Abstract
Overview:
Dirofilaria immitis and Dirofilaria repens are the most important filarial worms, causing heartworm disease and subcutaneous dirofilariosis, respectively. D repens is currently considered an emerging zoonotic agent in Europe.
Life cycle and infection:
Filarial worms infect mainly dogs, but also cats, ferrets, wild carnivores and humans. The life cycle involves an intermediate mosquito host. Compared with dogs, cats are imperfect hosts for dirofilarial worms. After inoculation, only a low number of L3 larvae develop to the adult stage in a small percentage of cats. Heartworm disease in cats may be associated with severe pulmonary thromboembolism and an eosinophilic inflammatory response in the lungs, potentially leading to sudden death. Otherwise self-cure occurs in most cases after 18–48 months. Subcutaneous dirofilariosis may present as subcutaneous nodules or dermatitis.
Diagnosis and treatment:
Diagnosis in cats is more difficult compared with dogs and needs a multistep approach (antigen and antibody tests, as well as diagnostic imaging). Cats with acute heartworm disease require stabilisation within an intensive care unit. Cats with respiratory signs or suggestive radiographic changes should receive prednisolone and follow-up with a similar multistep approach. Adulticidal therapy is not safe in cats.
Prevention:
In endemic areas cats should receive year-round chemoprophylaxis from 2 months of age.

Agents and life cycle
Filarial worms (order Spirurida, family Onchocercidae) are vector-borne nematodes infecting mainly dogs but also cats, ferrets, wild carnivores (foxes, jackals, coyotes, wolves, raccoons, wild felids, sea lions, black bears) and humans.1-3 Dirofilaria immitis and Dirofilaria repens (subgenus Nochtiella) are the most important species causing heart-worm disease (HWD) and subcutaneous dirofilariosis, respectively. Both species host symbiotic bacteria of the genus Wolbachia, which play an important role in the biology and pathogenicity of these nematodes. These bacteria are transmitted vertically between generations of nematodes and are responsible for the long-term survival and reproduction of filarial worms. For example, Wolbachia species is involved in moulting, embryogenesis and survival of filariae, while the filariae provide amino acids for Wolbachia bacterial growth. 2
Adult D immitis worms (females 25–30 cm × ~1 mm; males 12–20 cm × 0.7–0.9 mm) reside in pulmonary arteries and right heart chambers (Figure 1) whereas those of D repens (females 10–17 cm × 4–6 mm; males 5–7 cm × ~4 mm) are located in subcutaneous tissues (Figure 2).1,2
Figure 1.

Right side of the heart of a cat with heartworm disease (caval syndrome). After removal of the pericardial sac, two adult Dirofilaria immitis nematodes were found (arrowheads), one in the caudal vena cava (CVC). RA = right atrium. Courtesy of Ilaria Biasato and Laura Chiappino
Figure 2.

Histological image showing an adult Dirofilaria repens nematode in a subcutaneous nodule on the trunk of a cat. Evenly spaced longitudinal ridges (red arrow) are visible on the surface of the cuticle. Haematoxylin and eosin, × 100 magnification. Courtesy of Simone Manzocchi
The lengthy life cycle of these nematodes involves an intermediate mosquito host (in Europe mainly Culex pipiens, Aedes vexans and Aedes albopictus) that becomes infected by taking a blood meal from a reservoir host carrying an adequate number of circulating LI larvae (microfilariae).1,2,4,5 The most common vector species feed on humans, cats and dogs; however, the reservoir role is played by dogs or some wild canids, as cats and humans are minimally microfilaraemic. In susceptible mosquitoes, larvae moult up to the L3 stage; these larvae are then infective for the definitive hosts. 2
Cats are imperfect hosts for Dirofilaria worms; thus, the parasite life cycle in cats and dogs differs in a number of important respects (see box).
A metastrongylid nematode (Angiostrongylus chabaudi) that is occasionally detected in the right ventricle and pulmonary arteries of European wildcats 8 was recently found in two domestic cats in Italy.9,10 The location of adult lungworms in the pulmonary arteries, as for D immitis, makes this relevant should postmortem examination of cats be undertaken. It is postulated that cats are occasional hosts for A chabaudi because small and non-fertile parasites were detected and L1 larvae were not found in the faeces of infected cats. 8 Moreover, pathological findings consistent with Angiostrongylus infection were not evident and, thus, this parasitic infection is not thought to be clinically relevant in domestic cats. 8 The life cycle of Angiostrongylus vasorum, the metastrongylid species affecting dogs and wild canids, involves slugs and snails, and infection occurs by oral ingestion of infected molluscs carrying infectious L3 larvae; however, the intermediate host of A chabaudi is still not known. 8
Epidemiology
Global distribution
D immitis has a worldwide distribution, involving both tropical and temperate regions, and is endemic in some countries in Europe and North and South America. Conversely, D repens is frequently reported from Europe and Asia, infrequently from Africa and has not been found in the Americas, Australia and Japan. 11 In Europe, endemic areas for both species exist in Mediterranean countries (Italy, Spain, France, Greece and Turkey), but recent extension within these countries, as well as to Central and Eastern Europe (Switzerland, Austria, Germany, the Netherlands, Croatia, Serbia, Hungary, Czech Republic, Poland and Russia), has been documented.6,12,13 This spread is particularly concerning with D repens, as this is currently considered an emerging zoonotic agent in Europe. 14
Increasing mosquito abundance and prolonged seasonal activity, as well as increased travelling of infected dogs, are causing the spread of filarial infection. A global rise in temperatures also contributes by prolonging the season suitable for maturation of larvae in infected mosquitoes up to year-round. 15 Also, higher humidity levels increase the risk of exposure for susceptible hosts, particularly when the canine population is not undergoing large-scale chemoprophylactic treatment. 16 According to predictive models developed for dirofilariosis, summer temperatures in Europe are able to support the life cycle of larvae in mosquitoes even in colder regions, such as the UK, where infected reservoirs are present.12,17,18 Thus far, Estonia is the northernmost European area where completion of the D repens life cycle has been demonstrated, and a human case has been diagnosed as far north as Finland. 14
