Abstract
Background:
Inflammation is thought to play an important role in hand osteoarthritis (HOA), which is associated with pain and increased limitation of hand function.
Objectives:
To explore the acceptability of therapy with intramuscular methylprednisolone in HOA among health-care providers (HCPs) and HOA patients. Additionally, the response to a single methylprednisolone injection was investigated.
Design:
We adopted a mixed-methods design.
Methods:
In a qualitative study, we asked HCPs and patients for their acceptability of intramuscular methylprednisolone. A prospective observational study was performed afterward in HOA patients who received a single 120-mg intramuscular methylprednisolone injection as part of off-label administration. Average pain, functional impairment, and occurrence of adverse events were assessed at baseline and at 4, 8, and 12 weeks after the injection.
Results:
Fourteen HCPs and 15 patients participated in the first part of the study. They considered intramuscular methylprednisolone potentially effective, yet expressed concerns about the risk for long-term adverse events. Among the 22 HOA patients who received intramuscular methylprednisolone, 13 patients reported 44 adverse events, with half of them occurring within the first 4 weeks after injection and being classified as nonserious. Mean hand pain decreased the most 4 weeks after injection and this effect persisted till week 12, though less pronounced. Similar results were seen with HOA-related functional impairment, which improved the most at week 4 and to a lesser extent at week 12.
Conclusion:
We found a good acceptability of intramuscular methylprednisolone treatment among HCPs and HOA patients, as well as a potential to reduce pain and improve hand function with a good safety profile for as long as 12 weeks after a single administration.
Keywords: corticosteroid, feasibility, methylprednisolone, osteoarthritis, therapy
Plain language summary
Use of methylprednisolone in the muscle for hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial
Hand osteoarthritis is very common among adults and older people, and being characterized by high levels of pain, stiffness and decreased function of the hand. Therapeutic options for hand osteoarthritis are limited. Methylprednisolone is sometimes used in clinical practice with good results. However, these results are mainly based on the experience of the physicians and do not rely on properly performed studies. That is why we intend to finally perform such a study. But before starting that, we wanted to see if patients with hand osteoarthritis and healthcare providers would accept the idea of injecting methylprednisolone into the buttocks muscle for the treatment of hand osteoarthritis. That is why we performed interviews with patients and healthcare providers, which brought up six factors to think about when discussing this therapeutic option: how well it works, how safe it is, the overall situation of the patient, how it’s given, use of shared decision-making and logistics aspects. Next to this, we examined the effect of methylprednisolone over time on pain and function of the hands, as well as the frequency and nature of side effects. We found a decrease in hand pain and an improvement in hand function persisting for 12 weeks after just one methylprednisolone injection. Half of the patients reported side effects, but they all were non-serious. Eventually, we concluded that the use of methylprednisolone as an injection in the buttocks muscle for the treatment of hand osteoarthritis is accepted by both patients and health-care providers, and could be a safe and helpful therapy. The results will be used in the next study designed to assess the effect and safety of this therapy in a much larger group of patients with hand osteoarthritis.
Introduction
Hand osteoarthritis (HOA) is the most common cause of joint-related complaints in the hands.1,2 It often involves multiple joints and is characterized by various levels of pain, stiffness, and impaired hand function, leading in its severe forms to high levels of disease burden and reduced quality of life.1,3,4 Therapeutic pharmacological options for HOA are limited and current practice using nonpharmacological therapy, analgesics, and topical therapy has limited efficacy in reducing pain and improving other aspects of the disease.4,5
Previous studies indicated that inflammation might play an important role in the development and disease progression of HOA.6–12 Consequently, inflammation is considered a potential treatment target in HOA, though effective suppression has many challenges in terms of the targeted pathway, degree of anti-inflammatory effect (low-grade inflammation), and duration of therapy (chronic versus on-demand). Corticosteroids are potent anti-inflammatory compounds that bind to the glucocorticoid receptor on the cell membrane, leading to the activation of intracellular pathways responsible for the production of anti-inflammatory cytokines as well as the suppression of the pro-inflammatory ones. 13 In the case of multiple joint involvement, as often in HOA, systemically administered therapies should be preferred against local ones. However, the knowledge of the use of systemic corticosteroids in osteoarthritis (OA) patients is rather limited. In a double-blind, randomized, placebo-controlled trial, a short-term course of orally administered prednisolone reduced pain over 6 weeks in patients with HOA and synovial inflammation. 3 Likewise, in an observational study, a single intramuscular injection of 120-mg methylprednisolone was followed by a decrease in all symptoms in a group of HOA patients. 14 In addition, a lower number of adverse events for intramuscular (i.m.) corticosteroids as compared to oral administration has also been previously reported, 15 suggesting a better risk–benefit ratio for the intramuscular approach.
