Canine leptospirosis and One Health
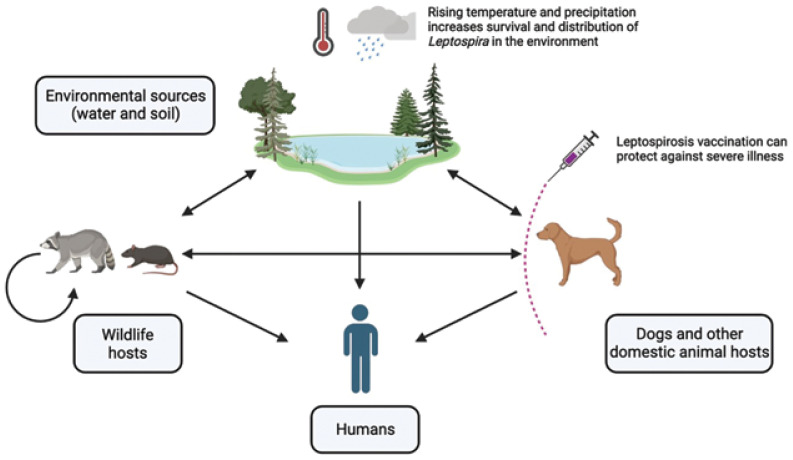
Leptospirosis is a global zoonotic disease that causes morbidity and mortality in dogs, humans, and other mammals (1). This disease is caused by pathogenic bacteria of the genus Leptospira, which are excreted in the urine of infected animals. Direct transmission occurs through contact with infected animal urine, whereas indirect transmission occurs through contact with urine-contaminated water, soil, or food (Figure 1) (1). Over 300 serovars exist, ranging in pathogenicity and host species (2).
Figure 1.
The main routes of transmission of Leptospira among wildlife, domestic animals, humans, and the environment (arrows). Leptospires are excreted through the urine of infected animals and can directly or indirectly infect other animals or cause environmental contamination. Increased temperature and precipitation can improve Leptospira survival and increase distribution in the environment. Vaccines are available for dogs, which may protect against illness and further transmission.
Image created using BioRender.com
Dogs are traditionally regarded as the maintenance host for Leptospira interrogans serovar Canicola, but are susceptible to infection from, or can be a carrier for, other serovars (3). Disease may present with a wide range of clinical signs and can be fatal (2). Accurate diagnosis of canine leptospirosis can be difficult, as dogs often present with non-specific clinical signs such as fever, lethargy, vomiting, and diarrhea (2,4). In addition, diagnostic testing has many limitations, including limited availability of resources and expertise in developing regions, varying sensitivities of testing methods, cross-reactivity in serological testing to vaccine-induced antibodies, and timeliness of diagnosis (2,4,5). In humans, leptospirosis is considered a neglected zoonotic disease due to these and other challenges (5).
Many factors are suspected in emergence and reemergence of leptospirosis in humans, including climate change, urbanization, land-use changes, and increased interactions among humans, wildlife, and domestic animals (6,7). However, changes in the epidemiology of canine leptospirosis due to anthropogenic and climatic influences are poorly understood (8). Due to multifaceted interactions among humans, animals, and the environment, effective surveillance and control of canine leptospirosis demands a One Health approach to reduce risk and improve health outcomes for dogs.
Global seroprevalence of canine leptospirosis was recently estimated at 18.5% (9). However, the true prevalence is likely higher as estimates are primarily based on studies using owned dogs, excluding the burden in unowned dogs where animal access and data are limited. Of the few studies that have examined unowned populations, stray and shelter dog populations have an estimated 27.6% average prevalence of leptospirosis globally (10). Stray dogs also have a higher Leptospira prevalence when compared to owned dog populations (11), possibly due to a lack of veterinary care, increased environmental exposures, etc. (10).
Studying canine leptospirosis using a One Health approach presents a unique opportunity to improve understanding of leptospirosis in animals and humans, surpassing its singular impact on canine health (12). Dogs have a distinct role in the transmission cycle of Leptospira, acting as both a host and a vector, due to their frequent interactions with humans, other animals, and the natural environment (13). Earlier work using a One Health approach to study canine leptospirosis demonstrated the potential for dogs to act as sentinels for leptospirosis and to detect early risk of disease in humans (14). Additional opportunities exist to assess risk factors and prevention strategies for canine leptospirosis within a One Health research context, such as completing environmental risk assessments, improving vaccine effectiveness, and evaluating social and behavioural determinants of canine leptospirosis. Increased communication among veterinary practitioners, diagnostic laboratories, veterinary epidemiologists, and other stakeholders invested in canine health is essential to identify areas where collaborative action can reduce the risk of leptospirosis in dogs. In this article, we highlight some opportunities for applying a One Health approach to inform effective surveillance, prevention, and control of canine leptospirosis.
