Abstract
Practical relevance:
Calcium is essential for many normal physiological processes within the body. Aberrations in calcium homeostasis leading to hypercalcaemia can result in clinical signs such as polyuriav and polydipsia, lethargy and weakness due to depressed excitability of muscle and nervous tissue, and gastrointestinal (GI) signs due to effects on GI smooth muscle. Hypercalcaemia in cats is mostly idiopathic, with chronic kidney disease and neoplasia also being common causes.
Clinical challenges:
Hypercalcaemia can be a diagnostic challenge and a good understanding of the regulation of calcium homeostasis can aid in interpreting results of diagnostic tests. Furthermore, the management approach may depend on the underlying cause of hypercalcaemia, and also its severity and chronicity.
Audience:
This review offers a comprehensive discussion of the regulation of calcium homeostasis, with a focus on the normal response to hypercalcaemia. It also discusses the diagnostic approach to, and management of, hypercalcaemia in cats, as well as specific aetiologies. This is relevant to all clinicians working with feline patients.
Evidence base:
The review draws evidence from peer-reviewed publications and also the author’s own clinical experience.
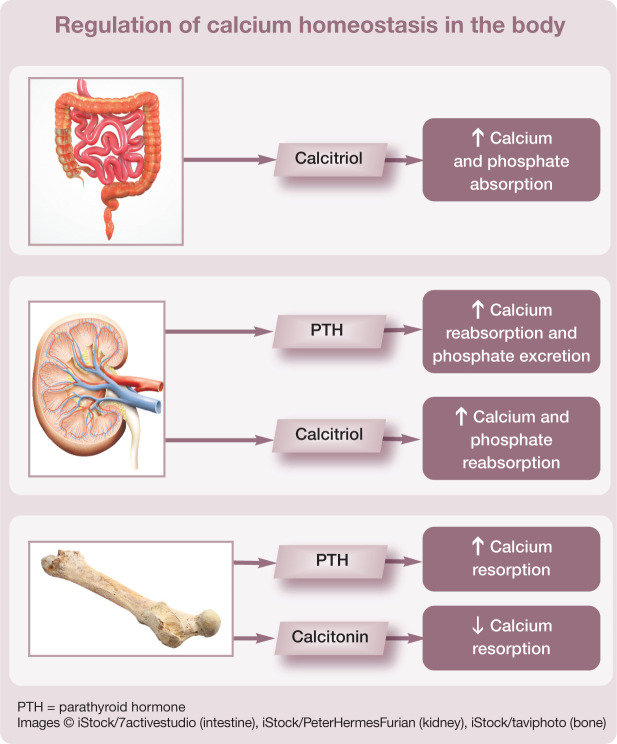
Calcium function and homeostasis
Calcium plays a key role in many normal physiological processes. It is important in neuromuscular transmission, enzyme activity, blood coagulation and muscle contraction (including skeletal, smooth and cardiac muscle), and is required for intracellular signalling and normal cellular function. Calcium mediates vascular smooth muscle contraction and maintains blood vessel tone and hence blood pressure. It enters vascular smooth muscle cells via voltage-dependent calcium channels (L-type calcium channels) or can be released from intracellular stores; the resultant increase in the intracellular concentration of calcium stimulates vascular smooth muscle contraction. Calcium is also the most abundant component of the skeleton, being required both for bone formation/resorption and for maintaining the structural integrity of bones and teeth.
Regulation of calcium is complex (see box on page 388). It is generally accepted that parathyroid hormone (PTH) is responsible for the minute-to-minute control of calcium and calcitriol for day-to-day control, with calcitonin having a relatively minor role in calcium regulation in the adult. The three organ systems responsible for calcium homeostasis are the gastrointestinal (GI) tract, kidneys and bone.
Hormones involved in regulation
Parathyroid hormone PTH increases calcium reabsorption in the distal convoluted tubules of the kidney and decreases phosphate reabsorption from the proximal tubules, resulting in reduced calcium and increased phosphate excretion in the urine. It is reported that approximately 98–99% of filtered calcium is reabsorbed in the kidneys of normal human patients, 1 with the predominant site of reabsorption being the proximal tubules. PTH also stimulates osteoclastic bone resorption and increases the number of osteoclasts on the bone surface to release calcium and phosphate from bone.
Calcitriol Otherwise known as the active form of vitamin D, calcitriol (1,25 dihydroxy-cholecalciferol) increases the absorption of calcium and phosphate from the GI tract and also reabsorption in the renal tubules. Intestinal absorption of calcium involves an active transport process in the duodenum. 2 A calcium pump system transports calcium from the mucosa into blood. Transport of calcium across the enterocyte requires the carrier protein calbindin, which is vitamin D dependent. If the diet is low in calcium this pump system becomes more active; if dietary calcium is high, the pump system becomes less active. Passive diffusion of calcium across the enterocyte can also occur in the jejunum and ileum, and to a lesser extent in the colon, when concentrations of calcium are high. 2 However, it has been suggested that calcium absorption is unaffected by calcium intake and that mechanisms other than GI absorption are more important for its homeostasis in the short term in cats and dogs. 3
Calcitonin Calcitonin is a potent calcium-lowering hormone and acts predominantly on bone to inhibit osteoclastic bone resorption.

Processes of homeostatic regulation
PTH is produced from chief cells in the parathyroid gland in response to ionised hypocalcaemia (decreased ionised calcium). Its actions to increase renal tubular reabsorption and bone resorption of calcium, and increase calcitriol activation in the kidneys, result in a net effect of increasing the ionised calcium concentration.
Calcitriol is activated in the kidneys under the influence of the enzyme 1-α-hydroxylase. It increases calcium absorption in the GI tract and reabsorption in the renal tubules, and enhances the ability of PTH for bone resorption. Therefore, the net effect is also to increase ionised calcium concentration. When PTH concentration is low, calcitriol increases bone uptake of mineral; this maintains calcium concentration within the bone to support plasma ionised calcium. Cats have low levels of vitamin D in their skin; and, unlike in humans, it is not synthesised in the skin in response to sunlight. 4 Therefore, cats are dependent on dietary intake and vitamin D is often supplemented in pet foods. It is important to remember that PTH and calcitriol are also influenced by phosphate concentrations.
