Abstract
Objectives
Rapid recovery from injectable anaesthesia benefits cat shelter neutering programmes. The effects of medetomidine, dexmedetomidine and atipamezole on recovery were evaluated in adult cats and kittens (⩽6 months old).
Methods
One hundred healthy male cats (age range 2–66 months, weight range 0.7–5.3 kg) admitted forneutering were randomly allocated to groups of 25. Anaesthesia was induced with 60 mg/m2 ketamine, 180 µg/m2 buprenorphine, 3 mg/m2 midazolam and either 600 µg/m2 medetomidine (groups M and MA) or 300 µg/m2 dexmedetomidine (groups D and DA) intramuscularly (IM). Groups MA and DA also received 1.5 mg/m2 atipamezole IM after 40 mins. Preparation time, surgical time, and times to sternal recumbency and standing were recorded. Data were analysed using the Kruskall–Wallis test, unpaired t-tests and ANOVA. Statistical significance was deemed to be P ⩽0.05.
Results
Groups did not differ significantly in age, body weight, preparation or surgical time. The time to sternal recumbency in group MA (64 ± 34 mins) was less than in group M (129 ± 32 mins), and in group DA it was less than in group D (54 ± 6 mins vs 110 ± 27 mins) (P <0.001). There were no differences in duration of recovery to sternal recumbency between groups M and D or MA and DA. The time to standing in group MA (79 ± 51 mins) was less than in group M (150 ± 38 mins) (P <0.001), and in group DA it was less than in group D (70 ± 22 mins vs 126 ± 27 mins) (P <0.01). Time to standing in group D (126 ± 27 mins) was less than in group M (150 ± 38 mins) (P <0.05). Time to standing in groups DA and MA were not different. Kittens recovered faster than adults after atipamezole. Minimal adverse effects were seen.
Conclusions and relevance
Atipamezole reliably reduced recovery time after anaesthesia incorporating either dexmedetomidine or medetomidine; however, the choice of dexmedetomidine or medetomidine had little effect. Recovery was faster in kittens.
Introduction
The Royal Society for the Prevention of Cruelty to Animals Greater Manchester Animal Hospital (GMAH) performs approximately 1500 cat castrations per annum (D Yates, unpublished data). Anaesthetic protocols that reduce recovery time are beneficial as the period when close monitoring is required is shorter and postanaesthetic hypothermia, 1 as well as the risk of anaesthetic mortality in the postoperative period, 2 are reduced. Rapid recovery would also benefit hospital management by enabling earlier postsurgical discharge, reducing staff time in monitoring recovery and potentially allowing a higher daily intake of cats for neutering.
A further consideration facing cat shelter neutering programmes is the need for surgery on very young animals. Up to 80% of cat litters in the UK are unplanned. 3 In an effort to improve cat population control and prevent unwanted litters, it is recommended that prepubertal gonadectomy is performed in kittens ⩽6 months old. 4 ‘Paediatric’ can be considered as <6 months old in terms of metabolic differences and analgesic requirements. 5 There are important physiological differences between kittens (‘paediatrics’) and adult cats that warrant consideration for anaesthesia for elective prepubertal neutering. Paediatric physiology and its implications for kitten anaesthesia include limited cardiovascular compensation, higher relative tissue oxygen consumption and a higher body surface area to mass ratio, resulting in a higher risk of hypothermia under anaesthesia. 4 Cardiac output is predominantly dependent on heart rate in paediatrics owing to a higher proportion of non-contractile tissue and hence a predetermined stroke volume.4,6 Paediatric animals also have an immature sympathetic nervous system. 7 These factors will affect the choice of anaesthetic protocol for paediatrics that have limited capacity to compensate for drug-induced bradycardia and are therefore likely to develop hypotension, particularly with α2-adrenoceptor agonists and opioids.4,7 Additionally, as tissue oxygen consumption is increased in paediatrics, it is recommended that oxygen is provided throughout surgery, even if anaesthetic depth is adequate without inhalational gases.4,7 Other pharmacological considerations for paediatric anaesthesia include hypoalbuminaemia, which may enhance the effects of highly protein-bound drugs such as propofol and diazepam, and immature hepatic enzyme systems, which may reduce the rate of drug metabolism.4,6 Benzodiazepines stimulate appetite and their use during anaesthesia may promote feeding on recovery. 8 This is particularly valuable for kittens in helping to prevent hypoglycaemia and hypothermia. 4 In spite of the apparent physiological disadvantage, there is recent evidence that kittens demonstrated fewer behavioural signs of pain and a faster return to normal behaviour postneutering than adult cats, supporting the move towards programmes for kitten neutering. 9
