Abstract
Six mutants (B1 to B6) that grew poorly in air on BG11 agar plates buffered at pH 8.0 were rescued after mutations were introduced into ndhB of wild-type (WT) Synechocystis sp. strain PCC 6803. In these mutants and a mutant (M55) lacking ndhB, CO2 uptake was much more strongly inhibited than HCO3− uptake, i.e., the activities of CO2 and HCO3− uptake in B1 were 9 and 85% of those in the WT, respectively. Most of the mutants grew very slowly or did not grow at all at pH 6.5 or 7.0 in air, and their ability to grow under these conditions was correlated with CO2 uptake capacity. Detailed studies of B1 and M55 indicated that the mutants grew as fast as the WT in liquid at pH 8.0 under air, although they grew poorly on agar plates. The contribution of CO2 uptake appears to be larger on solid medium. Five mutants were constructed by inactivating each of the five ndhD genes in Synechocystis sp. strain PCC 6803. The mutant lacking ndhD3 grew much more slowly than the WT at pH 6.5 under 50 ppm CO2, although other ndhD mutants grew like the WT under these conditions and showed low affinity for CO2 uptake. These results indicated the presence of multiple NAD(P)H dehydrogenase type I complexes with specific roles.
In cyanobacteria the type I NAD(P)H dehydrogenase complex (NDH-1) is a proton-pumping complex that has been shown to be involved in both the respiratory and photosynthetic electron transport chains (25). NDH-1 acts as a plastoquinone oxidoreductase with NADH or NADPH as a substrate. There is evidence that NDH-1 is located in the cytoplasmic membrane as well as the thylakoid membrane, but evidence for location in the cytoplasmic membrane has not been consistent. NDH-1 is composed of 12 recognized subunits, with the large, hydrophobic NdhB, NdhD, and NdhF subunits being core membrane components. The availability of a whole-genome database for Synechocystis sp. strain PCC 6803 has shown that most ndh genes are present as single copies; however, ndhD and ndhF are present as multiple copies with five and four members, respectively (note that NdhF4 has homology with NdhD5 and could be counted as an NdhD homolog). There is a marked degree of protein sequence divergence within the NdhD and NdhF families, and this has led to suggestions that several NDH-1 complexes may exist in cyanobacteria, each with different NdhD and/or NdhF subunits and with each potential complex having differing functions (21, 24). One main role for NDH-1 in the thylakoid membrane is to participate in cyclic electron flow around photosystem I and to pump protons into the lumen, thereby contributing to ΔpH-driven ATP generation at the expense of NADPH (11–13). However, other roles for NDH-1 are possible.
It has been demonstrated that NDH-1 is essential for inorganic carbon (Ci) transport in cyanobacteria (4, 5, 10, 16–18, 21). Inactivation of ndhB or ndhL in Synechocystis sp. strain PCC 6803 greatly reduced the activities of CO2 and HCO3− uptake, and in the past it has been assumed that the NDH-1-dependent cyclic electron transport supplies ATP to drive the Ci uptake (16–18). However, the question of why ATP produced by linear electron transport in these mutants does not drive the Ci transport, remains unresolved, and it was not certain whether ATP energizes the uptake of CO2 and HCO3−. The finding that the cmp operon encodes an ATP binding cassette transporter for HCO3− clearly indicates the presence of at least one type of ATP-dependent HCO3− transporter (22). Li and Canvin (8) reported that HCO3− uptake is supported by linear electron transport while CO2 uptake is supported by cyclic electron transport, based on the observation of differential effects of electron transport inhibitors and acceptors on uptake of the two carbon species. The results suggested that ATP produced by noncyclic electron transport energized HCO3− transport. Although the involvement of NDH-1 in CO2 uptake is clearly demonstrated, little is known about the mechanism of CO2 uptake and how it is energized.
Some ndh mutants, such as M55 (ndhB), show major inhibition of both CO2 and HCO3− uptake (16–18), whereas other mutants, such K22 and A41 (ndhD3), show an effect largely on high-affinity CO2 uptake (5). However, the previously described M55 mutant of Synechocystis sp. strain PCC 6803 is a highly disruptive insertional mutant of the single-copy ndhB gene (16–18), and it could be argued that removal of the NdhB protein could cause nonassembly of all NDH-1 complexes and production of secondary phenotypes. A less disruptive approach is to mutagenize ndhB with multiple single-base mutations and to select for mutants where the NdhD protein is still capable of assembly but lacks most functionality. To help understand the role of NDH-1 in Ci transport, we investigated whether random-site mutations within the ndhB gene of Synechocystis sp. strain PCC 6803 inhibit the uptake of CO2 and HCO3− to the same extent. We show in this paper by measurement of CO2 and HCO3− uptake in these mutants that CO2 uptake is much more strongly inhibited than HCO3− uptake, a result more consistent with the effect of ndhD3 mutations in Synechococcus sp. strain PCC 7002.
Synechocystis sp. strain PCC 6803 possesses five ndhD genes (3), with sll1733 (designated ndhD3) being homologous to ndhD3 in Synechococcus sp. strain PCC 7002. We describe in this paper the growth characteristics of mutants constructed by knocking out each of the ndhD genes in Synechocystis and show that ndhD3 has a specific role in inducing high-affinity CO2 uptake. Another purpose of the present work was to make some comparisons among the suite of ndhD mutants, as well as existing mutants such as M55 and new multiple-point mutants of ndhB, and attempt to provide a consensus view of the role of NDH-1 in Ci uptake in cyanobacteria.