Opportunities for a One Health approach
Environmental modelling and surveillance
The environment is critical to transmission and survival of Leptospira. Climatic factors are known to influence Leptospira transmission, with higher temperature and precipitation promoting bacterial survival in soil and water environments (1,6), contributing to greater burden in tropical and subtropical regions (15). Canine leptospirosis has a higher occurrence in low-income countries within Latin America and South Asia (9), and 73% of human cases occur in tropical regions (15). Recently, increasing occurrence of canine leptospirosis has been documented in nontropical areas, including Canada and Europe (16,17), and there is evidence for local weather events triggering human leptospirosis outbreaks across Europe (18).
Given increasing global temperatures and frequency and intensity of precipitation events (19), the prevalence of canine and human leptospirosis is expected to increase (6,7). Because leptospirosis is a climate-sensitive disease (6), it is necessary to predict how changing climates will influence canine disease to inform timely and effective prevention and control strategies. Epidemiological modelling is 1 tool to predict these effects on disease occurrence. Predictions for human leptospirosis in Indonesia using maximum entropy modelling showed increased risk and distribution of disease across the country with changes in climate (20). Vector-borne diseases among domestic dogs have also been forecasted using Bayesian spatiotemporal models (21,22), but none have examined canine leptospirosis specifically. Epidemiological forecasting of canine leptospirosis, facilitated through a One Health perspective, may provide important insights for both canine and human leptospirosis.
Further understanding of specific ecological and climatic influences of canine leptospirosis may be obtained by conducting environmental Leptospira surveillance. Exposure to contaminated water is a primary route of infection for dogs, humans, and other mammals (1). In controlled environments, Leptospira bacteria have maintained pathogenicity in water for at least 20 mo, even in unfavorable conditions (23). Currently, no standardized surveillance methods exist to monitor the presence and distribution of Leptospira in the environment. Conducting surveillance for the presence of Leptospira in water or soil samples would help identify high-risk areas and ascertain which serovars exist in the local environment. Complimentary surveillance done on wildlife would also identify local reservoir species and risks. For example, testing 8 wildlife species in Ontario revealed the highest Leptospira positivity among skunks and raccoons (24). Rats have also been identified as a reservoir species in British Columbia (25). Altogether, this information would be extremely valuable, particularly for the veterinary community, as it can be used to identify relevant transmission pathways to dogs. This will also aid in pet-owner education on local risks and provide veterinarians with information to use when advocating for leptospirosis vaccination to improve canine population health.
Improving vaccine effectiveness, protection, and uptake
Canine vaccination is currently the main strategy to prevent illness and urinary shedding of Leptospira (4). Unfortunately, many challenges exist with vaccination, including effectiveness and implementation. Despite good efficacy for dogs in experimental settings (26), vaccine effectiveness in natural settings is lower (27). Some vaccinated dogs will still experience disease, and asymptomatic carriers will continue to shed Leptospira (27). In a study to determine incidence of urinary shedding in dogs that were disease-free at baseline, almost 1/2 of dogs became infected within 1 y; and although most dogs were infected with highly virulent serovars, all remained asymptomatic (28). This asymptomatic shedding importantly contributes to continued circulation of Leptospira and may increase human and animal risk of exposure. Vaccine protection also depends on local serovar distribution, as commercial canine vaccines may not protect against all circulating pathogenic serovars in an area (29). This remains a large concern, as climate change may influence serovar distribution worldwide (30). Monitoring Leptospira serovar distribution can inform more effective vaccine development (31), provide more complete protection, and reduce risks of transmission to other animals, the environment, and humans.
