Abstract
Background:
Whereas clinical experience in dementia indicates high risk for financial mismanagement, there has been little formal study of real world financial errors in dementia.
Objective:
We aimed to compare caregiver-reported financial mistakes among people with Alzheimer’s disease, behavioral variant frontotemporal dementia (bvFTD), and primary progressive aphasia (PPA).
Methods:
Caregivers reported whether participants with dementia had made financial mistakes within the last year; and if so, categorized these as resulting from: (a) being too trusting or gullible, (b) being wasteful or careless with money, or (c) trouble with memory. In a pre-registered analysis (https://archive.org/details/osf-registrations-vupj7-v1), we examined the hypotheses that (1) financial mistakes due to impaired socioemotional function and diminished sensitivity to negative outcomes are more prevalent in bvFTD than in Alzheimer’s disease, and (2) financial mistakes due to memory are more prevalent in Alzheimer’s disease than in bvFTD. Exploratory analyses addressed vulnerability in PPA and brain-behavior relationships using voxel-based morphometry.
Results:
Concordant with our first hypothesis, bvFTD was more strongly associated than Alzheimer’s disease with mistakes due to being too trusting/gullible or wasteful/careless; contrary to our second hypothesis, both groups were similarly likely to make mistakes due to memory. No differences were found between Alzheimer’s disease and PPA. Exploratory analyses indicated associations between financial errors and atrophy in right prefrontal and insular cortex.
Conclusions:
Our findings cohere with documented socioemotional and valuation impairments in bvFTD, and with research indicating comparable memory impairment between bvFTD and Alzheimer’s disease.
Keywords: Alzheimer dementia, frontotemporal dementia, primary progressive aphasia, financial activities, financial management, decision making
BACKGROUND
Financial mismanagement is among the earliest and most consequential manifestations of dementia, with grave consequences for the functional independence of people with dementia and for their families’ current and future well-being. Pérès et al [1]. have reported that decline in financial instrumental activities of daily living may precede a formal diagnosis of dementia by over 10 years. Financial transactions are among the most cognitively demanding activities routinely undertaken by most people, and research on neural mechanisms underlying financial decision-making implicates many known to be vulnerable to normal and pathological processes of aging [2]. Interviews with social workers and reviews of Adult Protective Services cases also indicate that people with dementia are at high risk for financial abuse and victimization, with adverse outcomes including emotional pain, financial impoverishment, loss of independence and eviction [3, 4].
Unfortunately, there are several scientific gaps in our understanding of financial mismanagement by people with dementia [5]. Generally, population-based studies of elder financial abuse exclude respondents with cognitive impairment, who are precisely the population of greatest concern [6–8]. (“Generalization of our results to what may be the group most at-risk for mistreatment, the cognitively impaired elderly, is not appropriate” [8]. Though see Nicholas et al [9]. for an innovative population-based approach to investigating one form of financial mismanagement, credit payment delinquency.) Reported cases of financial abuse are subject to bias, as many cases are never reported due to impaired recognition, wishes to shield related perpetrators, or fears that involving public protective services will lead to the removal of people with dementia from their homes and families [10]. Impaired performance in dementia has been documented in formal laboratory-based measures of financial decisional capacity [11], but these do not assess real world transactions or errors. Finally, at the neurocognitive level financial transactions are complex, and effective financial management likely involves multiple interacting processes such as memory, executive function, risk attitudes, intertemporal choice, socioemotional function, theory of mind, mood and metacognition. Much work is still needed to link disease-related changes in subsystems that are differentially targeted in different neurodegenerative syndromes (often in multiple and intersecting ways) to the kinds of financial errors that are routinely made by people with dementia.
In previous work, we have examined dissociations in both real world financial decisions [12] and in formal laboratory-based measures of decision-making [13, 14] across neurodegenerative syndromes such as Alzheimer’s disease, behavioral variant frontotemporal dementia (bvFTD) and primary progressive aphasia (PPA), providing a link between the differential impairment of neurocognitive systems observed in these disorders and financial outcomes important to people with these disorders and their families. Conceptually, we have drawn on recent research in neuroeconomics to develop a mechanistic framework for susceptibility to financial errors in dementia, integrating the roles of cognitive and affective characteristics of the person with dementia (including changes referable to disease as well as premorbid knowledge and experience), interpersonal vulnerability (both situational and reflecting individuals’ impaired social judgment) and contextual factors such as family limitations on independence.
In the present pre-registered study we sought to prospectively apply our prior neuroeconomic conceptual framework for financial mismanagement in dementia [12]. We hypothesized (https://archive.org/details/osf-registrations-vupj7-v1) that, given differing patterns of cognitive and behavioral impairment observed in Alzheimer’s disease and bvFTD, people with these two disorders would exhibit different types of errors in financial decision-making. Specifically, we hypothesized (Hypothesis 1) greater frequency of errors referable to being too gullible or trusting (reflecting more impaired socioemotional function) and errors referable to being wasteful or careless with money (reflecting diminished sensitivity to negative outcomes [13]) in people with bvFTD than in people with Alzheimer’s disease; and we hypothesized (Hypothesis 2) greater frequency of errors referable to memory deficits in people with Alzheimer’s disease than in people with bvFTD. In planned exploratory analyses we also examined the frequency of such errors in PPA.
METHODS
Study participants
We recruited dyads of participants with Alzheimer’s disease, bvFTD, and PPA, and their caregivers, from existing longitudinal research cohorts at the University of California, San Francisco Memory and Aging Center. All participants with dementia were diagnosed by a multidisciplinary team of neurologists, neuropsychologists and nurses after a comprehensive evaluation including a clinical history, neurological examination and neuropsychological testing according to established research criteria [15–17]. In our pre-registered analysis (https://archive.org/details/osf-registrations-vupj7-v1), we planned to include 50 dyads in each category, with primary hypotheses addressing the planned contrasts between participants with Alzheimer’s disease and participants with bvFTD. Sample size calculations were based upon effect sizes observed in our prior exploratory study [12]. Existing research records from longitudinal research cohorts were reviewed to obtain demographic characteristics, including age, gender, handedness, and years of education; as well as Mini-Mental Status Examination (MMSE) and Clinical Dementia Rating (CDR) scores collected closest to the date of task administration.
