Abstract
We have isolated uracil-auxotrophic mutants of the hyperthermophilic archaeon Sulfolobus solfataricus in order to explore the genomic stability and mutational frequencies of this organism and to identify complementable recipients for a selectable genetic transformation system. Positive selection of spontaneous mutants resistant to 5-fluoroorotate yielded uracil auxotrophs with frequencies of between 10−4 and 10−5 per sensitive, viable cell. Four different, nonhomologous insertion sequences (ISs) were identified at different positions within the chromosomal pyrEF locus of these mutants. They ranged in size from 1,058 to 1,439 bp and possessed properties typical of known transposable elements, i.e., terminal inverted repeats, flanking duplicated target sequences, and putative transposase genes encoding motifs that are indicative of the IS4-IS5 IS element families. Between 12 and 25 copies of each IS element were found in chromosomal DNAs by Southern analyses. While characteristic fingerprint patterns created by IS element-specific probes were observed with genomic DNA of different S. solfataricus strains, no homologous sequences were identified in DNA of other well-characterized strains of the order Sulfolobales.
Small transposable DNA elements are integral parts of most prokaryotic genomes and are also frequently found in eukaryotes (14, 19). The mobile bacterial insertion sequence (IS) elements were initially identified by their capacity to generate mutations or to disseminate antibiotic resistance genes, but more recently, they have frequently been detected in association with pathogenic and virulence functions or degradative and catabolic pathways. Therefore, a general role of transposable elements in assembling and mobilizing sets of accessory functions in bacteria has been proposed (18, 19).
ISs appear also to be widely distributed in the domain Archaea. In three of the four completely sequenced archaeal genomes, all of which belong to (hyper)thermophilic organisms of the kingdom Euryarchaeota, many families of insertion elements have been identified (4, 15, 16, 31).
The best-studied archaeal IS elements are found in the extremely halophilic euryarchaeote Halobacterium salinarum (formerly Halobacterium halobium), which exhibits exceptionally high genetic variability due to insertional mutations and DNA rearrangements. Phenotypes of this organism mutate with frequencies of between 10−2 and 10−4 (21). The IS elements are nonrandomly distributed within the genome, residing mostly on plasmids and in the AT-rich chromosomal regions (21). In Halobacterium sp. strain NRC-1, an IS element-mediated process is proposed to account for the movement of chromosomal genes to the 200-kbp megaplasmid pNRC100, which seems to be evolving into a minichromosome (20). A transpositional burst of the IS element ISH27 was observed in Halobacterium after plates had been stored for long periods in the cold, suggesting that stress can lead to increased transposition activity (22).
Several IS elements have also been detected in the hyperthermophilic crenarchaeote Sulfolobus solfataricus. Spontaneous β-galactosidase mutants have been shown to arise with relatively high frequencies (about 10−4 per plated cell [26]). These mutants contained a transposable element in the lacS gene (β-galactosidase) with features typical of bacterial and archaeal ISs including terminal inverted repeats, a putative transposase gene, and short direct flanking repeats. Furthermore, in the ongoing genome sequencing project of S. solfataricus at least six more classically organized IS elements have so far been detected (28, 29). Finally, putative transposases and at least one insertion element have also been found in the sequence of the promiscuous conjugative plasmid pNOB8 of Sulfolobus sp., a close relative of S. solfataricus (30), and in strain MT-4 (2). Altogether, these data suggested that several IS elements might be active in Sulfolobus.
In this study, we were interested in exploring the genomic stability and mutational frequencies of S. solfataricus and in identifying stable auxotrophic mutants that can serve as complementable recipients in selectable genetic systems. S. solfataricus is becoming a model system to study cellular processes and gene functions in hyperthermophilic crenarchaeota, based on the isolation of several plasmids and viruses (34) and on the demonstration of genetic transformation of this organism (3, 5, 7, 25). Furthermore, the complete genomic sequence of S. solfataricus is currently being determined (29).
We have selected for pyrimidine-requiring auxotrophs with 5-fluoroorotic acid (5-FOA). Mutants that are resistant to this drug usually lack either orotidine-5′-monophosphate pyrophosphorylase (PyrE) or orotidine-5′-monophosphate decarboxylase (PyrF) and are thus unable to convert orotate to UMP, which represents the last step in de novo pyrimidine nucleotide biosynthesis. 5-FOA has previously been used for positive selection of uracil auxotrophs in different microorganisms, including the archaeon Sulfolobus acidocaldarius (10, 17). Although spontaneous mutational rates of S. acidocaldarius were found to be comparable to mutational rates of biosynthetic genes in Escherichia coli in a selection with 5-FOA (13), in this study we show that stable uracil-auxotrophic mutants of S. solfataricus arise spontaneously with frequencies at least 1 order of magnitude higher and are caused by transposition of insertion elements.
MATERIALS AND METHODS
Strains, media, and growth conditions.
S. solfataricus P1 (DSM 1616), P2 (DSM1617), and PH1 (26); Sulfolobus shibatae (DSM 5389); and S. acidocaldarius (DSM 639), PIT3, and KAW2 (gifts of Wolfram Zillig) were grown at 80°C and pH 3 in mineral medium supplemented with 0.2 or 0.1% tryptone (Oxoid) and 0.2% (either) d-xylose or l-arabinose (Sigma). Gelrite (0.7%; Kelco, San Diego, Calif.) was added to solidify the media for plating as described previously (9). For selection of pyrimidine-auxotrophic mutants, 50 μg of 5-FOA (Sigma) per ml was added as well as 10 μg of uracil per ml. Mutants were grown in media supplemented with 10 μg of uracil per ml.
Isolation of auxotrophic mutants.
Strains were grown in 50-ml liquid cultures to an optical density of A600 = 0.9 to 1.0 (late exponential phase), and approximately 106 to 107 cells were plated on solid medium supplemented with 5-FOA and uracil. Controls were plated on media solely containing 5-FOA but no uracil (no growth), and appropriate dilutions were plated on medium without inhibitor to determine viable cell counts as CFU. The plates were incubated for 5 to 7 days, and spontaneous 5-FOA-resistant mutants were isolated and tested by restreaking colonies on fresh plates and/or by inoculating liquid medium with and without uracil.
