Abstract
BACKGROUND
Technological advances and digital solutions have been proposed to overcome barriers to sustainable rehabilitation programs in patients with musculoskeletal disorders. However, to date, standardized telemonitoring systems able to precisely assess physical performance and functioning are still lacking.
AIM
To validate a new mobile telemonitoring system, named System for Tracking and Evaluating Performance (Step-App®), to evaluate physical performance in patients undergone knee and hip total arthroplasty.
DESIGN
Prospective cohort study.
METHODS
A consecutive series of older adults with knee and hip total arthroplasty participated in a comprehensive rehabilitation program. The Step-App®, a mobile telemonitoring system, was used to remotely monitor the effects of rehabilitation, and the outcomes were assessed before (T0) and after the rehabilitation treatment (T1). The primary outcomes were the 6-Minute Walk Test (6MWT), the 10-Meter Walk Test (10MWT), and the 30-Second Sit-To-Stand Test (30SST).
RESULTS
Out of 42 patients assessed, 25 older patients were included in the present study. The correlation analysis between the Step-App® measurements and the traditional in-person assessments demonstrated a strong positive correlation for the 6MWT (T0: r2=0.9981, P<0.0001; T1: r2=0.9981, P<0.0001), 10MWT (T0: r2=0.9423, P<0.0001; T1: r2=0.8634, P<0.0001), and 30SST (T0: r2=1, P<0.0001; T1: r2=1, P<0.0001). The agreement analysis, using Bland-Altman plots, showed a good agreement between the Step-App® measurements and the in-person assessments.
CONCLUSIONS
Therefore, we might conclude that Step-App® could be considered as a validated mobile telemonitoring system for remote assessment that might have a role in telemonitoring personalized rehabilitation programs for knee and hip replacement patients.
CLINICAL REHABILITATION IMPACT
Our findings might guide clinicians in remote monitoring of physical performance in patients with musculoskeletal conditions, providing new insight into tailored telerehabilitation programs.
Key words: Telemedicine, Aging, Rehabilitation, Mobile applications, Smartphone, Aged
In the recent years, growing attention has been rising on the effective and sustainable management of aging and age-related disorders.1-4 Joint replacement surgeries, such as hip and knee arthroplasties, are currently considered highly effective medical interventions for improving physical function, reducing pain, and enhancing the overall quality of life in individuals with degenerative joint diseases.5, 6 While the surgical procedures have consistently improved, the success of joint replacement is strictly linked to the effectiveness of post-operative rehabilitation.7, 8 Beyond its clinical importance, some economic considerations have been highlighted in the recent years and home-based rehabilitation programs have been recently proposed to improve functional recovery and increase cost-effectiveness.9, 10 Interestingly, the recent NICE guidelines underlined promising effects of home-based rehabilitation programs with intriguing implications for a sustainable and cost-effective therapeutic intervention.11 However, patients following hip and knee replacement might frequently be characterized by muscle weakness, reduced endurance, and impaired independence in activities of daily living. As a result, a patient-centered rehabilitation plan plays a pivotal role and it should be tailored to patients’ characteristics in accordance with the most recent guidelines for rehabilitation management of patients with knee and hip total arthroplasty.8, 11, 12
Thus, a standardized assessment of physical functioning and physical performance represents a crucial need also in home-based rehabilitation programs to both personalize the therapeutic intervention and monitoring benefits of the comprehensive rehabilitation program.13
In this context, technological advances and digital solutions might be considered sustainable tools for improving the remote assessment of elderly patients, overcoming barriers to in-person management and providing alternatives to inpatients and outpatient supervised rehabilitation.14-17
Despite these considerations, integrating validated technological devices in the remote assessment of older adults with musculoskeletal disorders is still a challenge. In more detail, the recent systematic review by Iovanel et al.18 assessed the role of wearable technology in patients undergoing total joint replacement, suggesting that wearable technology might have promising implications in the remote measure of patient’s functional outcomes. However, the authors underlined that studies in literature are now focusing on accelerometer and gyroscope devices, while no study used wearable devices to perform specific physical functioning rehabilitation tests.
Interestingly, the recent systematic review by Pires et al.19 assessed the role of technological devices in evaluating the 6-Minutes Walking Test (6MWT), a physical functioning test routinely used for assessing physical functioning. The authors reported that inertial and magnetic sensors were the more studied technological tools for telemonitoring 6MWT outcomes, with most of the studies focusing on patients with multiple sclerosis and pulmonary diseases. However, no previous study assessed the effect of a mobile application on patients’ musculoskeletal diseases.
Altogether, this evidence underlines that there is still a lack of technology to perform different functional tests, and expensive sensors are frequently needed to perform a remote assessment. In addition, technological tools currently available require technical expertise, are not portable, and data provided are time-consuming to analyze since that requires specialized personnel. Furthermore, technological solutions performing different physical functioning tests currently used in the rehabilitation management of patients with total knee or hip arthroplasty are still lacking.
As a result, telemonitoring systems are usually restricted to research settings, and digital implementation in clinical settings is currently limited by the several barriers previously mentioned.
Therefore, aim of the present study was to validate a new mobile telemonitoring system named System for Tracking and Evaluating Performance (Step-App®) in patients with knee and hip total arthroplasty to guide clinicians in the remote monitoring of patient’s performance and integrate technological solutions in the precise prescription of tailored rehabilitation programs.
Materials and methods
Study design
This prospective cohort study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines, and the STROBE checklist has been completed. The clinical trial protocol was realized in accordance with the Declaration of Helsinki20 and pertinent National and International regulatory requirements. The study was approved by the Institutional Review Board of the Azienda Ospedaliera Nazionale SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy (ASO.RRF.23.01; protocol number 14570). All the participants carefully read and signed a written informed consent before the study began.
Participants
A consecutive series of patients referred to the Physical and Rehabilitative Medicine Department of the Azienda Ospedaliera Nazionale SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy were assessed for eligibility from April 2023 to June 2023. To be eligible, the study participants had to meet the following inclusion criteria: 1) older people (aged more than 60 years), according to the definition by World Health Organization;21 2) recent hip or knee replacement surgery within the past two weeks; 3) ability to walk without assistance; 4) ability to stand up from a chair without operator assistance; 5) ability to read and understand the Italian language; 6) read and signed the informed consent.
Exclusion criteria were: 1) dementia or cognitive impairment assessed with a Mini-Mental State Examination (MMSE) score <24;22 2) pain at rest assessed with Visual Analogue Scale (VAS) Score23 over four points due to the potential effects of pain on functional assessment; 3) history of metabolic, cardiovascular, pulmonary, neurological or other pathological comorbidities affecting physical performance; 4) others surgical procedures potentially affecting walking, sitting, or standing within six months; 5) anemia; 6) premorbid bed-bound.
The eligibility was assessed by a multidisciplinary team composed of an expert physician specialized in physical medicine and rehabilitation (PMR) and a physiotherapist with years of expertise in orthopedic rehabilitation.
