Abstract
Background
Timely diagnosis and treatment of inflammatory bowel disease (IBD) may improve clinical outcomes.
Objective
Examine associations between time to diagnosis, patterns of prior healthcare use, and clinical outcomes in IBD.
Design
Using the Clinical Practice Research Datalink we identified incident cases of Crohn’s disease (CD) and ulcerative colitis (UC), diagnosed between January 2003 and May 2016, with a first primary care gastrointestinal consultation during the 3-year period prior to IBD diagnosis. We used multivariable Cox regression to examine the association of primary care consultation frequency (n=1, 2, >2), annual consultation intensity, hospitalisations for gastrointestinal symptoms, and time to diagnosis with a range of key clinical outcomes following diagnosis.
Results
We identified 2645 incident IBD cases (CD: 782; UC: 1863). For CD, >2 consultations were associated with intestinal surgery (adjusted HR (aHR)=2.22, 95% CI 1.45 to 3.39) and subsequent CD-related hospitalisation (aHR=1.80, 95% CI 1.29 to 2.50). For UC, >2 consultations were associated with corticosteroid dependency (aHR=1.76, 95% CI 1.28 to 2.41), immunomodulator use (aHR=1.68, 95% CI 1.24 to 2.26), UC-related hospitalisation (aHR=1.43, 95% CI 1.05 to 1.95) and colectomy (aHR=2.01, 95% CI 1.22 to 3.27). For CD, hospitalisation prior to diagnosis was associated with CD-related hospitalisation (aHR=1.30, 95% CI 1.01 to 1.68) and intestinal surgery (aHR=1.71, 95% CI 1.13 to 2.58); for UC, it was associated with immunomodulator use (aHR=1.42, 95% CI 1.11 to 1.81), UC-related hospitalisation (aHR=1.36, 95% CI 1.06 to 1.95) and colectomy (aHR=1.54, 95% CI 1.01 to 2.34). For CD, consultation intensity in the year before diagnosis was associated with CD-related hospitalisation (aHR=1.19, 95% CI 1.12 to 1.28) and intestinal surgery (aHR=1.13, 95% CI 1.03 to 1.23); for UC, it was associated with corticosteroid use (aHR=1.08, 95% CI 1.04 to 1.13), corticosteroid dependency (aHR=1.05, 95% CI 1.00 to 1.11), and UC-related hospitalisation (aHR=1.12, 95% CI 1.03 to 1.21). For CD, time to diagnosis was associated with risk of CD-related hospitalisation (aHR=1.03, 95% CI 1.01 to 1.68); for UC, it was associated with reduced risk of UC-related hospitalisation (aHR=0.83, 95% CI 0.70 to 0.98) and colectomy (aHR=0.59, 95% CI 0.43 to 0.80).
Conclusion
Electronic records contain valuable information about patterns of healthcare use that can be used to expedite timely diagnosis and identify aggressive forms of IBD.
Keywords: IBD CLINICAL, EPIDEMIOLOGY, IBD
WHAT IS ALREADY KNOWN ON THIS TOPIC
Diagnostic delay, from the point of first healthcare consultation, and increased healthcare utilisation may occur prior to inflammatory bowel disease (IBD) diagnosis, but their relationship to subsequent clinical outcomes is not yet established.
WHAT THIS STUDY ADDS
Increased primary care consultation frequency and intensity for gastrointestinal symptoms prior to diagnosis are associated with worse clinical outcomes in IBD, particularly risk of intestinal surgery.
Hospitalisation for gastrointestinal symptoms before diagnosis is also associated with an increased risk of intestinal surgery following diagnosis.
Longer time to diagnosis was associated with an increased risk of Crohn’s disease-related hospitalisation.
Paradoxically, a longer time to diagnosis was associated with a milder disease course in ulcerative colitis.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Expedited diagnostic approaches are required for patients who return repeatedly with unresolved gastrointestinal symptoms.
Electronic records contain valuable information about patterns of healthcare use that can be used to prompt targeted timely referral and identification of aggressive forms of IBD.
Introduction
Inflammatory bowel disease (IBD) is a chronic relapsing and remitting gastrointestinal condition, which in its initial stages can be challenging and time consuming to diagnose.1 2 Timely diagnosis enables early treatment to relieve patients’ symptoms and potentially reduces the risk of disease progression, hospitalisation and surgery.3–5 However, previous studies report that patients can wait for months to several years from symptom onset before receiving a diagnosis of IBD.1 6
Reasons for delay in diagnosis are likely complex. Patients may be unaware of the significance of their symptoms or be embarrassed to seek medical advice. One-tenth of patients report excess gastrointestinal symptoms 5 years before their eventual diagnosis with Crohn’s disease (CD) or ulcerative colitis (UC).1 However, symptoms of IBD may often be mistaken for more prevalent benign gastrointestinal conditions, such as irritable bowel syndrome (IBS) and haemorrhoids, particularly during the early stages of disease.7 8
Targeted investigation can expedite diagnosis.9 Set against this is the rising demand placed on healthcare services, which has been exacerbated in the wake of the COVID-19 pandemic. Individuals may be required to consult repeatedly before receiving a final diagnosis of IBD or, alternatively, need to access emergency hospital services.10
Previous studies have reported a higher than background prevalence of gastrointestinal symptoms and increased healthcare use and costs encountered in the years prior to IBD diagnosis, of which some encounters may be considered missed opportunities to diagnose, commence timely treatment and prevent disease progression.1 11 However, the association between patterns of healthcare use in the period prior to IBD diagnosis and subsequent clinical outcomes has not previously been thoroughly evaluated. In other chronic conditions, such as heart failure and malignancy, more frequent consultation, including emergency hospital admission prior to diagnosis, is associated with worse disease-related outcomes.12 13
The natural progression of IBD is variable and can range from indolent to an aggressive, rapidly evolving disease behaviour. While some studies have reported an association between diagnostic delay and the risk of disease complications, others have not.6 Most studies have relied on retrospective estimates of symptom duration before diagnosis, collected using patient questionnaires, from hospital cohorts, and are therefore subject to bias and are not representative.6
It is not clear which patients presenting with gastrointestinal symptoms will benefit from expedited investigation. To determine how patterns of consultation are predictive of worse IBD outcomes we designed a nationally representative population-based retrospective cohort study using linked primary care and hospital records. We aimed to examine the association between time to diagnosis, frequency/intensity of primary care and inpatient hospital episodes for gastrointestinal symptoms in the years before diagnosis, and the risk of subsequent adverse clinical outcomes in patients with IBD.
Methods
Data source
We analysed routinely collected primary care data from electronic health records from primary care practices that contributed to the Clinical Practice Research Datalink (CPRD), one of the largest validated primary care research databases in the world.14 It contains longitudinal, patient-level, deidentified electronic health records of 18 million patients from more than 700 general practices and is broadly representative of the UK population. The median follow-up for individuals registered in the CPRD is 9.4 years, allowing the study of long-term outcomes. We used CPRD GOLD version that contains data contributed by practices using Vision software. Primary care physicians use clinical codes to record symptoms, diagnoses, and prescriptions. Participating practices need to achieve and maintain ‘up to standard’ status to continue contributing to the dataset. The CPRD GOLD coding system has been extensively validated for use in IBD.15 16 CPRD primary care records are individually linked to the Hospital Episode Statistics (HES) database, which includes data on admissions and outpatient appointments in National Health Service hospitals in England.