Isolation of chromosomal DNA from Sulfolobus.
Three milliliters of a late-exponential culture was cooled and centrifuged in a minicentrifuge for 2 min at maximal speed. The cells were resuspended in 500 μl of Tris-EDTA buffer, and N-laurylsarcosine (0.8%) and Triton X-100 (0.06%) were added. After incubation at 20°C for 30 min, the lysed cells were extracted with phenol-chloroform and the DNA was precipitated with ethanol. About 50 ng of such preparations was used as templates in PCR amplifications, and about 3 μg was used for restriction enzyme analyses and subsequent Southern hybridizations.
PCR amplification, cloning, and sequence analysis.
Two primers derived from the DNA sequence located 5′ and 3′ adjacent to the pyrEF gene cluster in S. solfataricus P2 (see Fig. 2) that contained KpnI restriction sites at their 5′ ends were synthesized: pyr-R, GCA GGT ACC TCG TGT AGA TTT TCC CC, and pyr-L, GCG GTA CCA CTT GCG AAT AAT GCT GC. These primers specifically amplified a 1.7-kbp fragment from chromosomal DNA of S. solfataricus P1 and P2 at annealing temperatures of 52°C. Fragments of 2.9 to 3.1 kbp in size were obtained from auxotrophic mutants. Additional primer pairs complementary to regions in the pyrEF cluster were used to specifically amplify IS element sequences for the different mutants after their site of integration had been determined. One primer in each set contained a KpnI restriction site, and the other contained a SacII restriction site. Both enzymes did not cleave within the IS element sequences. The PCR products were purified, cleaved with KpnI (or KpnI and SacII, respectively), and ligated with pBluescript SK(+) cut with the same enzymes. The inserted DNA fragments of the resulting recombinant clones were identified by restriction mapping.
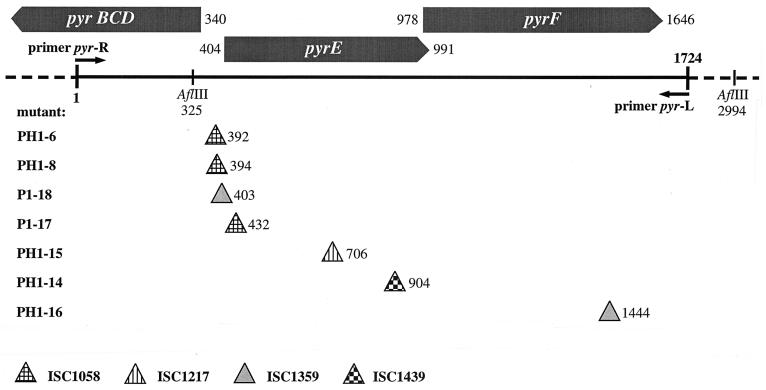
FIG. 2.
Genomic region of pyrimidine biosynthesis genes in S. solfataricus P2. The positions of the primers pyr-R and pyr-L, used to amplify the pyrEF genes from wt and mutant DNA, are indicated, as well as the sites of insertion of the four different IS elements found in seven different mutants. The two AflIII sites within this region are also shown. pyrE and pyrF are genes coding for putative orotidine-5′-monophosphate pyrophosphorylase (PyrE) or orotidine-5′-monophosphate decarboxylase (PyrF) as identified by homology to known protein sequences from the database.
Two primers, pyrE2Nhe (TTT TTG CTA GCT TTT TCC AAT ATT TTT C AC) and pyrE1CLA (TTT TTA TCG ATG GAT TTC GTG AAA GCT CTA C), were used to amplify the pyrE gene of S. acidocaldarius (accession no. Y12822, erroneously assigned in that submission to S. solfataricus).
Sequence analysis was performed on a Li-Cor DNA sequencer. The University of Wisconsin Genetics Computer Group package (version 10.0) was used for computer-aided editing of the DNA sequences. Amino acid sequences of the putative transposases were compared in a multiple alignment with PILEUP that was corrected manually. Only a few representatives of the transposases from the IS4-IS5 family were chosen for comparison, preferably those that were initially identified as relatives by BLASTP2 searches against the databases.
Southern hybridization.
Approximately 2 to 3 μg of chromosomal DNAs was cut with AflIII and electrophoresed in a 1% agarose gel using standard procedures (24). The DNA was blotted and hybridized according to standard procedures. The pyrEF- and IS element-specific probes were prepared by cutting out and purifying the respective inserts from recombinant pBluescript vectors and randomly labeling them using the digoxigenin-DNA labeling and detection kit (Boehringer Mannheim).
Nucleotide sequence accession numbers.
The DNA sequences of the three novel IS elements characterized in this study have been deposited in GenBank under accession no. AJ270982, AJ270983, and AJ270984.
RESULTS
Isolation of uracil-auxotrophic mutants.
To select spontaneous mutants resistant to 5-FOA, cultures were grown to mid- or late exponential phase and about 106 to 107 cells were plated on medium containing 50 μg of the inhibitor per ml and 10 μg of uracil per ml. The inhibitor concentration was about twice the MIC as determined for S. acidocaldarius (10). The same numbers of cells were also plated on medium without uracil. No growth was observed with the four Sulfolobus strains that were tested in these control experiments. Appropriate dilutions of the cultures were plated without inhibitor in order to determine viable cell counts and to calculate mutation frequencies per CFU. The results of some representative and independent experiments are shown in Table 1. Resistant colonies of S. acidocaldarius, S. shibatae, and Sulfolobus islandicus REN were recovered at frequencies of 10−6 to 10−7 per CFU. These results were comparable to those observed in earlier studies for S. acidocaldarius (2 × 10−7 [13]). In contrast, mutational frequencies of S. solfataricus appeared to be variable, but in all cases, they were at least 1 order of magnitude higher than those for the other strains. The highest frequencies were around 3 × 10−4. Enhanced frequencies were also obtained with S. solfataricus strain PH1, a β-galactosidase mutant of P1 that contains the insertion element ISC1217 stably integrated into the lacS gene (26).