Currently, i.m. methylprednisolone (also known as Depo-Medrol) is used in clinical practice, but the acceptability to offer this treatment differs among clinicians, due to lack of evidence about the efficacy and safety, especially when considering the chronicity of OA and thus the need for a long-term solution. In line with that, the dosage and interval of administration are still a matter of debate in daily practice while being of importance to design an adequate randomized double-blind, placebo-controlled trial to investigate the long-term use of i.m. methylprednisolone on HOA patients. Given these facts, we addressed some feasibility issues in the present study, by (i) exploring the acceptability of intramuscular methylprednisolone use in HOA among both health-care providers (HCPs) and HOA patients and (ii) studying the effect of a single intramuscular methylprednisolone injection in HOA patients over time in terms of frequency and nature of adverse events as well as effect on pain and hand function.
Methods
This mixed-methods study was approved by the local institutional review board of the Sint Maartenskliniek. The Medical Ethics Committee of Oost-Nederland confirmed that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this research (MEC-2022-13592). The reporting of this study conforms to the Consolidated criteria for Reporting Qualitative research statement 16 and the Strengthening the Reporting of Observational Studies in Epidemiology Statement. 17 First, a qualitative study was performed in order to examine the acceptability of intramuscular methylprednisolone injections in HOA as perceived by HCPs and patients. HCPs completed an online questionnaire with open-ended questions, while patients participated in short semistructured interviews, conducted by KALvK over the phone because of the COVID-19 pandemic. Second, an observational study of 12 weeks has been initiated to explore the nature and frequency of adverse events and the within-group effects on pain and hand functioning of a single i.m. methylprednisolone injection in patients with symptomatic HOA. All participants gave written informed consent prior to entering the study.
Population
HCPs [rheumatologists and physician assistants (PAs)] were recruited from the Rheumatology Department of the Sint Maartenskliniek Nijmegen. To avoid selection bias, we invited all 27 HCPs of our department by email to participate in this study. In addition, we invited two groups of HOA patients to participate in this study. During consultation patients were informed about the study by their treating rheumatologist and after showing interest they received information about the study by mail. The first group (HOA-group I) was invited to participate in the qualitative study and consisted of an a-selective group of HOA patients who visited the outpatient clinic of the Sint Maartenskliniek in the last year, were older than 40 years, fulfilled the 1990 American College of Rheumatology (ACR) diagnostic criteria for HOA, 18 and were able to read, write, and sufficiently communicate in Dutch. Patients who had previously received corticosteroids injections and those who suffered from other rheumatic diseases, fibromyalgia, or other conditions that could interfere with the assessment of pain were excluded. The second group of patients (HOA-group II) participated in the observational study and consisted of symptomatic HOA patients for whom an intramuscular injection of 120-mg methylprednisolone was indicated by their HCP as part of off-label administration. Patients were considered eligible if they fulfilled the same conditions as those in HOA-group I, as well as being able to complete online questionnaires via Castor Electronic Data Capture (EDC). 17 In addition, any glucocorticoid injection 12 weeks prior to inclusion constituted an exclusion criterion. Given the fact that this study was mainly focused on safety parameters, no power calculation has been done to determine the number of patients to be included.
Data collection
Regarding the participants of the qualitative study, following informed consent, HCPs completed an online questionnaire in Castor EDC. 19 The questionnaire contained three open-ended questions regarding the advantages, the disadvantages, and the terms and conditions of methylprednisolone injections for patients with HOA (Supplemental Appendix 1). HCPs also had the opportunity to write down any additional comments in the questionnaire. KALvK grasped the principles of qualitative research and interviewing, but did not undergo practical training. A semistructured interview guide was then developed to ensure that the perspectives of patients about methylprednisolone use in HOA were discussed (Supplemental Appendix 2). After consensus on a set of interview questions was reached, all interviews were conducted by one investigator (KALvK), who had no pre-existing relationship with any of the participants. Patients received a brochure with information about i.m. corticosteroid injections prior to taking part in the interviews. Interview questions had an open-ended format and remained flexible in order to be able to explore relevant topics as they arose during the interviews. In the interviews, of a maximum of 30 min, patients were asked to evoke under which terms and conditions they will consider having (multiple) i.m. methylprednisolone injections as therapy for their complaints, including advantages and disadvantages of the therapy. Interview conversations were audio recorded and afterward transcribed verbatim for analysis. Analysis was performed after approval of the interview summary by the patients. No field notes were made as interviews were performed by phone.
