Abstract
The parasite Trichomonas vaginalis (Tv) causes a highly prevalent sexually-transmitted infection. As an extracellular pathogen, the parasite mediates adherence to epithelial cells to colonize the human host. In addition, the parasite interfaces with the host immune system and the vaginal microbiota. Modes of Tv pathogenesis include damage to host tissue mediated by parasite killing of host cells, disruption of steady-state vaginal microbial ecology, and eliciting inflammation by activating the host immune response. Recent Tv research has uncovered new players that contribute to multifactorial mechanisms of host-parasite adherence and killing and has examined the relationship between Tv and vaginal bacteria. Mechanisms that may lead to parasite recognition and killing or the evasion of host immune cells have also been revealed.
Keywords: Trichomonas vaginalis, pathogenesis, microbiota, immunity, neutrophils, cytokines
Trichomonas vaginalis is the causative agent of trichomoniasis
Trichomonas vaginalis (Tv) is a unicellular, flagellated, microaerophilic (see Glossary) protist that causes trichomoniasis, a highly prevalent sexually-transmitted infection (STI) [1–3]. With a global prevalence of ~250 million, trichomoniasis is the most common non-viral sexually transmitted infection [5].
While >95% of Tv infections can be cleared using the 5- nitro-imidazole drug metronidazole, drug resistant strains are on the rise [1]. Common symptoms in infected women are vaginitis, and vaginal discharge, however serious adverse consequences include pelvic inflammatory disease, increased risk of HIV transmission and acquisition, and increased risk of malignant cervical cancers [4, 6–8]. Tv is also associated with female and male infertility, and with pre-term/ low weight infant births [4, 6, 9, 10]. Notably ~50% of women are asymptomatic [11]. Greater than 75% of men are asymptomatic [2], however men are carriers, and Tv can cause inflammation in the prostate.
To establish infection, the parasite must mediate contact with host epithelial cells, evade the onslaught of the host immune response, and contend with the vaginal microbiota (Figure 1, Key Figure). The parasite also has a microbiota of its own, harboring two mycoplasma species and a virus, which contribute to its pathogenesis [12]. In the wake of the Tv genome sequence publication, and with the adaptation of omics, in vitro adherence & cytotoxicity assays, and imaging technologies to interrogate Tv host-pathogen interactions, recent years have seen much progress in our understanding of how this important parasite causes disease.
Figure 1, Key Figure: Pathogenic behaviors of Trichomonas vaginalis:
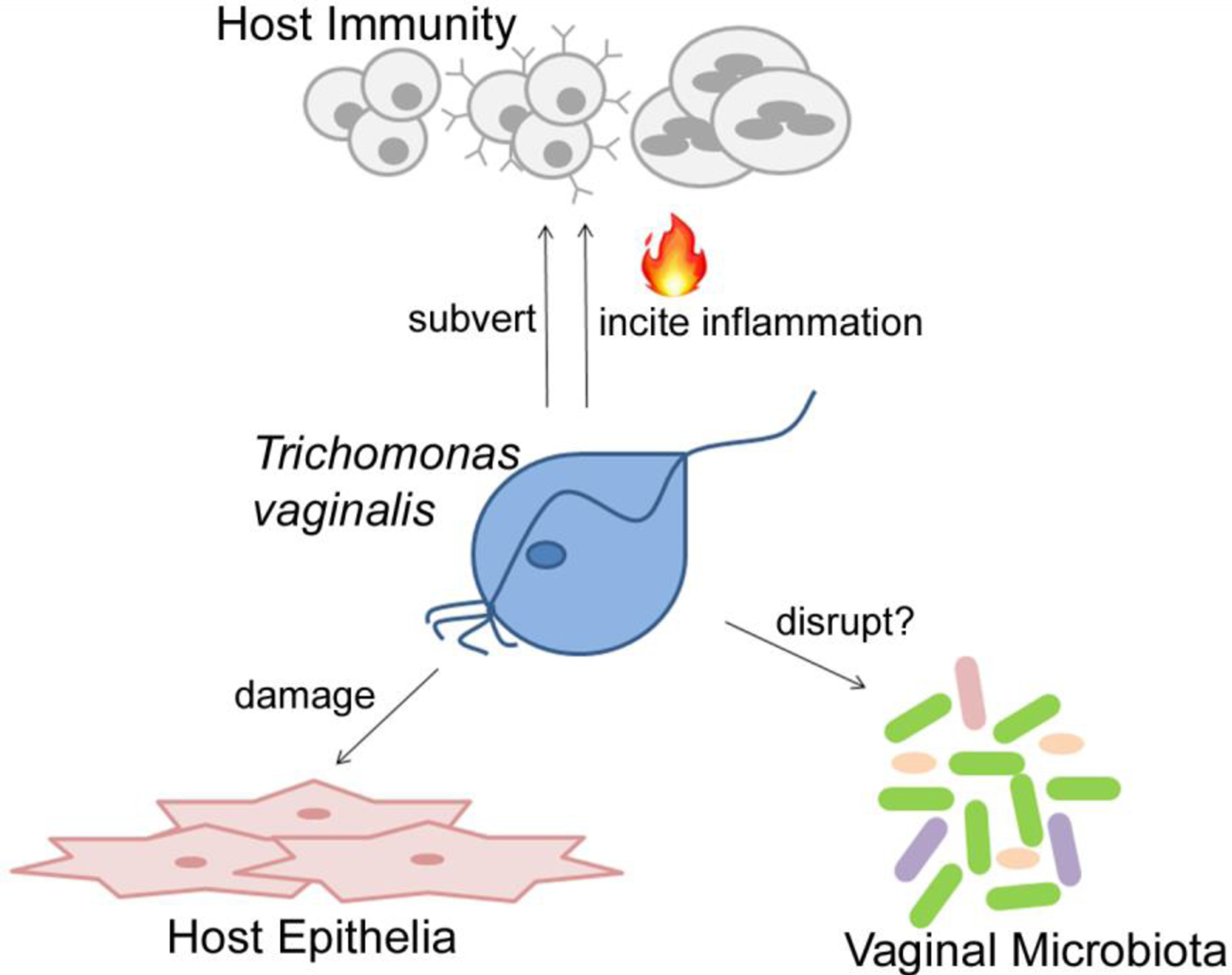
The parasite Trichomonas vaginalis (blue) is a sexually transmitted parasite, which primarily produces symptoms and pathology in the cervical and vaginal mucosa. The parasite can adhere to and lyse host epithelial cells (pink), causing tissue damage. Immune responses (grey) during Tv infection cause inflammation, which can also contribute to pathology, and the parasite has mechanisms to evade clearance by host immunity. Finally, the parasite lives alongside the vaginal microbiota, and is associated with pathogenic disruptions of vaginal bacteria.