Case definition and cohort construction
We identified incident cases of IBD diagnosed between January 2003 and May 2016 who had their first primary care consultation record for gastrointestinal symptoms in the 3-year period prior to their IBD diagnosis. We chose this interval since we previously found most individuals with IBD first consulted for gastrointestinal symptoms within this time period prior to diagnosis.1 All individuals required at least 4 years of follow-up from registering with their general practice before IBD diagnosis, with the first of these years free of any record of gastrointestinal symptoms (online supplemental appendices 1 and 2). We defined incident IBD cases, using a previously validated and published methodology, as individuals who had a first diagnostic Read code for either CD or UC registered with an ‘up to standard’ practice.17 18 We excluded individuals if they had codes for both CD and UC, or indeterminate codes such as ‘non-specific colitis’. All individuals included in the study had linkage between CPRD and HES. We identified individuals who consulted a primary care physician with their first gastrointestinal symptom(s), within the 3-year period before their IBD diagnosis, as we have previously shown a higher than background prevalence and incidence of gastrointestinal symptoms occur in this time frame and are therefore likely to be related to IBD.1 We used previously published and validated lists of Read codes to identify gastrointestinal symptoms of IBD, including abdominal or perianal pain, diarrhoea and rectal bleeding (online supplemental appendix 1).1 Patients were followed up from the date of IBD diagnosis until the first recorded outcome, deregistration, or death, if these occurred before that time, or the study endpoint defined as 5 years following IBD diagnosis.
bmjgast-2024-001371supp001.pdf (217.7KB, pdf)
Exposures
Time to IBD diagnosis, consultation frequency, consultation intensity and hospitalisation for gastrointestinal symptoms prior to IBD diagnosis were the primary exposure variables. We defined time to diagnosis as the number of months from the first recorded date of consultation for gastrointestinal symptom(s) to the date of IBD diagnosis, defined as the date of the first recorded code for an IBD diagnosis in CPRD. For consultation frequency, we allocated patients to groups according to the number of primary care consultations for gastrointestinal symptoms (1, 2, and >2) in the 3-year period before receiving a diagnosis of IBD. We examined the impact of consultation intensity, defined as consultation frequency per person in each individual year in the 3-year period prior to IBD diagnosis. Finally, we identified individuals who required hospital admission related to gastrointestinal symptoms prior to IBD diagnosis. This was defined as individuals who had a code (International Statistical Classification of Diseases and Related Health Problems, ICD-10) that included relevant gastrointestinal symptoms: abdominal pain, diarrhoea and per rectal bleeding, listed as their primary reason for admission (online supplemental appendix 1).
Outcomes
Study outcomes were oral corticosteroid use and dependency (surrogate measure of disease activity and severity), treatment escalation requiring immunomodulator use, IBD-related hospitalisation and IBD-related surgery.
We defined individuals as ‘exposed to oral corticosteroid’ if they had at least one prescription for corticosteroid during the study follow‐up period. Second, we identified individuals with corticosteroid dependency, adapted from European Crohn’s and Colitis Organisation guidelines criteria.19 An individual was defined as ‘corticosteroid‐dependent’ if they had either a prescription for corticosteroid that lasted longer than 3 months or required a repeat corticosteroid prescription within 3 months of stopping the previous corticosteroid course.19 20
Immunomodulator use was defined as the first prescription date of azathioprine, mercaptopurine or methotrexate following IBD diagnosis.
We used a previously published list of ICD‐10 codes to identify individuals where IBD was the primary reason for admission following diagnosis.21 We excluded day case activity and ‘zero-day admissions’, which can represent routine care such as endoscopic surveillance or administration of therapy.21
We used previously published Office of Population Censuses and Surveys Classification of Interventions and Procedures (OPCS) Version 4 codes to identify surgical procedures in the HES database.21 CD surgery was subcategorised as either major intra-abdominal (intestinal) surgery or perianal surgery. Colectomy was defined as any colectomy procedure following diagnosis of UC.17 21
Factors associated with time to diagnosis and patterns of consultation prior to IBD diagnosis
We identified potential factors associated with time to diagnosis, primary care consultation frequency, intensity, and hospital admission for gastrointestinal symptoms prior to IBD diagnosis, based on clinical knowledge and published literature. Age, low socioeconomic status, and smoking are associated with diagnostic delay in other chronic conditions.22 23 Younger age at diagnosis is also known to be associated with a more aggressive disease phenotype in IBD.23 We grouped individuals according to their age at diagnosis of IBD according to the Montreal classification (<17, 17–40 and >40 years). We used a postcode‐linked marker of social deprivation, the Index of Multiple Deprivation (IMD), to group patients by socioeconomic status from IMD 1 (least deprived) to 5 (most deprived).
IBS and depression have been reported to be associated with a longer time to specialist review in IBD1 and worse outcomes.24–26 Poor mental health has been associated with increased healthcare use in other chronic disease.27 We identified individuals who had codes for IBS, depression, anxiety or symptoms of depression or anxiety before their index presentation with gastrointestinal symptoms.
Individuals were classed as ‘smokers’, ‘ex-smokers’ or ‘non-smokers’ based on codes for smoking status in the 10 years before presentation with gastrointestinal symptoms using a previously reported methodology accounting for missing data.1 20 28 We considered the era of IBD diagnosis to account for secular change over the study period (era 1: 2003–2005; era 2: 2006–2008; era 3: 2009–2011; era 4: 2012–2016).
Statistical analysis
We used simple and multiple Cox regression analysis to calculate HRs and 95% CIs for our listed clinical outcome measures in the 5 years following diagnosis, given time to IBD diagnosis, gastrointestinal-related consultation frequency and hospital admission prior to IBD diagnosis. We also analysed the association between intensity of gastrointestinal consultations in primary care for each year in the 3 years prior to diagnosis and subsequent clinical outcomes. Within the multiple regression models, we adjusted for sex, age at diagnosis, social deprivation, smoking status, and era of diagnosis. Analysis was carried out separately for individuals diagnosed with CD and UC.
We used Kaplan‐Meier analysis to present time-to-event curves of IBD-related clinical outcomes in the 5 years following diagnosis given consultation frequency in primary care for gastrointestinal symptoms. We used multiple Cox regression to examine factors that may be associated with time to diagnosis; logistic regression was used to examine factors that may be associated with gastrointestinal-related consultation frequency in primary care and hospital admission prior to diagnosis of IBD. Analyses were performed using STATA V.17 (StataCorp, College Station, Texas, USA).
Results
We identified 2645 individuals with a new diagnosis of IBD between January 2003 and May 2016 who had their first gastrointestinal-related primary care consultation in the 3-year period prior to IBD diagnosis (table 1 and online supplemental appendix 2). The median time from the first consultation with gastrointestinal symptoms to diagnosis of CD was 7 months (IQR: 2–18 months) compared with 5 months (IQR: 2–16 months) for UC; 37% (n=288) and 31% (n=580) of individuals experienced gastrointestinal symptoms for more than a year before being diagnosed with CD and UC, respectively.
Table 1.