MATERIALS AND METHODS
Growth conditions.
WT and mutant cells of Synechocystis sp. strain PCC 6803 were grown at 30°C in BG11 medium (26) buffered with 20 mM N-Tris(hydroxymethyl)methyl-2-amino-ethanesulfonic acid (TES)-KOH (pH 8.0 and 7.0) or 2-(N-morpholino)ethanesulfonic acid (MES)-KOH (pH 6.5) and aeration with 3% (vol/vol) CO2 in air or with air. The solid medium was BG11 supplemented with 1.5% agar, 5 mM sodium thiosulfate, and 20 mM the same buffer. When air containing 50 or 20 ppm CO2 was used, the air was passed through Wako lime (Wako Co., Tokyo, Japan) packed in a metal tube (5-cm diameter and 20-cm length) at a flow rate of approximately 200 ml/min. Continuous illumination was provided by fluorescent lamps generating photosynthetically active radiation of 60 μmol of photons/m2/s.
Construction of mutants.
A clone, pUCEE5.1, derived from pUCEK11.8 (16), was used for random mutagenesis of ndhB. A kanamycin resistance cartridge (Kmr) cassette was inserted at the BglII site downstream of ndhB. Insertion of the cassette at this site did not change the phenotype of WT Synechocystis cells transformed with this construct (16). A fragment of 606 bp between the BamHI and SpeI sites was removed from the ndhB gene, and fragments containing the same DNA region were synthesized by a PCR method that introduces random mutations (7) and then reinserted between the two restriction sites. The sequences upstream of the BamHI site and downstream of the SpeI site were used to design the PCR primers, and pUCEE5.1 was used as a template. A library thus constructed, containing various mutated ndhB genes, was used to transform the WT cells of Synechocystis sp. strain PCC 6803 into Kmr mutants, using the protocol of Williams and Szalay (29). The transformants were spread on agar plates containing BG11 medium and kanamycin (10 μg/ml) buffered at pH 8.0, and the plates were incubated in air. Eight mutants were rescued as colonies that grew slowly under air. The mutated ndhB in the transformants was segregated to homogeneity (by successive-streak purification) as determined by PCR amplification. The ndhB gene in each mutant was amplified by PCR and used as a DNA-sequencing template.
M55 is the mutant constructed by inserting a Kmr cassette at the BamHI site in ndhB, as described previously (16).
The slr0331 (ndhD1), slr1291 (ndhD2), sll1733 (ndhD3), sll0027 (ndhD4), slr2007 (ndhD5), sll1732 (ndhF3), and sll1734 genes of Synechocystis sp. strain PCC 6803 (3) were amplified by PCR and then cloned into the pGEM-T vector (Promega, Madison, Wis.). The 801-bp BalI/BalI fragment in ndhD1, the 1,049-bp BalI/BalI fragment in ndhD2, the 51-bp EcoRV/KpnI fragment in ndhD3, the 1,029-bp BalI/BalI fragment in ndhD4, the 878-bp NheI/StuI fragment in ndhD5, the 336-bp EcoRI/PstI fragment in ndhF3, and the 45-bp EcoRI/PstI fragment in sll1734 were replaced with cassettes that confer resistance to spectinomycin (Spr) for ndhD1 mutants, chloramphenicol (Cmr) for ndhD2 mutants, kanamycin (Kmr) for ndhD3, ndhD4, ndhF3, and sll1734 mutants, and hygromycin (Hygr) for ndhD5 mutants. These cassettes were inserted in parallel or antiparallel to the direction of the genes. The constructs were used to transform the WT cells of Synechocystis sp. strain PCC 6803 to generate the ndhD1, ndhD2, ndhD3, ndhD4, ndhD5, ndhF3, and sll1734 mutants. The segregation of inactivated genes in each mutant was determined by the method described above.
Silicone oil-filtering centrifugation.
The uptake of 14CO2 and H14CO3− was measured in high-CO2-grown cells (aerated with air for 18 h in the light) using the silicone oil-filtering centrifugation method (28). The cells were suspended in BG11 medium buffered with 20 mM TES-KOH (pH 8.0) at a chlorophyll (Chl) concentration of 20 μg/ml. Ci uptake was initiated by the addition of 14CO2 or H14CO3− in the light and terminated by centrifugation.
Determination of growth characteristics.
WT and mutant strains grown under 3% CO2 were collected and resuspended in fresh BG11 medium to optical densities at 730 nm (OD730s) of 1.0, 0.1, and 0.01. Two microliters of the cell suspensions was spotted onto BG11 agar plates buffered at various pHs. The plates were incubated under 3% (vol/vol) CO2 in air or under air for 5 days with continuous illumination by fluorescent lamps at a photosynthetically active radiation intensity of 60 μmol of photons/m2/s. The OD730 was measured with a recording spectrophotometer, model UV2200 (Shimadzu Co., Kyoto, Japan).
Electrophoresis and Western analysis.
