Abstract
Objective:
Lysosome-associated membrane protein 3 (LAMP3) overexpression is implicated in the development and progression of Sjögren’s disease (SjD) by inducing lysosomal membrane permeabilization (LMP) and apoptotic cell death in salivary gland epithelium. The aim of this study is to clarify molecular details of LAMP3-induced lysosome-dependent cell death and to test lysosomal biogenesis as a therapeutic intervention.
Methods:
Human labial minor salivary gland biopsies were immunofluorescently analyzed for LAMP3 expression levels and galectin-3 puncta formation, a marker of LMP. Expression level of caspase-8, an initiator of LMP, was determined by Western blotting in cell culture. Galectin-3 puncta formation and apoptosis were evaluated in cell culture and a mouse model treated with glucagon-like peptidase-1 receptor (GLP-1R) agonists, a known promoter of lysosomal biogenesis.
Results:
Galectin-3 puncta formation was more frequent in SjD patients’ salivary glands compared to control glands. The proportion of galectin-3 puncta-positive cells was positively correlated with LAMP3 expression levels in the glands. LAMP3 overexpression increased caspase-8 expression, and knockdown of caspase-8 decreased galectin-3 puncta formation and apoptosis in LAMP3-overexpressing cells. Inhibition of autophagy increased caspase-8 expression, while restoration of lysosomal function using GLP-1R agonists decreased caspase-8 expression, which reduced galectin-3 puncta formation and apoptosis in both LAMP3-overexpressing cells and mice.
Conclusion:
LAMP3 overexpression induced lysosomal dysfunction, resulting in lysosome-dependent cell death via impaired autophagic caspase-8 degradation, and restoring lysosomal function by GLP-1R agonists could prevent this. These findings suggested that LAMP3-induced lysosomal dysfunction is central to disease development and a target for therapeutic intervention in SjD.
GRAPHICAL ABSTRACT
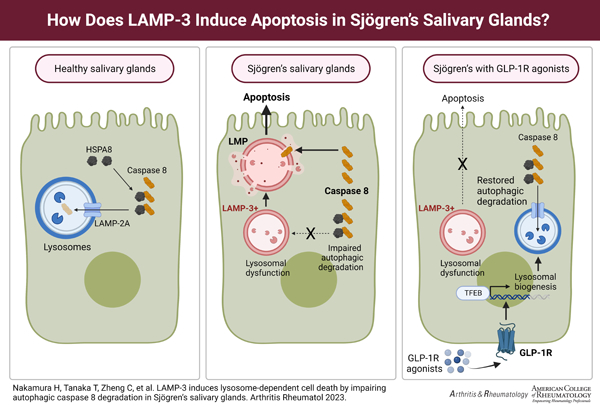
INTRODUCTION
Sjögren’s disease (SjD) is an autoimmune exocrinopathy characterized by progressive salivary and lacrimal gland hypofunction, lymphocytic infiltration into the affected glands, and the presence of serum anti-Ro/SSA antibodies (1).
Lysosomes are acidic membrane-bound organelles containing proteolytic enzymes, in which vacuolar-type ATPase (V-ATPase) maintains the acidic environment. As the cellular recycling system, the lysosomes are central to autophagy, the cellular self-degradation process. The autophagy-lysosomal pathways play an important role in energy metabolism and cell survival as a digestive system of the cell (2). Recent studies suggest lysosomal dysfunction, altered autophagy, and apoptotic cell death are involved in the development of salivary gland hypofunction and autoimmunity in SjD (3–12).
Lysosome-associated membrane protein (LAMP) 3 is upregulated in salivary glands of a subset of patients with SjD (3). Unlike LAMP1 and LAMP2, which are distributed in many cells and tissues, LAMP3 expression is limited in several cell types in immune system organs, such as dendritic cells in lymph nodes and tonsil (13, 14). Confocal immunofluorescent studies on SjD patients’ salivary glands revealed ectopic LAMP3 expression in salivary gland epithelial cells in addition to infiltrating immune cells forming lymphoid structures in the glands (3). Therefore, in subsequent studies, we have focused on the pathophysiological role of ectopic epithelial LAMP3 expression in salivary glands.
Overexpression of LAMP3 in salivary gland epithelial cells increases apoptotic cell death both in vitro (3) and in vivo (4). Mechanistically, LAMP3 overexpression destabilizes the lysosomal membrane via degradation of lysosome-associated membrane protein 1 (LAMP1), resulting in lysosomal dysfunction and impairment of autophagy (5). Eventually, LAMP3 overexpression induces lysosomal membrane permeabilization (LMP) and activates caspase-dependent apoptotic pathways following leakage of lysosomal proteases into cytoplasm (5). A critical step of this process is degradation of LAMP1 because overexpression of LAMP1 can prevent LMP and cell death (5). However, the detailed molecular mechanisms leading to LMP and lysosome-dependent cell death beyond LAMP1 degradation are still unclear.
In the current study, we found that increased expression of caspase-8 played an essential role in LAMP3-induced LMP and lysosome-dependent cell death. Furthermore, we showed that caspase-8 expression is increased by LAMP3 overexpression via impairment of autophagic flux. Finally, we demonstrated that restoration of lysosomal function prevented LAMP3-induced LMP and lysosome-dependent cell death by decreasing caspase-8 expression.
MATERIALS AND METHODS
Clinical samples
Labial minor salivary gland biopsies were obtained from 10 SjD patients who fulfilled the 2016 American College of Rheumatology/European League against Rheumatism classification criteria for primary Sjögren’s syndrome (15) and 5 control subjects who had sicca symptoms but did not meet the classification criteria at the Hokkaido University Hospital. All the participants provided informed consent to the study protocol approved by the Ethics Committee of the Hokkaido University Hospital (approval number: 014–0466). In addition, 80 labial minor salivary gland biopsies were obtained from the Sjögren’s International Collaborative Clinical Alliance (SICAA) specimen repository (16, 17). Their clinical characteristics are summarized in Supplementary Table 1. All procedures involving patients were conducted in accordance with the Declaration of Helsinki principles.
Animals
LAMP3 overexpression was induced in submandibular glands of female 6–8-week-old C57BL/6 mice (Charles River Laboratories, USA) as described previously (4). Briefly, adeno-associated virus vectors encoding LAMP3 were delivered into both submandibular glands (1011 particles/mouse in 100 μL) by retrograde ductal instillation through a thin cannula.
LAMP3-overexpressing mice received weekly subcutaneous injections of dulaglutide (Trulicity®, Lilly, USA) in a vehicle of phosphate-buffered saline (PBS) at 60 mg/kg or placebo (PBS only) for 3 months after developing salivary gland dysfunction. Treatment groups were blindly allocated to each cage of mice, but no randomization was performed for the allocation.
Body weight and pilocarpine-stimulated salivary flow rate in 20 minutes were determined at several time points post-cannulation. Blood and whole submandibular gland tissues were collected at the end of the study. Serum was separated by centrifugation, and then was stored at -80°C. The tissue was fixed with 10% (v/v) neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm. Serum anti-Ro/SSA antibodies were tested by solid-phase enzyme-linked immunosorbent assays, according to the manufacturer’s instructions (#5710, Alpha Diagnostic International, USA). Serum total IgG levels were not tested in this study. All mice were analyzed without any criterion for inclusion and exclusion. Outcomes were evaluated by a blinded researcher. Confounders were not controlled for this study.