Financial activities questionnaire
Based on a neuroeconomic conceptual framework presented in our prior exploratory study [12], we developed a questionnaire for dementia caregivers to provide information on their care recipients’ premorbid financial experiences, current engagement in financial management, and financial mistakes in the previous year, which has also been used in another research study (Supplementary Table 1) [18]. Questions regarding premorbid financial experiences and current engagement in financial management assessed past and present involvement in three domains of financial management (purchases, paying household bills, and preparing taxes or important documents) based on an established research framework for instrumental activities of daily living [19]. If participants with dementia continued to engage in any of these three domains of financial management, we then asked caregivers whether these participants had made mistakes in the previous year in managing their money or property. If caregivers reported any such mistakes in the previous year, we then asked caregivers to categorize such mistakes based on our prior conceptual framework (Fig. 3) [12], attributing them to: (a) being too trusting or gullible, (b) being wasteful or careless with money, (c) trouble with memory, (d) bad planning or organizing, or (e) worrying too much about bad things that could happen (i.e., paranoia).
Fig. 3. Voxel-based morphometry maps of grey matter associations with (A) financial mistakes of any subtype in the previous year (nonsignificant), (B) financial mistakes attributed to problems with memory in the previous year, and (C) financial mistakes attributed to problems with planning and organizing in the previous year.
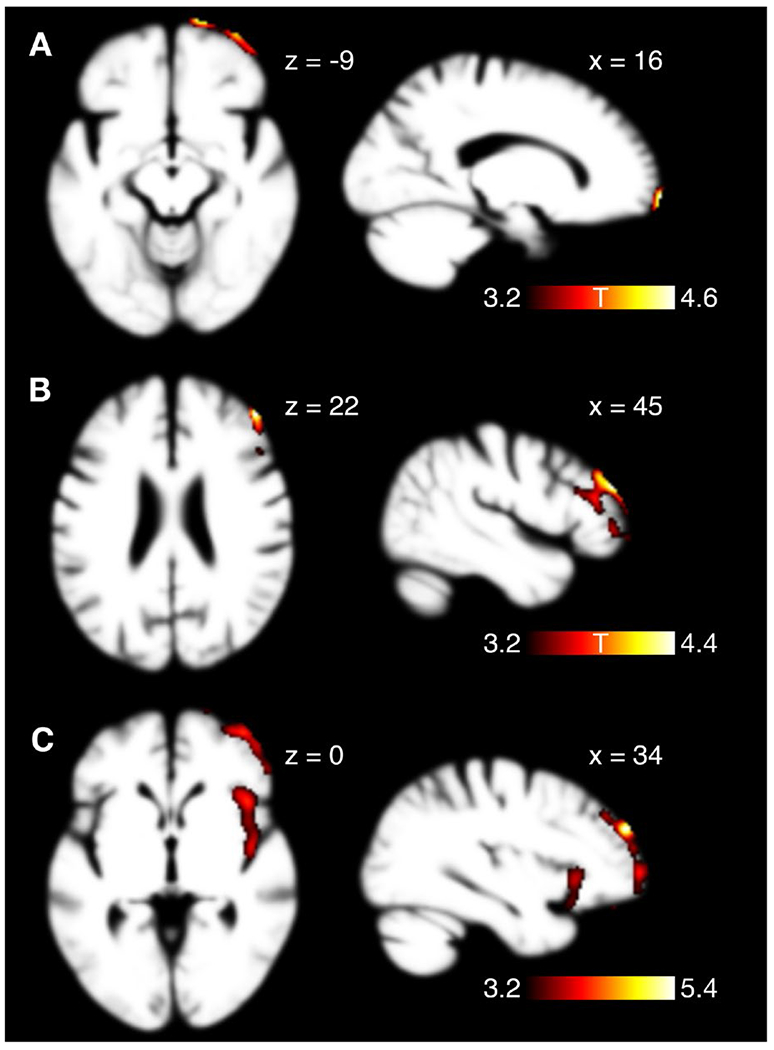
Images are displayed according to neurologic convention (right = right).
Some participant caregivers completed the questionnaire as a paper version administered during in-person research visits to our center. Other participant caregivers were provided with a study computer to complete a Qualtrics questionnaire during in-person research visits to our center. Other participant caregivers (including all caregivers who participated during the COVID-19 pandemic) received an individualized e-mail containing a unique web link to complete the Qualtrics questionnaire online; this link could be used to return to the task if it was not completed in one sitting.
Behavioral statistical analysis
In a pre-registered analytic plan (https://archive.org/details/osf-registrations-vupj7-v1), we articulated two hypotheses regarding financial mismanagement by people with Alzheimer’s disease or bvFTD. Hypothesis 1: Financial mistakes due to being too trusting or gullible and wasteful or too careless with money are more prevalent in people with bvFTD than in people with Alzheimer’s disease. Hypothesis 2: Financial mistakes due to trouble with memory are more prevalent in people with Alzheimer’s disease than in people with bvFTD. We also pre-registered an exploratory secondary aim to assess the prevalence of such mismanagement in people with PPA.
For Hypothesis 1, we produced multivariable logistic regression models with respondents’ reports of financial mismanagement due to (a) being too trusting/gullible, and due to (b) being wasteful/careless with money, respectively, as outcome variables. For Hypothesis 2, we conducted multivariable logistic regression models with respondents’ reports of financial mismanagement due to (c) trouble with memory as the outcome variable. For all models, we entered diagnostic category as the predictor of interest and participant age (mean-centered), gender, and educational attainment (mean-centered) as covariates. In addition to these pre-registered models, we conducted sensitivity analyses to assess whether our findings were influenced by disease severity, using multivariable logistic regression models including covariates above and adding MMSE total score as an additional covariate. We also created models with interactions terms including the diagnostic category and demographic variables to test for the specificity of reported associations.
As described in the pre-registered analytic plan, a priori power calculations were two-sided with alpha = 0.05, based on an expected 50 subjects in the Alzheimer’s disease and bvFTD groups and effect sizes observed in prior exploratory work [12]. For model (a) (Hypothesis 1), predicted power was 43%. For model (b) (Hypothesis 1), predicted power was 93-99%. For model (c) (Hypothesis 2), predicted power was 88%.
