Abstract

Practical relevance Numerous tests are available to the practitioner for quantifying proteinuria. It is important to understand the advantages and limitations of these tests and how the information gained can contribute to optimal patient management.

Patient group Cats with chronic kidney disease or systemic hypertension, as well as geriatric cats without overt evidence of disease (including renal dysfunction), are at particular risk of proteinuria. Evidence base Several longitudinal studies of cats seen in first opinion clinics have shown an association between proteinuria and decreased survival time. However, it is unknown whether the deaths that occur in proteinuric cats are due to progression of renal disease because it is often difficult to ascribe a cause of death to a single underlying aetiology in clinical patients. It is also unknown whether proteinuria is contributing to disease progression in these cats or whether proteinuric renal disease is intrinsically more rapidly progressive.
Clinical significance More aggressive investigation and management of patients with proteinuria may be appropriate since they are more likely to have progressive disease and/or increased mortality.
Why the recent interest in proteinuria?
All animals have small quantities of protein in their urine — when this is increased the patient is said to be proteinuric. Until recently, proteinuria was not a subject of particular interest to feline veterinarians because the occurrence of severe proteinuria — for example, sufficient to result in signs of nephrotic syndrome — is relatively uncommon in cats. More recently, however, the importance of low levels of proteinuria as a prognostic indicator and therapeutic target has been recognised. This article outlines the methods available to the feline practitioner for quantifying proteinuria and reviews the published evidence for its clinical significance.
Methods of assessment
Urine dipsticks
Urine dipstick tests provide a rapid, semi-quantitative assessment of proteinuria and are easy to perform in house. Dipsticks are more sensitive to albumin than to other proteins. They can be used to identify patients with proteinuria that is sufficiently severe to result in systemic hypoalbuminaemia (these cats will almost always have dipstick measurements of 3+ positive or more). Unfortunately, urine dipsticks cannot be used reliably to identify less severe proteinuria in cats, as both false positive and negative results may occur (see Fig 1, which illustrates the relationship between urine dipstick results and more precise quantification of proteinuria by measurement of urine protein:creatinine [UPC] ratios). In part, this is because urine dipsticks do not account for how dilute or concentrated a urine sample is. Generally speaking, any positive result on a urine dipstick is much more likely to be significant if the urine specific gravity is low than if it is high. However, when the result is either trace or 1+ positive (which most are) it is impossible to determine whether or not the patient is proteinuric. This limits the usefulness of urine dipsticks as a screening test in cats.
Fig 1.
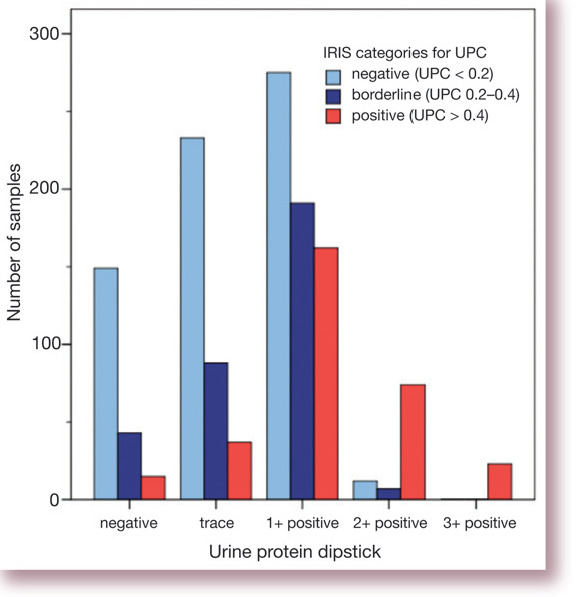
Bar chart illustrating the relationship between urine dipstick results and urine protein;creatinine (UPC) ratios. Data (author's unpublished observations) comprise measurements of UPC and dipstick protein in 1343 urine samples obtained from 531 cats (median 2, range 1–13, samples per cat)
SSA precipitation test
The sulfosalicylic acid (SSA) precipitation test is a semi-quantitative method for estimating proteinuria. Although it can be performed in house this is uncommon; its use is typically restricted to university and commercial laboratories. The test entails mixing equal volumes of urine and a 3–5% solution of SSA (5-sulfosal-icylic acid) and assessing the resulting turbidity, typically on a scale of 0 to 4+. Limited studies indicate that this test is specific but not sensitive so it may not be that useful in the clinical setting, even as a screening test. 1
Recent work has revealed that proteinuria may be of prognostic significance in certain groups of feline patients, even when it is relatively mild.