All procedures involving live animals were performed in accordance with institutional guidelines and standard operating procedures following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All the protocols were approved by the Ethics Committee of the National Institute of Dental and Craniofacial Research Veterinary Resources Core (approval number: 18–863).
Histology
Formalin-fixed paraffin embedded sections were deparaffinized, rehydrated and subjected to citric acid microwave antigen retrieval. Slides were blocked with 2% (w/v) bovine serum albumin (Sigma-Aldrich, USA) and permeabilized by 0.1% (v/v) Triton-X-100 (Sigma-Aldrich) for 30 minutes at 25℃. Slides were incubated with 10 μg/mL of primary antibodies overnight at 4℃ and then 10 μg/mL of secondary antibody for 1 hour at 25℃, followed by counterstaining with DAPI mounting medium (Abcam, USA). Mouse anti-galectin-3 (#ab2785, Abcam) and rabbit anti-ATP6V1A (ab199326) antibodies were purchased from Abcam; Rabbit anti-LAMP3 antibody (#12632–1-AP) was purchased from Proteintech (USA); Alexa Fluor-488 anti-rabbit IgG and Alexa Fluor-647 anti-mouse IgG antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (USA). Apoptotic cells were visualized using a terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay (#ab66110, Abcam), according to the manufacturer’s instructions. All images were acquired by a fluorescent microscope (Nikon, Japan) and analyzed using ImageJ software (in public domain source: National Institutes of Health, USA).
Histological characteristics of labial minor salivary gland biopsies were classified into the following four groups established by the SICCA: non-specific chronic sialadenitis (NSCS), focal lymphocytic sialadenitis (FLS), sclerosing chronic sialadenitis (SCS) and FLS/SCS (combination of FLS and SCS features) (17).
Cells and reagents
Mycoplasma-free A253 cells (ATCC, USA) were cultured in McCoy’5A Medium (Thermo-Fisher-Scientific, USA) supplemented with 10% fetal bovine serum (Thermo-Fisher-Scientific), and incubated at 37℃ with humidity in 5% CO2. Stably LAMP3-overexpressing A253 cells were established and propagated as described previously (5).
CASP8 (RC223744L4), pLenti-C-mGFP-P2A-Puro (PS100093), LAMP2A (SC118738), pCMV6-XL5 (PCMV6XL5) (all from Origene, USA) were purified using Endofree plasmid maxi kit (QIAGEN, USA) and transfected using Lipofectamine 3000 (Thermo-Fisher-Scientific). SiRNA for CASP8 (SR319563) and LAMP2 (SR320822) (both from Origene) were transfected using RNAi MAX (Thermo-Fisher-Scientific). Chloroquine, rapamycin, 3-methyladenine, and cycloheximide were purchased from Sigma-Aldrich. Liraglutide (Victoza®) was purchased from Novo Nordisk (Denmark).
Immunoprecipitation
A253 cells were washed three times in ice-cold PBS, lysed by incubation on ice for 40 minutes in 0.3% (v/v) Nonidet P-40 cell lysis buffer (Sigma-Aldrich) and then cleared by centrifugation at 14,000 g at 4℃ for 15 minutes (18). Supernatants (1300 μL) were incubated with 20 μL of Dynabeads Protein G for immunoprecipitation at 4℃ for 2 hours with gently shaking to eliminate protein which bind to the beads. The supernatants (50 μL) were then incubated with following antibodies-conjugated magnetic beads at 4℃ overnight with gently shaking: 4 μg anti-caspase-8 monoclonal antibody (#sc-81661; Santa cruz biotechnology, USA), or 4 μg anti-HSC70/HSP73 (HSPA8) monoclonal antibody (clone 1B5; Enzo Life Sciences, USA). After incubation, the beads were washed five times in the cell lysis buffer. The beads washed were heated for 10 min at 97℃ in 40 μL of NuPAGE LDS sample buffer (Thermo-Fisher-Scientific).
Western blot
Cells were washed in ice-cold PBS, lysed by RIPA buffer (Thermo-Fisher-Scientific) for 25 minutes on ice, and cleared by centrifugation at 17,000 g for 25 minutes at 4℃. Supernatants were heated for 10 min at 97℃ in NuPAGE LDS sample buffer. Total protein concentration in cell lysate was quantified using Bradford assay (Thermo-Fisher-Scientific).
Cell lysate samples containing equal amount of total protein were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes (Thermo-Fisher-Scientific). The membranes were blocked with 5% non-fat dried milk for 60 minutes at 25℃, and then incubated overnight at 4℃ with primary antibodies. After washing three times, the membranes were reacted with rabbit IgG HRP-linked whole antibody (Sigma-Aldrich) for 1 hour at 25℃. Signal was visualized using a Super Signal West Pico Chemiluminescent Substrate (Thermo-Fisher-Scientific) and quantified by densitometry using ImageJ software.
Anti-LC3 (#12741) and anti-caspase-8 (#4790) antibodies were purchased from Cell Signaling Technology (USA); Anti-LAMP1 (#21997– 1-AP) and anti-LAMP2 (#66301–1-Ig) antibodies were purchased from Proteintech; Anti-LAMP2A antibody (#ab125068) was purchased from Abcam; Anti-V-ATPaseV0a antibody (#sc-374475) was purchased from Santa Cruz biolotechnology; Anti- anti-HSC70/HSP73 (HSPA8) antibody (clone 1B5) was purchased from Enzo Life Sciences; Anti-α-tubulin antibody (#T6199) was purchased from Sigma-Aldrich.
Flow cytometry
Cells were replated at 3×105 cells per well in a 6-well plate for flow cytometry. Apoptotic cells were stained using the APC Annexin V Apoptosis Detection Kit with 7-AAD (#640930, BioLegend, USA) 48 hours after transfection with CASP8-GFP expression plasmid, 72 hours after treatment with CASP8 siRNA, and 18 hours after treatment with liraglutide. Acidic organelles were labelled by LysoTracker Deep Red (#L12492, Thermo-Fisher-Scientific) 18 hours after treatment with liraglutide. Signals were detected using the BD Accuri Flow Cytometer (BD Biosciences, USA), and obtained data were analyzed using FlowJo software (BD Bioscience).
Galectin-3 puncta assay
Cells were fixed with 4% (w/v) paraformaldehyde for 15 minutes, permeabilized using 0.1% (v/v) Triton-X-100 (Sigma-Aldrich) for 10 minutes, and blocked with 2% (w/v) bovine serum albumin for 30 minutes. Then, cells were incubated with a mixture of 10 μg/mL mouse anti-galectin-3 antibody (#ab2785, Abcam) at 4℃ overnight. The next day, cells were incubated with a mixture of 10 μg/mL Alexa Fluor-647 anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.) at 25℃ for 1 hour. Sections were subsequently counterstained with DAPI (#ab104139, Abcam). Images were acquired using a fluorescent microscope (Nikon, Japan) and analyzed using ImageJ software.
