Abstract
Background:
We performed a meta-analysis to investigate the effectiveness of telecardiac rehabilitation compared to center-based rehabilitation on cardiorespiratory fitness, blood pressure, blood lipids, body composition, and quality of life in patients with coronary heart disease.
Methods:
We searched the Medical Literature Analysis and Retrieval System Online, Cumulative Index to Nursing and Allied Health Literature, Cochrane, Embase, and Scopus databases and retrieved studies published until October 8, 2021. Randomized controlled trials were included to evaluate cardiorespiratory fitness, blood pressure, blood lipids, body composition, and quality of life after telecardiac rehabilitation and center-based rehabilitation in patients with coronary heart disease. The criteria of the Cochrane Handbook for Systematic Reviews of Interventions were used to evaluate the methodological quality of the studies. Funnel plot analysis and Egger test were performed to confirm the publication bias.
Results:
A total of 8 studies, including 750 participants, reported the effectiveness of the telecardiac rehabilitation and center-based rehabilitation included in the analysis. Except for total cholesterol and mental quality of life (P < .05), all parameters were not significantly different between telecardiac rehabilitation and center-based rehabilitation (P > .05).
Conclusion:
Telecardiac rehabilitation was similar to the effects of center-based rehabilitation. The overall prognosis of patients with coronary heart disease can be improved by increasing patients’ participation in cardiac rehabilitation through telerehabilitation.
Keywords: cardiac rehabilitation, coronary heart disease, meta-analysis, telerehabilitation
1. Introduction
Cardiac rehabilitation is an essential final step in the treatment of coronary heart disease.[1] It restores the physical and psychosocial functions of patients who have undergone acute medical treatment or cardiac surgery to a level equal to or greater than that before disease onset.[1,2] Cardiac rehabilitation not only effectively restores exercise capacity and increases psychological stability but also helps manage various risk factors for coronary heart disease, thereby reducing the recurrence, the need for rehospitalization and retreatment, and the cause of mortality.[1]
Cardiac rehabilitation is a comprehensive patient management program that consists of cardiac evaluation, treatment (individualized program), and risk factor management after the onset of heart disease.[3] The rehabilitation program involves various medical personnel (physical therapists, nurses, psychotherapists, occupational therapists, clinical nutritionists, and social workers), depending on the patient’s condition and risk factors under the supervision of the physician.[3]
Currently, cardiac rehabilitation is performed in many countries worldwide, and various studies have demonstrated its effectiveness and safety.[4–6] However, several factors, such as lack of facilities, busy work life, transportation, lack of patient will, and lack of cardiac rehabilitation specialists, have prevented the implementation of the program.[7,8]
Some previous studies have reported that home-based cardiac rehabilitation programs can help overcome such obstacles for cardiac rehabilitation and have similar effects in lowering mortality, risk of mortality, risk of recurrent coronary events, and risk factors for coronary heart disease compared to center-based cardiac rehabilitation programs.[9–11] However, in home-based cardiac rehabilitation programs, patients cannot be supervised, and optimal individualized exercise prescriptions are limited.
The recent development of information and communication technologies has helped overcome the shortcomings of home-based cardiac rehabilitation programs. Smartwatches and portable heart function measurement devices can measure heart rate, blood pressure, and oxygen saturation during exercise.[12,13] These data can be downloaded and sent to the attending physicians, allowing them to directly receive information on the patient’s condition and prescribe the intensity and type of exercise appropriate for each patient. In addition, remote assessment of the patient’s condition allows the patients to be evaluated in a convenient environment, wherein the physician can be queried and feedback can be provided.[12,13] Such cardiac rehabilitation programs that use information and communication technologies are called telecardiac rehabilitation. The effects of telecardiac rehabilitation have been investigated in various studies, especially after the rapid advancement of information and communication technologies.
Herein, we compared the effects of telecardiac rehabilitation with those of center-based cardiac rehabilitation through a meta-analysis.
2. Methods
2.1. Search strategy
In this study, the following search strategy was followed based on the patient/population, intervention, comparison, and outcome model: population, patients with coronary heart disease, intervention, telecardiac rehabilitation, (comparison, center-based rehabilitation, and outcome, cardiorespiratory fitness or exercise capacity, blood pressure, blood lipids, body composition, and quality of life. This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. Two researchers (Y.J.C. and M.C.C.) searched the literature published from January 1, 1990, to October 8, 2021, in the Medical Literature Analysis and Retrieval System Online, Cochrane, Embase, Scopus, and Cumulative Index to Nursing and Allied Health Literature databases. The following search terms were used: “telerehab,” “telerehabilitation,” “telecardiac rehab,” “telecardiac rehabilitation,” “telehealth,” “ehealth,” “coronary heart disease,” “atherosclerosis,” “angina pectoris,” “myocardial infarction,” and “coronary revascularization.”
2.2. Inclusion and exclusion criteria
The selection criteria were as follows: studies on telecardiac rehabilitation for patients with coronary heart disease, studies that performed center-based rehabilitation to compare the effectiveness of telecardiac rehabilitation, and randomized controlled studies. The exclusion criteria were as follows: review studies, studies that had been presented at any conference, and papers not written in English.
2.3. Data extraction
All search results were exported to EndNote X9 (Clarivate, London, United Kingdom), and duplications were checked. For the papers that remained after the duplication check, 2 reviewers (Y.J.C and M.C.C) independently evaluated those that met the selection criteria. The studies were selected by reviewing the titles and abstracts, and their conformation with the inclusion criteria was confirmed through a full-text review. Disagreements between the reviewers were resolved through discussion. Table 1 shows the information on the number of participants, age, types of disease, details of the exercise program, and information on the variables evaluated in each study. All data are presented as mean and standard deviation.
Table 1.
Characteristics of selected studies.