Injectable anaesthetic protocols incorporating α2-adrenoceptor agonists are frequently used for neutering programmes in both feral and domestic cat populations.4,10,11 Medetomidine, the 1:1 racemic mixture of the D-isomer dexmedetomidine and the L-isomer levomedetomidine, has been widely used in cats since its introduction in 1994. 12 Several studies have demonstrated that only the D-isomer is pharmacologically active at clinically relevant doses and that levomedetomidine has no biological activity.13–15 However, in vitro studies showed that the L-isomer possesses weak partial α2-adrenoceptor agonist or inverse α2-adrenoceptor agonist (negative antagonist) properties.16,17 Therefore, the pharmacological activity of levomedetomidine in a clinical setting is unclear. It has been suggested that the absence of levomedetomidine from commercial formulations of dexmedetomidine may provide more predictable analgesia and sedation than the racemic mixture. 15
A number of studies have compared the sedative and physiological effects of medetomidine and dexmedetomidine in dogs and cats. One investigation in 212 client-owned dogs demonstrated that medetomidine and dexmedetomidine produced comparable levels of sedation and analgesia but there was some suggestion that analgesia was marginally better with dexmedetomidine. 12 The same authors performed a study in 120 client-owned cats and concluded that medetomidine and dexmedetomidine (at half the medetomidine dose) produced similar sedation and analgesia. 18 They also reported that atipamezole fully reversed the clinical effects of dexmedetomidine. 18 These results are in agreement with an earlier laboratory study in cats comparing three intramuscular (IM) doses of dexmedetomidine with medetomidine at twice the respective dose (dexmedetomidine: 25, 50 and 75 µg/kg; medetomidine: 50, 100 and 150 µg/kg), which found that dexmedetomidine produced pharmacodynamic effects equal to those of medetomidine in cats. 19 This study concluded that the anaesthetic effects of medetomidine were most likely due to its dexmedetomidine isomer.
With particular relevance to anaesthesia of kittens, both medetomidine and dexmedetomidine negatively affect regulation of body temperature, which may delay recovery time, especially in younger animals.20,21 Both medetomidine and dexmedetomidine may cause a relative hyperglycaemia during anaesthesia by reducing endogenous insulin secretion.22,23 Prevention of hypoglycaemia is important as this could contribute to prolonged recovery.4,24
Our study had three objectives: firstly, to compare recovery times following medetomidine or dexmedetomidine when included in an established injectable general anaesthetic protocol for feline castration; secondly, to determine if inclusion of atipamezole altered the recovery time; and, thirdly, if recovery times of the above were varied in paediatric vs adult patients.
Materials and methods
The study was approved by the Animal Health Trust Clinical Research Ethics Committee, and written informed owner consent was obtained in all cases. One hundred entire male cats of any age (minimum 300 g body weight) or breed that were presented to GMAH for castration on four predesignated days in July 2013 were included. A full clinical examination was carried out on all cats at admission to ensure that they were healthy (American Society of Anesthesiologists’ classification I or II). Any sick animals, cryptorchid males and all females admitted for neutering on these predesignated dates were excluded.
The anaesthetic protocol was based on the GMAH ‘quad’ anaesthetic protocol for prepubertal cat neutering dosing according to body surface area (BSA): 60 mg/m2 ketamine (Ketaset; Zoetis), 180 µg/m2 buprenorphine (Vetergesic; Alstoe UK), 3 mg/m2 midazolam (Hypnovel; Roche) and 600 µg/m2 medetomidine (Domitor; Elanco Animal Health).4,25 This equates to equal volumes of each agent given in a single IM injection to induce anaesthesia. BSA was calculated as follows: BSA = (K × BW2/3)/100, where BSA is measured in m2, body weight (BW) in kg and K = 10.4 (cats). 4
Cats were randomly allocated into four groups of 25 using www.randomizer.org/form.htm as follows: group M – ‘quad’ with 600 µg/m2 medetomidine (Domitor 1 mg/ml; Elanco Animal Health); group MA – ‘quad’ with 600 µg/m2 medetomidine and 1.5 mg/m2 atipamezole (Antisedan; Elanco Animal Health) 40 mins later; group D – ‘quad’ with 300 µg/m2 dexmedetomidine in place of medetomidine (Dexdomitor 0.5 mg/ml; Elanco Animal Health); group DA – ‘quad’ with 300 µg/m2 dexmedetomidine in place of medetomidine and 1.5 mg/m2 atipamezole 40 mins later.
