Abstract
Presenting signs and initial investigations: An 8-year-old female spayed British shorthair cat was presented with a history of waxing and waning neurological signs. Neuroanatomical localisation was consistent with a diffuse forebrain disease. Blood ammonia concentration was increased. Abdominal ultrasonography and a bile acid stimulation test were normal. Magnetic resonance imaging (MRI) revealed hyperintense, bilaterally symmetrical, diffuse lesions on T2-weighted sequences, predominantly, but not exclusively, affecting the grey matter. Serum cobalamin (vitamin B12) concentration was low. Hypocobalaminaemia resulting in a urea cycle abnormality was considered a likely cause of the hyperammonaemia.
Treatment: Daily cobalamin injections resulted in a rapid clinical improvement. Eight weeks into treatment neurological examination was unremarkable and there was complete resolution of the MRI lesions.
Clinical importance: This is the first reported case of acquired feline hypocobalaminaemia resulting in an encephalopathy. Additionally, this case is unique in describing reversible brain MRI abnormalities in a cobalamin-deficient companion animal.

Clinical report
An 8-year-old female spayed British shorthair cat was presented with a 6-month history of waxing and waning neurological signs (Figure 1). The cat was fed a balanced, commercially available diet and had no known history of toxin exposure. Routine vaccinations were up to date. Video footage obtained the day before presentation showed abnormal mentation, head pressing behaviour and pacing (see accompanying supplementary video). The cat was in good body condition (body condition score 4/9) and physical examination was unremarkable. Neurological examination revealed an obtunded mental status, bilaterally decreased menace responses, normal visual placing, intact pupillary light reflexes and decreased facial sensation. Postural reactions were markedly reduced in all four limbs. Neuroanatomical localisation was consistent with a multifocal brain disease.
Figure 1.

The cat was observed by the owner to hypersalivate intermittently and this was commonly associated with periods of abnormal mentation
Results of a complete blood count and serum biochemistry were within normal limits. Blood ammonia concentration was increased (250 µmol/l; normal <50 µmol/l). Differential diagnoses for hyperammonaemia were hepatic dysfunction or a urea cycle abnormality. An acquired portosystemic shunt was suspected as a cause of the hyperammonaemia. An abdominal ultrasound scan did not reveal any abnormalities.
Symptomatic treatment for hepatic encephalopathy (HE) was commenced pending bile acid stimulation test results. Treatment consisted of amoxicillin/clavulanic acid (Synulox; Pfizer) (50 mg PO q12h for 7 days), lactulose (Lactulose Solution BP; Sandoz) (2 ml PO q8h), ranitidine (Zantac; GlaxoSmithKline) (0.5 ml PO q12h) and sucralfate (Antepsin; Chugai Pharma UK) (2 ml PO q12h). Intravenous fluid therapy with lactated Ringer’s solution (Aqupharm number 11; Animalcare) was started at 2 ml/kg/h.
Bile acid stimulation test results returned as normal. Urea cycle dysfunction secondary to hypocobalaminaemia was therefore considered a probable cause for the hyperammonaemia. Serum was taken to measure cobalamin concentration, and urine obtained by cystocentesis was submitted to determine methylmalonic acid (MMA) concentration by gas chromatography–mass spectrometry.
The cat displayed a marked deterioration in mental status over the following 3 days despite treatment for HE. Magnetic resonance imaging (MRI) of the brain was therefore performed, using a 0.4 T scanner (Hitachi Aperto; Tokyo, Japan), to investigate the progressive neurological abnormalities. T1- and T2-weighted (T1W/T2W) sequences were obtained in sagittal and transverse planes. Fluid-attenuated inversion recovery (FLAIR) sequences were not performed.
