Abstract

Practical relevance Although seizures occur less commonly in cats compared with dogs, they are one of the most common forms of neurological disease in the feline patient. Cats may experience both focal (partial) and generalized seizures and causes are divided into primary disorders, in which there is no underlying cause (ie, idiopathic epilepsy), and secondary disorders. Cats with secondary seizure disorders have either an underlying structural lesion or metabolic disease.

Patient group Seizures affect cats of all ages. Cats with idiopathic epilepsy tend to be younger (approximately 3.5 years) than cats with secondary seizure disorders (approximately 8 years).
Audience This review of feline seizures is directed at all veterinarians who treat cats, both in an emergency setting as well as in general practice.
Clinical challenges Refractory seizures are often a diagnostic and therapeutic challenge. A systematic approach to the seizuring cat is described, easing the task of diagnosing the cause of the seizures. In addition, novel antiepileptics are discussed, which can be used as add-on drugs in challenging feline seizure cases.
Evidence base Compared with the canine counterpart, the literature regarding treatment of feline seizures is less established. Recent clinical trials and studies are focusing on new treatment options for feline seizures. Specifically, these studies, some of which are ongoing, have led to the use of levetiracetam, zonisamide and pregabalin as add-on antiepileptics in cases that are refractory to phenobarbital.
Seizures and the feline patient
Seizure disorders are a well recognized neurological problem in cats and, although they occur less commonly than in dogs, seizures are one of the most common forms of neurological disease in the feline patient.1–5 Much of what is known regarding the etiology and diagnosis of feline seizure disorders can be extrapolated from dogs; however, care should be taken when comparing treatment options between the two species.
Prevalence and etiology
Seizure disorders are divided into primary and secondary disorders and seizures can occur in both focal (partial) and generalized forms.1,5
Focal seizures originate from a discrete epileptogenic focus within the cerebral cortex. Clinical features of focal seizures in cats are more variable than in dogs and may include drooling, facial movements, hippus, excessive vocalization/growling, random skittish behavior, and abnormal head, neck or limb movements.1,6 Focal seizures may progress to generalized seizures, though more often they remain isolated episodes. Focal seizures may occur several times throughout the day.
Generalized seizures originate from both cerebral hemispheres and are most often tonic-clonic seizures. During the pre-ictal phase, or aura, cats may display behavioral changes including aggressiveness, pacing, crying or vocalizing, restlessness, anxiety, salivation, increased affection, and growling. The ictus may be particularly violent in cats and is often accompanied by salivation, urination, defecation and pupil dilation. 6 Typically, the ictal phase lasts about 1–3 minutes in cats, though it may progress to a state of status epilepticus. The post-ictal phase is similar to what is recognized in the dog, lasting anywhere from minutes to days.
The primary versus secondary categorization of seizure disorders depends on whether or not there is an underlying cause.
MULTIMEDIA
Video recordings of cats showing partial epileptic seizures and severe myoclonic seizure activity are included in the online version of this article doi:10.1016/j.jfms.2009.03.006
Cats with idiopathic seizures tend to be younger than cats with secondary epilepsy.
Primary disorders
Primary seizure disorders are those conditions that have no underlying cause. Cats determined as having a primary seizure disorder are referred to as idiopathic epileptics. Idiopathic epilepsy in dogs is usually genetic in origin; however, there is little evidence of this in the cat. Historically, idiopathic epilepsy has been considered rare in cats. However, recent studies have disputed this. While the true incidence of idiopathic epilepsy in the cat is unknown, it has been suggested that between 21 and 59% of cats with seizures are idiopathic epileptics.6–8 In a recent study, 25% of cats were classified as having idiopathic epilepsy. 7 (This particular study further classified seizures into reactive [22% of cats] and symptomatic [50% of cats] categories, in which the seizure occurred secondarily to toxic and metabolic causes or structural brain disease, respectively; in this present review, these two categories are considered under the heading of secondary seizure disorders.)
