Abstract
Background.
The current study examined if early adversity was associated with accelerated biological aging, and if effects were mediated by the timing of puberty.
Methods.
In early mid-life, 187 Black and 198 White (, ) women reported on early abuse and age at first menstruation (menarche). Women provided saliva and blood to assess epigenetic aging, telomere length, and C-reactive protein. Using structural equation modeling, we created a latent variable of biological aging using epigenetic aging, telomere length, and C-reactive protein as indicators, and a latent variable of early abuse using indicators of abuse/threat events before age 13, physical abuse, and sexual abuse. We estimated the indirect effects of early abuse and of race on accelerated aging through age at menarche. Race was used as a proxy for adversity in the form of systemic racism.
Results.
There was an indirect effect of early adversity on accelerated aging through age at menarche (b = 0.19, 95% CI 0.03–0.44), in that women who experienced more adversity were younger at menarche, which was associated with greater accelerated aging. There was also an indirect effect of race on accelerated aging through age at menarche (b = 0.25, 95% CI 0.04–0.52), in that Black women were younger at menarche, which led to greater accelerated aging.
Conclusions.
Early abuse and being Black in the USA may both induce a phenotype of accelerated aging. Early adversity may begin to accelerate aging during childhood, in the form of early pubertal timing.
Keywords: aging, child abuse, epigenetic clock, inflammation, puberty, telomeres
Childhood adversity has lifelong consequences in the form of poor health in adulthood and premature mortality in that experiencing adverse events before age 18 reliably increases the risk for nine out of 10 leading causes of death in the USA (Hughes et al., 2017; Merrick et al., 2019). Health is consistently worse for Black Americans compared to White Americans, including higher rates of the leading causes of death in the USA (Adler & Rehkopf, 2008; Williams, 2012). Higher levels of early life adversity may help to explain health disparities between Black and White Americans (Slopen et al., 2016). To reduce health disparities, we need to understand the mechanisms that underlie the relationship between early adversity and health outcomes.
Early adversity has been associated with accelerated biological aging (i.e. older than chronological age) as indexed by epigenetic clocks, which are reliable predictors of disease and mortality (Field et al., 2018; Horvath & Raj, 2018). Previous studies have found childhood trauma predicts accelerated epigenetic aging for children and adults (Hamlat et al., 2021; Sumner, Colich, Uddin, Armstrong, & McLaughlin, 2019; Wolf et al., 2018; Zannas et al., 2015). Moreover, early adversity has been associated with other indices of cell aging that predict disease and mortality, including shorter telomere length (TL; Li, He, Wang, Tang, & Chen, 2017) and elevated C-reactive protein (CRP; Baumeister, Akhtar, Ciufolini, Pariante, & Mondelli, 2016). Childhood adversity predicts shorter TL (Ridout et al., 2018); in turn, shorter TL predicts the earlier onset of diseases of aging, such as cardiovascular disease (CVD; D’Mello et al., 2015; Haycock et al., 2014; Willeit et al., 2014). CRP, an index of systemic inflammation which increases with age and thought to be a mechanism of aging-related disease (Kaptoge et al., 2010), has been associated with childhood trauma in meta-analysis (Baumeister et al., 2016).
Early pubertal timing as accelerated aging
In a risky environment, pubertal maturation may accelerate to increase opportunities to reproduce before the individual dies or becomes compromised, potentially at the expense of investments in adult health and longevity (Belsky, Steinberg, & Draper, 1991; Ellis, 2004). Early adversity may recalibrate the hypothalamo-pituitary-adrenocortical (HPA) axis, which influences sexual development directly and affects the hypothalamic-pituitary-gonadal axis. Adversity may attenuate HPA-axis function, which results in the earlier onset of adrenal and gonadal hormones and has predicted earlier pubertal development in girls (Saxbe, Negriff, Susman, & Trickett, 2015).
Early pubertal timing has been associated with higher morbidity and earlier mortality (Charalampopoulos, McLoughlin, Elks, & Ong, 2014) and with accelerated biological aging as indexed by epigenetic clocks (Binder et al., 2018; Hamlat et al., 2021; Simpkin et al., 2017; Sumner et al., 2019) and TL (Koss et al., 2020). In meta-analysis, early adversity was associated with early pubertal timing as well as accelerated cellular aging, as indexed by both epigenetic age and TL (Colich, Rosen, Williams, & McLaughlin, 2020). The possibility that there is an indirect effect of early adversity on health through pubertal timing has rarely been empirically examined. In one study of 73 girls, maternal depression in infancy predicted higher basal cortisol, which predicted accelerated pubertal (adrenarcheal) development, which resulted in health problems at age 18 (Belsky, Ruttle, Boyce, Armstrong, & Essex, 2015). The recalibration of the HPA-axis may also speed up the pace of development (Colich & McLaughlin, 2022), which would result in earlier pubertal timing and faster aging of physiological systems, more generally, which would be captured in assessments of biological aging.
Multiple biomarkers as an index of biological aging
While a growing number of studies have examined how specific biomarkers may predict health outcomes, few studies have combined biomarkers. Epigenetic aging, TL, and CRP are usually studied independently from one another; however, integrating multiple markers of biological aging may allow for a more complete profile of multisystem-level aging (Liu et al., 2019). Different indices of biological aging are likely to have reciprocal effects on one another regulated by feedback loops (Gassen, Chrousos, Binder, & Zannas, 2017). Given their associations with ELA and interrelationships with one another, epigenetic aging, TL, and elevated CRP may be aggregated and operationalized as a phenotype of biological aging. Each biomarker may represent distinct pathways of aging (Belsky et al., 2018); their shared variance allows them to be used as indicators of a latent variable.
The current study examines if there is an indirect effect of adversity on such an aggregate composite of accelerated biological aging through pubertal timing. Using structural equation modeling, we created a latent variable of biological aging using epigenetic aging, TL, and CRP as indicators. Structural equation modeling (SEM)s partition variance unique to each indicator into separate residual variance estimates, purifying the structural path coefficients of measurement error, which is partitioned into the indicator-specific residual variances (Kline, 2011).