Statistical analysis
Quantitative variables were compared using a two-tailed Student’s t-test. When appropriate, multiple testing was corrected by Dunnett’s method. Categorical variables were compared using a chi square test. Relationship between two continuous variables were assessed by Pearson correlation coefficient. P values < 0.05 were considered statistically significant. All analyses were performed using GraphPad Prism 8.0 software (USA).
RESULTS
Epithelial LMP in salivary glands of SjD patients
Recent in vitro studies suggest LMP in salivary gland epithelial cells is associated with the pathophysiology of SjD (3–6). Galectin-3 puncta formation on the lysosomal membrane is associated with LMP and represents a convenient marker for examination of the state of lysosomes (19, 20). Immunofluorescent analysis of labial minor salivary glands showed that galectin-3 puncta was more frequently found in salivary gland epithelial cells of SjD patients (n = 10) compared to those of control non-SjD sicca subjects (n = 5) (23.8 ± 4.5% vs 2.6 ± 0.7%, p < 0.01, Figure 1). The finding extends the in vitro observations and supports the occurrence of LMP in salivary gland epithelial cells of SjD patients in vivo.
Figure 1. Epithelial LMP in salivary glands of SjD patients.
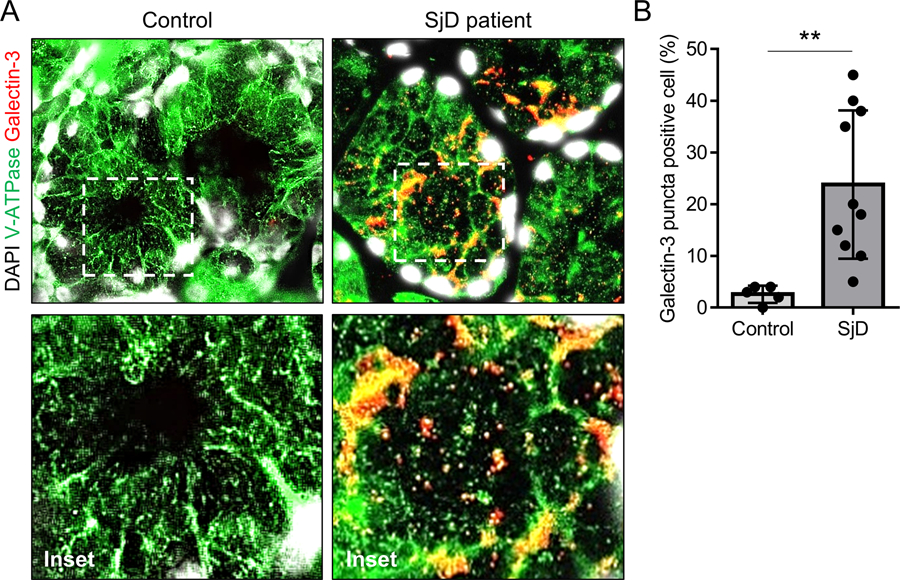
(A) Representative immunofluorescent images for V-ATPase (green) and galectin-3 (red) staining on labial minor salivary gland sections from SjD patients (n = 10) or control non-SjD sicca subjects (n = 5). Original magnification: 40x (inset: 100x). (B) Bar chart showing mean (± SD) percentage of galctin-3 puncta-positive cells. **P < 0.01, t-test.
LMP is correlated with LAMP3 expression in salivary glands of SjD patients
To further validate LMP in salivary gland epithelial cells of SjD patients, we analyzed 80 additional labial minor salivary glands from an independent cohort of SjD patients. Following staining, the samples were graded for LMP based on the proportion of galectin-3 puncta-positive cells (1: Normal < 10%, 2: Mild ≤ 30%, 3: Moderate ≤ 50%, 4: Severe > 50%) (Figure 2A) and for LAMP3 expression according to its mean fluorescent intensity (1: Low < 8000, 2: Middle ≤ 10000, 3: High ≤ 12000, 4: Very high > 12000) (Figure 2B). Among the 80 patients, 47 (59%) had moderate to severe LMP (grade ≥ 3) and 46 (58%) had high to very high LAMP3 expression (grade ≥ 3) in their salivary glands. The LMP grades had a significantly positive correlation with the LAMP3 expression grades (R = 0.65, P < 0.001, Figure 2C), supporting the previous in vitro finding that LAMP3 overexpression induced LMP in salivary gland epithelial cells (5).
Figure 2. LMP is correlated with LAMP3 expression in salivary glands of SjD patients.
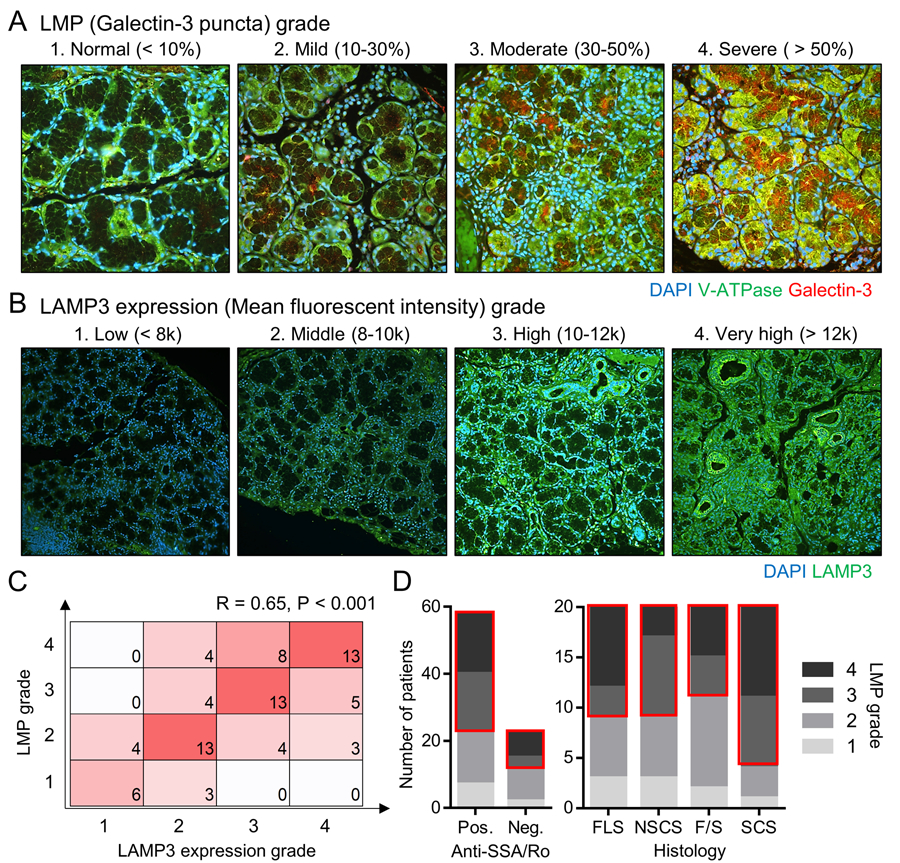
(A, B) Labial minor salivary gland sections from SjD patients (n = 80) were stained for (A) V-ATPase (green) and galectin-3 (red) (original magnification: 40x) or for (B) LAMP3 (green) (original magnification: 20x). Representative immunofluorescent image in each LMP and LAMP3 expression grade are shown. (C) Matrix shows the number of patients having indicated combination of LMP and LAMP3 expression grades. (D) Bar charts show the number of patients having indicated LMP grade based on positivity of serum anti-SSA/Ro antibodies or salivary gland histology. FLS, focal lymphocytic sialadenitis; NSCS, non-specific chronic sialadenitis; SCS, sclerosing chronic sialadenitis; F/S, combination of FLS and SCS features.