TABLE 1.
Estimation of mutation frequencies in Sulfolobus spp. by positive selection of uracil auxotrophs on 5-FOA
| Sulfolobus sp. (and strain where indicated) | No. of cellsa
|
Apparent frequency | |
|---|---|---|---|
| Sensitive | Resistant | ||
| S. solfataricus P1 | 4.7 × 106 | 113 | 2.4 × 10−5 |
| 3.3 × 105 | 103 | 3.1 × 10−4b | |
| S. solfataricus PH1(lacS) | 6.3 × 105 | 214 | 3.4 × 10−4b |
| 1.4 × 106 | 26 | 1.9 × 10−5 | |
| S. shibatae | 9.7 × 106 | 18 | 1.8 × 10−6 |
| S. islandicus REN | 8.7 × 106 | 64 | 7.4 × 10−6 |
| S. acidocaldarius | 3.2 × 106 | 6 | 1.8 × 10−6 |
| 4.5 × 105 | 2 | 4.4 × 10−6 | |
| 6.3 × 106 | 24 | 3.8 × 10−6 | |
Cell numbers were calculated from averages of CFU after serial dilutions on Gelrite plates containing tryptone and arabinose (sensitive) or tryptone-arabinose plus 50 μg of 5-FOA per ml and 10 μg of uracil per mL (resistant).
Experiments from which mutants were recovered for this study.
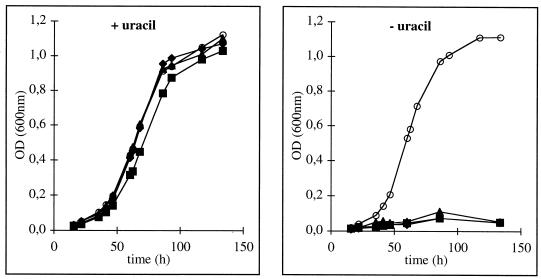
Sixteen isolates of S. solfataricus P1 or PH1 and two of S. acidocaldarius were subsequently checked for their auxotrophic phenotype in liquid medium. While all isolates grew in the presence of uracil with rates comparable to those of wild-type (wt) cells, 14 out of the 16 drug-resistant strains of S. solfataricus did not grow in uracil-free medium (Fig. 1), nor did the two isolated mutants of S. acidocaldarius. By analogy to earlier studies (10, 17), we therefore concluded that the FOA-resistant phenotype was most probably based on auxotrophs lacking biosynthetic conversion between orotate and UMP.
FIG. 1.
Growth of S. solfataricus P1 wt (open circles) and of 5-FOA-resistant mutants (filled symbols; triangles, P1-18; squares, P1-17; diamonds, PH1-15) on medium containing 0.2% d-arabinose and 0.1% tryptone as carbon sources with (10 μg/ml) or without uracil. OD, optical density.
On solid medium, however, to which no uracil was added, we observed weak growth of the mutants. Very small colonies developed after 4 days but ceased growth upon further incubation, while wt cells grew to large colonies (of about 2 to 3 mm in diameter) within 6 to 7 days. We therefore suspect that the gellan gum used for solidification of the medium might be slightly contaminated with nucleic acids that allowed some initial growth of the mutants until it was depleted. No revertants were observed from the auxotrophic isolates, either in liquid media or on plates (in the latter case, large colonies should have developed).
Genetic characterization of uracil-auxotrophic mutants.
We have used PCR amplification and chromosomal Southern analyses in order to study the pyrE-pyrF locus in the auxotrophic isolates. In the genomic sequence of S. solfataricus P2, the two genes lie adjacent to each other with the open reading frame (ORF) of pyrE overlapping pyrF by 14 bp (Fig. 2). Further homologs of pyrimidine synthesis genes (pyrBCD) were found directly upstream of pyrEF in the opposite orientation. The intergenic region of the two divergently oriented gene clusters was 63 bp long and contained a sequence in its center that follows the consensus of box A elements of archaeal promoters on both strands (TTTAAA). Based on the sequence information, two oligonucleotides were designed to amplify the pyrEF region from chromosomal DNA including 404 bp upstream of the putative pyrE gene and 78 bp downstream of pyrF (Fig. 2). The PCR amplification from S. solfataricus P1 DNA yielded a product of 1.7 kbp as predicted from the genomic sequence of the closely related strain P2. In contrast, all PCR products obtained from the auxotrophic isolates of strains P1 and PH1 were 1 to 1.4 kbp larger, suggesting that a genetic change caused by insertional mutations in the pyrEF region was responsible for the mutant phenotypes (data not shown). For comparison, two mutants of S. acidocaldarius were also analyzed by using primers derived from the pyrE gene sequence of this strain. In both cases, fragments of 550 bp were obtained, corresponding to the size of the predicted wt fragments (data not shown).
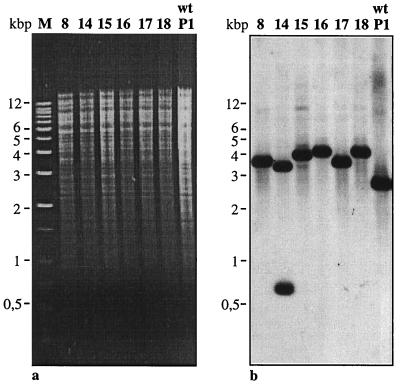
In order to verify these results, chromosomal DNA from seven mutants of S. solfataricus P1 and PH1 was isolated, and a probe spanning almost the complete pyrEF wt region (positions 394 to 1493 in Fig. 2) was used in Southern analysis (Fig. 3). While a single 2.7-kbp fragment was detected in wt DNA cut with AflIII, six of seven DNAs isolated from the mutants showed a hybridizing fragment that was enlarged by about 1 to 1.4 kbp, resulting in fragments of approximately 3.7 to 4.1 kbp in length. Mutant PH1-14 showed a hybridizing fragment of approximately 3.7 kbp and a second fragment of 750 bp (Fig. 3b) apparently caused by the presence of an additional AflIII site within the enlarged pyrEF locus.