Prevalence
The true prevalence of feline HWD is difficult to evaluate because of diagnostic difficulties, but infection is detected in the same areas as canine HWD at about 9–18% of the rate in unprotected dogs. 19 However, studies investigating anti-D immitis antibodies in cats show that the rate of exposure to infection is much higher.16,19-21 Outdoor cats have a three-fold higher risk of being antigen-positive, and male cats have been found to be more likely to develop mature infections in experimental studies. 22
Information about the prevalence of D repens infection in cats in Europe is very limited; however, a study performed in central Italy using both modified Knott and PCR tests found a prevalence of 1.6% in cats compared with 5.6% in dogs. 6 In central Poland, a prevalence of 0.7% in cats compared with 38% in dogs was determined by PCR. 23
Pathogenesis and clinical signs
Heartworm disease
Despite the low parasite load (one to six adult worms per cat), severe pathological changes are found early in cats and they can be life-threatening. Pulmonary endo-mesoarteritis with occlusive medial hypertrophy are observed as soon as immature worms arrive in the pulmonary vessels (about 3 months postinfection).24,25 Usually early death (3–4 months postinfection) of juvenile heartworms occurs soon after arrival in the pulmonary arteries; otherwise they survive about 2–4 years followed by self-cure in most cases. 26 Early or end-stage parasite death can be associated with acute severe lesions (severe pulmonary thromboembolism and eosino-philic inflammatory response in the lungs) causing the so-called heartworm-associated respiratory disease (HARD) characterised by acute-onset dyspnoea and an interstitial pattern on lung radiography.24,25,27 In experimental studies, the inflammatory response due to the death of immature adult heartworms is associated with chronic myofibrocyte proliferation, which is histologically evident in the lungs up to 18 months after infection. 28
Pulmonary arterial disease, associated with exposure to D immitis, is considered more severe in cats compared with dogs due to the increased activity of pulmonary intravascular macrophages in cats.29,30 In one case, pathological changes in the arterial wall caused fatal dissection of the pulmonary artery (Figures 3 and 4). 31 Moreover, the presence of circulating Wolbachia species antigens and anti-Wolbachia antibodies has been associated in experimentally infected cats with an inflammatory reaction in the host tissues that affects respiratory function.27,32
Figure 3.

Dorsolateral view of the pulmonary artery in a cat with heartworm disease. The right pulmonary artery (RPA) is diffusely red and tan in colour and severely dilated (*). Upon opening (inset picture), the wall of the RPA is split with haemorrhage (arrowhead) dissecting into the tunica media. Large and multifocal periadventitial haematomas (arrow) are also observed. AO = aorta; LPA = left pulmonary artery. Courtesy of Ilaria Biasato and Laura Chiappino
Sometimes sudden death is reported in apparently healthy cats as a consequence of severe pulmonary thromboembolic or haemorrhagic pathology, including haemothorax due to pulmonary artery dissection.21,31,33 According to experimental studies, an acute systemic anaphylaxis could be involved in this hyperacute course of feline HWD secondary to the release of large quantities of D immitis antigens from dead parasites. The usual clinical signs are severe respiratory insufficiency, hypotension, vomiting and diarrhoea.34,35 Most cats, however, have a less severe, transient or chronic course of HWD with mild to moderate respiratory signs, due to chronic bronchoalveolar inflammation (epithelial infiltration and proliferation of smooth muscle cells in bronchioles), persisting even after parasite death. Chronic vomiting, anorexia and cachexia are also reported and can be the most obvious clinical manifestations. 36 Caval syndrome is rarely observed in cats but can arise when one or two worms are located in the right side of the heart, causing tricuspid regurgitation (Figure 1).
Figure 4.
(a,b) Histopathological examination of the pulmonary artery (same cat as Figure 3). (a) The tunica media (TM) of the right pulmonary artery is dissected by haemorrhage (*). Haematoxylin and eosin stain, × 25 magnification. TA = tunica adventitia. (b) Marked and multifocal fibrosis (*) of the tunica intima with disorganisation/ fragmentation of the elastic fibres and fibrous tissue deposition (arrowhead) in the right pulmonary artery. Picrosirius red stain, × 100 magnification. Images courtesy of Ilaria Biasato and Laura Chiappino
Aberrant migrations (eg, to body cavities, central nervous system, femoral artery) are rare, but more frequently reported in cats than in dogs and are responsible for effusions or neurological manifestations (eg, blindness, ataxia, paraparesis, monoparesis, seizures).1,2,37,38 Acute-onset pelvic limb monoparesis due to femoral thromboembolism was caused in a cat by a 13 cm adult female heartworm extending from the caudal abdominal aorta into the right external iliac and femoral arteries. 38 Interestingly, HWD had been diagnosed in this cat 3 years earlier when it was evaluated for cough and successfully treated with prednisolone. 38
Laboratory diagnosis may reveal eosinophilia on complete blood count and on bronchoalveolar lavage cytology. Changes in concentrations of acute phase proteins have been described.25,39,40 Hyperglobulinaemia, hypoalbuminaemia and proteinuria can occur in cats with HWD, irrespective of their parasite burden. 41
Subcutanoeus dirofilariosis
D repens infection is often subclinical in affected hosts; however, in dogs, subcutaneous nodules and dermatitis are associated with the presence of adult worms. 42 In cats, incidental reports of D repens infection have included an adult parasite in the subcutaneous tissue of an asymptomatic queen during elective surgery and microfilariae in the blood of a stray cat and some of its 8-week-old kittens.7,43
Microfilaraemia or positive D repens-specific PCR have been reported in cats with papular and crusting dermatitis. 44 More recently, D repens adult worms were detected in a cat that was presented with three large (around 2 × 2 cm), firm and infiltrating subcutaneous nodules on the lateral thoracic wall and forelegs associated with axillary and inguinal lymphadenopathy (Figure 5). 45 Moderate absolute eosinophilia was the only clinico-pathological abnormality found and eosinophilic lymphadenitis (Figure 6) was detected on cytological evaluation of the enlarged lymph nodes. 45
Figure 5.