Typically, cats with idiopathic epilepsy have tonic-clonic and generalized seizures; however, focal seizures may also be a feature of idiopathic epilepsy. It is estimated that focal and generalized seizures occur with relatively equal frequency in cats. 7 Historically, focal seizures have been associated with structural lesions of the forebrain; 8 however, in the authors' experience, the presence of focal seizures does not rule out a diagnosis of idiopathic epilepsy.
Primary or secondary?
Differentiating between primary and secondary seizure disorders is critical both for determining a prognosis and devising an appropriate treatment plan. Cats with idiopathic seizures tend to have a longer median survival time compared with cats with secondary epilepsy. 1 The type of seizure (ie, focal versus generalized) does not appear to be helpful in differentiating between the categories of seizures; however, seizure etiology does appear to be associated with the cat's age at the first seizure. 7 Cats with idiopathic seizures tend to be younger (approximately 3.5 years) than cats with secondary epilepsy (approximately 8 years).
Secondary disorders
Secondary seizure disorders are conditions in which an underlying structural lesion or disease is identified. The major categories of secondary seizure disorders in cats include vascular events, inflammatory conditions/encephalitis, infectious etiologies, toxins, traumatic injuries, metabolic derangements, neoplasia, congenital malformations and degenerative conditions (Table 1).
Table 1.
Differential diagnosis list for the seizuring cat
| Category | Differentials |
|---|---|
| Vascular |
|
| Inflammatory/infectious |
|
| Toxic |
|
| Traumatic | Traumatic injury |
| Anomalous | Hydrocephalus Internal or external |
| Metabolic |
|
| Neoplastic |
|
| Degenerative | Storage diseases |
Regardless of the cause of the seizures, antiepileptic medications are needed to treat the seizure activity; additional therapies may be necessary for treating secondary seizure disorders.
Diagnostic work-up for the seizuring cat
History
The first step in determining a diagnosis for a seizuring cat is a thorough history. Seizures are often confused with syncopal episodes (though rare in cats), vestibular disease, pain/hyperesthesia or behavioral disorders. A detailed description — or, ideally, a video recording — of the episode is helpful in ascertaining if the patient is truly having a seizure. In situations when an episode is vague and unknown to be a true seizure, electroencephalography may be undertaken to evaluate brain function during the episode, although this is not commonly performed in a clinical setting. 9
Next, additional information must be gathered from the owner including the presence of any behavioral changes prior to the seizures; the cat's behavior in between the seizures; eating, drinking, urinating and defecating habits; current medications; environment (indoor or outdoor cat, exposure to other cats); vaccination status; retrovirus status (if known); travel history; and any potential exposure to toxins or history of traumatic events.
Physical and neurologic examinations
Once a thorough history has been obtained, physical and neurologic examinations are performed. Included in the physical examination is a fundic examination, as abnormalities may be associated with neurologic disease (eg, chorioretinitis with feline infectious peritonitis [FIP]). The neuroanatomic localization in all cats with seizures (regardless of the cause) is the forebrain, as seizures are the clinical manifestation of hypersynchronous abnormal neuronal activity originating in the cerebral cortex. The neurologic examination (see box below) helps to determine if one side of the cerebral cortex is more involved or if other parts of the nervous system, such as the brainstem or cerebellum, are involved.
Clinical features of forebrain disease in cats (in addition to seizures) include mentation changes, behavioral changes, visual deficits contralateral to the lesion, facial/nasal sensation deficits contralateral to the lesion, lip commissure droop contralateral to the lesion, a head turn towards the side of the lesion, anisocoria, propulsive (wide) circling with a normal gait towards the side of the lesion, general proprioceptive deficits contralateral to the lesion, and possibly head and neck hyperesthesia evident on palpation. 10
Neurological examination checklist
The neurologic examination is likely to be abnormal if the cat is examined shortly after a seizure has occurred, and is in the post-ictal phase. In these situations, the examination should be repeated when the patient is believed to have fully recovered.