Race differences in aging biomarkers
Race differences in health outcomes are largely due to social determinants and to systemic racism, which is a fundamental cause of racial inequities in health (Churchwell et al., 2020; Paradies et al., 2015; Phelan & Link, 2015; Williams & Rucker, 2000; Williams, Lawrence, & Davis, 2019). Systemic racism and related factors that contribute to health disparities between Black and White Americans may engender a phenotype or profile of accelerated aging (Hooten, Pacheco, Smith, & Evans, 2022). In one study, by age 30, Black women had a higher allostatic load (i.e. physiological wear on bodily systems due to adaptation to stress; McEwen, 1998) than Black men and White Americans of the same age (Geronimus, Hicken, Keene, & Bound, 2006). Although there is variance across studies, evidence supports Black Americans have longer TL than White Americans (Hunt et al., 2008; Needham et al., 2019), and that the rate of TL shortening for Blacks may be more rapid than for Whites across time (Rewak et al., 2014). In some studies, by ages 49–55, Black women have shorter TL than White women (Geronimus et al., 2010). Black adults have larger prospective increases in CRP over time than White adults (Zahodne, Kraal, Zaheed, Farris, & Sol, 2019) and Black women have higher CRP levels than Black men and White adults (Khera et al., 2005).
There is inconsistent evidence over whether Black Americans have accelerated, decelerated, or equivalent epigenetic aging as compared to White Americans; however, among postmenopausal women, Black women showed accelerated epigenetic aging in comparison to White women (Hamlat et al., 2022; Horvath et al., 2016; Liu et al., 2019). In addition to differences in aging biomarkers during adulthood, Black girls experience puberty at significantly younger ages than White girls (Bleil, Booth-LaForce, & Benner, 2017; Freedman et al., 2002), and so demonstrate accelerated aging as indexed by pubertal timing. The current study examined the indirect effect of race (used as a proxy for adversity in the form of systemic racism, which was not directly measured) on accelerated aging through pubertal timing.
The current study
The aims of the current study were to establish there if there was an indirect effect of early adversity on accelerated aging through pubertal timing. The current study makes use of a well-established and well-characterized longitudinal cohort from the National Heart, Lung, and Blood Institute Growth and Health Study (NGHS; Morrison, 1992) that followed young Black and White girls annually for over a decade starting at ages 9–10. As meta-analysis has concluded that specifically adversity characterized as abuse/threat in early life is related to earlier pubertal development (Colich et al., 2020; Sumner et al., 2019), we operationalized childhood adversity as abuse/threat. We also examined the indirect effect of race on accelerated aging through pubertal timing. To operationalize both early adversity and accelerated aging, we created two latent variables for the respective constructs using SEM. We used three indicators of abuse/threat to construct a latent variable of early life adversity (general abuse/threat before age 13, sexual abuse, and physical abuse). To operationalize accelerated aging, we created a latent variable using the indicators of epigenetic aging, TL, and CRP.
Methods and materials
Participants and procedure
The National Heart, Lung, and Blood Institute Growth and Health Study (NGHS) (1992) assessed Black and White girls annually for 10 years and re-recruited them as adults in early middle age. The initial aims were to track cardiovascular risk factors and other health-related variables annually from childhood through young adulthood in self-identified Black and White girls from Richmond (CA, USA), Cincinnati (OH, USA), and Washington (D.C, USA). In 1987–1988, the NGHS Contra Costa County cohort (887 girls) was recruited at ages 9 and 10 from public and parochial schools in the Richmond Unified School District area. Retention across the 10-year study period was 89%. More details about the initial study sample are available (NGHS, 1992).
In 2016, a follow-up study of the NGHS Contra Costa County cohort was initiated to assess health and well-being in midlife. Over 73% of eligible women (307 Black and 317 White) were enrolled in the follow-up study. Eligibility criteria for the follow-up study included: (1) being an original NGHS participant; (2) not pregnant at the time of recruitment, and had not experienced a pregnancy, miscarriage, or abortion within the last 3 months; and (3) not living abroad, nor incarcerated or otherwise institutionalized. Multiple recruitment strategies were used to re-recruit original NGHS participants from the Richmond site, including mailing and telephone follow-up, social media and electronic outreach, and door-to-door outreach. Eligible participants provided written informed consent and participated in (1) a baseline survey and (2) a home/clinic visit, including blood and saliva collection for biomarker assessment. This study was approved by the local Institutional Review Board.
The sample for the current study consisted of 385 participants (187 Black, 198 White, , ), who provided blood or saliva samples as part of the follow-up study. The 498 girls who were part of the original NGHS study, but who were not part of the current study, did not significantly differ in race, , p = 0.12, childhood income, , p = 0.87, or age at menarche, t(833) = 0.37, p = 0.71, from the 385 women of the current study.
Early life adversity
Early adversity was measured via a latent construct combining three measures (general abuse, sexual, and physical abuse) as described below. Creating a latent variable of adversity minimizes measurement error and allowed us to focus on the variance shared between the two measures.
General threat/abuse.
The Stress and Adversity Inventory (STRAIN, Slavich & Shields, 2018) was used to retrospectively measure the number of abuse/threat events before age 13. The STRAIN reliably assesses a person’s cumulative exposure to stress over the life course by systematically inquiring about a diverse array of acute life events and chronic difficulties. The STRAIN is widely used for the assessment of both retrospective and prospective life events. Cumulative stress exposure assessed with the STRAIN has been linked to poor metabolic health and mental health in young adulthood (Toussaint, Shields, Dorn, & Slavich, 2016). The general abuse/threat variable included stressors involving emotional abuse, sexual abuse, physical abuse, and prolonged harsh parental discipline. Respondents were also asked at what age the stressor occurred. The abuse/threat variable represented the total events that occurred during the ages of 0–12 years old.
