ABSTRACT
An emergence of multidrug-resistant (MDR) Staphylococcus haemolyticus has been observed in the neonatal intensive care unit (NICU) of Nîmes University Hospital in southern France. A case–control analysis was conducted on 96 neonates, to identify risk factors associated with S. haemolyticus infection, focusing on clinical outcomes. Forty-eight MDR S. haemolyticus strains, isolated from neonates between October 2019 and July 2022, were investigated using routine in vitro procedures and whole-genome sequencing. Additionally, five S. haemolyticus isolates from adult patients were sequenced to identify clusters circulating within the hospital environment. The incidence of neonatal S. haemolyticus was significantly associated with low birth weight, lower gestational age, and central catheter use (p < 0.001). Sepsis was the most frequent clinical manifestation in this series (20/46, 43.5%) and was associated with five deaths. Based on whole-genome analysis, three S. haemolyticus genotypes were predicted: ST1 (6/53, 11%), ST25 (3/53, 5.7%), and ST29 (44/53, 83%), which included the subcluster II-A, predominantly emerging in the neonatal department. All strains were profiled in silico to be resistant to methicillin, erythromycin, aminoglycosides, and fluoroquinolones, consistent with in vitro antibiotic susceptibility tests. Moreover, in silico prediction of biofilm formation and virulence-encoding genes supported the association of ST29 with severe clinical outcomes, while the persistence in the NICU could be explained by the presence of antiseptic and heavy metal resistance-encoding genes. The clonality of S. haemolyticus ST29 subcluster II-A isolates confirms healthcare transmission causing severe infections. Based on these results, reinforced hygiene measures are necessary to eradicate the nosocomial transmission of MDR strains.
KEYWORDS: Staphylococcus haemolyticus, neonatal intensive care unit, multidrug resistance, emergence, whole-genome sequencing, ST29, antiseptic resistance, healthcare associated infections
Introduction
Long considered as non-pathogenic commensal bacteria, Coagulase-Negative Staphylococci (CoNS) constitute the majority of species in the human skin microbiota, and their pathogenicity has been underestimated in clinical microbiology [1]. Based on clinical evidence, the contribution of CoNS to hospital-acquired bloodstream infections (BSI) increased from 9 to 27% in adult patients during the 1980s [2]. According to the report from the European Centre for Disease Prevention and Control (ECDC), CoNS were the most frequently isolated bacteria from BSI in Europe in 2019 [3]. This sharp increase is likely linked to medical advances, consisting of the increasing use of intravascular devices in both adults and infants, including extremely low birth weight newborns [4]. A recent French study showed that 31.6% of Staphylococcus BSIs are caused by CoNS, often due to intravascular catheters and immunodeficiency populations, including neonatal patients [5]. Staphylococcus haemolyticus is the second most frequently isolated CoNS after Staphylococcus epidermidis in clinical infections and sepsis [6–8].
The first S. haemolyticus outbreak was reported in 1984 in Spain and was documented as catheter-related sepsis [9]. Most healthcare-associated isolates of S. haemolyticus are classified as multidrug-resistant (MDR), limiting antibiotic therapy options [7,10]. This emerging bacterium has been widely detected in healthcare-associated infections (HCAI) such as BSIs, conjunctivitis, and catheter-related infections, leading to life-threatening conditions in immunocompromised patients, particularly in premature newborns [11–14].
The annual incidence of sepsis in neonatal intensive care units (NICUs) is around 3 million worldwide, with a mortality rate of 11–19% [15]. Moreover, late-onset sepsis (LOS), defined as non-maternal-fetal infection-related sepsis in newborns over three days old, is estimated at 1,000/100,000 infected newborns, causing 17.6% of neonatal deaths between 1979 and 2019 in 14 countries [16]. An American study reported that 29% of sepsis in NICUs was caused by CoNS between 2004 and 2013, notably categorized as LOS [17], largely due to S. epidermidis and S. haemolyticus infections [18,19]. Furthermore, preterm and very low birth weight were identified as risk factors increasing CoNS colonization or infection, especially in the presence of central venous catheters [20,21]. Epidemiological studies have shown that LOS is associated with the emergence of methicillin-resistant S. haemolyticus [14,22–24]. As described for methicillin-resistant S. epidermidis (MRSE) [25] and Staphylococcus capitis clone NRCS-A [26], virulence and antibiotic resistance can be related to bacteria genotypes. Recent European S. haemolyticus outbreaks in NICUs were due to ST2 and ST4 genotypes [10,22,24], while ST1, ST3, ST30, ST56, and ST65 were detected in Eastern European, African, Asian, and South American isolates [27]. However, even though an increase in catheter-related S. haemolyticus sepsis was observed across France in October 2021 by the national mission for the Surveillance and Prevention of Infections Associated with Implantable Devices (SPIADI), to our knowledge, no published study has yet documented a S. haemolyticus outbreak in France [28]. The pathogenicity of S. haemolyticus can be related to its genotype, but the virulence factors involved in the nosocomial spread of French S. haemolyticus isolates remain undocumented. Their persistence in the hospital environment could be due to biofilm formation and antimicrobial resistance, promoting HCAI [13,29].
In order to describe the S. haemolyticus genotypes circulating in the neonatology department, as well as their virulence and antimicrobial resistance involved in patient outcomes, we conducted a retrospective investigation of S. haemolyticus isolates collected in the NICU of Nîmes University Hospital using whole-genome sequencing (WGS).
Materials and methods
Study design, ethics, and case control description
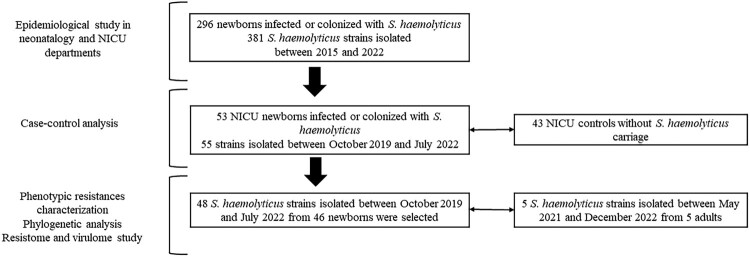
A time-series comparison of the number of routinely isolated bacteria in the neonatology and NICU departments at Nîmes University Hospital (France) was conducted between 2015 and 2022 (Figure 1). Over an eight-year period, a total of 381 S. haemolyticus strains were isolated from 296 newborns. The case–control analysis focused on 53 of the 296 neonates infected or colonized with S. haemolyticus between 2019 and 2022, with no S. haemolyticus carriage noted for the 43 neonatal controls from the intensive care unit. The control cohort included only newborns delivered on the same days as the case cohort to exclude any selection bias (Figure 2).
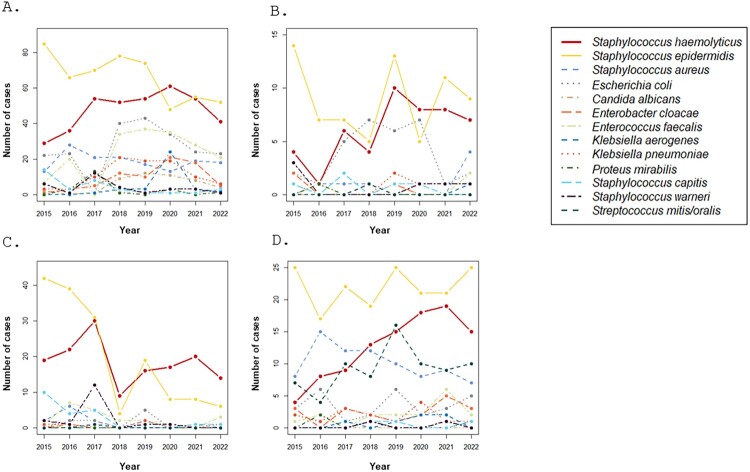
Figure 1.
Times series of bacteria frequently isolated in the NICU and neonatal departments at Nîmes University Hospital and their evolution between 2015 and 2022, including A. all samples; B. blood cultures; C. catheters; D. eyes swabs.
Figure 2.
Study design strategy including epidemiological screening, case-control analysis, and inclusion criteria for genomic analysis of isolates.
Patient consent was deemed unnecessary since the analysis of biological samples was obtained in the context of medical care and was considered non-interventional research, requiring only the non-opposition of the patient or parents during sampling (articles L1221-1.1, L1211-2, and N°DC-2020-4155 of the French Public Health Code). Our investigation focused solely on the analysis of anonymized patient data records and bacteria isolates. This study was approved by the Institutional Review Board of Nîmes University Hospital (23.08.07).