The determination of unilateral brain disease is often suggestive of structural disease such as a mass effect (tumor, granuloma or abscess) or a vascular event. Multifocal central nervous system disease is often associated with metabolic, degenerative, toxic, infectious, inflammatory and neoplasic lesions. It must be noted that the neurologic examination is likely to be abnormal if the cat is examined shortly after a seizure has occurred, and is in the post-ictal phase. In these situations, the examination should be repeated when the patient is believed to have fully recovered. It is also important to note that a normal neurologic examination does not entirely rule out structural brain disease, especially if the lesion is located in a ‘silent’ region of the brain. Additionally, cats with large cerebral masses (often meningiomas) may have increased intracranial pressure, and the cerebellum may partially herniate through the foramen magnum (Fig 1). These cats may present with an acute onset of cerebellar signs including a hypermetric/ataxic gait with normal general proprioception, intention tremors, and a decreased menace response with normal vision.
Fig 1.
Cat displaying opisthotonus, which may be a result of increased intracranial pressure and herniation of the brain, either under the tentorium cerebelli or through the foramen magnum. Note the extended neck and thoracic limbs
Clinical pathology
Baseline blood work, including a complete blood count (CBC), chemistry profile, thyroid profile, blood pressure monitoring, and bile acids as well as urinalysis should be performed in all cats with seizures. These non-invasive tests may help to diagnose metabolic causes of seizures and are useful in planning anesthesia for any advanced imaging.
Why the need for advanced diagnostics?
The typical evaluation of a dog with idiopathic epilepsy reveals normal neurologic and physical examination findings, a history of seizures with normal behavior and normal interictal periods, and normal blood work. These dogs typically begin to seizure between 1 and 5 years of age and, therefore, a dog meeting these criteria is presumptively diagnosed with idiopathic epilepsy and advanced diagnostics are often not necessary. Because such typical guidelines are not available for the feline idiopathic epileptic, advanced diagnostics are usually recommended for the seizuring cat, even when idiopathic epilepsy is suspected.
Advanced diagnostics
Advanced diagnostics typically include magnetic resonance imaging (MRI) (Fig 2) or computed tomography (CT), and potentially cerebrospinal fluid (CSF) analysis. A CSF tap is indicated if the imaging is normal or suggestive of intracranial disease and the cat is believed to have normal intracranial pressure. If there is a large space-occupying mass or evidence of brain herniation, a CSF tap is contraindicated. Normal CSF is clear and colorless with fewer than 5 cells/μl and less than 27 mg/dl protein (for a cisterna magna tap). Abnormalities in CSF are very sensitive indicators of intracranial disease, but often are not specific. However, when evaluated in conjunction with MRI/CT, CSF analysis can be a helpful diagnostic tool (Table 2). Cultures and infectious disease titers (cryptococcosis, toxoplasmosis and FIP) may also be useful tests to perform on CSF.11–14
Fig 2.
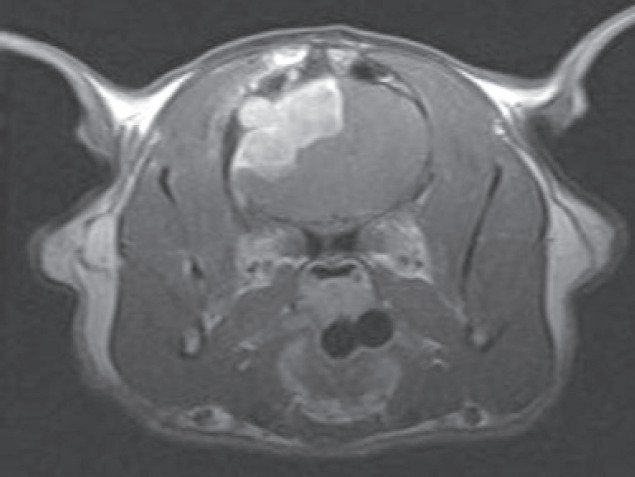
MRI scans from a cat with intracranial lymphoma, (a) T2-weighted axial image at the level of the interthalamic adhesion. The hyperintensity noted in the left cerebral hemisphere represents cerebral/peritumoral edema, (b) T1-weighted image at the same level acquired after the administration of gadolinium. A surface-oriented mass is identified
Table 2.