To clarify whether epithelial LMP is involved in the development and/or progression of the disease, we assessed an association between LMP grades and clinical features of SjD. Grade ≥ 3 of LMP was similarly found in both serum anti-Ro/SSA antibody-positive and antibody-negative patients (36/58 (62%) vs 11/22 (50%), P = 0.33, Figure 2D). The other serum markers, such as IgG levels (R = -0.11, P = 0.32) and C3 levels (R = 0, P = 0.99), had no significant correlations with LMP grades. On the other hand, LMP grades was associated with histological characteristics of salivary glands. Grade ≥ 3 of LMP was significantly more frequent in SCS glands compared to non-SCS glands (16/20 (80%) vs 31/60 (52%), P = 0.026, Figure 2D). SCS is characterized by interstitial fibrosis and acinar atrophy associated with severer salivary gland hypofunction (17). Taken together, these findings suggested LAMP3 overexpression could be involved in the development and progression of salivary gland hypofunction by causing epithelial LMP.
LAMP3-induced LMP and lysosome-dependent cell death are associated with increased caspase-8 expression
The above findings from patients’ salivary glands confirmed that LAMP3-induced LMP and subsequent lysosome-dependent cell death are an important pathophysiology of SjD. Next, we tested the molecular mechanisms associated with LAMP3-induced LMP in salivary gland epithelial cells using A253, a salivary ductal cell line.
Since uncleaved (full length) caspase-8 is known as an initiator of LMP by binding to V-ATPase on lysosomal membrane and blocking its function independent of the catalytic activity of the cleaved form (21), we compared uncleaved caspase-8 expression between LAMP3-overexpressing and control A253 cells. Analysis of Western blots showed that caspase-8 protein expression was increased in LAMP3-overexpressing A253 cells compared to control A253 cells (Figure 3A). To test an effect of increased caspase-8 expression on LMP and cell death, caspase-8 was overexpressed in A253 cells and knocked down in LAMP3-overexpressing A253 cells. Caspase-8 overexpression significantly increased the proportion of apoptotic cells (Figure 3B) and galectin-3 puncta-positive cells (Figure 3C). Knockdown of caspase-8 expression in LAMP3-overexpressing A253 cells using CASP8 siRNA significantly decreased the proportion of apoptotic cells in LAMP3-overexpressing A253 cells compared to treatment with negative control siRNA (Figure 3D and 3E). Galectin-3 puncta-positive cells were also decreased by caspase-8 knockdown (Figure 3F). These results demonstrate that LAMP3-induced LMP and lysosome-dependent cell death is associated with increased uncleaved caspase-8 expression.
Figure 3. LAMP3-induced LMP and lysosome-dependent cell death are associated with increased caspase-8 expression.
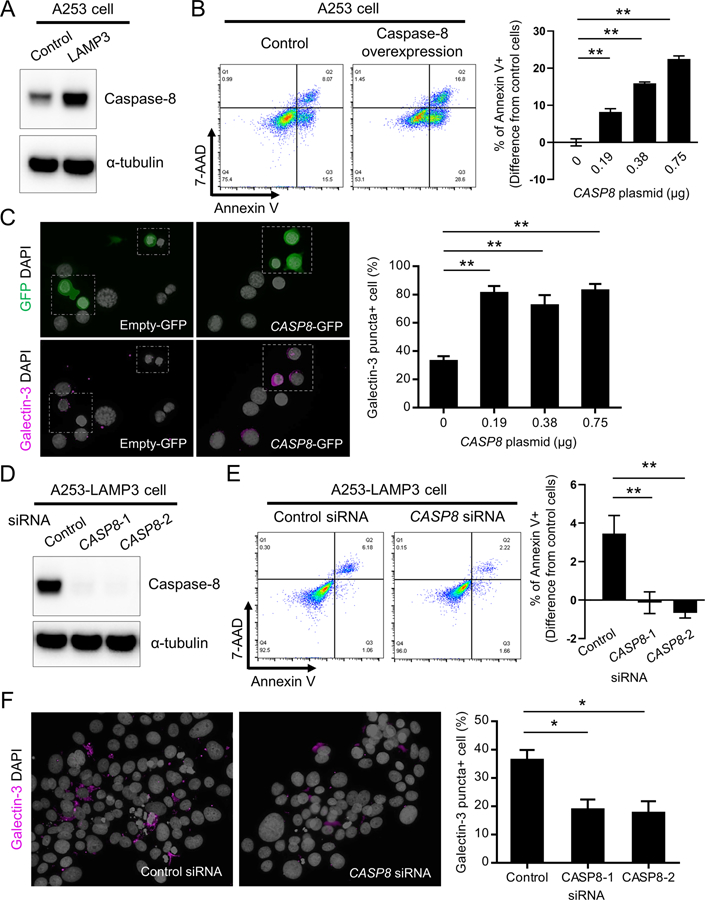
(A) Western blots showing caspase-8 and α-tubulin (internal control) expressions in control and LAMP3-overexpressing A253 cells. (B, C) A253 cells were transfected with CASP8-GFP and/or empty-GFP expression plasmids. (B) Flow cytometry using APC-annexin V and 7-AAD 48 hours after transfection, and bar chart showing the difference in the rate of annexin V-positive cells from that in control (n = 5). (C) Immunofluorescent images for GFP (green) or galectin-3 (magenta) staining, and bar chart showing percentage of galectin-3 puncta-positive cells (n = 5). (D-F) LAMP3-overexpressing A253 cells were treated with negative control or two types of CASP8 siRNA for 72 hours. (D) Western blots showing caspase-8 and α-tubulin expressions. (E) Flow cytometry using APC-annexin V and 7-AAD, and bar chart showing the difference in the rate of annexin V-positive cells from that in control A253 cells (n = 3). (F) Immunofluorescent images for galectin-3 (magenta) staining, and bar chart showing percentage of galectin-3 puncta-positive cells (n = 3). Original magnification: 40x. Values shown are mean ± SD. *p < 0.05, **p < 0.01, t-test with Dunnett’s correction.
Caspase-8 is degraded through the autophagy-lysosomal pathways
The above data suggested that increased caspase-8 expression could be associated with the change of lysosomal function. One hypothesis is that caspase-8 is normally degraded via the autophagy-lysosomal pathways and inhibition of the pathways by LAMP3 increases the intracellular pool of caspase-8. Treatment of A253 cells with chloroquine—an inhibitor of the autophagy-lysosomal pathways—significantly increased caspase-8 expression, while treatment with rapamycin—an activator of the autophagy-lysosomal pathways—significantly decreased caspase-8 expression (Figure 4A and Supplementary Figure 1). This finding supports the hypothesis that caspase-8 is degraded through the autophagy-lysosomal pathways.