UPC
The gold standard method for assessment of proteinuria is to collect all the urine excreted by the patient over a 24 h period and to accurately quantify the amount of protein that is contained within it. Since timed urine collections are simply not practical in the clinical setting this test is almost never performed. However, it has been shown that measurement of UPC ratios from spot urine samples are very closely correlated with results of 24 h collections and thus these measurements are most often used in clinical practice. 2 Indexing the protein measurement to creatinine in the urine provides a method for correcting for the volume of urine that is being produced over the day — if the volume is large the creatinine concentration will be low, and vice versa.
Measurement of UPC is commercially offered by almost all veterinary diagnostic laboratories and recently an in-house assay has also been developed. It has been reported that UPC measurements made using different methodologies (eg, wet and dry chemistries) may not be that comparable, so when measuring UPC serially in the same patient it is advisable to ensure that the same method of testing is used each time. 3
It is important to appreciate that the amount of protein excreted exists along a continuum. However, introducing cut-off points to compare groups of patients is very helpful. The International Renal Interest Society (IRIS) has introduced a system for staging renal disease according to the severity of azo-taemia, proteinuria and hypertension (see left). Wherever possible, this system will be used in this article.
IRIS staging of proteinuria in cats (www.iris-kidney.com)
UPC < 0.2 Non-proteinuric
UPC 0.2–0.4 Borderline proteinuric
UPC > 0.4 Proteinuric
Albuminuria
The relatively low levels of albumin present in urine (compared with blood) can be quantified by various forms of immunoassay. Most of the antibodies employed in these assays are not cross-reactive between species, meaning that human and canine assays cannot generally be used to test feline urine samples. The term ‘microalbuminuria’ has been coined to describe the detection of abnormal amounts of albumin in urine in quantities insufficient to be detected by conventional urine dipsticks. In human medicine, the term is usually considered to correspond to a concentration of 30–300 mg albumin/g creatinine. In the veterinary field no universally accepted values have been established to define microalbuminuria and cut-off points have tended to differ between studies.
In the past decade, immunological methods for quantifying albumin in the urine of veterinary patients have been developed. These have included ELISA methods, which provide a very precise quantification of albumin; the resulting measurement is either normalised to urine creatinine concentration, yielding the urine albumin:creatinine ratio (UAC), or to urine specific gravity. Protein measurements normalised by these two different methods have been shown to be very highly correlated. 4 Although measurement of UAC is offered by commercial laboratories in some areas these tests are not widely available and have mainly been employed in clinical research. A semi-quantitative test kit for detection of albumin in the urine of cats is also available. Marketed as a ‘cage-side’ test kit, it provides a method for diluting the urine sample to a specific gravity of 1.010. The test device is then inserted into the diluted urine sample and left for a minimum of 3 minutes. Coloured lines that develop on the device give an indication of whether the albumin concentration is < 1 mg/dl (negative) or > 1 mg/dl (further quantified into low, medium, high or very high positive).
Albumin is the predominant protein in urine and many of the tests employed clinically for quantification of proteinuria (dipsticks and UPCs, for example) are actually more sensitive tests for albumin than for other proteins. It is not surprising then that UAC and UPC values are fairly strongly correlated (Fig 2).
Fig 2.
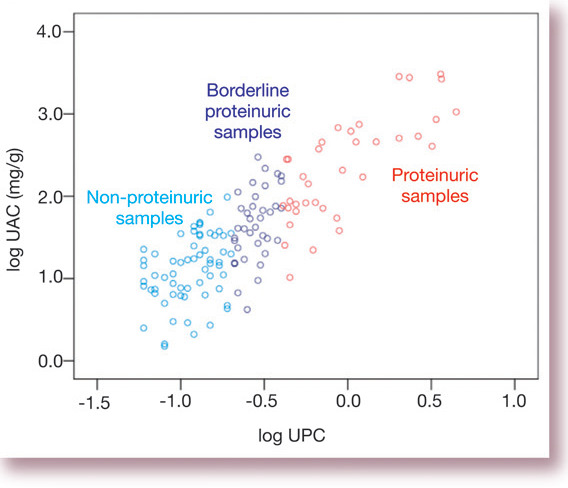
Correlation between total protein and albuminuria. Data are taken from a study by the author and colleagues of 136 cats. 5 Samples are categorised as non-proteinuric, borderline proteinuric and proteinuric according to IRIS staging criteria. UAC = urine albumin:creatinine ratio, UPC = urine protein: creatinine ratio
Localisation of proteinuria
The causes of proteinuria can be broadly categorised as pre-renal, renal or post-renal in origin.