Physical abuse and sexual abuse.
Adapted from Felitti et al., (1998), participants were asked if they had experienced physical abuse or sexual abuse. The physical abuse and sexual abuse variables represented if abuse before age 18 was endorsed.
Pubertal timing
Menarche.
During the initial NGHS study, each year from baseline (ages 9/10) for 10 years, participants self-reported age at menarche. When there were multiple reports of age at menarche, the average of all reports was used.
Accelerated aging
Epigenetic age acceleration (GrimAge).
DNA methylation analyses with saliva samples were performed at the Semel Institute UCLA Neurosciences Genomics Core (UNGC) using the Illumina Infinium HumanMethylation450 BeadChip (Illumina, Inc.). Genomic DNA was isolated using temperature denaturation and subjected to bisulfite conversion, PCR amplification, and DNA sequencing (EZ DNA Methylation-Gold Kit, Zymo Research, Tustin, California, USA). Methylation profiles were input to Horvath’s online calculator https://dnamage.genetics.ucla.edu/, which automatically imputes any missing CpGs. After selection of the advanced analysis option and normalization based on the BMIQ method (Teschendorff et al., 2013), the output files contain the estimated epigenetic age of each participant and measures of predictive accuracy and data quality (e.g. for identifying array outliers, ‘corSample VSgoldstandard’). Before data analysis began, participants (n = 26) were excluded due to quality control issues.
DNAm GrimAge†,1 is based on 1030 unique CpGs that robustly predict mortality as well as age-related conditions such as CVD (Li et al., 2020; Lu et al., 2019). We used the recent optimized version of GrimAge which uses new DNAm estimators of plasma proteins: high sensitivity CRP and hemoglobin A1C (Lu et al., 2019). ‘AgeAccelerationResidual’, the residual resulting from a linear model where DNAm age is regressed on chronological age, was the outcome variable. Positive residual values reflected an individual being older biologically than chronological age and negative residual values reflected the reverse.
Telomere length measurement.
Genomic DNA was extracted from 500 of saliva collected in the Oragene DNA kit (cat# OG-500, DNA Genotek Inc. Kanata, Ontario, Canada) with the DNA Agencourt DNAdvance kit (cat# A48705, Beckman Coulter Genomics Inc. Brea CA). DNA was stored at −80 °C and TL assays were performed between May and June of 2020. DNA was quantified by measuring OD260 with a NanoDrop 2000c Spectrophotometer (Nanodrop Products, Wilmington, DE, USA) and ran on 0.8% agarose gels to check DNA integrity. Samples that passed the quality control of OD260/OD280 between 1.7 and 2.0, concentration greater than 10 ng/ and no degradation were used for TL measurement. Five samples had concentration lower than 10 ng/ and nine samples were degraded.
The TL measurement assay was adapted from the published original method by Cawthon (Cawthon, 2002; Lin et al., 2010). After applying Dixon’s Q test to remove outliners, the average concentrations of T and S from the triplicate wells were used to calculate the T/S ratios. T/S ratio for each sample was measured twice. When the duplicate T/S value and the initial value varied by more than 7%, the sample was run the third time and the two closest values were reported. 26% samples were run a third time. The coefficient of variation for this study was 2.2 ± 1.6%. The PCR efficiencies for the T and S reactions were 92.9 ± 2.5% and 95.2 ± 2.4% respectively. DNA extraction and TL assays for the entire study were performed using the same lots of reagents. Lab personnel who performed the assays were provided with de-identified samples and were blind to all demographic and clinical data. The intra-class correlation TL values from duplicate DNA extraction from 48 randomly selected samples from the same cohort is 0.95 (CI 0.911–0.972).
C-reactive protein (CRP).
High-sensitivity CRP assays were performed by LabCorp (test 120766, CPT 86141). Adult participants had a fasting blood draw in the morning after a 10 h fast at local labs. Blood was collected into a green-top (heparin) tube, and sent to the nearest LabCorp assay lab, which in most cases was local to Richmond, CA. LabCorp has a strictly followed standardized automated protocol, certified by CLIA (Clinical Laboratory Improvement Amendments), and used nationally in each of their labs. The blood was separated into serum from cells within 1 h of drawing. A standard clinically used assay (immunochemiluminometric assay, ICMA on the Integra 800) was used.
Data analysis
To operationalize early adversity, we used three indicators of general abuse/threat before age 13, sexual abuse, and physical abuse to construct a latent variable. We used the three indicators of DNAm GrimAge, TL, and CRP to construct the latent variable of accelerated aging. The early adversity, menarche, and accelerated aging variables were regressed on race (Black = 1, White = 0). Annual income in childhood was regressed on adversity, age at menarche and accelerated aging. Age and current income were regressed on accelerated aging. The (a) direct and indirect (through age at menarche) effects of early adversity on accelerated aging as well as the (b) direct and indirect (through age at menarche) effects of race on accelerated aging were examined (Fig. 1). Global model fit was assessed using the robust test of exact model fit. Because the test may be sensitive to trivial amounts of data-model misfit in moderate to large samples (Browne & Cudeck, 1993), we also assessed model fit using the following approximate fit indices: Bentler’s comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean square residuals (SRMR). Satisfactory model fit was determined by Hu and Bentler’s (1999) recommended two-index strategy of either (a) CFI ⩾0.95 and SRMR ⩽0.08 or (b) RMSEA ⩽0.06 and SRMR ⩽0.08. Inferences for direct and indirect effects were based on the unstandardized regression estimates b and their 95% confidence intervals (CIs), with the latter computed via the non-parametric bias-corrected bootstrap based on 5000 replicate samples (MacKinnon, Lockwood, & Williams, 2004). Descriptive statistics were computed using SPSS version 28.0; latent variable models were fitted using Mplus version 8.7 (Muthén & Muthén, 2017).