Baseline characteristics of study population
| IBD status | Crohn’s disease n=782 |
Ulcerative colitis n=1863 |
| Gender, n (%) | ||
| Male | 390 (50) | 1021 (55) |
| Female | 392 (50) | 842 (45) |
| Age at diagnosis (years), n (%) | ||
| <17 | 86 (11) | 63 (3) |
| 17–40 | 380 (49) | 612 (33) |
| >40 | 316 (40) | 1188 (64) |
| Social deprivation, n (%) | ||
| IMD 1–3 | 512 (65) | 1311 (70) |
| IMD 4–5 | 270 (36) | 552 (30) |
| Time to diagnosis from first gastrointestinal consultation | ||
| Median (IQR), months | 7 (2–18) | 5 (2–16) |
| Primary care consultation frequency, n (%) | ||
| 1 | 264 (34) | 822 (44) |
| 2 | 200 (26) | 533 (29) |
| >2 | 318 (41) | 508 (27) |
| Hospitalisation for gastrointestinal symptoms before diagnosis, n (%) | 339 (43) | 623 (33) |
IMD 1 represents the least deprived and IMD 5 the most deprived.
IBD, inflammatory bowel disease; IMD, Index of Multiple Deprivation.
The median number of consultations for gastrointestinal symptoms prior to CD diagnosis was 3 (IQR: 1–3; total range: 1–17) compared with 2 (IQR: 1–3; total range: 1–15) in UC. We found 41% and 27% of individuals, who went on to be diagnosed with CD and UC, respectively, had a primary care consultation for gastrointestinal symptoms more than twice during the 3-year period prior to diagnosis. Among the whole cohort, 36% (n=962; CD=339 and UC=623) of individuals required gastrointestinal-related hospital admission prior to IBD diagnosis (online supplemental appendix 2).
Time to IBD diagnosis and clinical outcomes
Among individuals diagnosed with CD, we found that a longer time to diagnosis from first consultation for gastrointestinal symptoms was associated with increased risk of hospitalisation (adjusted HR (aHR)=1.03, 95% CI 1.01 to 1.68), but not surgery, in the 5 years following diagnosis (table 2a). Among individuals diagnosed with UC, a longer time to diagnosis was associated with a lower risk of corticosteroid use (aHR=0.87, 95% CI 0.79 to 0.97), UC-related hospitalisation (aHR=0.83, 95% CI 0.70 to 0.98) and colectomy (aHR=0.59, 95% CI 0.43 to 0.80) in the 5 years following diagnosis (table 2b).
Table 2.
Association of time to diagnosis, consultation frequency and hospitalisation for gastrointestinal symptoms before diagnosis with clinical outcomes following diagnosis of (a) Crohn’s disease and (b) ulcerative colitis*
| (a) Crohn’s disease | CS use | CS dependency | IM use | IBD hospitalisation | Intestinal surgery | Perianal surgery |
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Consultation frequency | ||||||
| 1 | – | – | – | – | – | – |
| 2 | 0.90 (0.68 to 1.18) | 1.11 (0.74 to 1.65) | 1.05 (0.78 to 1.43) | 1.35 (0.94 to 1.93) | 1.58 (0.99 to 2.51) | 1.08 (0.81 to 1.44) |
| ≥3 | 1.21 (0.94 to 1.56) | 1.23 (0.84 to 1.80) | 1.11 (0.84 to 1.52) | 1.80 (1.29 to 2.50) | 2.22 (1.45 to 3.39) | 1.00 (0.79 to 1.36) |
| Time to diagnosis | 0.89 (0.78 to 1.01) | 0.89 (0.73 to 1.07) | 0.92 (0.80 to 1.06) | 1.03 (1.01 to 1.68) | 0.87 (0.71 to 1.06) | 1.00 (0.88 to 1.15) |
| Prediagnosis hospital admission | 0.96 (0.76 to 1.21) | 1.05 (0.78 to 1.42) | 0.78 (0.60 to 1.01) | 1.30 (1.01 to 1.68) | 1.71 (1.13 to 2.58) | 1.19 (0.96 to 1.48) |
| Sex | ||||||
| Female | – | – | – | – | – | – |
| Male | 1.13 (0.55 to 0.86) | 0.85 (0.62 to 1.15) | 1.04 (0.83 to 1.32) | 1.12 (0.87 to 1.44) | 1.20 (0.86 to 1.66) | 1.05 (0.84 to 1.30) |
| Age at IBD diagnosis (years) | ||||||
| >40 | ||||||
| <17 | 1.45 (1.00 to 2.09) | 1.20 (0.68 to 2.12) | 3.60 (2.47 to 5.24) | 2.31 (1.51 to 3.56) | 0.71 (0.37 to 1.36) | 1.72 (1.18 to 2.51) |
| 17–40 | 1.46 (1.16 to 1.83) | 1.26 (0.90 to 1.76) | 1.89 (1.43 to 2.49) | 1.52 (1.14 to 2.03) | 1.18 (0.83 to 1.68) | 1.27 (0.99 to 1.62) |
| Era of IBD diagnosis | ||||||
| Era 1 | – | – | – | – | – | – |
| Era 2 | 1.07 (0.82 to 1.42) | 0.88 (0.59 to 1.29) | 1.50 (0.66 to 1.41) | 1.18 (0.83 to 1.69) | 1.34 (0.86 to 2.08) | 1.37 (0.98 to 1.92) |
| Era 3 | 0.90 (0.91 to 0.68) | 0.72 (0.47 to 1.11) | 2.02 (1.41 to 1.26) | 1.15 (0.80 to 1.65) | 1.29 (0.81 to 2.06) | 2.21 (1.58 to 3.09) |
| Era 4 | 1.32 (0.99 to 1.77) | 0.76 (0.49 to 1.18) | 3.32 (2.34 to 4.74) | 2.00 (1.35 to 2.98) | 1.25 (0.76 to 2.04) | 4.54 (3.28 to 6.28) |
| Smoking status* | ||||||
| Never | – | – | – | – | – | – |
| Ex-smoker | 0.85 (0.61 to 1.18) | 0.90 (0.55 to 1.48) | 0.91 (0.65 to 1.26) | 0.60 (0.38 to 0.93) | 0.96 (0.63 to 1.46) | 0.94 (0.67 to 1.31) |
| Current | 0.99 (0.75 to 1.31) | 1.22 (0.82 to 1.83) | 0.88 (0.65 to 1.19) | 1.05 (0.75 to 1.48) | 0.76 (0.50 to 1.16) | 0.90 (0.67 to 1.20) |
| Social deprivation | ||||||
| IMD 1–3 | – | – | – | – | – | – |
| IMD 4–5 | 1.12 (0.91 to 1.39) | 1.14 (0.83 to 1.56) | 0.97 (0.76 to 1.24) | 1.12 (0.86 to 1.46) | 1.11 (0.80 to 1.55) | 0.87 (0.69 to 1.08) |
| (b) Ulcerative colitis | CS use | CS dependency | IM use | IBD hospitalisation | Colectomy |
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Consultation frequency | |||||
| 1 | – | – | – | – | – |
| 2 | 1.26 (1.04 to 1.60) | 1.28 (0.94 to 1.75) | 1.12 (0.83 to 1.51) | 1.24 (0.93 to 1.66) | 0.93 (0.55 to 1.57) |