Pre-renal
Pre-renal proteinuria occurs when an increased amount of low molecular weight protein is presented to the glomerular barrier and then, due to its small size, passes relatively unimpeded into the primary glomerular filtrate. Although in the normal animal some small proteins will be filtered in this manner, they will usually be reabsorbed by the proximal tubular epithelium with the result that very little protein is present in the urine. If the amount of protein filtered is increased then this tubular reabsorption mechanism can be overwhelmed and proteinuria will result.
Examples of proteins that are filtered readily and may cause pre-renal proteinuria include immunoglobulin light chains, haemoglobin and myoglobin.
Renal
Proteinuria of renal origin is classically considered to be the hallmark of glomerular disease. Low-level proteinuria may also arise as a result of tubular dysfunction, which leads to a failure to process and reabsorb the small amount of protein, mainly albumin, which traverses the glomerular barrier even in normal animals.
Glomerular dysfunction can cause very severe proteinuria due to defects in the structure and/or charge of the filtration barrier. Broadly speaking, glomerular diseases can be divided into glomerulonephritis, amyloidosis and inherited glomerulopathies. Membrano-proliferative glomerulonephritis has been reported as a cause of severe proteinuria and nephrotic syndrome in cats but is an uncommon cause of renal disease in this species. 6 Amyloidosis has also been reported, most commonly in Abyssinians; however, deposition of amyloid in cats may occur solely within the renal medulla and, as a consequence, these patients are often not significantly proteinuric. 7 Specific inherited glomerulopathies, resulting in abnormalities in the glomerular basement membrane or slit diaphragm, have been described in humans and dogs but have not to date been documented in the cat.
The amount of protein that traverses the glomerular filtration barrier depends not only on the structural integrity of that barrier but also on the hydrostatic pressure that is applied to it. If the pressure is increased (so-called glomerular hypertension) the amount of protein that passes into the glomerular filtrate may increase. Glomerular hypertension may result from systemic hypertension if the autoregulatory mechanisms for maintaining normal glomerular capillary pressure are overcome, or impeded as with pre-existing renal disease. Alternatively, in the setting of renal compromise, where the number of functioning nephrons is severely diminished, the individual remaining nephrons will each filter a greater volume of plasma at increased pressure; this process (known as glomerular hyperfiltration) acts to preserve the global (ie, whole animal) glomerular filtration rate. It is postulated that glomerular hyperfiltration is a maladaptive mechanism that is beneficial in the short term to maintain the glomerular filtration rate, but over a longer period results in loss of nephrons and may contribute to the intrinsic progression of chronic kidney disease.
Post-renal
Post-renal proteinuria occurs when protein is added to the urine after its formation in the kidney. Inflammation of the bladder, for example due to bacterial urinary tract infection, is the most common cause of this category of proteinuria. Urolithiasis, tumours and, depending on the method of urine collection, contamination with proteins from the genital tract are other potential causes.
The magnitude of post-renal proteinuria is highly variable — and may be mild. Thus, in patients with lower urinary tract disease proteinuria should not invariably be dismissed as being post-renal in origin; if possible, the proteinuria should be re-quantified following resolution of the urinary tract disease to ensure that there is no concurrent problem causing renal proteinuria. Haematuria is often implicated as a cause of post-renal proteinuria but its importance has probably been over-emphasised. Blood does, of course, contain protein but considerable contamination must occur to result in significant proteinuria. As a general rule, if the urine sample is not visibly discoloured (ie, is not grossly haematuric) then the protein measurements will not be altered.
As a general rule, if the urine sample is not visibly discoloured with blood then the protein measurements will not be altered.
Clinical relevance of proteinuria
Historically only moderate to severe proteinuria, typically with a UPC > 1.0, has been considered to be of clinical significance in veterinary practice. However, recent work has revealed that proteinuria may be of prognostic significance in certain groups of feline patients, even when it is relatively mild.
Azotaemic cats
Proteinuria has been shown to be predictive of survival in azotaemic cats in three independent studies. The first study included 136 cats with variable renal function; some of these patients were also hypertensive. Proteinuria was found to be very highly related to survival, even when other clinically important factors, such as severity of azotaemia and the patient's age, were also included in the analysis. 5 Indeed, the hazard ratio (95% confidence Intervals) for death or euthanasia was 2.9 (1.4–6.3) and 4.0 (2.0–8.0) for UPC ratios of 0.2–0.4 and > 0.4, respectively, compared with the baseline group with UPC < 0.2, showing the prognostic significance of a degree of proteinuria that would previously have been considered trivial. Fig 3 shows the survival curves for the cats in that study.