Calcitonin is secreted from C cells in the thyroid gland in response to hypercalcaemia. Calcitonin inhibits osteoclastic activity, which in turn decreases bone resorption. It can also increase renal excretion of calcium via decreased tubular reabsorption. GI hormones such as gastrin can stimulate the secretion of calcitonin, 2 and this mechanism is considered to help regulate postprandial hypercalcaemia. Calcitonin may also have an important role in regulating bone turnover.
The relationship between calcium and its homeostatic regulators is presented in Figure 1.
Figure 1.
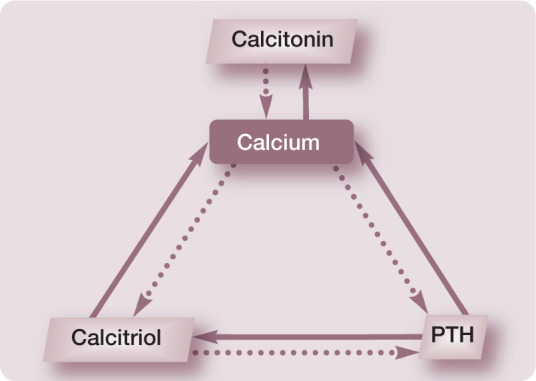
Relationship between calcium and its homeostatic regulators. Solid arrows indicate a positive (stimulatory) relationship and dotted arrows indicate a negative (inhibitory) relationship. PTH = parathyroid hormone
Physiological response to hypercalcaemia
The normal physiological response to hypercalcaemia includes decreased production of PTH from the parathyroid gland, increased production of calcitonin from C cells in the thyroid gland, and decreased calcitriol production in the kidneys due to direct inhibition and also decreased PTH production.
This results in:
Reduced release of calcium and phosphate from bone, due to reduced PTH concentrations;
Increased renal excretion of calcium, due to decreased PTH and calcitriol concentrations;
Decreased intestinal absorption of calcium, due to decreased calcitriol concentration.
The relationship between calcitropic hormones and calcium can be evaluated further by examining the PTH–calcium and calcitonin– calcium curves. The PTH–calcium curve generated in a study of healthy cats with induced acute hypercalcaemia shows a decrease in PTH as calcium increases, as would be the expected response (Figure 2). 5 When examining the calcitonin–calcium curve (Figure 3), it can be seen that there is a groupof cats that do not increase their calcitonin concentration in response to hypercalcaemia. This group has been termed ‘non-responders’. 6 This recent finding may suggest that certain cats are unable to evoke a normal physiological response in the face of hypercalcaemia to increase calcitonin and hence decrease the ionised calcium concentration. The study was performed in cats with acutely induced hypercalcaemia and further studies of cats with chronic, naturally occurring hypercalcaemia are required.
Figure 2.

The hypercalcaemic part of the parathyroid hormone (PTH)–calcium curve. PTH concentration decreases in response to increasing ionised calcium concentration. I-PTH is intact PTH and W-PTH is whole PTH (see page 391). Reproduced from Pineda et al, 5 with permission
Figure 3.
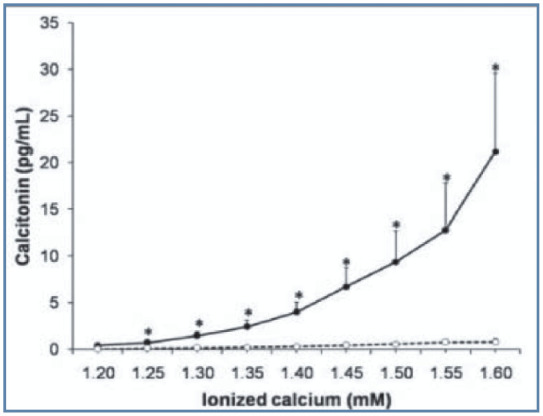
Calcitonin–calcium curve. Solid line represents the group of cats in which calcitonin concentration increases in response to increasing ionised calcium – that is the expected response. The dashed line represents a second group of ‘non-responder’ cats that do not increase their calcitonin concentration in response to hypercalcaemia. Reproduced from Pineda et al, 6 with permission
Pathophysiology
Identification of hypercalcaemia and implementation of appropriate therapy is important as soft tissue calcification is a likely sequela if the calcium x phosphate product becomes increased. Mineralisation of renal tissue may result in nephron injury or changes in renal blood flow, causing a decline in renal function and azotaemia. Hypercalcaemia may promote formation of calcium oxalate uroliths, which can lead to urinary tract obstruction. Whether acute hypercalcaemia induces pancreatitis remains controversial. Early studies examining the effects of calcium administration on the pancreas of cats identified necrosis of pancreatic acinar7,8 and ductal cells 8 after 12 h of intravenous calcium infusion, suggesting that hypercalcaemia may play a role in pancreatitis.
Measurement of calcium
Approximately 99% of total body calcium is stored in bones in the form of hydroxyapatite crystals. 9 These crystals, which contain calcium, phosphate and water, can act as a reservoir from which calcium is released when extracellular calcium concentration declines. Calcium can also be found intracellularly and, as mentioned, is important in normal cellular function. The smallest pool of calcium in the body is found in the extracellular space. This includes calcium in the blood, interstitium and accessible bone–calcium pool. It is calcium in the extracellular space that is measured in the clinical patient.
Extracellular calcium exists in three fractions (see below).
It is important when collecting a blood sample for measurement of calcium not to transfer the sample into a tube containing EDTA anticoagulant (which will chelate calcium and artificially decrease its concentration). Results for ionised calcium are also lower in heparinised blood samples compared with serum samples, so cannot be directly compared. 11 The age of the cat will additionally affect calcium concentration. Plasma calcium is higher in young animals during growth phases due to increased bone turnover. Kittens will have increased concentrations; both total and ionised calcium concentration can remain increased until 12 months of age (Figure 4). 12
Figure 4.