Cellular pathogenesis: Tv adherence to host cells and host-cell killing
Tv is an extracellular pathogen; it adheres to host cells as its mode of infection and pathogenesis [13], likely relying on adherence to genital epithelial cells to survive elimination through gravity and secretions. Tv also derives nutrients from lysing and feeding off host cells [13]. Tv can adhere to a broad range of hosts [14], and key cytoskeletal changes appear to facilitate host-cell binding. However, specific molecular moieties on the surface of parasite and host have also been shown to play a role in adherence and host-cell killing, including the parasite surface lipoglycan (LG), which binds to host cell galectin-1 [15], and Tv membrane proteins TVAG_244130 (TvBAP1) and TVAG_188850 (TvBAP2) [16]. Cellular pathogenesis is almost certainly multifactorial, as loss-of-function experiments with individual candidate players never completely inhibit adherence or host cell killing, and several surface molecules have been shown to play a role in adherence (Table 1).
Table 1:
Factors that promote Tv adherence to host cells
| Category | Parasite Effector | Mechanism | Reference |
|---|---|---|---|
| Surface glycoconjugate | Lipoglygan | Bind host Galectin-1 | [15] |
| Surface Proteins | TvBAP-1 | unknown | [16] |
| Tv BAP-2 | unknown | [16] | |
| Enzymes | TvPP1ϒ | unknown | [18] |
| TvLegu 1 | unknown | [20] | |
| TvROM1 | Processes surface proteins | [21] | |
| Vesicles | exosomes | Deliver protein & RNA to host cell | [32] |
| Modulating vaginal microbiota | unknown | Inhibit growth of lactobacillus | [39] |
Clinical isolates of the parasite vary considerably in their ability to adhere to and kill host cells. Work in our laboratory comparing adherence and host-cell killing among clinical isolates revealed that although the two behaviors do not share a strictly linear correlation, a threshold of sufficient adherence must be reached for host cell killing to occur [17]. Also, a few strains that are highly adherent, do not kill host cells [17], indicating that adherence per-se is not sufficient for host-cell killing, and that the latter is mediated by specific effectors that vary between strains. Recently, some new players have been shown to contribute to both processes (Table 1).
New players in adherence:
Recent experiments assaying the ability of parasites to adhere to host cells in vitro have identified new players. Knock-down of TvPP1 ϒ, a protein phosphatase, reduced host cell adherence by 50% [18]. Additionally, it has been reported that TvTim, a Tv glycolytic enzyme, binds laminin and fibronectin, suggesting that TvTim could play a role in ECM adherence during infection [19].
Tv proteases have also been shown to be important mediators of adherence. The cysteine protease TvLegu1 appears to play a role, as treatment of host cells with antisera raised against TvLegu1 showed a 45% decrease in adherence compared to pre-immune serum control. Furthermore, cysteine protease inhibitors reduce adherence by around 66%, indicating that TvLegu1 and other cysteine proteases play an important role in adherence [20]. Finally, we found that a serine protease inhibitor reduced adherence by ~ 60%, and rhomboid serine protease TvROM1, was shown to increase adherence by ~1.5 fold. A biochemical assay to identify TvROM1 substrates identified TvBAP-1, which is cleaved by TvROM1 and also mediates adherence [16, 21]. Interestingly, however, mutation in the TvROM1 cleavage site of TvBAP-1 further increased parasite adherence to host cells, indicating that Tv adherence is most-effective when a dynamic off-on approach is utilized, possibly in the context of parasite aggregates, as we discuss below [21].
Protein palmitoylation also likely plays a role in cytoadherence, as treatment with a palmitoylation inhibitor decreased host-cell adherence by 60% [22]. Players in adherence were also found to be regulated epigenetically, as treatment with a histone acetylation inhibitor increased adherence and also activated transcription at the promoters of genes shown to play a role in adherence [23]. Consistent with these results, a genome-wide approach found histone H3 modification associated with epigenetic upregulation of gene expression [24].
Effectors of host-cell killing:
Few studies have identified specific effectors in host-cell destruction. We showed that host-cell cytosol is released after co-culture with parasites [17], indicating that host-cell killing proceeds through necrosis, rather than induction of apoptosis. Spillage of cytosol was also observed in the killing of host lymphocytes [25]. Most recently, two proteases have been shown to play a role in host-cell killing. We found that overexpression of TvROM1 increased host-cell lysis by 4 fold, while only increasing adherence 1.5 fold, indicating that the protease may affect players of a lytic mechanism. Others have found that killing of epithelial cells was reduced 20% in the presence of polyclonal antibody to metalloprotease TvMP50 and that recombinant TvMP50 showed moderate lytic activity to host-cell monolayers [26].
High resolution imaging has also provided insight into host-cell killing mechanisms. Interestingly, very tight inter-digitations between Tv and host cell membranes have been observed in electron microscopy (EM) pictures [27], suggesting that Tv may utilize a similar trogocytic (trogo=to nibble) mechanism to kill host epithelial cells to that recently reported in E. histolytica [27, 28]. In contrast, whole-cell phagocytosis of smaller lymphoid cells and other microbes has been observed [29, 30].
Cellularity and cellular morphology during adherence:
It was recently observed that treatments with acetylation or palmitoylation inhibitors, which increase host-adherent behavior, also increase parasite clumping (parasite-parasite adherence) [22, 23]. Parasite clumping may facilitate social behavior for group mediated adherence and host cell killing. A scenario in which several parasites “hold on” to host cells to avoid being swept away, while others in the clump lyse host cells for nutrients or invasion can be envisioned. In this regard, it is interesting that Tv overexpressing the tetraspanin protein Tsp8 increase clumping ~ 8 fold [31]. We have also observed that exosomes with Tsp1 on their surface increase parasite adherence to host cells [32]. Together, these findings indicate that tetraspanins contribute to Tv virulence.
EM analyses have revealed striking images of amoeboid-shaped Tv adhered to host cells [33], suggesting that cytoskeletal rearrangement is involved in host-cell adherence. Investigators have therefore sought to determine players in this amoeboid transition, and have identified a fimbrin protein TvFim1 associated with actin bundles during amoeboid transition [34], consistent with TvFim1 playing a role in mediating host-cell adherence. It will be interesting to determine the relative contribution of cytoskeletal versus surface receptor factors in adherence to host cells. It is likely that the parasite employs different strategies in different in vivo circumstances or that different strategies are more effective on some cellular targets compared to others.
Effect of Tv extracellular vesicles on adherence:
We showed that Tv secretes exosomes, small extracellular vesicles that can fuse with host cells [32]. Remarkably, these exosomes were found to “prime” host cells for adherence; parasites adhered better to host cells that were pre-treated with exosomes, suggesting that Tv can “prepare the environment” for optimal colonization. Since exosomes deliver cargo into the host cell cytosol, Tv exosomes may affect host cell expression of adherence ligands. Moreover, host cell adherence was more enhanced when both host cells and parasites were pre-incubated with exosomes before the adherence assay. Combined with the observation that more adherent strains of parasites clump together, this suggests that parasites could use the same molecular moieties to bind to host cells as they do to bind each other, and exosomes serve as a way for the parasite to deposit a pre-fabricated ligand for its receptor onto the host cell. Further supporting this hypothesis is the striking phenotype that exosomes from more adherent strains of Tv can transfer their more-adherent phenotype to less adherent strains, through exosomes [32]. However, it remains to be determined whether exosomes from more-adherent strains facilitate enhanced adherence through their surface associated moieties, or through activity of the cytosolic cargo. Furthermore, ectosomes, larger extracellular vesicles that bud off the plasma membrane, are also produced by Tv and are upregulated upon host cell encounter, suggesting that they may also modulate host- cell pathogenesis [35].