Rehabilitation intervention
All the patients enrolled in the study received a standard rehabilitation treatment during their admission to the Rehabilitation Unit of the Azienda Ospedaliera SS. Antonio e Biagio e Cesare Arrigo, Alessandria, Italy. The standard rehabilitation treatment was similar to previous studies24, 25 and in accordance with the current guidelines.26-28 In particular, the standard rehabilitation intervention was composed by:
counseling and educational therapy: the enrolled patients performed multidisciplinary educational sessions performed by different healthcare operators (physiatrist, physiotherapist, and occupational therapist). The patients were trained to self-treatment during the day with specific exercises, daily strategies optimizing functional recovery and reducing the risk of complications, precautions, and activity to avoid (i.e., activity at risk of dislocating the hip).
physical rehabilitation therapy: the enrolled patients performed physical rehabilitation and occupational therapy sessions for five hours a day (three hours in the morning and two hours in the afternoon) for six days a week for four weeks. Physical therapy was composed not only of active mobilization, aerobic, and resistance training, but also of passive mobilization techniques, stretching exercises, and occupational therapy with a reduced impact on patients’ fitness. Aerobic training was performed with different exercise interventions targeting an exercise intensity between 60% and 85% of maximal heart rate, based on patients‘ personal tolerance. Resistance exercise training was based on weight-free exercises, TheraBand exercises, proprioceptive training, balance training, and core strengthening. Resistance training sessions were performed targeting 60-75% estimated one-repetition maximum (1RM). Occupational therapy sessions were mainly based on task-specific functional recovery based on transfer training, gait training, and activities of daily living (i.e., dressing and bathing activities). Exercise protocols were tailored to patients’ characteristics and were performed aiming at achieving pain-free rehabilitation. The exercise protocol was adapted daily based on the patients’ individual progressions. Each rehabilitation session was administered and supervised by the same physiotherapist with years of expertise in musculoskeletal rehabilitation. Adherence to the rehabilitation program was monitored by registering the access to the rehabilitation gym.
Step-App® mobile system
The Step-App® mobile application has been appositely realized for this study by the Department of Science and Technological Innovation (DISIT), University of Eastern Piedmont Amedeo Avogadro, Italy. An Android smartphone (Xiaomi Redmi Note 11 4/GY) with an Android 11 operating system was used to run the application. The accelerometer and gyroscope sensors of the smartphone were used to monitor patient movements, while the smartphone camera was used to identify markers needed for the 10-Meter Walking Test (10MWT). The device speakers were used to provide real-time feedback about the completed repetitions or the end of the tests performed. All tests can be started by a voice command detected by the smartphone microphone or by pressing the “Start” button on the user interface. The application was realized in both Italian and English interface. Due to language issues, all the study participants were assessed with the Italian Version of the Step-App® applications.
Outcomes
All the outcome measures were assessed at baseline (T0) and after 4 weeks of the comprehensive rehabilitation program (T1).
At T0 a physician specialized in Physical and Rehabilitation Medicine assessed sociodemographic, anthropometric, and clinical characteristics of the study population.
The primary outcomes of this study were:
6MWT: the 6MWT was performed following the American Thoracic Society guidelines.29 More in detail, the patient was asked to walk the longest possible distance alongside a 30-meter-long course marked by two cones for 6 minutes. Two different tests were performed on the same day with at least 30 minutes of rest between each other. The longest distance covered (measured in meters) was used for the analysis. In addition, the rate of perceived exertion (assessed by the Modified Borg Scale) and the peripheral capillary oxygen saturation (SpO2) were measured.30
6MWT with the Step-App® mobile system (6MWT-APP): the patient wore the device by a support belt positioned on the patient‘s thigh, in a lateral position (Figure 1A). To individualize the application parameters for each patient, a calibration session involving a minimum of 30 walking steps was necessary. A single calibration session was needed since the calibration parameters were stored on the device for each patient. After the initial calibration, a bracelet is connected to the smartphone to detect oxygen saturation and heart rate during the test (Figure 1B). Subsequently, the test can be started by pressing the “Start” button or by issuing a voice command which plays an acoustic feedback when detected. The 6MWT-APP has been performed as previously described for the assessment without the mobile application. The application automatically registered the number of rotations around the two cones used to mark the 30 meters distance used for the test (Figure 1C). Lastly, an acoustic feedback indicated that 6 minutes have elapsed. The application digitally calculated total meters, steps, HR, and SpO2 before, during, and after the test (Figure 1C).
10MWT: the patient was asked to walk a 10-meter straight line. The first two meters and the last two meters were marked with lines. The duration of the patient‘s walk from the initial line to the subsequent line was recorded. Walking speed (m/s) was calculated by the rate between the walked space divided by the time taken to travel it. The test was performed four times, two times at a comfortable speed (Slow 10MWT) and two times at the fastest possible speed (Fast 10MWT). Averages for the two types of speed were calculated.31
10MWT with the Step-App® mobile system (10MWT-APP): two colored markers were placed on a 10-meter straight line to mark the first two meters and the last two meters (Figure 2A). These markers allowed the mobile application the precise identification of distance by the camera. A support belt was placed on the patient‘s lateral thigh to frame the wall (Figure 2B).
Figure 1.

—6MWT with the Step-App® mobile system; A) the patient wore the device by a support belt positioned on the patient‘s thigh, in a lateral position; B) a bracelet is positioned on the wrist of the patient and connected to the smartphone to detect oxygen saturation and heart rate during the test; C. The application recorded the number of rotations around the two cones used to mark the 30 meters distance.
Figure 2.

—10MWT with the Step-App® mobile system. A) Two color markers were placed on a 10-meter straight line to mark the first two meters and the last two meters; B) the support belt was placed on the patient‘s lateral thigh to frame the wall.
The 10MWT-APP can be started by pressing the “Start” button or by a voice command. The test was performed as previously described without the mobile application. At the end of the test, the “End” button was pressed and the application calculated the walking speed during the 10MWT-APP. In accordance with the 10MWT, the 10MWT-APP was performed four times.
30SST: the 30SST was performed using a chair with a seat height of 43.2cm from the floor. The chair was positioned against a wall in order to prevent movements during the test. The patient was asked to get up repeatedly from the chair to reach a standing position and return to the previous seated position. Particular attention has been paid to joint dislocation prevention in patients with total hip replacement. In particular, hip flexion beyond 90° was avoided in accordance with the current guidelines.26 Cushions were used to adjust the seat height as needed. The patients had to complete as many repetitions as possible. The test lasted 30 seconds and the score was given as the total number of full stands (body erect and straight) performed by the patient.32
30SST with the Step-App® mobile system (30STS-APP): the device was placed on the front of the patient’s thigh (Figure 3A). The 30STS-APP was started by pressing the “Start” button or by voice command. During the 30 seconds of the test, the repetitions were performed as previously described for the 30STS. An acoustic feedback notified the patient on each completed repetition (Figure 3B). At the end of the 30 seconds, the application indicated the end of the test and the patient stopped. Lastly, the number of repetitions was shown on the mobile screen (Figure 3C).