Maintaining protective leptospirosis vaccination status can also be difficult due to owner socioeconomic factors (e.g., household income and education level), concern over adverse effects, and the inconvenience of annual boosters (32). In 2024, the World Small Animal Veterinary Association updated their vaccination guidelines to include leptospirosis as a core vaccine for dogs in areas where canine leptospirosis is endemic (33), and regional vaccine protocols often vary depending on local environmental risks and individual lifestyle. However, the likelihood a dog would never become exposed to wildlife, natural water sources, or other high-risk areas is quite low, and cases of canine leptospirosis have been documented even when no apparent exposure was observed (4). In their consensus statement on canine leptospirosis, the American College of Veterinary Internal Medicine declared that “all dogs are at risk of leptospirosis, regardless of signalment, geographic location, lifestyle, and the time of year” (2). However, adding leptospirosis vaccination to core vaccines will not necessarily increase vaccination levels. Even countries that do consider leptospirosis a core vaccine for dogs have suboptimal vaccination rates (~50%) (34). Many other factors may influence an owner’s decision to vaccinate their dog against leptospirosis, such as whether the vaccine is offered routinely or promoted by veterinarians, veterinary practice commitment to client education, owner risk perception, owner financial position, and the perceived value of the vaccine. Qualitative investigations of the social and behavioral elements influencing canine leptospirosis vaccination are required to better inform veterinary-client communication to improve vaccine uptake.
Improving disease reporting and data accessibility
Interdisciplinary and multisectoral collaboration are essential components of One Health (35). To improve understanding of the animal, human, and environmental risk factors for canine leptospirosis, advancements in disease reporting and data accessibility are necessary. The current lack of reliable and accessible epidemiological data is a major limitation to conducting adequate surveillance and modelling of canine leptospirosis. Veterinary diagnostic laboratories maintain considerable diagnostic data, which can be shared to conduct collaborative research. However, these data are usually not linked to corresponding case medical and activity history, which are essential to establish risk factors. This information is typically held by veterinary clinics or other practice-management software companies, with access subject to privacy and confidentiality regulations. Developing a system that integrates these data sources, along with improving communication and collaboration among veterinary, academic, and private animal-health stakeholders, would lead to more coordinated surveillance and improve understanding of individual, environmental, and socioeconomic risk factors for canine leptospirosis.
Conclusion
We have highlighted several potential benefits of applying a One Health approach to canine leptospirosis research. Knowledge gaps in canine leptospirosis transmission and epidemiology need to be comprehensively addressed by combining expertise from multiple disciplines in animal, environmental, and human sectors. Collaborative efforts from stakeholders such as veterinarians, animal shelters, and academia must also be made to increase knowledge and awareness of canine leptospirosis and communicate the importance of surveillance and prevention. Due to numerous interactions among dogs, humans, and the environment, the One Health opportunities we have outlined may also benefit our understanding of human leptospirosis. Employing a One Health approach provides a holistic understanding of canine leptospirosis that is essential for effective disease surveillance and risk-factor analysis, and for creating sustainable prevention and control strategies to reduce disease burden.
Funding Statement
Carys Vyn is a PhD student in Epidemiology and One Health at the University of Guelph. She is funded through the One Health Institute at the University of Guelph, the Ontario Veterinary College, and the Government of Ontario.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (kgray@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
Carys Vyn is a PhD student in Epidemiology and One Health at the University of Guelph. She is funded through the One Health Institute at the University of Guelph, the Ontario Veterinary College, and the Government of Ontario.