Regarding the observational study, after giving their informed consent, HOA patients (HOA-group II) were followed for 3 months. Data were collected at the following timepoints: baseline (B), that is, within 3 days after injection; at 4 (FU1), 8 (FU2), and 12 weeks (FU3) after methylprednisolone was administered. Patient and disease characteristics at baseline were obtained from patient files, including age, gender, years since diagnosis, C-reactive protein-levels, the presence of nodules (Heberden and/or Bouchard), the number of tender joints, and presence of inflammatory HOA according to the treating physician. Subsequently, patient files were examined to determine if patients met the ACR criteria. 18 At each time point, patients completed an online questionnaire investigating their average pain [Numerical Rating Scale (NRS); range 0–10; 0 = no pain], hand functionality (Functional Index for Hand OsteoArthritis; range 0–30; 0 = no functional impairment), 20 and use of analgesics (Yes/No; if yes, which analgesics do you use?). The occurrence of adverse events was assessed using a checklist based on the adverse event profile of corticosteroids, 21 with the possibility to indicate whether and what other adverse events had occurred (Supplemental Appendix 3). In case a patient repeatedly reported the same adverse event, this was recorded just once, in the period when it occurred for the first time.
Data analysis and statistical analysis
A thematic analysis was performed to identify themes that reflected the most important aspects regarding the acceptability of methylprednisolone use in HOA perceived by HCPs and HOA-group I. Two investigators (male rheumatologist CP and female master student KALvK) separately analyzed the answers to the questionnaires, collated codes, and grouped those codes into themes. By comparing and discussing a consensus on a set of themes was reached, with which the answers were analyzed. These themes are also supported with quotes in the Result section. Quotes were translated from Dutch into English. Quotes from HCPs were indexed by their identification number and their profession (e.g. HCP 27, Rheumatologist), and quotes from patients were indexed by the patient identification number and age (e.g. Patient 13, 57). Descriptive statistics were used to describe baseline parameters and outcome measures of the observational study. The Shapiro–Wilk test was used to check for normal distribution of data. Linear mixed models were used to assess differences between baseline and follow-up assessments for continuous data. Missing data in one or more variables were not replaced for statistical analysis, leading to different sample sizes for different follow-up moments. A two-sided p value <0.05 was considered statistically significant. In addition, the proportion of patients with improvements on NRS pain exceeding the minimal clinically important difference (MCID) was calculated. Farrar et al. 22 showed that for NRS pain a two-point decrease between baseline and follow-ups can be seen as the MCID. All statistical analyses were performed using R Statistical Software (v4.1.2; R Core Team 2021) within Rstudio version 2022.07.2 (Build 576).
Results
Acceptability of methylprednisolone injections among HCPs
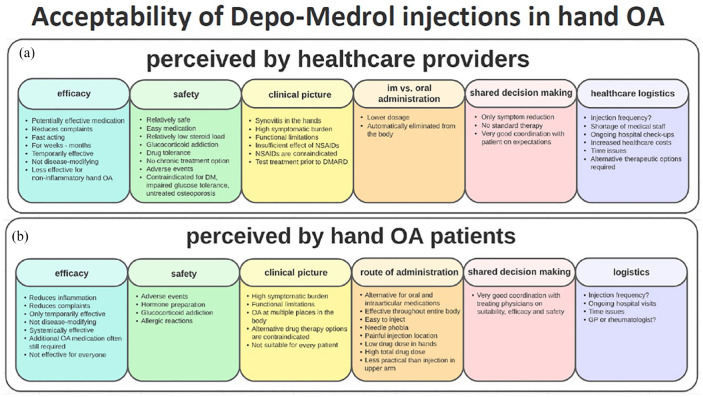
Of the 27 HCPs who were invited to participate in this qualitative study, 14 HCPs provided written informed consent and filled out the questionnaire. Based on their answers, we could identify six specific themes reflecting the acceptability of i.m. methylprednisolone use, including efficacy, safety, clinical picture, mode of administration, use of shared decision-making, and healthcare logistics [Figure 1(a)]. Within these themes, we further identified barriers and facilitators for i.m. methylprednisolone therapy in HOA. Some key elements have been mentioned by HCPs that would generally favor prescribing this medication to a HOA patient. First, the presence of high clinical burden like synovitis and therefore a higher chance for beneficial effect on pain and function together with a favorable safety profile: ‘Methylprednisolone is an “easy medication” that causes only few adverse events and it has a relatively low steroid load.’ (HCP 27, Rheumatologist). In addition, i.m. route was preferred over the oral one, due to a lower cumulative dosage and the possibility to administer the drug at large intervals. Finally, many HCPs highlighted the importance of shared decision-making in order to manage expectations in a balanced manner. Some concerns have been expressed as well, which may impede its administration. These included short duration of effect in some of the patients, lack of disease-modifying drug effect, and especially the chance of developing side effects (e.g. skin atrophy) when the drug would be repeatedly administered for longer periods of time. Also, the possibility to develop glucocorticoid addiction and drug tolerance on the long run were mentioned as concerns. Therefore, a low frequency of i.m. injections has been advocated by some HCPs, ranging from one to maximal three times a year: ‘I would only suggest methylprednisolone therapy if there are strict agreements that it can be given a maximum of X times only.’ (HCP 11, Rheumatologist). Finally, logistic issues also constitute barriers to the long-term use of this therapy due to monitoring the efficacy and safety of the therapy, potentially increasing the healthcare costs.