An antibody was raised against partial NdhB fused to glutathione-S-transferase (GST). The ndhB gene amplified by PCR was digested with MvoI and MbaI, and a fragment of 226 bp (from base 419 to 644 as numbered from the initiation codon of ndhB) was excised from a gel after electrophoresis of the digest. The fragment was blunted and ligated to the SmaI site of pUC119. The insert DNA was excised with EcoRI and BamHI and was ligated to pGEX-3X (Pharmacia, Uppsala, Sweden). The construct was used to transform Escherichia coli. GST-NdhB (partial) formed inclusion bodies, which were isolated, solubilized with 5% sodium dodecyl sulfate (SDS), and electrophoresed by SDS-polyacrylamide gel electrophoresis. A prominent band of GST-NdhB (partial) at 33 kDa was cut out from the gels and mashed with a pestle and mortar to be injected into rabbits.
Total membrane fractions were prepared from the WT and mutant cells as described by Nilsson et al. (15). SDS-polyacrylamide gel electrophoresis was performed in the system of Laemmli (6). Polypeptides were electrotransferred to nitrocellulose membrane and reacted with the antibody against GST-NdhB (partial). Goat anti-rabbit immunoglobulin G conjugated to peroxidase was used as the second antibody, and reacting bands were detected with an ECL kit (Amersham).
RT-PCR analysis.
RNA from air-grown Synechocystis cells was extracted by the method of Aiba et al. (1) and then subjected to reverse transcriptase (RT) PCR (14). Primers specific to the 3′ ends of ndhF3, ndhD3, sll1734, and sll1735, respectively, were used for the RT reaction. The subsequent PCR was performed with a pair of primers specific to ndhF3.
Mass spectrometric measurements.
Measurements of the initial CO2 uptake rate and kinetics for CO2 and HCO3− uptake under steady-state conditions were performed according to the method of Badger et al. (2).
Other methods.
Unless otherwise stated, standard techniques were used for DNA manipulation (9). Pigments in the cells were extracted in methanol, and the Chl concentration in the extract was determined (20).
RESULTS
Isolation of ndhB mutants and sites of mutations.
Eight mutants that grew poorly in air on agar plates buffered at pH 8.0 were rescued after mutations were introduced into ndhB of WT Synechocystis sp. strain PCC 6803 by low-fidelity PCR (7). Sequencing of impaired ndhB genes in these mutants (designated B1 to B8) revealed that B7 and B8 were identical to B1. Amino acid residues substituted in the B1 to B6 mutants are summarized in Table 1. There were substitutions of 2 to 8 amino acid residues in each of the ndhB mutants.
TABLE 1.
Substitution of amino-acid residues in NdhB of the B1-B6 mutants
| Mutant | Substitution of amino acid residuesa |
|---|---|
| B1 | 76N/Y, 104Q/R, 114I/V, 148Y/N, 149L/P, 151T/A, 197L/F, 241V/A |
| B2 | 104Q/R, 114I/V, 148Y/N, 149L/P, 151T/A, 197L/F, 241V/A |
| B3 | 76N/Y, 104Q/R, 114I/V, 161N/S, 166K/E, 193T/A |
| B4 | 148Y/N, 149L/P, 151T/A, 197L/F, 241V/A |
| B5 | 154M/T, 166K/E, 170I/V, 188L/S, 195L/M, 229I/V |
| B6 | 148Y/N, 166K/E |
Amino acid residues are expressed as letters. The amino acids were numbered from the N-terminal methionine residue. The letters with the numbers show the amino acids in the WT, and those after the shill indicate the residues in the mutants.
CO2 and HCO3− uptake in the ndhB mutants.
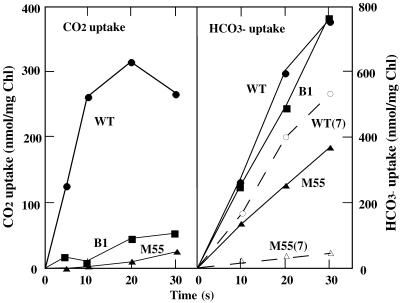
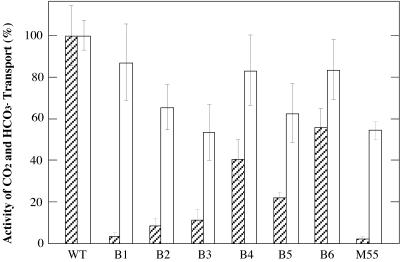
Figure 1 shows the time courses of CO2 uptake (left panel) and HCO3− uptake (right panel) for WT, B1, and M55 cells grown at pH 8.0 under 3% CO2 and then transferred to aeration with air for 18 h in the light. The activity of CO2 uptake was very low in the B1 and M55 mutants, but the activity of HCO3− uptake in B1 was as high as that in the WT, whereas M55 possessed half the WT activity. High-CO2-grown cells of these mutants also showed very low activity of CO2 uptake, indicating that the low activity is not a result of aeration with air in the light (data not shown). Thus, CO2 uptake was inhibited preferentially in these mutants. This was confirmed by the results summarized in Fig. 2, where the activities of CO2 and HCO3− uptake in the ndhB mutants are shown as percentages of the WT activities. Although the extent of inhibition varied, CO2 uptake was more strongly inhibited than HCO3− uptake in all the mutants examined. Thus, CO2 uptake is strictly dependent on NDH-1 whereas HCO3− uptake proceeds even in the absence of this enzyme. However, the result was that HCO3− uptake was partly inhibited in the mutants, although the extent of inhibition was at most 50%, even in M55. This suggests that the low activity of HCO3− transport is a secondary effect caused by the absence of NDH-1 or that NDH-1 has a supplemental role in the transport of HCO3−.