Subcutaneous nodules in a cat caused by Dirofilaria repens; (a) on the foreleg and (b) on the trunk. Images courtesy of Simone Manzocchi
Figure 6.
Fine-needle aspirate from an axillary lymph node of a cat with subcutaneous dirofilariosis (same cat as Figure 5), showing eosinophilic lymphadenitis. May-Grünwald–Giemsa, × 400 magnification. Courtesy of Simone Manzocchi
Diagnosis
The rare and short-term occurrence of microfilaraemia and the low number of adult worms make it difficult to diagnose HWD in cats, compared with dogs. A multistep approach, combining mainly antigen and antibody tests and diagnostic imaging, is required to overcome the limitations of each single diagnostic technique (Figure 7). 46 Moreover, HARD can occur during early death of parasites and this explains the possibility of discordant results between different tests even in symptomatic cats.
Figure 7.
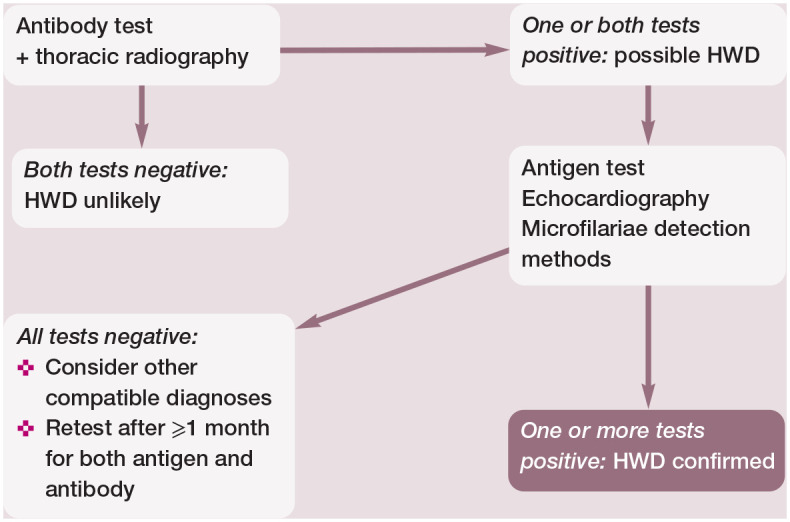
Combined use of different diagnostic tools is usually required to reach a diagnosis of feline heartworm disease (HWD) and in some cases confirmation is never obtained. Anti-Dirofilaria immitis antibody detection and thoracic radiography to look for compatible pulmonary lesions are sensitive methods for providing early information about possible feline HWD. However, antibody detection has limitations due to lower sensitivity in asymptomatic cats and lower specificity as time passes because antibodies persist for some time in cats that self-cure. A positive antigen test or the ultrasound visualisation of filarial worms are confirmatory, but their sensitivity is inadequate for ruling out HWD in the case of no antigen and worm detection. Occasionally, microfilariae are evident in blood (modified Knott test or millipore filter), and they have to be differentiated from those of Dirofilaria repens in regions where both species are found (eg, in many European countries)
In contrast with dogs, both microscopic detection of microfilariae and ELISA or immunochromatographic (IC)-based tests that detect circulating antigens of adult D immitis females have low sensitivity in cats.1,2,39,46 Albeit rarely, microfilariae of both species can be detected incidentally on feline routine blood smears and further investigations are always required for their identification. 7 When microfilariae are detected using routine techniques (modified Knott test or Millipore filtration), morphometric identification, cyto-chemical stain or PCR are needed to differentiate D immitis and D repens in regions where both species are found (eg, in many European countries). 39
Feline HWD is usually associated with the presence of few pre-adult or adult worms. When no or only one adult female worm is present, a false negative result is obtained on ELISA or IC antigen tests. 47 However, heat pretreatment of feline and canine serum or plasma samples improves the sensitivity of these commercial antigen assays in cats (and dogs) carrying only a few adult female worms by releasing antigens blocked within circulating immune complexes.48-51 Underlining the low negative predictive value of antigen assays in cats, it is suggested that any negative result is recorded as ‘no antigen detected’ instead of ‘negative’. 46
Anti-D immitis antibodies are found about 2 months postinfection and provide early confirmation of exposure to D immitis, irrespective of the parasite load. 52 However, a risk of false positive results exists because of antibody persistence even in cats that have cleared the parasites. 47 False negative results are also possible (particularly in asymptomatic cats), and a wide range of sensitivities exist among the different tests. 46 The combined use of antigen and antibody testing increases diagnostic accuracy of testing for feline HWD but negative results on both tests still cannot rule out a diagnosis in suspected HARD cases.
