Abstract
Acute respiratory distress syndrome (ARDS) represents a life-threatening inflammatory reaction marked by refractory hypoxaemia and pulmonary oedema. Despite advancements in treatment perspectives, ARDS still carries a high mortality rate, often due to systemic inflammatory responses leading to multiple organ dysfunction syndrome (MODS). Indeed, the deterioration and associated mortality in patients with acute lung injury (LI)/ARDS is believed to originate alongside respiratory failure mainly from the involvement of extrapulmonary organs, a consequence of the complex interaction between initial inflammatory cascades related to the primary event and ongoing mechanical ventilation-induced injury resulting in multiple organ failure (MOF) and potentially death. Even though recent research has increasingly highlighted the role of the gastrointestinal tract in this process, the pathophysiology of gut dysfunction in patients with ARDS remains mainly underexplored. This review aims to elucidate the complex interplay between lung and gut in patients with LI/ARDS. We will examine various factors, including systemic inflammation, epithelial barrier dysfunction, the effects of mechanical ventilation (MV), hypercapnia, and gut dysbiosis. Understanding these factors and their interaction may provide valuable insights into the pathophysiology of ARDS and potential therapeutic strategies to improve patient outcomes.
Introduction
ARDS represents a severe form of respiratory failure characterized by acute hypoxaemia and bilateral radiographic infiltrates due to lung inflammation and excessive alveolocapillary permeability, not attributed to cardiogenic pulmonary edema [1]. In 1821, Laennec documented cases of fatal "idiopathic pulmonary edema," marking the earliest known recording of the syndrome. During the First and Second World Wars, cases were observed in which different traumatic injuries could result in lung oedema over time. This led to the introduction of the term "shock lung" to describe the phenomenon. In 1967, Ashbaugh and colleagues published a case series involving 12 patients who developed respiratory failure in the context of various clinical conditions, representing the first systematic presentation of the syndrome [2, 3]. Since then, the clinical definition of ARDS has undergone significant revisions. Initially, an American–European consensus conference was convened in 1992 by the American Thoracic Society and the European Society of Intensive Care Medicine [4], followed by further revisions led by the ARDS Definition Task Force in Berlin in 2012 under the guidance of the European Society of Intensive Care Medicine [5, 6]. These revisions aimed to optimize the focus on the syndrome’s radiological manifestations and the severity of oxygenation impairment [3, 6]. The current definition, known as the Berlin definition, specifies that at the time of diagnosis, patients must receive at least 5 cmH2O of positive end-expiratory pressure (PEEP) [5, 6].
ARDS is a common condition, with epidemiological studies indicating that over three million individuals worldwide are diagnosed with it annually, constituting approximately 10% of those admitted to intensive care units (ICUs) [7]. Despite significant recent progress in treatment modalities and supportive care for ARDS patients, which includes the adoption of extracorporeal membrane oxygenation and protective lung ventilation strategies [8], the mortality rate associated with ARDS remains high, ranging from 34.9 to 46.1% [9]. In critically ill patients, hypoxia and/or hypercapnia seldom directly contribute to mortality. Today it is well established, that rather it emerges from the initiation of a systemic inflammatory response, leading to MODS and its associated complications. Notably, significant attention has been directed toward the role of the gastrointestinal (GI) tract in the pathogenesis of this syndrome [10]. Indeed, the significant challenge for clinicians and researchers is associated with the diverse nature of ARDS, which is evident in its various causes, manifestations, and responses to therapy. This complexity underscores the necessity for precise supportive care and the exploration of potential therapeutic strategies [11, 12].
The gut is recognized to have a substantial impact on critical illness, including trauma, pancreatitis, haemorrhagic shock, burns, and ARDS, by modifying systemic inflammation and pathogenesis of sepsis with a pivotal role in the pathophysiology of MODS in critically ill patients [13–17]. Previous theories suggested that intestinal hyperpermeability results in bacterial translocation into the systemic circulation in critical illness. However, today, it is widely recognized that the pathophysiological mechanisms involved are more complex than previously assumed. Indeed, critical illness impacts all aspects of the gut, including the epithelium, the immune system, and the microbiome, thus potentially initiating a pathological host response reaction [18]. Investigation of organ crosstalk in critical illness has revealed multifactorial biological communication between different organ systems, including gut-lung crosstalk [19]. Certainly, mounting evidence demonstrates the role of the gut microbiome in critical illness, including its impact on the onset, progression, and outcomes of the underlying disease [20]. It has been suggested that gut bacteria may enter the lungs through translocation during critical illness, facilitated by increased gut and alveolo-capillary permeability. This process can influence disease progression, drug response, and systemic inflammatory reactions and contribute to organ dysfunction. Among mechanically ventilated patients, there is an observed enrichment of lung microbiota with gut-associated microbes, which has been linked to the development of ARDS [21, 22]. However, the impact of LI/ARDS on gut homeostasis and function in patients with LI/ARDS has received limited attention. This review investigates recent advancements in understanding the interplay between the lungs and the gut in patients with LI/ARDS. Emphasis is placed on systemic and local inflammation, epithelial barrier dysfunction, effects of MV, hypercapnia, and gut microbiome dysbiosis, all of which are believed to influence lung-gut interactions significantly. In light of our recent comprehensive research into the gut-lung axis, as presented in our work on the triple hit hypothesis of LI in patients with acute brain injury [23], this review exclusively focuses on elucidating the lung-gut direction of the bidirectional communication between the lungs and the gut in LI/ARDS.
Impact of systemic inflammation on gut integrity in patients with ARDS
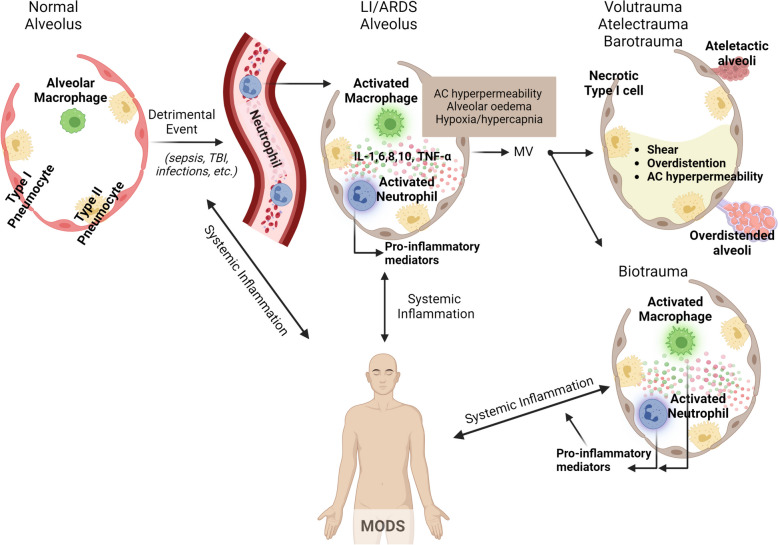
Severe acute inflammation plays a fundamental role in the pathogenesis of LI/ARDS [24]. Dysregulated systemic inflammation, marked by a sustained increase in circulating inflammatory cytokines and chemokines, is recognized as the central pathogenetic process leading to organ dysfunction and failure in ARDS patients (Fig. 1) [25]. Indeed, ARDS is characterized by two-way interactions between the lungs and other organ systems, rather than being localized solely to the pulmonary system, manifesting as a systemic inflammatory condition associated with elevated levels of inflammatory cytokines. Certainly, interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-8 are observed in both bronchoalveolar lavage fluid (BALF) and circulating plasma of individuals with ARDS [25]. This abnormal inflammatory response is a primary driver of both short-term and long-term morbidity and mortality in this patient population [25, 26], prompting tissue alterations in vital organs, culminating in the development of MODS (Fig. 1) [27]. Longitudinal evaluations of inflammatory cytokine levels have revealed that systemic and pulmonary inflammation persists for several weeks and may, in severe cases, extend for long periods of time after the clinical resolution of respiratory failure and extubation [25]. However, recent data regarding the duration of pro-inflammatory responses, particularly in the context of increased steroid use for septic shock, severe community-acquired pneumonia (CAP), and ARDS as per current recommendations and studies [28], remains unclear.