FIG. 1.
Time courses of uptake of 14CO2 (left panel) and H14CO3− (right panel) into WT (circles), B1 (squares), and M55 (triangles) cells, measured by the silicone oil-filtering centrifugation method. The cells were grown under 3% CO2 and then aerated with air for 18 h in the light at pH 8.0 (solid curves) or pH 7.0 (dashed curves). The cells were suspended in BG11 medium containing 15 mM NaCl buffered with 20 mM HEPES-KOH, pH 8.0. The concentrations of 14CO2 and H14CO3− were 7.9 and 61 μM, respectively.
FIG. 2.
Amount of Ci taken up by the mutant cells during incubation with 14CO2 (hatched bars) for 10 s or with H14CO3− (open bars) for 20 s in the light. Each value is shown as a percentage of the value obtained for WT cells (259 nmol of CO2/mg of Chl per 10 s and 473 nmol of HCO3−/mg of Chl per 20 s), and the error bars indicate standard deviations (n = 3). Cells grown at pH 8.0 were suspended in the same medium as for Fig. 1.
The time courses of HCO3− uptake in WT and M55 cells grown initially at pH 7.0 under 3% CO2 and then transferred to aeration with air are shown in Fig. 1. The result indicated that an increase in HCO3− uptake activity was not induced in M55 cells at this pH.
Growth characteristics of the ndhB mutants.
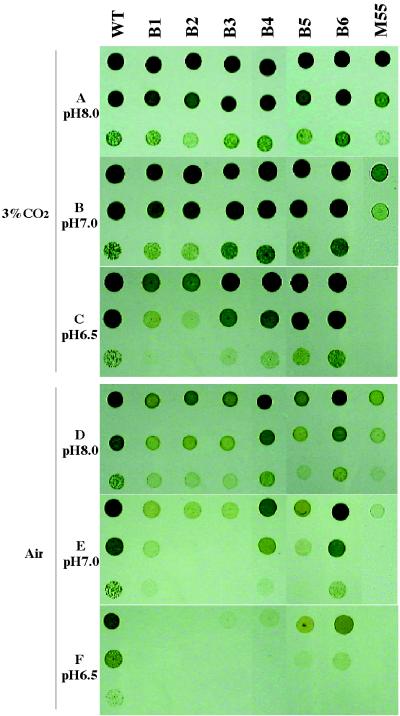
To explore how the inhibition of CO2 and HCO3− uptake affects the growth characteristics of the cells, growth of the mutant strains was examined on solid BG11 medium buffered at various pHs under 3% CO2 or air. At pHs 8.0 and 7.0 under 3% CO2, all the mutants except M55 grew as fast as the WT (Fig. 3A and B). M55 grew more slowly than the WT at pH 8.0 and was unable to grow at pH 6.5 under the high-CO2 conditions (Fig. 3A to C). The B1 and B2 mutants grew more slowly at pH 6.5 in 3% CO2 (Fig. 3C). All the mutants except B4 and B6 grew more slowly than the WT even at pH 8.0 under air (Fig. 3D) and very slowly at pH 7.0 (Fig. 3E). Most of the mutants were unable to grow at pH 6.5, and the growth of B6 was relatively poor under these conditions (Fig. 3F). The ability of the mutants to grow under atmospheric levels of CO2, especially at pH 7.0 or 6.5, was correlated with their activity for CO2 uptake (Fig. 2 and 3).
FIG. 3.
Effects of pH and CO2 concentration on the growth of the WT and mutants on agar plates. The WT and ndhB mutant cells of Synechocystis were pelleted by centrifugation and resuspended in BG11 medium, pH 8.0, 7.0, or 6.5. Two microliters of the cell suspensions, OD730 values of 1.0 (upper row of each panel), 0.1 (middle row), and 0.01 (lower row), were spotted on agar plates containing BG11 medium buffered at pH 8.0, pH 7.0, and pH 6.5. The plates were incubated under 3% (vol/vol) CO2 in air (A to C) or under air (D to F) for 5 days at 60 μmol of photons of photosynthetically active radiation/m2s−1.