Figure 3.

—30SST with the Step-App® mobile system. A) The device was placed on the front of the patient‘s thigh; B) during the test the application records the repetitions completed by the patient; C) at the end of the test, the number of repetitions is shown on the mobile screen.
All the functional tests were performed simultaneously by an expert physiotherapist with years of expertise in the functional assessment of patients with musculoskeletal conditions and by the smartphone application Step-App® described before.
The secondary outcomes were:
Pain intensity was assessed by the Numerical Pain Rating Scale (NPRS), a unidimensional scale from 0 to 10, corresponding to “no pain” and “worst pain imaginable”, respectively.33
Physical function was assessed by the Low Extremity Functional Scale (LEFS): this scale consists of 20 items, each of which can have a value from 0 to 4, respectively corresponding to “Extreme Difficulty” or “Unable to Perform Activity” and “No Difficulty”. The total maximum possible score of 80 indicates a higher functional level.34
Physical performance was assessed by the Short Physical Performance Battery (SPPB):35 this scale assessed physical performance by three tests: standing static balance in three different positions (feet together, semi-tandem, and tandem) for ten seconds, time to get up and sit down from a chair five times, and time to travel four meters.35 Each test is scored from zero to four, corresponding to “inability to perform the task” and “best test performance,” respectively. The SPPB total maximum score of 12 points corresponds to a good physical performance.35
Independence in activity of daily living was assessed by the Barthel Index.36 This index is composed of ten items regarding the activity of daily living. The value assigned to each item is based on the amount of physical assistance and supervision required to perform the activity. Scores assigned to the items are multiples of five: for two items zero or five points, for six items zero, five or ten, and for two items zero, five, ten or 15; zero indicates the inability to perform the activity and the need of total assistance and supervision. The total maximum score of 100 points indicates that the patient has an excellent independence in the activity of daily living.36
Lastly, the safety was recorded by the registration of adverse events, and Global Perceived Effect (GPE) Scale was used to characterize patient‘s satisfaction regarding this treatment.37 All the outcome measures were assessed in the morning before daily rehabilitation sessions in order to reduce the potential implications of fatigue. Outcome scales were performed before physical tests and the primary outcomes were assessed in the following order: Slow 10MWT, Fast 10MWT, 6MWT, and 30SST simultaneously by an expert physiotherapist and the Step-App® mobile application.
Statistical analysis
Sample size calculation has been performed considering primary outcome measures and correlation analysis. In accordance with the recommendation by Hobart et al.,38 a minimum of 20 patients is necessary to assess reliability tests (i.e. internal consistency, correlation, or test-retest) in observational studies. GraphPad Prism 9.5.1 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. Means±standard deviations were used for continuous variables, while categorical variables were represented as numbers and ratios. Due to the small sample, a non-Gaussian distribution of data was assumed. The Shapiro-Wilk statistic was used to confirm the non-Gaussian distribution. Intragroup differences have been assessed with Wilcoxon’s signed rank test. Spearman‘s correlation coefficient ® with 95% confidence intervals (CI) was used to assess the correlation between data assessed by the operator and the Step-App® device. Correlation was considered as poor (r<0.5), moderate (r ranging between 0.5 to<0.75), good (r ranging between 0.75 to 0.9), or excellent (r>0.90). A Bland-Altman plot was realized to identify potential systematic bias. A P value lower than 0.05 was considered statistically significant. Descriptive statistics were used to summarize the adverse effect of the treatment.
Results
Patient’s characteristics
Out of the 42 patients who were initially assessed for eligibility, 14 were excluded for not meeting the inclusion criteria (N.=12) or declining participation in the study (N.=2). As a result, a total of 28 eligible patients were included in this study. Lastly, three patients were lost during the study protocol due to discontinuation of the intervention (N.=1) or medical issues (N.=2). As a result, 25 patients completed the study protocol and were analyzed following the study intervention. Figure 4 shows in detail the study flow chart.
Figure 4.
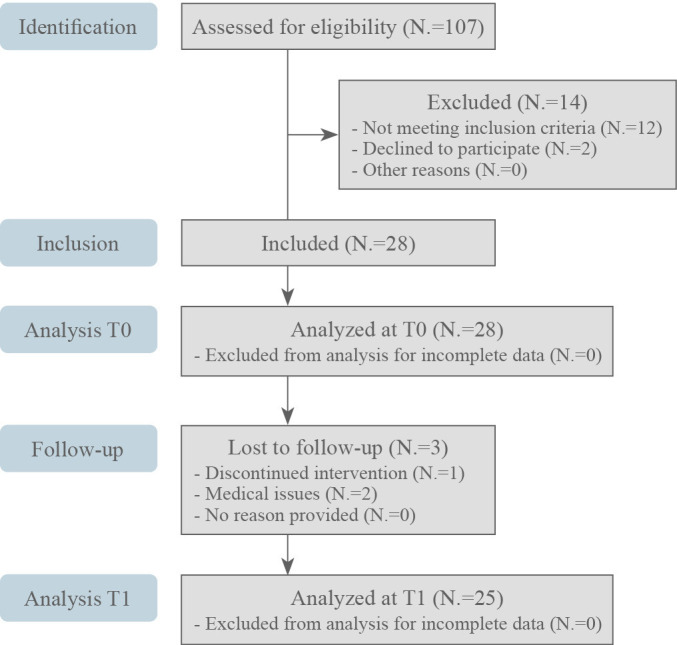
—CONSORT 2010 flow diagram.
The sample was composed of 13 females (52.0%) and 12 males (48.0%), characterized by a mean age of 69.0±11.5 years and mean BMI of 26.73±4.16 kg/m2 Most of the patients included were surgically treated by total hip arthroplasty (20 patients; 80.0%), while 5 patients (20.0%) were treated with knee arthroplasty. The cause of the surgery was osteoarthritis (21; 84.0%) or hip fracture (4; 16.0%). The mean time between the surgical procedure and study assessment was 11.96±4.30 days. Mean adherence to the rehabilitation program was 93.0%. Table I shows the anamnestic and demographic characteristics of study population.
Table I. —Anamnestic and demographic characteristics of study population.
| Total (N.=25) | |
|---|---|
| Age (years) | 69.00±11.5 |
| Sex (female/male) | 13/12 |
| Ethnicity (Caucasian) | 25 (100) |
| BMI (kg/m2) | 26.73±4.16 |
| MMSE | 30.4±2.71 |
| Days from surgery to hospitalization | 11.96±4.30 |
| Type of surgery | |
| Total hip replacement | 20 (80) |
| Total knee replacement | 5 (20) |
| Diagnosis | |
| Osteoarthrosis | 21 (84) |
| Hip fracture | 4 (16) |
| Side | |
| Left | 9 (36) |
| Right | 16 (64) |
| Bilateral | 0 |
Primary outcome
An excellent correlation was shown between 6MWT and 6MWT-APP both at T0 (r=0.9967; P<0.0001) and at T1 (r=0.9992; P<0.0001). In addition, a strong agreement between 6MWT and 6MWT-APP has been also confirmed by the Bland-Altman plot. Lastly, an excellent correlation was also shown in terms of distance improvement (T1-T0) measured after the rehabilitation treatments (r=0.9879; P<0.0001). Further details are shown in Figure 5.