Figure 4. Caspase-8 is degraded through chaperone-mediated autophagy.
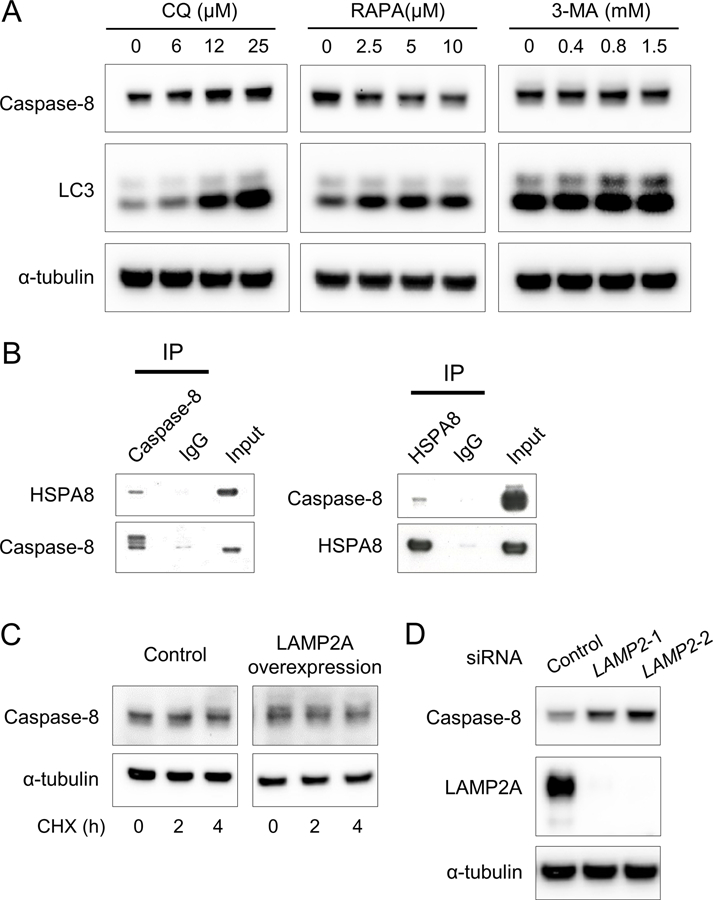
(A) Western blots showing caspase-8, LC3, and α-tubulin (internal control) expressions in A253 cells treated with chloroquine (CQ), rapamycin (RAPA), or 3-methyladenine (3-MA) at indicated concentration for 24 hours. (B) Western blots showing HSPA8 and caspase-8 expressions immunoprecipitated from A253 cell lysates using anti-caspase-8 antibodies or anti- HSPA8 antibodies, respectively (C) Western blots showing caspase-8 and α-tubulin expressions in A253 cells treated with cycloheximide (CHX) at 50 μg/mL for the indicated hours 48 hours after transfection with LAMP2A expression plasmids. (D) Western blots showing caspase-8, LAMP2A, and α-tubulin expressions in A253 cells treated with negative control or two types of LAMP2 siRNA for 48 hours.
We previously showed that LAMP3 overexpression inhibits macroautophagy (5), but treatment with 3-methyladenine—a macroautophagy inhibitor—did not affect caspase-8 expression (Figure 4A and Supplementary Figure 1).
Chaperone-mediated autophagy (CMA) is another type of selective protein degradation in lysosomes mediated by a chaperone, heat shock 70 kDa protein 8 (HSPA8) and LAMP type 2A (LAMP2A). Specifically, proteins with a peptide motif recognized and bound by HSPA8 in the cytosol are taken into lysosomes via LAMP2A (22–24). Immunoprecipitation showed binding between caspase-8 and HSPA8 (Figure 4B), indicating that caspase-8 can be recognized by HSPA8 as a substrate for CMA. Overexpression of LAMP2A significantly decreased caspase-8 expression following treatment with cycloheximide—a protein synthesis inhibitor (Figure 4C and Supplementary Figure 2), and knockdown of LAMP2A significantly increased caspase-8 expression (Figure 4D and Supplementary Figure 2). These results suggested that caspase-8 is degraded through CMA.
Restoration of lysosomal function prevents LAMP3-induced LMP and lysosome-dependent cell death via decrease in caspase-8 expression in vitro
The above data suggested that LAMP3 could increase caspase-8 expression via impairment of autophagic degradation, resulting in LMP and lysosome-dependent cell death. A novel therapeutic approach would be to restore autophagic flux by either stabilizing existing lysosomes or promoting the biogenesis of new lysosomes. Glucagon-like peptidase-1 receptor (GLP-1R) agonists are an anti-diabetic drug to promote insulin secretion from pancreatic β cells (25). Interestingly, it has been reported that GLP-1R agonists promote lysosomal biogenesis and restore lysosomal function by activating transcription factor EB (TFEB), a master regulator of lysosomes (26, 27). GLP-1Rs are expressed on many cell types including salivary gland epithelial cells (28, 29). To test whether restoration of lysosomal function and autophagic flux can decrease caspase-8 expression, LMP, and cell death, we treated LAMP3-overexpressing A253 cells with liraglutide, a GLP-1R agonist.
Consistent with these previous reports, flow cytometry analysis using Lysotracker for labeling acidic organelles showed that treatment with liraglutide significantly increased the number of functional lysosomes in LAMP3-overexpressing A253 cells (Figure 5A). Along with the restoration of lysosomal function, treatment with liraglutide significantly decreased caspase-8 expression in LAMP3-overexpressing A253 cells (Figure 5B), which reduced the proportion of galectin-3 puncta-positive cells (Figure 5C) and apoptotic cells (Figure 5D). Taken together, these results demonstrated that LAMP3 overexpression induced caspase-8-associated lysosome-dependent cell death by impairing autophagic flux, and that restoration of lysosomal function by GLP-1R agonists could reverse the process.
Figure 5. Restoration of lysosomal function prevents LAMP3-induced LMP and lysosome-dependent cell death via decrease in caspase-8 expression in vitro.
