-widespread-threat-fentanyl-mixed-xylazine〉.
- Evans, W.N., Lieber, E., Power, P., 2018. How the Reformulation of OxyContin Ignited the Heroin Epidemic. 〈https://wondercdc.gov/controller/datarequest/D77〉.
- Friedman J., Montero F., Bourgois P., Wahbi R., Dye D., Goodman-Meza D., Shover C. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022;233 doi: 10.1016/J.DRUGALCDEP.2022.109380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozdzialski L., Wallace B., Hore D. Point-of-care community drug checking technologies: an insider look at the scientific principles and practical considerations. Harm Reduct. J. 2023;20(1) doi: 10.1186/s12954-023-00764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T.C., Olson R., Jarczyk C., Erowid E., Erowid F., Thyssen S., Wightman R., del Pozo B., Michelson L., Consigli A., Reilly B., Ruiz S. Implementation and uptake of the massachusetts drug supply data stream: a statewide public health-public safety partnership drug checking program. J. Public Health Manag. Pract. 2022;28:S347–S354. doi: 10.1097/PHH.0000000000001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene S.A., Thurmon J.C. Xylazine – a review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 1988;11(4):295–313. doi: 10.1111/J.1365-2885.1988.TB00189.X. [DOI] [PubMed] [Google Scholar]
- Gupta R., Holtgrave D.R., Ashburn M.A. Xylazine — medical and public health imperatives. N. Engl. J. Med. 2023;388(24):2209–2212. doi: 10.1056/nejmp2303120. [DOI] [PubMed] [Google Scholar]
- Hayden J.A., Schmeling M., Hoofnagle A.N. Vol. 60. American Association for Clinical Chemistry Inc.; 2014. Lot-to-lot variations in a qualitative lateral-flow immunoassay for chronic pain drug monitoring; pp. 896–897. (Clinical Chemistry). [DOI] [PubMed] [Google Scholar]
- Haymann W. Xylazine (“tranq”): the potential for loss of life and limb. Dermatol. World Insights Inq. 2022;4(48) [Google Scholar]
- Kariisa M., O’donnell J., Kumar S., Mattson C.L., Goldberger B.A. Illicitly manufactured fentanyl–involved overdose deaths with detected xylazine — United States, January 2019–June 2022. Morb. Mortal. Wkly. Rep. 2023;72(26) doi: 10.15585/mmwr.mm7226a4. 〈https://www.dea.gov/sites/default/files/2022-12/The%20Growing%20〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krotulski A., Shinefeld J., DeBord J., Teixeira da Silva D., Logan B. NPS Discovery: CFSRE; 2023. Evaluation of BTNX Xylazine Test Strips for Drug Checking. [Google Scholar]
- Park J.N., Frankel S., Morris M., Dieni O., Fahey-Morrison L., Luta M., Hunt D., Long J., Sherman S.G. Evaluation of fentanyl test strip distribution in two Mid-Atlantic syringe services programs. Int. J. Drug Policy. 2021;94 doi: 10.1016/j.drugpo.2021.103196. [DOI] [PubMed] [Google Scholar]
- Park J.N., Sherman S.G., Sigmund V., Breaud A., Martin K., Clarke W.A. Validation of a lateral flow chromatographic immunoassay for the detection of fentanyl in drug samples. Drug Alcohol Depend. 2022;240 doi: 10.1016/j.drugalcdep.2022.109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.N., Tardif J., Thompson E., Rosen J.G., Lira J.A.S., Green T.C. A survey of North American drug checking services operating in 2022. Int. J. Drug Policy. 2023;121 doi: 10.1016/j.drugpo.2023.104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano T., Crowell J., Eggert K., Clark K., Alexander M., Grau L., Heimer R. Xylazine in the drug supply: emerging threats and lessons learned in areas with high levels of adulteration. Int. J. Drug Policy. 2023;120 doi: 10.1016/j.drugpo.2023.104154. [DOI] [PubMed] [Google Scholar]
- Reed M.K., Imperato N.S., Bowles J.M., Salcedo V.J., Guth A., Rising K.L. Perspectives of people in Philadelphia who use fentanyl/heroin adulterated with the animal tranquilizer xylazine; making a case for xylazine test strips. Drug Alcohol Depend. Rep. 2022;4 doi: 10.1016/j.dadr.2022.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfroe C.L. MDMA on the street: analysis anonymous®. J. Psychoact. Drugs. 1986;18(4):363–369. doi: 10.1080/02791072.1986.10472371. [DOI] [PubMed] [Google Scholar]
- Ruiz-Colón K., Chavez-Arias C., Díaz-Alcalá J.E., Martínez M.A. Vol. 240. Elsevier Ireland Ltd.; 2014. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: a comprehensive review of the literature; pp. 1–8. (Forensic Science International). [DOI] [PubMed] [Google Scholar]
- Sisco, E., Nestadt, D.F., Bloom, M.B., Schneider, K.E., Elkasabany, R.A., Rouhani, S., Sherman, S.G., 2023. Xylazine Test Strips For Drug Checking Understanding Sensitivity and Cross-Reactivity of Xylazine Lateral Flow Immunoassay Test Strips for Drug Checking Applications Xylazine test strips for drug checking. https://doi.org/10.26434/chemrxiv-2023-znb8j-v2. [DOI] [PubMed]
- Spadaro A., O’connor K., Lakamana S., Sarker A., Wightman R., Love J.S., Perrone J. Self-reported xylazine experiences: a mixed-methods study of reddit subscribers. J. Addict. Med. 2023;17(6):691–694. doi: 10.1101/2023.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren K.L., Colby J.M., Shugarts S.B., Wu A.H.B., Lynch K.L. Comparison of information-dependent acquisition on a tandem quadrupole TOF vs a triple quadrupole linear ion trap mass spectrometer for broad-spectrum drug screening. Clin. Chem. 2016;62(1):170–178. doi: 10.1373/clinchem.2015.241315. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime & Division for Treaty Affairs . United Nations; 2022. World Drug Report 2022. [Google Scholar]
- Żubrycka A., Kwaśnica A., Haczkiewicz M., Sipa K., Rudnicki K., Skrzypek S., Poltorak L. Illicit drugs street samples and their cutting agents. The result of the GC-MS based profiling define the guidelines for sensors development. Talanta. 2022;237 doi: 10.1016/j.talanta.2021.122904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material