Figure 5.
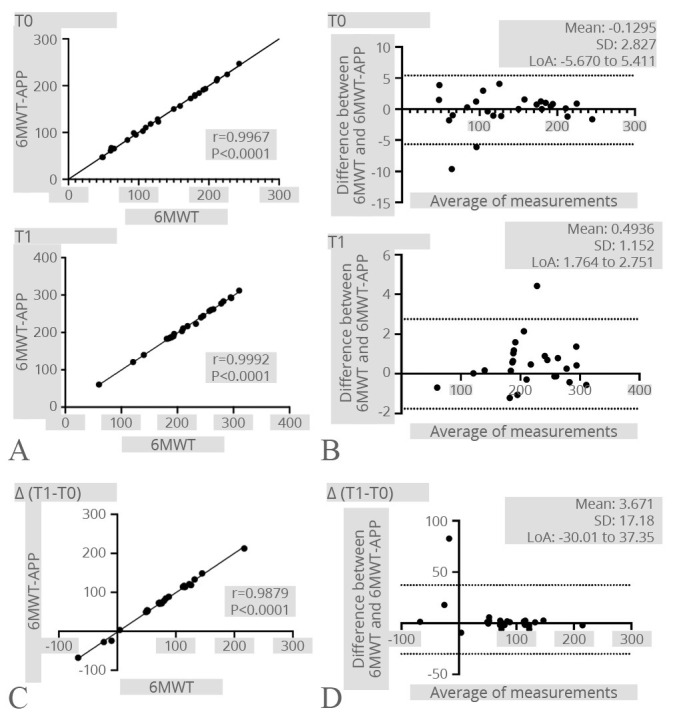
—6MWT. A) Correlation between the 6MWT and the 6MWT-APP at both time points; B) Bland-Altman plot showing the level of agreement and the consistency of the two measurements at both time points; C) correlation between the difference of the 6MWT results after the rehabilitation treatment registered by the operator and the Step-App® mobile system; D) Bland-Altman plot showing the level of agreement and the consistency of the two measurements difference after the rehabilitation treatment.
In accordance, an excellent correlation was found also between fast 10MWT and fast 10MWT-APP in both time points (T0: r=0.9676; P<0.0001; T1: r=0.9172; P<0.0001). Concurrently, an excellent correlation was also confirmed in terms of velocity improvements (T1-T0) after the rehabilitation intervention (r=0.9395; P<0.0001). The Bland-Altman plot confirmed the strong agreement between the two different methods. See Figure 6 for further details.
Figure 6.
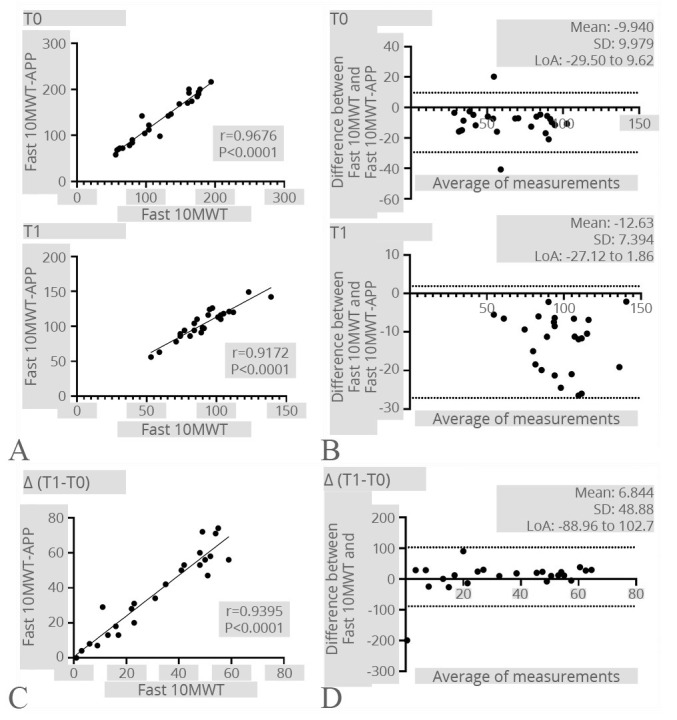
—Fast 10MWT. A) Correlation between the Fast 10MWT registered by the operator and the Fast 10MWT-APP at both time points; B) Bland-Altman plot showing the level of agreement and the consistency of the two measurements at both time points; C) correlation between the difference of the Fast 10MWT results after the rehabilitation treatment registered by the operator and the Step-App® mobile system; D) Bland-Altman plot showing the level of agreement and the consistency of the two measurement difference after the rehabilitation treatment.
Moreover, a strong correlation was observed between slow 10MWT and slow 10MWT-APP at both timepoints (T0: r=0.9871; P<0.0001; T1: r=0. 8948; P<0.0001). Furthermore, there was also a significant correlation in terms of velocity improvements (T1-T0) following the rehabilitation intervention (r=0.9546; P<0.0001). The Bland-Altman plot further demonstrated a robust agreement between the two distinct measurement methods. Figure 7 shows further details.
Figure 7.
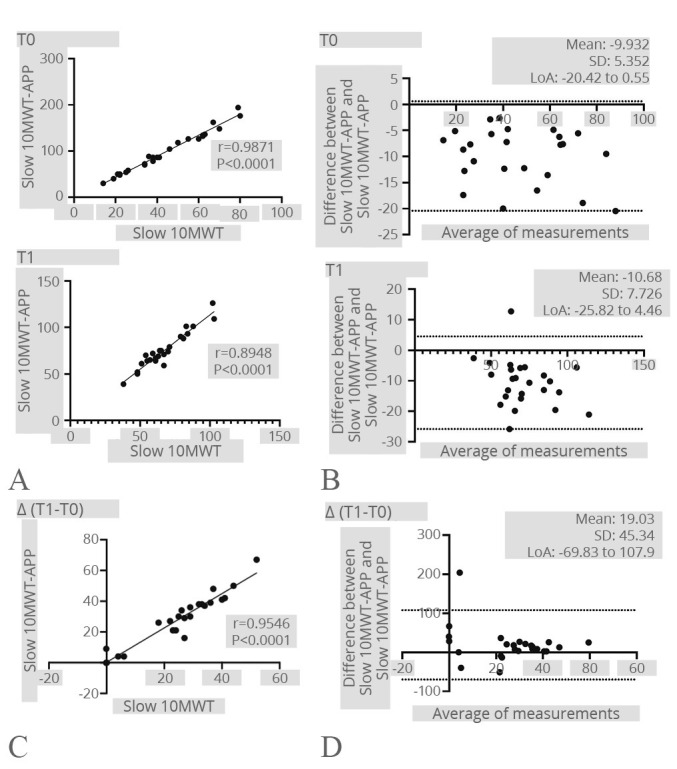
—Slow 10MWT. A) Correlation between the Slow 10MWT and the Slow 10MWT-APP at both time points; B) Bland-Altman plot showing the level of agreement and the consistency of the two measurements at both time points; C) correlation between the difference of the Slow 10MWT results after the rehabilitation treatment registered by the operator and the Step-App® mobile system; D) Bland-Altman plot showing the level of agreement and the consistency of the two measurement difference after the rehabilitation treatment.