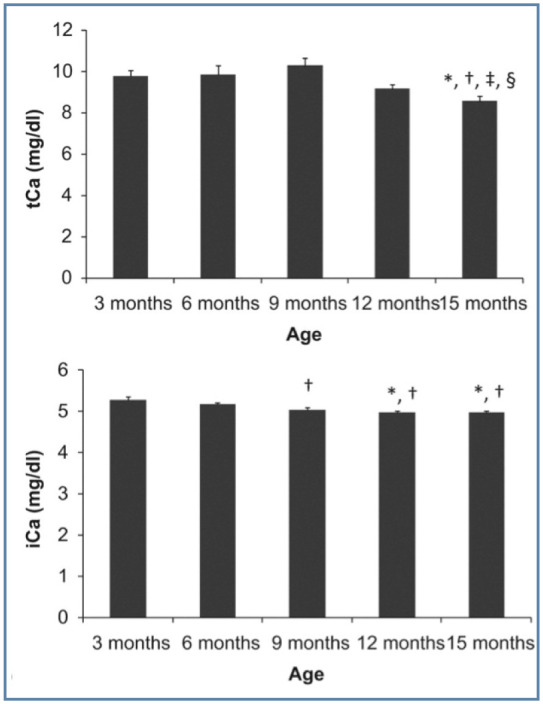
Total and ionised calcium (tCa and iCa, respectively) in growing cats. * = Significantly different to 3 months, † = significantly different to 6 months, ‡ = significantly different to 9 months, § = significantly different to 12 months. Reproduced from Pineda et al, 12 with permission
Use of adjustment formulae to correct total calcium for albumin or total protein concentration is not recommended. Moreover, it is not possible to accurately predict ionised calcium from total calcium. In one study, total calcium was inaccurate in predicting ionised calcium in 40% of cats, with the prevalence of hypercalcaemia and normocalcaemia being underestimated and hypocalcaemia being overestimated. 10
Ionised calcium concentration must be measured to accurately assess a patient’s calcium status, and requires analysis with ion-selective electrodes (Figure 5). Samples should be collected anaerobically if analysis cannot be performed immediately. Exposure to air will lead to loss of CO2 and an increase in pH; this promotes calcium binding to protein and results in a decreased ionised calcium concentration. The converse of this is seen in acidaemic patients; in this physiological situation hydrogen ions will displace protein-bound calcium ions and increase the ionised calcium concentration. If in-house analysis of ionised calcium is not possible, samples will need to be transported to a reference laboratory on ice to prevent formation of lactic acid resulting in acidosis. Submission of the sample should be discussed with the laboratory prior to collection. Commercial laboratories reporting results of samples that have been transported for analysis will usually note the pH of the sample alongside the ionised calcium concentration to aid interpretation of the result by the clinician.
Figure 5.

Ion-selective electrode analysers should be used for measurement of ionised calcium
Diagnostic approach
Blood testing
Biochemistry
Ionised calcium should be measured to confirm hypercalcaemia if total calcium is elevated. Hypercalcaemia should also be demonstrated to be persistent. A cat with an ionised calcium concentration >1.40 mmol/l is generally considered to be hypercalcaemic. Full biochemical analysis should be undertaken, ensuring the panel includes assessment of renal function and phosphate concentration. If available, acid–base analysis should also be performed.
Parathyroid hormone (PTH)
One useful approach is to assess if the hypercalcaemia is parathyroid dependent (ie, arising from the parathyroid gland) or parathyroid independent. This is achieved by measuring PTH. The majority of cats will have parathyroid-independent hypercalcaemia. Indeed, in one study only 8.4% of hypercalcaemic cats were reported to have parathyroid-dependent hypercalcaemia. 17 In normal patients, PTH production from the parathyroid glands will be suppressed in response to hypercalcaemia. Therefore, if PTH concentration is in the upper two-thirds of the reference interval or is increased, it suggests a parathyroid-dependent cause. A comprehensive review of feline hyperparathyroidism has recently been published, to which clinicians can refer for further information. 18
Caution is required in interpreting results of PTH assays. Appropriate sample handling must be ensured as PTH is relatively heat labile, which can affect results. Samples must be shipped on ice and the commercial laboratory to which the sample is to be submitted should be contacted to discuss sample transport.
Also, feline PTH is 84% homologous to human PTH, 19 which is important because assays used to measure PTH in cats are human assays. Furthermore, there are different assays available for measuring PTH. The second-generation (intact) PTH assays measure intact PTH (1-84) and also PTH (7-84) fragments. PTH fragments can accumulate in kidney disease, and may result in falsely elevated results. The third-generation (whole) PTH assays do not measure the fragments. Earlier studies validating PTH measurement in cats used intact PTH assays that are no longer commercially available.20,21 A recent study in cats validating human intact PTH and whole PTH assays found greater whole PTH concentrations compared with intact PTH. 6 This was thought to be related to reduced affinity of the antibody utilised in the assay (which was developed for use with human samples) against feline PTH. Given the variable results obtained with different assays, it is important to contact the laboratory to which the sample is to be submitted to discuss which assay is being used and whether appropriate validation and reference intervals have been established in the laboratory for the assay.
Parathyroid hormone-related peptide (PTH-rp)
Parathyroid hormone-related peptide (PTH-rp) can be measured if malignancy is suspected – but can be normal. Thus, a normal value does not fully exclude neoplasia. 17
Vitamin D metabolites
1,25 dihydroxycholecalciferol (calcitriol) and 25-hydroxyvitamin D3 (calcidiol) can be measured. Calcitriol reflects metabolically active vitamin D and calcidiol reflects cholecalciferol or ergocalciferol ingestion following hydroxylation. Calcidiol is the major circulating form of vitamin D.
Vitamin D metabolites are identical in all species; hence assays used to measure vitamin D perform better than those used to measure PTH. However, binding proteins can differ between species, which can interfere with assays; therefore, cross-species validation is required. Samples collected for measurement of vitamin D metabolites should be protected from light, to inhibit degradation.