Analyses were performed using the statistical programming language R [20].
Structural MRI analysis
T1-weighted MRI data for participants with dementia were acquired on a 3.0 Tesla Siemens (Siemens, Iselin, NJ) Prisma Fit scanner using a magnetization prepared rapid gradient echo (MPRAGE) sequence (160 sagittal slices, slice thickness 1.0 mm, field of view 256 × 230 mm2, matrix 256 × 230, voxel size 1.0 × 1.0 × 1.0 mm3, repetition time 2,300 ms, echo time 2.98 ms, flip angle 9°). Preprocessing was performed using Statistical Parametric Mapping 12 (SPM12) (Wellcome Department of Cognitive Neurology, London). To optimize intersubject registration, each participant’s image was warped to a template derived from 300 confirmed neurologically healthy older adults that had previously been collected at our research center (ages 44–86, M ± SD: 67.2 ± 7.3; 113 males, 186 females) scanned with one of three magnet strengths (1.5 T = 27.10%; 3 T = 62.88%; 4 T = 10.03% of participants) using affine and nonlinear transformations with the help of diffeomorphic anatomical registration through exponentiated lie algebra method (DARTEL) with standard implementation in SPM12. In all preprocessing steps, default parameters of the SPM12 toolbox were used [21].
To characterize regional atrophy in our three recruited disease cohorts (Alzheimer’s disease, bvFTD and PPA), whole brain voxel-based morphometry (VBM) analyses were conducted comparing them to age-, gender- and education-matched healthy controls with structural MRI scans performed at our center. First, a subset of participants with dementia in each cohort was identified that had T1-weighted MRI data obtained within 2 years of when their caregiver completed the financial activities questionnaire instrument. Next, healthy control participants with existing T1-weighted MRI data at our center were individually matched to each participant with dementia by gender and proximity in age (at the time MRI data were obtained) and educational attainment in years; no control participant was matched more than once. Age, gender and total intracranial volume were included as covariates. Resulting statistical significance maps were thresholded at voxelwise P < 0.001 and then thresholded at P < 0.05 based on cluster extent using a Monte Carlo simulation running 1,000 permutations.
In exploratory brain-behavior analyses, caregiver reports of participants’ financial mistakes of any subtype, financial mistakes due to being too trusting or gullible, financial mistakes due to being wasteful or careless, financial mistakes due to trouble with memory, financial mistakes due to bad planning or organizing, and financial mistakes due to worrying too much were entered as predictors of regional grey matter volumes using VBM across participants from all three neurodegenerative disease categories with available imaging data. Age, gender, total intracranial volume and time elapsed (in days) between the date that caregivers were queried about participants’ financial decisions and the date of the scan were included as covariates. Resulting statistical significance maps were thresholded at voxelwise P < 0.001 and then thresholded at P < 0.05 based on cluster extent using a Monte Carlo simulation running 1,000 permutations. All voxel-based statistical analyses were conducted using voxel-based lesion-symptom mapping (VLSM) software, version 2.55 [22].
RESULTS
Clinical characteristics
Responses to the questionnaire instrument were collected from caregivers for 146 participants with three neurodegenerative conditions: 50 from participants with Alzheimer’s disease, 58 from participants with bvFTD, and 38 from participants with PPA. Table 1 displays demographic and clinical characteristics of these participants, separately listing participants with three PPA variants: logopenic variant PPA (lvPPA), nonfluent variant PPA (nfvPPA) and semantic variant PPA (svPPA). In subsequent logistic regression models these variants were grouped together in a single PPA category to avoid estimation errors due to small cell counts, and because comparisons between PPA and the other groups were pre-registered as exploratory. Groups were relatively well-matched for age, gender and education; as expected, participants with bvFTD were slightly younger and those with PPA had less functional limitation. Disease severity as measured by MMSE and functional limitation as measured by Clinical Dementia Rating were quite mild, as participants with dementia were recruited in early disease stages. Reflecting the research cohorts from which the sample was derived, participants were highly educated and predominantly White. Most caregivers were either spouses or children of the participants (Supplementary Table 2).
Table 1.
Demographic and clinical features of participants with dementia
| Alzheimer, N = 50 | bvFTD, N = 58 | lvPPA, N = 16 | nfvPPA, N = 12 | svPPA, N = 10 | p-value | |
|---|---|---|---|---|---|---|
| Gender: | ||||||
| Female | 24 (48.0%) | 25 (43.1%) | 7 (43.8%) | 5 (41.7%) | 4 (40.0%) | |
| Male | 26 (52.0%) | 33 (56.9%) | 9 (56.3%) | 7 (58.3%) | 6 (60.0%) | |
| Age (y) | 68.1 (9.1) | 64.9 (9.7) | 67.1 (9.3) | 68.8 (7.5) | 64.6 (5.7) | 0.7 |
| Education (y) | 15.7 (2.6) | 16.2 (3.1) | 16.3 (2.4) | 16.0 (2.2) | 15.8 (1.4) | >0.9 |
| Race/Ethnicity: | 0.048 | |||||
| Hispanic | 6 (16.7%) | 2 (3.6%) | 2 (15.4%) | 0 (0.0%) | 0 (0.0%) | |
| non-Hispanic Asian | 4 (11.1%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 1 (10.0%) | |
| non-Hispanic Black | 1 (2.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| non-Hispanic White | 24 (66.7%) | 52 (94.5%) | 11 (84.6%) | 10 (90.9%) | 9 (90.0%) | |
| non-Hispanic Other Race | 1 (2.8%) | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| MMSE (max: 30) | 20.3 (5.2) | 21.7 (7.8) | 22.1 (5.1) | 25.5 (4.4) | 21.5 (5.0) | 0.008 |
| CDR-SB (max: 18) | 6.2 (2.9) | 6.6 (3.2) | 3.3 (1.7) | 1.8 (1.4) | 3.9 (2.3) | <0.001 |
Gender and Race/Ethnicity subgroupings are presented as N (%); whole group characteristics are presented as Mean (SD). Fisher’s exact test was used for categorical variables and Kruskal-Wallis rank sum test was used for continuous variables. bvFTD = behavioral variant frontotemporal dementia; lvPPA = logopenic variant primary progressive aphasia; nfvPPA = nonfluent variant primary progressive aphasia; svPPA = semantic variant primary progressive aphasia; MMSE = Mini-Mental State Examination; CDR-SB = Clinical Dementia Rating scale-Sum of Boxes.