Data collection for the 96 newborns studied in the case–control analysis included sex, birth weight, maternal age, prematurity grade, mode of delivery, premature rupture of membranes, threatened preterm labour, intrapartum fever, chorioamnionitis, twin pregnancy, appearance of amniotic fluid, duration of hospitalization, placement and duration of central and/or umbilical catheter, duration of parenteral nutrition, invasive and/or non-invasive ventilation and duration, antibiotic therapy at birth and/or after 72 h of life, necrotizing enterocolitis, and mortality (Table 1).
Table 1.
Comparison based on clinical and hospitalization characteristics of neonates infected or colonized with Staphylococcus haemolyticus (case cohort) versus non-carrier neonates (control cohort).
| Variables | Case cohort (n = 53) | Control cohort (n = 43) | p-value | |
|---|---|---|---|---|
| N (%) or median [interquartile range] | ||||
| Sex, Male/Female | 30 (56.6) / 23 (43.4) | 21 (48.8) / 22 (51.2) | 0.581 | |
| Birth information | ||||
| Maternal age (years) | 31 [25–37] | 31 [24–38] | 0.853 | |
| Birth weight (g) | 825 [650–1255] | 1550 [1252.5–2745] | < 0.001*** | |
| Prematurity stage | Extremely preterm < 28 weeks | 32 (60.4) | 10 (23.3) | < 0.001*** |
| Very preterm (28–32 weeks) | 14 (26.4) | 12 (27.9) | ||
| Moderate preterm (33–37 weeks) | 5 (9.4) | 17 (39.5) | ||
| Full term > 37 weeks | 2 (3.8) | 4 (9.3) | ||
| Delivery | Vaginal | 22 (41.5) | 16 (37.2) | 0.827 |
| Caesarean section | 31 (58.5) | 27 (62.8) | ||
| Premature rupture of membranes (PROM) | 23 (43.4) | 16 (37.2) | 0.686 | |
| Threatened preterm labour (TPL) | 37 (69.8) | 24 (55.8) | 0.229 | |
| Intrapartum fever | 4 (7.5) | 2 (4.7) | 0.688 | |
| Chorioamnionitis | Possible | 31 (58.5) | 20 (46.7) | 0.305 |
| Excluded | 22 (41.5) | 23 (53.5) | ||
| Twin pregnancy | 16 (30.2) | 4 (9.3) | 0.021* | |
| Appearance of amniotic fluid | Clear | 45 (84.9) | 33 (76.7) | 0.375 |
| Bloody | 5 (9.4) | 3 (7) | ||
| Meconium-stained | 1 (1.9) | 4 (9.3) | ||
| Tinted | 2 (3.8%) | 3 (7%) | ||
| Hospital data | ||||
| Length of hospitalization (days) | 52 [26–88] | 20 [13–39] | 0.004** | |
| Umbilical catheter placement | 44 (83) | 26 (60.5) | 0.025* | |
| Umbilical catheter placement time (days) | 4 [3–6] | 3 [0–5] | 0.012* | |
| Central catheter placement | 43 (81.1) | 18 (41.9) | < 0.001*** | |
| Central catheter placement time (days) | 10 [3–20] | 0 [0–6] | < 0.001*** | |
| Parenteral nutrition | 46 (86.8) | 33 (76.7) | 0.311 | |
| Duration of parenteral nutrition (days) | 10 [5–20] | 5 [2–9] | 0.001*** | |
| Invasive ventilation | 28 (52.8) | 11 (25.6) | 0.013* | |
| Duration of invasive ventilation (days) | 1 [0–5] | 0 [0–0.083] | 0.003** | |
| Non-invasive ventilation | 43 (53.8) | 37 (46.2) | 0.714 | |
| Duration of non-invasive ventilation (days) | 18 [5–45] | 8 [2–22.5] | 0.041* | |
| Antibiotic therapy at birth | 30 (56.6) | 22 (51.2) | 0.744 | |
| Antibiotic therapy after 72 hours of life | 32 (60.4) | 11 (25.6) | 0.001*** | |
| Necrotizing Enterocolitis | 7 (13.2) | 4 (9.3) | 0.749 | |
| Mortality | 7 (13.2) | 1 (2.3) | 0.071 | |
*, p-value < 0.1; **, p-value < 0.05; ***, p-value < 0.001.
Strains selection
Routine in vitro bacteriology investigation and WGS were conducted on 48 methicillin-resistant S. haemolyticus isolates successfully collected from 46 of 53 infected or colonized neonatal patients (Supplementary Table 1). Supplementary data were collected including neonates’ age, S. haemolyticus infection or colonization, type of infection, mortality in neonates with S. haemolyticus sepsis, and information on bacteriological samples (Supplementary Table 2). Moreover, considering the hypothesis of crossover colonization from other units of our hospital, five S. haemolyticus strains isolated between May 2021 and December 2022 from blood cultures of adult patients hospitalized in perioperative medical resuscitation (NSH055), the haematology-oncology department (NSH056), surgical (NSH057 and NSH058), and medical intensive care units (NSH059) were also analyzed (Figure 2, Supplementary Table 1).
Routine bacteriology investigation
Fifty-three S. haemolyticus strains isolated on routine bacteriology from blood, catheter, intraocular swab, and stool samples, isolated between October 2019 and July 2022, were handled according to the French Society of Microbiology recommendation (SFM) [30]. Bacterial identification was performed by mass spectrometry using Vitek MS® (Biomérieux, Marcy-l’Étoile, France). Antibiotic susceptibility testing was performed by the disc diffusion test according to the European Committee for Antimicrobial Susceptibility Testing (EUCAST) recommendations [31]. Vancomycin and teicoplanin MICs were determined using broth microdilution procedures (UMIC) (Bruker, Champs-sur-Marne, France). All isolates were stored in cryotubes at – 80°C for further investigations.
Whole-genome sequencing
All 53 S. haemolyticus strains isolated from neonates and adults were cultivated aerobically at 37°C for 24 h on Columbia sheep blood agar plates (5%) (Biomérieux). Following the manufacturer’s protocol, genomic DNA was extracted from a 200-µL culture suspension using the DNeasy UltraClean Microbial Kit (Qiagen, Aarhus, Denmark) and eluted in a 50-µL volume. For WGS, Illumina library preparation was constructed using 250 ng of the extracted DNA following the DNA Prep kit library paired-end protocol (Illumina, San Diego, USA) and sequenced in a 39 h run providing 2 × 250-bp reads on a Miseq sequencer (Illumina), as previously described [32]. Data quality control was applied directly on Miseq output Reads using QC platform (version 0.23.2).
In silico analysis
After data quality validation, the S. haemolyticus genomes were de novo assembled using Spades software (version 3.15.4) and blasted against the NCBI GenBank database, and also analyzed on the Type-Strain Genomes Server (https://tygs.dsmz.de/user_requests/new) online platform for more precision in bacteria identification. Genome annotation was performed on DDBJ Fast Annotation and Submission Tool online platform (https://dfast.ddbj.nig.ac.jp/), then the whole-genome sequence-based phylogenetic was constructed using Orthologous Average Nucleotide Identity Tool (OAT) software (version 0.93.10) [33]. Pangenome analysis was performed by comparison of the annotated S. haemolyticus genomes using Roary tools (version 3.13.0) available on Galaxy online software (https://www.usegalaxy.org.au/), then visualized on Phandango online tools. Single nucleotide polymorphism (SNP) analysis based on whole-genome alignment was performed using MAFFT (version 7), then quantified by snp-dists (version 0.6.3). Genome representation was performed using Geneious (version 23.0.4), and the SNP tree based on whole-genome alignment was visualized on Interactive Tree Of Life (ITOL) (https://itol.embl.de/upload.cgi). Antimicrobial resistance-, virulence-, pathogenicity – encoding genes, and plasmids were predicted using CGE online platform (http://www.genomicepidemiology.org/services/). Bacteria genotypes were predicted based on WGS on the MLST online platform (PubMLST – Public databases for molecular typing and microbial genome diversity).