FIG. 3.
Agarose gel electrophoresis (a) and corresponding Southern hybridization (b) of AflIII-digested genomic DNAs identifying the pyrEF loci in S. solfataricus P1 (wt) and in uracil-auxotrophic mutants. The pyrEF probe was obtained by PCR amplification of a 1,099-bp fragment (position 394 through 1493 in Fig. 2). M, markers.
Detection of IS elements in the pyrEF locus of uracil-auxotrophic mutants.
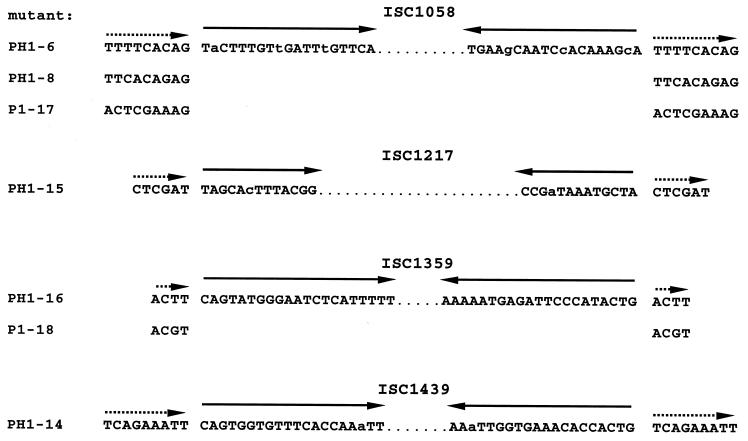
The pyrEF loci of seven mutants were directly amplified from chromosomal DNA and cloned into pBluescript. The recombinant fragments were cut with various restriction enzymes, and the patterns were compared to those of the wt fragment in order to localize insertions within the mutated pyrEF regions (data not shown). The exact positions of the insertions were then determined by sequence analysis and shown to be located in different sites in each of the seven mutants analyzed (Fig. 2). In three cases, the insertion was found within 12 bp immediately upstream of ORF pyrE (mutants PH1-6, PH1-8, and P1-18), three further insertions were found within ORF pyrE (mutants PH1-14, PH1-15, and P1-17), and one insertion was in ORF pyrF (mutant PH1-16). Oligonucleotides complementary to pyrEF sequences directly flanking each insertional site were used for PCR amplification of the ISs from the chromosomal DNA of the mutants. The seven fragments were cloned into pBluescript and sequenced from both sites. Four different ISs, of 1,058, 1,147, 1,359, and 1,439 bp in length, were identified (Fig. 4). The IS elements had terminal inverted repeats of 13, 19, 20, and 21 bp, respectively, and contained ORFs of 273 to 354 amino acids that potentially encoded transposases. Each IS was flanked by a directly repeated sequence of diagnostic length which was duplicated upon transposition of the respective element (Fig. 4 and Table 2). ISC1058 was found in three mutants at different target sites (PH1-6, PH1-8, and P1-17 [Fig. 2]). The 9-bp duplicated target sequences were different but shared identical bases in their last two positions (AG). ISC1359 was found in two mutants with different but similar flanks of only 4 bp (PH1-16, P1-18). ISC1439 was found in mutant PH1-14 with a 9-bp direct repeat. The insertion element of PH1-15 was identical to ISC1217, which had been detected earlier in a β-galactosidase mutant (26). The insertion elements were also found in the partial genome sequence of strain P2 (see below).
FIG. 4.
Sequences of structural features of the four IS elements. Dotted arrows, duplicated target sequences (direct repeats) in the pyrEF genes flanking the IS element in each mutant; solid arrows, inverted repeats at termini of IS elements (lowercase letters represent mismatches within the inverted repeat).
TABLE 2.
Features of IS elements isolated from pyrEF mutants of S. solfataricus
| Feature | ISC1058 | ISC1217a | ISC1359 | ISC1439 |
|---|---|---|---|---|
| Size (bp) | 1,058 | 1,147 | 1,359 | 1,439 |
| Size of inverted repeat (bp) | 19 | 13 | 21 | 20 |
| Size of direct repeat (bp) | 9 | 6 | 4 | 9 |
| Size of largest ORF (amino acids) | 299 | 354 | 273 | 321 |
| Mutated gene(s) | pyrE prom-pyrEFb | pyrE lacS | prom-pyrEF pyrF | pyrE |
| Copy no. in: | ||||
| S. solfataricus P1 | >17 | >12 | >13 | >20 |
| S. solfataricus P2 | >14 | >10 | >13 | >24 |
| PIT3 | >13 | >15 | >15 | >18 |
| KAW2 | 4 | 0 | 0 | 0 |
Data are from reference 26.
prom-pyrEF, IS element in promoter region.
Sequence similarity to known IS elements.
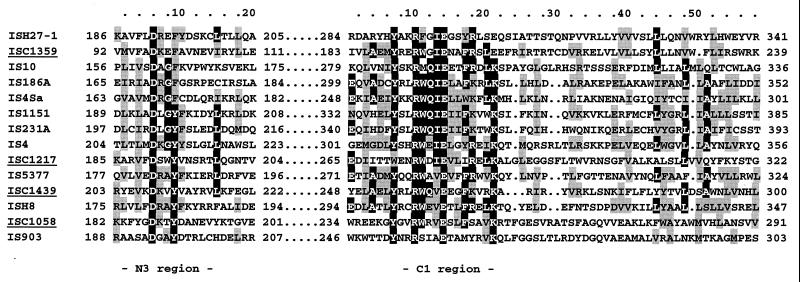
No apparent DNA sequence similarity of the four identified IS elements was found to other transposable elements available in the databases. Some similarities to transposases, however, were found when the deduced amino acid sequences of the largest ORF were used in the searches. While ISC1359 and ISC1439 showed limited similarities to elements of the IS4 family, ISC1058 was more similar to IS903 of the IS5 family. A closer inspection of the amino acid sequences revealed in all four cases the conserved motif Y-N2-R-N3-E-N6-K of the C1 region typical of transposases from the IS4-IS5 families (19, 23) (Fig. 5). Furthermore, the second conserved motif of the N3 region, which is also indicative of IS4 or IS5 elements (D-N-G/A-Y/F), was identified. Based on these two motifs, many of the known bacterial and archaeal IS elements, i.e., those of members of the genera Halobacterium and Haloferax, have been combined in these families. Members of these groups also share the conserved D-D-E motif of different families of prokaryotic and eukaryotic elements (i.e., transposases and retroviral integrases [19]).