Voxel-based morphometry by diagnostic group
Of the 146 participants, T1 MRI scans were available for 31 participants with Alzheimer’s disease, 53 participants with bvFTD, and 34 participants with PPA. Compared with matched healthy controls, these participants demonstrated distinct but overlapping patterns of atrophy that were consistent with clinical diagnoses (Fig. 1). In the Alzheimer’s disease cohort, atrophy was most prominent in the mesial temporal lobes, extending also to the middle and inferior temporal gyri, medial parietal lobes, and lateral parietal and frontal lobes. In the bvFTD cohort, atrophy was most prominent in a widely distributed network including the medial thalami, bilateral striatum, insulae and orbital gyri. In the PPA cohort (including together participants with lvPPA, nfvPPA and svPPA), atrophy was left-lateralized including the left temporal, frontal and parietal lobes and left insula. MNI coordinates, maximum T values and p values for significant clusters distinguishing each diagnostic group from matched controls are summarized in Supplementary Tables 3–5.
Fig. 1. Voxel-based morphometry maps of regional atrophy in participants with (A) Alzheimer’s disease, (B) behavioral variant frontotemporal dementia, and (C) primary progressive aphasia, as compared to matched healthy controls.
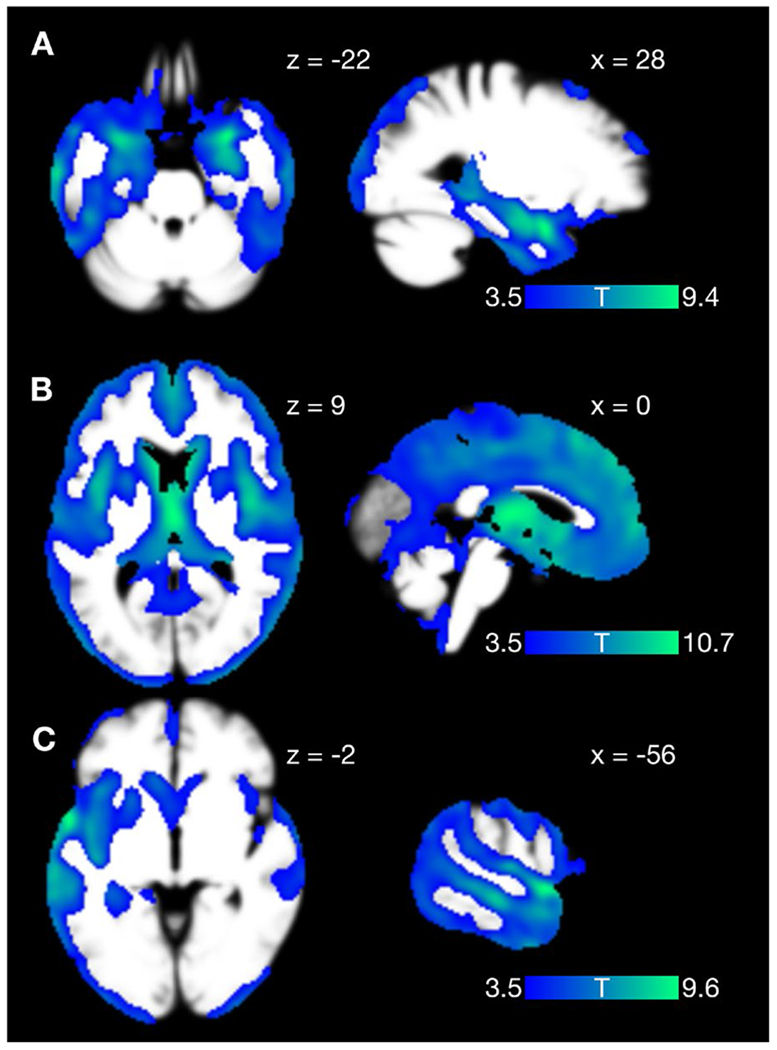
Images are displayed according to neurologic convention (right = right).
Financial management in the past year
Caregivers reported participants’ engagement in three categories of financial management (purchases, paying household bills, and preparing taxes or important documents) premorbidly and in the past year (Fig. 2). While most participants with dementia continued to make their own purchases, engagement diminished with increasing complexity of financial management activities (paying bills, preparing taxes and important documents). Participants with PPA were more likely to be involved in financial management than those with Alzheimer’s disease and bvFTD (making own purchases, p = 0.009; paying household bills, p = 0.058; preparing important documents, p = 0.002 [Fisher’s exact test]), consistent with the lower degree of functional impairment (Table 1) reported in this cohort.
Fig. 2. Continued involvement in financial management activities of three types in the past year, by diagnostic group.
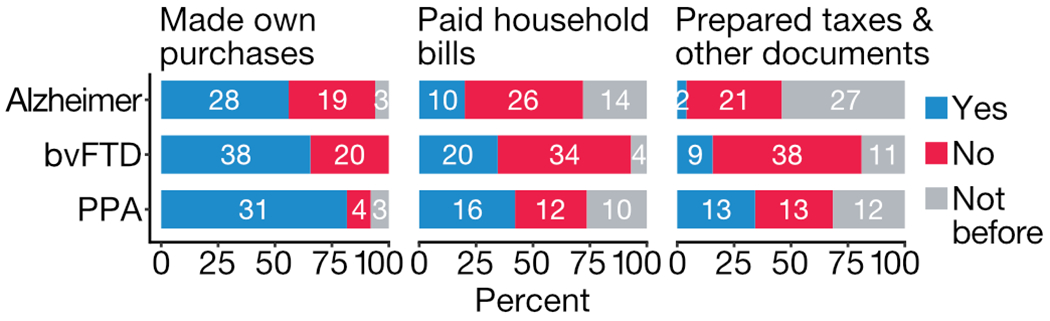
Overall counts and percentages, among participants with Alzheimer’s disease, bvFTD and PPA, for continued involvement in three domains of financial management. Grey bars indicate participants who did not engage in a given domain of financial management even prior to disease onset.