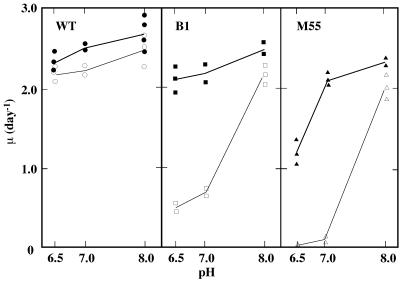
Figure 4 shows the growth rates of the WT, B1, and M55 cells in liquid medium plotted as a function of pH. There was not much effect of pH on growth rates of WT cells between pHs 8.0 and 6.5, either at 3% CO2 or in air (left panel). In contrast, the growth of B1 and M55 in air was strongly dependent on the pH of the medium. Under air, both mutants grew as fast as the WT at pH 8.0, but the growth of B1 was very poor and M55 was unable to grow at pH 7.0 or 6.5 (middle and right panels). These results indicate that, in liquid, inorganic carbon is predominantly supplied by CO2 uptake in WT cells in neutral- or acidic-pH media gassed with atmospheric levels of CO2. The high growth rates of the mutants at pH 8.0 in liquid medium in air, in spite of their slow growth on agar plates under the same pH and CO2 level, suggest that the contribution of HCO3− transport may be larger for growth in liquid medium than for growth on the surfaces of agar plates. The B1 mutant grew as fast as the WT under 3% CO2 for the pH range between 8.0 and 6.5 (Fig. 4, middle panel). There was a large drop in the growth rate of M55 at pH 6.5 even under 3% CO2 (right panel). It appears that NDH-1 has a role in the growth of cells at acidic pHs.
FIG. 4.
Effect of pH on growth rates of WT, B1, and M55 in liquid BG11 medium during aeration with 3% (vol/vol) CO2 in air (closed symbols) or under air (open symbols). Growth rates are shown by the μ values. Doubling time (in days), 0.693/μ.
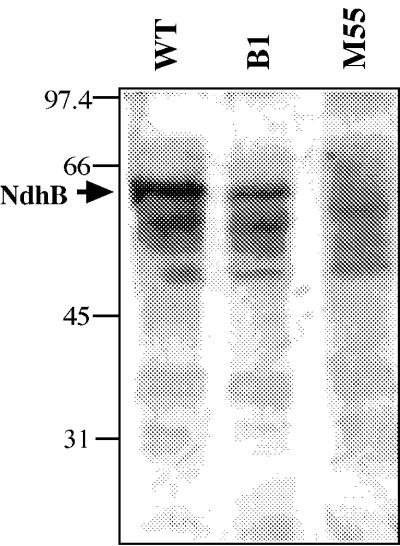
Identification of NdhB.
The antibody raised against GST-NdhB (partial) cross-reacted with several bands in the total cell membranes of the WT but most strongly with the band at 55 kDa, the molecular mass of NdhB estimated from the DNA database translation (Fig. 5, left lane). No cross-reacting band was present at 55 kDa in the membranes of M55 (right lane), indicating that the cross-reacting band in the WT is NdhB. Western analysis with the total cell membranes of B1 using the same antibody gave a band at 55 kDa (Fig. 5, middle lane). The density of the band in B1 membranes was lower than that in the WT membrane. The results indicated that NDH-1 is present in the B1 mutant, although the protein may not be fully functional.
FIG. 5.
Immunoblot of NdhB in the total cell membranes of Synechocystis sp. strain PCC 6803 WT, B1, and M55. Samples (10 μg of proteins) were solubilized at room temperature and boiled for several minutes and were run in a 10% gel. The antibody against GST-NdhB (partial) was used for immunoblotting.
Growth of ndhD mutants under limiting CO2 conditions.
ndhD1, ndhD2, ndhD3, ndhD4, and ndhD5 mutants grew as fast as the WT at pH 7.0 in air (data not shown). Measurement of the growth rates of these mutants at pH 6.5 and 50 ppm CO2 revealed that the ndhD3 mutant grew at about a third of the rate of WT cells, although the rest of the ndhD mutants showed virtually the same growth rate as the WT (Table 2). ndhF3 and sll1734 mutants constructed by inactivating the genes upstream and downstream of ndhD3, respectively, showed the same growth characteristics as the ndhD3 mutant at pH 6.5 and 50 ppm CO2, but inactivation of sll1735 downstream of sll1734 did not have a significant effect on the growth rate under these conditions. These results suggested that ndhD3, ndhF3, and sll1734 have a common and specific effect on the growth of cells under limiting CO2 conditions.
TABLE 2.
Growth rates of WT and mutants of Synechocystis sp. strain PCC 6803a
| Cells | μ (day−1) |
|---|---|
| WT | 1.06 ± 0.15 |
| ndhD1 | 1.12 ± 0.06 |
| ndhD2 | 1.05 ± 0.02 |
| ndhD3 (sll1733) | 0.35 ± 0.03 |
| ndhD4 | 0.95 ± 0.20 |
| ndhD5 | 0.90 ± 0.11 |
| ndhF3 (sll1732) | 0.36 ± 0.03 |
| sll1734 | 0.37 ± 0.02 |
| sll1735 | 0.90 ± 0.21 |
WT and mutant cells were grown at 30°C in BG11 medium with 20 mM MES-KOH (pH 6.5) under aeration with 50 ppm CO2 at a light intensity of 60 μmol PAR/m2/s. Specific growth rates (μ) are expressed per day with standard deviations (n = 3). To convert the growth rates to doublings per day, divide ln 2(0.693) by μ.
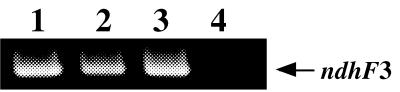
Expression of ndhD3 and neighboring genes.