Statistical analysis
Statistics were analyzed with R statistical software (version 4.2.0). Quantitative data were expressed as mean and standard deviation or median and quartiles according to the normality of distributions. Qualitative data were expressed as an absolute number and frequency (%). Either Mann–Whitney or Student tests were used to compare quantitative data according to normality. Chi-2 or Fisher exact tests were used for qualitative variables in an appropriate way. A p-value <0.05 was considered statistically significant. Microbial ecology and ST evolution over time have been managed as time series using the forecast R package [34].
Results
Infection overview in NICU and neonatal department
An epidemiological survey of the NICU and neonatology departments at Nîmes University Hospital was conducted between 2015 and 2022 to study their microbial ecology and its evolution. S. epidermidis and S. haemolyticus were the most common bacteria isolated in all clinical samples over the eight years (Figure 1A). S. epidermidis, S. haemolyticus and, Escherichia coli were the most isolated bacteria in blood cultures since 2017. A strong increase in S. haemolyticus prevalence was recorded from 2018 to 2021, which surpassed E. coli and S. epidermidis in 2020, associated with a decline in E. coli incidence (Figure 1B). Moreover, the number of catheters colonized or infected with S. haemolyticus since 2018 increased in parallel with a decrease in S. epidermidis (Figure 1C). Positive S. haemolyticus ocular swabs markedly increased from 2015 to 2021, with the dominance of S. epidermidis and S. haemolyticus since 2020 (Figure 1D).
Case–control analysis
To gain a deeper understanding of the factors associated with S. haemolyticus infection, we conducted a case–control analysis. This analysis included 53 newborns infected or colonized with S. haemolyticus and 43 NICU controls without S. haemolyticus carriage (Table 1). Infected or colonized newborns were significantly associated with lower birth weight (p < 0.001) and lower gestation age (p < 0.001). S. haemolyticus carriage was found to increase with twin pregnancy (p = 0.021). Its incidence was significantly correlated with central and umbilical catheter use in infected or colonized patients (p < 0.001 and p = 0.025, respectively), as well as their duration (p < 0.001 and p = 0.012, respectively). S. haemolyticus infection was significantly related to longer invasive ventilation (p = 0.003), antibiotic therapy initiated after 72 h of life (p = 0.001), longer duration of parenteral nutrition (p = 0.001), and longer hospitalization (p = 0.004). Although there was no significant difference in mortality between the two groups, seven newborns died in the case cohort, compared to one in the control cohort (p = 0.071).
Patient data and isolates
Shifting our focus to patient data and isolates, we collected 48 S. haemolyticus strains from 46 neonatal patients (two patients had two isolates) admitted to NICU from October 2019 through July 2022 for phenotypic and genomic analysis (Supplementary Tables 1 and 2). The median patient age at sampling was 11 days, and the sex ratio was 1:1. Twenty-nine neonates (63%) were extremely premature (<28 weeks), 27 (58.7%) were delivered by caesarean section with a median birth weight of 939 g. The presence of umbilical and central catheters, non-invasive ventilation, and parenteral nutrition was noted in 84.8%, 82.6%, 80.4%, and 89.1% of patients, respectively. Thirty-two (69.6%) newborns presented S. haemolyticus infections, including 20 sepsis (43.5%), eight conjunctivitis (17.4%), and four catheter-related infections (8.7%). The remaining 14 cases (30.4%) were clinically considered as colonization. The duration of hospitalization ranged from 48 to 71 days (median = 59.7). Twenty-eight (60.9%) patients received antibiotic therapy after 72 h of life, of which 27 (58.7%) were started at birth. Thirty-nine (84.2%) patients survived, and seven died after 30-day hospitalization, including five with S. haemolyticus sepsis.
Among the 48 collected isolates, 20 (41.7%) were from blood samples, 17 (35.4%) from catheters, eight (16.7%) from eye swabs, and three (6.2%) from stool samples. The 34 (70.8%) infection-related isolates included 22 (45.8%) sepsis, eight (16.7%) conjunctivitis, and four (8.3%) catheter-related infections. In particular, NSH005 and NSH005BIS strains were isolated from the same patient (Patient 05) in catheter and blood, while NSH049 and NSH049BIS strains were isolated from another patient (patient 49) in catheter and stool, respectively. For genomic comparative analysis, five S. haemolyticus strains isolated from adult patients were included in this study (Figure 2, Supplementary Table 1). All the 53 isolates were submitted to in vitro and WGS analysis to describe the clonality of S. haemolyticus strains circulating in the hospital.
Antimicrobial resistance profiles of S. haemolyticus isolates
Moving on to the analysis of the antimicrobial resistance profiles, we conducted in vitro antibiotic susceptibility tests on all S. haemolyticus isolates. These tests revealed a MDR phenotype for all these isolates (100%), including resistance to oxacillin, erythromycin, aminoglycosides, and ofloxacin. However, all strains were found to be susceptible to minocycline, linezolid, fusidic acid, and glycopeptides. Additionally, 51 out of 53 (96.2%) isolates were resistant to rifampicin, 47 out of 53 (88.7%) to clindamycin and cotrimoxazole, 17 out of 53 (32.1%) to fosfomycin, and 11 out of 53 (20.8%) to pristinamycin. No significant difference in antibiotic resistance profiles was observed between neonatal and adult strains, except for rifampicin susceptibility in only two adult isolates (p = 0.001) (Table 2).
Table 2.
Comparison of phenotypic antibiotic resistance between neonatal (Neo) and adult (A) Staphylococcus haemolyticus strains and according to neonatal isolates sequence type (ST).
| Antibiotics | All strains (n = 53) | Neonatal strains (n = 48) | Adult strains (n = 5) | p-value Neo vs A. | ST29 neonatal strains (n = 40) | ST25 neonatal strains (n = 2) | ST1 neonatal strains (n = 6) | p-value ST29 vs others |
|---|---|---|---|---|---|---|---|---|
| Oxacillin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Erythromycin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Clindamycin | 47 (88.7%) | 42 (87.5%) | 5 (100%) | 0.922 | 40 (100%) | 2 (100%) | 0 (0%) | <0.001*** |
| Pristinamycin | 11 (20.8%) | 11 (22.9%) | 0 (0%) | 0.533 | 11 (27.5%) | 0 (0%) | 0 (0%) | 0.240 |
| Minocyclin | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Kanamycin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Tobramycin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Gentamicin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Linezolid | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Ofloxacin | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Fusidic acid | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Fosfomycin | 17 (32.1%) | 17 (35.4%) | 0 (0%) | 0.266 | 13 (32.5%) | 0 (0%) | 4 (66.7%) | 0.149 |
| Rifampicin | 51 (96.2%) | 48 (100%) | 3 (60%) | 0.001 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Cotrimozaxole | 47 (88.7%) | 42 (87.5%) | 5 (100%) | 0.922 | 40 (100%) | 2 (100%) | 0 (0%) | <0.001*** |
| Vancomycin | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Teicoplanin | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
***, p-value < 0.001.
Genome analysis and annotation
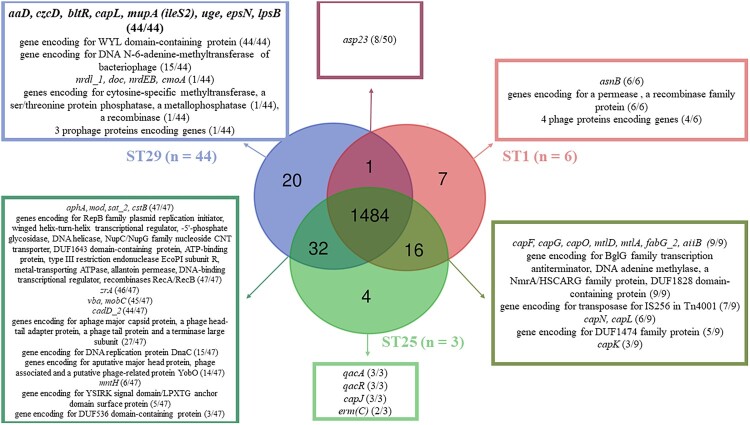
Delving into the genomic characteristics of S. haemolyticus, we conducted a comprehensive genome analysis and annotation. After sequence quality validation (QC < 30), Blast alignment of the assembled genomes against the NCBI GenBank database exhibited >98% sequence identity and >99% coverage with S. haemolyticus, and digital DNA–DNA hybridization (dDDH) > 70%. Genome assembly generated a median of 2,533,862 bp in size corresponding to a median coverage > 97% and a 0.030% gap ratio. Whole-genome annotation predicted a median of 32.6% GC content and a median of 84.7% coding ratio, including a median of 2,501 coding sequences (CDS), between three and six rRNA, 58–60 tRNA, while CRISPRs were predicted in only eight genomes. Multilocus sequence typing detected three Sequence Types (STs): ST29 (n = 44) and ST25 (n = 3) identified in both neonatal and adult strains, while ST1 (n = 6) was identified only in neonatal strains (Supplementary Table 3). Pangenome analysis of the annotated S. haemolyticus genomes yielded that, out of the 1,562 proteins successfully predicted in all strains, 1,484 proteins were shared among all STs. ST29 shared 32 CDS with ST25 and 3 with ST1, while ST1 shared only 16 CDS with ST25 (Supplementary Figures 1 and 2). In particular, seven unique genes were predicted in the ST1 genomes, including five phage-derived genes, four unique genes in the ST25 genomes, and 20 unique genes in ST29 genomes (Figure 3, Supplementary Table 4). In addition, plasmid rep20 was detected in all ST25 and ST29 strains, rep5b only in ST29 and one ST25 neonatal strain, and rep10 in two ST25 and two ST29, while no plasmid was detected in ST1 strains (Supplementary Table 3).