| ≥3 | 1.60 (1.31 to 1.96) | 1.76 (1.28 to 2.41) | 1.68 (1.24 to 2.26) | 1.43 (1.05 to 1.95) | 2.01 (1.22 to 3.27) |
| Time to diagnosis | 0.87 (0.79 to 0.97) | 0.95 (0.81 to 1.11) | 0.88 (0.76 to 1.03) | 0.83 (0.70 to 0.98) | 0.59 (0.43 to 0.80) |
| Prediagnosis hospital admission | 1.18 (0.99 to 1.39) | 1.04 (0.80 to 1.36) | 1.42 (1.11 to 1.81) | 1.36 (1.06 to 1.95) | 1.54 (1.01 to 2.34) |
| Sex | |||||
| Female | – | – | – | – | – |
| Male | 1.00 (0.85 to 1.17) | 1.37 (1.06 to 1.76) | 1.16 (0.92 to 1.48) | 1.01 (0.79 to 1.29) | 1.42 (0.93 to 2.16) |
| Age at IBD diagnosis (years) | |||||
| >40 | – | – | – | – | – |
| <17 | 1.82 (1.24 to 2.69) | 2.38 (1.37 to 4.12) | 3.35 (2.07 to 5.43) | 3.40 (1.47 to 1.89) | 2.54 (1.09 to 5.95) |
| 17–39 | 1.34 (1.14 to 1.60) | 1.52 (1.17 to 1.98) | 1.83 (1.42 to 2.34) | 1.47 (1.14 to 1.89) | 1.81 (1.17 to 2.79) |
| Era of IBD diagnosis | |||||
| Era 1 | – | – | – | – | – |
| Era 2 | 1.14 (0.92 to 1.43) | 1.05 (0.76 to 1.44) | 1.11 (0.78 to 1.57) | 0.82 (0.58 to 1.13) | 0.65 (0.38 to 1.09) |
| Era 3 | 1.35 (1.07 to 1.70) | 0.83 (0.58 to 1.20) | 1.53 (1.08 to 2.15) | 0.91 (0.66 to 1.29) | 0.62 (0.35 to 1.11) |
| Era 4 | 1.44 (1.14 to 1.83) | 0.76 (0.52 to 1.12) | 1.95 (1.36 to 2.80) | 1.15 (0.79 to 1.58) | 0.85 (0.46 to 1.54) |
| Smoking status* | |||||
| Never | – | – | – | – | – |
| Ex-smoker | 0.94 (0.99 to 1.54) | 0.88 (0.63 to 1.23) | 0.89 (0.64 to 1.22) | 0.79 (0.51 to 1.03) | 1.33 (0.75 to 2.34) |
| Current | 0.97 (1.13 to 1.79) | 0.59 (0.32 to 1.05) | 0.81 (0.49 to 1.32) | 0.78 (0.48 to 1.30) | 0.89 (0.36 to 2.20) |
| Social deprivation | |||||
| IMD 1–3 | – | – | – | – | – |
| IMD 4–5 | 1.06 (0.89 to 1.26) | 0.91 (0.69 to 1.1) | 0.79 (0.61 to 1.02) | 1.26 (0.98 to 1.62) | 0.65 (0.40 to 1.05) |
Bold indicates statistical significance in adjusted model.
IMD categories 4 and 5 (most deprived) versus IMD categories 1, 2 and 3 (least deprived).
Era 1: 2003–2005, Era 2: 2006–2008, Era 3: 2009–2011, Era 4: 2012–2016.
First CS use: time to first CS prescription following diagnosis.
CS dependency: corticosteroid dependency defined as a repeat steroid prescription within 3 months of the end of a previous steroid prescription or patients with steroid prescriptions for greater than 3 consecutive months.
Hospitalisation: IBD-related hospital admission following diagnosis.
Time to diagnosis: Time from first primary care consultation for gastrointestinal symptom(s).
*See online supplemental appendix 3 for unadjusted analyses.
CS, corticosteroid; IBD, inflammatory bowel disease; IM, immunomodulator; IMD, Index of Multiple Deprivation.
Gastrointestinal consultations before diagnosis and clinical outcomes
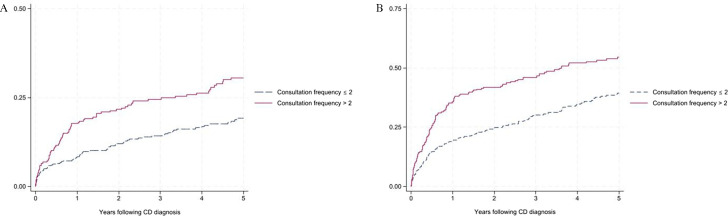
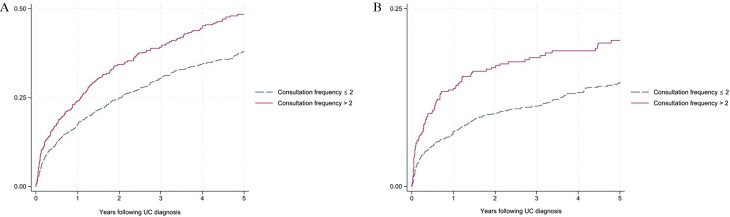
Among individuals diagnosed with CD, those who presented to primary care with gastrointestinal symptoms more than twice prior to diagnosis had an increased risk of CD-related hospitalisation (aHR=1.80, 95% CI 1.29 to 2.50) and intestinal surgery (aHR=2.22, 95% CI 1.45 to 3.39) in the 5 years following diagnosis, compared with those who had only one consultation (table 2a and figure 1). Among individuals diagnosed with UC, those who presented to primary care with gastrointestinal symptoms more than twice prior to diagnosis had an increased risk of corticosteroid use (aHR=1.60, 95% CI 1.31 to 1.96), corticosteroid dependency (aHR=1.76, 95% CI 1.28 to 2.14), immunomodulator use (aHR=1.68, 95% CI 1.24 to 2.26), UC-related hospitalisation (aHR=1.43, 95% CI 1.05 to 1.95) and colectomy (aHR=2.01, 95% CI 1.22 to 3.27) compared with those who had only one consultation (table 2b and figure 2).
Figure 1.
Probability of (A) Crohn’s disease (CD)-related intestinal surgery and (B) CD-related hospitalisation following diagnosis given consultation frequency for gastrointestinal symptoms prior to diagnosis.
Figure 2.
Probability of (A) corticosteroid use and (B) corticosteroid dependency in ulcerative colitis (UC) following diagnosis given consultation frequency for gastrointestinal symptoms prior to diagnosis.
Consultation intensity in primary care was highest in the year prior to diagnosis and was associated with worse clinical outcomes in both CD and UC. In the year before diagnosis, 26% and 17% of individuals diagnosed with CD and UC, respectively, consulted more than twice, compared with 4% and 2%, and 3% and 1%, in the second and third years before diagnosis, respectively.
In CD, individuals with a higher consultation intensity in the year prior to diagnosis had an increased risk of CD-related hospitalisation (aHR=1.19, 95% CI 1.12 to 1.28) and intestinal surgery (aHR=1.13, 95% CI 1.03 to 1.23) in the 5 years following diagnosis (table 3a). In UC, individuals with a higher consultation intensity in the year prior to diagnosis had an increased risk of corticosteroid use (aHR=1.08, 95% CI 1.04 to 1.13), corticosteroid dependency (aHR=1.05, 95% CI 1.00 to 1.11), and UC-related hospitalisation (aHR=1.12, 95% CI 1.03 to 1.21) (table 3b).
