Abstract
Background
Neuromyelitis optica spectrum disorder (NMOSD) is a relapsing, autoimmune, inflammatory astrocytopathy. Rituximab for B-cell suppression is a common treatment for NMOSD; however, large-scale randomised controlled trials are lacking.
Objective
Evaluate long-term efficacy and safety of rituximab for NMOSD.
Methods
Retrospective observational study of patients with NMOSD treated with rituximab. Annualised relapse rates (ARRs) before and during rituximab treatment were evaluated; Modified Rankin Scores (mRS) were measured as a marker of disability.
Results
In total, 37 patients were included: 27 aquaporin-4-IgG-seropositive and 10 seronegative NMOSD. The predominant rituximab dosing regimen was an initial 1000 mg, split over two 500 mg infusions, two weeks apart, followed by single 500 mg doses. Over a median follow-up of 54 months, ARR for the whole cohort was 0.136 (95% CI 0.088–0.201), significantly lower than the pretreatment ARR of 0.366 (95% CI 0.271–0.483, p < 0.001). There was a significant reduction in ARR for the seropositive subgroup, but not seronegative. Significant improvement in mRS was seen post-treatment. Infections were reported in 32% of patients during follow-up; most were mild.
Conclusion
Rituximab, at doses lower than traditionally used, may be an efficacious therapy for NMOSD, with a favourable safety profile.
Keywords: Neuromyelitis optica, rituximab, low-dose, long-term, efficacy, safety
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune astrocytopathy manifesting predominantly as optic neuritis and transverse myelitis. 1 The natural history of NMOSD is one of stepwise deterioration due to recurrent attacks; within five years of disease onset, half of patients require a wheelchair or have become legally blind.2,3 In the majority of patients, an IgG autoantibody binding to astrocytic aquaporin-4 (AQP4), the main water channel of the central nervous system, is detected. 4 AQP4-IgG has pathogenic potential: the antibody binds to the extracellular domain of AQP4 and activates complement, leading to complement-mediated destruction of astrocytes, internalisation of the water channel and antibody-dependent cell cytotoxicity. 5 In patients who are AQP4-IgG seronegative, a diagnosis of seronegative NMOSD can be made if specific clinical and radiological criteria are satisfied, as outlined by Wingerchuk and colleagues in the 2015 International Consensus Diagnostic Criteria. 6
As the pathogenesis of NMOSD involves humoral autoimmunity, B-cell depletion with rituximab was adopted as a therapeutic approach.7,8 Rituximab is a mouse and human chimeric IgG1 monoclonal antibody that binds to CD20, a protein expressed by B-lymphocytes from the pre-B-cell stage until plasma cell differentiation.5,9,10 NMOSD usually follows an aggressive relapsing course and B-cell re-emergence after rituximab therapy can precipitate relapses. 11 Thus, lifelong disease-modifying therapy is required and comes with the challenge of achieving sufficient immunosuppression whilst limiting adverse effects.
Although large-scale randomised controlled trials (RCTs) of rituximab are lacking, evidence from a small RCT and observational studies support the use of rituximab as a first-line therapy for NMOSD.5,12 However, the optimal dose remains unclear. Initial regimens of rituximab for NMOSD were based on the doses used for treating lymphoma – an induction dose of 375 mg/m2 infused once per week for four weeks or 1000 mg infused twice, two weeks apart, followed by 1000 mg maintenance doses – which were high-cost, off-label therapies, with a higher risk of adverse reactions.13,14 Recent studies assessed the safety and efficacy of different doses of rituximab.13,14 One meta-analysis suggested that a lower dose of rituximab – 100 mg weekly for three weeks – is as effective as higher doses at lowering relapse rate and reducing disability, although RCT data is lacking. 14
In recent years, additional immunotherapies have been found to be effective in the treatment of NMOSD, including eculizumab, inebilizumab and satralizumab.15–18 While approved by the US Food and Drug Administration, these are very expensive agents, and no head-to-head trials against rituximab have been conducted. When considering subsidising and prescribing a new and costly therapy, having established real-world evidence of the efficacy and safety of the benchmark therapy – in this case rituximab – is important for guiding decision-making.