The four drugs in the ‘quad’ protocol were mixed in one syringe immediately before administration into the quadriceps muscle. Surgery began as soon as anaesthetic depth was considered sufficient using normal clinical criteria. Although the ‘quad’ protocol provides multimodal analgesia in the form of medetomidine (or dexmedetomidine), ketamine and buprenorphine, all cats additionally received 3 mg/m2 meloxicam (Metacam; Boehringer Ingelheim) and 0.2 ml/kg long-acting amoxicillin (Betamox LA; Norbrook) subcutaneously immediately after induction.
Oxygen was provided via mask inhalation using an infant T-piece at 400 ml/kg/min throughout the procedure, and surgery was performed on an operating table preheated to 37°C. Isoflurane was administered if anaesthesia was insufficient. Bilateral scrotal incisions were made and open castration was performed. Scrotal skin incisions were left to heal by secondary intention. The surgical procedure was very short (generally <3 mins) and anaesthetic monitoring was restricted to observation of vital signs (respiration, mucous membrane colour and adequacy of anaesthesia) without formal recording.
Time of the ‘quad’ injection was recorded, as well as preparation time (time from injection to commencing surgery), total surgical time (from first scrotal incision to removal of second testis), time to first sternal recumbency and time to first standing. Cats in groups MA and DA received IM atipamezole 40 mins after the initial injection of the ‘quad’, and cats in groups M and D were allowed to recover spontaneously. Artificial tears (Viscotears; Novartis) were applied to the eyes to prevent corneal drying. All cats were allowed to recover in individual kennels preheated to 37ºC; once in sternal recumbency the temperature was reduced to 25ºC and food was offered.
One veterinary surgeon prepared and administered the anaesthetics (DY) and a second (NB) performed all the surgeries throughout the study. Recovery was monitored and recorded by a member of the hospital nursing staff under the direction of the anaesthetising vet. The treatment group allocation was not revealed to the nursing staff or the surgeon.
Statistical analyses
Data are described as mean ± SD unless otherwise stated. The treatment groups were compared as follows: group M with MA, group M with D, group MA with DA and group D with DA. ANOVA and post-hoc Tukey’s test were used to compare normally distributed data; that is, preparation time. Kruskall–Wallis and post-hoc Dunn’s multiple comparisons were used for the remaining data that were not normally distributed. Recovery time in kittens (⩽6 months old) was compared with adults (>6 months old) within each group using unpaired t-tests. Statistical significance was set at P ⩽0.05. Statistical analyses were performed using GraphPad Prism version 6.0b for Mac.
Power calculations (GraphPad Statmate 2) were based on data previously collected in the clinic. These indicated that 25 cats per group would give 80% power to detect a difference of 20 mins in recovery time.
Results
The majority (95%) of the cats presented for castration were domestic shorthair or longhair cats, and 5% were pedigree breeds (two Ragdolls, two Tonkinese and one Havana). Overall mean age was 13.5 months (range 8.0 weeks to 5.5 years). Details of abnormalities detected at preanaesthetic examination are shown in Table 1 (all cases were included in the study).
Table 1.
Abnormalities detected in study cats during the preanaesthetic examination
| Illness/injury | Cats affected (n) |
|---|---|
| Gingivitis | 1 |
| Cat bite abscess/wound | 3 |
| Mild diarrhoea | 3 |
| Mucoid conjunctivitis | 2 |
| Systolic heart murmur | 1 |
| Long bone fracture | 1 |
| Degloving paw injury | 1 |
| FIV positive on FASTest* | 3 |
| Fleas/flea dirt | 20 |
FASTest (Megacor)
FIV = feline immunodeficiency virus
Demographic data including mean ± SD age, body weight and BSA for the study cats is shown in Table 2. There was no significant difference in age or body weight of cats between the groups. Mean ± SD preparation time, surgery time, injection to sternal recumbency and injection to first standing times are shown in Table 3. There was no significant difference in preparation time or surgical time between the groups.
Table 2.