Urinalysis
Increased excretion of calcium in the urine of cats that are hypercalcaemic can predispose them to calcium oxalate urolith formation. Uroliths were identified in 15% of hypercalcaemic cats in a retrospective study, of which 73% were composed of calcium oxalate. 14
Diagnostic imaging
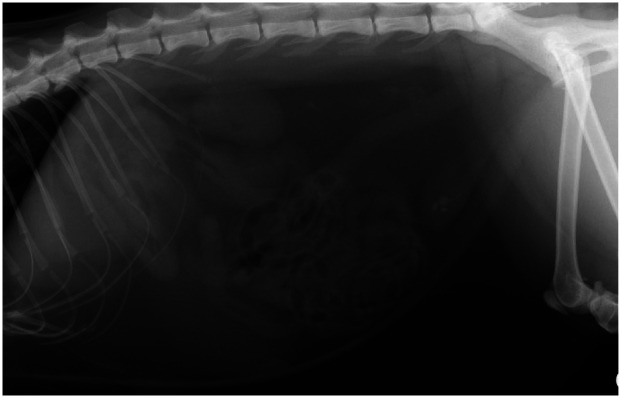
Thoracic and abdominal imaging can be helpful in screening for neoplasia or granulomatous lesions. Soft tissue calcification can occur when the calcium x phosphate product is >5.6 mmol2/l2. The kidneys and gastric mucosa are the predominant organs to be affected and this may be visualised on radiographs or CT images (Figure 6). Radiographs may also identify calcium oxalate nephroliths, ureteroliths or cystoliths (Figure 7). If primary hyperparathyroidism is suspected (which, as discussed above, is rare in cats), cervical ultrasonography can be performed to attempt to visualise a parathyroid mass, although this requires some expertise.
Figure 6.

Transverse CT image of the cranial abdomen and caudal thorax of a hypercalcaemic cat. The arrow indicates a mineralised gastric wal
Figure 7.
Right lateral radiograph of the abdomen of a cat with radiopaque calculi within the urinary bladder
General management
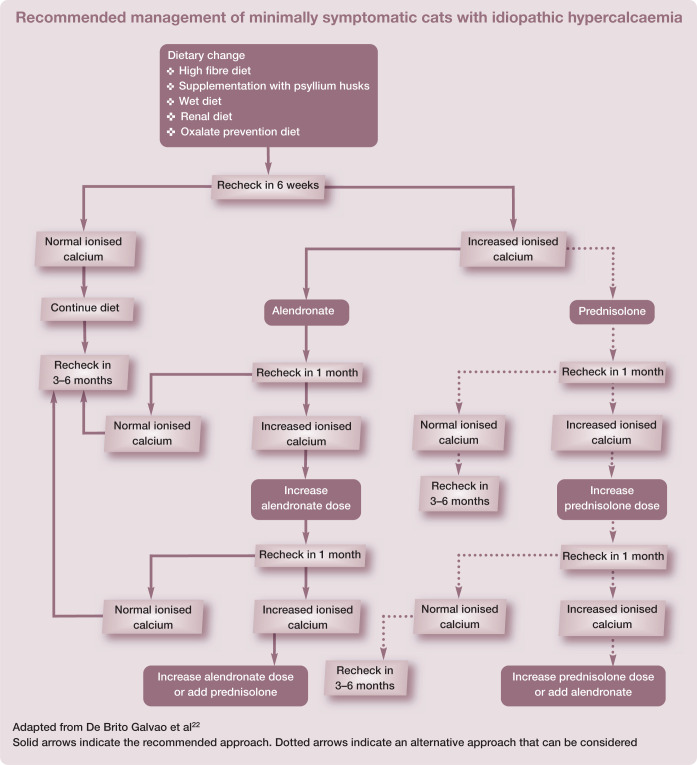
The management approach will depend not only on the severity of the hypercalcaemia but also the time frame of development (ie, acute vs chronic). Patients with mild hypercalcaemia (ionised calcium <0.25 mmol/l above the reference interval 22 ) that are asymptomatic and have a normal calcium x phosphate product may require no immediate treatment, whereas patients experiencing a severe acute rise in calcium concentration may require more aggressive treatment (see box on page 393).
No single treatment is recommended for managing all cases of hypercalcaemia and, therefore, the underlying cause should be addressed (see specific aetiologies section later). Supportive therapy is aimed at enhancing renal excretion of calcium and preventing calcium resorption from bone. Table 1 lists drugs that can be used for the management of hypercalcaemia and their doses.
Table 1.
Drugs for use in the management of hypercalcaemic cats
| Drug | Class of drug | Dose | Route of administration | Frequency of administration | Comments |
|---|---|---|---|---|---|
| Furosemide | Diuretic | 1–2 mg/kg | IV, SC, PO | q8–12h | Ensure the patient is fully hydrated before instigating furosemide therapy |
| Prednisolone | Glucocorticoid | 0.5–1 mg/kg | PO | q12–24h | Avoid use until a definitive diagnosis is reached |
| Dexamethasone | Glucocorticoid | 0.1–0.2 mg/kg | IV, SC | q24h | Avoid use until a definitive diagnosis is reached |
| Pamidronate | Bisphosphonate | 1.0–2.0 mg/kg | Slow (approx 4 h) infusion in 0.9% NaCl | May be repeated after 7–14 days | Nephrotoxicity possible; renal function should be monitored |
| Alendronate | Bisphosphonate | 5–20 mg/cat | PO | Every 7 days | Administer water or a small amount of food following medication to facilitate passage of the pill and reduce risk of oesophageal ulceration |
| Calcitonin | Hormone | 4–6 IU/kg | SC | q8–12h | Salmon calcitonin is the most potent form available |
IV = intravenously; SC = subcutaneously; PO = orally
Promoting calciuresis
Management can include intravenous fluid therapy (IVFT) and furosemide administration to promote calciuresis. It is important that a cat is well hydrated before initiating furosemide therapy. IVFT can be given to correct any fluid deficits and the associated volume expansion will aid in diluting circulating calcium concentrations. Isotonic saline (0.9% NaCl) is the fluid of choice as it does not contain calcium. If there is concern regarding a patient’s renal or cardiac function, fluid therapy should be approached cautiously as cats are susceptible to fluid volume overload given their small size.