| No. | Study | Participants (H/C) | Types of CHD | Exercise program | Outcomes |
|---|---|---|---|---|---|
| 1 | Arthur et al[14] | n: 120/122 mean age: 64.2 ± 9.4 yr/62.5 ± 8.8 yr | Coronary artery bypass graft | Home-based: monitor and revise workouts by phone every 2 wk for a total of 6 mo; exercise 5 times a week recommended; 10–15 min warm up, 40 min aerobic exercise (mainly at participants’ own pace), 10–15 min cool down (slow walking, stretching) | VO2 peak, peak METs, resting heart rate, peak heart rate, weight, waist-to-hip ratio |
| Center-based: directly supervised by exercise experts; supervised exercise sessions 3 times a week for 6 mo; 10–15 min warm up (walking, stretching), 40 min aerobic (cycle ergometer, arm cycle ergometer, treadmill, track walking, stair climbing), 10–15 min cool down (walking, stretching) | |||||
| 2 | Avila et al[12] | n: 26/29 mean age: 62.2 ± 7.1 yr/62.0 ± 7.4 yr | Coronary artery disease | Home-based: feedback by phone or email once a week for a total of 12 wk; individualized exercise prescription; 150 min per week of exercise recommended | VO2 peak, peak heart rate, peak respiratory exchange ratio, ventilatory thresholds, average steps per day, sit and rising test, handgrip strength, quadriceps maximal isometric knee extension strength and isokinetic total work, body mass index, waist and hip circumference, blood pressure, glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, health-related quality of life (SF-36) |
| Center-based: outpatient walking-based training for a total of 12 wk; 3 weekly sessions consisting of about 45 min of endurance training followed by relaxation | |||||
| 3 | Batalik et al[15] | n: 23/21 mean age: 56.1 ± 6.8 yr/57.1 ± 7.9 yr | Angina pectoris, myocardial infarction | Home-based: feedback, motivation, and education conducted once a week for a total of 12 wk; exercise 3 times a week for 60 min | VO2 peak, peak heart rate, peak respiratory exchange ratio, body mass index, waist circumference, health-related quality of life (SF-36) |
| Center-based: phase 2 rehabilitation program under the supervision of a physical therapist and cardiologist at the hospital for a total of 12 wk; exercise 3 times a week for 60 min | |||||
| 4 | Frederix et al[16] | n: 81/85 mean age: 58 ± 9 yr/63 ± 10 yr | Acute coronary syndrome for which a percutaneous coronary intervention, coronary artery bypass graft | Home-based: automated feedback is provided by email or SMS every week for a total of 18 wk; first 6 wk were in the hospital’s rehabilitation center, and was changed to home-based starting from the 7th week; no common routine between participants; for all physical activities such as walking, running, and biking, it was recommended that the patient increase the number of steps by 10% per week at baseline | VO2 peak, peak heart rate, peak respiratory exchange ratio, weight, body mass index, glucose, HbA1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides |
| Center-based: a standard phase 2 rehabilitation program for a total of 18 wk | |||||
| 5 | Gordon et al[17] | n: 52/45 mean age: 61 ± 10 yr/60 ± 9 yr | Previously documented acute myocardial infarction, coronary artery bypass graft surgery, transcatheter coronary artery intervention, and/or clinical diagnosis of angina pectoris | Home-based: a total duration of 12 wk; phone consultations at weeks 2, 4, 8, and 10; on-site counseling at week 6; no common routine among participants; individualized exercise program was prescribed | VO2 peak, body mass index, weight, blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides |
| Center-based: a total duration of 12 wk; exercise training sessions occurred for 3 consecutive days per week | |||||
| 6 | Kraal et al[18] | n: 25/25 mean age: 60.6 ± 7.5 yr/56.1 ± 8.7 yr | Myocardial infarction, unstable angina, or a revascularisation procedure (percutaneous coronary intervention or coronary artery bypass grafting) | Home-based: the physical therapist called once a week to provide feedback on training frequency, duration, and intensity for a total of 12 wk; exercising at least twice a week for 45–60 min per session | VO2 peak, maximal workload, peak heart rate, peak respiratory exchange ratio, health-related quality of life (MacNew questionnaire) |
| Center-based: conduct group-based training sessions with a treadmill or cycle ergometer under the supervision of a physical therapist and exercise specialist for a total of 12 wk; exercising at least twice a week for 45–60 min per session | |||||
| 7 | Maddison et al[13] | n: 82/80 mean age: 61.0 ± 13.2 yr/61.5 ± 12.2 yr | Atherosclerosis, angina pectoris, myocardial infarction, coronary revascularisation | Home-based: individual audio coaching and feedback in real time; without real-time interaction, participants reviewed automatic goal achievement feedback; a total duration of 12 wk with exercise 3 times per week; encouraged activity at least 5 d per week 30–60 min each session, including warm up and cool down; no common routine among participants, and individualized exercise program was prescribed | VO2 peak, height, weight, body mass index, waist and hip circumference, blood pressure, glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, accelerometry, self-efficacy, intention, confidence, locus of causality, health-related quality of life (EQ-5D) |
| Center-based: 12 wk of supervised exercise provided by a clinical exercise physiologist | |||||
| 8 | Varnfield et al[19] | n: 53/41 mean age: 54.9 ± 9.6 yr/56.2 ± 10.1 yr | Myocardial infarction | Home-based: patients motivate and educate and feedback was provided through SMS and audio and video files; weekly phone consultations; a total duration of 6 wk; the main exercise was walking, with at least 30 min of moderate activity (Borg scale: moderate) on most days of the week; personalized feedback provided | VO2 peak, peak heart rate, weight, body mass index, HbA1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, health-related quality of life (EQ-5D), mental health (Depression, Anxiety and Stress Scale 21) |
| Center-based: performed an individualized and supervised circuit-based exercise program of light to moderate intensity according to Borg scale for a total of 6 wk; 2 weekly supervised workouts and 1-h education sessions |
C = center-based, CHD = coronary heart disease, EQ-5D = Euro-Quality of Life-5 Dimension, H = home-based, HbA1c = hemoglobin A1 c, MET = metabolic equivalent of task, SF-36 = 36-Item Short-Form Survey.
2.4. Quality assessments
Methodological quality was evaluated using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess for potential bias.[20] Potential sources of bias included the following: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias.
2.5. Analyses
The extracted data were statistically analyzed using the Review Management Software (RevMan 5.3; Cochrane, London, United Kingdom). For each analysis, a heterogeneity test was performed using I2 statistics, which measures the extent of inconsistency in the results. When I2 values were <50%, the pooled data were considered homogeneous, and a fixed-effects model was used. In contrast, if I2 values were ≥50%, the pooled data were considered to have substantial heterogeneity, and the random-effects model was used for data analyses. The analyzed data were continuous variables; hence, we calculated the standardized mean differences (SMDs) and 95% confidence intervals (CIs). The data value used in the analysis was the amount of change calculated before and after the intervention. Statistical significance was set at P value of <.05. A meta-analysis was performed only when ≥2 studies were compared for each survey item.
2.6. Publication bias
Publication bias was assessed visually using a funnel plot showing the relationship between sample size and effect size. In addition, Egger test was used to test for symmetry in the funnel plot. Egger test was conducted using R software (Version 4.0.3, R Core Team), and an alpha of 0.05 was used as the cutoff for significance. Evaluation of publication bias through funnel plot and Egger test was performed only when there were ≥3 comparable studies.