Fig 3.
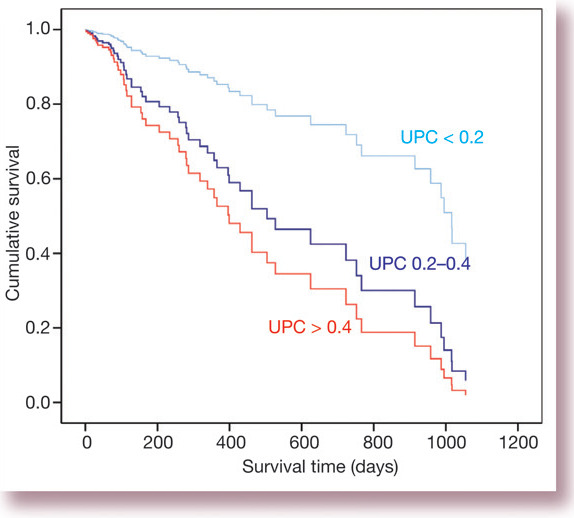
Survival curves for 136 cats with variable renal function stratified according to UPC. The effects of azotaemia and patient age are controlled for as co-dependent variables in this graph. Data are from Syme and others (2006) 5 showing deaths due to any cause during follow-up
The BENRIC study, which investigated the effects of angiotensin converting enzyme (ACE) inhibitor therapy in cats with chronic renal failure, has also demonstrated an inverse relationship between proteinuria and survival time.8,9 A further study demonstrated that UPC was significantly higher (median 1.33, range 0.50–6.47) in cats that died within 1 month of diagnosis of chronic renal failure, than in cats that survived for longer (median 0.22, range 0.01–1.44). 10
Non-azotaemic cats
Proteinuria has also been found to have prognostic value in two small studies of non-azotaemic cats, neither of which has yet been published in full. The first study was of 61 apparently healthy cats that had been presented for wellness examinations and from which stored urine samples were available for retrospective measurement of UPC. This showed that proteinuria was associated with reduced survival. 11 Interestingly, none of the other factors that were investigated (plasma creatinine concentration, patient age, urine specific gravity or systolic blood pressure) were predictive of survival. The median [25th, 75th percentile] UPC of the cats that died (n=15) was 0.30 [0.26, 0.37] compared with 0.11 [0.16, 0.21] in the 46 cats that were lost to follow-up (n=10) or still alive at the termination of the study (n=36).
In a second study, apparently healthy, non-azotaemic geriatric cats were enrolled in a prospective clinical trial to determine whether proteinuria is predictive of the development of azotaemia. Of the 80 cats enrolled, data from 68 cats was available for follow-up after 1 year, which showed that 23/68 (34%) had developed azotaemia. 12 The cats that developed azotaemia had significantly higher plasma creatinine concentration (152.5 ± 17.7 versus 135.0 ± 21.0 μmol/l) and UPC (0.25 [0.13, 0.39] versus 0.16 [0.12, 0.21]) at entry to the study than the cats that did not develop azotaemia.
Hypertensive cats
A further population of cats in which the prognostic significance of proteinuria has been investigated is those with systemic hypertension. 13 In a study of 141 hypertensive cats, all were treated with amlodipine at a dose of 0.625 or 1.25 mg/cat with the aim of maintaining the systolic blood pressure < 160 mmHg. Fifty-eight percent of the cats were azotaemic when the hypertension was diagnosed. The only variable in the study that was predictive of survival was proteinuria. The median survival time of the cats that died or were euthanased during the study was 490 days (range 217–1169), 313 days (range 124–607) and 162 days (range 73–406) for cats that were non-proteinuric (UPC < 0.2), borderline proteinuric (UPC 0.2–0.4) and proteinuric (UPC > 0.4), respectively (Fig 4). Time-averaged blood pressure (calculated from measurements made over the entire period of follow-up), age and plasma creatinine concentration were among the other pretreatment predictive variables that were investigated in that study, but none was significantly associated with survival.
Fig 4.