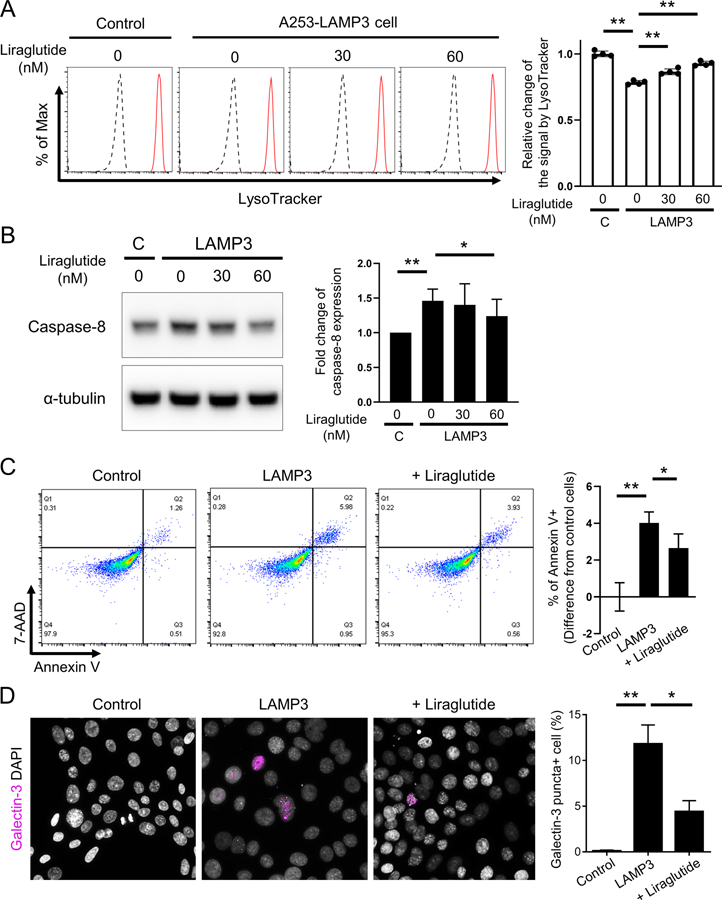
Control or LAMP3-overexpressing A253 cells were treated with liraglutide, an inducer of lysosomal biogenesis, at indicated concentration or vehicle control for 18 hours. (A) Flow cytometry using Lysotracker Deep Red (Black line: without Lysotracker; Red line: with Lysotracker) and bar chart showing mean fluorescent intensity. (B) Western blots showing caspase-8 and α-tubulin (internal control) expressions and bar chart showing relative change of caspase-8 expression normalized by α-tubulin expression. (C, D) Control or LAMP3-overexpressing A253 cells were treated with liraglutide at 60 nM or vehicle control for 18 hours. (C) Immunofluorescent images for galectin-3 (magenta) staining and bar chart showing percentage of galectin-3 puncta-positive cells. Original magnification: 40x. (D) Flow cytometry using APC-annexin V and 7-AAD and bar chart showing the difference in the rate of annexin V-positive cells from that in control cells. Values shown are mean ± SD (n = 4 for all experiments). *p < 0.05, **p < 0.01, t-test with Dunnett’s correction.
Restoration of lysosomal function prevents LAMP3-induced LMP and lysosome-dependent cell death in murine salivary glands
To confirm that restoration of lysosomal function can inhibit LAMP3-induced LMP and cell death in vivo, we treated LAMP3-overexpressing mice (an SjD mouse model that progressively develops lymphocytic infiltrates, salivary gland hypofunction, and serum anti-SSA/Ro antibodies (4)) with weekly subcutaneous injections of dulaglutide, a long-acting type of GLP-1R agonists.
Histological analysis on murine submandibular glands showed that treatment with dulaglutide significantly decreased the number of galectin-3 puncta-positive cells (Figure 6A) and apoptotic cells (Figure 6B), and size of lymphocyte infiltration area (Figure 6C) compared to placebo treatment. Recovery of salivary flow rate (Figure 6D) and decrease in serum anti-SSA/Ro antibody levels (Figure 6E) by dulaglutide treatment were not found during the observational period. Overall, these findings confirmed that restoration of lysosomal function could prevent LAMP3-induced LMP and lysosome-dependent cell death in vivo.
Figure 6. Restoration of lysosomal function prevents LAMP3-induced LMP and lysosome-dependent cell death in murine salivary glands.
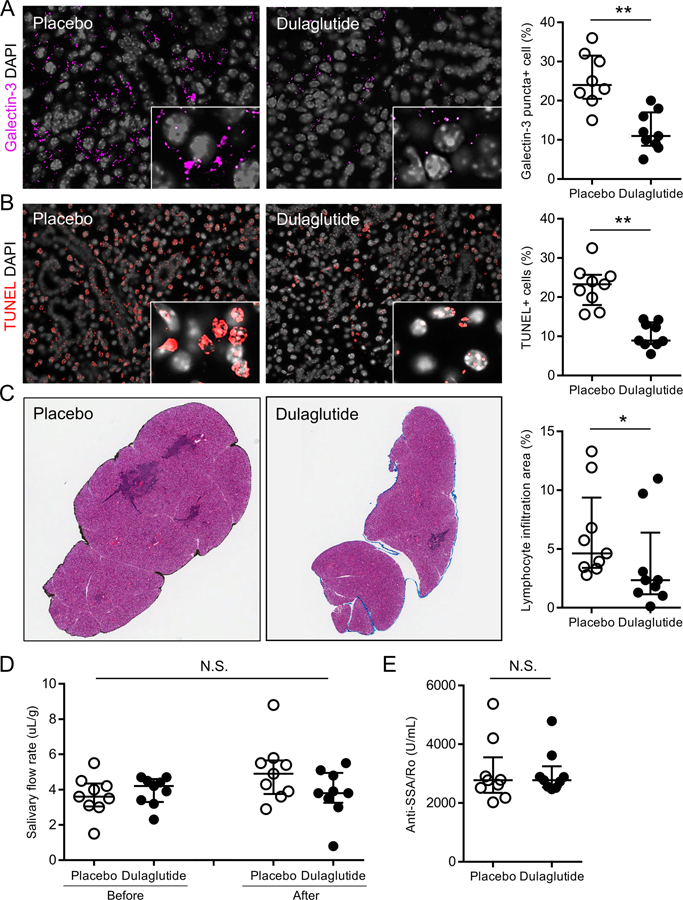
LAMP3-overexpressing mice received weekly subcutaneous injections of dulaglutide, an inducer of lysosomal biogenesis, at 0.6 mg/kg or placebo for 3 months after developing an SjD-like phenotype. (A) Immunofluorescent images of murine submandibular glands for galectin-3 (magenta) staining and bar chart showing the percentage of galectin-3 puncta-positive cells. (B) Immunofluorescent images of submandibular glands with terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL, red) and bar chart showing the percentage of TUNEL-positive apoptotic cells. (C) Hematoxylin and eosin staining of glands and the percentage of lymphocytic infiltration area in whole glands. (D) Pilocarpine-stimulated salivary flow per body weight in 20 minutes before and after treatment. (E) Serum anti-Ro/SS-A antibody levels. Original magnification: 40x (inset: 100x) for (A) and (B), and 4x for (C). Values shown are median and quartile (n = 9, each group). *p < 0.05, **p < 0.01, Wilcoxon test.
DISCUSSION
SjD is referred to as an epithelitis in salivary glands and the exact etiology is unknown (30). Salivary gland hypofunction of SjD is thought to be associated with increased epithelial apoptosis as a result of primary epithelitis and/or secondary damage by autoimmune response (16, 31, 32). Previous studies showed that LAMP3 overexpression induces LMP and lysosome-dependent cell death in salivary gland epithelial cells (3–5). In the present study, we have extended the finding and demonstrated that LAMP3 overexpression could impair autophagic flux, resulting in an increase in caspase-8 expression, which plays a pivotal role in LAMP3-induced LMP and lysosome-dependent cell death.