Thoracic radiography is always indicated for the diagnosis of HWD because of suggestive changes and for prognostication. 39 Characteristic abnormalities include a vascular pattern (enlargement, loss of tapering, tortuosity and truncation of the right or both caudal pulmonary arteries on ventrodorsal radiographs) and patchy infiltrates around the caudal lobar arteries resulting from plasma leakage and perivascular inflammation (often peripheral in caudal lobes) (Figure 8) or a diffuse bronchointerstitial pattern. 39 Radiographic changes can be the only abnormality detected in suspected HARD cases, but lungworm disease should be excluded in these cats as a differential diagnosis for the radiographic changes. 53 Pulmonary thromboembolism, arterial changes and bronchial collapse are common findings when cats with HWD undergo CT or angiography investigations of the thorax.25,54
Figure 8.
Thoracic radiograph (left lateral view) of a cat with acute dyspnoea caused by heartworm disease. A focal interstitial pattern (caudal lung lobes) is present. Courtesy of Luigi Venco
Filariae can sometimes be seen in the right heart chambers, pulmonary artery or distal caudal vena cava by echocardiography. 21 The body wall of adult heartworms appears as double hyperechoic parallel lines (‘railway lines’) because of reflection of ultrasound waves by the cuticle of the worm (Figure 9). However, diagnostic sensitivity is operator dependent, quantification of the worm burden is difficult and false negative as well as false positive results are possible. 39
Figure 9.
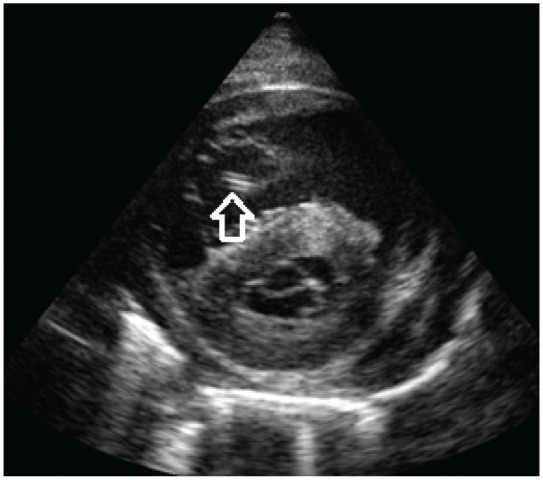
Echocardiography (right parasternal short axis view) in a cat with heartworm disease. The body wall of an adult heartworm appears as double hyperechoic parallel lines (arrow). Courtesy of Luigi Venco
Necropsy is the only diagnostic method available in cases of sudden death but is also used to confirm the disease when negative results of ante-mortem diagnostic methods are obtained in suspected cases. 31 The right side of the heart, pulmonary arteries and ectopic sites, including the brain and spinal cord in the case of neurological signs, must be carefully examined for adult worms.1,2,46
With respect to subcutaneous dirofilariosis, a diagnosis was obtained as an unexpected finding in one report. CT features of nodules caused by D repens were considered compatible with a diagnosis of metastatic fibrosarcoma, and the diagnosis of subcutaneous dirofilariosis was only obtained through cytological assessment, which revealed the presence of both adult worms and microfilariae (Figures 10 and 11). 45 Ultrasonographic examination of the nodules showed hyperechoic lines, compatible with filarial nematode parasites (Figure 12). 45
Figure 10.

CT image of the nodule pictured in Figure 5b. An ovular neoformation is visible in the subcutis of the trunk (arrow). Courtesy of Simone Manzocchi
Figure 11.
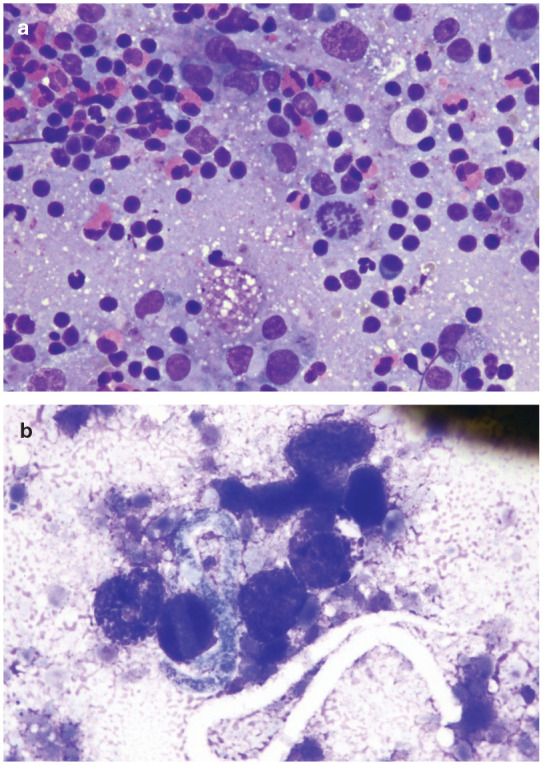
(a,b) Fine-needle aspiration of the nodule pictured in Figure 5b. (a) Mixed inflammatory population dominated by small lymphocytes, eosinophils and macrophages. May-Grünwald–Giemsa, × 400 magnification. (b) One microfilaria admixed with several round morulated eggs is present. May-Grünwald–Giemsa, × 600 magnification. Images courtesy of Simone Manzocchi
Figure 12.

