Abstract
Aim: The aim of this meta-analysis was to investigate the relationship between the baseline systemic immune inflammatory index (SII) and prognosis in patients with NSCLC. Materials & methods: The relation between pretreatment SII and overall survival, disease-free survival, cancer-specific survival, progression-free survival and recurrence-free survival in NSCLC patients was analyzed combined with hazard ratio and 95% CI. Results: The results showed that high SII was significantly correlated with overall survival and progression-free survival of NSCLC patients, but not with disease-free survival, cancer-specific survival and recurrence-free survival. Conclusion: The study suggests that a higher SII has association with worse prognosis in NSCLC patients.
PROSPERO registration number: CRD42022336270.
Keywords: : baseline, meta-analysis, NSCLC, prognosis, systemic immune inflammation index (SII)
Plain language summary
Summary points.
Predictors strongly associated with NSCLC survival can help clinicians develop prevention and treatment strategies for patients.
SII, a composite indicator based on peripheral blood lymphocyte, neutrophils, and platelet counts, has been found to be a novel prognostic factor for inflammation.
SII is a simple clinical indicator of inflammation, low cost, easy to operate, and quickly available.
The aim of this meta-analysis was to investigate the relationship between the baseline systemic immune inflammatory index (SII) and prognosis in patients with NSCLC.
High SII was significantly correlated with overall survival and progression-free survival of NSCLC patients.
Elevated SII could not predict worse disease-free survival, recurrence-free survival, cancer-specific survival, possibly due to the relatively small sample.
Despite heterogeneity, subgroup analyses by country, SII cut-off value, sample size, and analysis type did not attenuate most of the prognostic significance.
Our analysis suggested that the high pretreatment SII might be a negative prognostic indicator for patients diagnosed with NSCLC.
Lung cancer is one of the most common tumors, with a high incidence and mortality rate among all tumor types [1]. It is the dominating reason of death in cancer patients, killing an estimated 1.8 million people worldwide [2]. It includes two prime categories: NSCLC and SCLC. NSCLC is primarily classified into two categories: adenocarcinoma and squamous cell lung carcinoma accounting for approximately 85% among the whole types of lung cancer [3]. Predictors that are closely associated with NSCLC survival could help clinicians develop prevention and treatment strategies for patients. Consequently, it is of great meaningfulness to confirm novel factors for prognosis to improve long-term prognosis.
For the past few years, biomarkers of inflammation, such as platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), as well as C-reactive protein (CRP), have been confirmed to have the relevance to tumor prognosis [4]. The systemic immune inflammatory index (SII) has been discovered to be a new prognostic factor of inflammation that is an integrated index on the grounds of the peripheral blood lymphocyte, neutrophil and platelet counts. Its definition was following: SII = platelet count × neutrophil count/lymphocyte count [5]. As a simple clinical indicator of inflammation, it was at a low cost, convenient to operate, and has rapid availability. In recent years, more and more investigators have researched the connection between SII and prognosis of patients with NSCLC; but these consequences are controversial.
In this paper, we carried out the current analysis to systematically expound the correlation between SII and prognosis of NSCLC patients.
Methods
Search strategy
We searched the Cochrane Library, EMBASE, PubMed and Web of Science databases to filtrate the related published and unpublished articles based on the next terms: ‘systemic-immune-inflammation index’ or ‘SII’, ‘lung cancer’ or ‘NSCLC’ or ‘lung tumor’ or ‘lung neoplasms’. The document retrieval deadline was 3 June 2022, without geographical restrictions.
Study selection
The following inclusion criteria were set: literature exploring the correlation between SII and prognosis of patients diagnosed with NSCLC pathologically; neutrophil counts, lymphocyte counts and platelet counts were collected prior to any therapies, such as targeted therapy, chemoradiotherapy and surgery; the results involved overall survival (OS), cancer-specific survival (CSS), recurrence-free survival (RFS), disease-free survival (DFS) or progression-free survival (PFS); and the optimal cut-off value of SII was given.
The following exclusion criteria were set: meeting reports, case series, editorials, expert opinions, meta-analyses and reviews; repetitive studies; nonhuman studies; and studies where full text or data could not be obtained.
Data extraction & quality assessment
Two reviewers separately collected data from articles. The information extracted from the obtained data included: publication year, name of the first author, sample size, country, study period, therapeutic method, follow-up period, tumor–node–metastasis (TNM) stage, outcomes, the optimal cut-off value of SII, as well as HRs with 95% CIs. Excel spreadsheet (Microsoft) was used for data acquisition and summary. If there was any disagreement that could not be resolved after two-person discussion, the third researcher and two investigators jointly analyzed and discussed to solve the difference.
Quality assessment of the literatures was appraised by two investigators separately on the grounds of the NOS [6] in terms of comparability, selection, and exposure. If the NOS score was not less than 6, the literature was thought to be of high quality.
Statistical analysis
The prognostic effect of SII on OS, CSS, RFS, DFS and PFS in NSCLC patients was evaluated using the hazard ratio (HR) and 95% CI. The Higgins I-squared statistic and Cochran's Q test were chosen to analyze any heterogeneity amidst studies. If there was significant between-study heterogeneity (I2 >50% and/or p < 0.10), random-effects model (REM) was chosen to generate the pooled HRs and 95% CIs; if not, the fixed-effects model (FEM) was chosen [7]. The source of heterogeneity was explored by carrying out subgroup analyses. Begg's funnel plot was used to test publication bias, and p < 0.05 represents significant publication bias [8]. All analyses were performed using STATA V. 15.0 (Stata, TX, USA).