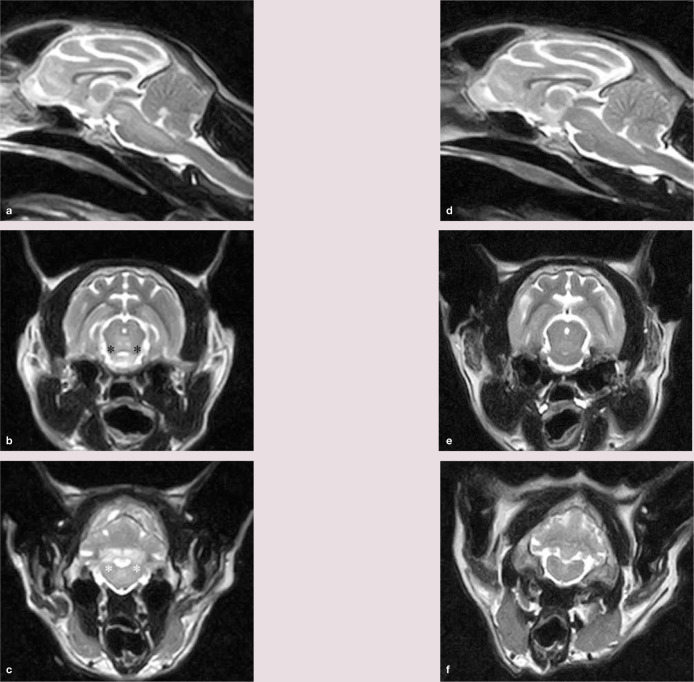
MRI revealed hyperintense, bilaterally symmetrical, diffuse lesions on T2W sequences, predominantly, but not exclusively, involving the grey matter. The thalamus, mesencephalon, pons, caudal cerebellar peduncles and interposital nuclei were affected (Figure 2a–c). No lesions were evident on T1W sequences. Bilaterally symmetrical MRI abnormalities were consistent with a metabolic or toxic disorder. The latter was considered unlikely given the waxing and waning disease course and duration, and the cat’s subsequent deterioration in a hospital environment.
Figure 2.
(a) Midline sagittal section showing intra-axial regions of hyperintensity to grey matter in the mesencephalon and pons. (b and c) Transverse images at the level of the rostral colliculus (b) and caudal cerebellar peduncles (c). Bilaterally symmetrical hyperintense to grey matter lesions can be identified affecting the mesencephalic tegmentum (adjacent to the black asterisks) and white matter lesions affecting the caudal cerebellar peduncles (adjacent to the white asterisks). (d f) Magnetic resonance images corresponding to images (a c) following 8 weeks of cobalamin supplementation. The hyperintense lesions have completely resolved
Serum cobalamin subsequently returned as low (85 ng/l; reference interval 240–1499 ng/l) and urinary MMA concentration was normal. There was no history or clinical evidence of gastrointestinal disease and feline trypsin-like immunoreactivity test results were within normal limits. A rapid clinical improvement was seen in response to daily cobalamin injections (VitBee 250; Dechra Veterinary Products) (0.3 ml SC q24h).
At follow-up 8 weeks after presentation, neurological examination was unremarkable and the owners reported normal behaviour at home. Serum cobalamin was high (>2000 ng/l) and the blood ammonia concentration had normalised. MRI revealed complete resolution of the abnormalities on T2W sequences (Figure 2d–f). FLAIR sequences in the transverse plane were additionally performed and were unremarkable. The cat was discharged on weekly cobalamin injections (0.3 ml SC) and remained clinically normal 12 months later.
Discussion
Cobalamin metabolism
Cobalamin is an essential cofactor for two mammalian enzyme systems, methionine synthase and methylmalonyl CoA mutase.1,2 Deficiency is frequently associated with haematological abnormalities, lethargy and inappetence.3,4 Acquired cobalamin deficiency in cats results from intestinal lymphoma, inflammatory bowel disease, cholangitis or cholangiohepatitis, exocrine pancreatic insufficiency or malnutrition.4,5 Dietary cobalamin is dissociated from proteins in the stomach, and bound to haptocorrins and subsequently to intrinsic factor in the duodenum. 4 In cats, intrinsic factor is exclusively pancreatic in origin and cobalamin-intrinsic factor absorption is restricted to the ileum via specific receptors.6,7 Cobalamin is subsequently bound to transcobalamin II following its release into the portal circulation. 8 Disruption of any of these processes may result in cobalamin deficiency. 9 Since investigation of the cat in this report, spontaneous hyperthyroidism has been reported to be associated with hypocobalaminaemia. 10 However, the clinical signs, results of laboratory investigations and 12-month follow-up findings were not supportive of concurrent hyperthyroidism. Pathogenesis of hypocobalaminaemia was not elucidated in the present case although acquired gastrointestinal disease cannot be excluded given the cat’s age.