We previously reported LAMP3-induced cell death is associated with caspase-3 activity and that caspase-3 expression is decreased by LAMP3 overexpression (3, 5), in contrast to the increase in caspase-8 expression. In the current study, we found that caspase-8 accumulation is the first step to initiate LMP associated with LAMP3 expression. Caspase-3 is then cleaved in cytoplasm by leaked lysosomal proteases following LMP to activate the apoptotic pathway (3, 5).
Our in vitro experiments using chloroquine, rapamycin, and 3-methyladenine suggested that caspase-8 is degraded via the autophagy-lysosomal pathways. Because these compounds also target other cellular pathways (33), we further studied the effect of LAMP2A overexpression/knockdown and GLP-1R agonist treatment (as a promoter of lysosomal biogenesis) on caspase-8 expression. All the results consistently indicated autophagic capase-8 degradation. Furthermore, binding ability of capase-8 to HSPA8, involvement of LAMP2A in caspase-8 expression, and negative result of 3-methyladenine treatment suggested CMA rather than macroautophagy as a main pathway of capase-8 degradation.
Our studies on human salivary glands showed that galectin-3 puncta formation (indicating LMP) was found in SjD patients’ salivary glands positively correlated with LAMP3 expression levels, indicating that some epithelial abnormalities originate from lysosomal dysfunction in SjD. The finding implies that evaluation of lysosomal function on salivary gland specimens including galectin-3 puncta assay may be useful as an additional diagnostic tool for SjD, and that lysosome-targeted approach may be promising for SjD-associated salivary gland hypofunction.
Although various immunosuppressants have been tried for treating SjD, collectively immunosuppressive therapies have not shown efficacy with respect to SjD-associated salivary gland hypofunction (34, 35). Thus, a more direct approach to the salivary epithelium is needed to reverse salivary gland hypofunction in SjD. Both our in vitro and in vivo studies showed that restoration of lysosomal function in salivary epithelium using GLP-1R agonists could prevent LAMP3-induced LMP and lysosome-dependent cell death. Unlike salivation stimulators, such as pilocarpine and cevimeline, which are palliative and can provide relief from dry mouth symptoms in SjD patients (35–37), the present study suggested a possibility that GLP-1R agonists could modify the disease course by correcting lysosomal function in salivary gland epithelial cells. Treatment with a GLP-1R agonist improved tissue damage assessed by lymphocytic infiltration in murine salivary glands, consistent with the finding from human salivary glands that LMP was associated with histological characteristics as a result of tissue damage.
GLP-1Rs are expressed in various cells including salivary gland epithelial cells, but are only expressed at low levels in immune cells (29) and no immune-related adverse effects have been reported since GLP-1RAs started to be used as an anti-diabetic drug (38, 39). Indeed, opportunistic infection resulting from the use of immunosuppressants is a significant issue in clinical practice of rheumatic diseases including SjD (40). Because salivary gland hypofunction does not affect mortality of the patients, aggressive immunosuppressive therapy with the increased risk of life-threatening opportunistic infections is not desirable for treating salivary gland hypofunction in term of benefit-risk balance. Thus, GLP-1R agonists may represent a lower risk alternative.
As a limitation of this study, we could not find a significant recovery of salivary flow rate in LAMP3-overexpressing mice after GLP-1R agonist treatment, in spite of decreased epithelial apoptosis. It is possible that more time is required for the recovery of salivary flow rate through salivary gland tissue regeneration because the treatment started after the mice developed overt salivary gland hypofunction. Many forms of GLP-1R agonists are available with different biodistributions in vivo (41). Dulaglutide is a fusion protein that links the GLP-1 peptide to a human immunoglobulin class 4 constant fragment to reduce the renal clearance rate of the drug, and the amino acid sequence is also modified to promote stability (42). Experimentation with other more native forms of the peptide may improve salivary gland function.
Although over 80 SjD patient samples were studied for galectin-3 puncta formation, only a small number of control non-SjD sicca subject samples were studied. Further studies are required to assess diagnostic sensitivity and specificity of epithelial LMP in SjD, including samples from patients with hepatitis C virus (43) or IgG4-related disease (44), that are mimicking condition to be excluded (15).
In conclusion, we found that LAMP3 overexpression induced lysosomal dysfunction and impaired autophagic flux, resulting in increased caspase-8 expression, LMP, and lysosome-dependent cell death. Moreover, we showed that restoration of lysosomal function using GLP-1R agonists could prevent LAMP3-induced LMP and cell death both in vitro and in vivo. These findings suggest LAMP3 overexpression and subsequent lysosomal dysfunction as an essential pathophysiology of SjD-associated salivary gland hypofunction and a target for therapeutic intervention.
Supplementary Material
Acknowledgement:
Assistance with this project was provided by the NIDCR Imaging Core (ZIC DE000750-01) and the NIDCR Veterinary Resources Core (ZIC DE000740-05). This study was also supported in part by the following grants: Takeda Science Foundation Research Fellowship to HN and JSPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at the NIH to TT.
Funding:
NIH/NIDCR Division of Intramural Research award 1ZIADE000695 (JAC).
Financial support:
NIH/NIDCR: 1ZIADE000695 (to JAC).
Footnotes
Competing interests: Authors declare that they have no competing interests.