Patient variables measured for each of the four treatment groups
| Group (n = 25) |
|||||||
|---|---|---|---|---|---|---|---|
| Patient variable | M | MA | D | DA | |||
| Age (months) | 14 ± 13 | 13 ± 14 | 15 ± 15 | 13 ± 16 | |||
| Body weight (kg) | 3.2 ± 1.3 | 2.7 ± 1.4 | 3.2 ± 1.1 | 2.9 ± 1.4 | |||
| BSA (m2) | 0.22 ± 0.07 | 0.19 ± 0.07 | 0.22 ± 0.05 | 0.21 ± 0.07 | |||
Data are mean ± SD
Group M = quad with medetomidine, no atipamezole; group MA = quad with medetomidine, atipamezole after 40 mins; group D = quad with dexmedetomidine, no atipamezole; group DA = quad with dexmedetomidine, atipamezole after 40 mins; BSA = body surface area
Table 3.
Study variables measured for each of the four treatment groups
| Group (n = 25) |
|||||||
|---|---|---|---|---|---|---|---|
| Study variable | M | MA | D | DA | |||
| Preparation time (mins) | 16 ± 4 | 15 ± 4 | 17 ± 3 | 16 ± 5 | |||
| Surgery time (mins) | 2.4 ± 0.6 | 2.7 ± 0.8 | 2.5 ± 0.7 | 2.6 ± 0.8 | |||
| Injection to sternal recumbency (mins) | 129 ± 32 | 64 ± 34 | 110 ± 27 | 54 ± 6 | |||
| Injection to standing (mins) | 150 ± 38 | 79 ± 51 | 126 ± 27 | 70 ± 22 | |||
Data are mean ± SD
Group M = quad with medetomidine, no atipamezole; group MA = quad with medetomidine, atipamezole after 40 mins; group D = quad with dexmedetomidine, no atipamezole; group DA = quad with dexmedetomidine, atipamezole after 40 mins
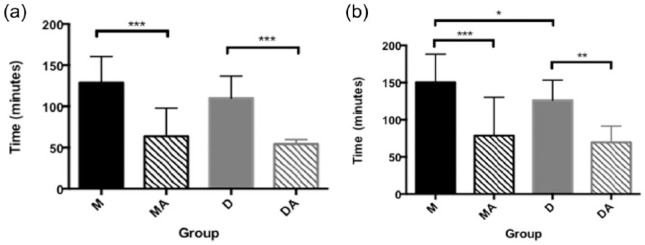
Time to sternal recumbency in group MA (64 ± 34 mins) was significantly shorter than in group M (129 ± 32 mins), and significantly shorter in DA (54 ± 6 mins) thanin group D (110 ± 27 mins) (P <0.001). There was no significant difference in time to sternal recumbency between groups M and D nor between groups MAand DA. Time to first standing in group MA (79 ± 51 mins) was significantly shorter than in group M (150 ± 38 mins) (P <0.001) and significantly shorter in group DA (70 ± 22 mins) than in group D (126 ± 27 mins) (P <0.01). Mean time to first standing in group D (126 ± 27 mins) was significantly shorter than in group M (150 ± 38 mins) (P <0.05). Time to standing in groups DA and MA was not significantly different (Figure 1). None of the cats in groups MA or DA reached sternal recumbency before administration of atipamezole.
Figure 1.
Time (mins; mean ± SD) to (a) sternal recumbency and (b) standing after intramuscular injection of the ‘quad’ anaesthetic. Mean ± SD shown for illustration only. Analysis performed using non-parametric methods. *Significant difference between groups joined by brackets (*P <0.05, **P <0.01, ***P <0.001). Group M = quad with medetomidine, no atipamezole; group MA = quad with medetomidine, atipamezole after 40 mins; group D = quad with dexmedetomidine, no atipamezole; group DA = quad with dexmedetomidine, atipamezole after 40 mins
Forty-one of the cats were ⩽6 months old; eight were in group M, 13 were in group MA, nine were in group D and 11 were in group DA. Of these 41 cats, three had mild diarrhoea within 10 mins of IM injection, one vomited on induction, one twitched the hindlimbs slightly after the first incision (no isoflurane required) and one of the kittens with diarrhoea required isoflurane because of purposeful hindlimb movement and pedal withdrawal reflex. No adverse effects were reported in the remaining 36 cats and all 41 recovered uneventfully.