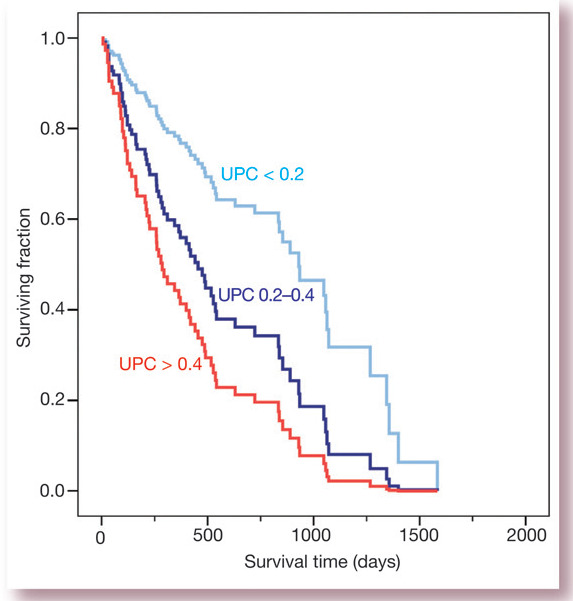
Survival curves for cats with hypertension stratified according to UPC. Data are taken from Jepson et al (2007) 13 showing deaths due to any cause during follow-up
Systemic hypertension may cause glomerular hypertension and therefore proteinuria. Hypertensive cats have been shown to be more proteinuric than normotensive cats with comparable renal function. 5 Glomerular hypertension and thus proteinuria could in theory be exacerbated by treatment with amlodipine since dilation of the afferent arteriole by this drug may allow the transmission of high systemic pressures to the glomerular capillaries. However, worsening of glomerular hypertension does not appear to happen in hypertensive cats since treatment with amlodipine tends to cause a reduction in proteinuria, as shown in Fig 5. This is presumably because of the profound decrease in blood pressure (typically around 50 mmHg) that occurs when treatment with amlodipine is initiated.
Fig 5.
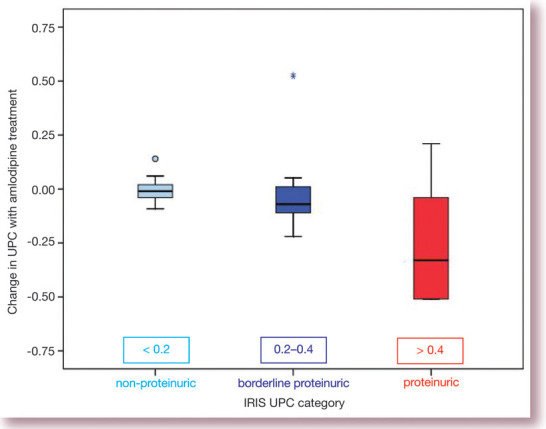
Change in proteinuria with amlodipine treatment of cats with systemic hypertension. Data are taken from the cats included in the study by Syme et al (2006), 5 although the data contained in this figure were not included in the original article
Hyperthyroid cats
Mild proteinuria is frequently present in cats with hyperthyroidism. 14 It is thought that the proteinuria is a reflection of the hyperfiltration that is known to occur in the hyperthyroid state. Additionally, changes in urinary protein excretion may reflect differences in tubular protein handling. The prognostic significance of proteinuria in hyperthyroidism is unknown but it does tend to resolve with treatment, even in cats that develop azotaemia.
Other conditions
Microalbuminuria is reported to be more common in sick than in healthy cats.15–17 A variety of infectious and inflammatory diseases have been implicated as causes of microalbuminuria. It has also, not surprisingly, been associated with lower urinary tract disease. In contrast to the situation in dogs, neoplastic diseases have not been found to be commonly associated with microalbuminuria in cats. 16
The prevalence of proteinuria (as evaluated by UPC measurement) in cats with systemic illness has been very discordant in different studies. One study found that 34/100 diseased cats had UPC measurements > 0.5, 17 while a second reported that none of 284 diseased cats had a UPC value > 0.4. 16 The inconsistency in these results may perhaps have arisen because of differences in the populations that were studied and in the methods used for UPC measurement. Although there is some evidence that apparently healthy cats can have a raised UPC, and that this in turn may be related to increased mortality, the value of microalbuminuria or UPC measurement as a non-specific screening test in individual patients that are not at high risk of developing renal disease seems doubtful.
Urinary albumin concentrations versus total protein
In human medicine, measurement of albumin in urine has gained credence since the seminal observation that this predicts the development of nephropathy in people with type 2 diabetes and the subsequent observation that it is also related to an increased risk of adverse cardiovascular events, such as stroke and myocardial infarction, in certain high-risk groups (for a review, see Basi et al 2008). 18 It is notable, however, that it has not been found to be that useful as a clinical marker in humans when applied to unselected populations.
In the studies conducted in cats to date, including those described in this article, the prognostic significance of albuminuria has not been shown to be any greater than that of UPC. No clear advantage or disadvantage of one of these methods over the other has been identified In cats.