FIG. 5.
Sequence similarity of the deduced amino acid sequences of the largest ORFs to representative transposases of the IS4-IS5 family of insertion sequences. The two conserved signatures, D-N-G/A-Y/F and Y-N2-R-N3-E-N6-K, of the N3 and the C1 region, respectively, as described by Rezsöhazy et al. (23), were found in the putative transposases of the S. solfataricus elements. Identical amino acids are shaded in black; similar amino acids are shaded in grey (with a 40% cutoff). Sources of known IS elements (accession numbers are given in brackets) from archaea: ISH27-1, H. salinarum PHH1 (X54432); ISH8, H. salinarum SD108 (M58557). Those for bacteria: IS10, Salmonella enterica serovar Typhimurium Tn10 (J01829); IS186A, E. coli RR1 (M11300); IS4Sa, Synechocystis sp. strain PCC6803 (U38915); IS1151, Clostridium perfringens NCTC2062 (Z18246); IS231A, Bacillus thuringiensis (X03397); IS4, E. coli K-12 (J01733); IS5377, Bacillus stearothermophilus CU21 (X67862); IS903, E. coli (J01839). IS elements from this study are underlined.
Abundance of insertion elements in the S. solfataricus genome and in auxotrophic mutants.
Southern analyses of chromosomal DNA were used to examine the copy number and distribution of the four IS elements in the S. solfataricus genome and mutant strains. Randomly labeled probes spanning the entire length of each IS element were prepared from the recombinant pBluescript plasmids and were used for hybridization against total DNA cut with AflIII (Fig. 6). The restriction enzyme did not cut within the sequences of ISC1058, -1217, and -1359. In ISC1439, two recognition sequences for AflIII were found at positions 1170 and 1330 of the element, but no hybridization was observed from the internal 160-bp fragment and from the remaining 109 bp of the labeled fragment (compare Fig. 3 and 6). We therefore assumed that the number of chromosomal fragments hybridizing to each IS element probe represented a minimal estimate of the copy number within the genomic DNAs.
FIG. 6.
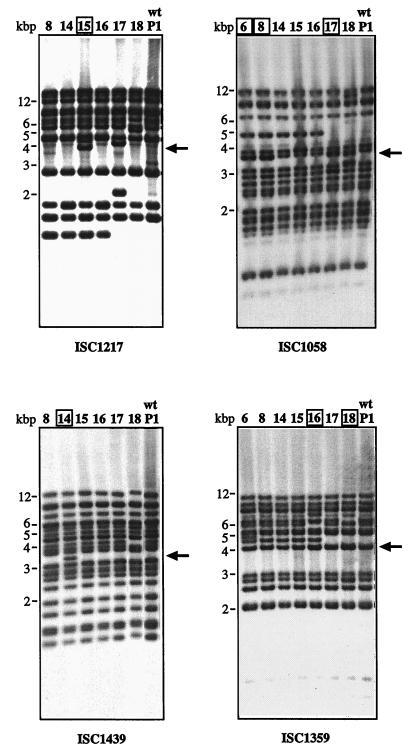
Southern hybridization using IS element-specific probes and AflIII-digested chromosomal DNA of S. solfataricus P1 (wt) and uracil-auxotrophic mutants derived from P1 (17 and 18) or PH1 (6, 8, 14, 15, and 16; β-galactosidase mutant with a copy of ISC1217 in lacS [26]). Each hybridizing fragment corresponds to at least one IS element copy, because AflIII does not cut within ISC1058, ISC1217, and ISC1359 and only close to the terminus of ISC1439 (Fig. 3b). Large arrows indicate the positions of fragments containing the IS element in the pyrEF region (as in Fig. 3, detected with the pyrEF probe). The respective mutants containing the IS element in this region are boxed. Note additional fragments in several mutants caused by multiple transposition events.
Hybridization with the ISC1217 probe (of mutant PH1-15) detected at least 11 fragments in chromosomal DNA of the wt strain (lane P1). An additional fragment of approximately 1.2 kbp was observed in the mutants derived from strain PH1 that contains an extra copy of this IS element within the lacS gene (Fig. 6) (PH1-8, PH1-14, PH1-15, and PH1-16). As expected, an extra fragment of 3.9 kbp hybridized with DNA of mutant PH1-15 showing the IS element copy within the pyrEF region (Fig. 6, arrow). However, an additional fragment of 4.5 kbp hybridized in PH1-15 and two additional fragments were also observed in mutant P1-17. Similarly, mutant P1-18 showed an additional hybridizing fragment (of approximately 5 kbp) at the expense of a larger one (approximately 6 kbp). We concluded that additional transposition events of ISC1217 apparently occurred in the chromosomal DNA of these mutants, as the latter two were identified through transposition of a different IS element into the pyrEF region (ISC1058 and ISC1359, respectively). Multiple transposition events were also observed with the other IS element-specific probes (see below).
About 20 fragments hybridized to probe ISC1439 in S. solfataricus P1, and the additional 3.4-kbp fragment of mutant PH1-14 showed the insertion of the element in pyrEF, while loss of one signal was observed in mutant P1-18.