3. Results
3.1. Study selection
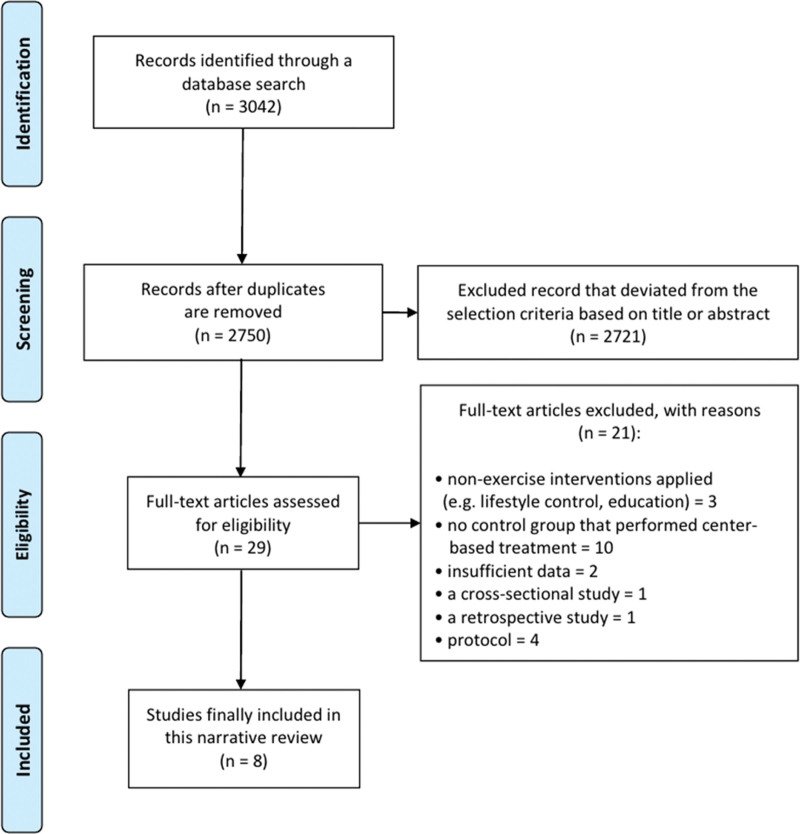
Out of a total of 3042 papers searched using the search terms, 8 papers[12–19] were finally selected after excluding duplicates and those that did not meet the inclusion criteria (Fig. 1). The analysis included studies confirming the effects of telecardiac rehabilitation and center-based rehabilitation and involved a total of 750 participants (367 in the telecardiac rehabilitation group and 383 in the center-based rehabilitation group).
Figure 1.
Flowchart showing the search results of the meta-analysis.
3.2. Study description
Eight studies[12–19] included in this meta-analysis randomly divided participants into a telecardiac rehabilitation group and a center-based rehabilitation group. Four studies[12,15–17] reported that they performed a phase II cardiac rehabilitation program as a center-based rehabilitation program. Phase II cardiac rehabilitation is part of a comprehensive outpatient program designed to improve heart health and quality of life in patients with heart disease.[21] The program is tailored to individual needs and includes instructions in supervised exercise, nutritional counseling, and lifestyle changes that reduce the risk of heart problems.[21]
Arthur et al[14] included participants who underwent coronary artery bypass graft surgery. Both telecardiac and center-based rehabilitation were performed for a total of 6 months, and the exercise sessions consisted of 10 to 15 minutes of warm up, 40 minutes of aerobic exercise, and 10 to 15 minutes of cool down. The aerobic exercise of the telecardiac rehabilitation was mainly walking at their own pace, and the center-based rehabilitation group performed track walking and stair climbing. Furthermore, they performed cycle ergometer, arm cycle ergometer, and treadmill exercises. The exercise prescription guidelines were the same for both groups as 60% of peak VO2 after baseline and 70% of peak VO2 after 3 months. The telecardiac rehabilitation group was recommended exercise 5 times a week and recorded exercise logs such as activity, exercise time, and heart rate during exercise. Feedback on monitoring and exercise modifications was provided through the phone every 2 weeks. The center-based rehabilitation group had 3 exercise sessions per week and was supervised by exercise specialists and kinesiologists.
Avila et al[12] included patients with coronary artery disease who had completed phase II cardiac rehabilitation under supervision. The telecardiac rehabilitation group was recommended to exercise for at least 150 minutes per week with 70% to 80% of the heart rate reserve. All exercise data were recorded with a smartwatch, and when the records were uploaded to the web application, the supervisor reviewed and planned an individualized exercise program. Feedback was provided to participants by phone or email once per week. The center-based rehabilitation group was trained on an ambulatory basis at the outpatient clinic, and sessions consisted of relaxation after approximately 45 minutes of endurance training at 70% to 80% of the heart rate reserve, 3 times a week. Both rehabilitations were conducted for a total of 12 weeks; after 12 weeks, both groups were advised to continue exercising. After that, there was no contact for 9 months, and follow-up was performed after 1 year.
Batalik et al[15] included patients with angina pectoris and myocardial infarction and performed telecardiac and center-based rehabilitation for a total of 12 weeks. In both rehabilitation groups, exercise was performed at a heart rate reserve of 70% to 80% in 60-minute sessions 3 times a week. In the telecardiac rehabilitation group, exercise feedback, motivation, and education were provided once a week over the phone. A center-based rehabilitation group performed a phase II rehabilitation program under the supervision of a physical therapist and cardiologist. After 12 weeks of rehabilitation, there was no contact for 1 year, and follow-up was performed at 15 months.
Frederix et al[16] included patients with acute coronary syndrome who underwent percutaneous coronary intervention or coronary artery bypass graft. All patients participated in the phase II rehabilitation program for 6 weeks, and the telecardiac rehabilitation group started telerehabilitation from the seventh week. Participants wore motion sensors all day and recorded all activities, which were uploaded weekly to the web application. Patients received weekly automated feedback on physical activity via email or SMS. Patients were encouraged to increase their daily step count by 10% each week. A center-based rehabilitation group participated in a phase II rehabilitation program for a total of 18 weeks. The center-based rehabilitation group wore the motion sensor for 7 days at 1, 6, and 18 weeks during the study. The motion sensor was worn all day, and data on all activities were recorded. Data from participants were uploaded by a clinician, and no feedback was provided upon reviewing the data. Additionally, participants in the center-based rehabilitation group did not have access to the recorded data.
Gordon et al[17] included patients with previously documented acute myocardial infarction, coronary artery bypass graft surgery, transcatheter coronary artery intervention, and/or clinical diagnosis of angina pectoris. Both telecardiac and center-based rehabilitation were performed for a total of 12 weeks. Telecardiac rehabilitation was a doctor-supervised program with case management by nurses, and telerehabilitation participants visited the office in-person with a manager at baseline and at 6 weeks. A phone consultation was conducted during weeks 2, 4, 8, and 10. Participants in the telecardiac rehabilitation group were provided with an individualized home-based exercise plan. The center-based rehabilitation group participated in a phase II rehabilitation program 3 times a week at the hospital. In addition to exercise training, education was provided to both telecardiac and center-based rehabilitation groups on coronary heart disease, coronary heart disease risk factors, and lifestyle modifications.