Measurement of cobalamin
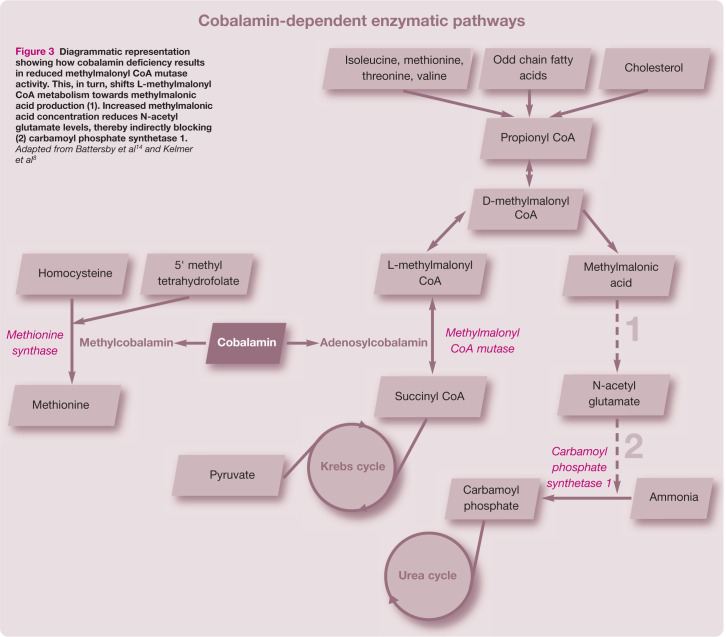
Reduced serum cobalamin concentrations do not necessarily reflect cellular cobalamin availability.11,12 MMA concentrations are considered a more accurate marker of feline cobalamin cellular deficiency than serum cobalamin. 1 Hypocobalaminaemia results in reduced concentrations of adenosylcobalamin. Deficiencies in this essential cofactor for the enzyme methylmalonyl CoA mutase result in reduced isomerisation of L-methylmalonyl CoA to succinyl CoA. 8 Consequently L-methylmalonyl CoA metabolism is shifted towards production of MMA (Figure 3). 12 Normal urinary concentrations of MMA detected in the present case prior to cobalamin supplementation were, therefore, surprising but were attributed to incorrect sample handling as the urine was not frozen prior to shipment for laboratory analysis. MMA concentrations are reported to normalise following cobalamin supplementation; 13 therefore repeat testing was not performed in this case.
Figure 3.
Diagrammatic representation showing how cobalamin deficiency results in reduced methylmalonyl CoA mutase activity. This, in turn, shifts L-methylmalonyl CoA metabolism towards methylmalonic acid production (1). Increased methylmalonic acid concentration reduces N-acetyl glutamate levels, thereby indirectly blocking (2) carbamoyl phosphate synthetase 1. Adapted from Battersby et al 14 and Kelmer et al 8
Pathogenesis of the encephalopathy
Potential causes considered for the encephalopathy were hyperammonaemia, increased MMA concentrations or a pathogenesis directly related to cobalamin deficiency. Hyperammonaemic encephalopathy secondary to hypocobalaminaemia was described in a cobalamin-deficient juvenile Border collie. 14 Increased MMA concentrations resulting in indirect inhibition of the rate-limiting enzyme of the urea cycle, carbamoyl phosphate synthetase 1, were proposed as a cause of the hyperammonaemia (Figure 3). 15 In contrast to this canine case, however, the current feline case showed no improvement on symptomatic treatment of HE. Hyperammonaemia was, therefore, considered an unlikely primary cause of the encephalopathy.
Elevated MMA concentrations in dogs and humans have been reported to cause a variety of neurological symptoms ranging from intermittent neurological abnormalities to acute encephalopathy.16,17 Clinical signs have commonly been attributed to hyperammonaemia, metabolic acidosis and ketonuria. 16 An organic acidaemia resulting in severe metabolic acidosis and ketonuria was reported in a cobalamin-deficient young cat. 8 Significantly elevated MMA concentrations were detected in both urine and serum samples by gas chromatography–mass spectrometry. Waxing and waning neurological signs attributed to the cerebrum displayed some similarities with the cat in our report. Elevated MMA concentrations were not detected in the present case but the proposed pathogenesis and poor urine sample handling mean they cannot be excluded. Therefore, the pathogenesis for the encephalopathy in the current feline case may relate to a primary cobalamin deficiency, elevated MMA concentrations, or a combination of the two.
Neuropathological effects of hypocobalaminaemia remain to be fully elucidated. In humans, cobalamin deficiency manifests primarily as a demyelinating disease of the spinal cord called subacute combined degeneration (SCD). 18 A leukoencephalopathy has also been described. 19 Two theories have been suggested to explain this pathogenesis. Senol et al 20 proposed that an accumulation of methylmalonyl CoA indirectly causes reduced myelin synthesis. However, a second study, performed in rats, demonstrated that demyelination results from increased synthesis of myelinotic tumour necrosis factor and decreased production of epidermal growth factor and interleukin-6. 21 By contrast, MRI abnormalities in the present feline case primarily reflected a polioencephalopathy, which has not been documented in cobalamin-deficient humans. This may be suggestive of an alternative neuropathogenesis to the putative role of hypocobalaminaemia causing demyelination in humans. In methylmalonic acidurias in humans, MMA, or its alternative metabolites malonic acid, propionyl CoA and 2-methylcitric acid, have been demonstrated to cause inhibition of the Krebs cycle and respiratory chain. 22 Grey matter changes revealed in this case may therefore have been related to high MMA concentrations.