Results
Study search
Totally, 350 studies that met standard requirements were obtained according to the search strategy. After deleting duplicate literature and filtrating headlines and abstracts, 57 studies were evaluated. Finally, a total of 25 studies were included and 11,195 patients were involved [9-33]. The methods of the literature screening are shown in Figure 1.
Figure 1.
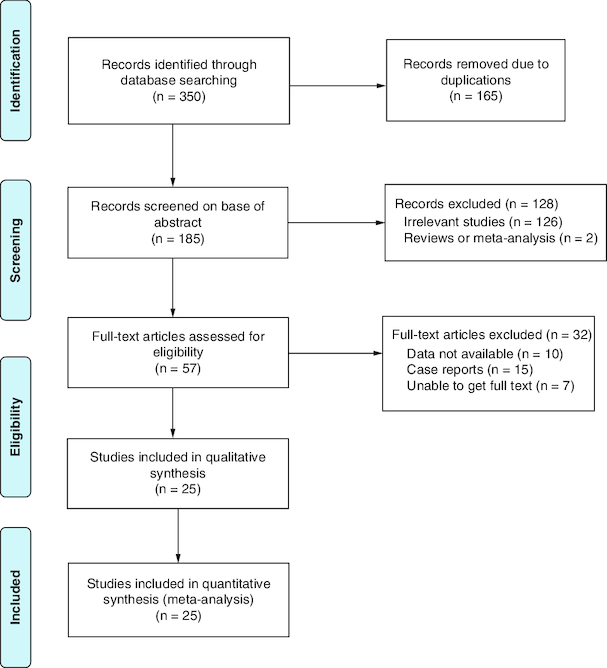
Flow chart of the included studies.
Study features
All eligible studies were designed retrospectively that were published during 2017 and 2022, with sample capacities ranging from 42 to 3,984. Of the 25 studies, one was carried out in Italy, two in Turkey, two in USA, three in Japan, and the rest in China. In total, 21 studies researched the prognostic effect of SII for OS, 11 studies researched the relationship between SII and PFS, three studies reported DFS, two studies reported RFS and two studies reported CSS. The maximum of SII cut-off values was 1343.67 and the minimum was 395.40. The primary features of the cohorts are shown in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
| Study | Year | Study period | Country | Sample size | Sex (F/M) | TNM stage | Treatment | Follow-up, median (range) | Cut-off value | Outcome | Analysis type | NOS | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Berardi et al. | 2019 | May 2006 – June 2015 | Italy | 311 | 95/216 | III–IV | CT/T | NA | 1270 | OS/PFS | M/U | 7 | [9] |
| Bilgetekin and Basal | 2020 | Jan 2010 – Mar 2020 | Turkey | 123 | 27/96 | NA | CT/T | 14.36 (9.13–23.69) | 730 | OS/PFS | M | 8 | [10] |
| Coutu et al. | 2022 | 2004 – 2019 | USA | 81 | 36/45 | III | CRT+S | 68.4 | 1260 | OS/DFS | M | 8 | [11] |
| Delikgoz Soykut et al. | 2022 | Jan 2012 – Dec 2017 | Turkey | 392 | 29/363 | IIIA–IIIB | CRT | NA | 817 | OS/PFS | M | 8 | [12] |
| Deng et al. | 2019 | Jan 2013 – Nov 2018 | China | 203 | 114/89 | IV | T | NA | 1343.665/1066.935 | OS/PFS | M | 6 | [13] |
| Fu et al. | 2021 | Apr 2008 – Dec 2015 | China | 3984 | 1845/2139 | I–IIIA | S | 45.1 | 479 | OS/RFS | M | 8 | [14] |
| Gao et al. | 2018 | Jan 2009 – Dec 2011 | China | 410 | 143/267 | I–IIIA | S | NA | 395.4 | OS | M | 7 | [15] |
| Guo, D. | 2018 | Aug 2013 – Jan 2016 | China | 140 | 45/95 | IIIB–IV | CRT/T | NA | 521 | OS/PFS | M | 7 | [16] |
| Guo, W. | 2019 | Jul 2006 – May 2012 | China | 569 | 144/425 | I–III | S | 60.3 (0.9–146.7) | 419.6 | OS | M | 8 | [17] |
| Ju et al. | 2021 | Jan 2014 – Dec 2016 | China | 102 | 61/41 | IIIB–IV | T | NA | 841.03 | OS/PFS | M | 8 | [18] |
| Keit et al. | 2021 | 2010 – 2019 | USA | 125 | 61/64 | III | CRT | NA | 1266 | OS/PFS | M | 7 | [19] |