Tv interactions with the vaginal microbiota
The vaginal microbiota likely plays a key role in Tv pathogenesis. In 2011, five main types of vaginal microbiota were established, ranging in their relative abundance of lactobacillus and overall taxa diversity, with type IV being the most diverse, and most commonly associated with pathogenic dysbioses, including bacterial vaginosis (BV), which is characterized by a major shift from lactobacillus-dominance, to mostly anaerobic species [36]. Interestingly, Tv is associated with type IV microbiome. One report found that 72% of Tv+ women have type IV vaginal microbiome [37]. Another study showed that Tv is present in 30% of cases of BV [38]. While it is not clear whether anaerobic microbiomes predispose to acquisition of Tv, or whether Tv disrupts the vaginal microbiome causing dysbioses, some recent studies have examined outcomes of Tv-interactions with common vaginal residents. Notably, in a vaginal ectocervical cell -microbiome-Tv culture model, Tv was shown to inhibit growth of lactobacillus, but not BV associated bacteria [39], suggesting Tv-driven modification of vaginal microbes. On the other hand, lactobacillus was found to decrease Tv adherence to host epithelial cells by 60% [40], suggesting that lactobacillus-dominated vaginas are less hospitable to the parasite. Also, in the presence of Candida albicans, Tv was found to be more cytopathic towards fibroblast cultures [41], suggesting that Tv pathogenesis is increased in Tv− C. albicans concomitant infections.
Another factor in Tv niche establishment is likely to be the human vaginal pH, which is typically 4.5 [39]. One study reported elevated vaginal pH as a key correlate of Tv infection [42]. Similarly, the type IV microbiome, which is most commonly associated with Tv, has an elevated pH compared to other vaginal microbiome types [37]. In vitro, the optimal pH for axenic Tv growth is 6.2, consistent with Tv either preferring a higher than normal vaginal pH, or with the parasite contributing to elevating vaginal pH. Future work to better discern vaginal environments that favor or discourage Tv growth and pathogenesis may inform design of probiotic or other suppository therapies to treat infection.
Tv symbionts contribute to pathogenesis
Tv is known to commonly harbor Trichomonas vaginalis virus (TVV), a double stranded RNA totivirus, and 2 different mycoplasma species, both of which likely contribute to Tv pathogenesis [12].
Trichomonas vaginalis virus (TVV):
TVV can be classified into types I-IV; the parasite may harbor none, one, or multiple types. TVV and the percentage of global isolates harboring it has been recently reviewed [12]. Analysis of TVV+ versus TVV− clinical isolates is consistent with adherence to host cells being increased in TVV+ strains [12], however caution is required in interpreting these results as adherence of isogenic strains differing only in the presence of TVV has not been examined. Anti-virals to clear strains of TVV to determine whether these correlations are strictly due to the virus, are also lacking. However, one report suggests a 2 fold increase in symptoms in TVV+ compared to TVV− cases, consistent with the virus possibly contributing to enhanced pathogenesis [43]. Interestingly, TVV+ isolates were also found to elicit greater inflammatory cytokine production from host cells, in a TLR3-dependent manner [44], suggesting that host recognition of TVV could enhance inflammation. As an anti-viral program of immunity is not likely effective in clearing Tv, this inflammation may rather contribute to infection-associated pathologies. Consistent with this hypothesis, structural analysis of TVV revealed a capsid with unusually large channels posited to facilitate release of the viral genome [45], which could enhance immunogenicity, since dsRNA is the ligand for TLR-3. Furthermore, metronidazole treatment was shown to increase inflammatory cytokine induction by TVV+ strains, likely due to Tv lysis releasing virus and allowing enhanced access to TLR3 [44].
Tv− associated mycoplasmas:
Mycoplasma hominis (Mh) is another common symbiont of Tv that is highly prevalent in clinical isolates, as recently reviewed [12]. Mh can increase Tv fitness, as a recent study reports that Mh is beneficial to the parasite by increasing its growth rate and ATP production by around 50% [46]. Furthermore, Mh has also been shown to contribute to inflammation [47]. Mh alone or in combination with Tv was shown to upregulate inflammatory cytokine secretion from a monocytic cell line [47]. Similarly, we found that isogenic strains harboring, or cleared of Mh, show dramatically different induction of key inflammatory cytokines from primary human monocytes [25]. It is not clear whether enhanced immune responses caused by Mh presence in Tv promote parasite clearance or merely contribute to inflammation- associated pathologies.
Recently, a new mycoplasma species, Mycoplasma girerdii (Mg) was identified in association with Tv [48] [49] [50]. One study reported this species to be present in 44% of Tv clinical isolates [48], and notably, 96% of women carrying Mg were positive for Tv [48]. It will be interesting to determine whether Mg similarly lives within Tv, similarly benefits Tv growth and metabolism, and whether it can also induce inflammatory cytokine secretion in the context of Tv symbiosis.
Tv recognition by host immunity and cytokine induction
It has long been known that the pleiotropic cytokine IL-8 is released in response to Tv infection [51–54]. Galectin 3 has recently been shown to modulate IL-8 secretion through engagement of the lipoglycan (LG) predominant on the parasite surface [55]. Since host cell galectins are becoming appreciated as Pattern Recognition Receptors (PRRs) [56], the LG-Galectin interaction is an exciting area of future research into parasite-derived immune signaling. Recent reports have also shown IL-1β, CCL2, IL-17, IL-22, IFNβ, IL-6, RANTES, MIP-3α, TNF, and IL-23 to be associated with Tv infection either in vivo or in vitro [25, 47, 55, 57–59]. Furthermore, parasite-produced exosomes were shown to induce IL-8 and IL-6 from host cells [32]. However, as mentioned above, a striking percentage of the cytokine abundance observed in vitro appears to be due to Tv symbionts. In comparing 6 strains harboring TVV to 3 strains without TVV, Fichorova et. al. observed that TVV+ strains were greatly enhanced in their ability to induce cytokines [44], with strains lacking the virus showing very little, if any induction. Similarly, Fiori et. al. reported that Mh induced several cytokines from THP-1 macrophages, while Tv alone only stimulated small amounts of IL-1B. However, the Tv− Mh consortium was able to induce greater amounts of cytokine than Mh alone, suggesting a synergism between the two microbes in inducing cytokines. We also observed an Mh- dependent induction of cytokines from primary human monocytes [25]. Interestingly, each of these studies indicate that no, or very little cytokine was induced from the parasite alone, however, these studies may not be sensitive enough to detect parasite-alone induced cytokine, or may not capture damage-associated cytokine induction that may occur in vivo. Furthermore, none of these studies considered both symbionts at that same time. Future work should determine cytokine induction from strains that are cleared of virus and mycoplasma, as well as having both symbionts present to determine the relative contribution of each and the immunogenicity of the parasite alone.