Various CSF findings and the diseases associated most commonly with them
| CSF finding | Description | Diseases |
|---|---|---|
| Lymphocytic pleocytosis | 5 WBC/μl > 50% lymphocytes Increased protein |
|
| Albuminocytologic dissociation | Increased protein with normal WBC count | Neoplasia Trauma |
| Mixed cell pleocytosis |
|
Fungal encephalitis (cryptococcosis, blastomycosis) Chronic FIP Infarction (mild) |
| Neutrophilic pleocytosis |
|
|
| Eosinophilic pleocytosis |
|
|
| Presence of intact RBC |
|
|
| Xanthochromic CSF | Hemorrhage prior to CSF collection | Primary bleeding disorders/coagulopathies |
WBC = white blood cell(s), RBC = red blood cells, FIP = feline infectious peritonitis, CSF = cerebrospinal fluid
Therapy for feline seizures
Due to the fact that each seizure episode has the potential to increase the number of epileptic foci in the brain, and that the response to therapy, rather than the severity of seizure activity, is an indication of prognosis, aggressive therapy is recommended for cats with seizures. 3
Phenobarbital
Phenobarbital is the current drug of choice in cats with multiple seizure episodes,4,14 and is available in both oral and intravenous formulations. The elimination half-life in cats is 34–50 h and, when given at a dose of 2.5 mg/kg body weight PO q 12 h, phenobarbital is a very effective antiepileptic drug. Side effects associated with phenobarbital use in cats are minimal and include sedation and ataxia, weight gain secondary to polyphagia, and polydipsia and polyurea. Hepatotoxicosis, a well recognized complication in dogs, has not been reported in cats.15,16 Occasionally, cats treated with phenobarbital have been reported with clinicopathologic abnormalities including leukopenia and thrombocytopenia, as well as immunemediated hypersensitivity reactions (severe cutaneous eruptions and marked lymphadenopathy), all of which are reversible with discontinuation of the drug.14,17
Serum drug concentrations should be measured 2–3 weeks after starting phenobarbital therapy. Therapeutic levels are similar to those reported in dogs (25–40 μg/ml). The ideal therapeutic range in cats is approximately 23–30 μg/ml. In dogs, repeated phenobarbital administrations are known to alter estimated steady state serum concentration as a consequence of enzyme induction. This results in the need to progressively increase oral dosage with time in order to maintain steady state therapeutic levels. This phenomenon of enzyme induction following repeated administration of phenobarbital is negligible in cats.
Serum drug concentrations should be monitored every 6 months, or 2–3 weeks after any change in dosage.2,5 In addition, it is recommended that CBC, chemistry profiles and bile acids are monitored once to twice yearly in cats on maintenance phenobarbital therapy.
When to treat?
Maintenance therapy is specifically recommended when a cat presents with more than one seizure within 6 months (either generalized or focal), has more than one cluster event (defined as more than one seizure in a 24 h period), or experiences status epilepticus (ie, continuous seizure activity that lasts more than 5 minutes or the presence of multiple seizures without returning to normal in between the seizures).
Therapy with antiepileptic drugs is recommended when seizures occur post trauma, particularly if they begin within the first week post trauma.
It is strongly advised to treat cats with antiepileptic drugs when there is evidence of structural forebrain disease (obtained via MRI/CT, CSF analysis, etc) such as neoplasia, infectious or non-infectious encephalitis, or congenital disease. Additional therapy is also warranted to treat the primary cause of the seizures; however, even after appropriate therapy (ie, surgery to remove a meningioma), therapy for the seizures is often necessary and is typically lifelong.