Neuroimaging findings
The present report is unique in describing MRI abnormalities in a hypocobalaminaemic companion animal. Bilaterally symmetrical hyperintense grey matter lesions on T2W images have also previously been described in cases of feline thiamine deficiency.23,24 Thiamine deficiency was nevertheless considered an unlikely cause of the grey matter MRI abnormalities in this cat as it had been fed a balanced commercial diet and improved following cobalamin supplementation without the need to supplement thiamine or alter its dietary regimen. An MRI study on hyperammonaemic dogs and cats with portosystemic shunts displaying signs of HE reported no abnormalities on T2W images. 25 Conversely, it described
T1W lentiform nuclei hyperintensities attributed to manganese deposition. 25 Hyperammonaemia is, therefore, an improbable cause of observed T2W hyperintensities in the present case.
In humans, high MMA concentrations have been linked with bilaterally symmetrical hyperintensities of the globi pallidi on T2W images. 16 While these changes were not revealed in the present case, elevated MMA concentrations, as discussed above, may have been associated with observed grey matter lesions. SCD is the most frequent neurological manifestation of cobalamin deficiency in humans. T2W MR sequences show high signal intensities within the posterior and lateral columns, whereas brain MR images are often unremarkable.18,20 Degenerative myelopathy secondary to cobalamin deficiency has also been documented in one cat. 5 A leukoencephalopathy with multifocal, confluent periventricular hyperintense signal changes on T2W MR images has been described in humans. 19 MRI cerebellar abnormalities, as in the present feline case, have only rarely been documented in humans. 26
Reversal of both T2W MRI hyperintensities and clinical signs on thiamine supplementation has previously been described in cases
of feline thiamine deficiency. 24 The present report is unique in describing reversible MRI changes in a cobalamin-deficient companion animal. Complete resolution of both imaging and neurological abnormalities was evident at an 8-week follow-up. Similarly, resolution or improvement of clinical and MRI abnormalities in humans has been achieved with early cobalamin supplementation.18,19,20,26 This is typically observed following 2–3 months of treatment.18,20,27
Conclusions
Resolution of neurological signs subsequent to cobalamin supplementation supports a diagnosis of encephalopathy secondary to acquired cobalamin deficiency, of which this is the first reported case in a cat. Hypocobalaminaemia should be considered a differential diagnosis in cats displaying compatible neurological signs. Diagnostic confirmation may be achieved through reversible brain MRI abnormalities subsequent to cobalamin supplementation. It is necessary to recognise, however, that rapid resolution of MRI lesions precludes a definitive retrospective diagnosis from being made once cobalamin treatment has commenced.
Footnotes
Date accepted: 24 January 2012
Funding
The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1. Ruaux CG, Steiner JM, Williams DA. Metabolism of amino acids in cats with severe cobalamin deficiency. Am J Vet Res 2001; 62: 1852–1858. [DOI] [PubMed] [Google Scholar]
- 2. Reed N, Gunn-Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 2007; 9: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaden SL, Wood PA, Ledley FD, Cornwell PE, Miller RT, Page R. Cobalamin deficiency associated with methylmalonic acidemia in a cat. J Am Vet Med Assoc 1992; 15: 1101–1103. [PubMed] [Google Scholar]
- 4. Simpson KW, Fyfe J, Cornetta A, Sachs A, Strauss-Ayali D, Lamb SV, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 5. Salvadori C, Cantile C, de Ambrogi G, Arispici M. Degenerative myelopathy associated with cobalamin deficiency in a cat. J Vet Med A Physiol Pathol Clin Med 2003; 50: 292–296. [DOI] [PubMed] [Google Scholar]
- 6. Fyfe JC. Feline intrinsic factor (IF) is pancreatic in origin and mediates ileal cobalamin (CBL) absorption. J Vet Intern Med 1993; 7: 133. [Google Scholar]