Mature IL-1β secretion is promoted by the inflammasome, a protein complex in the cytosol downstream of PRRs. Recently, it was suggested that Tv can activate the inflammasome, as silencing of Capsase-1, and NLRP3, two players in inflammasome signaling, decreased the amount of IL-1β observed in response to Tv at commensurate levels to their knockdowns [57]. Interestingly, the recently discovered murine gut trichomonad, Tritrichomonas muris was observed to induce an inflammasome- dependent program of immunity in the mouse gut [60]. However, it remains to be determined whether inflammasome activation associated with trichomonads is dependent on their symbionts.
Parasite-derived cytokine mimic:
We also reported that Tv secretes TvMIF, a homolog of human MIF (Macrophage migration inhibitory factor), which can bind the human MIF receptor, and increase host inflammatory cytokine production [61]. In addition, TvMIF could be involved in promotion of some metastatic cancers, as we found it to promote cell growth and invasion in a tissue culture model [61].
Immune recognition of Tv could increase spread of HIV:
In vitro work observing immune activation by Tv has also shed light on how the parasite might elicit increased HIV susceptibility. SLPI, an antimicrobial and immunoregulatory peptide in the vaginal mucosa was shown to be decreased by ~50 percent in Tv+ compared to Tv− controls. Elevated SLPI levels have been associated with greater control of HIV [62, 63], indicating that reduced SLPI could be a contributing factor in the increased transmission of HIV among Tv+ individuals. Tv-induced inflammatory cytokines, especially RANTES and MIP3α could contribute to recruitment of HIV targets. Interestingly, these two cytokines were found to be released in response to strains harboring TVV only [44], indicating that TVV could act as a co-factor in increasing Tv-associated HIV spread.
Immune clearance of Tv and Tv evasion of host immunity
Tv infection is known to activate the immune system through cytokine induction and inducing antibody production [64–67]. However, it is not clear why many infections persist, and why partner re-infection appears to occur in some cases [68]. It remains to be seen how symbionts contribute to antibody responses to the parasite. However, recent adaptations of flow cytometric analyses including Imaging Flow Cytometry and of live imaging conditions for super-high-resolution videos of Tv interactions with immune cells have allowed better characterization of effective anti-trichomonal responses, and how parasites may evade these. Potential evasion strategies are noted below and outlined in Table 2.
Table 2:
Tv immune-evasive behaviors
| Behavior | Function or Effector | Predicted Outcome | Reference |
|---|---|---|---|
| Immune cell lysis | Kill T-cells | Dampen adaptive immunity | [25] |
| Kill B-cells | Dampen humoral immunity | [25] | |
| DNase II Production | Degrade NETs (putative) | Evade neutrophil NETosis | [74] |
| Cysteine Protease production | Degrade antibody | Evade neutrophil trogocytosis | [75], [70] |
| Clumping | Tetraspanin 8, palmitoylated proteins, others unknown | Evade Neutrophil trogocytosis Enhance adherence to host cells | [22], [23], [31], [70] [22], [23], [21] |
| Exosome Secretion | Increase IL-10, decrease IL-8 and IL-17 | Promote immunological tolerance | [32], [78], [59] |
Neutrophil killing of Tv:
Neutrophils have long been known to be a critical cell-type recruited to the site of Tv infection [42, 69]. Additionally, IL-8, which predominantly functions in neutrophil recruitment is consistently the most abundant cytokine induced by Tv [51–54]. New experiments also show that supernatants from Tv-epithelial cell cultures induce neutrophil migration over a filter [58]. We therefore recently tested and confirmed that human neutrophils are able to kill Tv in vitro, and found this to occur rapidly, within 20 minutes [70]. To investigate the mechanisms that neutrophils use to kill Tv, we individually blocked each of the known neutrophil killing pathways at a time: phagocytosis, extracellular degranulation, and NETosis [71]. We found that neither extracellular degranulation, nor NETosis were involved in rapid Tv killing, but that killing was inhibited in the presence of engulfment inhibitors. Interestingly however, when we imaged this engulfment process, we found that in contrast to neutrophils phagocytosing Tv whole, they were found to be “nibbling” on the parasite; taking up to 25 bites before the parasite died [70] (Figure 2). This is consistent with killing using trogocytosis (trogo=to nibble) [72, 73], and is the first demonstration of neutrophils using trogocytosis to kill a pathogen. We found trogocytosis and Tv-killing to be almost completely inhibited when neutrophils were pre-incubated with a serine protease inhibitor, indicating that one of 4 known neutrophil serine proteases may contribute to degradation of the parasite surface into “bites.” We hypothesize that Tv size and motility preclude whole parasite engulfment; the parasite is rather killed by nibbling. It is not clear why Tv is not susceptible to killing by extracellular degranulation, or whether the parasite is also eventually killed using NETosis, a later-stage killing process. However it is interesting that a DNase was recently discovered to be part of the Tv constitutive secretome [74].
Figure 2:
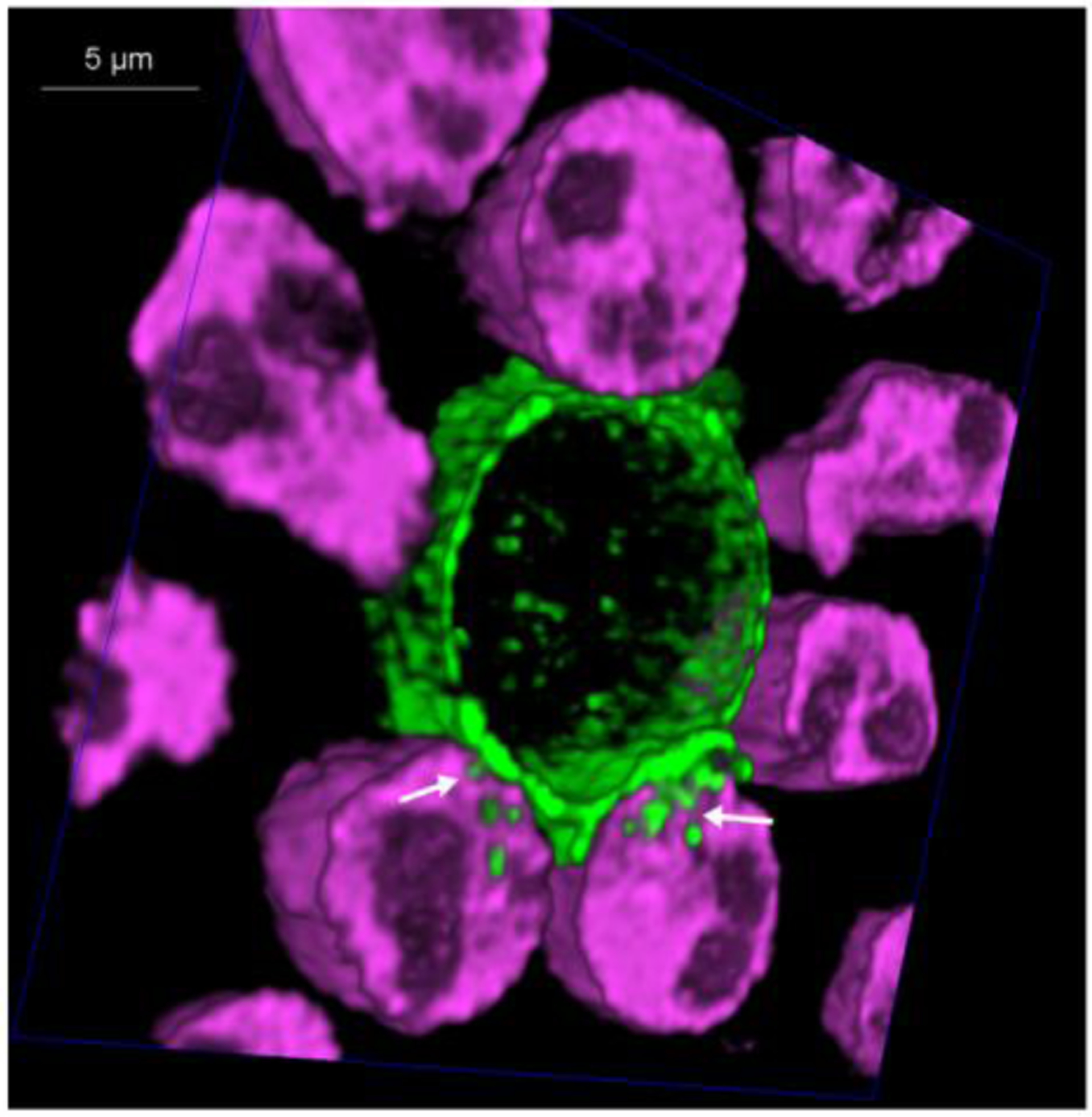
Human neutrophils kill Trichomonas vaginalis using trogocytosis: Primary human neutrophils were labelled with Cell Tracker Deep Red (magenta) and the Trichomonas vaginalis surface was biotinylated and labelled with streptavidin-488 (green). Neutrophils and parasites were allowed to interact at a ratio of 10 neutrophils: 1 parasite in the presence of 10% human AB serum at 370C. HyVolution, super-resolution live confocal imaging (Leica Microsystems) was used to take 60 cross-sections of the cells interacting in real-time. A cross-section of a 3 –dimensional image is shown, revealing neutrophil trogocytosis (nibbling) of the parasite. Location of “nibbling” in action is noted with white arrows. Complete data and methods are published [70].