Marker or mediator?
Although the studies described above have confirmed the significance of proteinuria as a prognostic indicator in the cat, what is not clear is whether proteinuria is simply a marker or whether in fact it is a mediator in this process. Proteinuria could, for example, be more likely to occur with certain types of kidney disease that are inherently more rapidly progressive, so serving as a marker for a poor prognosis. It is also not entirely certain that the increased death rate in proteinuric patients is entirely related to progressive kidney disease; proteinuric humans are at increased risk of dying from various forms of cardiovascular disease in addition to development of end-stage renal failure. 18
The association of proteinuria with decreased survival in azotaemic and/or hypertensive cats, and with the development of azotaemia in apparently healthy geriatric cats, has lent weight to the notion that proteinuria is directly injurious to the feline kidney. There is some evidence to support this from animal models and studies using cell culture. Exposure of proximal tubular cells to albumin and transferrin in concentrations that overwhelm the cells’ ability to process the protein results in a cascade of intracellular events culminating in the release of inflammatory and profibrotic cytokines from their basolateral surfaces. 19 Expression and secretion of these mediators can also be demonstrated in vivo and interventions that reduce proteinuria in these animal models also reduce inflammation and fibrosis. 20
Case notes
George, a 3-year old male neutered domestic shorthair cat, was presented for investigation of left hindlimb lameness that had been present for 48 h prior to referral.
Case work-up On physical examination no femoral pulse could be detected in the affected limb and the paw pads appeared pale and felt cold. Cardiac auscultation and the remainder of the physical examination were unremarkable. Thoracic radiographs obtained prior to referral were reviewed and appeared normal.
Blood samples were collected for haematology and biochemistry, and a urine sample was obtained by cystocentesis for complete urinalysis. An echocardiographic examination was performed on George because heart disease is the most common cause of arterial thrombus formation in cats and abnormalities may not always be detected on auscultation; however, this was unremarkable.
Diagnosis On receipt of the biochemistry and urinalysis results (Tables 1 and 2) the likely cause of George's lameness became apparent — these revealed that he was severely proteinuric and, as a result, was hypoalbuminaemic and hypercholesterolaemic. The reduced total calcium concentration was attributed to the hypoalbuminaemia. It was concluded that George was suffering from a protein-losing nephropathy; as a result he had become hypercoagulable and a thrombus had occluded the femoral artery in his left hindlimb.
Further diagnostic testing was performed; a UPC ratio measured on the previously submitted urine sample was 9.37; urine culture was negative; the kidneys appeared normal on ultrasound examination but a thrombus was detected at the level of the aortic trifurcation (see image below); systolic blood pressure measured by the Doppler method was 140 mmHg; FeLV and FIV tests were both negative; and his antithrombin level was reduced to 66% of normal.
Table 1.
Serum biochemistry results at presentation
| Parameter | Result | Reference range |
| Total protein | 50 g/l | 61–80 |
| Albumin | 16.5 g/l | 28–42 |
| Globulin | 33.5 g/l | 25–46 |
| Sodium | 155 mmol/l | 153–162 |
| Potassium | 4.4 mmol/l | 3.8–5.3 |
| Chloride | 117 mmol/l | 110–121 |
| Calcium | 1.85 mmol/l | 2.07–2.8 |
| Inorganic phosphorus | 1.09 mmol/l | 0.92–2.16 |
| Urea | 7.3 mmol/l | 6.1–11.2 |
| Creatinine | 116 μmol/l | 107–193 |
| Cholesterol | 10.6 mmol/l | 2.2–6.7 |
| Total bilirubin | 0.7 μmol/l | 0–3 |
| Alanine aminotransferase (ALT) | 77 U/l | 25–130 |
| Creatine kinase | 500 U/l | 52–506 |
| Alkaline phosphatase (ALP) | 15 U/l | 11–58 |
Table 2.
Urinalysis results at presentation
| Parameter | Result |
| Specific gravity | > 1.050 |
| pH | 7 |
| Nitrite | -ve |
| Protein | >3+ |
| Glucose | -ve |
| Ketones | -ve |
| Bilirubin | -ve |
| Blood | Trace |
| Sediment | Unremarkable |
George's owners would not permit renal biopsy so treatment for a protein-losing nephropathy of unknown cause was instituted. An immune-mediated glomerulonephritis was presumed. 1
Treatment and outcome A low-protein diet (Hill's K/D) with omega-3 fatty acid supplementation was introduced, and 18.75 mg (total dose) aspirin given three times weekly and benazepril (Fortekor; Novartis) 0.5 mg/kg once daily. George was discharged with instructions to confine him indoors and within a week his owners reported a notable improvement in lameness. Over the following weeks there was a reduction in the severity of proteinuria and a corresponding increase in serum albumin concentration and reduction in cholesterol concentration (Table 3). George is currently asymptomatic.