Fig. 1.
In patients with LI/ARDS, damage to the endothelium of pulmonary capillaries occurs, leading to the migration of activated immune cells into the lungs, thereby exacerbating the pulmonary inflammatory response. Within the air space, alveolar macrophages locally secrete cytokines to induce chemotaxis and activate neutrophils, which, in turn, release various pro-inflammatory molecules. The use of MV may further worsen lung injury, potentially resulting in excessive alveolar distension (volutrauma), the repetitive cyclic opening and closure of alveoli (atelectrauma), and the initiation of a complex inflammatory cascade, leading to both local and systemic inflammation (biotrauma). This inflammation can extend to distant organs and systems, exacerbating multiple organ dysfunction. ARDS acute respiratory distress syndrome, IL interleukin, LI lung injury, MODS multiple organ dysfunction syndrome, MV mechanical ventilation, TBI traumatic brain injury, TNF tumor necrosis factor
It has been suggested that specific etiologies of ARDS are associated with an elevated incidence of systemic inflammation. Particularly, sepsis, traumatic brain injury (TBI), and burn injury are identified as primary instigators of LI/ARDS within the ICU, with severe acute inflammation playing a pivotal role in its pathogenesis [29–33].
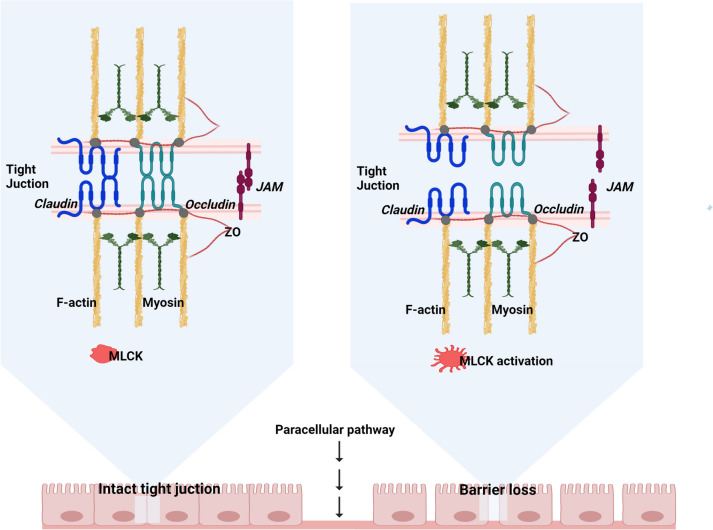
In the context of sepsis-associated LI/ARDS, enhanced production and augmentation of inflammatory cytokines have been associated with intestinal barrier dysfunction [34] and a significant increase in intestinal permeability, closely linked to the onset and progression of sepsis [35], and disrupted mucous layer integrity, characterized by compromised adhesion, reduced thickness, and decreased lumen coverage [36]. Indeed, cytokine storm leads to intestinal hyperpermeability through functional alterations of claudins, particularly up-regulation of junctional adhesion molecules (JAM) and claudin 2 and down-regulation of claudin 5 [37] and distribution of claudins 1, 3, 4, 5, and 8 as well, resulting in intestinal barrier dysfunction [38], disrupted paracellular transport of solutes and increased permeability to macromolecules (Fig. 2) [39]. Moreover, paracellular hyperpermeability is further mediated by cytokine-associated activation of myosin light chain kinase (MLCK), with cytokines further amplifying MLCK activity through a feed-forward mechanism, partly via alterations in claudin 15 [40] (Fig. 2). Similarly, in prospective cohort studies, patients with TBI displayed severe hypercytokinaemia (IL-1β, IL-6, IL-8, IL-10, and TNF-α) [33]. Elevated levels of TNF-α, IL-1β, and IL-6 in patients with TBI can impact the permeability of tight junctions (TJ) in the intestine [41]. Indeed, TNF-α binds to TNF receptors (TNFRs) on intestinal epithelial cells, initiating the upregulation of molecular pathways associated with pro-inflammatory cytokines like nuclear factor kB (NF-kB). Additionally, sympathetic hyperactivation following acute brain injury induces splanchnic hypoperfusion, leading to modifications in intestinal TJ proteins such as occludin and zonula occludens-1 (ZO-1), ultimately corresponding to intestinal hyperpermeability (Fig. 2) [42, 43].
Fig. 2.
The role of the gut in both health and critical illness. In critical illness, alterations in TJ integrity, which is vital for maintaining homeostasis, lead to loss of gut integrity and increased permeability. JAM junctional adhesion molecules, MLCK myosin light chain kinase, ZO zonula occludens
Recent research supports the hypothesis that impaired intestinal permeability leads to the bacterial translocation of gut microbes, such as Bacteroidetes and Enterobacteriaceae, entering the lungs of ARDS patients [44]. The translocation of bacteria initiates local activation of the mucosal immune system (MIS), prompting the production of inflammatory compounds (danger-associated molecular patterns-DAMPs) that traverse the mesenteric lymphatics to enter the lung and systemic circulation. Recognition of these molecules by innate immune cells fosters additional pro-inflammatory pathways, accelerating the progression of organ damage and MODS, including the gut [35, 45, 46].
Impact of mechanical ventilation on gut integrity
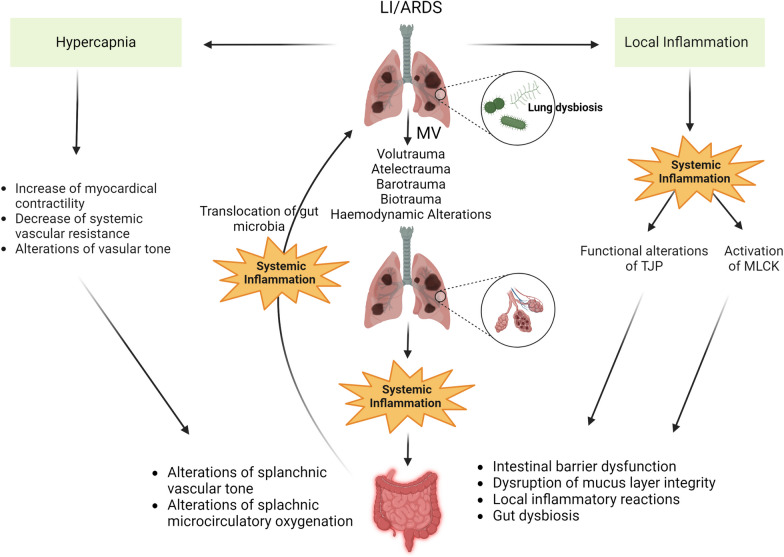
Although MV is considered one of the cornerstones of ARDS management, the recognition that it can contribute to LI represents a significant advancement in ARDS research [23, 24, 47]. Termed ventilator-induced lung injury (VILI), this phenomenon involves a range of mechanisms, including exposure to elevated inflation transpulmonary pressures (barotrauma), excessive alveolar distension (volutrauma), and repetitive cyclic opening and closure of alveoli (atelectrauma). Furthermore, beyond causing direct structural alterations, these mechanical forces can trigger a complex inflammatory cascade, resulting in both local and systemic inflammation (biotrauma) [48], potentially extending to distant organs and systems, exacerbating multiple system organ dysfunction and, ultimately, contributing to elevated mortality (Figs. 1, 3).
Fig. 3.