Kraal et al[18] included patients with myocardial infarction, unstable angina, percutaneous coronary intervention, or coronary artery bypass grafting. Telecardiac and center-based rehabilitation consisted of at least 2 training sessions per week for 12 weeks. Patients exercised for 45 to 60 minutes per session at 70% to 85% of their maximum heart rate. The telecardiac rehabilitation group learned how to use a wearable heart rate monitor and upload exercise records through the initial 3 supervised training sessions. After 3 supervised training sessions, home-based training was conducted. A physical therapist called once a week to provide feedback on training frequency, duration, and intensity and recommended terminating the phone feedback after 12 weeks but with continued training. The center-based rehabilitation group performed group-based training sessions on a treadmill or cycle ergometer under the supervision of a physical therapist and exercise specialist.
Maddison et al[13] included patients with atherosclerosis, angina pectoris, myocardial infarction, and coronary revascularization. The telecardiac rehabilitation group performed 3 exercise sessions per week for 12 weeks. Sessions lasted for a total of 30 to 60 minutes, including a warm- up and cool down, and patients exercised at an intensity of 40% to 65% of the heart rate reserve. The heart rate, respiration rate, and location data were uploaded to a web application through wearable sensors worn by the participants. Experts reviewed the uploaded data to monitor and coach workouts in real time. Outside of the real-time interaction, the participants were allowed to self-monitor and review feedback. The individualized and progressive exercise program was provided according to maximal aerobic capacity, exercise-induced signs and symptoms, demographic characteristics, and preferences. The center-based rehabilitation group performed 12 weeks of supervised exercise provided by a clinical exercise physiologist at a cardiac rehabilitation clinic.
Varnfield et al[19] performed 6 weeks of telecardiac and center-based rehabilitation in patients with myocardial infarction. During telecardiac rehabilitation, the patients were motivated and educated by SMS and audio/video files, and they had a smartphone installed with a health diary and activity monitoring application. The web application was instructed to upload data, and consultations were conducted based on the uploaded contents. The main exercise was walking, with at least 30 minutes of moderate-intensity activity (Borg scale 11–13) on most days of the week, and weekly telephone counseling was provided. The center-based rehabilitation group performed a rehabilitation program that included supervised exercise twice a week and a 1-hour education session. It was an individualized and supervised circuit-based program of light (6–10) to moderate (11–13) intensity according to Borg scale. Exercises included treadmills, resistance bands, rowers, weights, squats, and pushups.
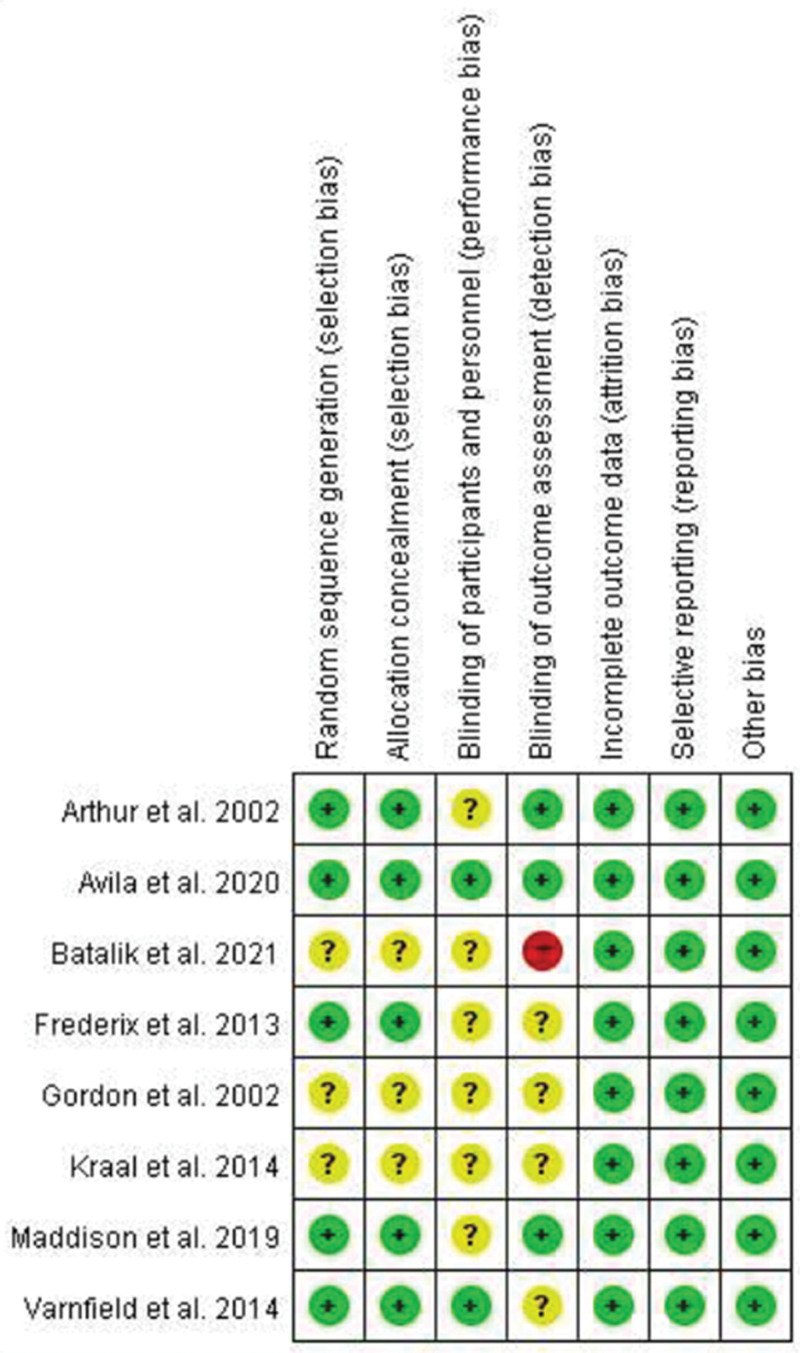
3.3. Risk of bias
Of all the papers included in this review, 3 studies[15,17,18] had an unclear risk of bias in random sequence generation and allocation concealment, while the other 5 studies[12–14,16,19] had a low risk of bias. In blinding of participants and personnel, 2 studies[12,19] had a low risk of bias, and 6 studies[13–18] had an unclear risk of bias. With respect to blinding of the outcome assessment, 3 studies[12–14] had a low risk of bias, 4 studies[16–19] had an unclear risk of bias, and 1 study conducted by Batalik et al[15] had a high risk of bias. With respect to incomplete outcome data, selective reporting, and other bias categories, all studies[12–19] had a low risk of bias (Fig. 2).
Figure 2.
Results of quality assessment of the selected randomized controlled trial studies.