Ultrasound image of the nodule pictured in Figure 5b. Many double and parallel hyperechoic lines are visible (arrow). Courtesy of Simone Manzocchi
Molecular investigations can be performed on DNA obtained from blood and collected worms. Parasite species-specific PCR assays are available for discriminating D immitis and D repens. 55 A panfilarial (six species) PCR has been developed and, in some cats, DNA of filarial worms not yet reported in feline hosts was detected.45,56
Treatment and follow-up
There is no adulticide approved for the treatment of feline HWD, and adulticidal therapy is not recommended in asymptomatic cats because self-cure occurs in most cases within 18–48 months.26,46 Infected cats should be followed up by thoracic radiography, echo-cardiography, and antigen and antibody testing for decision-making regarding treatment and to detect self-cure.
Prednisolone (2 mg/kg PO q24h, declining over a few weeks) should be given to cats with respiratory signs that test positive on antigen and/or antibody testing. 46 Prednisolone treatment should also be administered in asymptomatic cats with suggestive radiographic changes, and treatment should be followed up by the same clinical, radiographic and antigen/antibody testing method. 46 Improvement in radiographic changes in conjunction with previous positive antigen tests becoming negative are markers of recovery despite the persistence of positive antibody tests. 46 Hyperacute cases of HWD require intensive care unit stabilisation because of the risk of respiratory failure and shock.
Adulticide therapy used in dogs (melarsomine) is not safe in cats and carries a high risk of triggering pulmonary thromboembolism and anaphylactic reactions following parasite death.33,46 Surgical procedures to mechanically remove adult parasites visualised in the right heart and main pulmonary arteries have been proposed in symptomatic cats, but worms have to be retrieved intact to avoid anaphylaxis.46,57 Successful surgical removal of an adult heartworm that had migrated to the femoral artery, achieved via direct access to the worm from the arterioto-my site, has been reported. 38
Slow kill protocols combining monthly administration of heartworm-preventive drugs with a 1-month course of doxycycline therapy are used in dogs when melarsomine is contraindicated, 58 and have been reported in cats, 21 but their efficacy and safety are unknown and therefore these protocols are not recommended. Nevertheless, all cats diagnosed with HWD should undergo monthly application of preventive drugs (see below).
There is no adulticidal drug approved for the treatment of feline subcutaneous dirofilariosis. There is, however, a report of successful surgical removal of nodules containing adult worms and microfilariae in one affected cat. 45
Prevention
Because of the unpredictable and potentially fatal course of HWD in cats, and the lack of safe and effective treatments, all cats in endemic areas – irrespective of their access to the outdoors – should be given monthly chemoprophylaxis from 2 months of age year-round for killing infective larvae in the L3–L4 stages. 46 Although a recent study found that outdoor cats had a three-fold increased risk of being D immitis antigen-positive, it is known that about one-third of all antigen-positive cats actually live indoors. 22 The same recommendation is given for cats that live in areas that do not have heartworm but that are travelling to endemic areas. Chemoprophylaxis should be performed within 30 days of arrival in the risk area.59,60 Unfortunately, awareness of the importance of feline HWD prevention seems to be low; and/or prophylaxis is often neglected, especially in cat shelters, for economic reasons.22,61,62
Adequate preventive treatment covering the whole of the transmission season is crucial for effective prevention, and a year-round approach is often the best option because of climatic differences in humidity levels having a great impact on the risk of exposure for susceptible hosts, as discussed earlier. 16 Five preventive drugs are currently available for cats and are given monthly orally or topically as spot-ons (Table 1).25,63-65 Antigen- or antibody-positive cats can be safely given preventive therapy, but both tests should be carried out before starting chemoprophylaxis to obtain a risk assessment for HWD in cats living in endemic areas. 46
Table 1.
Drugs available for chemoprophylaxis of feline heartworm disease
| Macrocyclic lactone | Monthly dose | Administration route |
|---|---|---|
| Ivermectin | 24 ug/kg | Oral |
| Milbemycin oxime (in combination with praziquantel) | 2.0 mg/kg | Oral |
| Moxidectin (in combination with imidacloprid) | 1.0 mg/kg | Topical, spot-on |
| Selamectin (may be in combination with sarolaner) | 6.0 mg/kg | Topical, spot-on |
| Eprinomectin (in combination with praziquantel and (S)-methoprene and fipronil) | 0.48 mg/kg | Topical, spot-on |
Selamectin administration was not found to be effective in preventing subcutaneous dirofilariosis in one cat, but this apparent failure could have been due to prophylaxis being stopped before the end of the transmission season. 45

Key Points
D immitis and D repens are the most important filarial worms, causing heartworm disease and subcutaneous dirofilariosis, respectively.
The life cycle involves an intermediate mosquito host.
Filarial worms infect mainly dogs, but also cats, ferrets, wild carnivores and humans. Compared with dogs, cats are imperfect hosts for Dirofilaria worms. After inoculation, only a low number of L3 larvae develop to the adult stage in a small percentage of cats.
Heartworm disease in cats may be associated with severe pulmonary thromboembolism and eosinophilic inflammatory response in the lungs, potentially leading to sudden death. Otherwise, self-cure occurs in most cases within 18–48 months.
Subcutaneous dirofilariosis may present as subcutaneous nodules or dermatitis.
Diagnosis in cats is more difficult compared with dogs and needs a multistep approach (antigen and antibody tests as well as diagnostic imaging).
Cats with acute heartworm disease need hospitalisation in an intensive care unit for stabilisation.
Cats with respiratory signs or suggestive radiographic changes should receive prednisolone and diagnostic follow-up should be performed. Adulticide therapy is not safe in cats.
In endemic areas, cats should be given year-round chemoprophylaxis from 2 months of age.
D repens infection is currently considered an emerging zoonosis in Europe.