Table 3.
Association of consultation intensity with gastrointestinal symptoms in the years before diagnosis with clinical outcomes following (a) Crohn’s disease diagnosis and (b) ulcerative colitis*
| (a) Crohn’s disease | ||||||
| Year before diagnosis | CS use | CS dependency | IM use | IBD hospitalisation | Intestinal surgery | Perianal surgery |
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Year 1 | 1.03 (0.98 to 1.08) | 1.01 (0.96 to 1.07) | 1.00 (0.95 to 1.06) | 1.19 (1.12 to 1.28) | 1.13 (1.03 to 1.23) | 1.05 (0.98 to 1.13) |
| Year 2 | 1.00 (0.92 to 1.09) | 0.99 (0.90 to 1.09) | 1.03 (0.94 to 1.12) | 1.13 (1.01 to 1.25) | 0.86 (0.71 to 1.03) | 1.00 (0.90 to 1.12) |
| Year 3 | 0.96 (0.95 to 1.08) | 0.99 (0.88 to 1.11) | 0.90 (0.80 to 1.02) | 1.10 (0.91 to 1.33) | 1.25 (0.99 to 1.48) | 0.98 (0.87 to 1.11) |
| (b) Ulcerative colitis | |||||
| Year before diagnosis | CS use | CS dependency | IM use | IBD hospitalisation | Colectomy |
| Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) | |
| Year 1 | 1.08 (1.04 to 1.13) | 1.05 (1.00 to 1.11) | 1.03 (0.98 to 1.08) | 1.12 (1.03 to 1.21) | 1.12 (0.99 to 1.26) |
| Year 2 | 1.03 (0.96 to 1.11) | 1.07 (0.98 to 1.15) | 1.03 (0.95 to 1.13) | 1.05 (0.92 to 1.20) | 0.91 (0.68 to 1.20) |
| Year 3 | 1.02 (0.93 to 1.12) | 1.05 (0.96 to 1.16) | 1.03 (0.93 to 1.13) | 1.00 (0.81 to 1.23) | 1.00 (0.73 to 1.28) |
Bold indicates statistical significance in adjusted model.
IMD categories 4 and 5 (most deprived) versus IMD categories 1, 2 and 3 (least deprived).
Era 1: 2003–2005, Era 2: 2006–2008, Era 3: 2009–2011, Era 4: 2012–2016.
First CS use: time to first CS prescription following diagnosis.
CS dependency: corticosteroid dependency defined as a repeat steroid prescription within 3 months of the end of a previous steroid prescription or patients with steroid prescriptions for greater than 3 consecutive months.
Hospitalisation: IBD-related hospital admission following diagnosis.
Consultation intensity: consultation frequency per person, as a continuous variable, in each individual year over the 3-year period before diagnosis.
*See online supplemental appendix 4 for unadjusted analyses.
CS, corticosteroid; IBD, inflammatory bowel disease; IM, immunomodulator; IMD, Index of Multiple Deprivation.
Hospitalisation before diagnosis and subsequent clinical outcomes
Individuals who required hospitalisation for gastrointestinal symptoms prior to CD diagnosis had an increased risk of CD-related hospitalisation (aHR=1.30, 95% CI 1.01 to 1.68) and intestinal surgery (aHR=1.71, 95% CI 1.13 to 2.58) in the 5 years following CD diagnosis, compared with individuals who had none (table 2a). Among individuals diagnosed with UC, gastrointestinal-related hospital admission prior to diagnosis was associated with an increased risk of immunomodulator use (aHR=1.42, 95% CI 1.11 to 1.81), UC-related hospitalisation (aHR=1.36, 95% CI 1.06 to 1.95) and colectomy (aHR=1.54, 95% CI 1.01 to 2.34) in the 5 years after diagnosis, compared with individuals who had none (table 2b).
Factors associated with time to diagnosis and patterns of consultation before IBD diagnosis
Females and individuals with a diagnosis of IBS or depression and/or anxiety were more likely to have a longer time to diagnosis of IBD compared with those without. Similarly, individuals with a diagnosis of IBS, depression and/or anxiety were more likely to consult more than twice with gastrointestinal symptoms compared with those who presented only once. Individuals under 17 years of age at diagnosis were more likely to consult primary care more than twice and require gastrointestinal-related hospital admission prior to diagnosis, when compared with individuals over 40 years. Smokers were 42% more likely to consult more than twice with gastrointestinal symptoms than never smokers. Individuals aged <17 and between 17 and 39 years were associated with higher consultation intensity in the year prior to diagnosis. Those living in areas of greater socioeconomic deprivation were 29% more likely to require hospitalisation for gastrointestinal symptoms prior to diagnosis when compared with individuals living in more affluent postcodes. Compared with individuals diagnosed during 2003–2005, those diagnosed in the era 2012–2016 were 61% more likely to have hospitalisation for gastrointestinal symptoms prior to IBD diagnosis (table 4).
Table 4.
Factors associated with time to diagnosis, consultation frequency, consultation intensity and hospitalisation before diagnosis of IBD*
| Time to diagnosis | Consultation frequency | Consultation intensity | Prior GI hospitalisation | |
| Adjusted HR (95% CI) | Adjusted OR (95% CI) |
Adjusted
coefficient (95% CI) |
Adjusted OR (95% CI) | |
| Age | ||||
| >40 | – | – | – | – |
| <17 | 0.99 (0.82 to 1.17) | 2.32 (1.40 to 2.01) | 0.44 (0.20 to 0.67) | 1.74 (1.21 to 2.48) |
| 17–39 | 0.99 (0.91 to 1.07) | 1.68 (1.60 to 3.38) | 0.37 (0.25 to 0.48) | 0.95 (0.80 to 1.14) |
| Sex | ||||
| Male | – | – | – | – |
| Female | 0.89 (0.82 to 0.96) | 1.12 (0.94 to 1.33) | 0.00 (−0.11 to 1.11) | 0.96 (0.81 to 1.13) |
| Social deprivation | ||||
| IMD 1–3 | – | – | – | – |
| IMD 4–5 | 1.01 (0.93 to 1.10) | 1.09 (0.91 to 1.30) | 0.10 (−0.02 to 0.22) | 1.29 (1.09 to 1.54) |
| Smoking status* | ||||
| Never | – | – | – | – |
| Ex-smoker | 0.91 (0.82 to 1.01) | 1.06 (0.84 to 1.34) | 0.03 (−0.10 to 0.25) | 1.16 (0.93 to 1.46) |
| Current | 0.93 (0.88 to 1.08) | 1.42 (1.07 to 1.88) | 0.34 (0.16 to 0.51) | 1.23 (0.94 to 1.63) |
| Premorbid depression—anxiety | 0.87 (0.78 to 0.96) | 1.28 (1.02 to 1.60) | 0.12 (−0.22 to 0.27) | 1.17 (0.91 to 1.52) |
| Premorbid IBS | 0.66 (0.58 to 0.75) | 1.87 (1.44 to 2.41) | 0.08 (−0.10 to 0.25) | 1.18 (0.95 to 1.46) |
| Era of diagnosis | ||||
| Era 1 | – | – | – | – |
| Era 2 | 1.06 (0.95 to 1.18) | 1.04 (0.82 to 1.32) | −0.74 (−0.22 to 0.07) | 1.31 (1.04 to 1.64) |
| Era 3 | 1.01 (0.91 to 1.13) | 1.05 (0.83 to 1.32) | −1.13 (−0.28 to 0.27) | 1.57 (1.23 to 1.99) |
| Era 4 | 1.00 (0.89 to 1.12) | 0.88 (0.68 to 1.12) | −0.22 (−0.37 to 0.06) | 1.61 (1.26 to 2.03) |
Bold indicates statistical significance in adjusted model. Multiple regression includes all variables and covariates of simple regression.