Seventeen fragments hybridized to probe ISC1058 in S. solfataricus P1 (wt) DNA, and 13 fragments were detected with probe ISC1359. The additional copies of these two IS elements in the pyrEF region were seen as bands of stronger intensities at 3.7 kbp for ISC1058 (in mutants PH1-6, PH1-8, and P1-17) and at 4.0 kbp for ISC1359 (in mutants PH1-16 and P1-18). They could be visualized as separate bands when the chromosomal DNAs were cut with a different restriction enzyme (HincII [data not shown]). At least one additional copy of ISC1058 and ISC1359 was detected in the mutants derived from strain PH1 (4.9- and 4.5-kbp fragments, respectively, in Fig. 6), suggesting that both elements were active in the lacS mutant at an earlier stage. Two or three additional fragments hybridized to ISC1058 in mutant P1-18. One additional fragment was observed with ISC1359 in mutant PH1-6, while a larger fragment appeared in PH1-16 and P1-18 at the expense of a smaller one.
In summary, four out of the seven mutants showed transpositional events or chromosomal variations in addition to the insertional recombination of the pyrEF region. Only the patterns of mutants PH1-8 and PH1-14 (Fig. 6) appeared to be identical to that of the original strain PH1 (as shown for ISC1058 in Fig. 7b, lane 2).
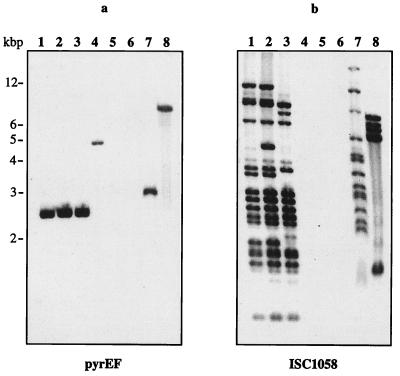
FIG. 7.
Southern hybridization with pyrEF (a)- and IS element (b)-specific probe of ISC1058 to AflIII-digested chromosomal DNAs of S. solfataricus P1 (lanes 1), the β-galactosidase mutant PH1 (lanes 2), strain P2 (lanes 3), S. shibatae (lanes 4), S. acidocaldarius (lanes 5), A. ambivalens (lanes 6), isolate PIT3 (lanes 7), and isolate KAW2 (lanes 8).
Distribution of the four IS elements in Sulfolobus strains.
Known strains of the order Sulfolobales (i.e., S. shibatae, S. acidocaldarius, and Acidianus ambivalens) and novel isolates that most probably represent close relatives of S. solfataricus were investigated in hybridization experiments to study their phylogenetic relationships and the occurrence of homologous IS elements. Strain PIT3 was isolated in 1998 from the same solfataric field in Pisciarelli, Italy, from which S. solfataricus P1 and P2 had been obtained in 1980 (33; personal communication). Strain KAW2 was isolated in Kawaiu, northern Japan. Under stringent hybridization conditions, fragments homologous to pyrEF and of equal size were detected in the chromosomal DNA of S. solfataricus strains P1, PH1, and P2 (Fig. 7a, lanes 1 to 3). While no hybridization was observed with DNA from S. acidocaldarius and A. ambivalens, fragments of different sizes derived from S. shibatae and isolates PIT3 and KAW2 hybridized to this probe (Fig. 7a, lanes 4, 7, and 8). This result reflects the closer phylogenetic relationship of the three latter organisms to S. solfataricus as determined by 16S rRNA analysis or chromosomal restriction fragment patterns (8; W. Zillig, personal communication).
With each of the four IS element-specific probes, similar but slightly distinct hybridization patterns were observed against chromosomal DNA of S. solfataricus strains P1, PH1, and P2 (as shown for ISC1058 in Fig. 7b, lanes 1 to 3). The differences in their patterns could be accounted for by restriction fragment length polymorphism in the two strains as well as by different activities of the IS elements.
No hybridization to IS element-specific probes was observed with DNA from other known members of the Sulfolobales, i.e., S. shibatae, S. acidocaldarius, and A. ambivalens. However, hybridization patterns of complexity similar to that found in S. solfataricus were observed with DNA from isolate PIT3 (as shown for ISC1058 in Fig. 7b, lane 7), suggesting that homologous IS elements of all four types occur with similar frequency in this strain. Interestingly, hybridization of ISC1058 to DNA of KAW2 (Fig. 7b, lane 8) and other isolates (data not shown) suggests that members of this IS element family or an element with high similarity occurs in relatives from Japanese hot springs. The other three ISC element-specific probes did not hybridize to DNA of these isolates (data not shown). No hybridization was observed with DNA of 20 other strains recently isolated from solfataras in New Zealand and Iceland (data not shown).
DISCUSSION
The results in this study suggest that transposon mutagenesis is a dominant mechanism of mutation in the hyperthermophile S. solfataricus and that IS elements may play an important role in genome dynamics of this organism. We have found four different active IS elements that inserted stably into the pyrEF locus in seven independent transposition events. In six out of seven cases, the insertion was found in the pyrE gene or immediately upstream of it, and only in one case was it found in pyrF. Similarly, Jacobs and Grogan (13) described an up-to-fivefold-higher mutational rate for pyrE than for pyrF in S. acidocaldarius. Mutants of S. acidocaldarius have not yet been analyzed on the genetic level, and IS elements have not been described for this organism. The apparent mutational frequency of pyrEF in that organism was 5 × 10−6 (10; this study). In contrast, spontaneous mutations in S. solfataricus that were created by IS elements arose with variable frequencies of between 10−4 and 10−5 per plated cell, i.e., they were at least 50 to 60 times higher than those in S. acidocaldarius. It is not clear yet which factors have influenced the rates of transposition events in our experiments. Potentially, small differences in growth conditions or conditions that cause stress reactions might induce transpositions. Such observations have been made earlier in the study of β-galactosidase mutants, where spontaneous mutants arose with frequencies of around 10−4 (26).