Aim
The aim of this study was to retrospectively examine the cohort of patients with AQP4-IgG-seropositive and seronegative NMOSD, treated with rituximab at two major tertiary hospitals in Brisbane, Australia. The focus was on exploring the dosing of rituximab and evaluating its efficacy and safety in this patient population, with the hypothesis being that rituximab would reduce relapse frequency and long-term disability.
Methods
This was a retrospective observational study. Pharmacy dispensing records of the participating hospitals were searched for cases of rituximab use for neurological conditions. The search extended from 1 January 2005 to 31 July 2021. Records encompassed both inpatient and outpatient dispensing. A chart review of each case was undertaken by a single author (MTGH) to identify patients diagnosed with NMOSD. The clinical, laboratory and magnetic resonance imaging (MRI) characteristics of these patients were examined against the 2015 International Panel for NMO Diagnosis criteria for diagnosing seropositive and seronegative NMOSD. 6 Patients who met the criteria for a diagnosis of NMOSD and had received at least one dose of rituximab were included in the study. If there was any uncertainty regarding the diagnosis, the case was also reviewed by an additional author (SB) and a consensus decision reached through discussion. Patients with myelin oligodendrocyte glycoprotein (MOG) antibodies were excluded. All seronegative NMOSD cases included in the study were tested for MOG antibodies, except for one who had a brain biopsy that was consistent with NMO. Cases were also excluded if the clinical data was inadequate or there had been less than nine months follow-up after the first dose of rituximab.
All data were extracted from patients’ medical records using a standardised data collection form and included demographics, other therapies received for the treatment of NMOSD, rituximab infusion dates and doses, adverse reactions, relapses as determined by the treating neurologist and laboratory and neuroimaging information. Modified Rankin scores (mRS) were determined by MTGH based on patients’ clinical records. The last date for which data was collected was 31 July 2022. Patients who ceased rituximab were followed for six months post-cessation. Ethics approval was obtained from Metro South Health Human Research Ethics Committee (LNR/2019/QMS/52617) with a waiver of consent.
Relapses were graded as mild, moderate or severe. A relapse was mild if it involved sensory symptoms that did not affect functional status or optic neuritis with a decline in visual acuity of less than 6/12 at worst. A relapse was moderate if it involved motor weakness, new functional impairment or optic neuritis with a decline in visual acuity of greater than 6/12 but less than 6/60 at worst. A relapse was severe if it rendered the patient immobile, involved optic neuritis with a reduction in visual acuity to 6/60 or greater, or led to complete impairment in another significant functional domain, including loss of bowel or bladder control requiring urinary catheterisation.
Relapse with B-cell reconstitution was defined by a CD19 B-cell count of greater than 0.01 × 109/L (adult reference range for reporting laboratory: 0.08–0.43 × 109/L) at the time of relapse. For some patients, rituximab was readministered once there was evidence of B-cell reconstitution. The method for early identification of B-cell reconstitution involved monthly monitoring of CD19 B-cell count, beginning approximately four months after the last rituximab infusion. All individual rituximab infusions contained 500 mg; dosing regimens identified will be discussed in the results section. Annualised relapse rates (ARRs) before and during the rituximab treatment period were evaluated. Aligning with prior studies, the ARR calculation required at least three months of observation before rituximab initiation to avoid an artificially inflated pre-treatment ARR. 19
Statistical analysis
Continuous data is presented as mean and standard deviation (SD) if normally distributed and as median and interquartile range (IQR) if not normally distributed. The distribution of each variable was tested using the Shapiro–Wilk test. Categorical data is presented as counts with their corresponding percentage. ARR and 95% confidence intervals (CIs) were calculated using the person-years method with weighted summing accounting for the heterogeneous length of follow-up. 20 Differences between the pre- and post-rituximab ARR and mRS were tested using the Mann–Whitney U test. Statistical significance was set at 5%.