Errors in managing money or property in past year
Caregivers also reported whether participants with dementia had made mistakes in managing their money or property in the previous year, and for reported mistakes characterized them within subtypes based on our previously published conceptual framework. 32% of Alzheimer’s disease caregivers, 52% of bvFTD caregivers, and 32% of PPA caregivers reported financial errors in the last year. Raw counts and percentages for the five queried subtypes of financial errors are reported in Table 2.
Table 2.
Reported errors in managing money or property in the past year
| Type of financial mistake | Alzheimer, N = 50 | bvFTD, N = 58 | PPA, N = 38 |
|---|---|---|---|
| Mistakes from being too trusting or gullible | 4 (8%) | 15 (26%) | 1 (3%) |
| Mistakes from being wasteful or careless with money | 5 (10%) | 19 (33%) | 4 (11%) |
| Mistakes from trouble with memory | 13 (26%) | 15 (26%) | 8 (22%) |
| Mistakes from bad planning or organizing | 7 (14%) | 16 (28%) | 3 (8%) |
| Mistakes from worrying too much about bad things that could happen | 2 (4%) | 2 (3%) | 0 (0%) |
| Errors of any type | 16 (32%) | 30 (52%) | 12 (32%) |
Values are presented as N (%). bvFTD = behavioral variant frontotemporal dementia; PPA = primary progressive aphasia. Column totals do not sum to errors of any type because many participants were reported to have made more than one type of error.
Pre-registered analyses: being too trusting/gullible, being wasteful/careless, and memory
While we collected data on five subtypes of financial errors, our pre-registered hypotheses concerned three subtypes. For Hypothesis 1: mistakes due to (a) being too trusting/gullible, and due to (b) being wasteful/careless with money. For Hypothesis 2: mistakes due to (c) trouble with memory. In analyses adjusted for age, gender and educational attainment (Table 3), participants with bvFTD were more likely than participants with Alzheimer’s disease to make mistakes due to being too trusting/gullible (OR 4.90, 95% CI 1.55-19.2, p = 0.012) and too wasteful/careless with money (OR 4.41, 95% CI 1.55-14.7, p = 0.009), consistent with our pre-registered Hypothesis 1. However, participants with Alzheimer’s disease were not more likely than participants with bvFTD to make mistakes due to trouble with memory (bvFTD OR 1.05, 95% CI 0.43-2.59, p > 0.9), contrary to our pre-registered Hypothesis 2. In post hoc sensitivity analyses including MMSE total score as an additional covariate to account for a potential influence of disease severity on our findings, p values and model coefficients were essentially unchanged. Also, to assess the specificity of the reported associations, we tested for interaction effects between the final diagnosis and demographic variables and these interaction terms did not attain significance.
Table 3.
Predictors of three subtypes of financial error
| Trusting or gullible | Wasteful or careless | Memory | ||||
|---|---|---|---|---|---|---|
| OR (95% CI)1 | p-value | OR (95% CI)1 | p-value | OR (95% CI)1 | p-value | |
| Diagnosis | ||||||
| Alzheimer | Reference | Reference | Reference | |||
| bvFTD | 4.90 (1.55 – 19.2) | 0.012 | 4.41 (1.55 – 14.7) | 0.009 | 1.05 (0.43 – 2.59) | >0.9 |
| PPA | 0.33 (0.02 – 2.39) | 0.3 | 1.06 (0.24 – 4.35) | >0.9 | 0.72 (0.25 – 1.98) | 0.5 |
| Age | 1.04 (0.98 – 1.11) | 0.2 | 0.99 (0.94 – 1.05) | 0.8 | 1.01 (0.96 – 1.05) | 0.8 |
| Gender | ||||||
| Female | Reference | Reference | Reference | |||
| Male | 1.35 (0.48 – 3.97) | 0.6 | 1.23 (0.50 – 3.07) | 0.7 | 1.74 (0.79 – 3.94) | 0.2 |
| Education | 1.04 (0.87 – 1.25) | 0.7 | 1.12 (0.95 – 1.33) | 0.2 | 1.00 (0.86 – 1.16) | >0.9 |
As part of our pre-registered exploratory analysis of financial errors in PPA, we compared financial errors in participants with PPA to participants with Alzheimer’s disease. No significant differences were found (Table 3).
Beyond our hypothesized associations, we tested the association of diagnosis with financial mistakes due to bad planning or organizing, and due to worrying too much about bad things that could happen (paranoia). Errors due to bad planning or organizing were more common in bvFTD than in Alzheimer’s disease in models adjusted for age, gender and educational attainment (Supplementary Table 6; OR 2.88, 95% CI = 1.07-8.47, p = 0.043), but this association was no longer significant in models additionally adjusted for MMSE total score (p = 0.17).
Exploratory brain-behavior analyses
In an exploratory brain-behavior analysis comparing participants from all three diagnostic categories who were reported to have made financial mistakes in the past year to participants who were reported to have managed money in the past year without making such mistakes, a cluster in the right frontal pole approached statistical significance (p = 0.057; Fig. 3A). Mistakes due to memory were associated with atrophy in the right middle frontal gyrus (p = 0.030; Fig. 3B), while mistakes due to problems with planning and organizing were associated with atrophy in the right middle frontal gyrus and right anterior insula (p = 0.011 and 0.025; Fig. 3C). No associations were found with mistakes due to being too trusting or gullible, due to being wasteful or careless, or due to worrying too much. MNI coordinates, maximum T values and p values for these reported clusters are summarized in Supplementary Tables 7–9.
DISCUSSION
In a pre-registered study using reports from 146 caregivers of people with three neurodegenerative conditions, we found support for our Hypothesis 1, that people with bvFTD are more likely than people with Alzheimer’s disease to mismanage finances due to impaired socioemotional function and due to diminished sensitivity to adverse outcomes; but did not find support for our Hypothesis 2, that people with Alzheimer’s disease are more likely than people with bvFTD to mismanage finances due to poor memory. (Indeed, both the raw prevalence and adjusted model estimates representing susceptibility to financial errors due to memory between Alzheimer’s disease and bvFTD were effectively equal.)