| Li, A. et al. | 2020 | Oct 2013 – Jan 2018 | China | 252 | 107/145 | NA | Mixed | 25.9 (1–63) | 630.85 | OS | M | 7 | [20] |
| Li, B. et al. | 2019 | Jun 2011 – Feb 2018 | China | 161 | 71/90 | IIIB–IV | T | 16.6 (2.3–86.3) | 824 | OS/PFS | U | 7 | [21] |
| Li, H. et al. | 2019 | May 2013 – May 2016 | China | 310 | 148/162 | NA | Mixed | NA | 1218.81 | OS | M | 6 | [22] |
| Li, W. et al. | 2021 | Jan 2009 – Dec 2018 | China | 214 | 94/120 | I–IV | R | 61 (1–138) | 696.52 | OS | M | 7 | [23] |
| Li, X. et al. | 2020 | Dec 2012 – Dec 2018 | China | 345 | 90/255 | IIIB–IV | RT/CRT | NA | 555.59 | OS | M | 9 | [24] |
| Liu et al. | 2019 | Mar 2016 – Jul 2018 | China | 44 | 11/33 | IIIB–IV | T | 6.9 (0.6–28.5) | 603.5 | OS/PFS | M | 6 | [25] |
| Shen et al. | 2021 | Jan 2014 – Dec 2015 | China | 1431 | 824/607 | I | S | 63 (1–82) | 580.671 | CSS/DFS | M | 9 | [26] |
| Takeda et al. | 2021 | Sep 2015 – Mar 2021 | Japan | 42 | 20/22 | NA | T | NA | 1000 | PFS | U | 6 | [27] |
| Tomita et al. | 2018 | 2008 – 2012 | Japan | 341 | 168/173 | I–III | S | NA | 471.2 | CSS | U | 7 | [28] |
| Tong et al. | 2017 | Jan 2006 – May 2012 | China | 332 | 126/206 | III | CT/CRT | NA | 660 | OS | M | 7 | [29] |
| Watanabe et al. | 2021 | 2010 – 2019 | Japan | 387 | 154/233 | I | S | 39.2 (3–117) | 715 | RFS | M | 8 | [30] |
| Xu et al. | 2021 | Jan 2008 – May 2010 | China | 234 | 97/137 | NA | CT/R | NA | 618.3 | OS | M | 8 | [31] |
| Yan et al. | 2020 | Jan 2009 – Dec 2011 | China | 538 | 195/343 | I–IIIA | S | NA | 402.37 | OS/DFS | M | 8 | [32] |
| Zhang et al. | 2021 | May 2015 – Jun 2018 | China | 124 | 68/56 | NA | RT | NA | 480 | OS/PFS | M | 8 | [33] |
CRT: Chemoradiotherapy; CSS: Cancer-specific survival; CT: Chemotherapy; DFS: Disease-free survival; M: Multivariable; NA: Not available; NOS: Newcastle-Ottawa scale; OS: Overall survival; PFS: Progression-free survival; RFS: Recurrence-free survival; RT: Radiotherapy; S: Surgery; T: Targeted therapy; U: Univariable.
SII & OS in NSCLC
The total number of studies was 21, with 8994 patients reporting the correlation between SII and OS in NSCLC patients. The consequences suggested that high pretreatment SII had the connection with worse OS (HR: 1.79, 95% CI: 1.58–2.02; p < 0.001), despite there was heterogeneity among literature (I2 = 58.0%, p < 0.001) (Figure 2A). Moreover, subgroup analyses by sample size, country, analysis type, and SII cut-off value also demonstrated the importance of SII in NSCLC patients (Table 2).
Figure 2.
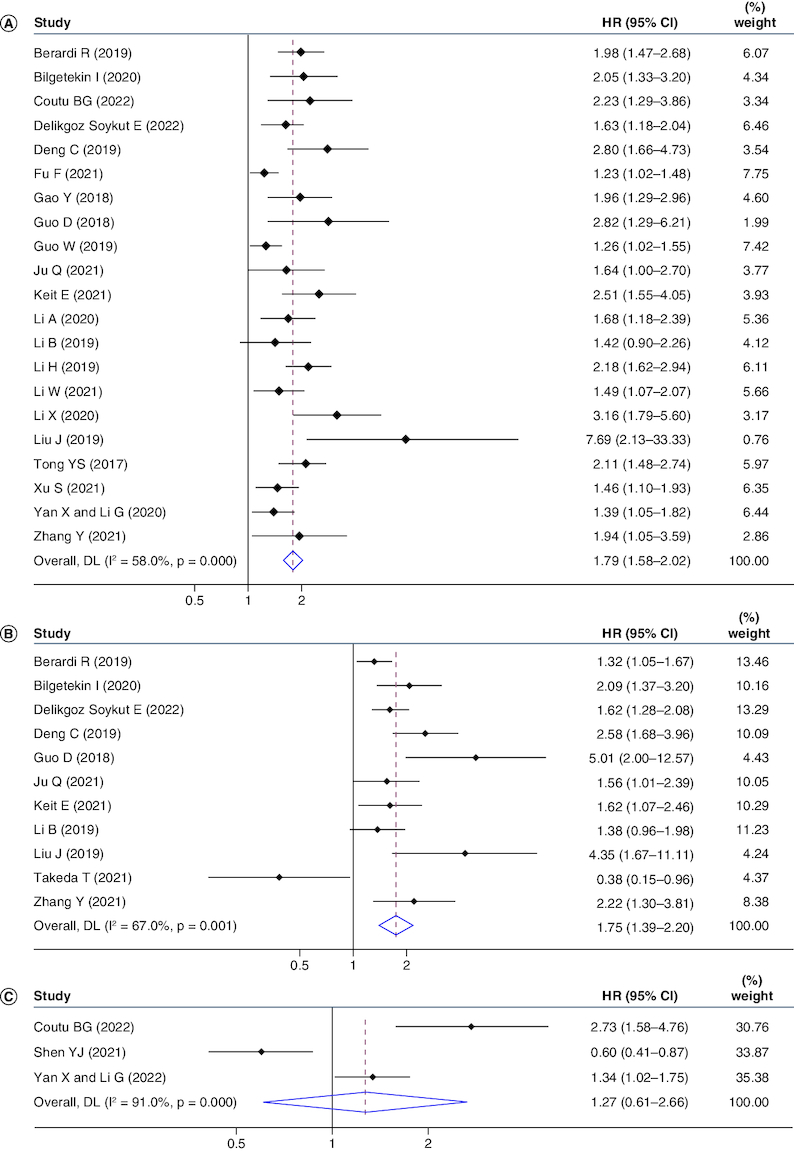
Forrest plots of studies evaluating hazard ratio with 95% CI of systemic immune inflammatory index for overall survival (A), progression-free survival (B) and disease-free survival (C).