- 7. Barron PM, Mackie JT, Evans NA, Langer N. Serum cobalamin concentrations in healthy cats and cats with non-alimentary tract illness in Australia. Aust Vet J 2009; 87: 280–283. [DOI] [PubMed] [Google Scholar]
- 8. Kelmer E, Shelton GD, Williams DA, Ruaux CG, Kerl ME, O’Brien DP. Organic acidemia in a young cat associated with cobalamin deficiency. J Vet Emerg Crit Care 2007; 17: 299–304. [Google Scholar]
- 9. Ibarrola P, Blackwood L, Graham PA, Evans H, German AJ. Hypocobalaminaemia is uncommon in cats in the United Kingdom. J Feline Med Surg 2005; 7: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cook AK, Suchodolski JS, Steiner JM, Robertson JE. The prevalence of hypocobalaminaemia in cats with spontaneous hyperthyroidism. J Small Anim Pract 2011; 52: 101–106. [DOI] [PubMed] [Google Scholar]
- 11. Ruaux CG, Steiner JM, Williams DA. Relationships between low serum cobalamin concentrations and methylmalonic acidemia in cats. J Vet Intern Med 2009; 23: 472–475. [DOI] [PubMed] [Google Scholar]
- 12. Berghoff N, Suchodolski JS, Steiner JM. Association between serum cobalamin and methylmalonic acid concentrations in dogs. Vet J 2012; 191: 306–311. [DOI] [PubMed] [Google Scholar]
- 13. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminemia. J Vet Intern Med 2005; 19: 155–160. [DOI] [PubMed] [Google Scholar]
- 14. Battersby IA, Giger U, Hall EJ. Hyperammonaemic encephalopathy secondary to selective cobalamin deficiency in a juvenile Border collie. J Small Anim Pract 2005; 46: 339–344. [DOI] [PubMed] [Google Scholar]
- 15. Dimski DS. Ammonia metabolism and the urea cycle: function and clinical implications. J Vet Intern Med 1994; 8: 73–78. [DOI] [PubMed] [Google Scholar]
- 16. Deodato F, Boenzi S, Santorelli FM, Dionisci-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet 2006; 142C: 104–112. [DOI] [PubMed] [Google Scholar]
- 17. Podell M, Shelton GD, Nyhan WL, Wagner SO, Genders A, Oglesbee M, et al. Methylmalonic and malonic aciduria in a dog with progressive encephalomyelopathy. Metab Brain Dis 1996; 11: 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee WJ, Hsu HY, Wang PY. Reversible myelopathy on magnetic resonance imaging due to cobalamin deficiency. J Chin Med Assoc 2008; 71: 368–372. [DOI] [PubMed] [Google Scholar]
- 19. Stojsavljevic N, Levic Z, Drulovic J, Dragutinovic GA. A 44-month clinical-brain MRI follow-up in a patient with B12 deficiency. Neurology 1997; 49: 878–881. [DOI] [PubMed] [Google Scholar]
- 20. Senol MG, Sonmez G, Ozdag F, Saracoglu M. Reversible myelopathy with vitamin B12 deficiency. Singapore Med J 2008; 49: 330–332. [PubMed] [Google Scholar]
- 21. Scalabrino G. Cobalamin (vitamin B12) in subacute combined degeneration and beyond: traditional interpretations and novel theories. Exp Neurol 2005; 192: 463–479. [DOI] [PubMed] [Google Scholar]
- 22. Kolker S, Schwab M, Hörster F, Sauer S, Hinz A, Wolf NI, et al. Methylmalonic acid, a biochemical hallmark of methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J Biol Chem 2003; 278: 47388–47393. [DOI] [PubMed] [Google Scholar]
- 23. Penderis J, McConnell JF, Calvin J. Magnetic resonance imaging features of thiamine deficiency in a cat. Vet Rec 2007; 160: 270–272. [DOI] [PubMed] [Google Scholar]
- 24. Palus V, Penderis J, Jakovljevic S, Cherubini GB. Thiamine deficiency in a cat: resolution of MRI abnormalities following thiamine supplementation. J Feline Med Surg 2010; 12: 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Torisu S, Washizu M, Hasegawa D, Orima H. Brain magnetic resonance imaging characteristics in dogs and cats with congenital portosystemic shunts. Vet Radiol Ultrasound 2005; 46: 447–451. [DOI] [PubMed] [Google Scholar]
- 26. Morita S, Miwa H, Kihira T, Kondo T. Cerebellar ataxia and leukoencephalopathy associated with cobalamin deficiency. J Neurol Sci 2003; 216: 183–184. [DOI] [PubMed] [Google Scholar]
- 27. Shyambabu C, Sinha S, Taly AB, Vijayan J, Kovoor JME. Serum vitamin B12 deficiency and hyperhomocystinemia: a reversible cause of acute chorea, cerebellar ataxia in an adult with cerebral ischemia. J Neurol Sci 2008; 273: 152–154. [DOI] [PubMed] [Google Scholar]