Our positive findings for Hypothesis 1 are consistent with prior work indicating specific impairment of socioemotional function and sensitivity to adverse outcomes in bvFTD [13, 23–27]. While prior studies have generally relied on laboratory-based measures of such behavioral changes, the present study documents consequential real world manifestations of these changes in illness. This finding also lends support to the neuroeconomic conceptual model [12] on which Hypothesis 1 was based, characterizing cognitive and affective sources of vulnerability (alongside contextual factors) that we hypothesized to be differentially affected in Alzheimer’s disease and bvFTD (Fig. 4). Finally, these differential responses indicate that caregiver reports can be used to distinguish mechanisms underlying financial mismanagement across different types of neurodegenerative illness.
Fig. 4. Neuroeconomic conceptual model of susceptibility to financial mismanagement in dementia.
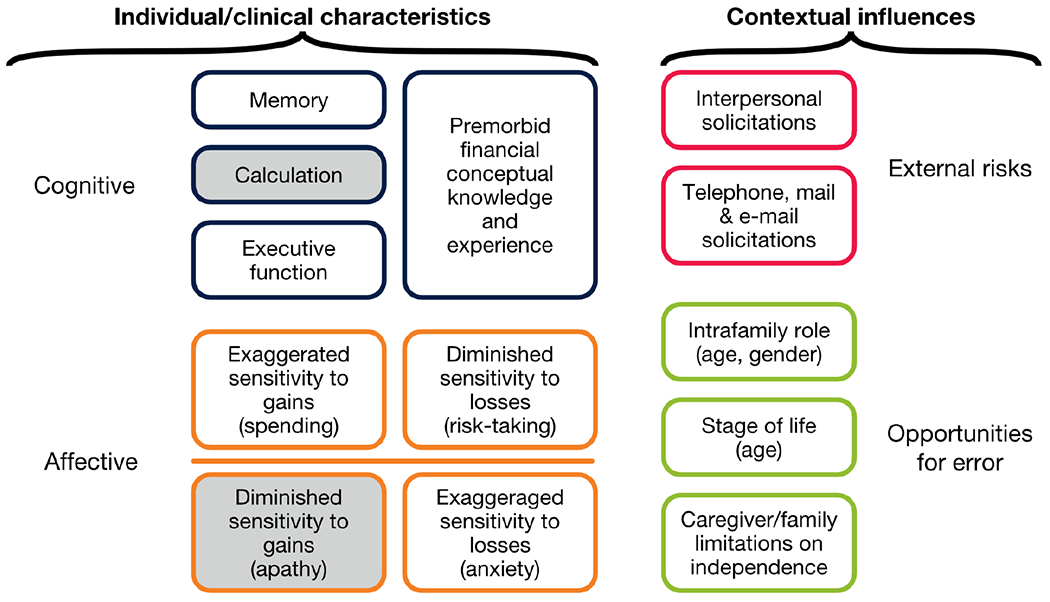
White boxes represent domains explicitly addressed in the research design; grey boxes represent domains not directly queried. (In prior exploratory research the prevalence of financial errors attributable primarily to calculation errors was small; in the case of diminished sensitivity to gains, we judged that we would not be able to distinguish pathological cases from other cases in which conservative decision-making reflects an adaptive response to diminished cognition.) Adapted[12] with permission by Taylor & Francis Ltd, www.tandfonline.com.
Our negative finding for Hypothesis 2 conflicts with a traditional conception of bvFTD as a disorder in which memory is unaffected; indeed, consensus diagnostic criteria for bvFTD require relative sparing of episodic memory. However, this criterion has been controversial [28, 29], and some experts have argued that treating memory impairment as an exclusion criterion for bvFTD hinders research on memory impairment in bvFTD. More recent work indicates significant overlap in standard memory measures between bvFTD and Alzheimer’s disease; one conjecture is that memory impairment is more variable in bvFTD than in Alzheimer’s disease and that some people with bvFTD have memory impairment comparable to people with Alzheimer’s disease [30]. This conjecture is compatible with a similar prevalence of memory-related financial errors in bvFTD and Alzheimer’s disease, especially if the subset of people with bvFTD and memory impairment also have other socioemotional impairments that make them less able to compensate for impaired memory. Indeed, one report suggests that while individuals with bvFTD retain intact memory of social interactions, this memory advantage does not protect them from financial mistreatment in a trust game paradigm. This observation is attributed to abnormal reward processing that impairs their ability to modify decisions based on prior knowledge [27].
Our exploratory brain-behavior associations relate decision-making to brain regions in cohorts affected by neurological disease. Recent work suggests that tests for brain-behavior associations amid normal variation in neurologically typical populations (“brain-wide association studies”) require thousands of participants to be reliable [31], in contrast with brain mapping studies in clinical populations. In our study, financial mismanagement due to memory problems is associated with right-sided prefrontal atrophy, while mismanagement attributable to trouble with planning or organizing is associated with insular regions. These anterior brain regions are implicated in cognitive control and emotion processing [32, 33]. Other research implicates anterior brain regions in episodic memory dysfunction in people with bvFTD and Alzheimer’s disease [34]. Importantly, atrophy in regions associated with dysfunction in episodic memory, including the prefrontal lobes, correlates with impairments in the ability to envision future outcomes [35]. Dysfunction in future-oriented thinking may manifest as blunted affective responses to negative consequences and result in riskier financial decision-making. Recognizing caveats about the reliability of past findings in neurologically typical populations, we note a general concordance between our findings and earlier studies linking financial vulnerability in aging with right-sided brain structures [36, 37].
Our study has several strengths. Participants with dementia were diagnosed at a multidisciplinary center with expertise in the differential diagnosis of neurodegenerative disease, increasing our confidence in diagnostic and pathological associations with reported behaviors. Our study design was informed by a prior neuroeconomic conceptual framework characterizing mechanisms underlying vulnerability to financial errors in dementia [12]. Our hypotheses and analytic methods were pre-registered, and our sample sizes were relatively large for disease-based behavioral research and based on explicit power calculations given effect sizes observed in prior exploratory research.
Limitations
Our study also has important limitations. Most centrally, our conceptual model of vulnerability to financial mismanagement (Fig. 4) includes not only individual/clinical characteristics of the person with dementia, but also contextual influences. These influences are highly dependent on living arrangements and socioeconomic factors; people with dementia who are isolated and/or have fewer financial and social resources are likely to be at greatest risk. Participants with dementia in our sample were all accompanied by caregivers who co-enrolled in research studies with them, indicating a high degree of involvement in their lives; they were also highly educated (a proxy for socioeconomic status) and predominantly White. Therefore, the vulnerabilities documented in our sample are likely to be unrepresentative of the vulnerabilities of the broader population of people with dementia, particularly those at greatest risk.