Diazepam
In situations where sole phenobarbital therapy is ineffective, or is contraindicated, additional antiepileptic drugs are needed. Oral diazepam has a longer elimination half-life in cats (15–20 h) than dogs (3–4 h) and is relatively effective at controlling seizures in cats. 2 Additionally, cats do not appear to develop a functional tolerance to the drug. Therefore, historically, diazepam has been the second-choice drug for treatment of feline epilepsy. 18 However, oral administration of diazepam in cats has been associated with a potentially fatal idiosyncratic hepatotoxicosis.19,20 Therefore, the use of oral diazepam should be limited; if used, diligent monitoring of clinical signs and serum alanine transaminase and aspartate transaminase activity must be performed so the drug can be immediately discontinued should any adverse reactions occur. However, it is strongly recommended that oral diazepam is not used in feline patients.
The authors consider oral diazepam use in cats to be contraindicated.
The intravenous formulation is not associated with hepatotoxicosis and is discussed later in the emergency therapy section.
Bromide
Bromide was initially thought to be a safe antiepileptic drug in cats due to its lack of hepatic metabolism. In addition, bromide's long elimination half-life (approximately 11 days), allowing for once-daily dosing, and the availability of therapeutic drug monitoring, made it a potentially promising antiepileptic drug in cats. 18 However, bromide has been associated with an idiosyncratic allergic pneumonitis, reportedly occurring in 35–42% of cats.21,22 The clinical signs are consistent with bronchial asthma and, although they abate with discontinuation of the drug, they may be life-threatening. Furthermore, bromide is not particularly effective as a feline antiepileptic as seizures are only controlled in approximately 35% of treated cats. 21
Levetiracetam
Levetiracetam is a novel antiepileptic drug. Its mechanism of action is not entirely known but appears to differ substantially from that of the more conventional antiepileptics.23–25 In vitro and in vivo recordings of epileptiform activity from the hippocampus have shown that levetiracetam inhibits burst firing without affecting normal neuronal excitability, suggesting that the drug may selectively prevent hyper-synchronization of epileptiform burst firing and propagation of seizure activity. Levetiracetam appears to have the ability to retard electrical kindling, a property that persists even after the drug has been discontinued. Therefore, levetiracetam may have antiepileptogenic properties and may assist in preventing the progression of epilepsy in at-risk patients.23,24 The synaptic vesicle protein SV2A is the binding site for levetiracetam. 26 It is present in high concentrations in the synaptic plasma membranes within the central nervous system. The molecular action of SV2A is unknown but it may be involved in transporting calcium or ATP. 27
Levetiracetam is considered an ideal antiepileptic drug with regard to its pharma-cokinetics. 28 It is rapidly absorbed following oral administration and has an oral bioavailability of approximately 100%. 29 Peak plasma concentrations are achieved at 2 h in the majority of cats. 30 Food does not affect the extent of absorption of levetiracetam, allowing it to be safely administered independent of the feeding schedule, which eases the task of giving the drug three times daily.28,31 The majority of levetiracetam is excreted unchanged in the urine and approximately 24% is excreted in the urine as an inactive metabolite. Renal elimination occurs primarily by glomerular filtration and correlates well with creatinine clearance. Therefore, the dose should be decreased accordingly in patients with impaired renal function. Levetiracetam is not significantly protein bound (< 10%) and, therefore, will not displace highly protein-bound drugs. 25
At a dose of 20 mg/kg PO q 8 h, levetiracetam is a safe and effective antiepileptic drug when given as an adjunct to phenobarbital in cats with suspected idiopathic epilepsy. 30 The half-life is approximately 3 h.29,30 Side effects are rare and may include transient inappetence and lethargy. In addition to treating suspected epileptics, the authors have used levetiracetam in two cats with intracranial meningiomas that were surgically removed, in which seizure control was not adequate with phenobarbital alone. Both cats showed a reduction in seizures and did not experience any side effects related to the drug. Levetiracetam also shows promise as a choice for sole antiepileptic drug therapy and has been used by the authors on occasions in which phenobarbital was not a viable option. Side effects were not noted in two cats treated with sole levetiracetam and the drug appeared to be effective in reducing the seizures.