Figure 1.
Acceptability of methylprednisolone injections in HOA. (a) Acceptability of methylprednisolone injections in HOA perceived by HCPs. (b) Acceptability of methylprednisolone injections in HOA perceived by patients.
HCP, health-care provider; HOA, hand osteoarthritis.
Acceptability of methylprednisolone injections among patients with HOA
Fifteen out of 19 invited HOA patients provided written informed consent and participated in interviews until data saturation had been reached (i.e. no new information emerged from the last two interviews). The median (IQR) age was 61 (53–69) years and 13 patients (87%) were female. Based on their answers we could identify six specific themes, reflecting the acceptability of i.m. methylprednisolone use. These themes were more or less similar with those of the HCPs and included efficacy, safety, clinical picture, route of administration, use of shared decision-making, and logistics aspects [Figure 1(b)]. Within these themes, we further identified barriers and facilitators perceived by the patients for i.m. methylprednisolone therapy in HOA.
Patients were willing to accept i.m. methylprednisolone injections as therapy if it ensures reduction of pain, improvement of hand function, reduce in use of concomitant analgesics, being a more convenient route than oral administration, as well as a lower frequency of administration. In addition, the severity of complaints, in particular severe pain impeding the use of hands, emerged as a crucial condition to accept this medication: ‘When in pain I really can’t use my hands, so before I would try a new medicine I have to know that it reduces the pain by minimally 50% (Patient 11, 62)’. The importance of shared decision-making was highlighted by the patients as well, indicating the importance of consulting their treating physicians prior to receiving the therapy: ‘If my rheumatologist is convinced that this medication is most likely to be effective for me and that it is not harmful to my body, I will gladly consider taking one or even more injections.’ (Patient 5, 70). Some drawbacks have been indicated that may discourage patients to accept this therapy. First, patients were concerned about the potential adverse events, especially skin atrophy, suppression of their immune system, development of infections, and gaining weight: ‘I’ve heard some benefits, but I’ve have also heard a lot of side effects. I understood that more bruising, a thinner skin, fever, respiratory distress, and an increase of certain blood levels might be possible. This is too much for me, making me doubt to use this medication.’ (Patient 4, 69). Second, the repeated visits to the hospital were named as a barrier, as patients would prefer to be treated in their own general practitioners practice rather than traveling to the outpatient clinic of our hospital.
Patient population
A total of 22 patients with symptomatic HOA were included in the observational part of the study, 11 of those were diagnosed with inflammatory HOA according to the treating rheumatologist. For eight patients, no mention of having synovitis/inflammation or not could be found in their medical records. The mean (SD) age of the patients was 67 (10) years (Table 1). Twenty-one patients (96%) were female and 21 of the patients were using one or more analgesics (Table 2). None of the patients was using oral anticoagulants. All participants completed all assessments (except for one participant who missed the 8-week follow-up assessment).
Table 1.
Patients’ characteristics at baseline.
| Variable | Baseline value (n = 22) |
|---|---|
| Age at baseline, years, mean (SD) | 67 (10) |
| Female, n (%) | 21 (96) |
| Years since diagnosis by rheumatologists, mean (SD) | 4 (5) |
| Presence of synovitis, n (%); n = 14 | 11 (79) |
| Presence of nodules of Heberden and/or Bouchard, n (%); n = 21 a | 21 (100) |
| Number of tender joints (0–30), mean (SD); n = 12 | 5 (3) |
| Average hand pain past week (0–10), mean (SD) | 6.0 (1.4) |
| Fulfilling ACR criteria HOA, n (%); n = 21 a | 21 (100) |
| hsCRP (mg/L), median (IQR); n = 13 | 2 (1–3) |
Unclear information in one patient file.
hsCRP, high-sensitivity C-reactive protein; HOA, hand osteoarthritis; IQR, interquartile range; SD, standard deviation.
Table 2.