The first attack, at disease onset, was not counted as a relapse when calculating ARR. The date of first attack was considered the starting date for being at risk of relapse. When exact dates were unclear, the following previously reported strategy was used for corrections: accuracy to month only was recorded as 15th of that month; accuracy to year only was recorded as 1st of July of that year. 21 Any conflicts, such as disease onset after start of treatment, were corrected by reference to other available data such as date of hospital admission or MRI. 21 Data was censored at the point of last follow-up or switch to alternative therapy. Statistical analyses were performed using R version 4.3.1.
Results
Study population
The inclusion criteria were met by 27 patients with AQP4-IgG-seropositive NMOSD and 10 patients with seronegative NMOSD. Demographic and baseline clinical characteristics are presented in Table 1. The majority were female (seropositive: 96%, seronegative: 60%). Mean age at first rituximab infusion was 45 years for the seropositive (SD: 12) and 35 years (SD: 13) for the seronegative group, with a median disease duration at rituximab initiation of 20 months for the seropositive (IQR: 4–68) and eight months for the seronegative group (IQR: 4–84). Prior to rituximab treatment, median mRS was 2 (IQR: 1–3) for the seropositive and 2 (IQR: 2–3) for the seronegative group.
Table 1.
Demographics and baseline clinical characteristics.
| Characteristic | Total (N = 37) | AQP4-IgG-positive (N = 27) | Seronegative (N = 10) |
|---|---|---|---|
| Age at disease onset (years), mean (SD) | 39 (14) | 42 (13) | 32 (13) |
| Age at rituximab initiation (years), mean (SD) | 42 (13) | 45 (12) | 35 (13) |
| Female, N (%) | 32 (86%) | 26 (96%) | 6 (60%) |
| Other autoimmune disease, N (%) | 13 (35%) | 12 (44%) | 1 (10%) |
| Location of clinical attacks pre-rituximab, N (%) | |||
| Optic neuritis | 27 (73%) | 18 (66%) | 9 (90%) |
| Longitudinally extensive transverse myelitis | 21 (57%) | 14 (52%) | 7 (70%) |
| Area postrema | 6 (16%) | 5 (19%) | 1 (10%) |
| Other brainstem lesion | 3 (8%) | 2 (7%) | 1 (10%) |
| Short segment transverse myelitis | 4 (11%) | 3 (11%) | 1 (10%) |
| Other therapies received pre-rituximab, N (%) | |||
| Azathioprine | 6 (16%) | 4 (15%) | 2 (20%) |
| Methotrexate | 3 (8%) | 2 (7%) | 1 (10%) |
| Mycophenolate | 2 (5%) | 1 (4%) | 1 (10%) |
| Other a | 5 (14%) | 3 (11%) | 2 (20%) |
| Other therapies received during rituximab treatment, N (%) | |||
| Azathioprine | 5 (14%) | 4 (15%) | 1 (10%) |
| Methotrexate | 0 | 0 | 0 |
| Mycophenolate | 4 (11%) | 3 (11%) | 1 (10%) |
| Disease duration at rituximab initiation (months), median (IQR) | 19 (4–85) | 20 (4–68) | 8 (4–84) |
| Duration of follow up after rituximab initiation (months), median (IQR) | 54 (29–89) | 59 (34–95) | 39 (19–62) |
| Number of rituximab infusions, median (IQR) | 10 (6–14) | 10 (6–14) | 6 (4–14) |
Other includes interferon beta-1a, glatiramer acetate and natalizumab.