Although a therapeutic range for levetiracetam has not been determined specifically for cats, it appears to be similar to that in humans (5–45 μg/ml). Monitoring is recommended approximately 1 week after commencing medication and then every 6–12 months. The goal of monitoring is more to aid in adjusting the drug dose, rather than to avoid side effects, due to levetiracetam's high safety margin. The authors have found that increasing the dose in 20 mg/kg increments is useful when the drug does not appear to be effective. In addition, monitoring routine blood work is recommended approximately every 6–12 months, as in all cats treated with antiepileptic medications.
At a dose of 20 mg/kg PO q 8 h, levetiracetam is a safe and effective antiepileptic when given as an adjunct to phenobarbital in cats with suspected idiopathic epilepsy.
Zonisamide
Zonisamide is a sulfonamide-based anticonvulsant drug that has been used successfully in dogs as an add-on antiepileptic drug.32,33 Suspected mechanisms of action include blockage of T-type calcium and voltage-gated sodium channels in the brain, facilitation of dopaminergic and serotonergic neurotransmission in the central nervous system, scavenging free radical species, enhancing GABA in the brain, inhibition of glutamate-mediated neuronal excitation in the brain, and inhibition of carbonic anhydrase activity. 14 Zonisamide is metabolized by hepatic microsomal enzymes and its half-life is significantly shorter in patients receiving phenobarbital or other drugs that increase the hepatic microsomal enzyme p450. 34
Status epilepticus and emergency therapy
Status epilepticus is considered a true medical emergency, requiring prompt recognition and treatment. If untreated, continuous seizure activity can lead to hyperthermia, hypoxia, hypotension, renal failure, disseminated intravascular coagulation, aspiration pneumonia and cardiopulmonary failure. A study of dogs with idiopathic epilepsy found that those experiencing episodes of status epilepticus had a significantly shorter mean survival time. 39
First-line emergency therapy
Intravenous diazepam, at 0.5–1.0 mg/kg body weight, is safe and has a rapid onset of action. If the bolus is successful in ceasing seizure activity, but additional seizures occur, further boluses or a continuous infusion may be administered. An infusion of diazepam at a rate of 0.5–2 mg/kg/h is used to maintain a seizure-free state. Careful monitoring of heart rate, blood pressure and respiratory rates, as well as diligent nursing care, are needed while cats are being treated with diazepam infusions. Once seizure-free for approximately 24 h, the infusion should be tapered and discontinued, while carefully monitoring for additional seizure activity.
Other traditional options
If diazepam is unsuccessful in ceasing seizure activity, other traditional options include pentobarbital, phenobarbital and propofol.
Pentobarbital is a barbiturate that is useful in treating intractable seizures at a dose of 2–15 mg/kg IV.14,40 It is not an anticonvulsant drug but rather ceases the motor activity that is associated with seizures. A complicating factor when using pentobarbital is that animals tend to paddle when recovering, which can easily be confused with seizure activity. Pentobarbital takes effect quickly, though not immediately like diazepam. It is potentially a severe respiratory depressant, and also causes hypothermia, so careful monitoring of the patient's breathing and temperature is indicated.