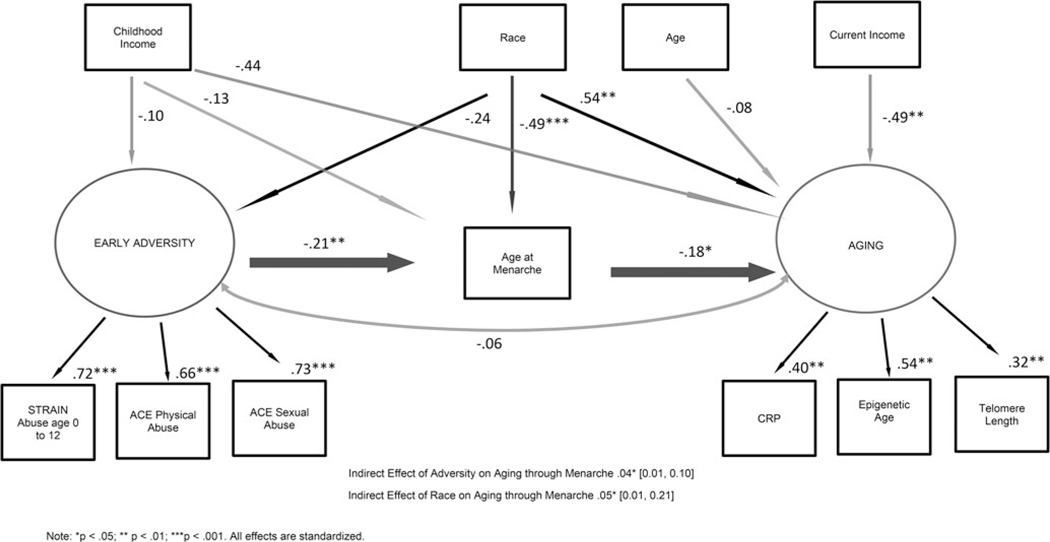
Figure 1.
Direct and indirect effects, through age at menarche, of early adversity and race on accelerated aging.
Results
Descriptive statistics are reported in Table 1 and correlations are reported in Table 2. Between Black and White women, there was no difference in the number of abuse or threat-related events before age 13 or number of sexual abuse experiences before age 18. White women (M = 1.30, S.D. = 0.46) reported significantly more experiences of physical abuse before 18 than Black women (M = 1.18, S.D. = 0.38). As expected, Black women were significantly younger at first menstruation, with a mean age at menarche of 11.96 years (S.D. = 1.22) compared to 12.47 years (S.D. = 1.23) for White women (Table 3).
Table 1.
Descriptives of study variables
| All | Black | White | t/χ2 | d | ||||
|---|---|---|---|---|---|---|---|---|
| Age years, M(S.D.) (Black = 187, White = 198) | 39.37 | (1.19) | 39.40 | (1.15) | 39.34 | (1.23) | 0.47 | 0.05 |
| Childhood income, % ⩾$60 000, n (Black = 187, White = 198) | 54.0 | (208) | 43.9 | (82) | 78.8 | (156) | 49.74*** | - |
| Annual income, % ⩾$60 000, n (Black = 187, White = 198) | 61.8 | (238) | 38.5 | (72) | 68.7 | (136) | 35.28*** | - |
| #Threat/abuse before age 13, M(S.D.) (Black = 145, White = 146) | 0.63 | (0.80) | 0.57 | (0.75) | 0.68 | (0.84) | 1.28 | 0.15 |
| #Physical abuse before age 18, M(S.D.) (Black = 181, White = 196) | 1.24 | (0.43) | 1.18 | (0.38) | 1.30 | (0.46) | 2.86** | 0.29 |
| #Sexual abuse before age 18, M(S.D.) (Black = 173, White = 189) | 1.25 | (0.43) | 1.24 | (0.43) | 1.25 | (0.44) | 0.25 | 0.03 |
| Age at menarche, years, M(S.D.) (Black = 177, White = 189) | 12.23 | (1.25) | 11.96 | (1.22) | 12.47 | (1.23) | 4.00*** | 0.42 |
| CRP, M(S.D.) (Black = 146, White = 161) | 3.29 | (3.81) | 3.95 | (4.17) | 2.69 | (3.34) | 2.90** | 0.34 |
| Epigenetic age acceleration, years, M(S.D.) (Black = 186, White = 198) | −0.07 | (5.10) | 1.23 | (5.07) | −1.29 | (4.83) | 5.00*** | 0.51 |
| Telomere length, M(S.D.) (Black = 181, White = 195) | 1.20 | (0.27) | 1.25 | (0.27) | 1.15 | (0.26) | 3.45*** | 0.36 |
Note:
p < 0.05
p < 0.01
p < 0.001.
Table 2.
Correlations of study variables
| Age | Threat/abuse | Physical abuse | Sexual abuse | Child income | Income | CRP | Epigenetic acceleration | Telomere length | Menarche | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | - | |||||||||
| Threat/abuse before 13 | 0.01 | - | ||||||||
| Physical abuse | 0.04 | 0.42*** | - | |||||||
| Sexual abuse | 0.05 | 0.45*** | 0.48*** | - | ||||||
| Childhood income | −0.05 | 0.11 | −0.06 | −0.06 | - | |||||
| Current income | 0.01 | −0.02 | 0.05 | −0.05 | 0.22*** | - | ||||
| CRP | <0.01 | −0.06 | 0.07 | 0.01 | −0.11* | −0.12* | - | |||
| Epigenetic age acceleration | −0.05 | 0.06 | <0.01 | 0.01 | −0.24*** | −0.23*** | 0.25*** | - | ||
| Telomere length | −0.05 | −0.11 | −0.06 | −0.09 | −0.09 | −0.13* | 0.07 | 0.13* | - | |
| Age at men arc he | 0.01 | −0.12 | −0.11* | −0.16** | 0.06 | 0.14** | −0.14* | −0.09 | −0.15** |
Note: Childhood income was categorized as less than $20 000/year or $20 000/year or more. Current income was categorized as less than $60 000/year or $60 000/year or more.
p < 0.05
p < 0.01
p < 0.001.