The products of RT reactions with primers specific to the 3′ ends of ndhF3, ndhD3, sll1734, and sll1735 were used as templates for the subsequent PCR with a set of primers specific to ndhF3. The PCR with three different templates gave the same products (Fig. 6, lanes 1 to 3). This indicated that ndhF3, ndhD3, and sll1734 are expressed together as an operon. No PCR product was found when a primer specific for sll1735 was used for the RT reaction (lane 4), indicating that the gene is expressed independently of the upstream genes.
FIG. 6.
RT-PCR analysis of total RNA from Synechocystis cells showing the expression of ndhF3, ndhD3, and sll1734 as an operon. RNA was prepared from cells grown at pH 8.0 under 50 ppm CO2. PCR amplification was performed with a set of primers specific to ndhF3 (ATTATCTGGCTAGTACC and GAATAGCTAAGAAAGGC), and the product was 1.8 kbp. The templates used for PCR were cDNAs which were synthesized by reverse transcription of mRNA by addition of RT with primers specific to ndhF3 (lane 1), ndhD3 (lane 2), sll1734 (lane 3), and sll1735 (lane 4), respectively.
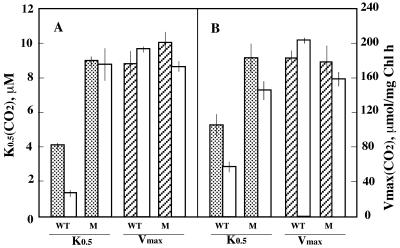
Initial and steady-state rates of CO2 uptake.
Cyanobacteria exhibit a transient and rapid uptake of CO2 following a dark-to-light transition. The CO2 uptake during steady-state photosynthesis and the relative affinity for gross CO2 uptake can be measured and calculated according to a method developed by Badger et al. (2). Using this method, the steady-state rate and initial rate of CO2 uptake were measured and calculated under various Ci concentrations, allowing K0.5(CO2), the concentration of CO2 required to reach the half-maximal uptake rate, to be determined. Figure 7 shows the K0.5(CO2) and Vmax values for the steady-state (Fig. 7A) and initial (Fig. 7B) rates of CO2 uptake obtained for the WT and ndhD3 mutant cells grown at 2% CO2 or induced overnight at 20 ppm CO2. There was no significant difference between the Vmax values of the WT and mutant cells. In contrast, the K0.5(CO2) values for initial and steady-state CO2 uptake were significantly higher in the mutant than in the WT, in cells both before and after 18 h of induction at 20 ppm CO2. The affinity of steady-state CO2 uptake for CO2 did not change in the mutant after induction at 20 ppm CO2, while the affinity increased about 2.5-fold in the WT after induction. Thus, ndhD3 appears to be essential for the induction of high-affinity CO2 uptake. In Synechocystis sp. strain PCC 6803, the Vmax of net CO2 uptake, upon illumination, was similar to the steady-state rate. The activation of initial CO2 uptake may not be as fast in this strain as in other cyanobacteria studied (e.g., Synechococcus sp. strain PCC 7942), leading to similar initial and gross steady-state rates.
FIG. 7.
The K0.5(CO2) and Vmax values for steady-state (A) and initial (B) CO2 uptake by the WT and ndhD3 mutant (M) strains of Synechocystis sp. strain PCC 6803 grown under 2% CO2 (shaded and hatched bars) and after 18 h of induction under 20 ppm CO2 (open bars). Each bar shows the average value of the results of four measurements, and the error bars indicate standard deviations.
DISCUSSION
As demonstrated previously (16), CO2 uptake was abolished in the mutant (M55) of Synechocystis sp. strain PCC 6803 that lacked ndhB. CO2 uptake was also inhibited in other ndhB mutants (B1 to B6 [Fig. 2]) that had been generated by random-site mutagenesis. However, in contrast to the previous observation with M55, inhibition of HCO3− uptake was not significant in these mutants and was at most 50%, even after complete inactivation of ndhB in M55 (Fig. 1 and 2). In other words, careful analysis of M55 indicates that inhibition of CO2 uptake is the major effect and that inhibition of HCO3− is a minor effect. In the less disruptive B1 to B6 mutants, the difference is more specifically related to CO2 uptake, indicating that the complete loss of the NdhB protein in M55, and potential loss of all NDH-1 complexes, may lead to secondary effects on HCO3− uptake. Moreover, mutants B1 and M55 grew as fast as the WT in liquid medium adjusted to pH 8.0 and gassed with air levels of CO2 (Fig. 4), unlike the previous observation that M55 was unable to grow under these conditions (16). Since WT cells grew at pH 7.0 as fast as at pH 8.0, we did not pay much attention to the pH of the medium for growth of M55 in previous studies, and a medium of pH 7.0 was frequently used. The discrepancy between the present and previous observations possibly arose from a mistake in growing the M55 cells at pH 7.0. To test this possibility, we grew M55 at pH 7.0 under 3% CO2 and then aerated with air overnight at the same pH. These M55 cells did not show HCO3− transport activity (Fig. 1), in contrast to the result obtained with the mutant cells grown and induced at pH 8.0 (Fig. 1 and 2). Thus, the slow growth or nongrowth of M55 under air reported in previous studies appears to be due to a failure to present the mutant with the more favorable conditions of pH 8.0 medium. In contrast, the B1 mutant grew almost as well as the WT at pHs 6.5, 7.0, and 8.0 under high-CO2 conditions (Fig. 4).