Phenobarbital is another viable emergency drug for treating status epilepticus. The bolus dose is 2–6 mg/kg body weight but it must be stressed that the effect is not immediate, but rather can take 15–25 minutes, so overdosing needs to be avoided. Use of phenobarbital as an emergency drug is useful when phenobarbital is the chosen maintenance drug. Phenobarbital can also be administered as a continuous infusion at a rate of 2–4 mg/kg/h. 14
-
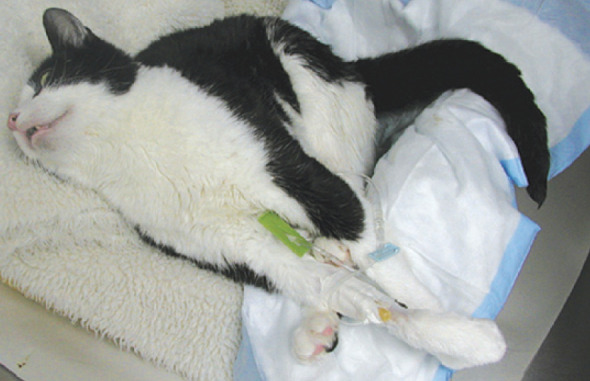
Propofol is an injectable anesthetic agent that has been shown to have GABA agonist activity in the central nervous system. In addition, it is helpful in lowering intracranial pressure as well as brain metabolic activity. 14 The drug must be given as a slow bolus (1–6 mg/kg body weight) as apnea is a common side effect if given too quickly. 41 Therefore, precautions must be taken and intubation must be available should the patient become apneic. Propofol is very successful in ceasing seizure activity and an infusion may be used at a rate of 0.1–0.6 mg/kg/min (Fig 3). In addition to causing apnea, propofol is also a cardiovascular depressant, so this too requires careful monitoring. Similar to pentobarbital, paddling may be seen when the patient is recovering from propofol treatment, and this may be confused with seizure activity. Although a propofol infusion may be expensive for larger dogs, this is rarely an issue in cats and, therefore, this is a very useful drug to treat status epilepticus.
Fig 3.
 Cat being treated with a propofol infusion for cluster seizures. Note that she is intubated during treatment and kept on a well padded surface. She was turned regularly, kept clean and dry, and had her lungs auscultated frequently to monitor breath sounds and effort
Cat being treated with a propofol infusion for cluster seizures. Note that she is intubated during treatment and kept on a well padded surface. She was turned regularly, kept clean and dry, and had her lungs auscultated frequently to monitor breath sounds and effort
Where sedation is a risk …
All of the above drugs, though useful emergency medications, cause sedation of varying degrees, which is undesirable in certain situations such as in post-craniotomy patients, patients with aspiration pneumonia, or patients in which the neurological status needs to be continuously monitored. In these situations, intravenous levetiracetam is a viable option. An injectable formulation has been developed for use in humans with partial-onset (focal) seizures and is well tolerated by humans, dogs and cats. The pharmacokinetics for the intravenous formulation are similar to the oral one.29,42 Side effects have not been noted in dogs and cats, though humans report dizziness, somnolence, fatigue and headaches. 43
Although additional work is needed, the authors have had success in ceasing seizure activity with levetiracetam administered in 20 mg/kg body weight boluses. The anticonvulsant effect is rapid and is maintained for several hours (longer than a single diazepam intravenous dose). More importantly, patients do not become sedate and appear to recover from the seizure episode more quickly than with diazepam administration. The patient can then be maintained on levetiracetam or transitioned onto phenobarbital depending on the individual case.
The half-life of zonisamide in cats is 33 h. 35 Due to this long half-life, once-daily administration of a 5–10 mg/kg body weight dose is likely to be appropriate in cats, although additional studies are needed to determine this. Approximately half of cats receiving a 20 mg/kg dose of zonisamide experience adverse reactions such as anorexia, diarrhea, vomiting, somnolence and ataxia. 35 The authors have used zonisamide in two cats that experienced seizures that were refractory to phenobarbital; one cat developed anorexia, necessitating removal of the drug, and the other cat responded very well, showing a dramatic reduction in seizure frequency.