Table 3.
Direct and indirect effects, through age at menarche, of early adversity on accelerated aging (N = 385)
| Predictor | Outcome | b | S.E. | β | 95% CI | P |
|---|---|---|---|---|---|---|
| Childhood income | Early adversity | −0.06 | 0.09 | −0.10 | [−0.22 to 0.12] | 0.48 |
| Childhood income | Age at menarche | −0.16 | 0.14 | −0.13 | [−0.45 to 0.11] | 0.25 |
| Childhood income | Accelerated aging | −1.22 | 0.62 | −0.44 | [−2.47 to −0.05] | 0.049 |
| Current income | Accelerated aging | −1.35 | 0.46 | −0.49 | [−2.04 to −0.50] | 0.003 |
| Age | Accelerated aging | −0.19 | 0.20 | −0.08 | [−0.59 to 0.18] | 0.34 |
| Race | Early adversity | −0.14 | 0.08 | −0.24 | [−0.30 to 0.02] | 0.10 |
| Race | Age at menarche | −0.62 | 0.14 | −0.49 | [−0.90 to −0.34] | <0.001 |
| Race | Accelerated aging | 1.50 | 0.52 | 0.54 | [0.51–2.61] | 0.004 |
| Early adversity | Age at menarche | −0.46 | 0.14 | −0.21 | [−0.75 to −0.20] | 0.001 |
| Early adversity | Accelerated aging | −0.31 | 0.53 | −0.06 | [−1.33 to 0.91] | 0.55 |
| Age at menarche | Accelerated aging | −0.40 | 0.17 | −0.18 | [−0.73 to −0.04] | 0.02 |
| Indirect effect of adversity on aging through menarche | 0.19 | 0.09 | 0.04 | [0.03–0.44] | 0.04 | |
| Indirect effect of race on aging through menarche | 0.25 | 0.12 | 0.05 | [0.04–0.52] | 0.04 | |
Note: Confidence intervals (CI) were generated via the non-parametric bias-corrected bootstrap based on 5000 replicate samples. Childhood income was categorized as less than $20 000/year or $20 000/year or more. Current income was categorized as less than $60 000/year or $60 000/year or more.
Relative to Black women, White women were significantly more likely to have current annual incomes of at least $60 000 (68.7% v. 38.5%) and to have had household incomes of at least $20 000 annually when aged 9 or 10 (78.8% v. 43.9%).2 Black women had significantly higher epigenetic age acceleration (epigenetic age relative to chronological age) than White women. On average, Black women were epigenetically older, 1.24 (5.07), than chronological age and White women were younger, −1.29 (4.83), than chronological age. Black women had significantly higher CRP than White women with a mean of 3.95 (4.17) mg/L compared to 2.69 (3.34) mg/L for White women. Black women had significantly longer TL with a mean T/S ratio of 1.25 (0.27) compared to 1.15 (0.26) for White women.
Model of early abuse and accelerated aging
Model fit was satisfactory: χ2(36) = 63.85, p = 0.001; CFI = .90; RMSEA = 0.05 (95% CI 0.03–0.07), SRMR = 0.04. The observed variables of early adversity (abuse before age 13, physical abuse, and sexual abuse) and accelerated aging (epigenetic age acceleration, CRP, and TL) loaded significantly onto their appropriate latent variables of adversity and accelerated aging, respectively (Fig. 1).
Race was not significantly associated with early adversity (b = −0.14, 95% CI −0.30 to 0.02, β = −0.24). Race was significantly associated with age at menarche (b = −0.62, 95% CI −0.90 to −0.34, β = −0.49), and with accelerated aging (b =1.50, 95% CI 0.51–2.61, β = 0.54), in that Black women had a younger age at menarche and higher accelerated aging. Childhood income was significantly negatively associated with accelerated aging (b = −1.22, 95% CI −2.47 to −0.05, β = −0.44), but not with early adversity (b = −0.06, 95% CI −0.22 to 0.12, β = −0.10), or age at menarche (b = −0.16, 95% CI −0.45 to 0.11, β = −0.13). Current income was significantly negatively associated with accelerated aging (b = −1.35, 95% CI −2.04 to −0.50, β = −0.49).
Early adversity was significantly negatively associated with age at menarche (b = −0.46, 95% CI −0.75 to −0.20, β = −0.21) and age at menarche was significantly negatively associated with accelerated aging (b = −0.40, 95% CI −0.73 to −0.04, β = −0.18), in that higher adversity was related to younger age at menarche and younger age at menarche was related to higher accelerated aging. There was a significant positive indirect effect of early abuse on accelerated aging through age at menarche (b = 0.19, 95% CI 0.03–0.44, β = 0.04), in that woman who experienced more abuse were younger at menarche, which was associated with greater accelerated aging. The direct association between adversity and accelerated aging was not significant (b = −0.31, 95% CI −1.33 to 0.91, β = −0.06). There was also a significant positive indirect effect of race on accelerated aging through age at menarche (b = 0.25, 95% CI 0.04–0.52, β = 0.09), in that Black women were younger at menarche, which was associated with greater accelerated aging.
Discussion
In the current study, adversity in childhood was associated with an earlier age of first menstruation (i.e. menarche), which was in turn associated with accelerated biological aging in mid-life for both Black and White women. As theorized, there was an indirect effect of childhood adversity on accelerated biological aging through age at menarche. Further, Black women had an earlier age at menarche and greater accelerated aging in comparison to White women and there was an indirect effect of race on accelerated aging through age at menarche. Accelerated biological aging may begin in childhood (i.e. early pubertal timing may be a form of accelerated aging) and the accelerated aging process set in motion by abuse during childhood. In line with the biological embedding model (Ehrlich, Ross, Chen, & Miller, 2016; Miller, Chen, & Parker, 2011), adversity may act through epigenetic changes, inflammation, and other biological processes to accelerate biological aging (Finkel & Holbrook, 2000).