Weights are from random-effects model.
Table 2.
Multivariate regression analysis with interaction terms.
| Outcome | Subgroup factor | Divided standard | Numbers of studies | HR (95% CI) | p-value | Heterogeneity | Effects model | |
|---|---|---|---|---|---|---|---|---|
| I2 (%) | Phet | |||||||
| OS | Country | Italy | 1 | 1.98 (1.47–2.67) | <0.001 | – | – | FEM |
| Turkey | 2 | 1.74 (1.38–2.19) | <0.001 | 0.0 | 0.385 | FEM | ||
| USA | 2 | 2.38 (1.66–3.42) | <0.001 | 0.0 | 0.750 | FEM | ||
| China | 16 | 1.73 (1.49–2.02) | <0.001 | 62.3 | <0.001 | REM | ||
| Sample size | ≥200 | 13 | 1.70 (1.47–1.96) | <0.001 | 64.9 | 0.001 | REM | |
| <200 | 8 | 2.02 (1.66–2.45) | <0.001 | 14.5 | 0.317 | FEM | ||
| SII cut-off value | ≥900 | 5 | 2.22 (1.86–2.63) | <0.001 | 0.0 | 0.813 | FEM | |
| <900 | 16 | 1.65 (1.44–1.88) | <0.001 | 52.4 | 0.008 | REM | ||
| Analysis type | MVA | 20 | 1.81 (1.59–2.06) | <0.001 | 59.8 | <0.001 | REM | |
| UVA | 1 | 1.42 (0.90–2.25) | 0.135 | – | – | FEM | ||
| PFS | Country | Italy | 1 | 1.32 (1.05–1.67) | 0.019 | – | – | FEM |
| Turkey | 2 | 1.73 (1.40–2.13) | <0.001 | 4.2 | 0.307 | FEM | ||
| China | 6 | 2.21 (1.55–3.16) | <0.001 | 62.1 | 0.022 | REM | ||
| USA | 1 | 1.62 (1.07–2.46) | 0.023 | – | – | FEM | ||
| Japan | 1 | 0.38 (0.15–0.96) | 0.041 | – | – | FEM | ||
| Sample size | ≥200 | 3 | 1.69 (1.23–2.32) | 0.001 | 72.9 | 0.025 | REM | |
| <200 | 8 | 1.80 (1.27–2.53) | 0.001 | 68.8 | 0.002 | REM | ||
| Analysis type | MVA | 8 | 1.10 (0.71–1.72) | 0.671 | 70.6 | 0.033 | REM | |
| UVA | 3 | 1.91 (1.64–2.21) | <0.001 | 44.9 | 0.08 | FEM | ||
| SII cut-off value | ≥900 | 4 | 1.38 (0.84–2.26) | 0.2 | 81.0 | 0.001 | REM | |
| <900 | 7 | 1.92 (1.49–2.47) | <0.001 | 51.3 | 0.055 | REM | ||
FEM: Fixed-effects model; M: Multivariable; OS: Overall survival; PFS: Progression-free survival; REM: Random-effects model; U: Univariable.
SII & PFS in NSCLC
Eleven studies involving 1767 patients reported the connection between SII and PFS in patients diagnosed with NSCLC. Pooled data gathering from 11 studies indicated that SII was in significant connection with PFS, with a combined HR estimate of 1.75 (95% CI: 1.39–2.20; p < 0.001), although there was heterogeneity (I2 = 67.0%, p = 0.001) (Figure 2B). Subgroup analyses were stratified by the country, SII cut-off value, and analysis type, suggesting the effect of SII on NSCLC prognosis (Table 2).
SII & DFS/RFS/CSS in NSCLC
Only three cohorts involving 2050 patients analyzed the association between SII and DFS. The pooled result was as follows: HR: 1.27, 95% CI: 0.61–2.66, p < 0.001, suggesting that increased SII had no significantly interrelation with worse DFS in NSCLC patients (Figure 2C).
The pretreatment SII did not appear to be associated with RFS in patients diagnosed with NSCLC, with only 2 cohorts (HR: 1.16, 95% CI: 1.02–1.32, p > 0.05).
Another two studies reported an interrelation between the baseline SII and CSS in patients with NSCLC, suggesting that the pretreatment SII had nothing to do with CSS (HR: 1.39, 95% CI: 0.22–8.78, p < 0.001).
Sensitivity analysis
Because of the significant heterogeneity among the literature, we conducted the sensitivity analyses on OS and PFS studies separately to evaluate whether individual studies influenced the integral analysis (Figure 3A & B). Sensitivity analysis indicated that the remanant HRs of the pooled studies were within the 95% CI of the consolidated HRs of OS and PFS in the analysis after the removal of any single study by turn, which showed that the pooled results had good stability.
Figure 3.