We reported brain-behavior analyses using MRI data obtained within two years of questionnaire completion. While 62% of the MRI data were acquired within two months from the time of questionnaire completion, MRI data further from the time of questionnaire completion may not accurately reflect participants’ disease severity. In an effort to limit the effects of rapid disease progression, we excluded participants who were not involved in making financial decisions in the past year.
Due to constraints of caregiver-reported assessments, our study was not able to delineate between different types of memory or to examine the interplay between episodic, semantic, and prospective memory. Additionally, we grouped PPA subtypes in the analysis due to limited sample sizes for each subtype. Recent evidence suggests that svPPA exhibit alterations in reward processing and hedonic valuation, possibly stemming from degeneration in the semantic appraisal network [13, 38, 39]. Impairments of this network in participants with svPPA may manifest as dysfunction in socioemotional processing and decreased sensitivity to negative outcomes. Nevertheless, our small sample sizes do not provide sufficient statistical power to draw meaningful conclusions about subtype differences in patients with PPA.
Also, documentation and characterization of financial errors depended on retrospective caregiver report. It is therefore likely that the prevalence of financial mismanagement documented here represents an underestimate, and the reporting of types of error may be biased by availability to recall (i.e., with more dramatic or consequential errors more likely to be reported). To minimize recall bias we limited reporting to financial errors in the previous year; for a research question of this nature recall bias is likely to be unavoidable and caregivers will generally be less susceptible to this bias than participants with dementia or other parties. Lastly, in most cases of financial exploitation among elderly populations, the perpetrator is a family member [40]. Complex familial dynamics and potential reluctance from caregivers to disclose instances of exploitation pose additional challenges to accurate documentation in our study. We would expect this limitation to apply to all studies that rely upon caregiver report, while studies relying on patient report could be compromised by memory impairments in dementia.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank our participants in research. VBM analyses were performed using the open source Brainsight system, developed at University of California San Francisco Memory and Aging Center by Katherine P. Rankin, Cosmo Mielke, and Paul Sukhanov, and powered by the VLSM script written by Stephen M. Wilson, with funding from the Rainwater Charitable Foundation and the UCSF Chancellor’s Fund for Precision Medicine.
Funding sources
This work was supported by the National Institutes of Health (NIA R01AG058817, NIA R01AG022983, NIA P01AG019824, NIA P30AG062422). The sponsors had no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflicts
The authors report no competing interests.
Consent statement
Written informed consent was provided by all participants who had decisional capacity; if participants did not have decisional capacity, consent was obtained from a legally authorized representative. The study protocol was approved by the UCSF Committee on Human Research.
Data availability
The pre-registered analytic plan for this study is available at https://archive.org/details/osf-registrations-vupj7-v1. Because the financial activities questionnaire instrument specifically includes items indicating vulnerability to financial abuse (e.g., whether in the past year a participant with dementia has made financial mistakes due to being too gullible or trusting with others), participant-level data are not publicly available. Academic, not-for-profit investigators may request access to data, subject to approval from the UCSF Human Research Protection Program and the UCSF Memory and Aging Center Executive Committee. Applications can be made via an online resource request form accessible at https://memory.ucsf.edu/research-trials/professional/open-science#Human-Studies and require completion of a Data Use Agreement accessible at the same address. Analytic code used to generate the behavioral results are available at https://osf.io/97vwd/.
REFERENCES
- [1].Pérès K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, Barberger-Gateau P. (2008) Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc 56, 37–44. [DOI] [PubMed] [Google Scholar]
- [2].Lighthall NR (2019) Neural mechanisms of decision-making in aging. Wiley Interdiscip Rev Cogn Sci 1–22. [DOI] [PubMed] [Google Scholar]
- [3].Manthorpe J, Samsi K, Rapaport J (2012) Responding to the financial abuse of people with dementia: A qualitative study of safeguarding experiences in England. Int Psychogeriatr 24, 1454–64. [DOI] [PubMed] [Google Scholar]
- [4].Huang Y, Lawitz A (2016) The New York State cost of financial exploitation study. Office of Children and Family Services. [Google Scholar]
- [5].Lichtenberg PA (2016) Financial exploitation, financial capacity, and Alzheimer’s disease. Am Psychol 71, 312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laumann EO, Leitsch SA, Waite LJ (2008) Elder mistreatment in the United States: Prevalence estimates from a nationally representative study. J Gerontol. Series B, Psychological Sciences and Social Sciences 63, S248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Beach SR, Schulz R, Castle NG, Rosen J (2010) Financial exploitation and psychological mistreatment among older adults: Differences between African Americans and non-African Americans in a population based survey. Gerontologist 50, 744–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Acierno R, Hernandez MA, Amstadter AB, Resnick HS, Steve K, Muzzy W, Kilpatrick DG (2010) Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health 100, 292–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nicholas LH, Langa KM, Bynum JPW, Hsu JW (2021) Financial presentation of Alzheimer disease and related dementias. JAMA Intern Med 181, 220–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jackson SL, Hafemeister TL (2012) APS investigation across four types of elder maltreatment. J Adult Prot 14, 82–92. [Google Scholar]
- [11].Marson D (2016) Commentary: A role for neuroscience in preventing financial elder abuse. Public Policy & Aging Report 26, 12–14. [Google Scholar]