3.4. Meta-analysis results
3.4.1. Cardiorespiratory fitness.
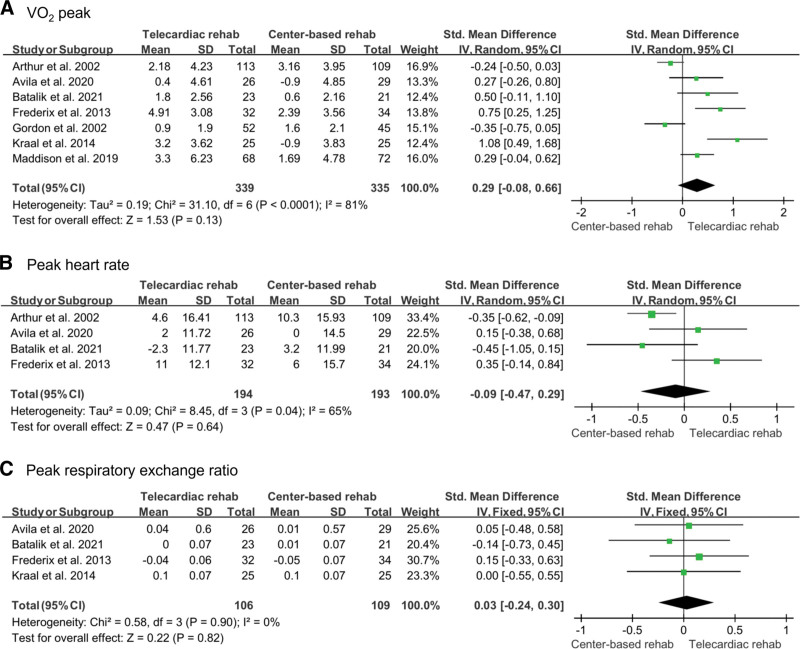
The VO2 peak, peak heart rate, and peak respiratory exchange ratio were measured to investigate cardiorespiratory fitness. Seven studies[12–18] evaluated the VO2 peak, of which 339 participants in the telecardiac rehabilitation group and 335 participants in the center-based rehabilitation group were included. A random-effect model was used for the analysis, and the improvement of VO2 peak was not significantly different between the 2 groups (SMD: 0.29; 95% CI: –0.08 to 0.66; P = .13; I2: 81%). Peak heart rate was investigated in 4 studies (194 in the telecardiac rehabilitation group and 193 in the center-based rehabilitation group).[12,14–16] A random-effect model was used for analysis, and there was no significant difference between the 2 groups (SMD: –0.09; 95% CI: –0.47 to 0.29; P = .64; I2: 65%). The peak respiratory exchange ratio was measured in 4 studies (106 in the telecardiac rehabilitation group and 109 in the center-based rehabilitation group).[12,15,16,18] A fixed-effect model was used for the analysis, and there was no significant difference between the 2 groups (SMD: 0.03; 95% CI: –0.24 to 0.30; P = .82; I2: 0%; Fig. 3).
Figure 3.
Forest plot showing the results of (A) VO2 peak, (B) peak heart rate, and (C) peak respiratory exchange ratio before and after telecardiac and center-based rehabilitation. CI = confidence interval, SD = standard deviation.
3.4.2. Blood pressure.
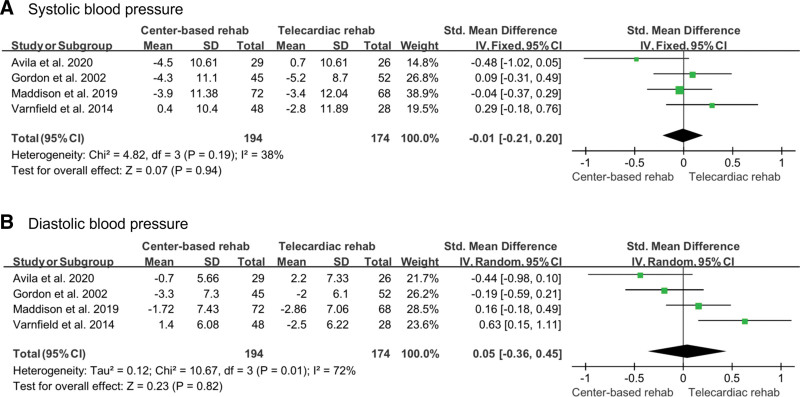
To investigate improvement in blood pressure, systolic and diastolic blood pressures were measured. These were measured in 4 studies (174 in the telecardiac rehabilitation group and 194 in the center-based rehabilitation group).[12,13,17,19] A random-effects model was used to analyze the effects of rehabilitation on systolic and diastolic blood pressures, which found no significant difference between telecardiac rehabilitation and center-based rehabilitation (systolic blood pressure [SMD: –0.01; 95% CI: –0.21 to 0.20; P = .94; I2: 38%]; diastolic blood pressure, [SMD: 0.05; 95% CI: –0.36 to 0.45; P = .82; I2: 72%]; Fig. 4).
Figure 4.
Forest plot showing the results of (A) systolic blood pressure and (B) diastolic blood pressure before and after telecardiac and center-based rehabilitation. CI = confidence interval, SD = standard deviation.
3.4.3. Blood lipids.
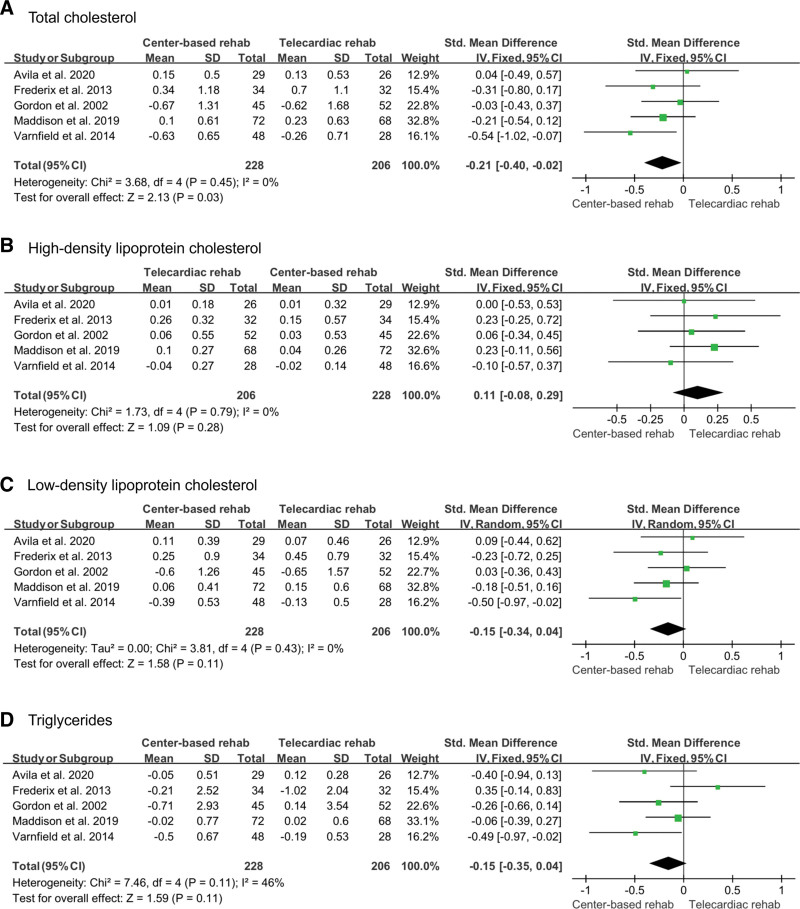
Total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides were included as variables to determine the degree of improvement in blood lipid levels. All variables were identified in 5 studies,[12,13,16,17,19] and a fixed-effects model was used for the analysis. The number of participants included in the analysis was 206 in the telecardiac rehabilitation group and 228 in the center-based rehabilitation group. Total cholesterol was significantly reduced after center-based rehabilitation than after telecardiac rehabilitation (SMD: –0.21; 95% CI: –0.40 to –0.02; P = .03; I2: 0%). For high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides, there was no significant difference between the 2 groups (high-density lipoprotein cholesterol [SMD: 0.11; 95% CI: –0.08 to 0.29; P = .28; I2: 0%]; low-density lipoprotein cholesterol, [SMD: –0.15; 95% CI: –0.34 to 0.04; P = .11; I2: 0%]; triglycerides, [SMD: –0.15; 95% CI: –0.35 to 0.04; P = .11; I2: 46%]; Fig. 5).