Acknowledgments
The authors are very grateful to Ilaria Biasato, Laura Chiappino, Simone Manzocchi and Luigi Venco for providing the images included in this article.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article. The ABCD is supported by Boehringer Ingelheim (the founding sponsor of the ABCD) and Virbac, but is a scientifically independent body and the members of this expert board receive no stipends from sponsors.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not necessarily required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
References
- 1. McCall JW, Genchi C, Kramer LH, et al. Heartworm disease in animals and humans. Adv Parasit 2008; 66: 193–285. [DOI] [PubMed] [Google Scholar]
- 2. Simon F, Siles-Lucas M, Morchon R, et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clin Microbiol Rev 2012; 25: 507–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Otranto D, Deplazes P. Zoonotic nematodes of wild carnivores. Int J Parasitol Parasites Wildl 2019; 9: 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cancrini G, Magi M, Gabrielli S, et al. Natural vectors of dirofilariasis in rural and urban areas of the Tuscan region, central Italy. J Med Entomol 2006; 43: 574–579. [DOI] [PubMed] [Google Scholar]
- 5. Cancrini G, Scaramozzino P, Gabrielli S, et al. Aedes albopictus and Culex pipiens implicated as natural vectors of Dirofilaria repens in central Italy. J Med Entomol 2007; 44: 1064–1066. [DOI] [PubMed] [Google Scholar]
- 6. Traversa D, Aste G, Milillo P, et al. Autochthonous foci of canine and feline infections by Dirofilaria immitis and Dirofilaria repens in central Italy. Vet Parasitol 2010; 169:128–132. [DOI] [PubMed] [Google Scholar]
- 7. Diugosz E, Szmidt A, Dobrzynski A, et al. Molecular investigation of possible Dirofilaria repens vertical transmission from queen to offspring – case report from Poland [abstract]. Parasit Vectors 2016; 10 Suppl 1. DOI: 10.1186/s13071-016-1902-x. [DOI] [Google Scholar]
- 8. Giannelli A, Kirkova Z, Abramo F, et al. Angiostrongylus chabaudi in felids: new findings and a review of the literature. Vet Parasitol 2016; 228: 188–192. [DOI] [PubMed] [Google Scholar]
- 9. Varcasia A, Tamponi C, Brianti E, et al. Angiostrongylus chaubadi Biocca, 1957: a new parasite for domestic cat? Parasit Vectors 2014; 7: 588. DOI: 10.1186/s13071-014-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Traversa D, Lepri E, Veronesi F, et al. Metastrongyloid infection by Aeluro strongylus abstrusus, Troglostrongylus brevior and Angiostrongylus chaubadi in a domestic cat. Int J Parasitol 2015; 45: 685–690. [DOI] [PubMed] [Google Scholar]
- 11. Yilmaz E, Wongkamchai S, Ramunke S, et al. High genetic diversity in the Dirofilaria repens species complex revealed by mitochondrial genomes of feline microfilaria samples from Narathiwat, Thailand. Transbound Emerg Dis 2019; 66: 389–399. [DOI] [PubMed] [Google Scholar]
- 12. Genchi C, Kramer LH, Rivasi F. Dirofilarial infections in Europe. Vector Borne Zoonotic Dis 2011; 11: 1307–1317. [DOI] [PubMed] [Google Scholar]
- 13. Morchon R, Carreton E, Gonzalez-Miguel J, et al. Heartworm disease (Dirofilaria immitis) and their vector in Europe -new distribution trends. Front Physiol 2012; 3: 196. DOI: 10.3389/fphys.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Capelli G, Genchi C, Baneth G, et al. Recent advances on Dirofilaria repens in dogs and humans in Europe. Parasit Vectors 2018; 11: 663. DOI: 10.1186/s13071-018-3205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Simon L, Afonin A, Lopez-Diez LI, et al. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops. The case of Spain. Vet Parasitol 2014; 200: 257–264. [DOI] [PubMed] [Google Scholar]
- 16. Montoya-Alonso JA, Carreton E, Morchon R, et al. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Vet Parasitol 2016; 216: 66–71. [DOI] [PubMed] [Google Scholar]
- 17. Genchi C, Rinaldi L, Cascone C, et al. Is heartworm disease really spreading in Europe? Vet Parasitol 2005; 133: 137–148. [DOI] [PubMed] [Google Scholar]
- 18. Genchi C, Rinaldi L, Mortarino M, et al. Climate and Dirofilaria infection in Europe. Vet Parasitol 2009; 163: 286–292. [DOI] [PubMed] [Google Scholar]
- 19. Venco L, Genchi M, Genchi C, et al. Can heartworm prevalence in dogs be used as provisional data for assessing the prevalence of the infection in cats? Vet Parasitol 2011; 176: 300–303. [DOI] [PubMed] [Google Scholar]
- 20. Montoya-Alonso JA, Carreton E, Corbera JA, et al. Current prevalence of Dirofilaria immitis in dogs, cats and humans from the island of Gran Canaria, Spain. Vet Parasitol 2011; 176: 291–294. [DOI] [PubMed] [Google Scholar]
- 21. Diakou A, Soubasis N, Chochlios T, et al. Canine and feline dirofilariosis in a highly enzootic area: first report of feline dirofilariosis in Greece. Parasitol Res 2019; 118: 677–682. [DOI] [PubMed] [Google Scholar]
- 22. Levy JK, Burling AN, Crandall MM, et al. Seroprevalence of heartworm infection, risk factors for seropositivity and frequency of prescribing heartworm preventives for cats in the United States and Canada. J Am Vet Med Assoc 2017; 250: 873–880. [DOI] [PubMed] [Google Scholar]