- [12].Chiong W, Hsu M, Wudka D, Miller BL, Rosen HJ (2014) Financial errors in dementia: testing a neuroeconomic conceptual framework. Neurocase 20, 389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chiong W, Wood KA, Beagle AJ, Hsu M, Kayser AS, Miller BL, Kramer JH (2016) Neuroeconomic dissociation of semantic dementia and behavioural variant frontotemporal dementia. Brain 139, 578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beagle AJ, Zahir A, Borzello M, Kayser AS, Hsu M, Miller BL, Kramer JH, Chiong W (2020) Amount and delay insensitivity during intertemporal choice in three neurodegenerative diseases reflects dorsomedial prefrontal atrophy. Cortex 124, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M (2011) Classification of primary progressive aphasia and its variants. Neurology 76, 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Manivannan M, Heunis J, Hooper SM, Bernstein Sideman A, Lui KP, Braley TL, Possin KL, Chiong W (2022) Use of telephone- and internet-based support to elicit and address financial abuse and mismanagement in dementia: Experiences from the Care Ecosystem study. J Alzheimers Dis 86, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community. J Gerontol 37, 323–9. [DOI] [PubMed] [Google Scholar]
- [20].R Core Team (2022) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- [21].Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K, Gitelman D, Glauche V, Henson R, Hutton C, Jafarian A, Kiebel S, Kilner J, Litvak V, Mattout J, Moran R, Penny W, Phillips C, Razi A, Stephan K, Tak S, Tyrer A, Zeidman P (2014) SPM12 manual. London: Wellcome Trust Centre for Neuroimaging. [Google Scholar]
- [22].Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6, 448–50. [DOI] [PubMed] [Google Scholar]
- [23].Chiong W, Wilson SM, D’Esposito M, Kayser AS, Grossman SN, Poorzand P, Seeley WW, Miller BL, Rankin KP (2013)The salience network causally influences default mode network activity during moral reasoning. Brain 136, 1929 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O’Callaghan C, Bertoux M, Irish M, Shine JM, Wong S, Spiliopoulos L, Hodges JR, Hornberger M (2016) Fair play: social norm compliance failures in behavioural variant frontotemporal dementia. Brain 139, 204–16. [DOI] [PubMed] [Google Scholar]
- [25].Sturm VE, Perry DC, Wood K, Hua AY, Alcantar O, Datta S, Rankin KP, Rosen HJ, Miller BL, Kramer JH (2017) Prosocial deficits in behavioral variant frontotemporal dementia relate to reward network atrophy. Brain Behav 7, e00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Toller G, Brown J, Sollberger M, Shdo SM, Bouvet L, Sukhanov P, Seeley WW, Miller BL, Rankin KP (2018) Individual differences in socioemotional sensitivity are an index of salience network function. Cortex 103, 211–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wong S, Irish M, O’Callaghan C, Kumfor F, Savage G, Hodges JR, Piguet O, Hornberger M (2017) Should I trust you? Learning and memory of social interactions in dementia. Neuropsychologia 104, 157–167. [DOI] [PubMed] [Google Scholar]
- [28].Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR (2010) How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology 74, 472–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hornberger M, Piguet O (2012) Episodic memory in frontotemporal dementia: A critical review. Brain 135, 678–92. [DOI] [PubMed] [Google Scholar]
- [30].Poos JM, Jiskoot LC, Papma JM, Van Swieten JC, Van Den Berg E (2018) Meta-analytic review of memory impairment in behavioral variant frontotemporal dementia. J Int Neuropsychol Soc 24, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, Malone SM, Kandala S, Feczko E, Miranda-Dominguez O, Graham AM, Earl EA, Perrone AJ, Cordova M, Doyle O, Moore LA, Conan GM, Uriarte J, Snider K, Lynch BJ, Wilgenbusch JC, Pengo T, Tam A, Chen J, Newbold DJ, Zheng A, Seider NA, Van AN, Metoki A, Chauvin RJ, Laumann TO, Greene DJ, Petersen SE, Garavan H, Thompson WK, Nichols TE, Yeo BTT, Barch DM, Luna B, Fair DA, Dosenbach NUF (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nomura EM, Gratton C, Visser RM, Kayser AS, Perez F, D’Esposito M (2010) Double dissociation of two cognitive control networks in patients with focal brain lesions. Proc Natl Acad of Sci 107, 12017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wu T, Schulz KP, Fan J (2021) Activation of the cognitive control network associated with information uncertainty. Neuroimage 230, 117703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Irish M, Addis DR, Hodges JR, Piguet O (2012) Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 135 (Pt 7), 2178–91. [DOI] [PubMed] [Google Scholar]
- [35].Irish M, Piguet O. The pivotal role of semantic memory in remembering the past and imagining the future (2013) Front Behav Neurosci 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han SD, Boyle PA, Yu L, Arfanakis K, James BD, Fleischman DA, Bennett DA (2016) Grey matter correlates of susceptibility to scams in community-dwelling older adults. Brain Imaging Behav 10, 524–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Han SD, Boyle PA, Arfanakis K, Fleischman D, Yu L, James BD, Bennett DA (2016) Financial literacy is associated with white matter integrity in old age. Neuroimage 130, 223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rijpma MG, Montembeault M, Shdo S, Kramer JH, Miller BL, Rankin KP (2023) Semantic knowledge of social interactions is mediated by the hedonic evaluation system in the brain. Cortex 161, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chokesuwattanaskul A, Jiang H, Bond RL, Jimenez DA, Russell LL, Sivasathiaseelan H, Johnson JCS, Benhamou E, Agustus JL, van Leeuwen JEP, Chokesuwattanaskul P, Hardy CJD, Marshall CR, Rohrer JD, Warren JD (2023) The architecture of abnormal reward behaviour in dementia: multimodal hedonic phenotypes and brain substrate. Brain Commun 5, fcad027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Weissberger GH, Goodman MC, Mosqueda L, Schoen J, Nguyen AL, Wilber KH, Gassoumis ZD, Nguyen CP, Han SD (2020) Elder abuse characteristics based on calls to the National Center on Elder Abuse Resource Line. J Appl Gerontol 39, 1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The pre-registered analytic plan for this study is available at https://archive.org/details/osf-registrations-vupj7-v1. Because the financial activities questionnaire instrument specifically includes items indicating vulnerability to financial abuse (e.g., whether in the past year a participant with dementia has made financial mistakes due to being too gullible or trusting with others), participant-level data are not publicly available. Academic, not-for-profit investigators may request access to data, subject to approval from the UCSF Human Research Protection Program and the UCSF Memory and Aging Center Executive Committee. Applications can be made via an online resource request form accessible at https://memory.ucsf.edu/research-trials/professional/open-science#Human-Studies and require completion of a Data Use Agreement accessible at the same address. Analytic code used to generate the behavioral results are available at https://osf.io/97vwd/.