In patients with LI/ARDS, severe acute inflammation leads to functional alterations of tight junction proteins and activation of MLCK, which are associated with intestinal barrier dysfunction, disruption of the mucus layer integrity, exacerbation of intestinal inflammatory reactions, and gut dysbiosis. MV may cause VILI, further exacerbating systemic inflammation and intestinal damage. Haemodynamic alterations during low-tidal volume MV and hypercapnia induced by protective MV are associated with increased myocardial contractility, decreased systemic vascular resistance, and alterations of vascular tone, leading to changes in splanchnic vascular tone and splanchnic microcirculatory oxygenation. Intestinal dysfunction and loss of gut integrity enhance systemic inflammation and promote the translocation of gut bacteria into the lungs, worsening pre-existing LI-mediated lung dysbiosis. ARDS acute respiratory distress syndrome, LI lung injury, MLCK myosin light chain kinase, MV mechanical ventilation, TJP tight junction proteins, VILI ventilator-induced lung injury
The pathophysiologic participation of biotrauma in the pathogenesis of VILI remained unclear until 1998 [48]. In recent years, it has become increasingly evident that under specific conditions, MV can provoke inflammatory responses and subsequent lung injury while effectively maintaining gas exchange. These inflammatory cascades have the potential to disseminate throughout the systemic circulation, exerting effects on extrapulmonary organs and systems, ultimately culminating in MOF [49]. Indeed, it is assumed that by increasing alveolar–vascular permeability, MV facilitates the decompartmentalization of the inflammatory response and is instrumental in the propagation of the injury from the lung to the distal organs, ultimately resulting in MOF and impacting mortality (Figs. 1, 3) [49]. Moreover, it is hypothesized that the MV-induced inflammatory response (biotrauma) arises from two distinct pathophysiologic mechanisms. Firstly, direct cellular trauma disrupts cell walls, leading to the release of cytokines both locally at the alveolar level and into the systemic circulation [50]. Secondly, a mechanism termed “mechanotransduction” has been identified, referring to the phenomenon that cells detect mechanical forces through a process known as "mechanosensation" and convert them into a series of cellular signaling events through "mechanotransduction," In vitro studies have highlighted that the majority of pulmonary cells can produce cytokines in response to cyclic stretch [51]. Furthermore, mechanical injury results in the release of DAMPs, which in turn trigger the recruitment of immune cells that produce pro-inflammatory cytokines. This, coupled with the activation of signaling cascades in alveolar epithelial and vascular endothelial cells due to overstretching, as well as dysregulation of the neuroinflammatory reflex, leads to a potent systemic inflammatory response. The continuous exposure to harmful mechanical forces during MV, especially high tidal volume (TV) MV, leads to a vicious cycle by exacerbating this inflammatory process, leading to increased permeability of the alveolar-capillary barrier, and enhancing susceptibility to additional lung damage [52].
Clinical studies have shown associations between MV and increased levels of inflammatory mediators such as TNF-α, IL-1, IL-6, IL-8, soluble TNF-α receptor 75, IL-1β, and IL-1 receptor antagonist (IL-1ra) in both plasma and BALF [47, 53, 54]. These findings are further supported by experimental studies demonstrating that MV may trigger the release of various proinflammatory mediators including IL-1β, IL-6, IL-8, TNF-α, C-X-C motif ligand 1 (CXCL1), and CXCL10, as well as macrophage inflammatory protein-2 [55], and intercellular adhesion molecule levels [56–59].
Several studies have attempted to elucidate the pathophysiology of distal organ injury in ARDS; however, the exact mechanisms remain incompletely understood. These potential factors include MV, hypoxaemia, the deleterious effects of bacteria or bacterial products, and systemic inflammation [60, 61]. Indeed, as mentioned above, accumulating evidence supports the hypothesis that ventilator-mediated inflammatory reactions and injury may extend to distal organs such as the liver, kidney, and brain [62, 63]. Nevertheless, little is known about the injurious effects of VILI on gut integrity. Moreover, the existing evidence regarding gut damage and dysfunction as possible detrimental consequences of MV-induced systemic inflammation is limited. As a component of the inflammatory/sepsis cascade, increased levels of TNF-α, IL-1β, and IL-6 have been shown to impact the permeability of TJ in the intestine [41]. Guery and coworkers (2003) have shown that MV elevates plasma TNF levels and gut permeability and that the administration of a TNF-neutralizing antibody intravenously reversed gut hyperpermeability [64]. The detrimental effects of TNF-α on the integrity of the intestinal epithelial barrier—independently or in conjunction with other proinflammatory cytokines—have been indicated by numerous prior investigations focusing on both experimental models and cell culture settings [65–68]. The underlying mechanisms of TNF-α-induced dysfunction of the intestinal epithelial barrier are complex and enclose variant processes: the induction of apoptosis in intestinal epithelial cells, alterations in the lipid composition of cell membranes, activation of MLCK by calcium-calmodulin, stimulation of myosin light chain (MLC) phosphorylation through increased expression of MLCK protein, and suppression of TJ protein expression. Notably, as pivotal in developing TNF-α-induced dysfunction in the intestinal epithelial barrier, the MLCK-mediated MLC phosphorylation pathway has been widely recognized [69]. Moreover, IL-6 is a multifunctional pro-inflammatory cytokine with pleiotropic effects, participating significantly in inflammatory responses both locally in the gut and systemically. However, despite its crucial role in inflammatory processes, the exact impact of IL-6 on the modulation of intestinal epithelial barrier function remains uncertain, as there is controversy regarding whether IL-6 exerts a protective or disruptive effect on the intestinal barrier [41]. Very recently, using an experimental mice model of VILI, Ding et al. [70] demonstrated elevated levels of TNF-α, IL-1β, and IL-6 in both serum and gut tissues, as assessed by enzyme-linked immunosorbent assay (ELISA). Remarkably, VILI mice exhibited significant increases in gut injury and a phenomenon known as PANoptosis, referring to the simultaneous occurrence of apoptosis, pyroptosis, and necroptosis in the gut, which is associated with MLC activation and TJ disruption. These pathological changes were associated with elevated TNF-α, IL-1β, and IL-6 serum levels. Moreover, VILI mice present impairments in intestinal barrier integrity, characterized by reduced expression of occludin and ZO-1, along with increased expression of claudin-2 and activation of MLC. Notably, intratracheal administration of Importin-7 (Imp7) small interfering RNA (siRNA) nanoparticles effectively suppressed the production of cytokines. It mitigated gut injury induced by VILI, suggesting the pathophysiologic effects of systemic inflammation in exacerbating gut injury following VILI and indicating the potential therapeutic utility of Imp7 siRNA nanoparticles for cytokine inhibition as a treatment strategy for VILI-associated complications [70]. In addition, experimental studies using a rabbit model of VILI illustrate a potential mechanism for the influence of biotrauma on MOF. By utilizing an acid aspiration model of ARDS, Imai and co-workers (2003) revealed that harmful ventilatory strategies, such as high TV and zero PEEP, markedly elevated the apoptosis of epithelial cells in the kidneys and small intestine, elevation which correlated significantly with organ dysfunction [71].
Impact of mechanical ventilation from a mechanistic point of view
The progressive deterioration of other organ systems, ultimately leading to the onset of MODS, is the primary factor contributing to elevated mortality among patients experiencing LI/ARDS [72]. Indeed, in critically ill patients, mortality is less frequently associated with hypoxia and/or hypercapnia but rather arises from the development of a systemic inflammatory response leading to MODS with related complications and even death. Notably, significant attention has been directed toward the role of the GI tract in the pathogenesis of this syndrome [10].