Lastly, the analysis revealed a perfect line result between 30SST and 30SST-APP at T0 (r=1; P<0.0001) and after the rehabilitation intervention (r=1; P<0.0001), indicating a strong and direct correlation between the two variables. In addition, the improvements in terms of repetition number between 30SST and 30SST-APP were significantly correlated (r=1; P<0.0001), indicating a clear and consistent relationship between the variables. The Bland-Altman plot showed a perfect line result, indicating an ideal agreement between the two variables (see Figure 8 for further details).
Figure 8.
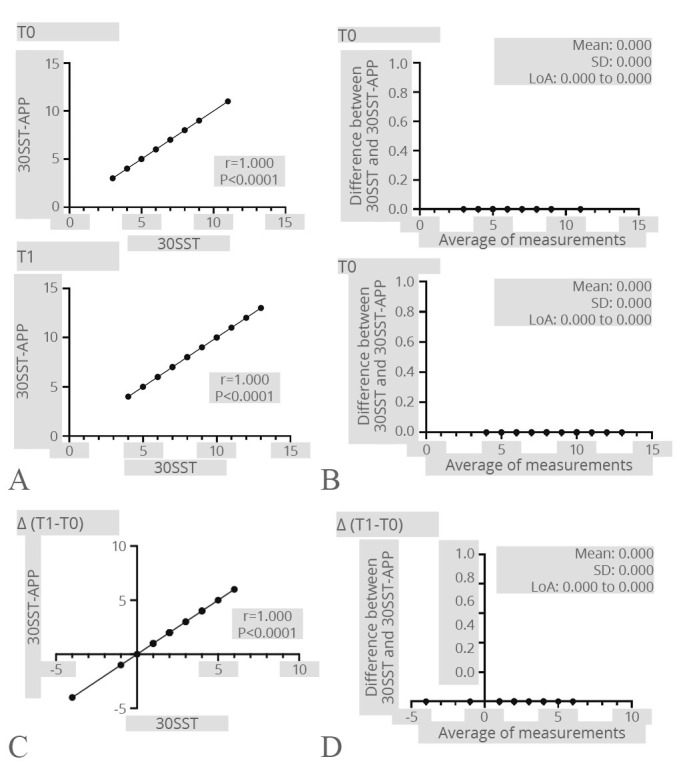
—30SST. A) Correlation between the 30SST test and the 30SST-APP at both time points; B) Bland-Altman plot showing the level of agreement and the consistency of the two measurements at both time points; C) correlation between the difference of the 30SST results after the rehabilitation treatment registered by the operator and the Step-App® mobile system; D) Bland-Altman plot showing the level of agreement and the consistency of the two measurement difference after the rehabilitation treatment.
Secondary outcomes
After the rehabilitation intervention, significant improvements were reported in terms of 6MWT (P<0.0001), 10MWT (P<0.0001), and 30STS (P<0.0001) were shown in both operator and Step-App® measures. On the other hand, no significant differences were reported between Step-App® mobile system assessment and operator assessment in terms of 6MWT, 10MWT, and 30STS.
Pain intensity assessed by NPRS showed a significant reduction (P=0.0421). The LEFS scores showed a significant improvement in functional ability (P<0.0001), in accordance with the physical performance assessed by the SPPB (P<0.0001). Lastly, the Barthel Index scores underlined a significant improvement in functional independence after the intervention (P<0.0001). More details are shown in Table II.
Table II. —Clinical outcome measures assessed at the baseline and after the study intervention.
| Operator | Step-App® mobile system | Between-group differences | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | ΔT(T1-T0) | P | T0 | T1 | ΔT(T1-T0) | P | T0 | T1 | ΔT(T1-T0) | ||
| 6MWT | Meters | 128.0 (88.5 to 189.0) |
210.0 (187.5 to 262.0) |
80.0 (51.0 to 119.5) |
<0.0001 | 128.4 (89.3 to 187.6) |
210.6 (182.4 to 261.1) |
78.7 (52.1 to 119.5) |
<0.0001 | 0.9886 | 0.8475 | 0.9195 |
| RPE Borg | 6.0 (4.0 to 7.0) |
4.0 (3.0 to 6.0) |
-1 (-2.5 to 1) |
0.1397 | 6.0 (4.0 to 7.0) |
4.0 (3.0 to 6.0) |
-1 (-2.5 to 1) |
0.1397 | >0.9999 | >0.9999 | >0.9999 | |
| SpO2 | 92.0 (89.5 to 95.0) |
93.0 (90.5 to 97.0) |
2.00 (-6 to 4.5) |
0.5957 | 92.0 (89.5 to 95.0) |
93.0 (90.5 to 97.0) |
2.00 (-6 to 4.5) |
0.5957 | >0.9999 | >0.9999 | >0.9999 | |
| 10MWT | Slow | 0.37 (0.24 to 0.58) |
0.40 (0.26 to 0.62) |
0.02 (0.0 to 0.04) |
<0.0001 | 0.43 (0.28 to 0.67) |
0.71 (0.64 to 0.89) |
0.30 (0.10 to 0.39) |
<0.0001 | 0.4045 | 0.1022 | 0.4670 |
| Fast | 0.60 (0.39 to 0.82) |
0.90 (0.79 to 1.03) |
0.35 (0.15 to 0.50) |
<0.0001 | 0.71 (0.41 to 0.94) |
1.04 (0.90 to 1.19) |
0.42 (0.16 to 0.56) |
<0.0001 | 0.3042 | 0.2080 | 0.3324 | |
| SST | 6.0 (5.0 to 7.5) |
8.0 (6.0 to 10.0) |
2 (1 to 3.5) |
<0.0001 | 6.0 (5.0 to 7.5) |
8.0 (6.0 to 10.0) |
2 (1 to 3.5) |
<0.0001 | >0.9999 | >0.9999 | >0.9999 | |
| NPRS | 2.0 (1.0 to 3.0) |
1.0 (0.0 to 2.0) |
1.0 (0.0 to 2.0) |
0.0421 | ||||||||
| LEFS | 28.0 (25.0 to 37.0) |
46.0 (39.5 to 50.0) |
14.0 (9.0 to 20.0) |
<0.0001 | ||||||||
| SPPB | 5.0 (4.0 to 5.0) |
7.0 (6.0 to 7.0) |
2.0 (1.0 to 2.5) |
<0.0001 | ||||||||
| Barthel Index | 80.0 (65.0 to 87.5) |
95.0 (90.0 to 95.0) |
15.0 (10.0 to 25.0) |
<0.0001 | ||||||||
Continuous variables are expressed as Median (IQR). 6MWT: 6-minute Walk Test; 10MWT: 10-Meter Walk Test; P: P-value; LEFS: Low Extremity Functional Scale; NPRS: Numerical Pain Rating Scale; SpO2: peripheral oxygen saturation; SPPB: short physical performance battery; SST: Sit to Stand Test; T0: at hospitalization, before treatment; T1: at discharge, after treatment.