References
- 1.Goarant C, Trueba G, Bierque E, Thibeaux R, Davis B, De La Pena-Moctezuma A. Leptospira and Leptospirosis. In: Pruden A, Ashbolt N, Miller J, editors. Water and Sanitation for the 21st Century: Health and Microbiological Aspects of Excreta and Wastewater Management (Global Water Pathogen Project) Michigan State University, East Lansing. Michigan: UNESCO; 2019. Part 3— Section 2. [Google Scholar]
- 2.Sykes JE, Francey T, Schuller S, Stoddard RA, Cowgill LD, Moore GE. Updated ACVIM consensus statement on leptospirosis in dogs. J Vet Intern Med. 2023;37:1966–1982. doi: 10.1111/jvim.16903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.André-Fontaine G. Canine leptospirosis — do we have a problem? Vet Microbiol. 2006;117:19–24. doi: 10.1016/j.vetmic.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Schuller S, Francey T, Hartmann K, et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 2015;56:159–179. doi: 10.1111/jsap.12328. [DOI] [PubMed] [Google Scholar]
- 5.Karpagam KB, Ganesh B. Leptospirosis: A neglected tropical zoonotic infection of public health importance — an updated review. Eur J Clin Microbiol Infect Dis. 2020;39:835–846. doi: 10.1007/s10096-019-03797-4. [DOI] [PubMed] [Google Scholar]
- 6.Lau CL, Smythe LD, Craig SB, Weinstein P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans R Soc Trop Med Hyg. 2010;104:631–638. doi: 10.1016/j.trstmh.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith AM, Stull JW, Moore GE. Potential drivers for the re-emergence of canine leptospirosis in the United States and Canada. Trop Med Infect Dis. 2022;7:377. doi: 10.3390/tropicalmed7110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinetz JM, Wilcox BA, Aguirre A, et al. Beyond disciplinary boundaries: Leptospirosis as a model of incorporating transdisciplinary approaches to understand infectious disease emergence. EcoHealth. 2005;2:291–306. [Google Scholar]
- 9.Ricardo T, Previtali MA, Signorini M. Meta-analysis of risk factors for canine leptospirosis. Prev Vet Med. 2020;181:105037. doi: 10.1016/j.prevetmed.2020.105037. [DOI] [PubMed] [Google Scholar]
- 10.Costa ACTRB, Colocho RAB, Pereira CR, Lage AP, Heinemann MB, Dorneles EMS. Canine leptospirosis in stray and sheltered dogs: A systematic review. Anim Health Res Rev. 2022;23:39–58. doi: 10.1017/S1466252321000190. [DOI] [PubMed] [Google Scholar]
- 11.Khamesipour F, Doosti A, Emadi MF, Awosile B. Detection of Brucella sp. and Leptospira sp. in dogs using conventional polymerase chain reaction. Bull Vet Inst Pulawy. 2014;58:527–531. [Google Scholar]
- 12.Zinsstag J, Utzinger J, Probst-Hensch N, Shan L, Zhou XN. Towards integrated surveillance-response systems for the prevention of future pandemics. Infect Dis Poverty. 2020;9:140. doi: 10.1186/s40249-020-00757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gay N, Soupé-Gilbert ME, Goarant C. Though not reservoirs, dogs might transmit Leptospira in New Caledonia. Int J Environ Res Public Health. 2014;11:4316–4325. doi: 10.3390/ijerph110404316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohn-Hausner N, Kmetiuk LB, Biondo AW. One Health approach to leptospirosis: Human–dog seroprevalence associated to socioeconomic and environmental risk factors in Brazil over a 20-year period (2001–2020) Trop Med Infect Dis. 2023;8:356. doi: 10.3390/tropicalmed8070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: A systematic review. PLoS Negl Trop Dis. 2015;9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major A, Schweighauser A, Francey T. Increasing incidence of canine leptospirosis in Switzerland. Int J Environ Res Public Health. 2014;11:7242–7260. doi: 10.3390/ijerph110707242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stull JW, Evason M, Weese JS, Yu J, Szlosek D, Smith AM. Canine leptospirosis in Canada, test-positive proportion and risk factors (2009 to 2018): A cross-sectional study. PLoS One. 2022;17:e0270313. doi: 10.1371/journal.pone.0270313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupouey J, Faucher B, Edouard S, et al. Human leptospirosis: An emerging risk in Europe? Comp Immunol Microbiol Infect Dis. 2014;37:77–83. doi: 10.1016/j.cimid.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Romero J, editors. IPCC Core Writing Team. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2023. pp. 35–115. [Google Scholar]
- 20.Dhewantara PW, Riandi MU, Wahono T. Effect of climate change on the geographical distribution of leptospirosis risk in western Java, Indonesia. IOP Conf Ser Earth Environ Sci. 2022;1089:012074. [Google Scholar]