Numerous studies indicate that lung protective techniques involving low TV and elevated PEEP levels lead to decreased mortality rates, thereby becoming the standard management approach for patients with ARDS [24, 73], yet, existing data regarding the impact of PEEP on splanchnic perfusion and systemic haemodynamics are contradictory. Indeed, despite enhancing arterial oxygenation, PEEP's application may adversely affect systemic haemodynamics by decreasing venous return and cardiac output, with the extent of these effects correlating with the level of PEEP administered [74]. Indeed, experimental and clinical investigations have revealed that among mechanically ventilated individuals without lung injury, PEEP induces a decrease in venous return, consequently impacting cardiac output [75–77]. Moreover, it has been demonstrated that during abdominal surgery, increasing PEEP from 0 to 15 cm H2O is associated with a simultaneous reduction in mixed-venous and hepatic venous oxygen saturations, with significant changes observed only at a PEEP of 15 cm H2O [78]. Additionally, the application of PEEP in patients who underwent abdominal surgery, along with the decrease in cardiac output, also impairs portal blood flow. At the same time, splanchnic oxygen consumption remained maintained due to compensatory increases in splanchnic oxygen extraction [79]. Similarly, in patients with LI resulting from septic shock, elevating PEEP levels to 15 cm H2O led to reductions in both cardiac output and hepatic vein oxygen saturation [80], which was significantly prominent at a PEEP level of 15 cm H2O compared to 10 cm H2O [81]. Moreover, PEEP can autonomously affect regional perfusion, particularly hindering splanchnic perfusion. Impaired splanchnic perfusion may compromise the gut`s barrier function, resulting in intestinal hyperpermeability and bacterial translocation and facilitating the initiation of MOF [74]. Experimental studies have shown a dose-dependent impact of PEEP on splanchnic blood flow. At PEEP levels below 10 cm H2O, the reduction in splanchnic blood flow is usually limited, and it becomes more prominent at levels ranging from 15 to 20 cm H2O [82]. Conversely, a number of clinical studies in patients with ARDS have demonstrated that high PEEP levels, up to 20 cmH2O, do not compromise gastric mucosal perfusion, as evaluated by tonometry, and are not related to significant impairments of systemic haemodynamics in the majority of ARDS patients [74, 81, 83]. However, it should be emphasized that the impact of PEEP is intricate and difficult to predict, particularly in the context of heterogeneous ARDS lungs. The effects of PEEP are influenced not only by the selected PEEP level but also by how it interacts with and alters lung status [84]. Therefore, managing patients at the bedside requires achieving a careful equilibrium between lung recruitment and preventing hyperinflation, with close monitoring of the haemodynamic response [85].
While this section primarily focuses on the mechanistic influence of MV on splanchnic perfusion and systemic haemodynamic alterations affecting gut integrity, the effects of MV on gut function are multifactorial. Elevated plasma-renin–angiotensin–aldosterone activity and enhanced catecholamine levels due to sympathetic activation are among the additional mechanisms that notably contribute to splanchnic hypoperfusion [86]. Moreover, commonly used medications to support MV, like opiates and sedatives—particularly benzodiazepines—can decrease gastrointestinal motility and hinder venous return through mechanisms such as venodilation or decreased responsiveness to vasopressor agents [86].
Considering the data provided above and the necessity of lung protective strategies, such as employing low TV and high levels of PEEP, in patients with ARDS, it is crucial to closely monitor both systemic and regional perfusion when administering high PEEP levels. Furthermore, future investigations should focus on assessing the prolonged effects of PEEP on splanchnic perfusion. Understanding the complexity of interactions between critical illness and the mechanistic impact of MV on the GI tract enables appropriate management and prospective use of preventive practices.
The role of hypercapnia within the lung-gut axis
Acute respiratory failure not only interrupts the process of gas exchange but also results in significant stress on cardiovascular function, necessitating higher cardiac output to maintain sufficient oxygen delivery. This challenge is exacerbated by enhanced oxygen demand resulting from increased breathing work and inefficient gas exchange. Additionally, lung collapse due to hypoxic pulmonary vasoconstriction contributes to increased right ventricular afterload, further complicating cardiovascular function [87]. Lung protective ventilation, characterized by using a small TV, is frequently associated with hypercapnia in patients with LI/ARDS [24, 26]. In the context of acute respiratory acidosis, pleiotropic cardiovascular consequences may trigger increased myocardial contractility (Fig. 3). This may be done through sympathetic nerve activation and simultaneous reduction in systemic vascular resistance. Moreover, changes in local vascular tone leading to vasoconstriction in the lungs and kidneys, alongside vasodilation in the brain show that regional perfusion may undergo dynamic alterations [10, 88]. In a biphasic manner, hepatic and splanchnic blood flow seems to be influenced by hypercapnia. In the beginning, sympathetic stimulation leads to a reduction in blood flow, followed by a subsequent increase attributed to the direct vasodilatory effect of carbon dioxide (CO2). Furthermore, it has been found that moderate fluctuations in CO2 arterial partial pressure (PCO2) correlate with elevated systemic perfusion in mechanically ventilated patients under stable conditions. Splanchnic perfusion, however—assessed by the difference in gastric mucosal/arterial PCO2 (DPCO2)—remained unaltered [10]. Experimental studies support these findings further by showing that in septic animal models, splanchnic microcirculatory oxygenation is enhanced by both acute hypercapnic acidosis and buffered hypercapnia, effectively mitigating the negative impacts induced by sepsis [89, 90]. On the contrary, further clinical studies have shown that lung-protective ventilation strategies in patients with ARDS failed to enhance gastric mucosal perfusion despite leading to increased cardiac output. It is hypothesized that the variability noted in the individual alterations of gastric mucosal perfusion resulting from low TV MV suggests that the direct local vasodilation caused by elevated tissue PCO2 may be counteracted by the augmented release of catecholamines in the systemic circulation [91].
Impact of ischaemia/reperfusion injury on gut integrity
Proposed in 2002 by Deitch, the "three-hit model" theory suggests a sequence of events in which an initial injury induces visceral hypoperfusion (first hit), prompting the gut to generate and release proinflammatory mediators. Subsequent haemodynamic resuscitation results in reperfusion, causing ischemia–reperfusion injury to the gut (second hit). This leads to loss of gut barrier function and an enhanced inflammatory reaction of intestinal origin, independent of bacterial or toxin translocation. Upon crossing the mucosal barrier, bacteria, and endotoxin further stimulate the immune response by releasing chemokines, cytokines, and other inflammatory mediators (Fig. 3). These substances exert their effects on the immune system, both locally and systemically, ultimately precipitating systemic inflammatory response syndrome (SIRS) and MODS, referred to as the third hit [13, 92]. Finally, as already mentioned, in the context of critical illness, damage occurs to the mucus layer, resulting in epithelial cell dysfunction. Ischaemia/reperfusion events further exacerbate this issue by diminishing the hydrophobicity of the mucus layer and causing changes in intestinal permeability [93].
Impact of altered lung microbiome on gut integrity
The widespread notion of lung sterility posed a barrier to the systematic investigation of the lung microbiome, resulting in a slowdown of research progress [94, 95]. However, research has revealed the prevalence of microaspiration or gastroesophageal reflux, even among apparently healthy individuals, leading to microbial colonization of the alveoli [96–99]. Bronchoscopic studies have highlighted that the carina represents the densest site of bacterial deoxyribonucleic acid (DNA) among healthy individuals, with a gradually diminishing density with further bifurcations, suggesting the impact of micro-aspiration on microbial immigration in the respiratory tract of healthy adults [100]. Using culture-independent molecular techniques, a diverse bacterial community has been revealed in the lower airways of asymptomatic individuals, predominantly characterized by species such as Prevotella, Veillonella, Streptococcus, and Fussobacterium [101–103].