The hybridization patterns of the uracil-auxotrophic mutants revealed that insertions in pyrEF were not the only recent transposition events. In five out of seven pyrEF mutants analyzed, we detected between one and five additional or different fragments hybridizing to IS-specific probes besides the additional IS element copy in pyrEF (Fig. 6). Similarly, an additional fragment hybridized to ISC1058 or ISC1359 in the genome of all PH1 mutants, indicating earlier transposition events during the selection of the lacS mutant, which was created by insertion of ISC1217 into the lacS gene (Fig. 7b). This represents a minimal number of transposition events, since multiple insertions into the same large DNA fragment or into large DNA fragments of similar size would not have been detectable in our study. Our findings suggest that the activity of each of these IS elements may be regulated by similar factors, making simultaneous transposition events more probable.
All four IS elements were found in high copy numbers not only in the genomes of S. solfataricus P1 and P2 but also in those of related strains that have only recently been isolated from the same solfataric field (Fig. 7 for strain PIT3; others are not shown), suggesting that the IS elements occur in high abundance also in natural strains and not only in isolates that have been propagated over several years under artificial laboratory conditions. The partial genome sequence of S. solfataricus P2 (29) so far has revealed at least three more IS elements that do not show any homology to each other and to the four elements described here. Within about 2.7 Mbp of the genome sequence, there were 12 copies of ISC1058, 5 copies of ISC1359, 13 copies of ISC1439, and 8 copies of ISC1217. Except for one region, we did not see any areas in the genome that contained a significant accumulation of IS elements. It seemed rather that transposition targets were randomly distributed (see also the website http://niji.imb.nrc.ca/sulfolobus). As reported earlier for other elements (29), individual copies of the different IS elements or IS families vary in their sequences either by carrying a few point mutations or by showing larger deletions. This suggests that the elements decay over long periods in the genome. It may explain how the organism copes with the large number of IS elements in the genome and is reminiscent of the occurrence of the IS element families in halobacteria (6, 21).
The IS elements seem to be specific for S. solfataricus or closely related strains and could be useful as markers in restriction fragment length polymorphism studies for strain typing and for the study of geographical distributions, because they detect relatively recent evolutionary divergence. Interestingly, one element, ISC1058 or a close relative thereof, was also detected in isolates from Japanese hot springs (Fig. 7b). Horizontal spread of IS elements among different strains is quite possible, because a number of viruses and plasmids have been described for Sulfolobus (34) and conjugation has been demonstrated for S. acidocaldarius and S. solfataricus (12, 27). Furthermore, IS elements and transposases have been identified on several large plasmids that were shown to propagate by conjugation (30; W. Zillig, personal communication). Also, the relatively high G+C content of the IS elements (up to 15% higher than the average G+C content of the genome) suggests that IS elements in S. solfataricus may have been acquired by horizontal gene transfer from another organism.
The isolation of auxotrophic mutants from S. solfataricus opens new possibilities for the development of genetic tools. So far, two selectable markers, an alcohol dehydrogenase (3) and a hygromycin pyrophosphatase (5), that confer resistance to specific inhibitors have been used in Sulfolobus. Similarly, the wt pyrEF genes could now be used as a selectable marker together with uracil-auxotrophic mutants. This marker should function in low-copy-number vectors as well as in single copy upon integration into the chromosome. Although insertional transposition was the cause of the mutant phenotypes, all auxotrophs seemed to be quite stable and should therefore be suitable recipients. Initial transformation experiments based on a recombinant plasmid derived from the S. shibatae virus SSV1 (32) allowed complementation to prototrophy with this self-spreading vector (unpublished data). With this system, it should be possible to demonstrate and optimize conditions for transformation by homologous recombination in Sulfolobus and to use the pyrEF marker in low-copy-number vectors. In addition, conjugation mechanisms and transfer of chromosomal markers can now be studied in S. solfataricus by mating different uracil auxotrophs with strains containing conjugative plasmids. It might also be possible to develop a transposon-based mutagenesis procedure by using transformation vectors that contain IS elements in combination with a selectable marker.
ACKNOWLEDGMENTS
We are grateful to Felicitas Pfeifer for laboratory support and encouragement. We thank Dave Edgell for primers pyr-L and pyr-R and Gaby Liebing for technical assistance. Many thanks are due to Wolfram Zillig for providing various strains and chromosomal DNA from his collection and for fruitful discussions. Thanks are due to Andrew Wedel for faithful linguistic help.
Footnotes
NRC publication no. 42312.