Table 3.
Sequential changes in albumin, cholesterol and UPC over time
| Date | Albumin | Cholesterol | UPC |
| 2.1.07 | 16.5 | 10.6 | 9.37 |
| 11.4.07 | 28.3 | 7.1 | 4.65 |
| 9.8.07 | 33.6 | 4.5 | 0.48 |
| 30.1.08 | 36.7 | 3.8 | 0.35 |
See Table 1 for reference ranges for albumin and cholesterol
* WHAT THIS CASE DEMONSTRATES
Some cats with protein-losing nephropathy may actually show a good clinical response to treatment. Responses seem more likely to be favourable when, as in George's case, there is no azotaemia present. Although thrombus formation is a potential complication of protein-losing nephropathy, in the author's experience cats are more often presented due to the development of subcutaneous
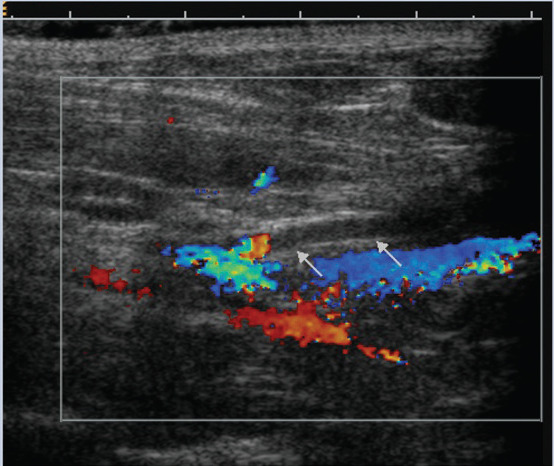
Ultrasound examination of the area of the iliac trifurcation revealing an absence of blood flow (arrows) in the left internal iliac artery. George's head is to the left of the image, tail to the right
Treatment with ACE inhibitors has been shown to be beneficial in slowing progression of renal damage in rodent models and in humans with naturally occurring kidney disease. 21 The benefit is most marked in patients that are proteinuric and appears to be greater than would be explained simply by a reduction in blood pressure. This benefit is thought to be due to the preferential dilation of the efferent arterioles within the kidney, which act to reduce glomerular hypertension and therefore also reduce proteinuria. The benefits may therefore be a result of the intraglomerular haemodynamic effects of the ACE inhibitors and/or due to a reduction in proteinuria if this does indeed act as a significant mediator of renal injury. Published studies of ACE inhibitor therapy in cats with azotaemic chronic kidney disease have demonstrated that these drugs do cause a significant reduction in proteinuria but have not been able to demonstrate a statistically significant survival benefit, although favourable trends were evident.8,22
The debate as to whether proteinuria is most important as a marker or a mediator of renal disease in the cat therefore currently remains unresolved.
Acknowledgements
The case notes on page 217 were provided by Kirsty Roe, senior clinical training scholar at the Royal Veterinary College.
KEY POINTS
UPC measurements > 0.2 in the cat should be considered as potentially abnormal.
Proteinuria is predictive of survival time in cats with chronic kidney disease and/or systemic hypertension.
Preliminary studies also suggest that proteinuria predicts the development of azotaemia in normal geriatric cats.
It is as yet unknown whether proteinuria is a marker or a mediator of renal injury in the cat.