- 23. Bajer A, Rodo A, Mierzejewska EJ, et al. The prevalence of Dirofilaria repens in cats, healthy dogs and dogs with concurrent babesiosis in an expansion zone in central Europe. BMC Vet Res 2016; 12: 183. DOI: 10.1186/s12917-016-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dillon AR, Blagburn B, Tillson DM, et al. Immature heart-worm infection produces pulmonary parenchymal, airway, and vascular disease in cats. J Am Vet Med Assoc 2007; 21: 608–609. [Google Scholar]
- 25. Dillon R, Tillson DM, Woolridge A, et al. Effect of pre-cardiac and adult stages of Dirofilaria immitis in pulmonary disease of cats: CBC, bronchial lavage cytology, serology, radiographs, CT images, bronchial reactivity, and histopathology. Vet Parasitol 2014; 206: 24–37. [DOI] [PubMed] [Google Scholar]
- 26. Venco L, Genchi C, Genchi M, et al. Clinical evolution and radiographic findings of feline heartworm infection in asymptomatic cats. Vet Parasitol 2008; 158: 232–237. [DOI] [PubMed] [Google Scholar]
- 27. Garcia-Guasch L, Caro-Vadillo A, Manubens-Grau J, et al. Is Wolbachia participating in the bronchial reactivity of cats with heartworm associated respiratory disease? Vet Parasitol 2013; 196: 130–135. [DOI] [PubMed] [Google Scholar]
- 28. Dillon RA, Blagburn BL, Tillson M, et al. The progression of heartworm associated respiratory disease (HARD) in SPF cats 18 months after Dirofilaria immitis infection. Parasit Vectors 2017; 10 Suppl 2: 533. DOI: 10.1186/s13071-017-2425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Browne LE, Carter TD, Levy JK, et al. Pulmonary arterial disease in cats seropositive for Dirofilaria immitis but lacking adult heartworms in the heart and lungs. Am J Vet Res 2005; 66: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 30. Dillon AR, Warner AE, Brawner W, et al. Activity of pulmonary intravascular macrophages in cats and dogs with and without adult Dirofilaria immitis. Vet Parasitol 2008; 158: 171–176. [DOI] [PubMed] [Google Scholar]
- 31. Biasato I, Tursi M, Zanet S, et al. Pulmonary artery dissection causing haemothorax in a cat: potential role of Dirofilaria immitis infection and literature review. J Vet Cardiol 2017; 19: 82–87. [DOI] [PubMed] [Google Scholar]
- 32. Morchon R, Ferreira AC, Martin-Pacho JR, et al. Specific IgG antibody response against antigens of Dirofilaria immitis and its Wolbachia endosymbiont bacterium in cats with natural and experimental infections. Vet Parasitol 2004; 125: 313–321. [DOI] [PubMed] [Google Scholar]
- 33. Alho AM, Giannelli A, Otranto D, et al. Dirofilaria immitis: a silent cause of pulmonary thromboembolism and sudden death in cats. J Am Vet Med Assoc 2016; 249: 751–753. [DOI] [PubMed] [Google Scholar]
- 34. Lister A, Atwell R. Physiological and haematological findings and clinical observations in a model of acute systemic anaphylaxis in Dirofilaria immitis-sensitised cats. Aust Vet J 2006; 84: 151–157. [DOI] [PubMed] [Google Scholar]
- 35. Lister A, Atkins C, Atwell R. Acute death in heartworm-infected cats: unravelling the puzzle. Vet Parasitol 2007; 158: 196–203. [DOI] [PubMed] [Google Scholar]
- 36. Dillon AR, Brawner AR, Jr, Robertson-Plouch CK, et al. Feline heartworm disease: correlations of clinical signs, serology, and other diagnostic results of a multicentre study. Vet Ther 2000; 1: 176–182. [PubMed] [Google Scholar]
- 37. Favole P, Cauduro A, Opreni M, et al. Epidural dirofilariosis in a paraparetic cat: case report of Dirofilaria immitis infection. J Feline Med Surg 2013; 10: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oldach MS, Gunther-Harrington CT, Balsa IM, et al. Aberrant migration and surgical removal of a heartworm (Dirofilaria immitis) from the femoral artery of a cat. J Vet Intern Med 2018; 32: 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Venco L, Marchesotti F, Manzocchi S. Feline heartworm disease: a ‘Rubik’s-cube like’ diagnostic and therapeutic challenge. J Vet Cardiol 2015; 17: S190–S201. [DOI] [PubMed] [Google Scholar]
- 40. Silvestre-Ferreira AC, Vieira L, Vilhena H, et al. Serum acute phase proteins in Dirofilaria immitis and Wolbachia seropositive cats. J Feline Med Surg 2016; 19: 693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atkins CE, Vaden SL, Arther RG, et al. Renal effects of Dirofilaria immitis in experimentally and naturally infected cats. Vet Parasitol 2011; 176: 317–323. [DOI] [PubMed] [Google Scholar]
- 42. Venco L, Valenti V, Bertazzolo W, et al. A mini-invasive procedure for removal of adult Dirofilaria repens from subcutaneous nodules in dogs. Intern J Appl Res Vet Med 2011; 9: 217–223. [Google Scholar]
- 43. Mazurkevich A, Vasylyk A, Avramenko T, et al. Adult Dirofilaria repens nematodes in a cat from Kiev, Ukraine. Vet Rec 2004; 155: 638–639. [DOI] [PubMed] [Google Scholar]