Results suggest women who experienced abuse in childhood may mature earlier and present as biologically older in adulthood than same-age peers. Findings align with work showing that both early abuse and earlier age at menarche were associated with faster epigenetic age acceleration (Hamlat et al., 2021). Black women demonstrated another path of vulnerability by which earlier age at menarche resulted in accelerated biological aging. Along with a younger age at menarche, Black women had greater accelerated aging compared to White women, including higher levels of CRP and faster epigenetic age acceleration. There was no difference in early life adversity, as assessed by a composite of three abuse variables, between Black and White women. Race is a proxy for systemic racism (which was not directly captured in the current study), which is a fundamental cause of racial inequities in health (Phelan & Link, 2015; Williams et al., 2019). Black women may ‘weather’ or experience greater biological aging due to race-related stressors (Geronimus, 1992), and weathering may begin to influence aging-related biomarkers beginning in childhood. Black girls experience pubertal onset at significantly younger ages than White girls (Bleil et al., 2017; Freedman et al., 2002) and so demonstrate accelerated aging as indexed by pubertal timing, which may contribute to health disparities between Black and White women in later life.
The current study provides support for the psychosocial acceleration hypothesis, which holds that in an early environment characterized by high adversity, pubertal maturation may accelerate to maximize opportunities for reproduction. The focus of resources on the acceleration of puberty may be at the expense of investments for adult health and lead to premature aging decades before the development of serious disease and dysfunction. Taken together, study findings support that early pubertal timing and accelerated biological aging represent similar evolutionary-developmental processes (Belsky, 2019; Belsky & Shalev, 2016) and that early puberty may be conceptualized as accelerated aging that begins in childhood.
Accelerated biological aging may serve as a mechanism by which early adversity contributes to health disparities, and girls who experience early adversity, especially Black girls, may benefit from intervention. Puberty is a sensitive period for recalibration of the HPA axis in children who have experienced early life adversity (Gunnar, DePasquale, Reid, & Donzella, 2019). Intervention during the pubertal transition could protect against accelerated aging and lead to healthier outcomes during adulthood. For example, supportive family environments have been found to buffer against further epigenetic age acceleration for Black youth experiencing high levels of racial discrimination (Brody, Miller, Yu, Beach, & Chen, 2016).
Strengths and limitations
The current study has notable strengths including a sufficiently large sample of Black and White women to examine the indirect effects of early adversity on accelerated biological aging through age at menarche. Secondly, this is the first study to utilize an accelerated aging latent variable using CRP, epigenetic clocks, and TL to investigate the relationship between early adversity and biological aging.3 Thirdly, we also used multiple measures of early adversity to construct a latent variable, which included abuse before age 13 as well as childhood physical and sexual abuse. To better isolate effects of early abuse, we also included a measure of early financial adversity (household income in childhood) as a covariate. Finally, examining biological aging as an outcome in midlife allows us to evaluate the influence of early adversity on biological aging decades before the onset of major disease or disability.
Study results should be considered within the context of several limitations. For one, adversity that took place in childhood was assessed retrospectively in adulthood. The accuracy of retrospective recall of childhood experiences should not be assumed (Hardt & Rutter, 2004; Reuben et al., 2016); however, retrospective report of maltreatment is practical with an adult sample (Baldwin, Reuben, Newbury, & Danese, 2019; Newbury et al., 2018). Secondly, the current study evaluated the associations between early adversity, age at menarche, and accelerated biological aging in Black and White women and did not include men. Future studies should examine similar associations in both men and women of other racial and ethnic backgrounds. For instance, children in disadvantaged neighborhoods and those with advance pubertal development had faster pace of biological aging as measured by DunedinPoAm,4 and Latinx-identifying children had a faster pace of aging than White children (Raffington et al., 2021). Thirdly, our research did not have multiple assessments of biological aging. Prospective research should evaluate the effects of early adversity and age of menarche on longitudinal changes of biological aging across the lifespan. Our research did not have multiple assessments of biological aging. Prospective research should evaluate the effects of early adversity and age of menarche on longitudinal changes of biological aging across the lifespan. Finally, in the current study, the direct effect of adversity on aging was not significant. An indirect effect may be statistically significant when the total or direct effect is not significant, and the significance of the total effect should not be used as a requirement for mediation (Fritz, Cox, & MacKinnon, 2015; Hayes, 2009; Kenny & Judd, 2014; O’Rourke & MacKinnon, 2015).
Supplementary Material
Acknowledgements.
This research was supported by the National Institute of Mental Health grant 5T32MH019391, National Institute on Aging grant 1R01AG059677 to E. S. E., National Institute on Aging diversity supplement (R01AG059677; PI: Epel) to E. J. H, and the National Institute of Child Health and Human Development grant 1R01HD073568 to B. L. This work was supported by the National Institute on Aging for the National Institutes of Health under grant number P30AG015272 (University of California San Francisco, Center for Aging in Diverse Communities). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests.
Elissa J. Hamlat, Torsten B. Neilands, Barbara Laraia, Joshua Zhang, Ake T. Lu, Jue Lin, Steve Horvath, and Elissa S. Epel reported no biomedical financial interests or potential conflicts of interest.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723001629
We a priori selected DNA GrimAge over other clocks at the time of study conception for several reasons. DNAm GrimAge has a strong association with age at menopause (Lu et al., 2019) and thus may be more likely to be associated with reproductive transitions such as age at menarche. When relationships between trauma and age at menarche with four clocks (Horvath DNAm Age, Hannum DNAm Age, DNAm PhenoAge, DNAm GrimAge), GrimAge was the only clock to have significant associations with trauma and age at menarche (Hamlat et al., 2021).
$20 000 was the approximate median household income in Richmond, CA when the initial study began in 1985. $60 000 was the approximate median household income in Richmond, CA when follow-up data collection began in 2016.