Critically ill patients could necessitate a range of interventions in the ICU, such as MV, antibiotic therapy, continuous blood purification, and immunosuppressive regimens [100], which potentially may influence the microbial composition and diversity of these patients [104–106]. Alterations of the lung microbiome associated with critical illness significantly correlate with systemic and local inflammation [44, 101, 104, 107]. A reduction in bacterial diversity occurs, and potential pathogens, often originating from alternative ecosystems like the GI tract and the skin, may displace commensal microbial populations [101, 108]. Examining BALF from patients with ARDS, Kyo et al. [109] discovered tendencies toward increased lung bacterial burden, evidenced by elevated 16S rRNA gene copy numbers. Furthermore, they observed a notable reduction in alpha diversity among ARDS patients, encompassing both copy numbers and the relative abundance of betaproteobacteria [109]. Moreover, patients with LI exhibit augmented contamination of gut-associated bacteria within their lung microbiome, including species from the Enterobacteriaceae family [44], which have been associated with the progression to ARDS [44]. Although the enrichment of the lung microbiome with gut-derived bacteria may suggest a broader dysbiosis in critically ill patients, a study conducted by Panzer et al. (2018) highlights the significant role of these microbes in ARDS pathophysiology [44]. The authors emphasize a substantial involvement of these bacteria in ARDS pathogenesis, particularly in patients who underwent MV, where early lung dysbiosis coincides with a notable increase in inflammatory mediators (IL-6, IL-8), thereby predisposing patients to subsequent ARDS development [44].
The composition of gut microbiota could be influenced by lung dysbiosis (Fig. 3). In a pre-clinical study, influenza infection was found to cause a rise in the presence of Enterobacteriaceae and a decline in the levels of Lactobacilli and Lactococci in the gut [110]. Moreover, a recent study by Gu and colleagues (2020) demonstrated that Coronavirus disease (COVID)-19 patients exhibited substantially decreased intestinal bacterial diversity compared to healthy controls. Additionally, there was a notable increase in the relative abundance of opportunistic pathogens like Streptococcus, Rothia, Veillonella, and Actinomyces, alongside a decrease in the relative excess of beneficial symbionts [111].
The intestinal microbiome undergoes significant alterations due to both the physiological impacts of critical illness, including sepsis and ARDS, and the clinical interventions employed in intensive care settings [104]. In a clinical study including 52 participants, rectal swabs were collected to assess the composition of the gut microbiome using 16S rRNA gene sequencing over their ICU stay. Additional research, including patients’ mortality rate at 28 days after admission to the ICU, revealed dysbiosis in the gut of critically ill patients. The observed dysbiosis was identified as an independent risk factor for increased mortality at 28 days, and, according to the authors, lower mortality was associated with genera (e.g., Parasutterella and Campylobacter), while high mortality with taxa from the Anaerococcus genus and Enterobacteriaceae family [112].
The profound impact of changes in the gut microbiome as a risk factor for LI/ARDS was acknowledged as early as the late 1970s. Cuevas and colleagues demonstrated in animal models of shock that the onset of lung injury could be avoided through pretreatment with enteric antibiotics [113]. Furthermore, Wang and colleagues (2014) demonstrated that in experimental influenza infection, the interferon (IFN)-γ produced by lung-derived CCR9 + CD4 + T cells altered the composition of the gut microbiota and induced intestinal immune injury [114]. Additional experimental studies further support the hypothesis that influenza pulmonary infection can substantially modify intestinal microbiota profile depending on a mechanism involving type I interferons (IFN-Is) [115]. However, data are scarce concerning the pathophysiology of how lung dysbiosis may influence the gut microbiome in patients with LI/ARDS. Given that the compromised permeability of the alveoli-capillary membrane increases in LI/ARDS due to direct (mainly epithelial) or indirect (mainly endothelial) damage, it is reasonable to speculate that alveoli-capillary permeability represents a significant risk factor for gut–lung bacterial translocation (Figs. 1, 3) [116]. Moreover, during critical illness, there is a significant restructuring of the environmental conditions that support gut bacterial growth, leading to a profound impact on the reproductive rates of microbial community members [104].
In host defense, mucus plays a fundamental role by forming a barrier that protects the gut epithelium from direct contact with bacteria and digestive enzymes. Due to its hydrophobic properties, mucus substantially hinders the passage of positively charged, water-soluble toxic molecules across its surface [93]. In addition, the proliferation of microbes within the Proteobacteria phylum is facilitated by elevated nitrate levels and a modified mucosal oxygen gradient. Included in this phylum are several widely recognized gram-negative rods such as Pseudomonas aeruginosa and Escherichia coli, along with specific members of the Firmicutes phylum like Staphylococcus aureus and Enterococcus spp [117–121]. Resulting from the transition to a “disease-promoting microbiome” or “pathobiome” in the context of critical illness, subsequent pro-inflammatory processes occur within the intestinal epithelial cells. This can present as enhanced permeability of TJ and breakdown of mucus integrity, both factors implicated in gastrointestinal injury and the development of MODS [122–124].
Ultimately, while mounting evidence suggests that changes in the lung microbiome could impact gut integrity, there remains a significant gap in our understanding of how lung dysbiosis specifically influences the gut microbiome in ARDS. Further investigation is crucial to elucidate these pathways and their clinical relevance.
Therapeutic perspectives
Given the significant impact of the gut-lung axis and intestinal integrity on critical illness, interventions aimed at preserving and restoring the microbiota hold promise for enhancing outcomes in critically ill patients with ARDS. Bacterial metabolites from the intestine, particularly short-chain fatty acids (SCFAs), play a crucial role in influencing both local and systemic immune responses, thereby contributing significantly to the immunomodulatory effects of probiotics and prebiotics [125, 126]. The term “prebiotics” refers to dietary components, predominantly indigestible oligosaccharides, that specifically promote the growth and activity of beneficial gut microbiota, enhancing the balance of intestinal flora and contributing to improvements in human health [127]. Prebiotics include various dietary nutrients, such as carbohydrate-based dietary fibers composed of monosaccharide polymers, which undergo microbial fermentation in the intestines. This process produces molecules like SCFAs and peptidoglycan, which impact the innate immune system. It is suggested that prebiotics can reduce intestinal inflammation, endotoxaemia, and hypercytokinaemia, which might benefit critical illness. Additionally, they positively influence mucosal immunological homeostasis and barrier integrity [128, 129]. Probiotics, defined as live microorganisms that, when administered in adequate quantities, confer health benefits to the host, encompass various strains such as bifidobacteria, lactic acid bacteria, enterococci, and yeast [130, 131]. Their contributions include protecting the intestinal barrier, inhibiting pathogen proliferation, reducing bacterial translocation, improving lipid profiles, reducing uraemic toxins, modulating levels of serum pro-inflammatory cytokines, enhancing levels of serum anti-inflammatory cytokines, and promoting host immunomodulation to prevent infections [132–137].
Fecal microbiota transplantation, where fecal material from a healthy donor is transferred to a patient with disrupted gut microbiota, has shown therapeutic efficacy during critical illness by promoting the recovery of intestinal diversity, suppressing the growth of pathogenic bacterial communities in the gut, fostering competitive exclusion of pathogenic bacteria by the local intestinal microbiota, and restoring the host immune response. [138–142].
Indeed, shifting the focus of future research from conventional symptomatic treatments like lung protective MV is essential. Efforts should be redirected towards implementing more precise interventions to restore immune balance, mitigate inflammatory responses, and reestablish the ecological balance of the gut-lung axis while also preventing alterations of the lung and gut microbiome. Moreover, by adjusting the gut microbiota and fortifying the integrity of the intestinal barrier, crucial mechanisms can be activated to decrease bacterial migration, diminish exposure to endotoxins, and maintain overall immune stability, thereby having the potential to reduce both the frequency and severity of ARDS and the associated intestinal dysfunction.
Conclusions
The strong correlation between the severity of nonpulmonary organ failure and ARDS underscores the importance of considering extrapulmonary manifestations in ARDS management and prognosis. With increasing ARDS severity, organ dysfunction intensifies, involving multiple systems. The gut-lung axis is acknowledged as a crucial component in the pathophysiology of ARDS, highlighting the bidirectional communication that entails bacterial and immune interactions between the gut and lungs within each respective compartment. Every kingdom and compartment plays a pivotal role in this interplay, thus influencing the prognosis of LI/ARDS. Among lung protective ventilation and maintenance of systemic haemodynamics, potential therapies aiming at restoring intestinal integrity, microbiome, and homeostasis balance between the two systems could significantly contribute to LI/ARDS management. While the gut-lung axis is acknowledged as crucial in ARDS pathophysiology, additional research is needed to clarify the bidirectional pathways of gut-lung interactions and their role in lung and extra-pulmonary injury. This understanding is essential for developing therapies that target maintaining intestinal integrity, microbiome, and homeostasis to manage LI/ARDS effectively.