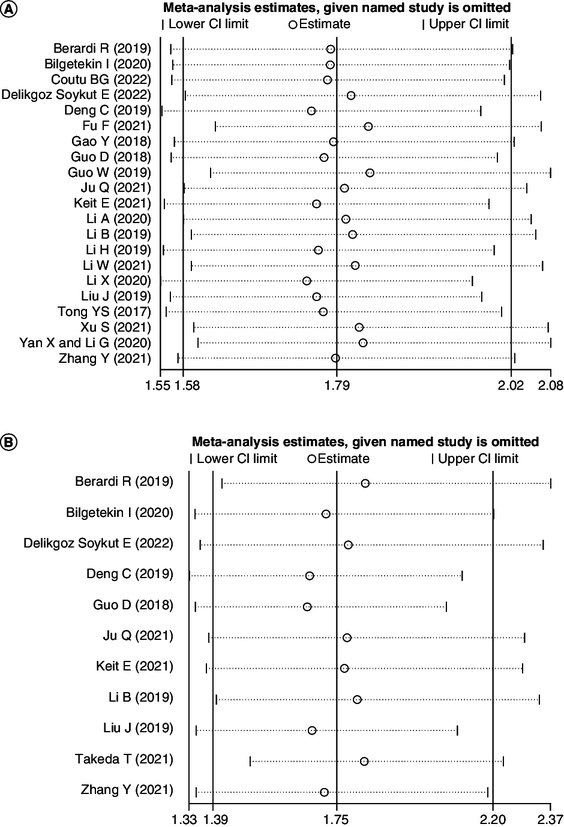
Sensitivity analysis of (A) overall survival and (B) progression-free survival.
Publication bias
Bias estimation was published principally with the purpose of evaluating the dependability of analysis consequences, particularly those which were statistically significant [34]. The possibility of bias was assessed by performing Begg's funnel plot (p < 0.05). It proved that there was significant publication bias among studies about OS (Figure 4A). So, we employed the trim and fill methods to evaluate the dissymmetry of funnel plot. The funnel plot showed symmetry by calculating and filling in seven unpublished studies (Figure 4B). No statistically significant changes were observed. The funnel plot (p = 0.285) demonstrated that was no prominent publication bias in PFS studies (Figure 4C).
Figure 4.
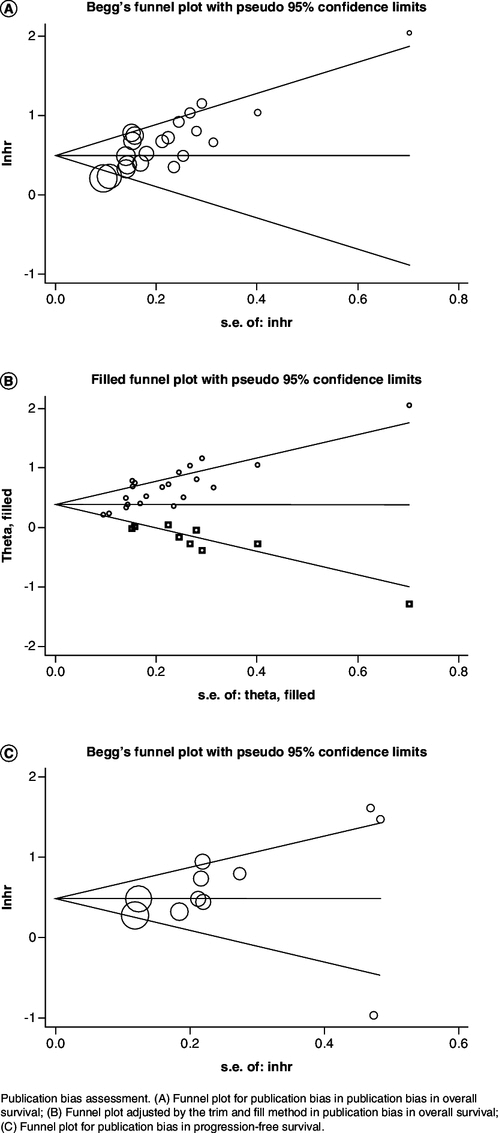
Publication bias assessment.
(A) Funnel plot for publication bias in overall survival; (B) Funnel plot adjusted by the trim and fill method in overall survival; (C) Funnel plot for publication bias in progression-free survival.
Discussion
Inflammation tends to promote cancer development and promotes tumorigenesis at all stages. Cancer cells and their surrounding stromal and inflammatory cells participate in a carefully orchestrated interaction that forms the inflammatory tumor microenvironment (TME) [35]. It has been found that tumor-associated inflammation takes an irreplaceable role in the tumor microenvironment [36,37]. Inflammatory cytokines and their receptors have an indispensable influence in the growth of tumor and the response of inflammation [38]. In addition, inflammatory factors have the association with poor prognosis in cancer patients. For the past few years, some prognostic indicators, such as CRP, interleukin-6 (IL-6), NLR and PLR have been proven to have prognostic effects on NSCLC patients [39-45]. Compared with CRP and IL-6, biomarkers that were related to inflammation based on the inflammatory cells throughout the body which have rapid availability, Such as NLR and PLR, are low-cost, easy to operate and obtain in clinic. However, these markers of inflammation generally consist of two kinds of inflammatory cells, while SII, a novel non-invasive biomarker based on three inflammatory factors has been demonstrated to be more objective that was in possession of better prognostic reliability [46,47].