The mutation of ndhB had much less effect on HCO3− uptake than on CO2 uptake (Fig. 1 and 2), consistent with the observation that HCO3− transport appears to be driven by linear electron transport (8). The activity of HCO3− uptake in M55 was much lower than the WT rate (Fig. 1 and 2). This could be due to secondary effects of stress caused by exposing the cells to light under low-CO2 conditions in the absence of CO2 uptake. The possibility that NDH-1 has a secondary role in HCO3− transport in supplying additional ATP by an NDH-1-dependent cyclic electron transport around photosystem I cannot be ruled out (11–13). The CO2 uptake reaction is postulated to be an energy-dependent unidirectional conversion of CO2 to HCO3− (reviewed in reference 4). However, this reaction may be ATP independent, since CO2 uptake is not operational in the presence of linear electron transport alone (8).
The inability of the ndhB mutants to grow at pH 7.0 or 6.5 under atmospheric levels of CO2 correlated with their low capacities for CO2 uptake (Fig. 2 and 3). Thus, the inorganic carbon source is mainly supplied by CO2 uptake under these conditions. These mutants grew as fast as the WT in liquid medium at pH 8.0 under air, which indicated that at this pH, HCO3− transport was a sufficient carbon source for the cells. On agar plates, the mutant colonies grew more slowly than the WT at pH 8.0 under air.
The presence of five ndhD genes in the Synechocystis sp. strain PCC 6803 genome (3) and their differential expression under different CO2 concentrations (21) suggested that there are multiple types of NDH-1 complexes with different functions. In this series of mutants, the ndhD3 mutant was the only one that displayed the phenotype of slow growth at limiting CO2 (i.e., 50 ppm CO2) and reduced affinity for CO2 uptake, whereas the other mutants lacking ndhD (ndhD1, ndhD2, ndhD4, and ndhD5 mutants) did not show this mutant phenotype (Table 2 and Fig. 7). The K0.5 value for CO2 uptake was higher in the ndhD3 mutant than in the WT (Fig. 7), which may explain the low growth rate of ndhD3 at pH 6.5 and 50 ppm CO2 (Table 2). Similar results have recently been reported with a mutant of Synechococcus sp. strain PCC 7002 in which the ndhD3 gene was inactivated (5). None of the ndhD mutants showed the accentuated phenotype of M55 (i.e., complete loss of CO2 uptake activity). Recently, we found that an ndhD3-ndhD4 double mutant did not show any CO2 uptake activity, although the mutant grew as fast as the WT under photoheterotrophic conditions at atmospheric levels of CO2 (unpublished results). It appears evident that there are functionally distinct multiple NDH-1 complexes and that an NDH-1 complex having ndhD3 or ndhD4 as a subunit is essential to CO2 uptake. The exact function of this NDH-1 complex in CO2 uptake is not known and is being explored.
The information presented in this paper, however, does allow for limited speculation on how a particular type of NDH-1 complex might be specifically involved in CO2 uptake but not HCO3− uptake. One previous model for CO2 uptake in cyanobacteria suggested that a vectorial carbonic-anhydrase-like moiety located within a notional plasma membrane-based transporter could function in the transport of CO2 by providing a localized source of hydroxyl ions to drive the conversion of CO2 to HCO3− followed by release of HCO3− on the cytosolic face of the transporter (23). In this model, the CO2 transporter would operate as a type of active, facilitated diffusion and the necessary OH− ions were envisaged to be supplied by some component of the respiratory electron transport chain. A new speculative model has arisen (4) that postulates that a type of facilitated diffusion could operate at the level of the thylakoid and result in active accumulation of HCO3−. Here, a vectorial carbonic-anhydrase-like reaction could be closely associated with an NDH-1 complex such that OH− ions might be produced in a “localized pocket” and used to drive the conversion of CO2 to HCO3−. In this model, initial entry of CO2 into the cell would be passive, whereas direct HCO3− uptake would occur via separate transporters, such as the cmp transporter, BCT1 (22). The results of this paper are consistent with this new model in that elimination or mutation of all potential NDH-1 complexes (i.e., ndhB modification) or modification of specific subcomplexes containing NdhD3 or NdhD4 result in partial or complete loss of CO2 uptake capacity without major effects on HCO3− uptake. The recent finding that the NdhB protein is missing from highly purified cytoplasmic membranes of WT cells (results not shown) but is present in thylakoid preparations (Fig. 5) is also consistent with this new thylakoid-based model for CO2 uptake.