Acknowledgements
Author contributions
The study was designed by MZ and AE. MZ searched the articles and drafted the manuscript, to which AE contributed and revised. Both authors read and approved the final manuscript.
Funding
Publication costs for this article were funded by the authors' institutions.
Availability of data and materials
Not applicable.
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637. doi: 10.1016/S0140-6736(21)00439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montgomery AB. Early description of ARDS. Chest. 1991;99(1):261–262. doi: 10.1378/chest.99.1.261. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Bernard GR, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- 5.Force ADT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson ND, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 7.Abe T, et al. Epidemiology and patterns of tracheostomy practice in patients with acute respiratory distress syndrome in ICUs across 50 countries. Crit Care. 2018;22(1):195. doi: 10.1186/s13054-018-2126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan EKY, Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir Res. 2018;19(1):50. doi: 10.1186/s12931-018-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernando SM, et al. Diagnosis and management of acute respiratory distress syndrome. CMAJ. 2021;193(21):E761–E768. doi: 10.1503/cmaj.202661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putensen C, Wrigge H, Hering R. The effects of mechanical ventilation on the gut and abdomen. Curr Opin Crit Care. 2006;12(2):160–165. doi: 10.1097/01.ccx.0000216585.54502.eb. [DOI] [PubMed] [Google Scholar]
- 11.Calfee CS, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calfee CS, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131(3):241–244. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 14.Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20(4):214–223. doi: 10.1016/j.molmed.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill–evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25(7):741–757. doi: 10.1111/j.1365-2036.2006.03174.x. [DOI] [PubMed] [Google Scholar]
- 16.Reintam A, Kern H, Starkopf J. Defining gastrointestinal failure. Acta Clin Belg. 2007;62(Suppl 1):168–172. doi: 10.1179/acb.2007.62.s1.022. [DOI] [PubMed] [Google Scholar]
- 17.Louis K, et al. Bacterial translocation in an experimental model of multiple organ dysfunctions. J Surg Res. 2013;183(2):686–694. doi: 10.1016/j.jss.2013.01.064. [DOI] [PubMed] [Google Scholar]
- 18.Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Crit Care Clin. 2016;32(2):203–212. doi: 10.1016/j.ccc.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nath S, Kitsios GD, Bos LDJ. Gut-lung crosstalk during critical illness. Curr Opin Crit Care. 2023;29(2):130–137. doi: 10.1097/MCC.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 20.Adelman MW, et al. The gut microbiome's role in the development, maintenance, and outcomes of sepsis. Crit Care. 2020;24(1):278. doi: 10.1186/s13054-020-02989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukherjee S, Hanidziar D. More of the gut in the lung: how two microbiomes meet in ARDS. Yale J Biol Med. 2018;91(2):143–149. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P, et al. The gut-lung axis in critical illness: microbiome composition as a predictor of mortality at day 28 in mechanically ventilated patients. BMC Microbiol. 2023;23(1):399. doi: 10.1186/s12866-023-03078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziaka M, Exadaktylos A. Pathophysiology of acute lung injury in patients with acute brain injury: the triple-hit hypothesis. Crit Care. 2024;28(1):71. doi: 10.1186/s13054-024-04855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziaka M, Exadaktylos A. Brain–lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care. 2021;25(1):358. doi: 10.1186/s13054-021-03778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meduri GU, et al. Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy. Chest. 2009;136(6):1631–1643. doi: 10.1378/chest.08-2408. [DOI] [PubMed] [Google Scholar]
- 26.Ziaka M, Exadaktylos A. ARDS associated acute brain injury: from the lung to the brain. Eur J Med Res. 2022;27(1):150. doi: 10.1186/s40001-022-00780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranieri VM, et al. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA. 2000;284(1):43–44. doi: 10.1001/jama.284.1.43. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri D, et al. Focused update: guidelines on use of corticosteroids in sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med. 2024;52(5):e219–e233. doi: 10.1097/CCM.0000000000006172. [DOI] [PubMed] [Google Scholar]
- 29.Bellani G, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 30.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–427. doi: 10.1038/s41581-018-0005-7. [DOI] [PubMed] [Google Scholar]
- 31.Vermeij JD, et al. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J Neurotrauma. 2013;30(24):2073–2079. doi: 10.1089/neu.2013.3060. [DOI] [PubMed] [Google Scholar]
- 32.Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukoc Biol. 2008;84(3):607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziaka M, Exadaktylos A. The heart is at risk: understanding stroke-heart–brain interactions with focus on neurogenic stress cardiomyopathy—a review. J Stroke. 2023;25(1):39–54. doi: 10.5853/jos.2022.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westrom B, et al. The immature gut barrier and its importance in establishing immunity in newborn mammals. Front Immunol. 2020;11:1153. doi: 10.3389/fimmu.2020.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Assimakopoulos SF, et al. Gut-origin sepsis in the critically ill patient: pathophysiology and treatment. Infection. 2018;46(6):751–760. doi: 10.1007/s15010-018-1178-5. [DOI] [PubMed] [Google Scholar]
- 36.Chang M, et al. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS ONE. 2012;7(6):e40087. doi: 10.1371/journal.pone.0040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoseph BP, et al. Mechanisms of intestinal barrier dysfunction in sepsis. Shock. 2016;46(1):52–59. doi: 10.1097/SHK.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, et al. Disruption of tight junctions during polymicrobial sepsis in vivo. J Pathol. 2009;218(2):210–221. doi: 10.1002/path.2525. [DOI] [PubMed] [Google Scholar]
- 39.Gunzel D. Claudins: vital partners in transcellular and paracellular transport coupling. Pflugers Arch. 2017;469(1):35–44. doi: 10.1007/s00424-016-1909-3. [DOI] [PubMed] [Google Scholar]
- 40.Lorentz CA, et al. Myosin light chain kinase knockout improves gut barrier function and confers a survival advantage in polymicrobial sepsis. Mol Med. 2017;23:155–165. doi: 10.2119/molmed.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Sadi R, et al. Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene. PLoS ONE. 2014;9(3):e85345. doi: 10.1371/journal.pone.0085345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, Jiang X. Effects of enteral nutrition on the barrier function of the intestinal mucosa and dopamine receptor expression in rats with traumatic brain injury. JPEN J Parenter Enteral Nutr. 2015;39(1):114–123. doi: 10.1177/0148607113501881. [DOI] [PubMed] [Google Scholar]
- 43.Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger–damage control by the immune system. J Leukoc Biol. 2012;92(3):539–551. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panzer AR, et al. Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically ill trauma patients. Am J Respir Crit Care Med. 2018;197(5):621–631. doi: 10.1164/rccm.201702-0441OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10(6):350–356. doi: 10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anthony DC, et al. The systemic response to brain injury and disease. Brain Behav Immun. 2012;26(4):534–540. doi: 10.1016/j.bbi.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Ziaka M, et al. High-tidal-volume mechanical ventilation and lung inflammation in intensive care patients with normal lungs. Am J Crit Care. 2020;29(1):15–21. doi: 10.4037/ajcc2020161. [DOI] [PubMed] [Google Scholar]
- 48.Tremblay LN, Slutsky AS. Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians. 1998;110(6):482–488. [PubMed] [Google Scholar]
- 49.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157(6 Pt 1):1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 50.Plotz FB, et al. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med. 2004;30(10):1865–1872. doi: 10.1007/s00134-004-2363-9. [DOI] [PubMed] [Google Scholar]