Safety and feasibility
No major adverse events were reported during the study. The most common adverse events were fatigue during the tests (N.=5) and pain (N.=6). The GPE score assessed after the rehabilitation treatment was 6.2 considering patients’ perspectives and 4.0 considering physical therapists’ perspectives.
Discussion
The most important finding of the present study was a significant correlation between the three tests assessed by the operators and Step-App®, suggesting that this promising digital tool might be effectively integrated into the telerehabilitation monitoring of patients with total hip and knee arthroplasty. In addition, an excellent agreement between operator assessment and Step-App® assessment was confirmed by the Bland-Altman plot with potential implications for the interchangeability of the two different methods.
Interestingly, growing efforts have been recently paid to rehabilitation delivery at home.39, 40 In this scenario, the recent review by Bäcker et al.41 assessed the role of mobile applications in telerehabilitation of total hip arthroplasty and total knee arthroplasty, showing promising results in integrating technological solutions to conventional rehabilitation programs. However, little evidence is currently available in physical performance tests assessment by digital solutions.
In line with these considerations, the recent RCT by Li et al.42 assessed the role of an app-based exercise program in terms of 10MWT and 6MWT. Although the rehabilitation program was successfully compared to usual care, it is interesting to notice that all functional tests performed were monitored in person. These findings underline a large gap of knowledge about wearable technological solutions assessing the effects of home-based interventions.
On the other hand, it should be noted that previous studies assessed the role of mobile applications in 6MWT assessment.43-46 On the other hand, most of these studies focused on patients with cardiopulmonary diseases43, 46 or healthy subjects.44, 45 As a result, Step-App® mobile application is the first wearable digital tool validated on patients surgically treated for total hip or knee arthroplasty. Moreover, the Spearman‘s correlation coefficient registered in our study was the highest registered in current literature.
In accordance, few studies have already validated mobile applications for iOS systems assessing gait speed with 10MWT.47, 48 However, these studies were performed on healthy subjects47, 48 and disease-specific data were still unavailable. In addition, the mobile application already validated were based on step count and step length estimation,47 with a potential source of systematic bias due to the step length asymmetry and alterations commonly registered during the postsurgical management of patients with total hip and knee arthroplasty.49, 50
Lastly, STS is a simple test routinely used in rehabilitation clinical practice to assess lower limb muscle strength and physical performance, and different mobile applications have been proposed to digitally evaluate this outcome measure.51-53 On the other hand, the currently available applications were not wearable, required different instruments,53 or were validated for a single or five repetitions.51, 52 Thus, to our knowledge, this is the first mobile application for the assessment of the 30STS test.
Altogether, these data suggested that mobile applications might be effectively integrated into a tailored assessment of patient’s performance. However, to the best of our knowledge, Step-App® is the first mobile application allowing to perform different physical test measures and potentially improving the remote monitoring of patients with musculoskeletal conditions.
Lastly, the results of this study highlighted a good patients satisfaction assessed by GPE, underlining that Step-App® might be effectively integrated in a patient-centered care of older adults. In this context, several barriers might affect telehealth implementation in older adults, including impaired visual and haring performance, access to technology, and reduced trust with technology.54 The results of the present study showed promising implications for actively engage older adults in their rehabilitation intervention, promoting self-management and reducing healthcare costs.
In addition, our study highlighted a significant correlation between operators’ assessment and Step-App® mobile system also in terms of improvement after the rehabilitation treatment. These results suggested that Step-App® might allow the objectification of clinical results and improve evidence about the effectiveness of a comprehensive rehabilitation approach. Moreover, objective and digital data might pave the way of integrating machine learning and artificial intelligence in the precise monitoring of patients with disability, providing valuable insight for the digital innovation in rehabilitation settings.55
Limitations of the study
Despite these promising results, this study is not free from limitations. More in detail, the cost-effectiveness and the long-term effects of the rehabilitation program performed have not been assessed. However, it should be noted that the aim of the study was to validate the Step-App® mobile system in a rehabilitation setting. To our knowledge, this is the first freely available application performing different performance analyses in older adults. On the other hand, this is a monocentric study assessing patients with hip and knee arthroplasty. Thus, it is not possible to generalize results to other specific populations. On the other hand, the promising data suggests potential implementations in different patients characterized by different domains of disability. Further studies are necessary on this topic to elucidate the role of this cutting-edge mobile application in the telemonitoring of patients with different musculoskeletal conditions.
Conclusions
Step-App® mobile system showed to be the first digital tool allowing a precise measurement of different performance tests routinely used in rehabilitation. Our findings might promote the digital implementation in rehabilitation management of musculoskeletal conditions. We retain that further studies are needed to characterize the role of digital data provided by this application that might be implemented with machine learning and artificial intelligence improving a more detailed assessment of patients with musculoskeletal conditions.
Acknowledgments
The authors acknowledge Alessandro Pelosi for his support to this work.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: This publication is part of the project NODES which has received funding from the MUR – M4C2 1.5 of PNRR with grant agreement no. ECS00000036.