- 44. Tarello W. Clinical aspects of dermatitis associated with Dirofilaria repens in pets: a review of 100 canine and 31 feline cases (1990-2010) and a report of a new clinic case imported from Italy to Dubai. J Parasitol Res 2011; 2011: 1–7. DOI: 10.1155/2011/578385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manzocchi S, Lendner M, Piseddu E, et al. Nodular presentation of Dirofilaria repens infection in a cat mimicking a fibrosarcoma. Vet Clin Pathol 2017; 46: 158–163. [DOI] [PubMed] [Google Scholar]
- 46. Jones S, Graham W, von Simon C, et al. Current feline guidelines for the prevention, diagnosis and management of heart-worm (Dirofilaria immitis) infection in cats. Batavia, Ill: American Heartworm Society, 2014. [Google Scholar]
- 47. Berdoulay M, Levy JK, Snyder PS, et al. Comparison of serological tests for the detection of natural heartworm infection in cats. J Am Anim Hosp Assoc 2004; 40: 376–384. [DOI] [PubMed] [Google Scholar]
- 48. Little SE, Raymond MR, Thomas JE, et al. Heat treatment prior to testing allows detection of antigen of Dirofilaria immitis in feline serum. Parasit Vectors 2014; 7: 1. DOI: 10.1186/1756-3305-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Velasquez L, Blagburn BL, Duncan-Decoq R, et al. Increased prevalence of Dirofilaria immitis antigen in canine samples after heat treatment. Vet Parasitol 2014; 206: 67–70. [DOI] [PubMed] [Google Scholar]
- 50. Gruntmeir JM, Adolph CB, Thomas JE, et al. Increased detection of Dirofilaria immitis antigen in cats after heat pre-treatment of samples. J Feline Med Surg 2016; 19: 1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Little SE, Saleh M, Wohltjen M, et al. Prime detection of Dirofilaria immitis: understanding the influence of blocked antigen on heartworm test performance. Parasit Vectors 2018; 11: 186. DOI: 10.1186/s13071-018-2736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prieto G, Ceciliani F, Venco L, et al. Feline dirofilariosis: antibody response to antigenic fractions containing specific 20 to 30 kDa polypeptides from the adult Dirofilaria immitis somatic antigen. Vet Parasitol 2002; 103: 341–353. [DOI] [PubMed] [Google Scholar]
- 53. Pennisi MG, Hartmann K, Addie D, et al. Lungworm disease in cats. ABCD guidelines on prevention and management. J Feline Med Surg 2015; 17: 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panopoulos I, Specchi S, Soubasis N, et al. Multidetector computed tomographic pulmonary angiography in a cat with fatal heartworm disease. Vet Radiol Ultrasound 2018; 59: E71–E75. [DOI] [PubMed] [Google Scholar]
- 55. Favia G, Lanfrancotti A, Della Torre A, et al. Polymerase chain reaction identification of Dirofilaria repens and Dirofilaria immitis. Parasitology 1996; 113: 567–571. [DOI] [PubMed] [Google Scholar]
- 56. Rishniw M, Barr SC, Simpson KW, et al. Discrimination between six species of canine microfilariae by a single polymerase chain reaction. Vet Parasitol 2006; 135: 303–314. [DOI] [PubMed] [Google Scholar]
- 57. Iizuka T, Hoshi K, Ishida Y, et al. Right atriotomy using total venous inflow occlusion for removal of heartworms in a cat. J Vet Med Sci 2009; 71: 489–491. [DOI] [PubMed] [Google Scholar]
- 58. Savadelis MD, Ohmes CM, Hostetler JA, et al. Assessment of parasitological findings in heartworm-infected beagles treated with Advantage Multi® for dogs (10% imidacloprid + 2.5% moxidectin) and doxycycline. Parasit Vectors 2017; 10: 245. DOI: 10.1186/s13071-017-2190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. European Scientific Counsel Companion Animal Parasites. GL5: control of vector-borne diseases in dogs and cat. www.esccap.org/page/GL5+Control+of+VectorBorne+Diseases+in+Dogs+and+Cats/29/#.WSisA2jyiUl (2012, accessed February 27, 2020). [Google Scholar]
- 60. European Society of Dirofilariosis and Angiostrongylosis. Guidelines for clinical management of feline heartworm disease. www.esda.vet (2017, accessed February 27, 2020). [Google Scholar]
- 61. Polak KC, Smith-Blackmore M. Animal shelters: managing heartworms in resource-scarce environments. Vet Parasitol 2014; 206: 78–82. [DOI] [PubMed] [Google Scholar]
- 62. Genchi M, Rinaldi L, Venco L, et al. Dirofilaria immitis and D repens in dog and cat: a questionnaire study in Italy. Vet Parasitol 2019; 267: 26–31. [DOI] [PubMed] [Google Scholar]
- 63. Baker CF, Tielemans E, Pollmeier MG, et al. Efficacy of a single dose of a novel topical combination product containing eprinomectin to prevent heartworm infections in cats. Vet Parasitol 2014; 202: 49–53. [DOI] [PubMed] [Google Scholar]
- 64. Little SE, Hostetler J, Thomas JE, et al. Moxidectin steady state prior to inoculation protects cats from subsequent, repeated infection with Dirofilaria immitis. Parasit Vectors 2015; 8: 107. DOI: 10.1186/s13071-015-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McTier TL, Pullins A, Chapin S, et al. The efficacy of a novel topical formulation of selamectin plus sarolaner (Revolution® Plus/Stronghold® Plus) in preventing the development of Dirofilaria immitis in cats. Vet Parasitol 2019; 270: 56–62. [DOI] [PubMed] [Google Scholar]
- 66. Pampiglione S, Rivasi F, Angeli G, et al. Dirofilariasis due to Dirofilaria repens in Italy, an emergent zoonosis: report of 60 new cases. Histopathology 2001; 38: 344–354. [DOI] [PubMed] [Google Scholar]