- 51.Vlahakis NE, et al. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277(1):L167–L173. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 52.Madahar P, Beitler JR. Emerging concepts in ventilation-induced lung injury. F1000Res. 2020;9:F1000 Faculty Rev-222. doi: 10.12688/f1000research.20576.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ranieri VM, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282(1):54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 54.Pinheiro de Oliveira R, et al. Mechanical ventilation with high tidal volume induces inflammation in patients without lung disease. Crit Care. 2010;14(2):R39. doi: 10.1186/cc8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, et al. Molecular mechanisms of ventilator-induced lung injury. Chin Med J. 2018;131(10):1225–1231. doi: 10.4103/0366-6999.226840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuipers MT, et al. High levels of S100A8/A9 proteins aggravate ventilator-induced lung injury via TLR4 signaling. PLoS ONE. 2013;8(7):e68694. doi: 10.1371/journal.pone.0068694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nickles HT, et al. Mechanical ventilation causes airway distension with proinflammatory sequelae in mice. Am J Physiol Lung Cell Mol Physiol. 2014;307(1):L27–37. doi: 10.1152/ajplung.00288.2013. [DOI] [PubMed] [Google Scholar]
- 58.Lex D, Uhlig S. One-hit models of ventilator-induced lung injury: benign inflammation versus inflammation as a by-product. Anesthesiology. 2017;126(5):909–922. doi: 10.1097/ALN.0000000000001605. [DOI] [PubMed] [Google Scholar]
- 59.Wang X, et al. High-mobility group box 1 protein is involved in the protective effect of Saquinavir on ventilation-induced lung injury in mice. Acta Biochim Biophys Sin (Shanghai) 2017;49(10):907–915. doi: 10.1093/abbs/gmx085. [DOI] [PubMed] [Google Scholar]
- 60.Darmon M, et al. Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol. 2014;9(8):1347–1353. doi: 10.2215/CJN.08300813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joannidis M, et al. Lung-kidney interactions in critically ill patients: consensus report of the Acute Disease Quality Initiative (ADQI) 21 Workgroup. Intensive Care Med. 2020;46(4):654–672. doi: 10.1007/s00134-019-05869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hegeman MA, et al. Ventilator-induced endothelial activation and inflammation in the lung and distal organs. Crit Care. 2009;13(6):R182. doi: 10.1186/cc8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turon M, et al. Mechanisms involved in brain dysfunction in mechanically ventilated critically ill patients: implications and therapeutics. Ann Transl Med. 2018;6(2):30. doi: 10.21037/atm.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guery BP, et al. Ventilation-induced lung injury is associated with an increase in gut permeability. Shock. 2003;19(6):559–563. doi: 10.1097/01.shk.0000070738.34700.bf. [DOI] [PubMed] [Google Scholar]
- 65.Clayburgh DR, et al. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Investig. 2006;116(10):2682–2694. doi: 10.1172/JCI29218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marchiando AM, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189(1):111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer A, et al. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-alpha on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304(11):G970–G979. doi: 10.1152/ajpgi.00183.2012. [DOI] [PubMed] [Google Scholar]
- 68.Feng Y, Teitelbaum DH. Tumour necrosis factor-induced loss of intestinal barrier function requires TNFR1 and TNFR2 signalling in a mouse model of total parenteral nutrition. J Physiol. 2013;591(15):3709–3723. doi: 10.1113/jphysiol.2013.253518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye D, Ma TY. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-alpha-induced regulation of myosin light chain kinase gene activity. J Cell Mol Med. 2008;12(4):1331–1346. doi: 10.1111/j.1582-4934.2008.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding N, et al. Systemic cytokines inhibition with Imp7 siRNA nanoparticle ameliorates gut injury in a mouse model of ventilator-induced lung injury. Biomed Pharmacother. 2023;165:115237. doi: 10.1016/j.biopha.2023.115237. [DOI] [PubMed] [Google Scholar]
- 71.Imai Y, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289(16):2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 72.Haglund U, Fiddian-Green RG. Assessment of adequate tissue oxygenation in shock and critical illness: oxygen transport in sepsis, Bermuda, April 1 + 2, 1989. Intensive Care Med. 1989;15(7):475–477. doi: 10.1007/BF00255606. [DOI] [PubMed] [Google Scholar]
- 73.Acute Respiratory Distress Syndrome, N et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 74.Bruhn A, et al. Effects of positive end-expiratory pressure on gastric mucosal perfusion in acute respiratory distress syndrome. Crit Care. 2004;8(5):R306–R311. doi: 10.1186/cc2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liebman PR, et al. The mechanism of depressed cardiac output on positive end-expiratory pressure (PEEP) Surgery. 1978;83(5):594–598. [PubMed] [Google Scholar]
- 76.Dorinsky PM, Whitcomb ME. The effect of PEEP on cardiac output. Chest. 1983;84(2):210–216. doi: 10.1378/chest.84.2.210. [DOI] [PubMed] [Google Scholar]
- 77.Pick RA, et al. The cardiovascular effect of positive end-expiratory pressure. Chest. 1982;82(3):345–350. doi: 10.1378/chest.82.3.345. [DOI] [PubMed] [Google Scholar]
- 78.Berendes E, et al. Effects of positive end-expiratory pressure ventilation on splanchnic oxygenation in humans. J Cardiothorac Vasc Anesth. 1996;10(5):598–602. doi: 10.1016/S1053-0770(96)80136-4. [DOI] [PubMed] [Google Scholar]
- 79.Winso O, et al. Portal blood flow in man during graded positive end-expiratory pressure ventilation. Intensive Care Med. 1986;12(2):80–85. doi: 10.1007/BF00254516. [DOI] [PubMed] [Google Scholar]
- 80.Trager K, Radermacher P, Georgieff M. PEEP and hepatic metabolic performance in septic shock. Intensive Care Med. 1996;22(11):1274–1275. doi: 10.1007/BF01709351. [DOI] [PubMed] [Google Scholar]
- 81.Kiefer P, et al. Effect of positive end-expiratory pressure on splanchnic perfusion in acute lung injury. Intensive Care Med. 2000;26(4):376–383. doi: 10.1007/s001340051170. [DOI] [PubMed] [Google Scholar]
- 82.Beyer J, et al. The effect of PEEP ventilation on hemodynamics and regional blood flow with special regard to coronary blood flow. Thorac Cardiovasc Surg. 1980;28(2):128–132. doi: 10.1055/s-2007-1022063. [DOI] [PubMed] [Google Scholar]
- 83.Akinci IO, et al. Gastric intramucosal pH is stable during titration of positive end-expiratory pressure to improve oxygenation in acute respiratory distress syndrome. Crit Care. 2003;7(3):R17–23. doi: 10.1186/cc2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suarez-Sipmann F, Ferrando C, Villar J. PEEP titration guided by transpulmonary pressure: lessons from a negative trial. J Thorac Dis. 2019;11(Suppl 15):S1957–S1962. doi: 10.21037/jtd.2019.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heunks L, Piquilloud L, Demoule A. How we approach titrating PEEP in patients with acute hypoxemic failure. Crit Care. 2023;27(1):415. doi: 10.1186/s13054-023-04694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mutlu GM, Mutlu EA, Factor P. GI complications in patients receiving mechanical ventilation. Chest. 2001;119(4):1222–1241. doi: 10.1378/chest.119.4.1222. [DOI] [PubMed] [Google Scholar]
- 87.Myeong LJ, Pinsky MR. Cardiovascular interactions in respiratory failure. In: Webb A, editor. Oxford textbook of critical care. Oxford: Oxford Academic; 2016. [Google Scholar]
- 88.Carvalho CR, et al. Temporal hemodynamic effects of permissive hypercapnia associated with ideal PEEP in ARDS. Am J Respir Crit Care Med. 1997;156(5):1458–1466. doi: 10.1164/ajrccm.156.5.9604081. [DOI] [PubMed] [Google Scholar]
- 89.Stubs CC, et al. Acute, short-term hypercapnia improves microvascular oxygenation of the colon in an animal model of sepsis. Microvasc Res. 2013;90:180–186. doi: 10.1016/j.mvr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Beck C, et al. The beneficial effects of acute hypercapnia on microcirculatory oxygenation in an animal model of sepsis are independent of K(+)ATP channels. Microvasc Res. 2015;99:78–85. doi: 10.1016/j.mvr.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Sitbon P, et al. Effects of tidal volume reduction in acute respiratory distress syndrome on gastric mucosal perfusion. Intensive Care Med. 2001;27(5):911–915. doi: 10.1007/s001340100931. [DOI] [PubMed] [Google Scholar]