Figure 5.
Forest plot showing the results of (A) total cholesterol, (B) high-density lipoprotein cholesterol, (C) low-density lipoprotein cholesterol, and (D) triglycerides before and after telecardiac and center-based rehabilitation. CI = confidence interval, SD = standard deviation.
3.4.4. Body composition.
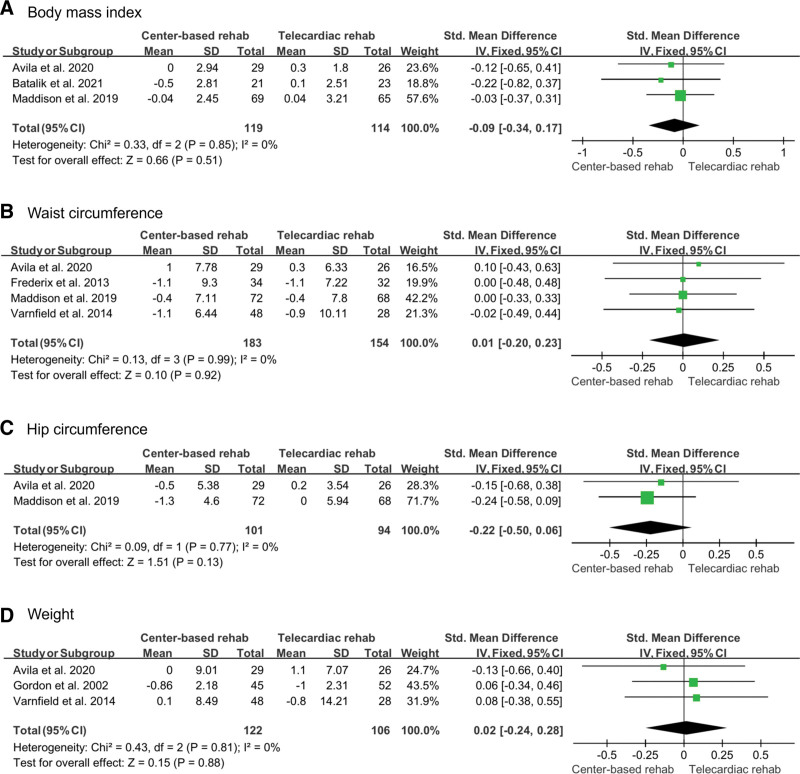
Body composition included body mass index, waist circumference, hip circumference, and weight, which was investigated in 3,[12,13,15] 4,[12,13,16,19] 2,[12,13] and 3[12,17,19] studies, respectively. The number of participants included 114, 154, 94, and 106 in the telecardiac rehabilitation group and 119, 183, 101, and 122 in the center-based rehabilitation group, respectively. A fixed-effect model was used to analyze the effect sizes of body mass index, waist circumference, hip circumference, and weight, which showed no significant intergroup difference (body mass index [SMD: –0.09; 95% CI: –0.34 to 0.17; P = .51; I2: 0%]; waist circumference, [SMD: 0.01; 95% CI: –0.20 to 0.23; P = .92; I2: 0%]; hip circumference, [SMD: –0.22; 95% CI: –0.50 to 0.06; P = .13; I2: 0%]; weight, [SMD: 0.02; 95% CI: –0.24 to 0.28; P = .88; I2: 0%; Fig. 6).
Figure 6.
Forest plot showing the results of (A) body mass index, (B) waist circumference, (C) hip circumference, and (D) weight before and after telecardiac and center-based rehabilitation. CI = confidence interval, SD = standard deviation.
3.4.5. Quality of life.
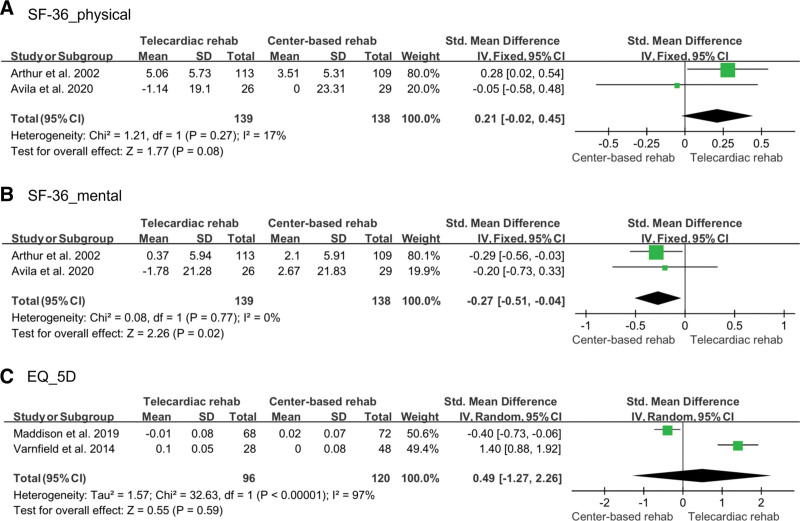
Quality of life was confirmed using the Short-Form 36-Item Health Survey and Euro-Quality of Life-5 Dimension. Short-Form 36-Item Health Survey was evaluated separately for physical and mental health, which were evaluated in 2 studies (139 in the telecardiac rehabilitation group and 138 in the center-based rehabilitation group).[12,14] A fixed-effect model was adopted for the analysis, and there was no significant difference between the 2 groups in the physical health domain (SMD: 0.21; 95% CI: –0.02 to 0.45; P = .08; I2: 17%), but the center-based rehabilitation showed a significant improvement in the mental health domain compared to telecardiac rehabilitation (SMD: –0.27; 95% CI: –0.51 to –0.04; P = .02; I2: 0%). Euro-Quality of Life-5 Dimension was assessed in 2 studies,[13,19] with 96 participants in the telecardiac rehabilitation group and 120 participants in the center-based rehabilitation group. A random-effect model was used for the analysis, and there was no significant difference between the 2 groups (SMD: 0.49; 95% CI: –1.27 to 2.26; P = .59; I2: 97%; Fig. 7).