For the majority of patients (seropositive: 74%, seronegative: 70%), rituximab was the first disease-modifying therapy administered for NMOSD. The rituximab dose was either 500 mg or 1000 mg administered as two separate 500 mg infusions two weeks apart. No other rituximab dose was used. The most common practice was to give the 1000 mg split dose as the first treatment (seropositive: 78%, seronegative: 100%), followed by 500 mg doses thereafter. Rituximab redosing was initially based on B-cell reconstitution in 23 patients (62%). Of these, nine were switched to routine redosing, either six-monthly (eight patients) or nine-monthly (one patient), during follow-up. In 14 cases (38%), a routine redosing schedule was used from the start of rituximab treatment, with 13 patients being dosed six-monthly and one patient being dosed four-monthly due to an early relapse. Median number of rituximab doses was 10 (IQR: 6–14) for the seropositive and 6 (IQR: 4–14) for the seronegative group and median follow-up was 59 months (IQR: 34–95) and 39 months (19–62), respectively.
Relapses and disability
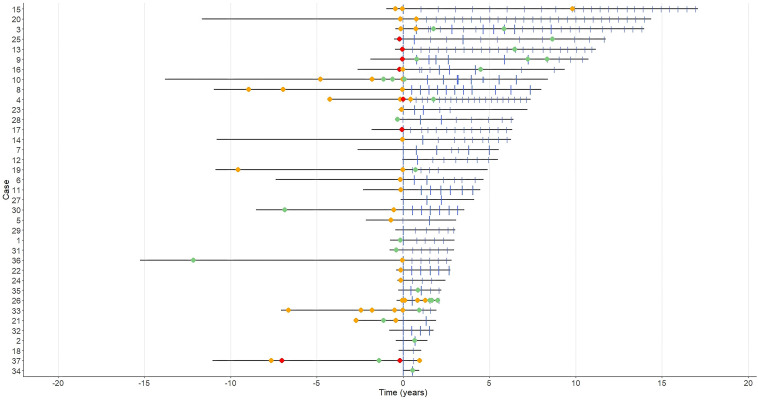
Treatment and relapse timelines for each patient are displayed in Figure 1 and further relapse details in Table 2. After rituximab treatment, 12 patients in the AQP4-IgG-positive (44%) and four patients in the seronegative group (40%) experienced relapses. Of the 15 relapses in the seropositive group post-rituximab, three (20%) occurred within six months of starting rituximab and of the 11 relapses in the seronegative group, one (9%) occurred within six months of rituximab commencement. Eight relapses in the seropositive group (53%) and six relapses in the seronegative group (55%) occurred within six months of last rituximab dose. In both groups, 27% of relapses occurred in the setting of B-cell reconstitution, at a median of 275 days after the last rituximab infusion (IQR: 252.5–330.5), with a median CD19 B-cell count of 0.17 × 109 (IQR: 0.11–0.26 × 109). Relapses post-rituximab initiation were more often mild (69% of all relapses for both groups were mild) compared to pre-rituximab (18%). There were no severe relapses post-rituximab, whereas 16% of relapses were severe pre-rituximab.
Figure 1.
Relapse timelines spanning up to 15 years pre-rituximab and 17 years post-rituximab. Time point “0” on the x-axis represents rituximab commencement. Green circle = mild relapse, yellow circle = moderate relapse, red circle = severe relapse. Large blue vertical line = 1000 mg dose of rituximab (two 500 mg doses two weeks apart). Small blue vertical line = 500 mg dose of rituximab.
Table 2.
Pre- and post-rituximab relapse details.