Mean ± SD times to sternal recumbency and first standing between kittens ⩽6 months old and cats >6 months in each group are shown in Table 4. Kittens reached sternal recumbency in group DA sooner (51 ± 5 mins) than adults (57 ± 5 mins) (P = 0.017). Time to sternal recumbency was not different in the other groups (P = 0.8099 in group M; P = 0.1416 in group MA; and P = 0.6859 in group D). Kittens in group MA stood sooner (56 ± 8 mins) than adults (103 ± 67 mins) (P = 0.002) but there was no difference in the other groups (P = 0.4319 in group M; P = 0.2305 in group D; and P = 0.1338 in group DA).
Table 4.
Time to sternal recumbency and time to first standing for cats ⩽6 months old and cats >6 months old in each of the four treatment groups
| Group | Time to sternal recumbency (mins) |
Time to standing (mins) |
||
|---|---|---|---|---|
| ⩽6 months old | >6 months old | ⩽6 months old | >6 months old | |
| M | 127 ± 36 (n = 8) | 130 ± 31 (n = 17) | 141 ± 32 (n = 8) | 155 ± 41 (n = 17) |
| MA | 54 ± 8 (n = 13) | 74 ± 47 (n = 12) | 56 ± 8 * (n = 13) | 103 ± 67 (n = 12) |
| D | 107 ± 37 (n = 9) | 112 ± 20 (n = 16) | 117 ± 38 (n = 9) | 131 ± 18 (n = 16) |
| DA | 51 ± 5 * (n = 11) | 57 ± 5 (n = 14) | 62 ± 19 (n = 11) | 75 ± 23 (n = 14) |
Data are mean ± SD
Significant difference (P <0.05) between kittens and cats in that treatment group
Group M = quad with medetomidine, no atipamezole; group MA = quad with medetomidine, atipamezole after 40 mins; group D = quad with dexmedetomidine, no atipamezole; group DA = quad with dexmedetomidine, atipamezole after 40 mins; n = number of cats within each age bracket in each group
Adverse effects and complications that occurred during anaesthesia or surgery are reported in Table 5. The most common adverse effect during this study was vomiting within 5 mins of anaesthetic injection (5% incidence), despite the recommendation of an overnight fast. Animals that vomited were equally distributed between the groups. One cat developed a small scrotal haematoma postoperatively but recovered uneventfully and was discharged that evening with no further problems. No lasting side effects or complications were noted in any of the cats after recovery from general anaesthesia and all were discharged the same day.
Table 5.
Adverse effects/complications reported in study cats
| Adverse effect/complication | Cats affected (n) | Treatment group(s) of affected cats |
|---|---|---|
| Vomiting | 5 | MA, MA, D, DA, DA |
| Diarrhoea | 3 | MA, D, D |
| Hindlimb twitchafter incision | 3 | M, DA, DA |
| Ataxia on standing | 2 | MA, MA |
| Requirement for isoflurane | 1 | D |
| Scrotal haematoma | 1 | DA |
| Panting during recovery | 1 | DA |
Group M = quad with medetomidine, no atipamezole; group MA = quad with medetomidine, atipamezole after 40 mins; group D = quad with dexmedetomidine, no atipamezole; group DA = quad with dexmedetomidine, atipamezole after 40 mins
Discussion
This study fulfilled our first two aims of evaluating the effects on recovery of incorporating medetomidine, dexmedetomidine or atipamezole into the ‘quad’ protocol. In this study, atipamezole markedly reduced recovery times but only minor differences were detected between the effects of medetomidine and dexmedetomidine. There was also a trend towards faster recoveries in cats ⩽6 months old than in adults, fulfilling our third aim of evaluating recovery in paediatric animals and further allaying some of the apprehension concerning anaesthesia of kittens for prepubertal neutering.