References
- 1.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, et al. The World report on ageing and health: a policy framework for healthy ageing. Lancet 2016;387:2145–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26520231&dopt=Abstract 10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell 2014;159:709–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25417146&dopt=Abstract 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi L, de Sire A, Mezian K, Curci C, Perrero L, Turco A, et al. Impact of exercise training on muscle mitochondria modifications in older adults: a systematic review of randomized controlled trials. Aging Clin Exp Res 2022;34:1495–510. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35079977&dopt=Abstract 10.1007/s40520-021-02073-w [DOI] [PubMed] [Google Scholar]
- 4.Lippi L, Uberti F, Folli A, Turco A, Curci C, d’Abrosca F, et al. Impact of nutraceuticals and dietary supplements on mitochondria modifications in healthy aging: a systematic review of randomized controlled trials. Aging Clin Exp Res 2022;34:2659–74. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35920994&dopt=Abstract 10.1007/s40520-022-02203-y [DOI] [PubMed] [Google Scholar]
- 5.Ferguson RJ, Palmer AJ, Taylor A, Porter ML, Malchau H, Glyn-Jones S. Hip replacement. Lancet 2018;392:1662–71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30496081&dopt=Abstract 10.1016/S0140-6736(18)31777-X [DOI] [PubMed] [Google Scholar]
- 6.Price AJ, Alvand A, Troelsen A, Katz JN, Hooper G, Gray A, et al. Knee replacement. Lancet 2018;392:1672–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30496082&dopt=Abstract 10.1016/S0140-6736(18)32344-4 [DOI] [PubMed] [Google Scholar]
- 7.de Sire A, Invernizzi M, Baricich A, Lippi L, Ammendolia A, Grassi FA, et al. Optimization of transdisciplinary management of elderly with femur proximal extremity fracture: A patient-tailored plan from orthopaedics to rehabilitation. World J Orthop 2021;12:456–66. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34354934&dopt=Abstract 10.5312/wjo.v12.i7.456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mistry JB, Elmallah RD, Bhave A, Chughtai M, Cherian JJ, McGinn T, et al. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J Knee Surg 2016;29:201–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26963074&dopt=Abstract 10.1055/s-0036-1579670 [DOI] [PubMed] [Google Scholar]
- 9.Janhunen M, Katajapuu N, Paloneva J, Pamilo K, Oksanen A, Keemu H, et al. Effects of a home-based, exergaming intervention on physical function and pain after total knee replacement in older adults: a randomised controlled trial. BMJ Open Sport Exerc Med 2023;9:e001416. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36896366&dopt=Abstract 10.1136/bmjsem-2022-001416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatoye F, Gebrye T, Fatoye C, Mbada C. A systematic review of economic models for cost effectiveness of physiotherapy interventions following total knee and hip replacement. Physiotherapy 2022;116:90–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35605563&dopt=Abstract 10.1016/j.physio.2022.01.002 [DOI] [PubMed] [Google Scholar]
- 11.National Guideline C. NICE Evidence Reviews Collection. Evidence review for outpatient hip and knee postoperative rehabilitation: Joint replacement (primary): hip, knee and shoulder: Evidence review R. London: National Institute for Health and Care Excellence (NICE) Copyright © NICE 2020; 2020. [PubMed] [Google Scholar]
- 12.Jette DU, Hunter SJ, Burkett L, Langham B, Logerstedt DS, Piuzzi NS, et al. American Physical Therapy Association . Physical therapist management of total knee arthroplasty. Phys Ther 2020;100:1603–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32542403&dopt=Abstract 10.1093/ptj/pzaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsdotter AK, Roos EM, Westerlund JP, Roos HP, Lohmander LS. Comparative responsiveness of measures of pain and function after total hip replacement. Arthritis Rheum 2001;45:258–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11409667&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 14.Lippi L, Turco A, Folli A, D’Abrosca F, Curci C, Mezian K, et al. Technological advances and digital solutions to improve quality of life in older adults with chronic obstructive pulmonary disease: a systematic review. Aging Clin Exp Res 2023;35:953–68. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36952118&dopt=Abstract 10.1007/s40520-023-02381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippi L, D’Abrosca F, Folli A, Dal Molin A, Moalli S, Maconi A, et al. Closing the Gap between Inpatient and Outpatient Settings: Integrating Pulmonary Rehabilitation and Technological Advances in the Comprehensive Management of Frail Patients. Int J Environ Res Public Health 2022;19:9150. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35954506&dopt=Abstract 10.3390/ijerph19159150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marotta N, Calafiore D, Curci C, Lippi L, Ammendolia V, Ferraro F, et al. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med 2022;58:818–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36169933&dopt=Abstract 10.23736/S1973-9087.22.07643-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paolucci T, de Sire A, Ferrillo M, di Fabio D, Molluso A, Patruno A, et al. Telerehabilitation proposal of mind-body technique for physical and psychological outcomes in patients with fibromyalgia. Front Physiol 2022;13:917956. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36091366&dopt=Abstract 10.3389/fphys.2022.917956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iovanel G, Ayers D, Zheng H. The Role of Wearable Technology in Measuring and Supporting Patient Outcomes Following Total Joint Replacement: review of the Literature. JMIR Perioper Med 2023;6:e39396. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36633891&dopt=Abstract 10.2196/39396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pires IM, Denysyuk HV, Villasana MV, Sá J, Marques DL, Morgado JF, et al. Development technologies for the monitoring of six-minute walk test: a systematic review. Sensors (Basel) 2022;22:581. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35062542&dopt=Abstract 10.3390/s22020581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Association WM, World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24141714&dopt=Abstract 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Organization WH. Decade of Healthy Ageing 2020–2030 [Internet]. Available from: https://www.who.int/initiatives/decade-of-healthy-ageing [cited 2022, Feb 1]
- 22.Kurlowicz L, Wallace M. The mini-mental state examination (MMSE). Thorofare, NJ: SLACK Incorporated; 1999. p. 8, 9. [DOI] [PubMed]
- 23.Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int 1985;5:145–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=4048757&dopt=Abstract 10.1007/BF00541514 [DOI] [PubMed] [Google Scholar]
- 24.Gaweł J, Fibiger W, Starowicz A, Szwarczyk W. Early assessment of knee function and quality of life in patients after total knee replacement. Ortop Traumatol Rehabil 2010;12:329–37. [Polish.] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20876926&dopt=Abstract [PubMed] [Google Scholar]
- 25.Hadamus A, Białoszewski D, Błażkiewicz M, Kowalska AJ, Urbaniak E, Wydra KT, et al. Assessment of the Effectiveness of Rehabilitation after Total Knee Replacement Surgery Using Sample Entropy and Classical Measures of Body Balance. Entropy (Basel) 2021;23:164. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33573057&dopt=Abstract 10.3390/e23020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith TO, Jepson P, Beswick A, Sands G, Drummond A, Davis ET, et al. Assistive devices, hip precautions, environmental modifications and training to prevent dislocation and improve function after hip arthroplasty. Cochrane Database Syst Rev 2016;7:CD010815. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27374001&dopt=Abstract 10.1002/14651858.CD010815.pub2 [DOI] [PMC free article] [PubMed]
- 27.van Doormaal MC, Meerhoff GA, Vliet Vlieland TP, Peter WF. A clinical practice guideline for physical therapy in patients with hip or knee osteoarthritis. Musculoskelet Care 2020;18:575–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32643252&dopt=Abstract 10.1002/msc.1492 [DOI] [PubMed]
- 28.Colibazzi V, Coladonato A, Zanazzo M, Romanini E. Evidence based rehabilitation after hip arthroplasty. Hip Int 2020;30(suppl):20–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33267691&dopt=Abstract 10.1177/1120700020971314 [DOI] [PubMed] [Google Scholar]