Figure 3.
Comparison of the annotated genomes according to the sequence type. 1,484 genes were shared between the three STs, 32 genes between ST25 and ST29, 20 only in ST29, 16 between ST1 and ST25, seven only in ST1, four only in ST25, and only one gene shared between ST1 and ST29 strains.
Phylogenetic analysis
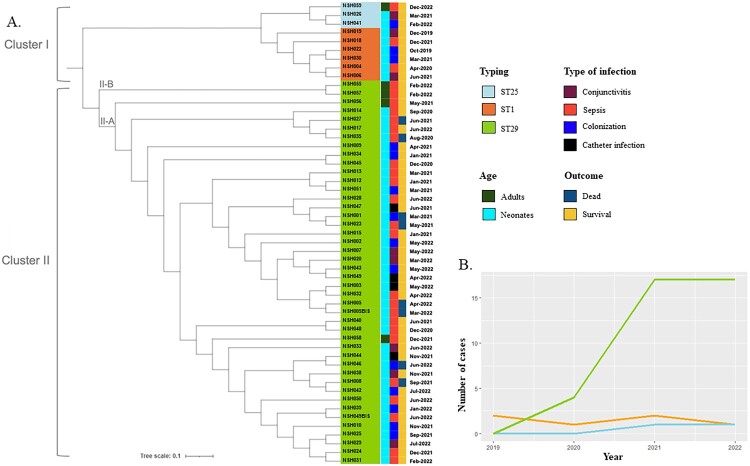
Transitioning to the analysis of phylogenetic relationships, we conducted a comprehensive phylogenetic analysis based on whole-genome sequence alignment of the 48 neonatal and 5 adult S. haemolyticus isolates. We identified two major clusters (Figure 4A), which were confirmed by pangenome analysis and ANI score (Supplementary Figures 1 and 2). Cluster I comprised all ST1 and ST25 strains. Cluster II included only ST29 isolates. Two sub-clusters constituted the ST29 lineage; sub-cluster II-A included 40 neonatal strains and one adult strain (NSH058), isolated from a blood culture of a patient admitted to perioperative intensive care. Subcluster II-B was composed only of adult strains (NSH055, NSH056, and NSH057). Combining whole-genome phylogeny and chronology analysis revealed that the ST29 lineage was predominantly identified up to 2022, revealing the emergence of S. haemolyticus in the NICU, while ST1 and ST25 genotypes remained stable throughout this period (Figure 4B).
Figure 4.
S. haemolyticus genome comparison and chronology. (a) Whole-genome phylogenetic tree of the 53 S. haemolyticus clinical isolates collected between 2019 and 2022 was generated using the ITOL online platform (https://itol.embl.de/). The scale bar represents a 10% sequence divergence. Strains are colour-coded according to their ST. Cluster I includes ST1 (orange) and ST25 strains (light blue). Cluster II includes all ST29 (green) isolates distributed into two subclusters (II-A and II-B). (b) Evolution of the annual incidence according to S. haemolyticus genotype with ST1 in orange, ST25 in light blue and ST29 in green.
Additionally, ST29 SNP distance analysis revealed that all neonatal strains and the adult strain NSP058 had less than 15 SNPs, while the other adult strains NSH055, NSH056, and NSH057 were more distant, confirming the emergence and dissemination of cluster II-A in the NICU (Supplementary Table 5). The strains NSH055 and NSH057, belonging to cluster II-B and isolated from blood cultures six days apart, were genetically identical. These strains were isolated from two patients who were hospitalized in the same room. Among the eight ST29 strains isolated from seven deceased patients, NSH001 (isolated from the catheter) and NSH023 (isolated from the blood culture) were detected one month apart and were highly similar, confirming the persistence of this strain in the NICU (Figure 4A, Supplementary Table 1). Two particular strains, NSH005 and NSH005BIS, isolated from a catheter and bloodstream of the same deceased patient, were genetically identical, providing clear proof of catheter-related sepsis.
In silico prediction of antibiotic resistance-encoding genes
Building upon the genomic analysis, we utilized in silico methods to predict the presence of antibiotic resistance-encoding genes in all 53 S. haemolyticus strains. These strains were predicted to be MDR due to the presence of blaZ, mecA, mph(C), msr(A), aac(6’)-aph(2’’), and norA genes, combined with (S84L) and (S80L) mutations in gyrA and parC genes, respectively. These findings were in complete agreement with in vitro phenotypes (Table 3). Resistance to streptogramin A and related antibiotics was due to vga(A)LC and erm(C) genes detected in 46 (86.7%) and 2 (3.8%) isolates. The aminoglycosides resistance encoding gene aadD and the mupirocin resistance encoding gene mupA were only detected in ST29 strains (p < 0.001), while aph(3’)-III gene encoding for an aminoglycoside phosphotransferase, and the dfrG gene responsible for cotrimoxazole resistance, were present in ST29 and ST25 strains.
Table 3.
Comparison of in silico resistance to antibiotics and antiseptics between neonatal and adult Staphylococcus haemolyticus strains and according to neonatal isolates sequence type (ST).
| Resistance associated genes | All strains (n = 53) | Neonatal strains (n = 48) | Adult strains (n = 5) | p-value Neo vs A | ST29 neonatal strains (n = 40) | ST25 neonatal strains (n = 2) | ST1 neonatal strains (n = 6) | p-value ST29 vs others |
|---|---|---|---|---|---|---|---|---|
| blaZ | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| mecA | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| mph(C) | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| msr(A) | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| vga(A)LC | 46 (86.7%) | 42 (87.5%) | 4 (80%) | 1 | 40 (100%) | 1 (50%) | 1 (16.7%) | <0.001*** |
| erm(C) | 2 (3.8%) | 2 (4.2%) | 0 (0%) | 1 | 0 (0%) | 2 (100%) | 0 (0%) | <0.001*** |
| aph(3’)-III | 47 (88.7%) | 42 (87.5%) | 5 (100%) | 0.922 | 40 (100%) | 2 (100%) | 0 (0%) | <0.001*** |
| aadD | 44 (83%) | 40 (83.3%) | 4 (80%) | 1 | 40 (100%) | 0 (0%) | 0 (0%) | <0.001*** |
| aac(6’)-aph(2'’) | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| gyrA (S84L) mutation | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| parC (S80L) mutation | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| norA | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| dfrG | 47 (88.7%) | 42 (87.5%) | 5 (100%) | 0.922 | 40 (100%) | 2 (100%) | 0 (0%) | <0.001*** |
| rpoB (D471E), (I527M), (S532N) mutations | 50 (94.3%) | 46 (95.8%) | 4 (80%) | 0.34 | 40 (100%) | 0 (0%) | 6 (100%) | <0.001*** |
| mupA | 44 (83%) | 40 (83.3%) | 4 (80%) | 1 | 40 (100%) | 0 (0%) | 0 (0%) | <0.001*** |
| qacE | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| qacH | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| qacA/qacR | 3 (5.7%) | 2 (4.2%) | 1 (20%) | 0.659 | 0 (0%) | 2 (100%) | 0 (0%) | <0.001*** |
| mepA/mepR | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| sepA | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| mdeA | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| bltR | 44 (83%) | 40 (83.3%) | 4 (80%) | 1 | 40 (100%) | 0 (0%) | 0 (0%) | <0.001*** |
***, p-value < 0.001.
The three classic mutations (D471E), (I527M) and (S532N) usually predicted in the rpoB gene involved in rifampicin resistance were detected in S. haemolyticus rpoB gene at positions (I180V) and (S796A) in both ST1 and ST29 strains, while no specific mutations were detected in ST25 strains. Fosfomycin resistance phenotype could be due to the efflux pumps MDR activity encoded by mepA/mepR and sepA.