Atipamezole was administered only after 40 mins to allow the effect of ketamine to abate. This was in accordance with the Ketaset (Zoetis) datasheet, which recommends that atipamezole is administered 45 mins after induction when using medetomidine in combination with ketamine and butorphanol. This is expected to ensure a smooth recovery. 26
Previous studies have focused on clinical efficacy and safety of dexmedetomidine in cats, and have measured sedation, analgesia and muscle relaxation, as well as monitoring heart rate, respiratory rate and rectal temperature after administration.18,19,27,28 The aim of our study was to focus on the effect of the two isomers and atipamezole on recovery times. There was some suggestion from our results that replacing medetomidine with dexmedetomidine in the ‘quad’ reduced recovery time. Mean time to sternal recumbency was reduced by 19 mins (without atipamezole) and 10 mins (with atipamezole). Mean time to standing was reduced by 24 mins (without atipamezole) and 9 mins (with atipamezole). However, only time to standing without atipamezole was significantly different. The study had 80% power to detect a real difference in time to sternal recumbency and to standing of 20 mins, and was therefore underpowered to establish whether the difference in recovery time to sternal recumbency was real. Although shortening the recovery time by approximately 20 mins using dexmedetomidine could be beneficial, a reduction of much less than this seems unlikely to have obvious clinical benefit. A larger study may have answered the question of whether time to sternal recumbency was really shorter with dexmedetomidine but would have added little to the clinical relevance. Regardless of whether dexmedetomidine or medetomidine was used, atipamezole reduced recovery times by approximately 1 h in this study. Clearly, this has more clinical relevance than the difference between medetomidine and dexmedetomidine. Shorter recovery time reduces the duration of anaesthesia thereby reducing the risk of anaesthesia-related mortality; the postoperative period has been identified as the most common point of anaesthetic-related mortality, particularly within the first 3 h after surgery. 2
The results from this investigation concur with findings in earlier studies that atipamezole is effective whether or not levomedetomidine is present with dexmedetomidine. 12 Previous studies have demonstrated almost identical clinical effects of dexmedetomidine and medetomidine, and so it would be logical to expect recovery time to be similar, as demonstrated in the present study. 18 However, Granholm et al suggested that although levomedetomidine is pharmacologically inactive, its presence in racemic medetomidine might impact upon both the pharmacodynamic and pharmacokinetic effects of dexmedetomidine. 18 They reported that 90–180 mins after IM administration, cats given dexmedetomidine (without atipamezole) overall had higher heart rates and more normal pulse characteristics than those receiving medetomidine. Additionally, once peak effect had developed, the analgesia and sedation scores were consistently lower for dexmedetomidine than medetomidine. 18 Although none of these differences were statistically significant, Granholm et al suggested that these data may have clinical relevance as they might reflect a difference in metabolism and hence a faster recovery, after the peak effect, from dexmedetomidine compared with medetomidine. 18 It is conceivable that the trend for faster recovery after dexmedetomidine found in our study may have been a result of differences in metabolism between dexmedetomidine and the racemic mixture.
Adverse effects were limited and none were life threatening. Vomiting at induction of general anaesthesia was the most frequent adverse event, with an incidence of 5%. Vomiting and nausea are commonly reported side effects in cats after administration of α2-adrenoceptor agonists due to central sensitisation of the area postrema. 29 The incidence in this study was much lower than previously reported after dexmedetomidine in cats.28,30 The reason for this is unknown but could be a result of the lower doses of α2-adrenoceptor agonists used in this study compared with others. Fasting prior to general anaesthesia was requested for all cats in this study but there is little firm evidence to date to suggest that withholding food affects the incidence of α2-adrenoceptor agonist-induced vomiting in cats. 28
Almost half of the cats in the present study were ⩽6 months old. They were randomly allocated between the four treatment groups, and there was no evidence that they were more adversely affected than the adults overall. Of the 16 reported occurrences of adverse effects in this study, only six occurred in cats ⩽6 months old. Additionally, their recovery time was consistently shorter than in adults, reaching significance in both groups given atipamezole. Adverse effects occurring in the younger animals – vomiting, diarrhoea, requirement for isoflurane and hindlimb twitching – were of too low an incidence to associate with any drug protocol. Three had diarrhoea after induction and one of these three was the only animal in the study to require isoflurane due to inadequate anaesthetic depth. Of the three cats with diarrhoea, one was in group MA and two were from group D. The two in group D were 14-week-old Tonkinese kittens from the same litter with a previous history of diarrhoea, so this was probably unrelated to the anaesthetic. It is not clear why the one cat needed isoflurane; the most likely explanation is that it did not receive its full ‘quad’ dose IM. It is unlikely that one cat receiving 1% isoflurane for 3 mins would affect the mean recovery time of the group. Isoflurane is rapidly exhaled and had little time for accumulation in the body. 31 Additionally, this cat’s recovery to both sternal recumbency and standing fell within 2 SD of the group mean.