REFERENCES
- 1.Aagaard C, Leviev I, Aravalli R N, Forterre P, Prieur D, Garrett G A. General vectors for archaeal hyperthermophiles: strategies based on a mobile intron and a plasmid. FEMS Microbiol Rev. 1996;18:93–104. doi: 10.1111/j.1574-6976.1996.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 2.Ammendola S, Politi L, Scandurra R. Cloning and sequencing of ISC1041 from the archaeon Sulfolobus solfataricus MT-4, a new member of the IS30 family of insertion elements. FEBS Lett. 1998;428:217–223. doi: 10.1016/s0014-5793(98)00530-4. [DOI] [PubMed] [Google Scholar]
- 3.Aravalli R N, Garrett R A. Shuttle vectors for hyperthermophilic Archaea. Extremophiles. 1997;1:183–191. doi: 10.1007/s007920050032. [DOI] [PubMed] [Google Scholar]
- 4.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, et al. Complete genome sequence of the methanogenic archaeon Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 5.Cannio R, Contursi P, Rossi M, Bartolucci S. An autonomously replicating transforming vector for Sulfolobus solfataricus. J Bacteriol. 1998;180:3237–3240. doi: 10.1128/jb.180.12.3237-3240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charlebois R L, Doolittle W F. Transposable elements and genome structure in halobacteria. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 297–307. [Google Scholar]
- 7.Elferink M G, Schleper C, Zillig W. Transformation of the extremely thermoacidophilic archaeon Sulfolobus solfataricus via a self-spreading vector. FEMS Microbiol Lett. 1996;137:31–35. doi: 10.1111/j.1574-6968.1996.tb08078.x. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs T, Huber H, Burggraf S, Stetter K O. 16S rDNA-based phylogeny of the archaeal order Sulfolobales and reclassification of Desulfurolobus ambivalens as Acidianus ambivalens comb. nov. Syst Appl Microbiol. 1996;19:56–60. [Google Scholar]
- 9.Grogan D W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989;171:6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grogan D W. Selectable mutant phenotypes of the extremely thermophilic archaebacterium Sulfolobus acidocaldarius. J Bacteriol. 1991;173:7725–7727. doi: 10.1128/jb.173.23.7725-7727.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grogan D W, Gunsalus R P. Sulfolobus acidocaldarius synthesizes UMP via a standard de novo pathway: results of a biochemical-genetic study. J Bacteriol. 1993;175:1500–1507. doi: 10.1128/jb.175.5.1500-1507.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grogan D W. Exchange of genetic markers at extremely high temperatures in the archaeon Sulfolobus acidocaldarius. J Bacteriol. 1996;178:3207–3211. doi: 10.1128/jb.178.11.3207-3211.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs K, Grogan D W. Rates of spontaneous mutation in an archaeon from geothermal environments. J Bacteriol. 1997;179:3298–3303. doi: 10.1128/jb.179.10.3298-3303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jurka J. Repeats in genomic DNA: mining and meaning. Curr Opin Struct Biol. 1998;8:333–337. doi: 10.1016/s0959-440x(98)80067-5. [DOI] [PubMed] [Google Scholar]
- 15.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, et al. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 16.Klenk H P, Clayton R, Tomb J, White O, Nelson K E, et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 17.Kondo S, Yamagishi A, Oshima T. Positive selection for uracil auxotrophs of the sulfur-dependent thermophilic archaebacterium Sulfolobus acidocaldarius by use of 5-fluoroorotic acid. J Bacteriol. 1991;173:7698–7700. doi: 10.1128/jb.173.23.7698-7700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahillon J. Transposons as gene haulers. APMIS Suppl. 1998;84:29–36. doi: 10.1111/j.1600-0463.1998.tb05645.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng W V, Ciufo S A, Smith T M, Bumgarner R E, Baskin D, Faust J, Hall B, Loretz C, Seto J, Slagel J, Hood L, DasSarma S. Snapshot of a large dynamic replicon in a halophilic archaeon: megaplasmid or minichromosome? Genome Res. 1998;8:1131–1141. doi: 10.1101/gr.8.11.1131. [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer F. Genetics of halobacteria. In: Rodriguez-Valera F, editor. Halophilic bacteria. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 105–133. [Google Scholar]
- 22.Pfeifer F, Blaseio U. Transposition burst of the ISH 27 insertion element family in Halobacterium halobium. Nucleic Acids Res. 1990;18:6921–6925. doi: 10.1093/nar/18.23.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezsöhazy R, Hallet B, Delcour J, Mahillon J. The IS4 family of insertion sequences: evidence for a conserved transposase motif. Mol Microbiol. 1993;9:1283–1295. doi: 10.1111/j.1365-2958.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Schleper C, Kubo K, Zillig W. The particle SSV1 from the extremely thermophilic archaeon Sulfolobus is a virus: demonstration of infectivity and of transfection with viral DNA. Proc Natl Acad Sci USA. 1992;89:7645–7649. doi: 10.1073/pnas.89.16.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schleper C, Röder R, Singer T, Zillig W. An insertion element of the extremely thermophilic archaeon Sulfolobus solfataricus transposes into the endogenous β-galactosidase gene. Mol Gen Genet. 1994;243:91–96. doi: 10.1007/BF00283880. [DOI] [PubMed] [Google Scholar]
- 27.Schleper C, Holz I, Janekovic D, Murphy J, Zillig W. A multicopy plasmid of the extremely thermophilic archaeon Sulfolobus effects its transfer to recipients by mating. J Bacteriol. 1995;177:4417–4426. doi: 10.1128/jb.177.15.4417-4426.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sensen C W, Klenk H P, Singh R K, Allard G, Chan C C, et al. Organizational characteristics and information content of an archaeal genome: 156 kb of sequence from Sulfolobus solfataricus P2. Mol Microbiol. 1996;22:175–191. doi: 10.1111/j.1365-2958.1996.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 29.Sensen C W, Charlebois R L, Chow C, Clausen I G, Curtis B, et al. Completing the sequence of the Sulfolobus solfataricus P2 genome. Extremophiles. 1998;2:305–312. doi: 10.1007/s007920050073. [DOI] [PubMed] [Google Scholar]
- 30.She Q X, Phan H E, Garrett R A, Albers S V, Stedman K, Zillig W. Genetic profile of pNOB8 from Sulfolobus: the first conjugative plasmid from an archaeon. Extremophiles. 1998;2:417–425. doi: 10.1007/s007920050087. [DOI] [PubMed] [Google Scholar]
- 31.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierz-bowski J, Gibson R, Jiwani N, Caruso A, Bush D, Safer H, Patwell D, Prabhakar S, McDougall S, Shimer G, Goyal A, Pietrokovski S, Church G M, Daniels C J, Mao J-I, Rice P, Nölling J, Reeve J N. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stedman K M, Schleper C, Rumpf E, Zillig W. Genetic requirements for the function of the archaeal virus SSV1 in Sulfolobus solfataricus: construction and testing of viral shuttle vectors. Genetics. 1999;152:1397–1405. doi: 10.1093/genetics/152.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zillig W, Stetter K O, Wunderl S, Schulz W, Priess H, Scholz I. The Sulfolobus-“Caldariella” group: taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol. 1980;125:259–269. [Google Scholar]
- 34.Zillig W, Prangishvilli D, Schleper C, Elferink M, Holz I, et al. Viruses, plasmids and other genetic elements of thermophilic and hyperthermophilic Archaea. FEMS Microbiol Rev. 1996;18:225–236. doi: 10.1111/j.1574-6976.1996.tb00239.x. [DOI] [PubMed] [Google Scholar]