References
- 1.Welles EG, Whatley EM, Hall AS, Wright JC. Comparison of Multistix PRO dipsticks with other biochemical assays for determining urine protein (UP), urine creatinine (UC) and UP: UC ratio in dogs and cats. Vet Clin Pathol 2006; 35: 31–36. [DOI] [PubMed] [Google Scholar]
- 2.Adams LG, Polzin DJ, Osborne CA, O'Brien TD. Correlation of urine protein/creatinine ratio and twenty-four-hour urinary protein excretion in normal cats and cats with surgically induced chronic renal failure. J Vet Intern Med 1992; 6: 36–40. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes PK, Yang V, Weilbacher A. Comparison of methods used for determining urine protein-to-creatinine ratio in dogs and cats [abstract]. J Vet Intern Med 2005; 19: 431. [Google Scholar]
- 4.Syme HM, Elliott J. Comparison of urinary albumin excretion normalized by creatinine concentration or urine specific gravity [abstract]. J Vet Intern Med 2005; 19: 460. [Google Scholar]
- 5.Syme HM, Markwell PJ, Pfeiffer D, Elliott J. Survival of cats with naturally occurring chronic renal failure is related to severity of proteinuria. J Vet Intern Med 2006; 20: 528–35. [DOI] [PubMed] [Google Scholar]
- 6.Nash AS, Wright NG, Spencer AJ, Thompson H, Fisher EW. Membranous nephropathy in the cat: A clinical and pathological study Vet Rec 1979; 105: 71–77. [DOI] [PubMed] [Google Scholar]
- 7.Chew DJ, DiBartola SP, Boyce JT, Gasper PW. Renal amyloidosis in related Abyssinian cats. J Am Vet Med Assoc 1982; 181: 139–142. [PubMed] [Google Scholar]
- 8.King JN, Gunn-Moore DA, Tasker S, Gleadhill A, Strehlau G. Tolerability and efficacy of benazepril in cats with chronic kidney disease. J Vet Intern Med 2006; 20: 1054–64. [DOI] [PubMed] [Google Scholar]
- 9.King JN, Tasker S, Gunn-Moore DA, Strehlau G, BENRIC Study Group Prognostic factors in cats with chronic kidney disease. J Vet Intern Med 2007; 21: 906–16. [PubMed] [Google Scholar]
- 10.Kuwahara Y, Ohba Y, Kitoh K, Kuwahara N, Kitagawa H. Association of laboratory data and death within one month in cats with chronic renal failure, J Small Anim Pract 2006; 47: 446–50. [DOI] [PubMed] [Google Scholar]
- 11.Walker DS, Syme HM, Markwell P, Elliott J. Predictors of survival in healthy, non-azotaemic cats, J Vet Intern Med 2004; 18: 417. [Google Scholar]
- 12.Jepson RE, Syme HM, Vallance C, Elliott J. Proteinuria, albuminuria, creatinine concentration and urine specific gravity as prospective predictors for the development of azotaemia in cats. J Vet Intern Med 2007; 21: 598. [Google Scholar]
- 13.Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension, J Vet Intern Med 2007; 21: 402–9. [DOI] [PubMed] [Google Scholar]
- 14.Syme HM, Elliott J. Evaluation of proteinuria in hyperthyroid cats. J Vet Intern Med 2001; 15: 299. [Google Scholar]
- 15.Langston C. Microalbuminuria in cats. J Am Anim Hosp Assoc 2004; 40: 251–54. [DOI] [PubMed] [Google Scholar]
- 16.Whittemore JC, Miyoshi Z, Jensen WA, Radecki SV, Lappin MR. Association of microalbuminuria and the urine albumin-to-creati-nine ratio with systemic disease in cats. J Am Vet Med Assoc 2007; 230: 1165–69. [DOI] [PubMed] [Google Scholar]
- 17.Mardell EJ, Sparkes AH. Evaluation of a commercial in-house test kit for the semi-quantitative assessment of microalbuminuria in cats. J Feline Med Surg 2006; 8: 269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Basi S, Fesler P, Mimran A, Lewis JB. Microalbuminuria in type 2 diabetes and hypertension: A marker, treatment target, or innocent bystander? Diabetes Care 2008; 31: S194–201. [DOI] [PubMed] [Google Scholar]
- 19.Benigni A, Remuzzi G, How renal cytokines and growth factors contribute to renal disease progression. Am J Kidney Dis 2001; 37: S21–24. [DOI] [PubMed] [Google Scholar]
- 20.Donadelli R, Abbate M, Zanchi C, et al. Protein traffic activates NF-kB gene signaling and promotes MCP-1-dependent interstitial inflammation. Am J Kidney Dis 2000; 36: 1226–41. [DOI] [PubMed] [Google Scholar]
- 21.Maschio G, Alberti D, Janin G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N Engl J Med 1996; 334: 939–45. [DOI] [PubMed] [Google Scholar]
- 22.Mizutani H, Koyama H, Watanabe T, et al. Evaluation of the clinical efficacy of benazepril in the treatment of chronic renal insufficiency in cats. J Vet Intern Med 2006; 20; 1074–79. [DOI] [PubMed] [Google Scholar]
- 23.Lees GE, Brown SA, Elliott J, Grauer GE, Vaden SL, American College of Veterinary Internal Medicine. Assessment and management of proteinuria in dogs and cats: 2004 ACVIM Forum Consensus Statement (small animal). J Vet Intern Med 2005; 19: 377–85. [DOI] [PubMed] [Google Scholar]