Furosemide acts to inhibit calcium reabsorption in the loop of Henle. Thiazide diuretics should not be used as these enhance the reabsorption of calcium in the renal distal tubules (based on data in human patients, albeit has not been studied in cats or dogs).
Glucocorticoids
Glucocorticoids act to reduce bone resorption, decrease intestinal absorption and increase renal excretion of calcium. Their mechanism of action is thought to be through inhibiting prostaglandin E, osteoclastic activating factor and vitamin D production. 23 It is important to avoid steroids if the underlying aetiology remains unclear and further investigations to determine a definitive diagnosis are to be pursued.
Bisphosphonates
Bisphosphonates exert their effect by reducing the number and activity of osteoclasts. There are few reports on the use of these drugs in cats. Pamidronate is available and is administered as an intravenous infusion. Recently alendronate has been evaluated in a small study of cats with idiopathic hypercalcaemia. 24 Approximately two-thirds of the cats in the study achieved normocalcaemia during the 6 month study period and no adverse effects were noted. The advantage of alendronate over other bisphosphonates is its oral route of administration. In addition, it requires only once weekly administration. To optimise GI absorption it is best administered after fasting.
Calcitonin
Although calcitonin is one of the normal physiological regulators of calcium (decreasing its concentration), its effects are short-lived, requiring multiple daily administration; therefore, it does not lend itself to use in clinical patients. In dogs it has also been associated with side effects, such as anorexia and vomiting, as well as development of tolerance to therapy. 25 However, calcitonin is rapid acting following intravenous administration and, thus, may be appropriate for use in the emergency management of acute hypercalcaemia.
Dietary change
Dietary change is perhaps one of the most important aspects of management in cats with idiopathic hypercalcaemia. High fibre diets can be fed to bind intestinal calcium, thus reducing its absorption. Wet diets can also be helpful in promoting diuresis and generally have a lower calcium content than dry diets. Renal diets may be successful in some cases as these are low in calcium and phosphate, although calcium availability is higher compared with maintenance diets. Renal diets are also more alkalising than normal maintenance diets. However, hypercalcaemia has been noted in cats fed a renal diet and so this diet should be discontinued if hypercalcaemia worsens. Diets formulated for management of calcium oxalate urolithiasis may also be considered, as these are restricted in calcium and decrease urinary acidification.
Specific aetiologies
Idiopathic hypercalcaemia
Idiopathic hypercalcaemia is considered the most common diagnosis in hypercalcaemic cats and the cause remains unknown. There may be genetic factors or possibly dietary factors involved. Some studies have reported hypercalcaemia associated with the feeding of an acidifying diet or use of urinary acidifiers, which resolved after dietary change or discontinuation of the urinary acidifying therapy. 28 These dietary modifications may result in a chronic metabolic acidosis that increases bone resorption and hence promotes hypercalcaemia and urinary calcium excretion,29,30 although this remains unproven in healthy cats. 31
Idiopathic hypercalcaemia can be seen in cats of any age and there is no sex predisposition; however, longhaired cats appear to be overrepresented. 13 Clinical signs are often vague or absent and hypercalcaemia may be detected as an incidental finding. In some cases calcium may be increased for months without overt clinical signs. If clinical signs do develop these may include weight loss, diarrhoea, constipation, vomiting or anorexia. In patients with idiopathic hypercalcaemia, the total and ionised calcium levels are elevated but phosphate, PTH-rp and vitamin D metabolites are normal. PTH may be normal or decreased due to the suppressive effect of hypercalcaemia. The magnitude of hypercalcaemia in affected cases is generally mild to moderate. In one retrospective study, 10% of cats with idiopathic hypercalcaemia were reported to have mild ionised hypercalcaemia (1.40–1.50 mmol/l), 80% had moderate ionised hypercalcaemia (1.50–1.75 mmol/l) and 10% had severe ionised hypercalcaemia (>1.75 mmol/l). 13
Dietary management may be successful in many cases. Feeding a high fibre diet, supplementing with psyllium husks or feeding a wet diet is recommended as an initial first step, particularly in cats with only mild or moderate hypercalcaemia. A renal diet or diet formulated for management of calcium oxalate urolithiasis can be considered as an alternative, as discussed above.
An algorithm detailing the approach to managing minimally symptomatic cats with idiopathic hypercalcaemia is presented on page 395.
Chronic kidney disease
Hypercalcaemia and renal azotaemia can be a diagnostic challenge as hypercalcaemia can cause kidney damage or develop as a consequence of CKD. The prevalence of hypercalcaemia increases with the severity of azotaemia, with one study reporting 8% of cats with early compensated kidney disease to have a total hypercalcaemia, compared with 18% of cats with uraemic kidney disease and 38% of cats with end-stage kidney disease. 32 Generally speaking, in cats with early and mid-stage CKD, ionised calcium is normal or low and total calcium is normal or increased. This is the result of increased phosphate concentration associated with reduced renal clearance forming complexes with ionised calcium. Indeed, in the study reporting total hypercalcaemia with early compensated, uraemic and end-stage kidney disease, 0%, 9% and 6% of cats had ionised hypercalcaemia for each group, respectively. 32 It is thought that the total hypercalcaemia that may be seen in cats with CKD is associated with increased complexed calcium, although this remains unstudied.
In late-stage CKD in human patients, tertiary hyperparathyroidism can develop from progression of renal secondary hyperparathyroidism. Whether this occurs in cats is unclear, although hyperplastic parathyroid glands have been noted in some cats with IRIS stage 4 CKD. Tertiary hyperparathyroidism is thought to result from an altered set point of the calcium-sensing receptor and uncontrolled secretion of PTH from the parathyroid gland.