Figure 7.
Forest plot showing the results of (A) physical domain of SF-36, (B) mental domain of SF-36, and (C) EQ-5D index before and after telecardiac and center-based rehabilitation. CI = confidence interval, EQ-5D = Euro-Quality of Life-5 Dimension, SD = standard deviation, SF-36 = Short-Form 36-Item Health Survey.
3.4.6. Publication bias.
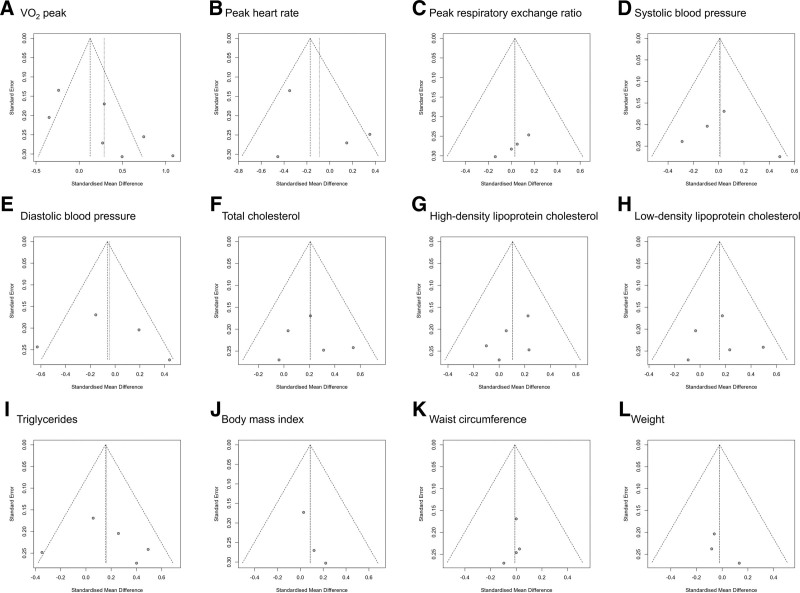
Funnel plot analysis and Egger test were performed to evaluate the publication bias. The graphic funnel plots of all variables were symmetrical (Fig. 8). Moreover, the P value of Egger test was >.05, except for 1 variable (peak respiratory exchange ratio), indicating an insignificant publication bias (VO2 = 0.058, peak heart rate = 0.471, peak respiratory exchange ratio = 0.011, body mass index = 0.169, systolic blood pressure = 0.719, diastolic blood pressure = 0.829, total cholesterol = 0.876, high-density lipoprotein cholesterol = 0.341, low-density lipoprotein cholesterol = 0.953, triglyceride = 0.758, waist circumference = 0.538, and weight = 0.429).
Figure 8.
Graphic funnel plots showing the differences in each assessment before and after telecardiac and center-based rehabilitation.
4. Discussion
In the current meta-analysis, we found that telecardiac rehabilitation has an effect similar to that of center-based rehabilitation. Both rehabilitation methods showed similar positive effects on cardiorespiratory fitness (VO2 peak, peak heart rate, and peak respiratory exchange ratio), systolic and diastolic blood pressures, body composition (body mass index, waist circumference, hip circumference, and weight), and physical quality of life. However, total cholesterol was lower after center-based rehabilitation than after telecardiac rehabilitation, although the effects on high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides did not differ between both rehabilitation methods. In addition, the mental quality of life improved more after center-based rehabilitation than after telecardiac rehabilitation.
Currently, cardiac rehabilitation is a class 1 recommendation for patients with coronary heart disease.[22] Several previous studies have demonstrated the positive therapeutic effects of cardiac rehabilitation.[23–25] Cardiac rehabilitation improves functional status, exercise capacity, and quality of life.[25] In addition, it decreases the occurrence of coronary heart events and mortality rates.[23,24] Usually, cardiac rehabilitation is performed using a center-based rehabilitation program; however, despite evidence of positive therapeutic effects, it is underutilized. Typically, 30% to 40% of patients with coronary heart disease participate in cardiac rehabilitation.[7,8,26] Recently developed information and communication technologies are expected to help increase compliance with cardiac rehabilitation. For telecardiac rehabilitation, a smart wearable device is attached to a patient’s body to monitor physical activity and body condition, and the device can measure the patient’s movement, cardiorespiratory fitness, blood pressure, and blood lipids.[13] When all the measured data are uploaded to the web application, medical staff can determine the patient’s condition based on the data and provide individualized feedback to each patient.[13] This ensures that the patient is given appropriate advice and reassurance.[27] Moreover, telerehabilitation facilitates participation in rehabilitation, especially for people with reduced mobility or environmental restrictions. In support of this, previous studies have reported that prior to coronavirus disease 2019, >50% of patients did not complete any type of cardiac rehabilitation after discharge from the hospital, but after coronavirus disease 2019, 69% participated in telerehabilitation after completing an in-person outpatient program.[28]
In our meta-analysis, although patients did not visit the hospital in-person, telecardiac rehabilitation had similar improvement effects with center-based rehabilitation in most of the evaluated domains, including cardiorespiratory fitness, systolic and diastolic blood pressure, blood lipids (high- and low-density lipoprotein cholesterol, triglycerides), body composition, and physical quality of life.
In addition, despite no significant intergroup difference in high- and low-density lipoprotein and triglycerides, total cholesterol was lower after center-based rehabilitation. We think that integrated slight or minimal intergroup differences in high- and low-density lipoprotein and triglycerides resulted in significant intergroup differences in their sum (total cholesterol). In addition, mental quality of life was significantly improved after center-based rehabilitation than after telecardiac rehabilitation. Participants in center-based rehabilitation can feel supported by direct contact with physicians. Moreover, participants would feel psychological stability and bonding or be motivated by other patients in the same rehabilitation center.[29,30]
This meta-analysis had some limitations. First, reoccurrence and mortality rates were not compared between groups. A meta-analysis of reoccurrence and mortality rates could not be performed because no related data were reported except by Batalik et al.[15] Second, the cardiac rehabilitation duration was not considered. For each study, the outcomes were evaluated over various periods, from the immediate effect after cardiac rehabilitation to 15 months later. When the rehabilitation period was classified in detail, the inclusion of too few studies prevented a meta-analysis. A future meta-analysis to compensate for these limitations is warranted.
In conclusion, we compared telecardiac rehabilitation and center-based rehabilitation in patients with coronary heart disease and found that the overall effects of both rehabilitation methods were similar, except for total cholesterol and mental quality of life. Telecardiac rehabilitation can improve access to healthcare services for patients who are unable to visit a hospital due to time or location problems. Therefore, increasing the participation rate of patients with coronary heart disease by actively introducing remote cardiac rehabilitation can help improve prognosis after coronary heart disease.