- 92.Sertaridou E, et al. Gut failure in critical care: old school versus new school. Ann Gastroenterol. 2015;28(3):309–322. [PMC free article] [PubMed] [Google Scholar]
- 93.Qin X, et al. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29(3):372–376. doi: 10.1097/SHK.0b013e3181453f4e. [DOI] [PubMed] [Google Scholar]
- 94.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10(2):299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dickson RP, et al. The microbiome and the respiratory tract. Annu Rev Physiol. 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111(5):1266–1272. doi: 10.1378/chest.111.5.1266. [DOI] [PubMed] [Google Scholar]
- 97.Huxley EJ, et al. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64(4):564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 98.Hilty M, et al. Disordered microbial communities in asthmatic airways. PLoS ONE. 2010;5(1):e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Budden KF, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 100.Martin-Loeches I, et al. The importance of airway and lung microbiome in the critically ill. Crit Care. 2020;24(1):537. doi: 10.1186/s13054-020-03219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kelly BJ, et al. Composition and dynamics of the respiratory tract microbiome in intubated patients. Microbiome. 2016;4:7. doi: 10.1186/s40168-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shukla SD, et al. Microbiome effects on immunity, health and disease in the lung. Clin Transl Immunol. 2017;6(3):e133. doi: 10.1038/cti.2017.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yagi K, et al. The lung microbiome during health and disease. Int J Mol Sci. 2021;22(19):10872. doi: 10.3390/ijms221910872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dickson RP. The microbiome and critical illness. Lancet Respir Med. 2016;4(1):59–72. doi: 10.1016/S2213-2600(15)00427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agudelo-Ochoa GM, et al. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes. 2020;12(1):1707610. doi: 10.1080/19490976.2019.1707610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Szychowiak P, et al. The role of the microbiota in the management of intensive care patients. Ann Intensive Care. 2022;12(1):3. doi: 10.1186/s13613-021-00976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zakharkina T, et al. The dynamics of the pulmonary microbiome during mechanical ventilation in the intensive care unit and the association with occurrence of pneumonia. Thorax. 2017;72(9):803–810. doi: 10.1136/thoraxjnl-2016-209158. [DOI] [PubMed] [Google Scholar]
- 108.Akrami K, Sweeney DA. The microbiome of the critically ill patient. Curr Opin Crit Care. 2018;24(1):49–54. doi: 10.1097/MCC.0000000000000469. [DOI] [PubMed] [Google Scholar]
- 109.Kyo M, et al. Unique patterns of lower respiratory tract microbiota are associated with inflammation and hospital mortality in acute respiratory distress syndrome. Respir Res. 2019;20(1):246. doi: 10.1186/s12931-019-1203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Enaud R, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol. 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gu S, et al. Alterations of the gut microbiota in patients with Coronavirus disease 2019 or H1N1 influenza. Clin Infect Dis. 2020;71(10):2669–2678. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salameh TJ, et al. Gut microbiome dynamics and associations with mortality in critically ill patients. Gut Pathog. 2023;15(1):66. doi: 10.1186/s13099-023-00567-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cuevas P, et al. The lung lesion in four different types of shock in rabbits. Arch Surg. 1972;104(3):319–322. doi: 10.1001/archsurg.1972.04180030067015. [DOI] [PubMed] [Google Scholar]
- 114.Wang J, et al. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–2410. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deriu E, et al. Influenza virus affects intestinal microbiota and secondary salmonella infection in the gut through type I interferons. PLoS Pathog. 2016;12(5):e1005572. doi: 10.1371/journal.ppat.1005572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou X, Liao Y. Gut-lung crosstalk in sepsis-induced acute lung injury. Front Microbiol. 2021;12:779620. doi: 10.3389/fmicb.2021.779620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winter SE, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grootjans J, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176(5):2283–2291. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Albenberg L, et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147(5):1055–1063. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Honda K, Littman DR. The microbiome in infectious disease and inflammation. Annu Rev Immunol. 2012;30:759–795. doi: 10.1146/annurev-immunol-020711-074937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2(3):204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 122.Reintam Blaser A, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24(1):224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Reintam Blaser A, et al. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39(5):899–909. doi: 10.1007/s00134-013-2831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45(2):337–347. doi: 10.1097/CCM.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marrocco F, et al. Short-chain fatty acids promote the effect of environmental signals on the gut microbiome and metabolome in mice. Commun Biol. 2022;5(1):517. doi: 10.1038/s42003-022-03468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vernocchi P, Del Chierico F, Putignani L. Gut microbiota metabolism and interaction with food components. Int J Mol Sci. 2020;21(10):3688. doi: 10.3390/ijms21103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Everard A, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–2786. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vinolo MA, et al. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 129.Wei Y, et al. The intestinal microbial metabolite desaminotyrosine is an anti-inflammatory molecule that modulates local and systemic immune homeostasis. FASEB J. 2020;34(12):16117–16128. doi: 10.1096/fj.201902900RR. [DOI] [PubMed] [Google Scholar]
- 130.Hill C, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 131.Gagliardi A, et al. Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health. 2018;15(8):1679. doi: 10.3390/ijerph15081679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Manzanares W, et al. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016;19:262. doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suez J, et al. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 134.Guan ZW, Yu EZ, Feng Q. Soluble dietary fiber, one of the most important nutrients for the gut microbiota. Molecules. 2021;26(22):6802. doi: 10.3390/molecules26226802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lopes R, et al. Modulation of intestinal microbiota, control of nitrogen products and inflammation by pre/probiotics in chronic kidney disease: a systematic review. Nutr Hosp. 2018;35(3):722–730. doi: 10.20960/nh.1642. [DOI] [PubMed] [Google Scholar]
- 136.Milajerdi A, et al. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. 2020;59(2):633–649. doi: 10.1007/s00394-019-01931-8. [DOI] [PubMed] [Google Scholar]
- 137.Seifi N, et al. Effects of synbiotic supplementation on the serum endotoxin level, inflammatory status, and clinical outcomes of adult patients with critical illness: a randomized controlled trial. Nutr Clin Pract. 2022;37(2):451–458. doi: 10.1002/ncp.10758. [DOI] [PubMed] [Google Scholar]
- 138.Kim KO, Gluck M. Fecal microbiota transplantation: an update on clinical practice. Clin Endosc. 2019;52(2):137–143. doi: 10.5946/ce.2019.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Du D, et al. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury. Oxid Med Cell Longev. 2021;2021:5816837. doi: 10.1155/2021/5816837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Limketkai BN, et al. Fecal microbiota transplantation for the critically ill patient. Nutr Clin Pract. 2019;34(1):73–79. doi: 10.1002/ncp.10228. [DOI] [PubMed] [Google Scholar]
- 141.Keskey R, et al. The use of fecal microbiota transplant in sepsis. Transl Res. 2020;226:12–25. doi: 10.1016/j.trsl.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.He S, et al. Gut microbiome-based therapeutics in critically ill adult patients—a narrative review. Nutrients. 2023;15(22):4734. doi: 10.3390/nu15224734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.