| Total (N = 37) | AQP4-IgG-positive (N = 27) | Seronegative (N = 10) | |
|---|---|---|---|
| Relapse severity pre-rituximab, N (%) | |||
| Mild | 9 (18%) | 6 (16%) | 3 (25%) |
| Moderate | 33 (66%) | 26 (68%) | 7 (58%) |
| Severe | 8 (16%) | 6 (16%) | 2 (17%) |
| Relapse severity post-rituximab | |||
| Mild | 17 (65%) | 12 (80%) | 5 (45.5%) |
| Moderate | 9 (35%) | 3 (20%) | 6 (55.5%) |
| Severe | 0 | 0 | 0 |
| Relapses within 6 months of starting rituximab, N (% of relapses post-rituximab) | 4 (15%) | 3 (20%) | 1 (9%) |
| Post-rituximab relapses associated with B-cell reconstitution, N (%) | 7 (27%) | 4 (27%) | 3 (27%) |
| mRS pre-rituximab, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (2–3) |
| mRS at end of follow-up post-rituximab, median (IQR) | 1 (1–2) | 1 (0–2) | 1 (1–2) |
ARR pre- and post-rituximab are displayed in Table 3. There was a significant lowering of ARR post-rituximab for the whole cohort (ARR 0.136 [95% CI 0.088–0.201] vs 0.366 [95% CI 0.271–0.483] pre-rituximab; p < 0.001). There was also a significant lowering of ARR for the seropositive group (p < 0.001); with the pre-rituximab ARR being 0.375 (95% CI: 0.264–0.517) and the post-rituximab ARR being 0.097 (95% CI: 0.053–0.163). For the seronegative group, ARR was calculated with and without patient 26. Patient 26 was an outlier who experienced six relapses within two years of commencing rituximab, met diagnostic criteria for seronegative NMO, but had some features in keeping with MS, although no oligoclonal bands in the CSF. Whilst the post-rituximab ARR was lower without patient 26 (ARR 0.133 [95% CI: 0.043–0.311] vs 0.278 [95% CI: 0.139–0.497] with patient 26), the change from pre-rituximab ARR did not reach statistical significance (pre-rituximab ARR 0.314 [95% CI: 0.157–0.562] without patient 26 vs 0.339 [95% CI: 0.175–0.592] with patient 26; p = 0.313 and 0.432, respectively). Following rituximab therapy, there was improvement in disability as measured by mRS. For the total cohort, median mRS was 2 (IQR: 1–3) prior to rituximab and improved to 1 (IQR: 1–2) at the time of last follow-up (p < 0.001).
Table 3.
Comparison of pre- and post-rituximab ARR.
| Group | N | Pre-rituximab | Post-rituximab | p value | ||
|---|---|---|---|---|---|---|
| Relapses | Relapses | |||||
| Events | ARR (95% CI) | Events | ARR (95% CI) | |||
| Total | 37 | 50 | 0.366 (0.271–0.483) | 26 | 0.136 (0.088–0.201) | <0.001 |
| AQP-4-IgG-positive | 27 | 38 | 0.375 (0.264–0.517) | 15 | 0.097 (0.053–0.163) | <0.001 |
| Seronegative with patient 26 | 10 | 12 | 0.339 (0.175–0.592) | 11 | 0.278 (0.139–0.497) | 0.432 |
| Seronegative without patient 26 | 9 | 11 | 0.314 (0.157–0.562) | 5 | 0.133 (0.043–0.311) | 0.313 |
Adverse events
The frequency of adverse events is described in Table 4. Allergic reactions occurred in two patients (5%) leading to rituximab cessation. Infections were reported in 32% of patients. Most were mild, with urinary tract infection in the setting of a urinary catheter being particularly common. There was one fatal case of urosepsis in a patient with tetraparesis and one case of severe SARS-CoV-2 pneumonia in an unvaccinated patient. Secondary hypogammaglobulinaemia (IgG < 6.0 g/L) occurred in three patients (8%), with one patient (3%) requiring treatment with IVIg due to recurrent infections. All three cases of hypogammaglobulinaemia were mild based on IgG level (mild being 4.0–5.99 g/L, moderate 2.0–3.99 g/L and severe < 2.0 g/L). 22 Three patients were diagnosed with malignancies after commencing rituximab: one with small cell neuroendocrine lung cancer, one with non-small cell lung cancer and one with myelodysplastic syndrome progressing to acute myeloid leukaemia (AML). Rituximab was ceased in four patients (11%) due to adverse events, including the two patients who experienced allergic reactions, one patient who developed liver cirrhosis and the one patient who developed AML.
Table 4.