We also showed that neutrophil trogocytosis and killing of Tv is dependent on human serum, and that ~50% of killing is inhibited in the presence of an Fc-Receptor blocking agent [70], suggesting a role for adaptive immunity in enhancing Tv killing by neutrophils. Interestingly, old reports identified cysteine proteases of the parasites that showed ability to degrade immunoglobulin in vitro [75], suggesting that the parasite could evade trogocytosis by degrading antibody. It is also interesting that the Fc-Receptor inhibitor only blocked 50% of killing, and we found that complement factors also coat the Tv surface, indicating that opsonization by complement factors could also mediate Tv killing by neutrophils [70]. This is consistent with old reports of complement-mediated parasite clearance [76].
We found that an average of 5 neutrophils were needed to achieve killing of a trichomonad, surrounding and sequestering the parasite before and during nibbling [70]. In this regard, it is highly likely that Tv clumping discussed above [22, 23, 31] could serve as a neutrophil- evasive behavior. It remains to be seen how symbionts contribute to neutrophil killing. Also, Tv has been reported to induce apoptosis of neutrophils in vitro [77], indicating that killing of neutrophils could be a strategy that some strains use to evade killing. However, we observed that neutrophil killing of Tv was completed within 20 minutes, while induction of neutrophil apoptosis took hours. However, as neutrophil killing of Tv is contact-dependent, it is possible that large clumps of Tv may induce apoptosis of neutrophils at a distance.
Tv has lymphotoxic activity:
In a broad-survey of challenge experiments between Tv and different immune-cell subsets, we found that Tv was able to kill T-cells and B-cells, but not monocytes [25]. We also found that in mixed lymphocyte cultures, B-cells were preferentially targeted for killing, and that this killing could be mediated by Tv soluble factors [25]. It is interesting that Tv shows a preference for killing B-cells, the producers of antibody, particularly in light of the fact that antibody enhances neutrophil killing of the parasite. It therefore appears that the parasite employs a program of humoral immunity evasion, by killing B-cells, and degrading antibody [75].
Immunomodulation by Tv exosomes:
Collectively, these data support a model by which inflammatory cytokine induced during Tv infection induced neutrophil recruitment and attack of Tv. In this regard, it is interesting that Tv exosomes were also found to be immunomodulatory. We demonstrated that pre-treatment with exosomes reduced subsequent IL-8 responses [32]. Moreover, another group found that 2 day pre-treatment of mice with exosomes, reduced IL-17 secretion, and reduced gross inflammation score, indicating that exosomes could be a way that parasites dampen the immune response [78]. Interestingly, IL-17 responses have been reported in Tv+ women [59], and a major function of IL-17 adaptive responses is neutrophil recruitment. Moreover, the same report found that exosome pretreatment increased levels of the anti-inflammatory cytokine IL-10 in response to subsequent infection. This is consistent with another report that secretory products from Tv induce IL-10 from dendritic cells [79], and with reports of IL-10 induction from bovine cells with the bovine trichomonad Tritrichomonas foetus [80]. Tv exosome generation and secretion could therefore be a mechanism that the parasite uses to subvert host immunity, allowing it to gain a foothold. It is not clear whether these anti-inflammatory properties of exosomes also have beneficial anti-inflammatory roles in the host, or whether they merely stymie immune clearance of the parasite, allowing more host damage and pathology.
Concluding remarks and future directions:
The publication of the Tv genome in 2007 paved the way to identify molecular players in Tv pathogenesis [33]. Our recent adaptation of the CRISPR/Cas9 gene editing system in Tv will potentiate more powerful interrogation of molecules that the parasite uses to establish infection and subvert host immunity [81]. In addition, datasets of the membrane proteome [16], exosome proteome [32], phosphoproteome [82], secretome [74], palmitoylated proteome [22], and genes regulated epigenetically [23, 24] are now all available for researchers to mine. However, a key limitation remains incomplete annotation of the genome, abundant repetition, predominant transposable elements [83], and absent genome sequences for more virulent parasite strains. Lack of robust genomic DNA models continue to hinder the use of RNAseq to identify and interrogate genes that are regulated upon host contact. Flow cytometric analyses of trichomonads has also allowed better quantitation for in vitro pathogenicity assays [25, 70, 84]. Implementation of the oxygen-independent fluorophore, iLOV, has improved fluorescent tagging in the microaerophilic organism [85], and should facilitate improved in vivo imaging and tracking. However, a major limitation to studying pathogenesis has been lack of a robust mouse model that sustains adequate Tv titers. The variation in microbiota and pH between human and mouse vaginas likely contributes to this. Future efforts to “humanize” the mouse vaginal environment using hormonal and microbial supplements may help to overcome this considerable challenge We outline some important open questions (see Outstanding Questions box) that need to be addressed to better characterize Tv pathogenesis and inform therapeutic and management strategies for this highly prevalent infection.