Management of hypercalcaemia in cats with CKD can include reducing phosphate retention through feeding a renal diet that is restricted in protein and phosphate. Intestinal phosphate binders can also be used. However, it important to note that some cats can develop hypercalcaemia when being fed a renal diet. The reasons for this remain unclear. In one study, 3/15 (20%) cats with CKD developed hypercalcaemia while being fed a renal diet. 33 Low PTH was documented in all three cats, indicating this was not the result of tertiary hyperparathyroidism. Increased phosphate intake led to resolution of hypercalcaemia in all three cases, which suggested that development of hypercalcaemia was a response to phosphate restriction. Some phosphate binders contain calcium carbonate that may, theoretically, contribute to hypercalcaemia.
Some clinicians advocate the use of calcitriol as part of the management of CKD in cats on the basis that it may be of benefit due to decreased calcitriol production in the diseased kidney. Calcitriol will also suppress PTH production, thus aiding in the management of renal secondary hyperparathyroidism. However, there is a risk of development of hypercalcaemia with its useand, if concurrent hyperphosphataemia is present, this may contribute to soft tissue mineralisation. Furthermore, despite studies, there is no proven clinical benefit and, therefore, use of calcitriol is not recommended at this time. It would also be contraindicated in a hypercalcaemic patient.
Hyperparathyroidism
Primary hyperparathyroidism is rare in cats, as discussed earlier. It is associated with increased total and ionised calcium concentrations, increased PTH concentration, decreased phosphate concentration and normal or increased calcitriol. If primary hyperparathyroidism is confirmed, the abnormal parathyroid tissue can be surgically resected, with close monitoring for development of hypocalcaemia or recurrent laryngeal nerve damage following surgery. Other management approaches that have been reported in dogs include ultrasound-guided radiofrequency heat ablation and ethanol ablation of the parathyroid gland. 34
Secondary hyperparathyroidism can be classified as nutritional or renal. In nutritional hyperparathyroidism it is low calcium or an altered calcium:phosphate ratio in the diet that stimulates production of PTH. Therefore, it is not generally associated with hypercalcaemia. It is something that may be recognised more commonly with the increasing popularity of feeding bone and raw food diets, with between 20–60% of such diets being unbalanced in calcium, vitamin D and phosphate. 2 Nutritional secondary hyperparathyroidism is typically associated with a predominantly meat diet, as these are low in calcium but high in phosphate which stimulates PTH production.
Renal secondary hyperparathyroidism results from reduced renal clearance of phosphate in patients with a decreased glomerular filtration rate. The increasing blood phosphate forms complexes with calcium, resulting in a corresponding decrease in calcium concentration and stimulating PTH production.
Tertiary hyperparathyroidism results from autologous secretion of PTH from the parathyroid gland and can lead to development of hypercalcaemia. It is rarely recognised in cats but can be associated with end-stage kidney disease.
Malignancy-associated hypercalcaemia
Hypercalcaemia of malignancy is less common in cats than in dogs. Neoplasia as the underlying cause is reportedly found in approximately two-thirds of hypercalcaemic dogs vs one-third of hypercalcaemic cats. 23
Hypercalcaemia can develop through humoral mechanisms or through osteolytic mechanisms. Osteolytic mechanisms can include metastatic spread to bone, haematological abnormalities in the bone marrow or local production of bone-resorbing factors. Primary bone neoplasia is rarely associated with hypercalcaemia. In humoral hypercalcaemia of malignancy, production of PTH-rp plays an important role in the pathogenesis; however, cytokines such as interleukin-1 (IL-1) and transforming growth factor-beta (TGF-β) may also play a role. 23 Both PTH-rp and cytokines have similar functions to PTH in stimulating bone resorption.
In cats, squamous cell carcinoma and lymphoma are the two most common neoplasms associated with hypercalcaemia, accounting for two-thirds of all cases of neoplasia in one retrospective study. 14 Other neoplasms that have been associated with hypercalcaemia in cats include multiple myeloma, 35 bronchocarcinoma, 14 osteosarcoma 14 and fibrosarcoma. 14
Both total and ionised calcium are increased in patients with malignancy-associated hypercalcaemia and, therefore, PTH concentration should be low. PTH-rp can be increased, but can also be normal. 17 Management involves specific therapy for the underlying neoplasia. The use of glucocorticoids prior to confirmation of the diagnosis should be avoided, as these drugs will interfere with the ability to make a diagnosis of lymphoma. There are no studies examining whether survival times for cats with neoplasia associated with hypercalcaemia are worse than for those without hypercalcaemia.
Hypervitaminosis D
Hypervitaminosis D refers to toxicity resulting from calcidiol or calcitriol as well as cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Iatrogenic causes include excessive dietary supplementation or excessive treatment. Certain plants such as jessamine (a variant of jasmine) contain glycosides of calcitriol and, therefore, ingestion can lead to hypercalcaemia. Some rodenticides also contain cholecalciferol. Topical ointments for management of psoriasis can contain vitamin D analogues and possible exposure to such creams should be discussed with owners. As these ointments contain vitamin D analogues, calcidiol or calcitriol concentrations will not generally be increased if measured.
There is typically a parallel increase in calcium and phosphate concentration in patients with hypervitaminosis D. Calcidiol can be normal or increased depending on which form of vitamin D is associated with the intoxication. PTH concentration will be low due to hypercalcaemia and also the suppressive effects of calcitriol. Calcidiol can remain elevated for weeks to months following intoxication due to lipid storage and slow release. The decline of calcitriol is more rapid. The presence of soft tissue calcification may be more suggestive of vitamin D toxicity than other causes of hypercalcaemia, as both calcium and phosphate rise in parallel, resulting in an increased calcium x phosphate product.