Moreover, qacE, qacH, and mdeA genes involved in antiseptic resistance activity were predicted in all S. haemolyticus genomes. The multidrug-efflux transporter 2 regulator gene (bltR) was detected only in ST29 strains, while the quaternary ammonium compound efflux MFS transporter gene and its regulator (qacA/qacR) encoding for chlorhexidine resistance were detected only in ST25 strains. Copper efflux pumps encoding genes (copA, copB, and copZ) and zinc transporters (crzB and crzA_2) involved in heavy metal resistance were identified in all S. haemolyticus genomes (Table 4). However, czrA encoding for an HTH-type transcriptional repressor CzrA was absent in ST1 strains. The ars operon (arsRDABC) involved in arsenite efflux arsB_2, arsR_1 and arsR_2 was detected in all strains, while arsC_2 was absent only in ST1 isolates. The czcD gene encoding for cadmium, cobalt, and zinc/H(+)-K(+) antiporter was only detected in ST29 isolates (p < 0.001). Additionally, whole-genome annotation detected 12 efflux pumps and 2 regulators of these pumps in all S. haemolyticus strains, potentially involved in drug release and vital activities (Table 3, Table 4, Supplementary Table 4).
Table 4.
Comparison of resistance to heavy metal-encoding genes between neonatal (Neo) and adult (A) Staphylococcus haemolyticus strains and according to neonatal isolates sequence type (ST).
| Resistance to heavy metals associated genes | All strains (n = 53) | Neonatal strains (n = 48) | Adult strains (n = 5) | p-value Neo vs A | ST 29 neonatal strains (n = 40) | ST 25 neonatal strains (n = 2) | ST 1 neonatal strains (n = 6) | p-value ST29 vs others |
|---|---|---|---|---|---|---|---|---|
| copZ | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| copA | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| copB | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| csoR | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| czrB | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| czrA_2 | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| czrA | 46 (86.8%) | 41 (85.4%) | 5 (100%) | 0.824 | 39 (97.5%) | 2 (100%) | 0 (0%) | <0.001*** |
| arsB_1 | 50 (94.3%) | 45 (93.8%) | 5 (100%) | 1 | 37 (92.5%) | 2 (100%) | 6 (100%) | 0.726 |
| arsB_2 | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| arsB_3 | 50 (94.3%) | 45 (93.8%) | 5 (100%) | 1 | 37 (92.5%) | 2 (100%) | 6 (100%) | 0.726 |
| arsC_1 | 50 (94.3%) | 45 (93.8%) | 5 (100%) | 1 | 37 (92.5%) | 2 (100%) | 6 (100%) | 0.726 |
| arsC_2 | 47 (88.7%) | 42 (87.5%) | 5 (100%) | 0.922 | 40 (100%) | 2 (100%) | 0 (0%) | <0.001*** |
| arsR_1 | 53 (100%) | 48 (100%) | 5 (100%) | 1 | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| arsR_2 | 53 (100%) | 48 (100%) | 5 (100%) | ND | 40 (100%) | 2 (100%) | 6 (100%) | ND |
| cadD_2 | 44 (83%) | 40 (83.3%) | 4 (80%) | 1 | 40 (100%) | 0 (0%) | 0 (0%) | <0.001*** |
| czcD | 44 (83%) | 40 (83.3%) | 4 (80%) | 1 | 40 (100%) | 0 (0%) | 0 (0%) | <0.001*** |
| cadD_1 | 34 (64.2%) | 32 (66.7%) | 2 (40%) | 0.488 | 32 (80%) | 0 (0%) | 0 (0%) | <0.001*** |
| cadD_3 | 31 (58.5%) | 29 (60.4%) | 2 (80%) | 0.685 | 29 (72.5%) | 0 (0%) | 0 (0%) | <0.001*** |
| cadD | 3 (5.7%) | 2 (4.2%) | 1 (20%) | 0.659 | 0 (0%) | 2 (100%) | 0 (0%) | <0.001*** |
***, p-value < 0.001; ND, not determined.
In silico prediction of virulence encoding genes
In light of the high morbidity associated with S. haemolyticus (seven deaths (15.2%) from the 46 newborns), which is likely due to the expression of virulence factors, we proceeded to predict the presence of virulence-encoding genes in S. haemolyticus using in silico methods. (Table 5, Supplementary Table 6). Autolysin (atl) and lytic transglycosylase (sceD) genes, which encode for surface proteins responsible for adherence to human keratinocytes, were detected in all S. haemolyticus genomes. The clpP gene, which encodes for ClpP protease involved in biofilm formation, along with agrA, agrB, and agrD encoding for biofilm regulation and Agr quorum sensing, rsbUVW-sigB operon, luxS, lytSR, alrRS, cidA, and lrgA were detected in all strains. A putative hemolysin III gene was detected as a unique toxin-related factor. katA, eno, and tuf genes, which encode for immune system evasion, were predicted in all genomes, while IS256 was only predicted in ST1 and ST25 genomes. Different classes of capsular polysaccharide proteins were predicted depending on the bacterial genotypes, including two only identified in ST25, one only in ST1, one only identified in ST29 and ST1, and seven in ST1 and ST25 genomes. Additional virulence factors included genes encoding proteases (clpC, clpX), virulence regulation (vraSR), and conserved virulence factor B and C were also predicted in all genomes.
Table 5.
Comparison of virulomes between neonatal and adult Staphylococcus haemolyticus isolates and according to the sequence type (ST).
| Putative Genetic Determinants of Virulence | ST29 neonatal strains (n = 40) | ST5 neonatal strains (n = 2) | ST1 neonatal strains (n = 6) | p-value | |
|---|---|---|---|---|---|
| Biofilm-related genes | |||||
| bap, bhp | Biofilm associated protein | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| fnbP | Fibronectin binding protein | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| atl | Bifunctional autolysin | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| icaABDC | Polysaccharide Intercellular Adhesion (PIA) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| group11 | YSIRK signal domain/ LPXTG anchor domain surface protein | 1 (2.5%) | 2 (100%) | 0 (0%) | <0.001*** |
| sceD | Lytic transglycosylase SceD | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| agrA | Accessory genes regulator A | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| agrB | Accessory genes regulator B | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| agrD | Accessory genes regulator D | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| agrC | Accessory genes regulator C | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| alrRS | ArlRS two-component system | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| lytRS | LytSR two component regulatory system | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| luxS | S-ribosylhomocysteine lyase | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| sarA | Transcriptional regulator SarA | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| mgrA | HTH-type transcription regulator MgrA | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| sigB | Sigma B | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| rsbUVW | / | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| cidA | Holin-like protein CidA | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| lgrA | Antiholin-like protein LrgA | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| clpP | Clp proteases | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| psmα, psmβ | Phenol soluble modulins (PSMs) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Toxins-related genes | |||||
| hly (hla), hlb, hlg | α – β – γ-hemolysins | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| group_1295 | Hemolysin III | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| sea, seb, sec, seg, and sei | Enterotoxins | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Avoid host immune response and Invasiveness | |||||
| katA | Catalase | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| eno | Enolase | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| tuf | Elongation factor tu | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| IS256 | Transposase for insertion sequence element IS256 in transposon Tn4001 | 0 (0%) | 2 (100%) | 4 (66.7%) | <0.001*** |
| capA | Capsular polysaccharide type 5/8 Biosynthesis protein CapA | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| capB | Tyrosine-protein kinase | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| capC | Tyrosine-protein phosphatase | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| capD | Polysaccharide biosynthesis protein | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| capL | UDP-N-acetyl-D-galactosamine dehydrogenase | 40 (100%) | 0 (0%) | 6 (100%) | <0.001*** |
| capE | NAD-dependent epimerase/dehydratase family protein | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capF | Capsular polysaccharide biosynthesis protein CapF | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capG | UDP-N-acetylglucosamine 2-epimerase (non-hydrolyzing) | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capO | Nucleotide sugar dehydrogenase | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capM | Sugar transferase | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capK | Capsular polysaccharide biosynthesis protein | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capN | Capsular polysaccharide type 5/8 Biosynthesis epimerase CapN | 0 (0%) | 2 (100%) | 6 (100%) | <0.001*** |
| capI | Glycosyltransferase | 0 (0%) | 0 (0%) | 6 (100%) | <0.001*** |
| capH | O-acetyltransferase | 0 (0%) | 2 (100%) | 0 (0%) | <0.001*** |
| capJ | O-antigen ligase family protein | 0 (0%) | 2 (100%) | 0 (0%) | <0.001* |
| Other virulence-associated genes | |||||
| clpC, clpX | Clp proteases | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| vraSR | Sensor Pt VraS/ Response regulator pt VraR | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| Group 1823 | Thermonuclease family protein | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
| cvfB, cvfC | Conserved virulence factor B and C | 40 (100%) | 2 (100%) | 6 (100%) | 1 |