Previous studies evaluating medetomidine and dexmedetomidine in cats all report a decrease in body temperature.18,19,27 This is due to both decreased heat production with reduced muscle activity and to the direct effects of α2-adrenoceptor agonists on thermoregulation. 20 This highlights the importance of maintaining body temperature during and after procedures using these drugs. Body temperature was not measured in this study, and its contribution to recovery time is unknown. However, preheated operating tables and recovery kennels were used for all cats in an attempt to prevent hypothermia throughout anaesthesia and to minimise the effect of this variable on recovery time. Surgical and hence anaesthetic time, another potential variable that would affect recovery time, was kept consistent by including only routine cat castrations in the study.
Two cats in the study had pre-existing pathological conditions: a fractured tibia and a forelimb degloving injury, respectively, which both required examination and application of supportive dressing under anaesthesia. These injuries may have delayed time to standing but exclusion of their data did not affect the results of statistical analysis. Both of these cats were randomly allocated to group DA. Recovery time to sternal recumbency and standing in the cat with the fractured tibia fell within 2 SD of the group mean but recovery time to standing in the cat with the degloving injury was longer.
Conclusions
Atipamezole given 40 mins after induction of anaesthesia with the ‘quad’ combination significantly reduced recovery time when either medetomidine or dexmedetomidine were incorporated in the protocol. Replacing medetomidine with dexmedetomidine had limited effect on the duration of recovery. Overall, there was a trend towards faster recovery in kittens compared with adults, particularly when atipamezole was used, thus providing some evidence to assuage concerns regarding feline paediatric anaesthesia. Further studies to assess alternative timing and dosage of atipamezole would be worthwhile.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 8 July 2015
References
- 1. Redondo JI, Suesta P, Gil L, et al. Retrospective study of the prevalence of postanaesthetic hypothermia in cats. Vet Rec 2012; 170: 206. [DOI] [PubMed] [Google Scholar]
- 2. Brodbelt DC, Blissit KJ, Hammond RA, et al. The risk of death: the confidential enquiry into perioperative small animal fatalities. Vet Anaesth Analg 2008; 35: 365–373. [DOI] [PubMed] [Google Scholar]
- 3. Welsh CP, Gruffydd-Jones TJ, Roberts MA, et al. Poor owner knowledge of feline reproduction contributes to the high proportion of accidental litters born to UK pet cats. Vet Rec 2014; 174: 118. [DOI] [PubMed] [Google Scholar]
- 4. Joyce A, Yates D. Help stop teenage pregnancy! Early-age neutering in cats. J Feline Med Surg 2011; 13: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mathews KA. Analgesia for the pregnant, lactating and neonatal to pediatric cat and dog. J Vet Emerg Crit Care 2005; 15: 273–284. [Google Scholar]
- 6. Holden D. Paediatric patients. In: Seymour C, Duke-Novakovski T. (eds). BSAVA manual of canine and feline anaesthesia and analgesia. 2nd ed. Gloucester: British Small Animal Veterinary Association, 2007, pp 296–302. [Google Scholar]
- 7. Grandy JL, Dunlop CI. Anesthesia of pups and kittens. J Am Vet Med Assoc 1991; 198: 1244–1250. [PubMed] [Google Scholar]
- 8. Morris HV, Nilsson S, Dixon CI, et al. Alpha 1- and alpha-2 containing GABA receptor modulation is not necessary for benzodiazepine-induced hyperphagia. Appetite 2009; 52: 675–683. [DOI] [PubMed] [Google Scholar]
- 9. Polson S, Taylor PM, Yates D. Effects of age and reproductive status on postoperative pain after routine ovariohysterectomy in cats. J Feline Med Surg 2014; 16: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams LS, Levy JK, Robertson SA, et al. Use of the anesthetic combination of tiletamine, zolazepam, ketamine, and xylazine for neutering feral cats. J Am Vet Med Assoc 2002; 220: 1491–1495. [DOI] [PubMed] [Google Scholar]