Outstanding Questions:
Is there a correlation between Tv strains that display high levels of pathogenic behavior in vitro and increased symptoms and complications in patients?
Does genomic variance and differential gene expression contribute to the broad range of pathogenic properties of Tv strains?
What are the major host cell receptors for Tv? Do specific protein receptors and proteoglycans play a role in adherence?
What induces cytoskeletal rearrangement when parasites bind to host cells? How critical is cytoskeletal reorganization to adherence and cytotoxicity?
How is host cell biology modified upon uptake of Tv exosomes? What mechanisms mediate host cell: exosome interaction?
What is the function of the human cytokine mimic, TvMIF in the parasite?
Does lactobacillus protect against acquisition of Tv, or favor displacement of the parasite from the vagina?
Does lactobacillus show utility as a probiotic in Tv infected women?
Is the co-occurrence of Tv and type IV vaginal microbiome due to specific metabolic conditions of Type IV microbiome that favor Tv growth?
Do Tv strains cleared of all symbionts, and lacking any host-lytic behavior display any immunogenicity?
Do TVV and mycoplasma symbionts increase adherence and cytolytic capacity of Tv in vitro and in vivo?
Does the recently identified Tv symbiont, Mycoplasma girerdii, contribute to Tv fitness, and affect pathogenesis or immunogenicity of Tv?
What is the effectiveness of neutrophil trogocytosis in killing Tv in vivo and what trogocytic-evasive behaviors may the parasite employ?
Is trogocytic killing of Tv an adaptive immune mechanism, or can it be employed as an innate immune mechanism?
What are the downstream immunological consequences of neutrophil killing of Tv using trogocytosis; does it promote or decrease inflammation?
What effector proteins does Tv use to kill T-cells and B-cells?
What are the conditions needed to establish a mouse model that will sustain adequate titers of Tv to study virulence and cellular immune responses in vivo?
Highlights:
New players in the multifactorial process of host-parasite interactions have been identified, including parasite secreted vesicles (exosomes) and various surface and secreted proteases and effector proteins.
Tv is associated with disruption of the vaginal microbiota; specific interactions with vaginal microbes representative of the healthy and disrupted-state are being examined.
Neutrophils were found to kill Tv using a novel antimicrobial mechanism, trogocytosis. Identification of the mechanisms immune cells use to kill Tv allows analysis of modes the parasite might use to evade immune elimination.
A holistic view of how Tv modulates host immunity is emerging. New roles for three microbial symbionts, a parasite-derived cytokine mimic (TvMIF), exosomes, a surface glycoconjugate, and the selective targeting of lymphocyte populations for killing have been revealed.
Glossary:
- bacterial vaginosis (BV)
an overgrowth of anaerobic vaginal bacteria, especially Gardnerella vaginalis
- Candida albicans
a fungi normally found in humans. Can overgrow and cause “yeast infections” in the vagina
- cytokine
a secreted protein used for communication among immune cells
- ECM (Extracellular Matrix)
secreted proteins and polysaccharides that bind cells together
- ectosomes
extracellular vesicles of moderate size (100–350 nm), which bud directly from the plasma membrane
- exosomes
small extracellular vesicles (30–100 nm), secreted from cells that transfer information to neighboring cells
- extracellular degranulation
the process by which neutrophils excrete vesicles containing toxic effector molecules onto a pathogen to elicit pathogen death
- humoral immunity
the arm of the immune response that generates antibodies and implements antibody-mediated pathogen attack
- Imaging Flow Cytometry
A technology that combines the speed and quantitation of Flow Cytometry with high-resolution microscopy, allowing localization and quantitation of fluorescent signals in single cells
- inflammasome
an intracellular multiprotein complex in immune cells that can recognize pathogens or stress, and activate some inflammatory cytokines including IL-1β
- inflammation
a state of immune-activation which can mediate pathogen clearance, but also become pathogenic
- lactobacillus
the most abundant bacteria in healthy vaginas, produces lactic acid
- metronidazole
an antibiotic commonly used to treat Tv and other anaerobic organisms
- microaerophilic
grows in the presence of limited oxygen
- microbiota
all microbes in a particular environment
- Mycoplasma
a bacterial genus lacking cell walls, and are the smallest known living cells
- MIF
(Macrophage migration Inhibitory Factor); a highly conserved secreted protein that functions as a pleiotropic inflammatory cytokine
- NETosis
(Neutrophil Extracellular Trap); the process by which neutrophils expel their nuclear DNA to form extracellular webs of nucleic acid that can ensnare, and lead to death of pathogens.
- neutrophils
the most abundant immune cells in mammals, and usually the first to arrive at the site of infection. Neutrophils have multiple mechanisms to kill pathogens
- opsonization
the process by which serum factors coat a pathogen surface, providing crosslinking to receptors on phagocytic or trogocytic cells, thus facilitating engulfment
- phagocytosis
the process by which one cell engulfs another cell whole. Neutrophils can phagocytose and subsequently degrade many pathogens
- PRRs (Pattern Recognition Receptors)
Molecules on immune cells that recognize specific microbial molecular signatures and relay a signal to the cytoplasm upon engagement, resulting in initiation of an immune response
- SLPI
(Secretory Leukocyte Protease Inhibitor); a protein secreted by host cells which is thought to protect host cells from proteases secreted by immune effector cells
- TLR3
(Toll-like Receptor 3); a member of the TLR family of PRR signaling proteins found on the surface of immune cells; recognizes dsRNA
- trogocytosis
(trogo= to nibble); a process by which one cell uptakes fragments of an adjacent cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Leitsch D, Recent Advances in the Trichomonas vaginalis Field. F1000Res, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirt RP and Sherrard J, Trichomonas vaginalis origins, molecular pathobiology and clinical considerations. Curr Opin Infect Dis, 2015. 28(1): p. 72–9. [DOI] [PubMed] [Google Scholar]
- 3.Secor WE, et al. , Neglected parasitic infections in the United States: trichomoniasis. Am J Trop Med Hyg, 2014. 90(5): p. 800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fichorova RN, Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol, 2009. 83(1–2): p. 185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization, W.H., Prevalence and Incidence of selected sexually transmitted infections, Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis, and Trichomonas vaginalis: methods and results used by the WHO to generate 2005 estimates. . 2011.