REFERENCES
- 1.Odani T, Chiorini JA. Targeting primary Sjögren’s syndrome. Mod Rheumatol 2019;29:70–86. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi M, Hirata N, Tanaka T, Suizu F, Nakajima H, Chiorini JA. Autophagy as a modulator of cell death machinery. Cell Death Dis 2020;11:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka T, Warner BM, Odani T, Ji Y, Mo YQ, Nakamura H, et al. LAMP3 induces apoptosis and autoantigen release in Sjögren’s syndrome patients. Sci Rep 2020;10:15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura H, Tanaka T, Pranzatelli T, Ji Y, Yin H, Perez P, et al. Lysosome-associated membrane protein 3 misexpression in salivary glands induces a Sjögren’s syndrome-like phenotype in mice. Ann Rheum Dis 2021;80:1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka T, Warner BM, Michael DG, Nakamura H, Odani T, Yin H, et al. LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy 2022;18:1629–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mo YQ, Nakamura H, Tanaka T, Odani T, Perez P, Ji Y, et al. Lysosomal exocytosis of HSP70 stimulates monocytic BMP6 expression in Sjögren’s syndrome. J Clin Invest 2022;132:e152780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colafrancesco S, Barbati C, Priori R. Maladaptive Autophagy in the Pathogenesis of Autoimmune Epithelitis in Sjögren’s Syndrome. Arthritis Rheumatol 2022;74:654–64. [DOI] [PubMed] [Google Scholar]
- 8.Barrera MJ, Aguilera S, Castro I, Matus S, Carvajal P, Molina C, et al. Tofacitinib counteracts IL-6 overexpression induced by deficient autophagy: implications in Sjögren’s syndrome. Rheumatology (Oxford) 2021;60:1951–62. [DOI] [PubMed] [Google Scholar]
- 9.Colafrancesco S, Vomero M, Iannizzotto V, Minniti A, Barbati C, Arienzo F, et al. Autophagy occurs in lymphocytes infiltrating Sjögren’s syndrome minor salivary glands and correlates with histological severity of salivary gland lesions. Arthritis Res Ther 2020;22(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voynova E, Lefebvre F, Qadri A, Muller S. Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren’s syndrome. J Autoimmun 2020;108:102418. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Wang F, Schall N, Muller S. Rescue of autophagy and lysosome defects in salivary glands of MRL/lpr mice by a therapeutic phosphopeptide. J Autoimmun 2018;90:132–45. [DOI] [PubMed] [Google Scholar]
- 12.Alessandri C, Ciccia F, Priori R, Astorri E, Guggino G, Alessandro R, et al. CD4 T lymphocyte autophagy is upregulated in the salivary glands of primary Sjögren’s syndrome patients and correlates with focus score and disease activity. Arthritis Res Ther 2017;19:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alessandrini F, Pezzè L, Ciribilli Y. LAMPs: Shedding light on cancer biology. Semin Oncol 2017;44:239–53. [DOI] [PubMed] [Google Scholar]
- 14.Cai Y, Chen MX, Deng YJ, Liu LL, Lin XP, Lu PF, et al. Clinical and Pathological Implications of Increases in Tonsillar CD19(+)CD5(+) B Cells, CD208(+) Dendritic Cells, and IgA1-positive Cells of Immunoglobulin A Nephropathy. Curr Med Sci 2022;42:93–9. [DOI] [PubMed] [Google Scholar]
- 15.Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis 2017;76:9–16. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Kalra L, Lai Z, Guimaro MC, Aber L, Warner BM. Inhibition of bone morphogenetic protein 6 receptors ameliorates Sjögren’s syndrome in mice. Sci Rep 2020;10:2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin H, Pranzatelli TJF, French BN, Zhang N, Warner BM, Chiorini JA. Sclerosing Sialadenitis Is Associated With Salivary Gland Hypofunction and a Unique Gene Expression Profile in Sjögren’s Syndrome. Front Immunol 2021;12:699722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capone S, Raggioli A, Gentile M, Battella S, Lahm A, Sommella A, et al. Immunogenicity of a new gorilla adenovirus vaccine candidate for COVID-19. Mol Ther 2021;29:2412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aits S, Kricker J, Liu B, Ellegaard AM, Hämälistö S, Tvingsholm S, et al. Sensitive detection of lysosomal membrane permeabilization by lysosomal galectin puncta assay. Autophagy 2015;11:1408–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aits S Methods to Detect Loss of Lysosomal Membrane Integrity. Methods Mol Biol 2019;1880:315–29. [DOI] [PubMed] [Google Scholar]
- 21.Zhong B, Liu M, Bai C, Ruan Y, Wang Y, Qiu L, et al. Caspase-8 Induces Lysosome-Associated Cell Death in Cancer Cells. Mol Ther 2020;28:1078–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 1989;246:382–5. [DOI] [PubMed] [Google Scholar]
- 23.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science 1996;273:501–3. [DOI] [PubMed] [Google Scholar]
- 24.Saha T LAMP2A overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy 2012;8:1643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura H, Fujieda Y. How should rheumatologists manage glucocorticoid-induced hyperglycemia? Mod Rheumatol 2021;31:519–28. [DOI] [PubMed] [Google Scholar]
- 26.Fang Y, Ji L, Zhu C, Xiao Y, Zhang J, Lu J, et al. Liraglutide Alleviates Hepatic Steatosis by Activating the TFEB-Regulated Autophagy-Lysosomal Pathway. Front Cell Dev Biol 2020;8:602574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zummo FP, Cullen KS, Honkanen-Scott M, Shaw JAM, Lovat PE, Arden C. Glucagon-Like Peptide 1 Protects Pancreatic β-Cells From Death by Increasing Autophagic Flux and Restoring Lysosomal Function. Diabetes 2017;66:1272–85. [DOI] [PubMed] [Google Scholar]
- 28.Ono R [GLP-1 receptor expression in rat major salivary glands and the effects of bilateral maxillary molar extraction on its expression]. Kokubyo Gakkai Zasshi 2015;81(3)-82(1):8–14. [PubMed] [Google Scholar]
- 29.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science 2015;347:1260419. [DOI] [PubMed] [Google Scholar]
- 30.Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. Semin Arthritis Rheum 2003;33:49–65. [DOI] [PubMed] [Google Scholar]
- 31.Yin H, Cabrera-Perez J, Lai Z, Michael D, Weller M, Swaim WD, et al. Association of bone morphogenetic protein 6 with exocrine gland dysfunction in patients with Sjögren’s syndrome and in mice. Arthritis Rheum 2013;65:3228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai Z, Yin H, Cabrera-Pérez J, Guimaro MC, Afione S, Michael DG, et al. Aquaporin gene therapy corrects Sjögren’s syndrome phenotype in mice. Proc Natl Acad Sci U S A 2016;113:5694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 2021;17:1–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos-Casals M, Brito-Zerón P, Bombardieri S, Bootsma H, De Vita S, Dörner T. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 2020;79:3–18. [DOI] [PubMed] [Google Scholar]
- 35.Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA 2010;304:452–60. [DOI] [PubMed] [Google Scholar]
- 36.Yamada H, Nakagawa Y, Wakamatsu E, Sumida T, Yamachika S, Nomura Y, et al. Efficacy prediction of cevimeline in patients with Sjögren’s syndrome. Clin Rheumatol 2007;26:1320–7. [DOI] [PubMed] [Google Scholar]
- 37.Noaiseh G, Baker JF, Vivino FB. Comparison of the discontinuation rates and side-effect profiles of pilocarpine and cevimeline for xerostomia in primary Sjögren’s syndrome. Clin Exp Rheumatol 2014;32:575–7. [PubMed] [Google Scholar]
- 38.Laurindo LF, Barbalho SM. GLP-1a: Going beyond Traditional Use. Int J Mol Sci 2022;23:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Yao D, Xia J. Efficacy and safety of dulaglutide compared with glargine in patients with type 2 diabetes: A systematic review and meta-analysis. J Clin Pharm Ther 2021;46:1245–53. [DOI] [PubMed] [Google Scholar]
- 40.Singh JA, Cleveland JD. Serious infections in Sjögren’s syndrome patients: a national U.S. study. Clin Exp Rheumatol 2020;38 Suppl 126:47–52. [PubMed] [Google Scholar]
- 41.Salameh TS, Rhea EM, Talbot K, Banks WA. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem Pharmacol 2020;180:114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson AM, Trujillo JM. Dulaglutide: the newest GLP-1 receptor agonist for the management of type 2 diabetes. Ann Pharmacother 2015;49:351–9. [DOI] [PubMed] [Google Scholar]
- 43.Maldonado JO, Beach ME, Wang Y. HCV Infection Alters Salivary Gland Histology and Saliva Composition. J Dent Res 2022;101:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto M, Takahashi H, Shinomura Y. Mechanisms and assessment of IgG4-related disease: lessons for the rheumatologist. Nat Rev Rheumatol 2014;10:148–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