Granulomatous disease
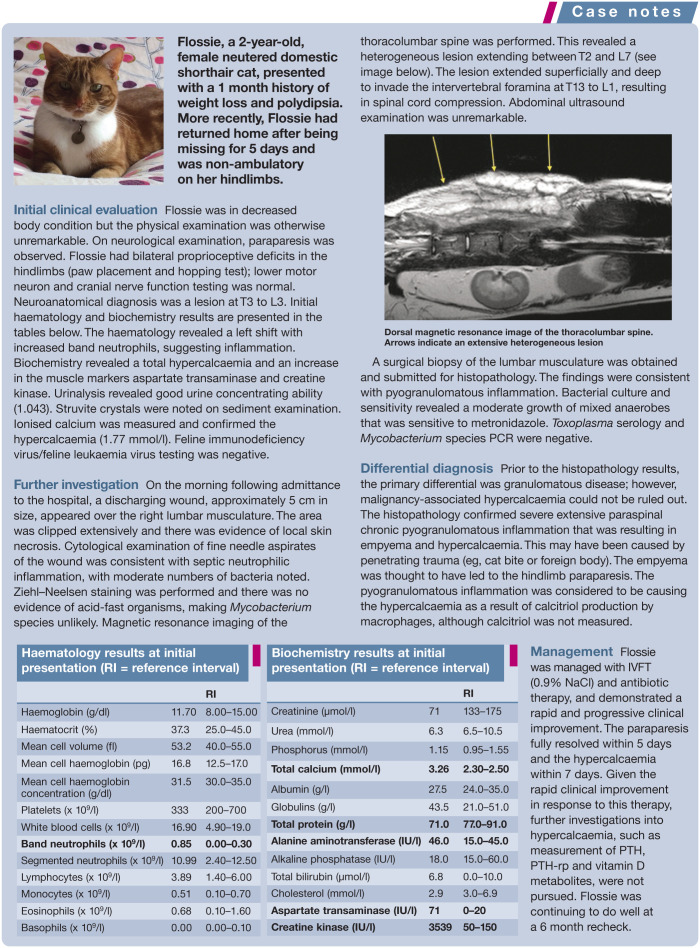
Granulomatous inflammation is a potential, but uncommon, cause of hypercalcaemia in cats. Hypercalcaemia can result from granulomatous inflammation because macrophages can synthesise calcitriol from calcidiol without negative feedback regulation. Therefore, calcitriol concentrations can be high and calcidiol concentrations normal. Hypercalcaemia has been associated with mycobacteria, 35 feline infectious peritonitis, 14 toxoplasmosis, 14 Nocardia species, 36 Cryptococcus species 14 and Actinomyces rhinitis 14 in cats.
It may be difficult to distinguish between hypercalcaemia associated with granulomatous disease and neoplasia if abnormalities are found on physical examination or imaging. Increased calcitriol concentration may be helpful; however, a definitive diagnosis should be based on cytological or histopathological examination of tissue.
Hypoadrenocorticism
Hypoadrenocorticism (Addison’s disease) is much less common in cats than in dogs. The mechanism by which hypercalcaemia develops is poorly understood. It may be associated with increased renal reabsorption of calcium secondary to hypovolaemia, the presence of a metabolic acidosis or increased bone resorption. The hypercalcaemia resolves with successful management of hypoadrenocorticism. Standard treatment generally involves the use of fludrocortisone ± prednisolone.
Key Points
The important regulators of calcium are PTH, calcitriol and calcitonin.
Hypercalcaemia in cats is mostly idiopathic, with chronic kidney disease and neoplasia also being common causes.
Diagnosis of hypercalcaemia should be based on a persistently increased ionised calcium concentration (>1.40 mmol/l).
The diagnostic approach should include determination of whether the hypercalcaemia is parathyroid dependent or independent. The presence of high or inadequately suppressed PTH suggests parathyroid-dependent disease. This is rare in cats.
Although it is the most commonly made diagnosis in hypercalcaemic cats, the underlying aetiology of idiopathic hypercalcaemia remains unknown. It is generally associated with mild to moderate hypercalcaemia.
Management of hypercalcaemia is dependent on the underlying cause, and its severity and chronicity. Cats with an increased calcium x phosphate product (>5.6 mmol2/l2) are at risk of soft tissue mineralisation and require treatment. Patients with only mild or moderately increased calcium concentration that are asymptomatic may not require immediate therapy. Severe hypercalcaemia requires emergency treatment.
Acknowledgments
The author would like to acknowledge Jenny Reeve who was the clinician with primary responsibility for the management of Flossie.
Footnotes
Funding: The author received no financial support for the research, authorship and/or publication of this article.
The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- 1. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015; 10: 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cline J. Calcium and vitamin d metabolism, deficiency, and excess. Top Companion Anim Med 2012; 27: 159–164. [DOI] [PubMed] [Google Scholar]
- 3. Mack JK, Alexander LG, Morris PJ, et al. Demonstration of uniformity of calcium absorption in adult dogs and cats. J Anim Physiol Anim Nutr (Berl) 2015; 99: 801–809. [DOI] [PubMed] [Google Scholar]
- 4. Morris JG. Ineffective vitamin D synthesis in cats is reversed by an inhibitor of 7-dehydrocholestrol-delta7-reductase. J Nutr 1999; 129: 903–908. [DOI] [PubMed] [Google Scholar]
- 5. Pineda C, Aguilera-Tejero E, Raya AI, et al. Feline parathyroid hormone: validation of hormonal assays and dynamics of secretion. Domest Anim Endocrinol 2012; 42: 256–264. [DOI] [PubMed] [Google Scholar]
- 6. Pineda C, Aguilera-Tejero E, Raya AI, et al. Assessment of calcitonin response to experimentally induced hypercalcemia in cats. Am J Vet Res 2013; 74: 1514–1521. [DOI] [PubMed] [Google Scholar]
- 7. Frick TW, Spycher MA, Heitz PU, et al. Hypercalcaemia and pancreatic ultrastructure in cats. Eur J Surg 1992; 158: 289–294. [PubMed] [Google Scholar]
- 8. Frick TW, Hailemariam S, Heitz PU, et al. Acute hypercalcemia induces acinar cell necrosis and intraductal protein precipitates in the pancreas of cats and guinea pigs. Gastroenterology 1990; 98: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 9. Greco DS. Endocrine causes of calcium disorders. Top Companion Anim Med 2012; 27: 150–155. [DOI] [PubMed] [Google Scholar]
- 10. Schenck PA, Chew DJ. Prediction of serum ionized calcium concentration by serum total calcium measurement in cats. Can J Vet Res 2010; 74: 209–213. [PMC free article] [PubMed] [Google Scholar]
- 11. Ceron JJ, Martinez-Subiela S, Hennemann C, et al. The effects of different anticoagulants on routine canine plasma biochemistry. Vet J 2004; 167: 294–301. [DOI] [PubMed] [Google Scholar]