Neutrophils are the important source of matrix metalloproteinase (MMP-9), and MMP-9 expression in tumor regions is increased in NSCLC. Neutrophils can release highly active MMP-9 to participate in the generation of tumor new blood vessels, and promote the migration, invasion and metastasis of tumor cells. A study on lung cancer showed that neutrophil count is an effective indicator of prognosis and has certain clinical value [48]. Lymphocytes are produced by the lymphatic organ and are the main cells of the immune system. It includes subgroups such as T cells and B cells and NK cells. Cellular immunity is mainly mediated by T cells, which become sensitized by antigen stimulation and directly contact and attack foreign bodies with specific antigenicity, such as tumor cells. B cells are mainly involved in humoral immune function of the body. In lung, tumor-associated B cells can differentiate into plasma cells and produce tumor-specific antibodies, which recognize and respond to tumor-associated antigens. Lung cancer patients with a higher proportion of tumor-associated antigen reactive immunoglobulin tend to have a higher density of follicular B cells. Both follicular B cells and tumor-infiltrating plasma cells are associated with better long-term survival in lung cancer patients. NK cells mainly mediate the killing function of tumor cells and virus-infected cells. They initiate cell death process directly and indirectly by recognizing, binding and forming immune synapse of target cells. They share a killing mechanism with cytotoxic T lymphocytes that involves tumornecrosis factor related apoptosis-induced ligand. TRAIL or activation of the receptor after the fas-fas-ligand interaction to activate the apoptotic pathway. Another mechanism involves the release of cytotoxic vesicles that contain apoptosis-inducing enzymes such as serine protease granase B and perforin. Target cell receptors bind to and absorb these vesicles, triggering apoptosis. One study in lung cancer showed that low lymphocyte percentage was an indicator of poor prognosis in patients, providing a higher clinical benefit [49]. Platelets have a role in angiogenesis, and their angiogenesis promoting function can play a role in tumor blood vessels, while in lung cancer patients, platelets tend to play a role in the systemic system. Activated platelets can secrete a variety of immunomodulatory molecules such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), etc., which are beneficial to the proliferation and metastasis of tumor cells by inhibiting NK cell activity and reducing NK cell toxicity. An analysis showed that elevated pretreated platelet count was an independent predictor of OS, DFS, PFS, time to progression (TTP) in lung cancer patients [50].
Our study was carried out in order to investigate the interrelation between the elevated SII and OS, PFS, DFS, RFS and CSS of NSCLC. In this study of 11195 subjects from 25 cohorts, it was proved that high SII was an important prognostic factor on NSCLC patients, whereas elevated SII could not predict worse DFS, RFS, CSS, possibly due to the relatively small sample. Despite heterogeneity, subgroup analyses by country, SII cut-off value, sample size, and analysis type did not attenuate most of the prognostic significance. In addition, subgroup analysis showed an accordant prognostic effect of SII for patients diagnosed with NSCLC of OS with a cut-off value ≥900, which was accordant with the result of a study about the relationship between SII and pancreatic cancer [51]. This result suggested that the OS possibly could have better prognostic value when its cut-off value was not less than 900.
As far as we know, a study by Wang Y et al. [52] also demonstrated that SII was an effective prognostic marker for NSCLC patients. The result was roughly consistent with the present study. However, based on it, our analysis included additional studies from different regions of the world: China, Turkey, Italy, and the United States, and we also explored the association between RFS and SII in NSCLC patients, although the literature included was limited. Subgroup analysis by country, SII cut-off value, sample size, and analysis type remained significant for prognosis. There was a meta-analysis involving seven studies on the prognostic value of SII in lung cancer, five of which focused on NSCLC, one on SCLC, and another one focused on both NSCLC and SCLC [53]. The results showed that high SII was significantly associated with poor OS in patients with lung cancer, and SII could predict the prognosis of lung cancer.
In an analysis of patients with urinary cancer, higher pretreatment SII was associated with worse OS and PFS [54]. And it was also associated with poorer CSS although only four studies were included, a finding that was in direct contrast to our analysis. In addition, this analysis found that patients with elevated SII may have adverse pathological features, including large tumor size, poor differentiation, and advanced tumor stage. Another analysis in patients with gastric cancer found that high SII was significantly associated not only with OS and DFS, but also with poor RFS, which was contrary to the result of this study [55].
So far, it has been widely recognized that inflammatory markers are closely related to the formation and progression of lung cancer. NLR can reflect the balance of pro-tumor and anti-tumor factors in NSCLC patients. The increase of NLR can reflect the immune imbalance of the body to a certain extent, that is, the anti-inflammatory effect is weakened, the inflammatory response is enhanced, and the tumor cells are in a state of positive growth [56]. A foreign meta-analysis found that high NLR was associated with poorer survival in patients with NSCLC. Patients with higher NLR before treatment had shorter overall survival (OS). NLR, as an alternative marker of systemic inflammation, has prognostic value in patients with non-small-cell lung cancer undergoing treaty-intended radiotherapy [57]. PLR can be used as an indicator to evaluate the progression and prognosis of lung cancer, and can indicate the coagulation status, inflammatory response and anti-tumor immunity of the body. A study included 254 patients with NSCLC as research objects and retrospectively analyzed the correlation between PLR and patient prognosis. The results showed that the overall survival of the high PLR group was significantly lower than that of the low PLR group, suggesting that PLR level was negatively correlated with overall survival [58]. Compared with them, SII is more stable, and it takes into account white blood cell, platelet, and lymphocyte counts, so it can more fully reflect the patient's inflammatory and immune status.
The study has the several limitations. First, the sample capacity was quite small in general, and it varied greatly among different studies. We did not perform the subgroup analysis among studies for DFS, RFS and CSS because fewer data was collected in the analysis. Further studies are required to demonstrate the prognostic value on SII for DFS, RFS and CSS in sick people with NSCLC. Secondly, all studies were retrospectively designed and the majority of them were carried out in China and Japan which could have contributed to possible selection bias and high heterogeneity. But then again, the analysis of sensitivity and the bias of publication test suggested the reliability of the consequence, we could not exclude the reason for the differences between cohorts due to the different research criteria used between studies. Thirdly, there may be publication bias, and meaningful results were more likely to be published. It was important, however, that after trimming and filling, there was no change in the prognostic effect of pretreated SII for patients with NSCLC. Finally, due to the lack of raw data, we could not carry out additional subgroup analyses based on TNM stage, comorbidities, duration of follow-up and other factors.