IMD categories 4 and 5 (most deprived) versus IMD categories 1, 2 and 3 (least deprived).
Era 1: 2003–2005, Era 2: 2006–2008, Era 3: 2009–2011, Era 4: 2012–2016.
First CS use: time to first CS prescription following diagnosis.
CS dependency: corticosteroid dependency defined as a repeat steroid prescription within 3 months of the end of a previous steroid prescription or patients with steroid prescriptions for greater than 3 consecutive months.
Hospitalisation: first IBD-related hospital admission following diagnosis.
Time to diagnosis: time from first primary care consultation for gastrointestinal symptom(s).
Consultation intensity: consultation frequency per person in the year prior to IBD diagnosis.
*See online supplemental appendix 5 for unadjusted analyses.
CS, corticosteroid; GI, gastrointestinal; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IMD, Index of Multiple Deprivation.
Discussion
Main findings
In this large population-based study we found more frequent primary care consultation for gastrointestinal symptoms prior to IBD diagnosis was associated with worse clinical IBD outcomes, notably an increased risk of surgery, and, with respect to UC, an increased risk of steroid dependency. Primary care consultation intensity was highest in the 1 year prior to diagnosis and in this year was associated with worse clinical outcomes in both CD and UC. Likewise, hospitalisation for gastrointestinal symptoms before diagnosis was associated with an increased risk of subsequent IBD-related hospital admission and intestinal surgery following diagnosis. A longer time to diagnosis, from the point of first primary care consult with gastrointestinal symptoms, was associated with increased disease-related hospitalisation in CD, but not surgery, and a milder disease course in UC.
Findings in relation to previous studies
To the best of our knowledge, this is the first nationally representative study to demonstrate an association between consultation frequency and intensity for gastrointestinal symptoms prior to diagnosis with subsequent adverse clinical outcomes following the diagnosis of IBD. Previous studies report a relationship between delayed diagnosis and adverse IBD-related clinical outcomes such as surgery.2 29 However, the majority of these studies used retrospective questionnaires conducted in secondary healthcare settings, thus likely subject to both recall and referral centre bias.6
In our study, a longer time from first primary care consultation to diagnosis was associated with a subsequent increased hospitalisation for CD, but not surgery; in contrast, for UC, it was associated with a milder disease course. Our findings are similar to a previous study that also used UK primary care records, which reported no associated risk between time to diagnosis and worse clinical outcomes.30
We also considered the impact of primary care consultation intensity for gastrointestinal symptoms prior to diagnosis, which was highest in the 1-year period immediately before diagnosis, and a greater consultation intensity in this year was associated with worse IBD outcomes. This reflects our previous observation that individuals with CD and UC were four times more likely to visit their primary care physician for gastrointestinal symptoms when compared with age-sex matched control groups without IBD between 18 and 6 months before diagnosis.1 Repeat consultations may either be clinician or patient initiated, likely driven by both symptom frequency and severity. Our findings suggest that higher primary care consultation frequency and intensity before diagnosis are linked to a more aggressive/severe disease behaviour with worse outcomes, although the observed effects are relatively modest. This is in keeping with paediatric studies that show a short fulminant onset of symptoms is associated with worse clinical outcomes following UC diagnosis, including risk of colectomy.31 32
Hospitalisation for gastrointestinal symptoms prior to IBD diagnosis was more common in those from deprived postcodes and had an associated higher risk of adverse clinical outcomes following diagnosis. This is consistent with other findings that report emergency hospital presentation prior to diagnosis is associated with worse IBD-related clinical outcomes.30
Previous literature reporting the relationship between diagnostic delay and IBD outcomes is inconsistent, with several studies suggesting diagnostic delay based on self-reported symptom onset is associated with worse clinical outcomes following diagnosis,2 33 while others have not.30 34 The differences observed between this study and others may relate to how ‘diagnostic delay’ is defined. Most previous studies have measured total time to diagnosis, including both patient-related and healthcare-related delay, whereas our study measured the interval from first related primary care consult for gastrointestinal symptoms prior to IBD diagnosis. We found that a longer time to UC diagnosis was associated with a lower risk of subsequent hospitalisation and colectomy, suggesting this group may have a milder, more indolent disease course. Our findings are supported by the observation that asymptomatic or mildly symptomatic individuals, who are diagnosed with IBD at colonoscopy as part of bowel cancer screening initiatives, have a milder pattern of disease behaviour.35 In contrast, a longer time to CD diagnosis was associated with a small increased risk of hospitalisation but not surgery which contrasts with most reports evaluating delay from the point of symptom onset.
The concept of the ‘waiting time paradox’, the effect that patients with severe symptoms indicative of a more aggressive and fulminant disease phenotype present rapidly over a short period of time, are diagnosed, and treated early, thereby leading to an apparent association between longer waits and better outcomes, has been reported for cancer diagnoses. It is considered an important source of bias in studies investigating the impact of diagnostic and treatment delays on cancer survival, where the biology of the disease may outweigh the impact of diagnostic delay when determining clinical outcomes.36 37 Such a phenomenon may also be at play with regard to IBD whereby a fulminant disease course prior to diagnosis, rather than a long symptomatic period prior to diagnosis, may predict a more aggressive/severe disease course. This may be reflected in our findings, particularly regarding UC.
Guidelines recommend that clinicians investigate persistent non-specific gastrointestinal symptoms, which are also prevalent in other common gut disorders such as IBS.38 Our study found that individuals with a prior diagnosis of IBS were more likely to have experienced a longer time to diagnosis and higher consultation frequency for gastrointestinal symptoms in the period before IBD diagnosis. It is possible individuals with undiagnosed IBD who receive a clinical diagnosis of IBS are less likely to be investigated, resulting in a longer time to diagnosis.7 Similarly, we found that a prior diagnosis or symptoms of depression-anxiety were associated with both a longer time to diagnosis and increased consultation frequency for gastrointestinal symptoms in the period prior to IBD diagnosis. Gastrointestinal symptoms may be considered more likely to be of functional origin in these patients. In this respect, we have previously reported increased rates of depression following the onset of undiagnosed gastrointestinal symptoms in the lead up to a diagnosis of IBD.15
Strengths and limitations
We used data drawn from a large, validated, nationally representative, linked primary care and hospital database. CPRD data are collected at the time of consultation and therefore, unlike most previous studies that have relied on retrospective self-reported data from specialist centres, are free from recall and selection bias. There are limitations to the study design. We estimated time to diagnosis using captured data from primary care consultations and therefore cannot account for the duration of unreported symptoms prior to consultation. When interpreting the findings of our study, it is worth reflecting that they relate to patients with gastrointestinal symptoms presenting to primary care but other extraintestinal symptoms may also herald the onset of IBD.