- 11. Harrison KA, Robertson SA, Levy JK, et al. Evaluation of medetomidine, ketamine and buprenorphine for neutering feral cats. J Feline Med Surg 2011; 13: 896–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Granholm M, McKusick BC, Westerholm FC, et al. Evaluation of the clinical efficacy and safety of intramuscular and intravenous doses of dexmedetomidine and medetomidine in dogs and their reversal with atipamezole.Vet Rec 2007; 160: 891–897. [DOI] [PubMed] [Google Scholar]
- 13. Virtanen R, Savola JM, Saano V, et al. Characterization of the selectivity, specificity and potency of medetomidine as an α2-adrenoreceptor agonist. Eur J Pharmacol 1988; 150: 9–14. [DOI] [PubMed] [Google Scholar]
- 14. Savola JM, Virtanen R. Central α2-adrenoreceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol 1991; 195: 193–199. [DOI] [PubMed] [Google Scholar]
- 15. Kuusela E, Raekallio M, Antilla M, et al. Clinical effects and pharmacokinetics of medetomidine and its enantiomers in dogs. J Vet Pharmacol Ther 2000; 23: 15–20. [DOI] [PubMed] [Google Scholar]
- 16. Jansson CC, Marjamäki A, Luomala K. Coupling of human α2-adrenoreceptor subtypes to regulation of cAMP production in transfected S115 cells. Eur J Pharmacol 1994; 266: 165–174. [DOI] [PubMed] [Google Scholar]
- 17. Jansson CC, Kukkonen JP, Näsman J. Protean agonism at α2-adrenoreceptors. Mol Pharmacol 1998; 53: 963–968. [PubMed] [Google Scholar]
- 18. Granholm M, McKusick BC, Westerholm FC, et al. Evaluation of the clinical efficacy and safety of dexmedetomidine in cats and their reversal with atipamezole. Vet Anaesth Analg 2006; 33: 214–223. [DOI] [PubMed] [Google Scholar]
- 19. Ansah OB, Raekallio M, Vainio O. Comparison of three doses of dexmedetomidine with medetomidine in cats following intramuscular administration. J Vet Pharmacol Ther 1998; 21: 380–387. [DOI] [PubMed] [Google Scholar]
- 20. MacDonald E, Scheinin H, Scheinin M. Behavioural and neurochemical effects of medetomidine, a novel veterinary sedative. Eur J Pharmacol 1988; 158: 119–127. [DOI] [PubMed] [Google Scholar]
- 21. Clarke-Price S. Inadvertent perianesthetic hypothermia in small animal patients. Vet Clin Small Anim 2015; 45: 983–994. [DOI] [PubMed] [Google Scholar]
- 22. Ambrisko TD, Hikasa Y. Neurohormonal and metabolic effects of medetomidine compared with xylazine in beagle dogs. Can J Vet Res 2002; 66: 42–49. [PMC free article] [PubMed] [Google Scholar]
- 23. Ghimire LV, Muszkat M, Sofowora GG, et al. Variation in the α(2A) adrenoceptor gene and the effect of dexmedetomidine on plasma insulin and glucose. Pharmacogenet Genomics 2013; 23: 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sinclair RCF, Faleiro RJ. Delayed recovery of consciousness after anaesthesia. Contin Educ Anaesth Crit Care Pain 2006; 6: 114–118. [Google Scholar]
- 25. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Office of Animal Health. Ketaset 100 mg/ml solution for injection. NOAH compendium datasheet. http://www.noahcompendium.co.uk (2015, accessed June 20, 2015)
- 27. Selmi AL, Mendes GM, Lins BT, et al. Evaluation of the sedative and cardiorespiratory effects of dexmedetomidine, dexmedetomidine-butorphanol, and dexmedetomidine-ketamine in cats. J Am Vet Med Assoc 2003; 222: 37–41. [DOI] [PubMed] [Google Scholar]
- 28. McSweeney PM, Martin DD, Ramsey DS, et al. Clinical efficacy and safety of dexmedetomidine used as a preanesthetic prior to general anesthesia in cats. J Am Vet Med Assoc 2012; 240: 404–412. [DOI] [PubMed] [Google Scholar]
- 29. Hikasa Y, Akiba T, Iino Y, et al. Central alpha-adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol 1992; 229: 241–251. [DOI] [PubMed] [Google Scholar]
- 30. Santos LCP, Ludders JW, Erb HN, et al. A randomized, blinded controlled trial of the antiemetic effect of ondansetron on dexmedetomidine-induced emesis in cats. Vet Anaesth Analg 2011; 38: 320–327. [DOI] [PubMed] [Google Scholar]
- 31. Dugdale A. Veterinary anaesthesia: principles to practice. Oxford: Wiley-Blackwell, 2010, p 69. [Google Scholar]