- 12. Pineda C, Aguilera-Tejero E, Guerrero F, et al. Mineral metabolism in growing cats: changes in the values of blood parameters with age. J Feline Med Surg 2013; 15: 866–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Midkiff AM, Chew DJ, Randolph JF, et al. Idiopathic hypercalcemia in cats. J Vet Intern Med 2000; 14: 619–626. [DOI] [PubMed] [Google Scholar]
- 14. Savary KC, Price GS, Vaden SL. Hypercalcemia in cats: a retrospective study of 71 cases (1991–1997). J Vet Intern Med 2000; 14: 184–189. [DOI] [PubMed] [Google Scholar]
- 15. Schenck PA. Calcium homeostasis in thyroid disease in dogs and cats. Vet Clin North Am Small Anim Pract 2007; 37: 693–708, vi. [DOI] [PubMed] [Google Scholar]
- 16. Williams TL, Elliott J, Berry J, et al. Investigation of the pathophysiological mechanism for altered calcium homeostasis in hyperthyroid cats. J Small Anim Pract 2013; 54: 367–373. [DOI] [PubMed] [Google Scholar]
- 17. Bolliger AP, Graham PA, Richard V, et al. Detection of parathyroid hormone-related protein in cats with humoral hypercalcemia of malignancy. Vet Clin Pathol 2002; 31: 3–8. [DOI] [PubMed] [Google Scholar]
- 18. Parker VJ, Gilor C, Chew DJ. Feline hyperparathyroidism: pathophysiology, diagnosis and treatment of primary and secondary disease. J Feline Med Surg 2015; 17: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toribio RE, Kohn CW, Chew DJ, et al. Cloning and sequence analysis of the complementary DNA for feline preproparathyroid hormone. Am J Vet Res 2002; 63: 194–197. [DOI] [PubMed] [Google Scholar]
- 20. Finch NC, Syme HM, Elliott J. Parathyroid hormone concentration in geriatric cats with various degrees of renal function. J Am Vet Med Assoc 2012; 241: 1326–1335. [DOI] [PubMed] [Google Scholar]
- 21. Barber PJ, Elliott J, Torrance AG. Measurement of feline intact parathyroid-hormone: assay validation and sample handling studies. J Small Anim Pract 1993; 34: 614–620. [Google Scholar]
- 22. De Brito Galvao JF, Chew DJ, Schenck PA. Feline idiopathic hypercalcemia. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy. St Louis, MO: Elsevier Saunders, 2014, pp 242–248. [Google Scholar]
- 23. Bergman PJ. Paraneoplastic hypercalcemia. Top Companion Anim Med 2012; 27: 156–158. [DOI] [PubMed] [Google Scholar]
- 24. Hardy BT, de Brito Galvao JF, Green TA, et al. Treatment of ionized hypercalcemia in 12 cats (2006–2008) using PO-administered alendronate. J Vet Intern Med 2015; 29: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groman RP. Acute management of calcium disorders. Top Companion Anim Med 2012; 27: 167–171. [DOI] [PubMed] [Google Scholar]
- 26. Major P, Lortholary A, Hon J, et al. Zoledronic acid is superior to pamidronate in the treatment of hypercalcemia of malignancy: a pooled analysis of two randomized, controlled clinical trials. J Clin Oncol 2001; 19: 558–567. [DOI] [PubMed] [Google Scholar]
- 27. Wypij JM, Fan TM, Fredrickson RL, et al. In vivo and in vitro efficacy of zoledronate for treating oral squamous cell carcinoma in cats. J Vet Intern Med 2008; 22: 158–163. [DOI] [PubMed] [Google Scholar]
- 28. McClain HM, Barsanti JA, Bartges JW. Hypercalcemia and calcium oxalate urolithiasis in cats: a report of five cases. J Am Anim Hosp Assoc 1999; 35: 297–301. [DOI] [PubMed] [Google Scholar]
- 29. Ching SV, Norrdin RW, Fettman MJ, et al. Trabecular bone remodeling and bone mineral density in the adult cat during chronic dietary acidification with ammonium chloride.J Bone Miner Res 1990; 5: 547–556. [DOI] [PubMed] [Google Scholar]
- 30. Ching SV, Fettman MJ, Hamar DW, et al. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J Nutr 1989; 119: 902–915. [DOI] [PubMed] [Google Scholar]
- 31. Bartges JW, Kirk CA, Cox SK, et al. Influence of acidifying or alkalinizing diets on bone mineral density and urine relative supersaturation with calcium oxalate and struvite in healthy cats. Am J Vet Res 2013; 74: 1347–1352. [DOI] [PubMed] [Google Scholar]
- 32. Barber PJ, Elliott J. Feline chronic renal failure: calcium homeostasis in 80 cases diagnosed between 1992 and 1995. J Small Anim Pract 1998; 39: 108–116. [DOI] [PubMed] [Google Scholar]
- 33. Barber PJ, Rawlings JM, Markwell PJ, et al. Hypercalcaemia in naturally occurring feline chronic renal failure [abstract]. J Vet Intern Med 1998; 12: 223. [Google Scholar]
- 34. Rasor L, Pollard R, Feldman EC. Retrospective evaluation of three treatment methods for primary hyperparathyroidism in dogs. J Am Anim Hosp Assoc 2007; 43: 70–77. [DOI] [PubMed] [Google Scholar]
- 35. Sheafor SE, Gamblin RM, Couto CG. Hypercalcemia in two cats with multiple myeloma. J Am Anim Hosp Assoc 1996; 32: 503–508. [DOI] [PubMed] [Google Scholar]
- 36. Mealey KL, Willard MD, Nagode LA, et al. Hypercalcemia associated with granulomatous disease in a cat. J Am Vet Med Assoc 1999; 215: 959–962. [PubMed] [Google Scholar]