- 21.Liu Y, Lund RB, Nordone SK, Yabsley MJ, McMahan CS. A Bayesian spatio-temporal model for forecasting the prevalence of antibodies to Ehrlichia species in domestic dogs within the contiguous United States. Parasit Vectors. 2017;10:138. doi: 10.1186/s13071-017-2068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson SC, Liu Y, Lund RB, et al. A Bayesian spatio-temporal model for forecasting the prevalence of antibodies to Borrelia burgdorferi, causative agent of Lyme disease, in domestic dogs within the contiguous United States. LaDeau SL. PLoS One. 2017;12:e0174428. doi: 10.1371/journal.pone.0174428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andre-Fontaine G, Aviat F, Thorin C. Waterborne leptospirosis: Survival and preservation of the virulence of pathogenic Leptospira spp. in fresh water. Curr Microbiol. 2015;71:136–142. doi: 10.1007/s00284-015-0836-4. [DOI] [PubMed] [Google Scholar]
- 24.Shearer KE, Harte MJ, Ojkic D, DeLay J, Campbell D. Detection of Leptospira spp. in wildlife reservoir hosts in Ontario through comparison of immunohistochemical and polymerase chain reaction genotyping methods. Can Vet J. 2014;55:240–248. [PMC free article] [PubMed] [Google Scholar]
- 25.Himsworth CG, Bidulka J, Parsons KL, et al. Ecology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, Canada. PLoS Negl Trop Dis. 2013;7:e2270. doi: 10.1371/journal.pntd.0002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esteves SB, Santos CM, Salgado FF, et al. Efficacy of commercially available vaccines against canine leptospirosis: A systematic review and meta-analysis. Vaccine. 2022;40:1722–1740. doi: 10.1016/j.vaccine.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Sant’Anna da Costa R, Di Azevedo MIN, Dos Santos Baptista Borges AL, Aymée L, Martins G, Lilenbaum W. Effect of vaccination against Leptospira on shelter asymptomatic dogs following a long-term study. Animals. 2022;12:1788. doi: 10.3390/ani12141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sant’Anna da Costa R, Di Azevedo MIN, Dos Santos Baptista Borges AL, Carvalho-Costa FA, Martins G, Lilenbaum W. Persistent high leptospiral shedding by asymptomatic dogs in endemic areas triggers a serious public health concern. Animals. 2021;11:937. doi: 10.3390/ani11040937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griebsch C, Ward MP, Norris JM. Canine leptospirosis: Global distribution, diagnosis, and treatment. Adv Small Anim Care. 2022;3:177–220. [Google Scholar]
- 30.Lau CL, Skelly C, Smythe LD, Craig SB, Weinstein P. Emergence of new leptospiral serovars in American Samoa — ascertainment or ecological change? BMC Infect Dis. 2012;12:19. doi: 10.1186/1471-2334-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertasio C, Boniotti MB, Lucchese L, et al. Detection of new Leptospira genotypes infecting symptomatic dogs: Is a new vaccine formulation needed? Pathogens. 2020;9:484. doi: 10.3390/pathogens9060484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sánchez-Vizcaíno F, Muniesa A, Singleton DA, et al. Use of vaccines and factors associated with their uptake variability in dogs, cats and rabbits attending a large sentinel network of veterinary practices across Great Britain. Epidemiol Infect. 2018;146:895–903. doi: 10.1017/S0950268818000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squires RA, Crawford C, Marcondes M, Whitley N. 2024 Guidelines for the vaccination of dogs and cats — compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA) J Small Anim Pract. 2024;65:277–316. doi: 10.1111/jsap.13718. [DOI] [PubMed] [Google Scholar]
- 34.Eschle S, Hartmann K, Rieger A, Fischer S, Klima A, Bergmann M. Canine vaccination in Germany: A survey of owner attitudes and compliance. PLoS One. 2020;15:e0238371. doi: 10.1371/journal.pone.0238371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adisasmito WB, Almuhairi S, Behravesh CB, et al. One Health: A new definition for a sustainable and healthy future. PLoS Pathog. 2022;18:e1010537. doi: 10.1371/journal.ppat.1010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Interdisciplinary and multisectoral collaboration are essential components of One Health (35). To improve understanding of the animal, human, and environmental risk factors for canine leptospirosis, advancements in disease reporting and data accessibility are necessary. The current lack of reliable and accessible epidemiological data is a major limitation to conducting adequate surveillance and modelling of canine leptospirosis. Veterinary diagnostic laboratories maintain considerable diagnostic data, which can be shared to conduct collaborative research. However, these data are usually not linked to corresponding case medical and activity history, which are essential to establish risk factors. This information is typically held by veterinary clinics or other practice-management software companies, with access subject to privacy and confidentiality regulations. Developing a system that integrates these data sources, along with improving communication and collaboration among veterinary, academic, and private animal-health stakeholders, would lead to more coordinated surveillance and improve understanding of individual, environmental, and socioeconomic risk factors for canine leptospirosis.