- 6.Mielczarek E and Blaszkowska J, Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection, 2016. 44(4): p. 447–58. [DOI] [PubMed] [Google Scholar]
- 7.Tao L, et al. , Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing. BMC Public Health, 2014. 14: p. 1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh I, et al. , Association between high risk human papillomavirus infection and co-infection with Candida spp. and Trichomonas vaginalis in women with cervical premalignant and malignant lesions. J Clin Virol, 2017. 87: p. 43–48. [DOI] [PubMed] [Google Scholar]
- 9.Nakubulwa S, et al. , Genital infections and risk of premature rupture of membranes in Mulago Hospital, Uganda: a case control study. BMC Res Notes, 2015. 8: p. 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamilton H, et al. , Trichomonas vaginalis Brain Abscess in a Neonate. Clin Infect Dis, 2018. 66(4): p. 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton M, et al. , The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis, 2007. 45(10): p. 1319–26. [DOI] [PubMed] [Google Scholar]
- 12.Fichorova R, et al. , Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res Microbiol, 2017. 168(9–10): p. 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan CM, de Miguel N, and Johnson PJ, Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem, 2011. 51: p. 161–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addis MF, Rappelli P, and Fiori PL, Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect Immun, 2000. 68(7): p. 4358–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okumura CY, Baum LG, and Johnson PJ, Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol, 2008. 10(10): p. 2078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Miguel N, et al. , Proteome analysis of the surface of Trichomonas vaginalis reveals novel proteins and strain-dependent differential expression. Mol Cell Proteomics, 2010. 9(7): p. 1554–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lustig G, et al. , Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun, 2013. 81(5): p. 1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz C, et al. , A protein phosphatase 1 gamma (PP1gamma) of the human protozoan parasite Trichomonas vaginalis is involved in proliferation and cell attachment to the host cell. Int J Parasitol, 2012. 42(8): p. 715–27. [DOI] [PubMed] [Google Scholar]
- 19.Miranda-Ozuna JF, et al. , The Glycolytic Enzyme Triosephosphate Isomerase of Trichomonas vaginalis Is a Surface-Associated Protein Induced by Glucose That Functions as a Laminin- and Fibronectin-Binding Protein. Infect Immun, 2016. 84(10): p. 2878–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rendon-Gandarilla FJ, et al. , The TvLEGU-1, a legumain-like cysteine proteinase, plays a key role in Trichomonas vaginalis cytoadherence. Biomed Res Int, 2013. 2013: p. 561979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riestra AM, et al. , A Trichomonas vaginalis Rhomboid Protease and Its Substrate Modulate Parasite Attachment and Cytolysis of Host Cells. PLoS Pathog, 2015. 11(12): p. e1005294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nievas YR, et al. , Protein palmitoylation plays an important role in Trichomonas vaginalis adherence. Mol Cell Proteomics, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pachano T, et al. , Epigenetics regulates transcription and pathogenesis in the parasite Trichomonas vaginalis. Cell Microbiol, 2017. 19(6). [DOI] [PubMed] [Google Scholar]
- 24.Song MJ, et al. , Epigenome mapping highlights chromatin-mediated gene regulation in the protozoan parasite Trichomonas vaginalis. Sci Rep, 2017. 7: p. 45365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mercer F, et al. , Leukocyte Lysis and Cytokine Induction by the Human Sexually Transmitted Parasite Trichomonas vaginalis. PLoS Negl Trop Dis, 2016. 10(8): p. e0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puente-Rivera J, et al. , The 50kDa metalloproteinase TvMP50 is a zinc-mediated Trichomonas vaginalis virulence factor. Mol Biochem Parasitol, 2017. 217: p. 32–41. [DOI] [PubMed] [Google Scholar]
- 27.Midlej V and Benchimol M, Trichomonas vaginalis kills and eats--evidence for phagocytic activity as a cytopathic effect. Parasitology, 2010. 137(1): p. 65–76. [DOI] [PubMed] [Google Scholar]
- 28.Fiori PL, Rappelli P, and Addis MF, The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes Infect, 1999. 1(2): p. 149–56. [DOI] [PubMed] [Google Scholar]
- 29.Rendon-Maldonado JG, et al. , Trichomonas vaginalis: in vitro phagocytosis of lactobacilli, vaginal epithelial cells, leukocytes, and erythrocytes. Exp Parasitol, 1998. 89(2): p. 241–50. [DOI] [PubMed] [Google Scholar]
- 30.Pereira-Neves A and Benchimol M, Phagocytosis by Trichomonas vaginalis: new insights. Biol Cell, 2007. 99(2): p. 87–101. [DOI] [PubMed] [Google Scholar]
- 31.Coceres VM, et al. , The C-terminal tail of tetraspanin proteins regulates their intracellular distribution in the parasite Trichomonas vaginalis. Cell Microbiol, 2015. 17(8): p. 1217–29. [DOI] [PubMed] [Google Scholar]
- 32.Twu O, et al. , Trichomonas vaginalis exosomes deliver cargo to host cells and mediate hostratioparasite interactions. PLoS Pathog, 2013. 9(7): p. e1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlton JM, et al. , Draft genome sequence of the sexually transmitted pathogen Trichomonas vaginalis. Science, 2007. 315(5809): p. 207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kusdian G, et al. , The actin-based machinery of Trichomonas vaginalis mediates flagellate-amoeboid transition and migration across host tissue. Cell Microbiol, 2013. 15(10): p. 1707–21. [DOI] [PubMed] [Google Scholar]
- 35.Nievas YR, et al. , Membrane-shed vesicles from the parasite Trichomonas vaginalis: characterization and their association with cell interaction. Cell Mol Life Sci, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravel J, et al. , Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A, 2011. 108 Suppl 1: p. 4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brotman RM, et al. , Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex Transm Dis, 2012. 39(10): p. 807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Sayed Zaki M, et al. , Correlation of Trichomonas vaginalis to bacterial vaginosis: a laboratory-based study. J Infect Dev Ctries, 2010. 4(3): p. 156–63. [DOI] [PubMed] [Google Scholar]
- 39.Fichorova RN, et al. , The villain team-up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect, 2013. 89(6): p. 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phukan N, et al. , The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex Transm Infect, 2013. 89(6): p. 455–9. [DOI] [PubMed] [Google Scholar]
- 41.Ozcelik S, et al. , The cytopathic effects of Trichomonas vaginalis on fibroblast cell culture alone and with C. albicans and E. coli. Turkiye Parazitol Derg, 2012. 36(4): p. 193–7. [DOI] [PubMed] [Google Scholar]
- 42.Lazenby GB, Soper DE, and Nolte FS, Correlation of leukorrhea and Trichomonas vaginalis infection. J Clin Microbiol, 2013. 51(7): p. 2323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jehee I, et al. , Direct detection of Trichomonas vaginalis virus in Trichomonas vaginalis positive clinical samples from the Netherlands. J Virol Methods, 2017. 250: p. 1–5. [DOI] [PubMed] [Google Scholar]
- 44.Fichorova RN, et al. , Endobiont viruses sensed by the human host - beyond conventional antiparasitic therapy. PLoS One, 2012. 7(11): p. e48418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parent KN, et al. , Structure of a protozoan virus from the human genitourinary parasite Trichomonas vaginalis. MBio, 2013. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Margarita V, et al. , Symbiotic Association with Mycoplasma hominis Can Influence Growth Rate, ATP Production, Cytolysis and Inflammatory Response of Trichomonas vaginalis. Front Microbiol, 2016. 7: p. 953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiori PL, et al. , Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect, 2013. 89(6): p. 44954. [DOI] [PubMed] [Google Scholar]