- 29.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002;166:111–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12091180&dopt=Abstract 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 30.Mahler DA, Horowitz MB. Perception of breathlessness during exercise in patients with respiratory disease. Med Sci Sports Exerc 1994;26:1078–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7808239&dopt=Abstract 10.1249/00005768-199409000-00002 [DOI] [PubMed] [Google Scholar]
- 31.Amatachaya S, Kwanmongkolthong M, Thongjumroon A, Boonpew N, Amatachaya P, Saensook W, et al. Influence of timing protocols and distance covered on the outcomes of the 10-meter walk test. Physiother Theory Pract 2020;36:1348–53. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30704332&dopt=Abstract 10.1080/09593985.2019.1570577 [DOI] [PubMed] [Google Scholar]
- 32.Jones CJ, Rikli RE, Beam WC. A. 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10380242&dopt=Abstract 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 33.Kahl C, Cleland JA. Visual analogue scale, numeric pain rating scale and the McGill pain Questionnaire: an overview of psychometric properties. Phys Ther Rev 2005;10:123–8. 10.1179/108331905X55776 [DOI] [Google Scholar]
- 34.Binkley JM, Stratford PW, Lott SA, Riddle DL, Network NA, North American Orthopaedic Rehabilitation Research Network . The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther 1999;79:371–83. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10201543&dopt=Abstract [PubMed] [Google Scholar]
- 35.de Fátima Ribeiro Silva C, Ohara DG, Matos AP, Pinto AC, Pegorari MS. Short physical performance battery as a measure of physical performance and mortality predictor in older adults: A comprehensive literature review. Int J Environ Res Public Health 2021;18:10612. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34682359&dopt=Abstract 10.3390/ijerph182010612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965;14:61–5. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14258950&dopt=Abstract [PubMed] [Google Scholar]
- 37.Meisingset I, Stensdotter AK, Woodhouse A, Vasseljen O. Predictors for global perceived effect after physiotherapy in patients with neck pain: an observational study. Physiotherapy 2018;104:400–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30477677&dopt=Abstract 10.1016/j.physio.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 38.Hobart JC, Cano SJ, Warner TT, Thompson AJ. What sample sizes for reliability and validity studies in neurology? J Neurol 2012;259:2681–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22729386&dopt=Abstract 10.1007/s00415-012-6570-y [DOI] [PubMed] [Google Scholar]
- 39.de Sire A, Marotta N, Agostini F, Drago Ferrante V, Demeco A, Ferrillo M, et al. A Telerehabilitation Approach to Chronic Facial Paralysis in the COVID-19 Pandemic Scenario: What Role for Electromyography Assessment? J Pers Med 2022;12:497. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35330496&dopt=Abstract 10.3390/jpm12030497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turolla A, Rossettini G, Viceconti A, Palese A, Geri T. Musculoskeletal Physical Therapy During the COVID-19 Pandemic: Is Telerehabilitation the Answer? Phys Ther 2020;100:1260–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32386218&dopt=Abstract 10.1093/ptj/pzaa093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bäcker HC, Wu CH, Pförringer D, Petersen W, Stöckle U, Braun KF. A Review of Functional Outcomes after the App-Based Rehabilitation of Patients with TKA and THA. J Pers Med 2022;12:1342. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36013291&dopt=Abstract 10.3390/jpm12081342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li I, Bui T, Phan HT, Llado A, King C, Scrivener K. App-based supplemental exercise in rehabilitation, adherence, and effect on outcomes: a randomized controlled trial. Clin Rehabil 2020;34:1083–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32508183&dopt=Abstract 10.1177/0269215520928119 [DOI] [PubMed] [Google Scholar]
- 43.Brooks GC, Vittinghoff E, Iyer S, Tandon D, Kuhar P, Madsen KA, et al. Accuracy and Usability of a Self-Administered 6-Minute Walk Test Smartphone Application. Circ Heart Fail 2015;8:905–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26283292&dopt=Abstract 10.1161/CIRCHEARTFAILURE.115.002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith-Turchyn J, Adams SC, Sabiston CM. Testing of a Self-administered 6-Minute Walk Test Using Technology: Usability, Reliability and Validity Study. JMIR Rehabil Assist Technol 2021;8:e22818. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34554105&dopt=Abstract 10.2196/22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jesus MO, Ostolin TL, Proença NL, Silva RP, Dourado VZ. Self-Administered Six-Minute Walk Test Using a Free Smartphone App in Asymptomatic Adults: reliability and Reproducibility. Int J Environ Res Public Health 2022;19:1118. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35162141&dopt=Abstract 10.3390/ijerph19031118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherrenberg M, Bonneux C, Yousif Mahmood D, Hansen D, Dendale P, Coninx K. A Mobile Application to Perform the Six-Minute Walk Test (6MWT) at Home: A Random Walk in the Park Is as Accurate as a Standardized 6MWT. Sensors (Basel) 2022;22:4277. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35684898&dopt=Abstract 10.3390/s22114277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurt CP, Lein DH, Jr, Smith CR, Curtis JR, Westfall AO, Cortis J, et al. Assessing a novel way to measure step count while walking using a custom mobile phone application. PLoS One 2018;13:e0206828. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30399162&dopt=Abstract 10.1371/journal.pone.0206828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito Y, Nakamura S, Tanaka A, Watanabe R, Narimatsu H, Chung UI. Evaluation of the validity and reliability of the 10-meter walk test using a smartphone application among Japanese older adults. Front Sports Active Living 2022;4:904924. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36267485&dopt=Abstract 10.3389/fspor.2022.904924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horstmann T, Listringhaus R, Haase GB, Grau S, Mündermann A. Changes in gait patterns and muscle activity following total hip arthroplasty: a six-month follow-up. Clin Biomech (Bristol, Avon) 2013;28:762–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23906936&dopt=Abstract 10.1016/j.clinbiomech.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 50.Cichy B, Wilk M, Sliwiński Z. Changes in gait parameters in total hip arthroplasty patients before and after surgery. Med Sci Monit 2008;14:CR159–69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18301360&dopt=Abstract [PubMed] [Google Scholar]
- 51.Marques DL, Neiva HP, Pires IM, Zdravevski E, Mihajlov M, Garcia NM, et al. An Experimental Study on the Validity and Reliability of a Smartphone Application to Acquire Temporal Variables during the Single Sit-to-Stand Test with Older Adults. Sensors (Basel) 2021;21:2050. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33803927&dopt=Abstract 10.3390/s21062050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teixeira E, Bohn L, Guimarães JP, Marques-Aleixo I. Portable Digital Monitoring System for Sarcopenia Screening and Diagnosis. Geriatrics (Basel) 2022;7:121. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36412610&dopt=Abstract 10.3390/geriatrics7060121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montemurro A, Ruiz-Cárdenas JD, Martínez-García MD, Rodríguez-Juan JJ. Validity of an iPhone App to Detect Prefrailty and Sarcopenia Syndromes in Community-Dwelling Older Adults: The Protocol for a Diagnostic Accuracy Study. Sensors (Basel) 2022;22:6010. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36015771&dopt=Abstract 10.3390/s22166010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kruse C, Fohn J, Wilson N, Nunez Patlan E, Zipp S, Mileski M. Utilization Barriers and Medical Outcomes Commensurate With the Use of Telehealth Among Older Adults: systematic Review. JMIR Med Inform 2020;8:e20359. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32784177&dopt=Abstract 10.2196/20359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Payedimarri AB, Concina D, Portinale L, Canonico M, Seys D, Vanhaecht K, et al. Prediction Models for Public Health Containment Measures on COVID-19 Using Artificial Intelligence and Machine Learning: A Systematic Review. Int J Environ Res Public Health 2021;18:4499. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33922693&dopt=Abstract 10.3390/ijerph18094499 [DOI] [PMC free article] [PubMed] [Google Scholar]