Adverse events during follow-up on rituximab.
| Adverse event, N (%) | N = 37 patients |
|---|---|
| Allergic reaction | 2 (5%) |
| Anaphylaxis | 0 |
| Secondary hypogammaglobulinaemia | 3 (8%) |
| Hypogammaglobulinaemia requiring IVIg | 1 (3%) |
| Liver cirrhosis | 1 (3%) |
| Patients with at least one infection | 12 (32%) |
| Type of infection | |
| Recurrent sinusitis | 1 (3%) |
| Pneumonia | 4 (11%) |
| Urinary tract infection | 7 (19%) |
| Recurrent giardiasis | 1 (3%) |
| Herpes zoster | 1 (3%) |
| Severe infections | 2 (5%) |
| Urosepsis | 1 (3%) |
| SARS-Cov-2 pneumonia | 1 (3%) |
| Malignancy | 3 (8%) |
| Small cell neuroendocrine lung cancer | 1 (3%) |
| Non-small cell lung cancer | 1 (3%) |
| Acute myeloid leukaemia | 1 (3%) |
| Rituximab discontinuation | |
| Total | 8 (22%) |
| Due to allergic reactions | 2 (5%) |
| Due to relapses | 1 (3%) |
| Due to liver cirrhosis | 1 (3%) |
| Due to malignancy | 1 (3%) |
| Switched to ocrelizumab due to availability | 3 (8%) |
Discussion
This study investigated the efficacy and long-term safety of rituximab treatment, in an observational two-centre cohort of patients with AQP-4-seropositive and seronegative NMOSD. The doses of rituximab used in these centres – predominantly a 1000 mg induction dose given as two 500 mg doses two weeks apart, followed by single 500 mg doses – are lower than the doses most commonly reported in the literature.12,19 Patients with NMOSD require lifelong preventive treatment given the potential for devastating relapses and as such, an aim of rituximab therapy should be to administer effective doses, but avoid overtreating which may increase adverse effects, including hypogammaglobulinaemia and infections. 14
The results of this study suggest that rituximab may be an effective therapy for NMOSD at lower doses than initially reported. Rituximab was associated with a significantly reduced relapse rate in the whole cohort and seropositive subgroup, but significance was not reached for the seronegative subgroup. This may be due to the small cohort of seronegative patients and a greater degree of disease heterogeneity in this cohort. Rituximab was also associated with a significant improvement in mRS. This is an encouraging finding in light of the long follow-up durations, where aging and medical comorbidities can begin to impair function.
In the present study, the earliest case of rituximab use dated back to 2005, providing 17 years of follow-up data. In most of the early cases, rituximab redosing was initially based on B-cell reconstitution. However, in the latter years of the study, there was a shift to routine six-monthly dosing for most patients. This may have been driven by clinician experience of relapses occurring in the setting of B-cell reconstitution. Previous studies have provided evidence to support both redosing strategies.7,23–25 Novi et al. found no significant difference in ARR between six-monthly dosing and dosing based on B-cell re-emergence. 24 However, to our knowledge, such a comparison has not been undertaken for the lower doses of rituximab used in our study. It would appear from our results, and those of other studies, that six-monthly 500 mg dosing is generally adequate to maintain B-cell suppression. Greenberg et al. studied time to B-cell return following two doses of rituximab: 100 mg and 1000 mg and found B-cell return after a mean of 140 and 258 days, respectively. 26 Thus, 500 mg dosing could be expected to provide an intermediate duration of B-cell suppression. In clinical practice, redosing based on early identification of B-cell reconstitution requires a particularly high level of pharmacovigilance. The difficulty of such monitoring is illustrated by our finding that 27% of relapses in each group occurred in the setting of B-cell return. These relapses all took place more than six months after the last dose of rituximab, strengthening the case for routine six-monthly dosing.