- 48.Fettweis JM, et al. , An emerging mycoplasma associated with trichomoniasis, vaginal infection and disease. PLoS One, 2014. 9(10): p. e110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin DH, et al. , Unique vaginal microbiota that includes an unknown Mycoplasma-like organism is associated with Trichomonas vaginalis infection. J Infect Dis, 2013. 207(12): p. 1922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costello EK, et al. , Candidatus Mycoplasma girerdii replicates, diversifies, and co-occurs with Trichomonas vaginalis in the oral cavity of a premature infant. Sci Rep, 2017. 7(1): p. 3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryu JS, et al. , Production of interleukin-8 by human neutrophils stimulated with Trichomonas vaginalis. Infect Immun, 2004. 72(3): p. 1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaio MF, et al. , Generation of interleukin-8 from human monocytes in response to Trichomonas vaginalis stimulation. Infect Immun, 1995. 63(10): p. 3864–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaio MF, et al. , Monocyte-derived interleukin-8 involved in the recruitment of neutrophils induced by Trichomonas vaginalis infection. J Infect Dis, 1994. 170(6): p. 1638–40. [DOI] [PubMed] [Google Scholar]
- 54.Jarrett OD, et al. , T. vaginalis Infection Is Associated with Increased IL-8 and TNFr1 Levels but with the Absence of CD38 and HLADR Activation in the Cervix of ESN. PLoS One, 2015. 10(6): p. e0130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fichorova RN, et al. , Trichomonas vaginalis Lipophosphoglycan Exploits Binding to Galectin-1 and −3 to Modulate Epithelial Immunity. J Biol Chem, 2016. 291(2): p. 998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasta GR, Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol, 2012. 946: p. 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gu NY, et al. , Trichomonas vaginalis induces IL-1beta production in a human prostate epithelial cell line by activating the NLRP3 inflammasome via reactive oxygen species and potassium ion efflux. Prostate, 2016. 76(10): p. 885–96. [DOI] [PubMed] [Google Scholar]
- 58.Seo MY, et al. , Inflammatory response of prostate epithelial cells to stimulation by Trichomonas vaginalis. Prostate, 2014. 74(4): p. 441–9. [DOI] [PubMed] [Google Scholar]
- 59.Makinde HM, et al. , IL-22 levels are associated with Trichomonas vaginalis infection in the lower genital tract. Am J Reprod Immunol, 2013. 70(1): p. 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chudnovskiy A, et al. , Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell, 2016. 167(2): p. 444–456 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Twu O, et al. , Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci U S A, 2014. 111(22): p. 8179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taborda NA, et al. , Higher SLPI expression, lower immune activation, and increased frequency of immune cells in a cohort of Colombian HIV-1 controllers. J Acquir Immune Defic Syndr, 2012. 60(1): p. 12–9. [DOI] [PubMed] [Google Scholar]
- 63.Pillay K, et al. , Secretory leukocyte protease inhibitor in vaginal fluids and perinatal human immunodeficiency virus type 1 transmission. J Infect Dis, 2001. 183(4): p. 653–6. [DOI] [PubMed] [Google Scholar]
- 64.Paintlia MK, et al. , Specific IgA response, T-cell subtype and cytokine profile in experimental intravaginal trichomoniasis. Parasitol Res, 2002. 88(4): p. 338–43. [DOI] [PubMed] [Google Scholar]
- 65.Kaur S, et al. , Antitrichomonas IgG, IgM, IgA, and IgG subclass responses in human intravaginal trichomoniasis. Parasitol Res, 2008. 103(2): p. 305–12. [DOI] [PubMed] [Google Scholar]
- 66.Bastida-Corcuera FD, et al. , Antibodies to Trichomonas vaginalis surface glycolipid. Sex Transm Infect, 2013. 89(6): p. 467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ton Nu PA, et al. , Kinetics of circulating antibody response to Trichomonas vaginalis: clinical and diagnostic implications. Sex Transm Infect, 2015. 91(8): p. 561–3. [DOI] [PubMed] [Google Scholar]
- 68.Forna F and Gulmezoglu AM, Interventions for treating trichomoniasis in women. Cochrane Database Syst Rev, 2003(2): p. CD000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rein MF, Sullivan JA, and Mandell GL, Trichomonacidal activity of human polymorphonuclear neutrophils: killing by disruption and fragmentation. J Infect Dis, 1980. 142(4): p. 575–85. [DOI] [PubMed] [Google Scholar]
- 70.Mercer F, et al. , Neutrophils kill the parasite Trichomonas vaginalis using trogocytosis. PLoS Biol, 2018. 16(2): p. e2003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolaczkowska E and Kubes P, Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol, 2013. 13(3): p. 159–75. [DOI] [PubMed] [Google Scholar]
- 72.Ralston KS, et al. , Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature, 2014. 508(7497): p. 526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilmartin AA, Ralston KS, and Petri WA Jr., Inhibition of Amebic Lysosomal Acidification Blocks Amebic Trogocytosis and Cell Killing. MBio, 2017. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stafkova J, et al. , Dynamic secretome of Trichomonas vaginalis: Case study of beta-amylases. Mol Cell Proteomics, 2018. 17(2): p. 304–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Provenzano D and Alderete JF, Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect Immun, 1995. 63(9): p. 3388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillin FD and Sher A, Activation of the alternative complement pathway by Trichomonas vaginalis. Infect Immun, 1981. 34(1): p. 268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song HO, et al. , Trichomonas vaginalis: reactive oxygen species mediates caspase-3 dependent apoptosis of human neutrophils. Exp Parasitol, 2008. 118(1): p. 59–65. [DOI] [PubMed] [Google Scholar]
- 78.Olmos-Ortiz LM, et al. , Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice. Parasite Immunol, 2017. 39(6). [DOI] [PubMed] [Google Scholar]
- 79.Song MJ, et al. , Modulation of dendritic cell function by Trichomonas vaginalis-derived secretory products. BMB Rep, 2015. 48(2): p. 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vilela RC and Benchimol M, IL-10 release by bovine epithelial cells cultured with Trichomonas vaginalis and Tritrichomonas foetus. Mem Inst Oswaldo Cruz, 2013. 108(1): p. 110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janssen BD, et al. , CRISPR/Cas9-mediated gene modification and gene knock out in the human-infective parasite Trichomonas vaginalis. Sci Rep, 2018. 8(1): p. 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeh YM, et al. , Phosphoproteome profiling of the sexually transmitted pathogen Trichomonas vaginalis. J Microbiol Immunol Infect, 2013. 46(5): p. 366–73. [DOI] [PubMed] [Google Scholar]
- 83.Bradic M, et al. , The Tc1/mariner transposable element family shapes genetic variation and gene expression in the protist Trichomonas vaginalis. Mob DNA, 2014. 5: p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brooks AE, et al. , An improved quantitative method to assess adhesive properties of Trichomonas vaginalis to host vaginal ectocervical cells using flow cytometry. J Microbiol Methods, 2013. 92(1): p. 73–8. [DOI] [PubMed] [Google Scholar]
- 85.Wang SE, et al. , The fluorescent protein iLOV outperforms eGFP as a reporter gene in the microaerophilic protozoan Trichomonas vaginalis. Mol Biochem Parasitol, 2017. 216: p. 1–4. [DOI] [PubMed] [Google Scholar]