***, p-value < 0.001.
Correlation between genomic and clinical outcomes
Having analyzed the genomic features and virulence factors, we then sought to understand the correlation between these genomic characteristics and the clinical outcomes observed in the NICU. All deaths reported in this series were associated with the S. haemolyticus ST29 cluster II-A, following its emergence in the NICU. This could be attributed to specific virulence factor-encoding genes present exclusively in ST29 genomes. Twenty unique genes were identified solely in ST29 genomes (Figure 3, Supplementary Table 4), including the uge gene involved in the integrity of smooth lipopolysaccharide and capsule, the epsN gene involved in exopolysaccharide biosynthesis during pellicle formation and swarming, and the lpsB encoding for core oligosaccharide glycosyl transferase involved in lipopolysaccharide biosynthesis. Capsular polysaccharides encoding genes (capA, capB, capC, capD, and capL) implicated in immune evasion and anti-phagocytosis activity, were also detected in ST29 genomes. Additionally, heavy metal resistance-associated genes cadD_2 and czcD were exclusively detected in ST29, along with antibiotic resistance-associated genes bltR and mupA (also known as the ileS2 gene). The plasmid-located ileS2 gene is flanked by two copies of the insertion sequence IS257, and all ST29 genomes harboured the azo1 gene, which encodes for an azoreductase, responsible for metabolizing azo dyes.
Discussion
S. haemolyticus infection is often underestimated in clinical microbiology due to its classification as a commensal skin bacterium, despite its high levels of antimicrobial resistance [13,35]. Hospital environments constitute perfect ecosystems for the selection and expansion of MDR and methicillin-resistant S. haemolyticus, leading to serious HCAI in immunocompromised patients such as preterm neonates [36]. In this study, we analysed the epidemiological, clinical, and microbiological characteristics of S. haemolyticus in a cohort of newborns admitted to the NICU over eight years. Our findings demonstrate a significant increase in the number of S. haemolyticus isolates, highlighting the emergence of this pathogen as an important cause of LOS and death in preterm infants. Consistent with previous reports [20,21], preterm birth, low birth weight, and invasive procedures were identified as major risk factors for S. haemolyticus infection (p < 0.001). This increase in S. haemolyticus incidence could be attributed to the natural selection of persistent MDR S. haemolyticus in the hospital ecology, while its spread may be facilitated by healthcare workers, shared equipment, and airborne transmission in intensive care units [36,37]. Notably, all S. haemolyticus isolates exhibited MDR (e.g. resistant to methicillin, aminoglycosides, fluoroquinolones, rifampicin, and macrolides), supporting the worldwide increase of MDR S. haemolyticus strains reported in the last decade [35,38,39]. The high resistance against antibiotics not typically used in the NICU further confirms this trend, in contrast to the worldwide emergence of MDR S. capitis clones specific to this ward and susceptible to fluoroquinolones [26]. Three rpoB gene mutations (D471E), (I527M), and (S532N), previously documented [40,41] and involved in rifampicin resistance phenotype, were detected in ST1 and ST29 isolates. All S. haemolyticus strains harboured fluoroquinolones resistance gyrA (S84L) and parC (S80L) mutations [42]. No resistance to glycopeptides and linezolid was detected. In silico prediction of multidrug efflux pumps and antiseptic resistance encoding genes in S. haemolyticus genomes revealed MDR strains resistant to disinfectants, including cationic antiseptic agents such as acriflavine, ethidium bromide, benzalkonium chloride, and chlorhexidine digluconate, widely used to disinfect hospital surfaces, explaining its persistence and cross-transmission in the NICU [43–45].
Based on phylogenetic analysis, ST29 was the dominant genotype circulating in Nîmes University Hospital (44/53), with subcluster II-A responsible for the S. haemolyticus NICU outbreak as noted by France's health authorities [46], resulting in severe sepsis and associated mortality. Despite the first clinical description of S. haemolyticus ST29 being reported in India in 2016 from a conjunctival swab [39,47], no clinical studies have been published on the emergence of this variant. The latest European documented outbreaks in the NICU were due to ST2 and ST4, ST2, and ST13 in Norway, Sweden, and Switzerland, respectively [22,24], while no studies have been published documenting the emergence of ST29 worldwide.
Considering ST29’s direct association with mortality in newborns, this high morbidity could be explained by the expression of virulence factors involved in immune evasion and anti-phagocytosis activity [48–52], as well as by biofilm formation-encoding genes predicted only in ST29 genomes. Moreover, the insertion sequence IS257, related to CoNS virulence and biofilm formation [53,54] was identified in all S. haemolyticus genomes, but with ≥3 copies in ST29 genomes. The persistence and expansion of S. haemolyticus in the hospital environment are potentially due to efflux pumps such as bltR, cadD_2, czcD, involved in multidrug release and heavy metals resistance [55], as well as the presence of rep5 and rep20 plasmids involved in methicillin resistance phenotype [56]. The identification of various virulence genes associated with biofilm formation and adhesion suggests the potential of S. haemolyticus ST29 to colonize medical devices and cause persistent infections, highlighting the adaptability and evolutionary success of S. haemolyticus as a nosocomial pathogen. Phylogenetic analysis of ST29 genomes showed that the adult strains were genetically distant from the neonatal strains, except the blood isolate NSH058 from a resuscitation surgery patient, supporting the hypothesis of healthcare transmission between departments either by nursing staff and/or shared medical instruments [45]. The similarity of NSH005 isolated from the catheter and NSH005BIS from blood of the same dead patient, confirmed the origin of the sepsis and the risk of catheter-related infection [57].
Our study has several limitations. Firstly, the retrospective design limits the availability of clinical data and may introduce selection bias. Secondly, the study was conducted at a single centre, potentially limiting the generalizability of the findings. Thirdly, the lack of WGS data from other national or international centres hinders global comparisons and limits our understanding of the population structure and evolutionary dynamics of S. haemolyticus, as well as their implication in severe infections and mortality. Finally, based on in silico findings, further phenotypic analysis should be performed to study biofilm formation and the expression of virulence genes, particularly in ST29 and death-associated strains. Antiseptic and new disinfectant in vitro susceptibility tests are needed to identify the tolerance thresholds and propose appropriate molecules to eradicate the persistence of S. haemolyticus in hospital environments.
In conclusion, our study provides important insights into the epidemiology, clinical characteristics, and genomic features of S. haemolyticus infections in newborns. The increasing incidence, high antimicrobial resistance rates, and the emergence of S. haemolyticus ST29 underscore the need for enhanced surveillance and infection control measures in NICUs. Further research is needed to elucidate the global distribution, population structure, and pathogenic potential of S. haemolyticus, providing effective preventive strategies and therapeutic interventions to combat this emerging threat in vulnerable neonates.
Supplementary Material
Acknowledgements
The authors thank the Nîmes University Hospital for its structural, human, and financial support through the award obtained by our team during the internal call for tenders ‘Thématiques phares’. We also thank Sarah Kabani for her editing assistance.
Funding Statement
This work is a part of routine bacteriology laboratory activity. No specific funding was received for this study.
Consent and research ethics
This study was conducted in accordance with the Declaration of Helsinki and had been reviewed and approved by the “Institutional Review Board” Ethics Committee of Nîmes University Hospital under the following number: 23.08.07. No specific clinical sampling was performed for this study, only bacteria isolates, and anonymized clinical records were analyzed.