As a sensitivity analysis (Table S1), the latent accelerated aging variable was replaced by the three biomarkers in the same model. The indirect effects of adversity and of race on CRP and on TL were significant; however, the indirect effects on epigenetic aging did not reach significance. This suggests CRP and telomere length may be driving the association between early adversity and biological aging.
In Table S2, we have substituted DunedinPoAm, a newer clock which measures the pace of epigenetic aging, for GrimAge. The primary findings of an indirect effect of childhood adversity on accelerated aging through age at menarche and an indirect effect of race on accelerated aging through age at menarche are supported when DunedinPoAm is substituted; in fact, both indirect effects are larger.
The notes appear after the main text.
References
- Adler NE, & Rehkopf DH (2008). US disparities in health: Descriptions, causes, and mechanisms. Annual Review of Public Health, 29, 235–252. [DOI] [PubMed] [Google Scholar]
- Baldwin JR, Reuben A, Newbury JB, & Danese A. (2019). Agreement between prospective and retrospective measures of childhood maltreatment: A systematic review and meta-analysis. JAMA Psychiatry, 76(6), 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, & Mondelli V. (2016). Childhood trauma and adulthood inflammation: A meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry, 21(5), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, … Caspi A. (2018). Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? American Journal of Epidemiology, 187(6), 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. (2019). Early-life adversity accelerates child and adolescent development. Current Directions in Psychological Science, 28(3), 241–246. [Google Scholar]
- Belsky J, Ruttle PL, Boyce WT, Armstrong JM, & Essex MJ (2015). Early adversity, elevated stress physiology, accelerated sexual maturation, and poor health in females. Developmental Psychology, 51(6), 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, & Shalev I. (2016). Contextual adversity, telomere erosion, pubertal development, and health: Two models of accelerated aging, or one? Development and Psychopathology, 28(4), 1367–1383. [DOI] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, & Draper P. (1991). Childhood experience, interpersonal development, and reproductive strategy: An evolutionary theory of socialization. Child Development, 62(4), 647–670. [DOI] [PubMed] [Google Scholar]
- Binder AM, Corvalan C, Mericq V, Pereira A, Santos JL, Horvath S, … Michels KB (2018). Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics, 13(1), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil ME, Booth-LaForce C, & Benner AD (2017). Race disparities in pubertal timing: Implications for cardiovascular disease risk among African American women. Population Research and Policy Review, 36(5), 717–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SRH, & Chen E. (2016). Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science, 27(4), 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, & Cudeck R. (1993). Alternative ways of assessing model fit. In Bollen K& Long K(Eds.), Testing structural equation models (pp. 136–162). Newbury Park: Sage. [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalampopoulos D, McLoughlin A, Elks CE, & Ong KK (2014). Age at menarche and risks of all-cause and cardiovascular death: A systematic review and meta-analysis. American Journal of Epidemiology, 180(1), 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, … American Heart Association. (2020). Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation, 142(24), e454–e468. [DOI] [PubMed] [Google Scholar]
- Colich NL, & Mclaughlin KA (2022). Accelerated pubertal development as a mechanism linking trauma exposure with depression and anxiety in adolescence. Current Opinion in Psychology, 46, 101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, & McLaughlin KA (2020). Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin, 146(9), 721–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Mello MJ, Ross SA, Briel M, Anand SS, Gerstein H, & Paré G. (2015). Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circulation: Cardiovascular Genetics, 8(1), 82–90. [DOI] [PubMed] [Google Scholar]
- Ehrlich KB, Ross KM, Chen E, & Miller GE (2016). Testing the biological embedding hypothesis: Is early life adversity associated with a later proinflammatory phenotype?. Development and Psychopathology, 28(4pt2), 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ (2004). Timing of pubertal maturation in girls: An integrated life history approach. Psychological Bulletin, 130, 920–958. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine, 14(4), 245–258. [DOI] [PubMed] [Google Scholar]
- Field AE, Robertson NA, Wang T, Havas A, Ideker T, & Adams PD (2018). DNA methylation clocks in aging: Categories, causes, and consequences. Molecular Cell, 71(6), 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, & Holbrook NJ (2000). Oxidants, oxidative stress and the biology of ageing. Nature, 408(6809), 239–247. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, & Berenson GS (2002). Relation of age at menarche to race, time period, and anthropometric dimensions: The Bogalusa Heart Study. Pediatrics, 110(4), e43. [DOI] [PubMed] [Google Scholar]
- Fritz MS, Cox MG, & MacKinnon DP (2015). Increasing statistical power in mediation models without increasing sample size. Evaluation & the Health Professions, 38(3), 343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassen NC, Chrousos GP, Binder EB, & Zannas AS (2017). Life stress, glucocorticoid signaling, and the aging epigenome: Implications for aging-related diseases. Neuroscience & Biobehavioral Reviews, 74, 356–365. [DOI] [PubMed] [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity and Disease, 2(3), 207–221. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J. (2006). ‘Weathering’ and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96(5), 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Cruz TD (2010). Do US black women experience stress-related accelerated biological aging? Human Nature, 21(1), 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, DePasquale CE, Reid BM, & Donzella B. (2019). Pubertal stress recalibration reverses the effects of early life stress in postinstitutionalized children. Proceedings of the National Academy of Sciences, 116(48), 23984–23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat EJ, Adler NE, Laraia B, Surachman A, Lu AT, Zhang J, … Epel ES (2022). Association of subjective social status with epigenetic aging among black and white women. Psychoneuroendocrinology, 141, 105748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat EJ, Prather AA, Horvath S, Belsky J, & Epel ES (2021). Early life adversity, pubertal timing, and epigenetic age acceleration in adulthood. Developmental Psychobiology, 63(5), 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M. (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 45(2), 260–273. [DOI] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, & Willeit P. (2014). Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. British Medical Journal, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2009). Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76(4), 408–420. [Google Scholar]
- Hooten NN, Pacheco NL, Smith JT, & Evans MK (2022). The accelerated aging phenotype: The role of race and social determinants of health on aging. Ageing Research Reviews, 73, 101536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, … Jamieson BD (2016). An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biology, 17(1), 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, & Raj K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. [DOI] [PubMed] [Google Scholar]