ACKNOWLEDGMENTS
This study was supported by the grants for Research for the Future Program (JSPS-RFTF97R16001) and the Human Frontier Science Program to T.O. and by RSBS core funding to G.D.P. and M.R.B.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Badger M R, Palmqvist K, Yu J W. Measurement of CO2 and HCO3− fluxes in cyanobacteria and microalgae during steady-state photosynthesis. Plant Physiol. 1994;90:529–536. [Google Scholar]
- 3.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan A, Reinhold L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:539–570. doi: 10.1146/annurev.arplant.50.1.539. [DOI] [PubMed] [Google Scholar]
- 5.Klughammer B, Sültemeyer D, Badger M R, Price G D. The involvement of NAD(P)H dehydrogenase subunits NdhD3 and NdhF3 in high affinity CO2 uptake in Synechococcus sp. PCC7002 gives evidence for multiple complexes with specific roles in cyanobacteria. Mol Microbiol. 1999;32:1316–1332. doi: 10.1046/j.1365-2958.1999.01457.x. [DOI] [PubMed] [Google Scholar]
- 6.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 7.Leung D W, Chen E, Goeddel D V. J. Methods Cell Mol Biol. 1989;1:11–15. [Google Scholar]
- 8.Li Q, Canvin D T. Energy sources for HCO3− and CO2 transport in air-grown cells of Synechococcus UTEX 625. Plant Physiol. 1998;116:1125–1132. doi: 10.1104/pp.116.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 10.Marco M N, Ohad I, Schwarz R, Lieman-Hurwitz J, Gabay C, Kaplan A. High CO2 concentration alleviates the block in photosynthetic electron transport in an ndhB-inactivated mutant of Synechococcus sp. PCC7942. Plant Physiol. 1993;101:1047–1053. doi: 10.1104/pp.101.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mi H, Endo T, Schreiber U, Ogawa T, Asada K. Electron donation from cyclic and respiratory flow to the phytosynthetic intersystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis PCC6803. Plant Cell Physiol. 1992;33:1233–1238. [Google Scholar]
- 12.Mi H, Endo T, Schreiber U, Ogawa T, Asada K. NAD(P)H dehydrogenase-dependent cyclic electron flow around photosystem I in the cyanobacterium Synechocystis PCC6803: a study of dark-starved cells and spheroplasts. Plant Cell Physiol. 1994;35:163–173. [Google Scholar]
- 13.Mi H, Endo T, Ogawa T, Asada K. Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediated cyclic electron transport in the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 1995;36:661–668. [Google Scholar]
- 14.Mullis K B, Ferre' F, Gibbs R A. The polymerase chain reaction. Boston, Mass: Birkhauser; 1994. pp. 97–109. [Google Scholar]
- 15.Nilsson F, Simpson D J, Jansson C, Andersson B. Ultrastructural and biochemical characterization of a Synechocystis 6803 mutant with inactivated psbA genes. Arch Biochem Biophys. 1992;295:340–347. doi: 10.1016/0003-9861(92)90526-3. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T. A gene homologous to the subunit-2 gene of NADH dehydrogenase is essential to inorganic carbon of Synechocystis PCC6803. Proc Natl Acad Sci USA. 1991;88:4275–4279. doi: 10.1073/pnas.88.10.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T. Cloning and inactivation of a gene essential to inorganic carbon transport of Synechocystis PCC6803. Plant Physiol. 1991;96:280–284. doi: 10.1104/pp.96.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa T. Identification and characterization of the ictA/ndhL gene product essential to inorganic carbon transport of Synechocystis PCC6803. Plant Physiol. 1992;99:1604–1608. doi: 10.1104/pp.99.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa T, Miyano A, Inoue Y. Photosystem-I-driven inorganic carbon transport in the cyanobacterium, Anacystis nidulans. Biochim Biophys Acta. 1985;808:77–84. [Google Scholar]
- 20.Ogawa T, Shibata K. A sensitive method for determining chlorophyll b in plant extracts. Photochem Photobiol. 1965;4:193–200. [Google Scholar]
- 21.Ohkawa H, Sonoda M, Katoh H, Ogawa T. The use of mutants in the analysis of the CO2-concentrating mechanism in cyanobacteria. Can J Bot. 1998;76:1035–1042. [Google Scholar]
- 22.Omata T, Price G D, Badger M R, Okamura M, Gohta S, Ogawa T. Identification of an ABC-type bicarbonate transporter of the cyanobacterium Synechococcus sp. strain PCC 7942. Proc Natl Acad Sci USA. 1999;96:13571–13576. doi: 10.1073/pnas.96.23.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price G D, Badger M R. Ethoxyzolamide inhibition of CO2 uptake in the cyanobacterium Synechococcus PCC7942 without apparent inhibition of internal carbonic anhydorase activity. Plant Physiol. 1989;89:37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price G D, Sültemeyer D, Klughammer B, Ludwig M, Badger M R. The functioning of the CO2 concentrating mechanism in several cyanobacteria strains: a review of general physiological characteristics, genes, proteins, and recent advances. Can J Bot. 1998;76:973–1002. [Google Scholar]
- 25.Schmetterer G. Cyanobacterial respiration. In: Bryant D, editor. The molecular biology of the cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 409–435. [Google Scholar]
- 26.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sültemeyer D, Klughammer B, Ludwig M, Badger M R, Price G D. Random insertional mutagenesis used in the generation of mutants of the marine cyanobacterium Synechococcus sp. strain PCC7002 with an impaired CO2 concentrating mechanism. Aust J Plant Physiol. 1997;24:317–327. [Google Scholar]
- 28.Volokita M, Zenvirth D, Kaplan A, Reinhold L. Nature of the inorganic carbon species actively taken up by the cyanobacterium Anabaena variabilis. Plant Physiol. 1984;76:599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams J G K, Szalay A A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene. 1983;24:37–51. doi: 10.1016/0378-1119(83)90129-4. [DOI] [PubMed] [Google Scholar]