Conclusion
In summary, our analysis suggested that the high pretreatment SII might be a negative prognostic indicator for patients diagnosed with NSCLC. Therefore, we suggested that SII could be used in clinical practice as a valid marker for the prognosis of NSCLC patients diagnosed with NSCLC, which could help to stratify patients and determine personalized treatment regimens. Nevertheless, the prognostic value of SII in NSCLC needs further research improvement and large-scale clinical trials to confirm.
Author contributions
Conceived and designed the analysis: W-H Chen, J-J Shao, H-Z Chen, Q Wang.
Collected the data: W-H Chen, J-J Shao, Y Yang.
Contributed data or analysis tools: J-J Shao, Y Yang, J-B Liu.
Performed the analysis: W-H Chen.
Wrote the paper: W-H Chen, J-J Shao.
Reviewed the draft: J-G Chen, Q Wang, R-F Xu, Y Meng, S Huang, H-Z Chen.
Study supervision: J-G Chen, Q Wang, Rong-Fang Xu, Ji-Bin Liu, Hai-Zhen Chen.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ferlay J, Soerjomataram I, Dikshit Ret al. . Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136(5), E359–E386 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RLet al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 3.de Goede OM, Nachun DC, Ferraro NMet al. . Population-scale tissue transcriptomics maps long non-coding RNAs to complex disease. Cell 184(10), 2633–2648; e19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang R, Chang Q, Meng Xet al. . Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J. Cancer 9(18), 3295–3302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B, Yang XR, Xu Yet al. . Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 20(23), 6212–6222 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25(9), 603–605 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Barili F, Parolari A, Kappetein PAet al. . Statistical Primer: heterogeneity, random- or fixed-effects model analyses? Interact. Cardiovasc. Thorac. Surg. 27(3), 317–321 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Herrmann D, Sinnett P, Holmes Jet al. . Statistical controversies in clinical research: publication bias evaluations are not routinely conducted in clinical oncology systematic reviews. Ann. Oncol. 28(5), 931–937 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Berardi R, Santoni M, Rinaldi Set al. . Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small-cell lung cancer. Ann. Transl. Med. 7(20), 572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilgetekin I, Basal FB. Systemic immune-inflammation index is the best prognostic factor in patients with advanced stage adenocarcinoma of the lung treated with pemetrexed. J. Coll. Physicians Surg. Pak. 30(9), 933–939 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Coutu BG, Johnson KC, Bhirud Aet al. . Systemic immune-inflammatory index association with survival in patients undergoing trimodality therapy for lung cancer. Oncology 100(5), 247–256 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Delikgoz SE, Kemal Y, Karacin Cet al. . Prognostic impact of immune inflammation biomarkers in predicting survival and radiosensitivity in patients with non-small-cell lung cancer treated with chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 66(1), 146–157 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Deng C, Zhang N, Wang Yet al. . High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine (Baltimore) 98(33), e16875 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu F, Deng C, Wen Zet al. . Systemic immune-inflammation index is a stage-dependent prognostic factor in patients with operable non-small-cell lung cancer. Transl. Lung Cancer Res. 10(7), 3144–3154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The study found that high systemic immune inflammatory index values were associated with an increase in other inflammatory markers, such as C-reactive protein and white blood cell counts, as well as the presence of malignant clinical features, such as lymph node metastasis and lung infiltration.
- 15.Gao Y, Zhang H, Li Yet al. . Preoperative increased systemic immune-inflammation index predicts poor prognosis in patients with operable non-small-cell lung cancer. Clin. Chim. Acta 484, 272–277 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Guo D, Zhang J, Jing Wet al. . Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol. 14(25), 2643–2650 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Guo W, Cai S, Zhang Fet al. . Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small-cell lung cancer. Thorac. Cancer 10(4), 761–768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The study concluded that systemic immune inflammatory index could be a useful biomarker for predicting survival outcomes in patients with surgically resected NSCLC.
- 18.Ju Q, Huang T, Zhang Yet al. . Systemic immune-inflammation index predicts prognosis in patients with different EGFR-mutant lung adenocarcinoma. Medicine (Baltimore) 100(6), e24640 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keit E, Coutu B, Zhen Wet al. . Systemic inflammation is associated with inferior disease control and survival in stage III non-small-cell lung cancer. Ann. Transl. Med. 9(3), 227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li A, Mu X, He Ket al. . Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol. 16(30), 2433–2444 (2020). [DOI] [PubMed] [Google Scholar]
- 21.Li B, Wang S, Li Cet al. . The kinetic changes of systemic inflammatory factors during bevacizumab treatment and its prognostic role in advanced non-small-cell lung cancer patients. J. Cancer 10(21), 5082–5089 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Wang G, Zhang Het al. . Prognostic role of the systemic immune-inflammation index in brain metastases from lung adenocarcinoma with different EGFR mutations. Genes Immun. 20(6), 455–461 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Li W, Qu Y, Wen Fet al. . Prognostic nutritional index and systemic immune-inflammation index are prognostic biomarkers for non-small-cell lung cancer brain metastases. Biomark Med. 15(13), 1071–1084 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Li X, Hu P, Liu Jet al. . Systemic immune-inflammation index predicted overall survival and radiosensitivity in advanced non-small-cell lung cancer. Future Oncol. 16(5), 103–115 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Li S, Zhang Set al. . Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J. Clin. Lab. Anal. 33(8), e22964 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen YJ, Qian LQ, Ding ZPet al. . Prognostic value of inflammatory biomarkers in patients with stage I lung adenocarcinoma treated with surgical dissection. Front Oncol. 