Clinical outcomes at weeks 4, 8, and 12 of the observational study.
| Variable | Baseline | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| Mean (SD) | Δ [95% CI] | Δ [95% CI] | Δ [95% CI] | |
| Average hand pain past week (0–10) | 6.0 (1.4) | −2.0 [−2.7 to −1.2] | −1.6 [−2.3 to −0.9] | −1.2 [−2.0 to −0.5] |
| Pain in other joints of worst moments past week (0–10) | 6.5 (2.4) | −1.4 [−2.3 to −0.6] | −0.4 [−1.3 to 0.4] | −0.4 [−1.2 to 0.5] |
| FIHOA score (0–30) | 13.0 (5.7) | −2.3 [−4.1 to −0.6] | −2.2 [−4.0 to −0.4] | −1.5 [−3.3 to 0.3] |
| n (%) | n (%) | n (%) | n (%) | |
| Analgesics use | 21 (96) | 19 (86) | 18 (82) | 20 (91) |
| Acetaminophen | 16 (73) | 12 (55) | 15 (68) | 15 (68) |
| NSAID | 8 (36) | 8 (36) | 5 (23) | 6 (27) |
| Opiates | 3 (14) | 2 (9) | 3 (14) | 2 (9) |
| Hydroxychloroquine | 3 (14) | 3 (14) | 2 (9) | 2 (9) |
CI, confidence interval; FIHOA, Functional Index for Hand OsteoArthritis; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; Δ, difference compared to baseline.
Adverse events
During the study period, a total of 13 patients reported 44 adverse events, of which almost half (19 adverse events) occurred within the first 4 weeks after methylprednisolone administration (Table 3). All adverse events were nonserious and resolved within 4 weeks after mentioning, except for the persisting adverse events. The persisting adverse events, reported by 6 of the 22 patients (27%), included mood changes, eye diseases, infection, insomnia, skin changes, and weight gain. Insomnia, heart palpitations, mood changes, and skin changes were the most frequently reported adverse events. No allergic reactions or comorbidities such as cardiovascular diseases or diabetes were reported. Reported local adverse events were pain around the injection site (one patient) and tingling or numbness (one patient). Finally, transitory muscle cramps, facial pain, the sensation of a cold leg, nerve root pain, attacks of dizziness and tightness of the chest, shortness of breath, fluctuating blood pressure, and hot flushes have been mentioned by six patients within the first 4 weeks after methylprednisolone administration.
Table 3.
Reported adverse events of the checklist in the observational study at weeks 4, 8, and 12.
| Reported adverse events | Week 4 (n = 22) | Week 8 (n = 21) | Week 12 (n = 22) | Total | Persisting adverse events |
|---|---|---|---|---|---|
| Local adverse events | |||||
| Pain around the injection site | 1 | 0 | 0 | 1 | 0 |
| Tingling or numbness | 1 | 0 | 0 | 1 | 0 |
| Systemic adverse events | |||||
| Reddening of the face | 3 | 0 | 0 | 3 | 0 |
| Mood changes | 4 | 1 | 2 | 7 | 1 |
| Eye diseases | 1 | 0 | 0 | 1 | 1 |
| High blood pressure | 0 | 3 | 1 | 4 | 0 |
| Digestive disorder | 0 | 1 | 2 | 3 | 0 |
| Infection | 0 | 1 | 0 | 1 | 1 |
| Insomnia | 1 | 2 | 2 | 5 | 2 |
| Heart palpitations | 1 | 3 | 2 | 6 | 0 |
| Round face | 1 | 1 | 0 | 2 | 0 |
| Skin changes | 4 | 2 | 1 | 7 | 4 |
| Weight gain | 2 | 1 | 0 | 3 | 3 |
| Total adverse events | 19 | 15 | 10 | 44 | 12 |
| Number of patients with ⩾1 adverse event, n (%) | 13 (59) | 12 (57) | 12 (55) | ||
Reported adverse events are shown as n. Persisting adverse events are defined as adverse events reported more than once by the same patient.
Clinical parameters
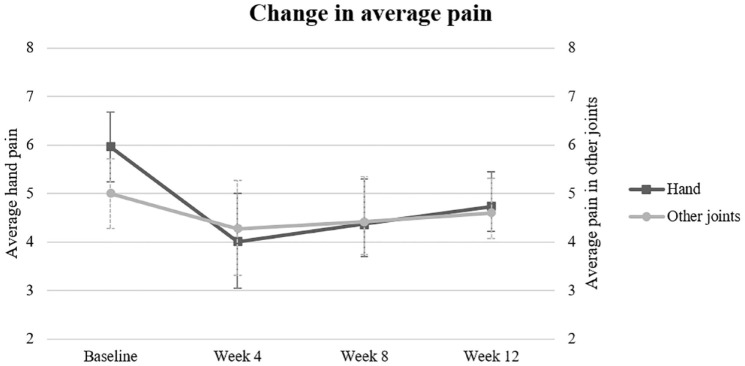
Four weeks after methylprednisolone administration a significant decrease of −2.0 (95% CI: −2.7 to −1.2) in the average hand pain was observed (Figure 2), yet this effect diminished over time to a decrease of −1.2 (−2.0 to −0.5) at the 12-week follow-up. Of note, during the first 8 weeks after injection, 14 (64%) patients exceeded the MCID, a two-point decrease between baseline and follow-ups, for NRS pain. 20 After 12 weeks 15 (68%) patients had exceeded the MCID. In addition, hand function and the pain in other joints on the worst moments also improved during the follow-up, in a similar way as hand pain (Figure 2). The improvement of the clinical parameters seem not to rely on an increased use of regular analgesics, as no changes were found in the use of these drugs during the follow-up period in patients (Table 2).