We were unable to capture data on medications prescribed in the hospital setting, meaning rates of corticosteroid and immunomodulator use reported in this study are likely to be underestimated. However, in the UK, hospital outpatient prescribing is highly regulated, and primary care practices using shared care protocols enable general practitioners to accept the responsibility for the safe prescribing and monitoring of specialist medicines for patients with chronic conditions in the community. Therefore, it is likely that we would have captured the large proportion of prescriptions, some of which may be only initiated in secondary care.
We were unable to identify episodes where individuals presented to the emergency department alone without requiring hospital admission, and thus the association between emergency hospital presentation and clinical outcomes may have been underestimated. Data defining endoscopic and radiological disease extent, or biochemical markers, such as C reactive protein and faecal calprotectin that are associated with disease severity, were not available for our analysis.
By choosing a methodology that included symptomatic individuals attending primary care in the 3 years before diagnosis, with no symptom in the preceding year, a small number of individuals may have been omitted but we chose this study design to minimise inclusion of consults for non-IBD-related gastrointestinal symptoms. This time interval was chosen since our previous findings revealed an excess of gastrointestinal symptoms in patients who later develop IBD compared with the background population emerged in this time frame.1 We found no secular relationship by era of diagnosis regarding IBD outcomes (although hospitalisation prior to diagnosis was more common in the most recent era studied). This suggests diagnostic approaches seemingly have not altered time to diagnosis in the study period. More recently, the wider adoption of faecal calprotectin testing in primary care may allow more timely diagnosis. While the association of deprivation was evaluated, ethnicity was not reliably coded in the dataset and warrants evaluation in future work. Further work is also needed to determine if our observed findings are replicated in other healthcare systems.
Implications
Our findings highlight the need for expedited diagnostic approaches for patients who consult more frequently or intensely in primary care or require hospital admission for gastrointestinal symptoms. We speculate that some individuals with IBD who have a more aggressive disease behaviour do not necessarily present with a long duration of symptoms but instead with a rapidly progressive fulminant disease course, leading to a higher frequency and intensity of consultation and urgent hospital attendance in the period prior to IBD diagnosis. Clinicians need to be alert to the possibility of IBD when patients return repeatedly with unresolved symptoms. Prior healthcare use can alert clinicians to those at risk of a more aggressive IBD course, prompting targeted timely assessment. Further, prospective studies using newly described diagnostic and prognostic biomarker may shed further light on the relationship between symptom onset and healthcare use in the years before diagnosis and subsequent disease prognosis. Our findings, and those of others, indicate a significant burden of disease and healthcare use in the years before IBD diagnosis.11 35 39 Diagnostic pathways that take account of patterns of healthcare consultation, alongside appropriate use of surrogate markers of inflammation such as faecal calprotectin, may enable expedited specialist referral and timely treatment.38 39
Conclusion
Consultation frequency, intensity and hospitalisation prior to diagnosis are associated with a subsequent risk of adverse IBD outcomes. Electronic healthcare records contain valuable information regarding patterns of consultation and may be used to expedite timely assessment and identify those at risk of aggressive forms of IBD.
Footnotes
@jaya_nish
Collaborators: Professor Richard C G Pollok, Professor Sonia Saxena, Professor Alex Bottle, Professor Irene Petersen, Dr Jonathan Blackwell, Dr Hanna Creese and Dr Nishani Jayasooriya
Contributors: The POP-IBD study group is a collaboration between St George’s University of London, Imperial College London, and University College London conducting population-based studies in the field of inflammatory bowel disease. NJ and JB prepared the data and carried out statistical analysis overseen by IP and AB. NJ and RCGP drafted the paper. All authors contributed to the concept, design, and interpretation of results, and commented on drafts of the manuscript. RCGP is the guarantor of this article.
Funding: JB was funded by Crohn’s and Colitis UK Grant [grant number: SP2018/3] SS is supported by an NIHR Senior investigator award (205021) and NIHR Applied Research Collaboration for Northwest London. SS and HC are supported by National Institute for Health and Care Research (NIHR) School for Public Health Research (SPHR), [Grant Reference Number NIHR 204000] The NIHR School for Public Health Research is a partnership between the Universities of Sheffield; Bristol; Cambridge; Imperial; and University College London; The London School for Hygiene and Tropical Medicine (LSHTM); LiLaC – a collaboration between the Universities of Liverpool and Lancaster; and Fuse - The Centre for Translational Research in Public Health a collaboration between Newcastle, Durham, Northumbria, Sunderland, and Teesside Universities. Professor Bottle’s Unit at Imperial College London is affiliated with the NIHR Imperial Patient Safety Translational Research Centre. The NIHR Imperial Patient Safety Translational Centre is a partnership between the Imperial College Healthcare NHS Trust and Imperial College London. The School for Public Health Imperial College London is also grateful for support from the Imperial NIHR Biomedical Research Centre. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health Department of Health and Social Care.
Disclaimer: The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Competing interests: RCGP—advisory board for Galapagos; Celltrion educational sponsorship.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: POP-IBD Collaboration, Richard CG Pollok, Sonia Saxena, Alex Bottle, Irene Petersen, Jonathan Blackwell, Hanna Creese, and Nishani Jayasooriya
Data availability statement
Data may be obtained from a third party and are not publicly available.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
CPRD and HES ethical and scientific approval for the study was granted by the Independent Scientific Advisory Committee (ISAC) on 8 January 2021 (Protocol approval number: 20_000248,website URL: https://cprd.com/protocol/impact-timely-diagnosis-and-recurrent-health-seeking-behaviour-subsequent-clinical-course-health-seeking-behaviour-subsequent-clinical-course).