Author contributions
All authors contributed significantly to the study and the creation of this manuscript; acquisition, analysis, and interpretation of data; and critical revision of the manuscript for important intellectual content. All authors reviewed the results and approved the final version of the manuscript.
Abbreviations:
- CI =
- confidence interval
- SF-36 =
- Short-Form 36-Item Health Survey
- EQ-5D =
- Euro-Quality of Life-5 Dimension
- SMD =
- standardized mean difference
How to cite this article: Jin Choo Y, Chang MC. Effects of telecardiac rehabilitation on coronary heart disease: A PRISMA-compliant systematic review and meta-analysis. Medicine 2022;101:28(e29459).
All data generated or analyzed during this study are included in this published article.
An ethics statement is not applicable because this study is based exclusively on published literature.
The present study was supported by a National Research Foundation of Korea grant funded by the Korean government (Grant No: NRF-2019M3E5D1A02069399).
The authors have no conflicts of interest to disclose.
References
- [1].Heran BS, Chen JM, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–740. [DOI] [PubMed] [Google Scholar]
- [3].Mampuya WM. Cardiac rehabilitation past, present and future: an overview. Cardiovasc Diagn Ther. 2012;2:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manzoor S, Hisam A, Aziz S, et al. Effectiveness of mobile health augmented cardiac rehabilitation on behavioural outcomes among post-acute coronary syndrome patients: a randomised controlled trial. J Coll Physicians Surg Pak. 2021;31:1148–53. [DOI] [PubMed] [Google Scholar]
- [5].Muthukrishnan R, Malik GS, Gopal K, et al. Power walking based outpatient cardiac rehabilitation in patients with post-coronary angioplasty: randomized control trial. Physiother Res Int. 2021;26:e1919. [DOI] [PubMed] [Google Scholar]
- [6].Tamulevičiūtė-Prascienė E, Beigienė A, Thompson MJ, et al. The impact of additional resistance and balance training in exercise-based cardiac rehabilitation in older patients after valve surgery or intervention: randomized control trial. BMC Geriatr. 2021;21:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eijsvogels TMH, Maessen MFH, Bakker EA, et al. Association of cardiac rehabilitation with all-cause mortality among patients with cardiovascular disease in the Netherlands. JAMA Netw Open. 2020;3:e2011686. [DOI] [PubMed] [Google Scholar]
- [8].Kotseva K, Wood D. De Bacquer D, EUROASPIRE investigators. determinants of participation and risk factor control according to attendance in cardiac rehabilitation programmes in coronary patients in Europe: EUROASPIRE IV survey. Eur J Prev Cardiol. 2018;25:1242–51. [DOI] [PubMed] [Google Scholar]
- [9].Moholdt T, Bekken Vold M, Grimsmo J, et al. Home-based aerobic interval training improves peak oxygen uptake equal to residential cardiac rehabilitation: a randomized, controlled trial. PLoS One. 2012;7:e41199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Oerkild B, Frederiksen M, Hansen JF, et al. Home-based cardiac rehabilitation is as effective as centre-based cardiac rehabilitation among elderly with coronary heart disease: results from a randomised clinical trial. Age Ageing. 2011;40:78–85. [DOI] [PubMed] [Google Scholar]
- [11].Piotrowicz E, Baranowski R, Bilinska M, et al. A new model of home-based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. Eur J Heart Fail. 2010;12:164–71. [DOI] [PubMed] [Google Scholar]
- [12].Avila A, Claes J, Buys R, et al. Home-based exercise with telemonitoring guidance in patients with coronary artery disease: does it improve long-term physical fitness? Eur J Prev Cardiol. 2020;27:367–77. [DOI] [PubMed] [Google Scholar]
- [13].Maddison R, Rawstorn JC, Stewart RAH, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105:122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arthur HM, Smith KM, Kodis J, et al. A controlled trial of hospital versus home-based exercise in cardiac patients. Med Sci Sports Exerc. 2002;34:1544–50. [DOI] [PubMed] [Google Scholar]
- [15].Batalik L, Dosbaba F, Hartman M, et al. Long-term exercise effects after cardiac telerehabilitation in patients with coronary artery disease: 1-year follow-up results of the randomized study. Eur J Phys Rehabil Med. 2021;57:807–14. [DOI] [PubMed] [Google Scholar]
- [16].Frederix I, Van Driessche N, Hansen D, et al. Increasing the medium-term clinical benefits of hospital-based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. Eur J Prev Cardiol. 2015;22:150–158. [DOI] [PubMed] [Google Scholar]
- [17].Gordon NF, English CD, Contractor AS, et al. Effectiveness of three models for comprehensive cardiovascular disease risk reduction. Am J Cardiol. 2002;89:1263–8. [DOI] [PubMed] [Google Scholar]
- [18].Kraal JJ, Peek N, Van den Akker-Van Marle ME, et al. Effects of home-based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short-term results of the FIT@Home study. Eur J Prev Cardiol. 2014;21:26–31. [DOI] [PubMed] [Google Scholar]
- [19].Varnfield M, Karunanithi M, Lee CK, et al. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–9. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons, 2019. [Google Scholar]
- [21].Pinto BM, Goldstein MG, Papandonatos GD, et al. Maintenance of exercise after phase II cardiac rehabilitation: a randomized controlled trial. Am J Prev Med. 2011;41:274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Simon M, Korn K, Cho L, et al. Cardiac rehabilitation: a class 1 recommendation. Cleve Clin J Med. 2018;85:551–8. [DOI] [PubMed] [Google Scholar]
- [23].Pack QR, Goel K, Lahr BD, et al. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community-based study. Circulation. 2013;128:590–7. [DOI] [PubMed] [Google Scholar]
- [24].Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Prabhu NV, Maiya AG, Prabhu NS. Impact of cardiac rehabilitation on functional capacity and physical activity after coronary revascularization: a scientific review. Cardiol Res Pract. 2020;2020:1236968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kotseva K, Wood D, De Backer G, et al. EUROASPIRE III Study Group. Use and effects of cardiac rehabilitation in patients with coronary heart disease: results from the EUROASPIRE III survey. Eur J Prev Cardiol. 2013;20:817–26. [DOI] [PubMed] [Google Scholar]
- [27].Dixon DR, Burns CO, Granpeesheh D, et al. A program evaluation of home and center-based treatment for autism spectrum disorder. Behav Anal Pract. 2016;10:307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nakayama A, Takayama N, Kobayashi M, et al. Remote cardiac rehabilitation is a good alternative of outpatient cardiac rehabilitation in the COVID-19 era. Environ Health Prev Med. 2020;25:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].King KM, Humen DP, Smith HL, et al. Psychosocial components of cardiac recovery and rehabilitation attendance. Heart. 2001;85:290–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shahsavari H, Shahriari M, Alimohammadi N. Motivational factors of adherence to cardiac rehabilitation. Iran J Nurs Midwifery Res. 2012;17:318–24. [PMC free article] [PubMed] [Google Scholar]