11, 711206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda T, Yamada T, Tanimura Ket al. . Prognostic markers of survival among japanese patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer receiving first-line alectinib. Diagnostics (Basel) 11(12), 2170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomita M, Ayabe T, Maeda Ret al. . Systemic immune-inflammation index predicts survival of patients after curative resection for non-small-cell lung cancer. In Vivo 32(3), 663–667 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong YS, Tan J, Zhou XLet al. . Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small-cell lung cancer. J. Transl. Med. 15(1), 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K, Noma D, Masuda Het al. . Preoperative inflammation-based scores predict early recurrence after lung cancer resection. J. Thorac. Dis. 13(5), 2812–2823 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Cao S, Yu Y. High systemic immune-inflammation index is a predictor of poor prognosis in patients with nonsmall cell lung cancer and bone metastasis. J. Cancer Res. Ther. 17(7), 1636–1642 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Li G. Preoperative systemic immune-inflammation index predicts prognosis and guides clinical treatment in patients with non-small-cell lung cancer. Biosci. Rep. 40(3), BSR20200352 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Chen Z, Jin Fet al. . The value of the systemic immune-inflammation index in predicting survival outcomes in patients with brain metastases of non-small-cell lung cancer treated with stereotactic radiotherapy. Mediators Inflamm. 2021, 2910892 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yajun E, He N, Wang Yet al. . Percutaneous transluminal angioplasty (PTA) alone versus PTA with balloon-expandable stent placement for short-segment femoropopliteal artery disease: a metaanalysis of randomized trials. J. Vasc. Interv. Radiol. 19(4), 499–503 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51(1), 27–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 357(9255), 539–545 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Mantovani A, Allavena P, Sica Aet al. . Cancer-related inflammation. Nature 454(7203), 436–444 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Cho WC, Kwan CK, Yau Set al. . The role of inflammation in the pathogenesis of lung cancer. Expert Opin. Ther. Targets 15(9), 1127–1137 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Liao C, Yu Z, Guo Wet al. . Prognostic value of circulating inflammatory factors in non-small-cell lung cancer: a systematic review and meta-analysis. Cancer Biomark. 14(6), 469–481 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Jin Y, Sun Y, Shi Xet al. . Prognostic value of circulating C-reactive protein levels in patients with non-small-cell lung cancer: a systematic review with meta-analysis. J. Cancer Res. Ther. 10(Suppl.), C160–C166 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Jiang J, Tang Set al. . Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Int. Immunopharmacol. 85, 106677 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Jiang T, Bai Y, Zhou Fet al. . Clinical value of neutrophil-to-lymphocyte ratio in patients with non-small-cell lung cancer treated with PD-1/PD-L1 inhibitors. Lung Cancer 130, 76–83 (2019). [DOI] [PubMed] [Google Scholar]; • This study suggests that measuring and monitoring neutrophil-to-lymphocyte ratio levels can help assess patient response to treatment with PD-1/PD-L1 inhibitors and possible risk of adverse events.
- 43.Zhang H, Gao L, Zhang Bet al. . Prognostic value of platelet to lymphocyte ratio in non-small-cell lung cancer: a systematic review and meta-analysis. Sci. Rep. 6, 22618 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu X, Sun S, Gao XSet al. . Prognostic value of platelet to lymphocyte ratio in non-small-cell lung cancer: evidence from 3,430 patients. Sci. Rep. 6, 23893 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao QT, Yuan Z, Zhang Het al. . Prognostic role of platelet to lymphocyte ratio in non-small-cell lung cancers: a meta-analysis including 3,720 patients. Int. J. Cancer 139(1), 164–170 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Tong YS, Tan J, Zhou XLet al. . Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small-cell lung cancer. J. Transl. Med. 15(1), 221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo W, Cai S, Zhang Fet al. . Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small-cell lung cancer. Thorac. Cancer 10(4), 761–768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teramukai S, Kitano T, Kishida Yet al. . Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur. J. Cancer 45(11), 1950–1958 (2009). [DOI] [PubMed] [Google Scholar]
- 49.Huang H, Li L, Luo Wet al. . Lymphocyte percentage as a valuable predictor of prognosis in lung cancer. J. Cell Mol. Med. 26(7), 1918–1931 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuan Y, Zhong H, Ye Let al. . Prognostic value of pretreatment platelet counts in lung cancer: a systematic review and meta-analysis. BMC Pulm. Med. 20(1), 96 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shui Y, Li M, Su Jet al. . Prognostic and clinicopathological significance of systemic immune-inflammation index in pancreatic cancer: a meta-analysis of 2,365 patients. Aging (Albany NY) 13(16), 20585–20597 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Li Y, Chen Pet al. . Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small-cell lung cancer: a meta-analysis. Ann. Transl. Med. 7(18), 433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Chen B, Wang Let al. . Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine (Baltimore) 98(3), e13788 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li X, Gu L, Chen Yet al. . Systemic immune-inflammation index is a promising non-invasive biomarker for predicting the survival of urinary system cancers: a systematic review and meta-analysis. Ann. Med. 53(1), 1827–1838 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Lin S, Yang Xet al. . Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J. Cell. Physiol. 234(5), 5555–5563 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy 122(7), 474–488 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Punjabi A, Barrett E, Cheng Aet al. . Neutrophil-lymphocyte ratio and absolute lymphocyte count as prognostic markers in patients treated with curative-intent radiotherapy for non-small-cell lung cancer. Clin. Oncol. (R Coll Radiol.) 33(8), e331–e338 (2021). [DOI] [PubMed] [Google Scholar]
- 58.Huang Q, Diao P, Li CLet al. . Preoperative platelet-lymphocyte ratio is a superior prognostic biomarker to other systemic inflammatory response markers in non-small-cell lung cancer. Medicine (Baltimore) 99(4), e18607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