Figure 2.
Change in average pain for hands and other joints on a 0–10 Numeric Rating Scale from baseline till week 12. High values represent more pain; low values represent less pain. Data points represent means; error bars, SD.
SD, standard deviation.
Discussion
To our knowledge, this is the first study that investigated the feasibility of methylprednisolone therapy in HOA. Both HCPs and patients indicated a high level of acceptability of therapy with i.m. methylprednisolone for HOA, although concerns about (the burden of) adverse events with repeated administration were expressed. Nevertheless, therapy with i.m. methylprednisolone seems to be a safe option as most adverse events were of short duration and all of them were classified as nonserious. A single i.m. injection of methylprednisolone in patients with symptomatic HOA significantly reduced pain up to 12 weeks after administration. Next to this, methylprednisolone i.m. administration resulted in a decrease in pain in other joints and improvement in hand function. Both quantitative and qualitative results indicated that the repeated use of i.m. methylprednisolone might be a treatment option in patients with HOA.
Qualitative results of our study indicated a high level of acceptability of therapy with i.m. methylprednisolone for HOA among both HCPs and patients. Most patients were very open-minded toward receiving a methylprednisolone injection, which emphasizes the high need and interest for a therapy that can reduce their complaints. According to HCPs, methylprednisolone could be a suitable therapeutic option in HOA patients with signs of synovitis in the hand joints, which is in line with previous reports.3,4 Moreover, HCPs indicated that they considered i.m. methylprednisolone an effective therapy for patients with HOA, for pain, hand function, and stiffness of the hands. This corresponds to patients’ expectations of this therapy. Patients consider pain as the most important complaint that needs to be addressed, since this will probably improve hand function as well. Overall, there appears to be a clear association between the amount of complaints and patients’ satisfaction of the therapy, namely severe pain is associated with satisfaction by small reduction in pain and vice versa. Nevertheless, both HCPs and patients acknowledge that i.m. methylprednisolone could be only temporarily effective in HOA. Our results tend to confirm this assumption, indicating that most of the patients experienced a rapid improvement in pain and function within the first 4 weeks after treatment was administered, but this effect diminished over time.
The dosage used in our study (120 mg) has also been suggested to be effective in HOA by a previous observational study, 14 whereas 40-mg triamcinolone was indicated to be effective 12 weeks after its intramuscular administration in patients with hip OA. 23 It is, therefore, very important that future trials consider exploring the effectiveness of 40-mg methylprednisolone in HOA patients. The search for effective therapies for HOA is of crucial importance considering the substantial disease burden encountered in HOA patients. When judging the analgesic potential, a reduction of approximately two points in the NRS has been termed as a clinically important difference. 22 In our study, we found a 2.0 reduction in mean NRS-reported hand pain 4 weeks after methylprednisolone administration. Noteworthy, the magnitude of our effect on pain and functioning of the hand is similar to the observed effect by Kroon et al. 3 on the effectiveness of 10-mg prednisolone orally for 6 weeks, suggesting that methylprednisolone therapy could be a promising intervention to decrease pain and improve hand function in patients with HOA. This is in line with other previous reports investigating i.m. methylprednisolone in HOA. 14 Taken together, our results indicate that i.m. methylprednisolone could be an effective therapeutic option in HOA patients.
Regarding the safety, HCPs and patients have concerns regarding the probability to develop adverse events and the type of them. This might negatively impact the inclusion and retention in future trials. The majority of adverse events that occurred in this study were classified as nonserious and were of a short-course in the majority of cases. From this perspective, repetitive administration of methylprednisolone injections could be considered as a long-term therapeutic option in HOA patients. Nevertheless, additional measures to reduce harms of this medication have to be considered when such a study will be undertaken, including glucocorticoid toxicity index and bone densitometry. In line with a previous study by Oray et al., 24 HCPs believe that adverse events can potentially be minimized by careful monitoring and using appropriate preventative strategies, thereby improving the risk/benefit ratio of corticosteroid therapy. Also, a good communication between physicians and patients is essential. Patients should be properly informed of what to expect from this therapy, including the efficacy and adverse events that may occur. Finally, logistical issues, such as who and where to administer methylprednisolone injections and perform check-ups, would need to be addressed if this therapy is going to be implemented in HOA care.