Authors contribution
CM and MM: Study design, experimental workflow, whole genomes sequencing, data collection, validation and writing the original draft of the manuscript. MM: bioinformatic investigation. BT: routine bacteriology investigation, whole genome sequencing, and data collection. FS and JA: data collection, validation, and statistical analysis. MDM, MS, TAT: patient inclusion, data collection, and sampling. CDR, AS: conceptualization and validation. JO, BRM, and AP: design of the study, routine investigation data collection, validation, and conceptualization. AP: isolates collection and routine bacteriology validation. JPL: Study design, study direction, validation, and conceptualization, authorization, funding acquisition and writing of the original manuscript. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The genomes sequences mentioned in the present study are available on the NCBI GenBank database under the accession number PRJNA1010742.
References
- 1.Becker K, Both A, Weißelberg S, et al. . Emergence of coagulase-negative staphylococci. Expert Rev Anti Infect Ther. 2020;18(4):349–366. doi: 10.1080/14787210.2020.1730813 [DOI] [PubMed] [Google Scholar]
- 2.Schaberg DR, Culver DH, Gaynes RP.. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91(3B):S72–S75. doi: 10.1016/0002-9343(91)90346-y [DOI] [PubMed] [Google Scholar]
- 3.ECDC . Healthcare-associated infections acquired in intensive care units Annual Epidemiological Report 2019. 2019; [cited 2023 Sep 12]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/healthcare-associated-infections-intensive-care-units-annual-epidemiological-report-2019.pdf.
- 4.Karchmer AW. Nosocomial bloodstream infections: organisms, risk factors, and implications. Clin Infect Dis. 2000;31(Suppl 4):S139–S143. doi: 10.1086/314078 [DOI] [PubMed] [Google Scholar]
- 5.N’Guyen Y, Baumard S, Vernet-Garnier V, et al. . Coagulase-negative Staphylococcus bacteraemia accounts for one third of Staphylococcus bacteraemia in a French university hospital. Scand J Infect Dis. 2012;44(2):79–85. doi: 10.3109/00365548.2011.617777 [DOI] [PubMed] [Google Scholar]
- 6.Falcone M, Giannella M, Raponi G, et al. . Teicoplanin use and emergence of Staphylococcus haemolyticus: is there a link? Clin Microbiol Infect. 2006;12(1):96–97. doi: 10.1111/j.1469-0691.2005.01307.x [DOI] [PubMed] [Google Scholar]
- 7.Hope R, Livermore DM, Brick G, et al. . Non-susceptibility trends among staphylococci from bacteraemias in the UK and Ireland, 2001-06. J Antimicrob Chemother. 2008;62(Suppl 2):ii65–ii74. doi: 10.1093/jac/dkn353 [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi F, Watanabe S, Baba T, et al. . Whole-genome sequencing of Staphylococcus haemolyticus uncovers the extreme plasticity of its genome and the evolution of human-colonizing staphylococcal species. J Bacteriol. 2005;187(21):7292–7308. doi: 10.1128/JB.187.21.7292-7308.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sitges-Serra A, Puig P, Liñares J, et al. . Hub colonization as the initial step in an outbreak of catheter-related sepsis due to coagulase negative staphylococci during parenteral nutrition. J Parenter Enter Nutr. 1984;8(6):668–672. doi: 10.1177/0148607184008006668 [DOI] [PubMed] [Google Scholar]
- 10.Cavanagh JP, Hjerde E, Holden MTG, et al. . Whole-genome sequencing reveals clonal expansion of multiresistant Staphylococcus haemolyticus in European hospitals. J Antimicrob Chemother. 2014;69(11):2920–2927. doi: 10.1093/jac/dku271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panda S, Kar S, Sharma S, et al. . Multidrug-resistant Staphylococcus haemolyticus isolates from infected eyes and healthy conjunctivae in India. J Glob Antimicrob Resist. 2016;6:154–159. doi: 10.1016/j.jgar.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 12.Sloos JH, Dijkshoorn L, Van Boven CP.. Septicaemias caused by a strain of Staphylococcus haemolyticus exhibiting intermediate susceptibility to teicoplanin in multiple intensive care unit patients. J Antimicrob Chemother. 2000;45(3):410–411. doi: 10.1093/jac/45.3.410 [DOI] [PubMed] [Google Scholar]
- 13.Barros EM, Ceotto H, Bastos MCF, et al. . Staphylococcus haemolyticus as an important hospital pathogen and carrier of methicillin resistance genes. J Clin Microbiol. 2012;50(1):166–168. doi: 10.1128/JCM.05563-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira PM, Binatti VB, Sued BP, et al. . Staphylococcus haemolyticus disseminated among neonates with bacteremia in a neonatal intensive care unit in Rio de Janeiro, Brazil. Diagn Microbiol Infect Dis. 2014;78(1):85–92. doi: 10.1016/j.diagmicrobio.2013.06.026 [DOI] [PubMed] [Google Scholar]
- 15.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, et al. . The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi: 10.1016/S2213-2600(18)30063-8 [DOI] [PubMed] [Google Scholar]
- 16.Fleischmann C, Reichert F, Cassini A, et al. . Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106(8):745–752. doi: 10.1136/archdischild-2020-320217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bizzarro MJ, Shabanova V, Baltimore RS, et al. . Neonatal sepsis 2004-2013: the rise and fall of coagulase-negative staphylococci. J Pediatr. 2015;166(5):1193–1199. doi: 10.1016/j.jpeds.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitriou G, Fouzas S, Giormezis N, et al. . Clinical and microbiological profile of persistent coagulase-negative staphylococcal bacteraemia in neonates. Clin Microbiol Infect. 2011;17(11):1684–1690. doi: 10.1111/j.1469-0691.2011.03489.x [DOI] [PubMed] [Google Scholar]
- 19.Björkqvist M, Söderquist B, Törnqvist E, et al. . Phenotypic and genotypic characterisation of blood isolates of coagulase-negative staphylococci in the newborn. APMIS. 2002;110(4):332–339. doi: 10.1034/j.1600-0463.2002.100408.x [DOI] [PubMed] [Google Scholar]
- 20.Piening BC, Geffers C, Gastmeier P, et al. . Pathogen-specific mortality in very low birth weight infants with primary bloodstream infection. PLoS One. 2017;12(6):e0180134. doi: 10.1371/journal.pone.0180134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craft A, Finer N.. Nosocomial coagulase negative staphylococcal (CoNS) catheter-related sepsis in preterm infants: definition, diagnosis, prophylaxis, and prevention. J Perinatol. 2001;21(3):186–192. doi: 10.1038/sj.jp.7200514 [DOI] [PubMed] [Google Scholar]
- 22.Westberg R, Stegger M, Söderquist B.. Molecular epidemiology of neonatal-associated Staphylococcus haemolyticus reveals endemic outbreak. Microbiol Spectr. 2022;10(6):e02452–22. doi: 10.1128/spectrum.02452-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta G, Kumari S.. Multi-resistant Staphylococcus haemolyticus in a neonatal unit in New Delhi. Ann Trop Paediatr. 1997;17(1):15–20. doi: 10.1080/02724936.1997.11747857 [DOI] [PubMed] [Google Scholar]
- 24.Klingenberg C, Rønnestad A, Anderson AS, et al. . Persistent strains of coagulase-negative staphylococci in a neonatal intensive care unit: virulence factors and invasiveness. Clin Microbiol Infect. 2007;13(11):1100–1111. doi: 10.1111/j.1469-0691.2007.01818.x [DOI] [PubMed] [Google Scholar]
- 25.Deplano A, Vandendriessche S, Nonhoff C, et al. . National surveillance of Staphylococcus epidermidis recovered from bloodstream infections in Belgian hospitals. J Antimicrob Chemother. 2016;71(7):1815–1819. doi: 10.1093/jac/dkw086 [DOI] [PubMed] [Google Scholar]
- 26.Butin M, Martins-Simões P, Rasigade J-P, et al. . Worldwide endemicity of a multidrug-resistant Staphylococcus capitis clone involved in neonatal sepsis. Emerg Infect Dis. 2017;23(3):538–539. doi: 10.3201/eid2303.160833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sands K, Carvalho MJ, Spiller OB, et al. . Characterisation of staphylococci species from neonatal blood cultures in low- and middle-income countries. BMC Infect Dis. 2022;22(1):593. doi: 10.1186/s12879-022-07541-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Mee-Marquet N, Valentin AS. National report. Surveillance of infections associated with invasive devices. [cited 2023 Sep 25]. Available from: https://www.spiadi.fr/app/files/SPIADI2022%20-%20Rapport%20National%20provisoire%20 -%20juillet%202023.f1c6bbc6102c992806cb30ebe079ea8d.pdf).