References
- 1. Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and duration of gastrointestinal symptoms before diagnosis of inflammatory bowel disease and predictors of timely specialist review: a population-based study. J Crohns Colitis 2021;15:203–11. 10.1093/ecco-jcc/jjaa146 [DOI] [PubMed] [Google Scholar]
- 2. Schoepfer A, Santos J, Fournier N, et al. Systematic analysis of the impact of diagnostic delay on bowel damage in paediatric versus adult onset crohn’s disease. J Crohns Colitis 2019;13:1334–42. 10.1093/ecco-jcc/jjz065 [DOI] [PubMed] [Google Scholar]
- 3. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1896–905. 10.1093/ibd/izz059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magro F, Rodrigues-Pinto E, Coelho R, et al. Is it possible to change phenotype progression in crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol 2014;109:1026–36. 10.1038/ajg.2014.97 [DOI] [PubMed] [Google Scholar]
- 5. Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of crohn’s disease in a population-based cohort from Cardiff (1986-2003): a study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. 10.1136/gut.2009.202101 [DOI] [PubMed] [Google Scholar]
- 6. Jayasooriya N, Baillie S, Blackwell J, et al. POP-IBD study group. systematic review with meta-analysis: time to diagnosis and the impact of delayed diagnosis on clinical outcomes in inflammatory bowel disease. Aliment Pharmacol Ther 2023;57:635–52. 10.1111/apt.17370 [DOI] [PubMed] [Google Scholar]
- 7. Card TR, Siffledeen J, Fleming KM. Are IBD patients more likely to have a prior diagnosis of irritable bowel syndrome? Report of a case-control study in the general practice research database. UEG Journal 2014;2:505–12. 10.1177/2050640614554217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stapley SA, Rubin GP, Alsina D, et al. Clinical features of bowel disease in patients aged &50 years in primary care: a large case-control study. Br J Gen Pract 2017;67:e336–44. 10.3399/bjgp17X690425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walker GJ, Moore L, Heerasing N, et al. Faecal calprotectin effectively excludes inflammatory bowel disease in 789 symptomatic young adults with/without alarm symptoms: a prospective UK primary care cohort study. Aliment Pharmacol Ther 2018;47:1103–16. 10.1111/apt.14563 [DOI] [PubMed] [Google Scholar]
- 10. Hawthorne AB, Glatter J, Blackwell J, et al. Inflammatory bowel disease patient‐reported quality assessment should drive service improvement: a national survey of UK IBD units and patients. Aliment Pharmacol Ther 2022;56:625–45. 10.1111/apt.17042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vadstrup K, Alulis S, Borsi A, et al. Cost burden of crohn’s disease and ulcerative colitis in the 10-year period before diagnosis-a Danish register-based study from 2003-2015. Inflamm Bowel Dis 2020;26:1377–82. 10.1093/ibd/izz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottle A, Kim D, Aylin P, et al. Routes to diagnosis of heart failure: observational study using linked data in England. Heart 2018;104:600–5. 10.1136/heartjnl-2017-312183 [DOI] [PubMed] [Google Scholar]
- 13. Arhi CS, Markar S, Burns EM, et al. Delays in referral from primary care are associated with a worse survival in patients with esophagogastric cancer. Dis Esophagus 2019;32:1–11. 10.1093/dote/doy132 [DOI] [PubMed] [Google Scholar]
- 14. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blackwell J, Saxena S, Petersen I, et al. Depression in individuals who subsequently develop inflammatory bowel disease: a population-based nested case–control study. Gut 2021;70:1642–8. 10.1136/gutjnl-2020-322308 [DOI] [PubMed] [Google Scholar]
- 16. Lewis JD, Brensinger C, Bilker WB, et al. Validity and completeness of the general practice research database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf 2002;11:211–8. 10.1002/pds.698 [DOI] [PubMed] [Google Scholar]
- 17. Chhaya V, Saxena S, Cecil E, et al. The impact of timing and duration of thiopurine treatment on colectomy in ulcerative colitis: a national population-based study of incident cases between 1989–2009. Aliment Pharmacol Ther 2015;41:87–98. 10.1111/apt.13017 [DOI] [PubMed] [Google Scholar]
- 18. Alexakis C, Saxena S, Chhaya V, et al. Smoking status at diagnosis and subsequent smoking cessation: associations with corticosteroid use and intestinal resection in crohn's disease. Am J Gastroenterol 2018;113:1689–700. 10.1038/s41395-018-0273-7 [DOI] [PubMed] [Google Scholar]
- 19. Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010;4:7–27. 10.1016/j.crohns.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 20. Blackwell J, Saxena S, Alexakis C, et al. The impact of smoking and smoking cessation on disease outcomes in ulcerative colitis: a nationwide population-based study. Aliment Pharmacol Ther 2019;50:556–67. 10.1111/apt.15390 [DOI] [PubMed] [Google Scholar]
- 21. Ahmad A, Laverty AA, Alexakis C, et al. Changing nationwide trends in endoscopic, medical and surgical admissions for inflammatory bowel disease: 2003–2013. BMJ Open Gastroenterol 2018;5:e000191. 10.1136/bmjgast-2017-000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dai Z, Ma Y, Zhan Z, et al. Analysis of diagnostic delay and its influencing factors in patients with chronic obstructive pulmonary disease: a cross-sectional study. Sci Rep 2021;11:14213:14213. 10.1038/s41598-021-93499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duricova D, Burisch J, Jess T, et al. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis 2014;8:1351–61. 10.1016/j.crohns.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 24. Fairbrass KM, Lovatt J, Barberio B, et al. Bidirectional brain-gut axis effects influence mood and prognosis in IBD: a systematic review and meta-analysis. Gut 2022;71:1773–80. 10.1136/gutjnl-2021-325985 [DOI] [PubMed] [Google Scholar]
- 25. Umar N, King D, Chandan JS, et al. The association between inflammatory bowel disease and mental ill health: a retrospective cohort study using data from UK primary care. Aliment Pharmacol Ther 2022;56:814–22. 10.1111/apt.17110 [DOI] [PubMed] [Google Scholar]
- 26. Blackwell J, Alexakis C, Saxena S, et al. Association between antidepressant medication use and steroid dependency in patients with ulcerative colitis: a population-based study. BMJ Open Gastroenterol 2021;8:e000588. 10.1136/bmjgast-2020-000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yohannes AM, Willgoss TG, Baldwin RC, et al. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry 2010;25:1209–21. 10.1002/gps.2463 [DOI] [PubMed] [Google Scholar]
- 28. Marston L, Carpenter JR, Walters KR, et al. Smoker, ex-smoker or non-smoker? The validity of routinely recorded smoking status in UK primary care: a cross-sectional study. BMJ Open 2014;4:e004958. 10.1136/bmjopen-2014-004958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hong Z, Ren J, Li Y, et al. Delayed diagnosis is associated with early and emergency need for first Crohn’s disease-related intestinal surgery. Med Sci Monit 2017;23:4841–6. 10.12659/msm.904238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walker GJ, Lin S, Chanchlani N, et al. Quality improvement project identifies factors associated with delay in IBD diagnosis. Aliment Pharmacol Ther 2020;52:471–80. 10.1111/apt.15885 [DOI] [PubMed] [Google Scholar]
- 31. Krishna M, Britto S, Qian J, et al. Diagnostic delay and colectomy risk in pediatric ulcerative colitis. J Pediatr Surg 2020;55:403–5. 10.1016/j.jpedsurg.2019.03.012 [DOI] [PubMed] [Google Scholar]
- 32. Rinawi F, Assa A, Eliakim R, et al. Risk of colectomy in patients with pediatric-onset ulcerative colitis. J Pediatr Gastroenterol Nutr 2017;65:410–5. 10.1097/MPG.0000000000001545 [DOI] [PubMed] [Google Scholar]
- 33. Kang HS, Koo JS, Lee KM, et al. Two-year delay in ulcerative colitis diagnosis is associated with anti-tumor necrosis factor alpha use. World J Gastroenterol 2019;25:989–1001. 10.3748/wjg.v25.i8.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zaharie R, Tantau A, Zaharie F, et al. Diagnostic delay in Romanian patients with inflammatory bowel disease: risk factors and impact on the disease course and need for surgery. J Crohns Colitis 2016;10:306–14. 10.1093/ecco-jcc/jjv215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodríguez-Lago I, Merino O, Azagra I, et al. Characteristics and progression of preclinical inflammatory bowel disease. Clin Gastroenterol Hepatol 2018;16:1459–66. 10.1016/j.cgh.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 36. Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371:m4087. 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neal RD, Tharmanathan P, France B, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015;112 Suppl 1:S92–107. 10.1038/bjc.2015.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–106. 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodríguez-Lago I, Agirre U, Intxaurza N, et al. Increased use of healthcare resources during the preclinical period of inflammatory bowel disease. Dig Liver Dis 2021;53:927–30. 10.1016/j.dld.2021.04.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2024-001371supp001.pdf (217.7KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available.