Our study has several strengths and limitations. Combining qualitative and quantitative research generated a valuable, more extensive understanding of methylprednisolone acceptability to inform the feasibility of a future trial. A previous study by Donovan et al. 25 demonstrated that qualitative research enabled improving acceptability and recruitment rates from 40% to 70% by changing the content and delivery of study information. Moreover, we examined both HCPs and patients’ views regarding the use of methylprednisolone in HOA, which made the perception of methylprednisolone acceptability even more comprehensive. The current study has several limitations as well. First, due to the small sample size as well as a selection bias due to only recruiting at the Sint Maartenskliniek, the results of the observational study must be interpreted with caution. Nevertheless, we believe that the population investigated lies within the target population for this intervention in the real-world practice and the trends observed in pain and hand function behavior during the follow-up period are valid, as previously reported by others. 14 Additionally, comparing the baseline characteristics of other relevant studies3,14 describing the effectiveness of interventions in secondary care, our study population exhibits similarities with those studies, despite a slightly higher proportion of female subjects. This demonstrates the generalizability of our results to the broader osteoarthritis population. Second, the generalization of our findings about HCPs from the qualitative study might be limited in the context of a mono-centric study, as this study was carried out only in the Sint Maartenskliniek in the Netherlands, while some cultural differences and habits of the Dutch population (both patients and doctors) might also further limit the generalization of these results. Moreover, due to the COVID-19 pandemic interviews were conducted over the phone, which made it impossible to respond to nonverbal cues, which might affect the internal validity of our qualitative results. On the other hand, the internal validity has been increased when two different researchers separately analyzed and interpreted the qualitative data from the questionnaires and interviews.
For the future randomized controlled trial, the safety and efficacy are the most important aspects considering the design. The observational study has shown that after 12 weeks hand pain was still lower than at baseline, indicating that the i.m. methylprednisolone dosage interval used in this study might be considered for repeated administrations on a long term clinical trial. Regarding the safety issues, mentioned in the qualitative study, it can be valuable to include an additional study arm of 40-mg methylprednisolone treatment every 3 months.
Conclusion
In general, HCPs and HOA patients are supportive for the use of methylprednisolone therapy in HOA, despite concerns regarding the risk of long-term adverse events. Quantitative findings suggest that i.m. methylprednisolone therapy in patients with symptomatic HOA is a safe treatment that could reduce pain and improve hand function. An adequately powered future randomized trial is required to further explore the long-term effects of i.m. methylprednisolone injections in terms of efficacy and safety.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors are grateful to all participating patients and staff of the Department of Rheumatology at the Sint Maartenskliniek of Nijmegen where the study was coordinated.
Appendix
Abbreviations
ACR American College of Rheumatology
EDC Electronic data capture
FIHOA Functional Index for Hand OsteoArthritis
HCP health-care providers
HOA hand osteoarthritis
i.m. intramuscular
MCID minimal clinically important difference
NRS Numerical Rating Scale
OA osteoarthritis
PA physician assistants
Footnotes
ORCID iD: Merel Hartog  https://orcid.org/0009-0006-1845-3544
https://orcid.org/0009-0006-1845-3544
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Merel Hartog, Radboudumc, Geert Grooteplein Zuid 10, Nijmegen 6525 GA, The Netherlands; Department of Research, Sint Maartenskliniek, Nijmegen, The Netherlands; Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands.
Kyra A. L. van Keeken, Department of Research, Sint Maartenskliniek, Nijmegen, The Netherlands Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands.
Cornelia H. M. van den Ende, Department of Research, Sint Maartenskliniek, Nijmegen, The Netherlands Department of Rheumatology, Radboudumc, Nijmegen, The Netherlands.
Calin D. Popa, Department of Rheumatology, Sint Maartenskliniek, Nijmegen, The Netherlands Department of Rheumatology, Radboudumc, Nijmegen, The Netherlands.
Declarations
Ethics approval and consent to participate: This mixed-methods study was approved by the local institutional review board of the Sint Maartenskliniek. The Medical Ethics Committee of Oost-Nederland confirmed that the rules laid down in Medical Research Involving Human Subjects Act do not apply to this research (MEC-2022-13592). All participants gave written informed consent prior to entering the study.
Consent for publication: Not applicable.
Author contributions: Merel Hartog: Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing.
Kyra A. L. van Keeken: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing.
Cornelia H. M. van den Ende: Conceptualization; Methodology; Supervision; Writing – review & editing.