In the present study, 8% of patients developed mild hypogammaglobulinaemia; one was treated with IVIg due to recurrent infections. A study by Kim et al. found that rituximab doses of 1000 mg given twice, two weeks apart or 375 mg/m2 given weekly for four weeks for induction, followed by individual doses of 375 mg/m2 based on CD27 memory B-cell recovery, resulted in 41% of patients developing hypo-IgG by 14 years of treatment. 27 This was concordant with the results of other studies of similar rituximab dosing regimens with shorter follow-up.19,28–30 One such study, by Avouac et al., also reported a relatively high frequency of symptomatic and severe infections – 54% and 19%, respectively – and found that hypogammaglobulinaemia was associated with the development of infections in patients with urinary dysfunction. 28
The ability to make direct comparisons between our study and prior studies involving higher doses of rituximab is limited for a number of reasons, including the retrospective nature of the majority of studies and variations in the proportion of patients exposed to other immunosuppressive agents. However, our study does suggest both efficacy and a favourable safety profile when rituximab is administered at the lower dose of 500 mg, with a relatively small proportion of patients developing hypogammaglobulinaemia and infections.
A particular strength of our study was the long follow-up duration (median 54 months). The study does have limitations however. As a retrospective study, it was limited to relapses and adverse effects that were documented in the medical record. This may have biased towards reduced recognition of mild adverse events including minor infections. All NMOSD relapses would be expected to come to medical attention and thus be documented in the medical record. We were also unable to use Expanded Disability Status Scale (EDSS) as a disability outcome measure, because the medical record often lacked the level of detail required to accurately estimate EDSS.
Conclusion
Our study provides long-term evidence that rituximab may be an efficacious therapy for NMOSD and carries a favourable safety profile, at lower doses than have been traditionally used. Improvement in relapse rate in the seronegative subgroup did not meet statistical significance, with interpretation limited by the small cohort size. The optimal dosing of rituximab for the treatment of NMOSD warrants further investigation, ideally with a RCT comparing different doses of rituximab for efficacy and safety.
Acknowledgements
The authors thank William Pinzon Perez, statistician with Queensland Cyber Infrastructure Foundation Ltd, for assistance with statistical analysis.
Footnotes
Data availability statement: The dataset analysed in this study is available from the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Michael T G Hayes https://orcid.org/0000-0003-0614-1800
Stefan Blum https://orcid.org/0000-0002-2702-3742
Contributor Information
Michael T G Hayes, Department of Neurology, Princess Alexandra Hospital, Woolloongabba, Australia.
Robert J Adam, Department of Neurology, Royal Brisbane and Women's Hospital, Herston, Australia; University of Queensland Centre for Clinical Research, Royal Brisbane and Women's Hospital, Herston, Australia.
Pamela A McCombe, Department of Neurology, Royal Brisbane and Women's Hospital, Herston, Australia; University of Queensland Centre for Clinical Research, Royal Brisbane and Women's Hospital, Herston, Australia.
Michael Walsh, Department of Neurology, Princess Alexandra Hospital, Woolloongabba, Australia.
Stefan Blum, Department of Neurology, Princess Alexandra Hospital, Woolloongabba, Australia.