- Hu LT, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: a Multidisciplinary Journal, 6(1), 1–55. [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, … Dunne MP (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2(8), e356–e366. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, … Aviv A. (2008). Leukocyte telomeres are longer in African Americans than in whites: The national heart, lung, and blood institute family heart study and the Bogalusa heart study. Aging Cell, 7(4), 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, & Danesh J. (2010). Emerging risk factors collaboration C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. The Lancet, 375(9709), 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny DA, & Judd CM (2014). Power anomalies in testing mediation. Psychological Science, 25(2), 334–339. [DOI] [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, … de Lemos JA (2005). Race and gender differences in C-reactive protein levels. Journal of the American College of Cardiology, 46(3), 464–469. [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Principles and practice of structural equation modeling (3rd ed.). New York, NY: The Guilford Press. [Google Scholar]
- Koss KJ, Schneper LM, Brooks-Gunn J, McLanahan S, Mitchell C, & Notterman DA (2020). Early puberty and telomere length in preadolescent girls and mothers. The Journal of Pediatrics, 222, 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ploner A, Wang Y, Magnusson PK, Reynolds C, Finkel D, … Hägg S. (2020). Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. Elife, 9, e51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, He Y, Wang D, Tang J, & Chen X. (2017). Association between childhood trauma and accelerated telomere erosion in adulthood: A meta-analytic study. Journal of Psychiatric Research, 93, 64–71. [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, … Blackburn E. (2010). Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. Journal of Immunological Methods, 352(1–2), 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen BH, Assimes TL, Ferrucci L, Horvath S, & Levine ME (2019). The role of epigenetic aging in education and racial/ethnic mortality disparities among older US Women. Psychoneuroendocrinology, 104, 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, … Whitsel EA (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, & Williams J. (2004). Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research, 39(1), 99–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, Guinn AS, Chen J, Klevens J, … Ottley P. (2019). Vital signs: Estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention – 25 States, 2015–2017. Morbidity and Mortality Weekly Report, 68(44), 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. (1992). Obesity and cardiovascular disease risk factors in black and white girls: The NHLBI Growth and Health Study. American Journal of Public Health, 82(12), 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2017). Mplus user’s guide (8th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Needham BL, Salerno S, Roberts E, Boss J, Allgood KL, & Mukherjee B. (2019). Do black/white differences in telomere length depend on socioeconomic status? Biodemography and Social Biology, 65(4), 287–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury JB, Arseneault L, Moffitt TE, Caspi A, Danese A, Baldwin JR, & Fisher HL (2018). Measuring childhood maltreatment to predict early-adult psychopathology: Comparison of prospective informant-reports and retrospective self-reports. Journal of Psychiatric Research, 96, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke HP, & MacKinnon DP (2015). When the test of mediation is more powerful than the test of the total effect. Behavior Research Methods, 47, 424–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, … Gee G. (2015). Racism as a determinant of health: a systematic review and meta-analysis. PloS ONE, 10(9), e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC, & Link BG (2015). Is racism a fundamental cause of inequalities in health? Annual Review of Sociology, 41, 311–330. [Google Scholar]
- Raffington L, Belsky DW, Kothari M, Malanchini M, Tucker-Drob EM, & Harden KP (2021). Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics, 147(6), e2020024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, … Danese A. (2016). Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry, 57(10), 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, … Kubzansky LD (2014). Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biological Psychology, 99, 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, … Tyrka AR (2018). Early life adversity and telomere length: A meta-analysis. Molecular Psychiatry, 23(4), 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Negriff S, Susman EJ, & Trickett PK (2015). Attenuated hypothalamicpituitary-adrenal axis functioning predicts accelerated pubertal development in girls 1 year later. Development and Psychopathology, 27(3), 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin AJ, Howe LD, Tilling K, Gaunt TR, Lyttleton O, McArdle WL, … Relton CL (2017). The epigenetic clock and physical development during childhood and adolescence: Longitudinal analysis from a UK birth cohort. International Journal of Epidemiology, 46 (2), 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Shonkoff JP, Albert MA, Yoshikawa H, Jacobs A, Stoltz R, & Williams DR (2016). Racial disparities in child adversity in the US: Interactions with family immigration history and income. American Journal of Preventive Medicine, 50(1), 47–56. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, & Beck S. (2013). A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics, 29(2), 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Heart Lung, and Blood Institute Growth and Health Study Research Group. (1992). Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. American Journal of Public Health, 82(12), 1613–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, & Slavich GM (2016). Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. Journal of Health Psychology, 21(6), 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeit P, Raschenberger J, Heydon EE, Tsimikas S, Haun M, Mayr A, … Kiechl S. (2014). Leucocyte telomere length and risk of type 2 diabetes mellitus: New prospective cohort study and literature-based meta-analysis. PLoS ONE, 9(11), e112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (2012). Miles to go before we sleep: Racial inequities in health. Journal of Health and Social Behavior, 53(3), 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Lawrence JA, & Davis BA (2019). Racism and health: Evidence and needed research. Annual Review of Public Health, 40, 105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Rucker TD (2000). Understanding and addressing racial disparities in health care. Health Care Financing Review, 21(4), 75. [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, … Logue MW (2018). Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology, 92, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Zaheed A, Farris P, & Sol K. (2019). Longitudinal effects of race, ethnicity, and psychosocial disadvantage on systemic inflammation. SSM-Population Health, 7, 100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, … Mehta D. (2015). Lifetime stress accelerates epigenetic aging in an urban, African American cohort: Relevance of glucocorticoid signaling. Genome Biology, 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.