Calin D. Popa: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Plotz B, Bomfim F, Sohail MA, et al. Current epidemiology and risk factors for the development of hand osteoarthritis. Curr Rheumatol Rep 2021; 23: 61. [DOI] [PubMed] [Google Scholar]
- 2. Veronese N, Smith L, Bolzetta F, et al. Efficacy of conservative treatments for hand osteoarthritis. Wien Klin Wochenschr 2021; 133: 234–240. [DOI] [PubMed] [Google Scholar]
- 3. Kroon FPB, Kortekaas MC, Boonen A, et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 394: 1993–2001. [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Teichtahl AJ, Jones G, et al. METHODS – a randomised controlled trial of METhotrexate to treat Hand Osteoarthritis with Synovitis: study protocol for a randomised controlled trial. BMC Musculoskelet Disord 2021; 22: 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kloppenburg M, Kroon FP, Blanco FJ, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis 2019; 78: 16–24. [DOI] [PubMed] [Google Scholar]
- 6. Haugen IK, Bøyesen P, Slatkowsky-Christensen B, et al. Associations between MRI-defined synovitis, bone marrow lesions and structural features and measures of pain and physical function in hand osteoarthritis. Ann Rheum Dis 2012; 71: 899–904. [DOI] [PubMed] [Google Scholar]
- 7. Haugen IK, Slatkowsky-Christensen B, Bøyesen P, et al. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann Rheum Dis 2016; 75: 117–123. [DOI] [PubMed] [Google Scholar]
- 8. Keen HI, Wakefield RJ, Grainger AJ, et al. An ultrasonographic study of osteoarthritis of the hand: synovitis and its relationship to structural pathology and symptoms. Arthritis Rheum 2008; 59: 1756–1763. [DOI] [PubMed] [Google Scholar]
- 9. Kortekaas MC, Kwok WY, Reijnierse M, et al. Inflammatory ultrasound features show independent associations with progression of structural damage after over 2 years of follow-up in patients with hand osteoarthritis. Ann Rheum Dis 2015; 74: 1720–1724. [DOI] [PubMed] [Google Scholar]
- 10. Kortekaas MC, Kwok WY, Reijnierse M, et al. Brief report: association of inflammation with development of erosions in patients with hand osteoarthritis: a prospective ultrasonography study. Arthritis Rheumatol 2016; 68: 392–397. [DOI] [PubMed] [Google Scholar]
- 11. Kortekaas MC, Kwok WY, Reijnierse M, et al. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann Rheum Dis 2010; 69: 1367–1369. [DOI] [PubMed] [Google Scholar]
- 12. Mathiessen A, Slatkowsky-Christensen B, Kvien TK, et al. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann Rheum Dis 2016; 75: 825–830. [DOI] [PubMed] [Google Scholar]
- 13. Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am 2016; 42: 15–31, vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keen HI, Wakefield RJ, Hensor EMA, et al. Response of symptoms and synovitis to intra-muscular methylprednisolone in osteoarthritis of the hand: an ultrasonographic study. Rheumatology (Oxford) 2010; 49: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 15. Kirkland SW, Cross E, Campbell S, et al. Intramuscular versus oral corticosteroids to reduce relapses following discharge from the emergency department for acute asthma. Cochrane Database Syst Rev 2018; 6: CD012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007; 19: 349–357. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 18. Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum 1990; 33: 1601–1610. [DOI] [PubMed] [Google Scholar]
- 19. Castor Electronic Data Capture. Castor electronic data capture, https://castoredc.com (2022, accessed October 5, 2022).
- 20. Dreiser RL, Maheu E, Guillou GB, et al. Validation of an algofunctional index for osteoarthritis of the hand. Rev Rhum Engl Ed 1995; 62(Suppl. 1): 43S–53S. [PubMed] [Google Scholar]
- 21. Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. StatPearls. Treasure Island, FL: StatPearls Publishing LLC., 2022. [PubMed] [Google Scholar]
- 22. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158. [DOI] [PubMed] [Google Scholar]
- 23. Dorleijn DMJ, Luijsterburg PAJ, Reijman M, et al. Intramuscular glucocorticoid injection versus placebo injection in hip osteoarthritis: a 12-week blinded randomised controlled trial. Ann Rheum Dis 2018; 77: 875–882. [DOI] [PubMed] [Google Scholar]
- 24. Oray M, Abu Samra K, Ebrahimiadib N, et al. Long-term side effects of glucocorticoids. Expert Opin Drug Saf 2016; 15: 457–465. [DOI] [PubMed] [Google Scholar]
- 25. Donovan J, Mills N, Smith M, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002; 325: 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-docx-2-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, sj-pdf-3-tab-10.1177_1759720X241253974 for Intramuscular methylprednisolone administration in hand osteoarthritis patients: a feasibility study to inform a randomized controlled trial by Merel Hartog, Kyra A. L. van Keeken, Cornelia H. M. van den Ende and Calin D. Popa in Therapeutic Advances in Musculoskeletal Disease