References
- 1.Wingerchuk DM, Lucchinetti CF. Neuromyelitis optica spectrum disorder. N Engl J Med 2022; 387: 631–639. [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Hogancamp WF, O'Brien PCet al. et al. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999; 53: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 3.Jacob A, Matiello M, Weinshenker BG, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol 2009; 66: 1128–1133. [DOI] [PubMed] [Google Scholar]
- 4.Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007; 69: 2221–2231. [DOI] [PubMed] [Google Scholar]
- 5.Damato V, Evoli A, Iorio R. Efficacy and safety of rituximab therapy in neuromyelitis optica spectrum disorders: a systematic review and meta-analysis. JAMA Neurol 2016; 73: 1342–1348. [DOI] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85: 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao D, Ren K, Lu J, et al. Rituximab at lower dose for neuromyelitis optica spectrum disorder: a multicenter, open-label, self-controlled, prospective follow-up study. Front Immunol 2023; 14: 1148632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zéphir H, Bernard-Valnet R, Lebrun C, et al. Rituximab as first-line therapy in neuromyelitis optica: efficiency and tolerability. J Neurol 2015; 262: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 9.Collongues N, de Seze J. An update on the evidence for the efficacy and safety of rituximab in the management of neuromyelitis optica. Ther Adv Neurol Disord 2016; 9: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlasova G, Mraz M. The regulation and function of CD20: an “enigma” of B-cell biology and targeted therapy. Haematologica 2020; 105: 1494–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S-H, Kim W, Li XF, et al. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol 2011; 68: 1412–1420. [DOI] [PubMed] [Google Scholar]
- 12.Tahara M, Oeda T, Okada K, et al. Safety and efficacy of rituximab in neuromyelitis optica spectrum disorders (RIN-1 study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2020; 19: 298–306. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S, Zhou H, Xu Q, et al. Efficacy of low-dose rituximab on neuromyelitis optica-associated optic neuritis. Front Neurol 2021; 12: 637932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei K, Nie Q, Zhu Y, et al. Different doses of rituximab for the therapy of neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Mult Scler Relat Disord 2022; 68: 104127. [DOI] [PubMed] [Google Scholar]
- 15.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4–positive neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 614–625. [DOI] [PubMed] [Google Scholar]
- 16.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–1363. [DOI] [PubMed] [Google Scholar]
- 17.Traboulsee A, Greenberg BM, Bennett JL, et al. Safety and efficacy of satralizumab monotherapy in neuromyelitis optica spectrum disorder: a randomised, double-blind, multicentre, placebo-controlled phase 3 trial. Lancet Neurol 2020; 19: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 2114–2124. [DOI] [PubMed] [Google Scholar]
- 19.Barreras P, Vasileiou ES, Filippatou AG, et al. Long-term effectiveness and safety of rituximab in neuromyelitis optica spectrum disorder and MOG antibody disease. Neurology 2022; 99: E2504–E2E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akaishi T, Ishii T, Aoki Met al. et al. Calculating and comparing the annualized relapse rate and estimating the confidence interval in relapsing neurological diseases. Front Neurol 2022; 13: 875456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke L, Bukhari W, O'Gorman CM, et al. Response to treatment in NMOSD: the Australasian experience. Mult Scler Relat Disord 2022; 58: 103408. [DOI] [PubMed] [Google Scholar]
- 22.Barmettler S, Ong M-S, Farmer JR, et al. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open 2018; 1: e184169. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ip VHL, Lau AYL, Au LWC, et al. Rituximab reduces attacks in Chinese patients with neuromyelitis optica spectrum disorders. J Neurol Sci 2013; 324: 38–39. [DOI] [PubMed] [Google Scholar]
- 24.Novi G, Bovis F, Capobianco M, et al. Efficacy of different rituximab therapeutic strategies in patients with neuromyelitis optica spectrum disorders. Mult Scler Relat Disord 2019; 36: 101430. [DOI] [PubMed] [Google Scholar]
- 25.Cree BAC, Lamb S, Morgan K, et al. An open label study of the effects of rituximab in neuromyelitis optica. Neurology 2005; 64: 1270–1272. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg BM, Graves D, Remington G, et al. Rituximab dosing and monitoring strategies in neuromyelitis optica patients: creating strategies for therapeutic success. Mult Scler 2012; 18: 1022–1026. [DOI] [PubMed] [Google Scholar]
- 27.Kim S-H, Park NY, Kim KH, et al. Rituximab-induced hypogammaglobulinemia and risk of infection in neuromyelitis optica spectrum disorders: a 14-year real-life experience. Neurol Neuroimmunol Neuroinflamm 2022; 9: e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avouac A, Maarouf A, Stellmann J-P, et al. Rituximab-induced hypogammaglobulinemia and infections in AQP4 and MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm 2021; 8: e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcinnò A, Marnetto F, Valentino P, et al. Rituximab-induced hypogammaglobulinemia in patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm 2018; 5: e498. e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tallantyre EC, Whittam DH, Jolles S, et al. Secondary antibody deficiency: a complication of anti-CD20 therapy for neuroinflammation. J Neurol 2018; 265: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]