- 29.Silva PV, Cruz RS, Keim LS, et al. . The antimicrobial susceptibility, biofilm formation and genotypic profiles of Staphylococcus haemolyticus from bloodstream infections. Mem Inst Oswaldo Cruz. 2013;108(6):812–813. doi: 10.1590/0074-0276108062013022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourlet T, Bouchara JP, Galinier JL, et al. REMIC V7. In : Société Française de Microbiologie, editor. Référentiel en Microbiologie Médicale, Paris; 2022.
- 31.EUCAST: Clinical Breakpoints and Dosing of Antibiotics . [cited 2022 Jun 1]. Available from: https://eucast.org/clinical_breakpoints/.
- 32.Magnan C, Ahmad-Mansour N, Pouget C, et al. . Phenotypic and genotypic virulence characterisation of Staphylococcus pettenkoferi strains isolated from human bloodstream and diabetic foot infections. Int J Mol Sci. 2022;23(24):15476. doi: 10.3390/ijms232415476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee I, Ouk Kim Y, Park SC, et al. . OrthoANI: an improved algorithm and software for calculating average nucleotide identity. Int J Syst Evol Microbiol. 2016;66(2):1100–1103. doi: 10.1099/ijsem.0.000760 [DOI] [PubMed] [Google Scholar]
- 34.Hyndman R, Athanasopoulos G, Bergmeir C, et al. Forecasting functions for time series and linear models. R package version 8.21.1.9000. [cited 2023 Aug 28]. Available from: https://pkg.robjhyndman.com/forecast/.
- 35.Manoharan M, Sistla S, Ray P.. Prevalence and molecular determinants of antimicrobial resistance in clinical isolates of Staphylococcus haemolyticus from India. Microb Drug Resist. 2021;27(4):501–508. doi: 10.1089/mdr.2019.0395 [DOI] [PubMed] [Google Scholar]
- 36.Noshak MA, Ahangarzadeh Rezaee M, Hasani A, et al. . Molecular detection and characterization of the Staphylococcus epidermidis and Staphylococcus haemolyticus isolated from hospitalized patients and healthcare workers in Iran. BioMed Res Int. 2023;2023:1–10. doi: 10.1155/2023/3775142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perdreau-Remington F, Stefanik D, Peters G, et al. . Methicillin-resistant Staphylococcus haemolyticus on the hands of health care workers: a route of transmission or a source? J Hosp Infect. 1995;31(3):195–203. doi: 10.1016/0195-6701(95)90066-7 [DOI] [PubMed] [Google Scholar]
- 38.Cavanagh JP, Wolden R, Heise P, et al. . Antimicrobial susceptibility and body site distribution of community isolates of coagulase-negative staphylococci. APMIS. 2016;124(11):973–978. doi: 10.1111/apm.12591 [DOI] [PubMed] [Google Scholar]
- 39.Bouchami O, Achour W, Mekni MA, et al. . Antibiotic resistance and molecular characterization of clinical isolates of methicillin-resistant coagulase-negative staphylococci isolated from bacteremic patients in oncohematology. Folia Microbiol (Praha). 2011;56(2):122–130. doi: 10.1007/s12223-011-0017-1 [DOI] [PubMed] [Google Scholar]
- 40.Bathavatchalam YD, Solaimalai D, Amladi A, et al. . Vancomycin heteroresistance in Staphylococcus haemolyticus : elusive phenotype. Future Sci OA. 2021;7(7):FSO710. doi: 10.2144/fsoa-2020-0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bakthavatchalam YD, Vasudevan K, Neeravi A, et al. . First draft genome sequence of linezolid and rifampicin resistant Staphylococcus haemolyticus. Jpn J Infect Dis. 2020;73(4):296–299. doi: 10.7883/yoken.JJID.2019.081 [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Lee GY, Lim JH, et al. . Species profiles and antimicrobial resistance of non-aureus staphylococci isolated from healthy broilers, farm environments, and farm workers. Food Sci Anim Resour. 2023;43(5):792–804. doi: 10.5851/kosfa.2023.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben Saida N, Marzouk M, Ferjeni A, et al. . A three-year surveillance of nosocomial infections by methicillin-resistant Staphylococcus haemolyticus in newborns reveals the disinfectant as a possible reservoir. Pathol Biol. 2009;57(3):e29–e35. doi: 10.1016/j.patbio.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Correa JE, De Paulis A, Predari S, et al. . First report of qacG, qacH and qacJ genes in Staphylococcus haemolyticus human clinical isolates. J Antimicrob Chemother. 2008;62(5):956–960. doi: 10.1093/jac/dkn327 [DOI] [PubMed] [Google Scholar]
- 45.Tong SYC, Holden MTG, Nickerson EK, et al. . Genome sequencing defines phylogeny and spread of methicillin-resistant Staphylococcus aureus in a high transmission setting. Genome Res. 2015;25(1):111–118. doi: 10.1101/gr.174730.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.French Society of Hospital Hygiene (SF2H) . Avis du 15 juillet 2022 Relatif aux bonnes pratiques de soins aux nouveau-nés pour prévenir les infections sur dispositifs invasifs et la transmission croisée en secteurs de soins de néonatologie (avis complété le 21 juillet 2022). 2022. [cited 2023 Sep 12]. Available from: https://www.cpias-ile-de-france.fr/docprocom/doc/sf2h-avis-bonnes-pratiques-neonat-150722.pdf.
- 47.Panda S, Jena S, Sharma S, et al. . Identification of novel sequence types among Staphylococcus haemolyticus isolated from variety of infections in India. PLoS One. 2016;11(11):e0166193. doi: 10.1371/journal.pone.0166193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regué M, Hita B, Piqué N, et al. . A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect Immun. 2004;72(1):54–61. doi: 10.1128/IAI.72.1.54-61.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagórska K, Ostrowski A, Hinc K, et al. . Importance of eps genes from Bacillus subtilis in biofilm formation and swarming. J Appl Genet. 2010;51(3):369–381. doi: 10.1007/BF03208867 [DOI] [PubMed] [Google Scholar]
- 50.Luke NR, Sauberan SL, Russo TA, et al. . Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun. 2010;78(5):2017–2023. doi: 10.1128/IAI.00016-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flahaut S, Vinogradov E, Kelley KA, et al. . Structural and biological characterization of a capsular polysaccharide produced by Staphylococcus haemolyticus. J Bacteriol. 2008;190(5):1649–1657. doi: 10.1128/JB.01648-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pain M, Hjerde E, Klingenberg C, et al. . Comparative genomic analysis of Staphylococcus haemolyticus reveals key to hospital adaptation and pathogenicity. Front Microbiol. 2019;10:2096. doi: 10.3389/fmicb.2019.02096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalantar-Neyestanaki D, Mansouri S, Tadjrobehkar O, et al. . The frequency of adherence, biofilm-associated, arginine catabolic mobile element genes, and biofilm formation in clinical and healthcare worker coagulase-negative staphylococci isolates. BMC Microbiol. 2023;23(1):222. doi: 10.1186/s12866-023-02959-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melchior MB, Van Osch MH, Lam TJ, et al. . Extended biofilm susceptibility assay for Staphylococcus aureus bovine mastitis isolates: evidence for association between genetic makeup and biofilm susceptibility. J Dairy Sci. 2011;94(12):5926–5937. doi: 10.3168/jds.2011-4243 [DOI] [PubMed] [Google Scholar]
- 55.Lawal OU, Fraqueza MJ, Worning P, et al. . Staphylococcus saprophyticus causing infections in humans is associated with high resistance to heavy metals. Antimicrob Agents Chemother. 2021;65(7):e02685–20. doi: 10.1128/AAC.02685-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mikhaylova Y, Shelenkov A, Chernyshkov A, et al. . Whole-genome analysis of Staphylococcus aureus isolates from ready-to-eat food in Russia. Foods. 2022;11(17):2574. doi: 10.3390/foods11172574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eltwisy HO, Twisy HO, Hafez MH, et al. . Clinical infections, antibiotic resistance, and pathogenesis of Staphylococcus haemolyticus. Microorganisms. 2022;10(6):1130. doi: 10.3390/microorganisms10061130 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genomes sequences mentioned in the present study are available on the NCBI GenBank database under the accession number PRJNA1010742.