Pregabalin
Pregabalin is a new anticonvulsant drug that is considered to be the ‘next generation’ of gabapentin. Its mechanism of action appears to be related to interaction with the α2δ subunit of neuronal voltage-gated calcium channels. Reducing calcium influx leads to reduction of the synaptic release of glutamate, an excitatory neurotransmitter. In dogs administered a 4 mg/kg bodyweight dose orally, pregabalin has a half-life of 6.8 hours; this dose appears to be well tolerated. 36 The major side effect noted in dogs is sedation and the dose must be gradually increased from 2 mg/kg to 3 or 4 mg/kg q 8–12 h as needed for effectiveness. 37
No information is available regarding the use of pregabalin in cats; however, it is likely to prove an effective antiepileptic drug in this species. Its predecessor, gabapentin, has been used with limited success in cats; anecdotally, the dose is 5–10 mg/kg body weight PO q 12 h. Sedation is the most common side effect noted with gabapentin therapy, and is likely to be a side effect associated with pregabalin in cats as well. The half-life and therapeutic range for both gabapentin and pregabalin are currently unknown in cats.
Monitoring and assessment of seizure control
All clients should be encouraged to maintain a seizure log, detailing the number of seizures as well as unique features of the seizure (ie, length of ictus period, post-ictal period, generalized versus focal versus cluster seizure, and any other events that may affect the cat's life). Regular physical and neurologic examinations should be performed, at a minimum of every 6 months. These visits are often more frequent in the initial stages of therapy. Monitoring of antiepileptic drug concentrations and a minimum database (CBC, chemistry profile and urinalysis) is indicated every 6 months once the cat is on maintenance therapy, and more frequently at the beginning of therapy.
When therapy is not effective (ie, the cat shows a reduction in seizure frequency of less than 50%, or no change, or an increase in seizure frequency), the treatment plan must be re-evaluated. Treatment failures are the result of progressive disease, refractory seizures, poor client compliance, inadequate drug dosing or drug interactions. Refractory cases should be treated either by increasing the dosage of the present drug or by adding another medication to the treatment regime. This decision is aided by assessing current antiepileptic drug blood concentrations, general blood work and side effects related to the drug. If the drug concentration is within the low-normal range and side effects are not noted, increasing the initial drug dosage is a viable option. If side effects are present or the drug concentration is high-normal or high, another antiepileptic drug should be added.
Successful response to therapy?
Determining how effective an antiepileptic drug is proving should be based on the individual cat's response. A drug is considered to be effective if it results in a reduction in seizure frequency of 50% or more. For example, a cat that seizured six times per month prior to therapy, and three or fewer times per month after therapy was instituted, is considered to have shown a favorable response to medication. By contrast, a cat that seizured once per month both prior to and after therapy is considered a treatment failure.
KEY POINTS
Differentiating between primary and secondary seizure disorders is critical for determining a prognosis as well as devising an appropriate treatment plan.
Cats with idiopathic epilepsy often have tonic-clonic and generalized seizures. However, focal seizures may also be a feature of idiopathic epilepsy.
Response to therapy, rather than the severity of seizure activity, is an indication of prognosis in cats with seizures; therefore, aggressive therapy with anticonvulsants is recommended.
Phenobarbital is the current drug of choice for cats with multiple seizure episodes.
Levetiracetam is a safe and effective antiepileptic when given as an adjunct to phenobarbital in cats with suspected idiopathic epilepsy, and may be considered a second-line anticonvulsant in cats.
Case notes
Allison, an 11-year-old female spayed domestic shorthair cat, presented with a recent onset of generalized seizures. She experienced two on the day of presentation and one earlier in the week. According to her owners, she had also been showing behavioral changes over the previous few weeks.
Case work-up No major abnormalities were noted on physical examination. On neurological examination, Allison was quiet, alert and responsive. She had a left head turn. Examination of her cranial nerves revealed a negative menace response in her right eye (OD), and normal palpebral and pupillary light reflexes in both eyes (OU). She had decreased nasal sensation on the right side of her face and a slight lip droop on the right. She was ambulatory with a normal gait, though she often walked in wide circles to the left side. Her proprioception was decreased in the right thoracic and right pelvic limbs, as evidenced by decreased hopping and decreased extensor postural thrust on the right side. Her spinal reflexes were all within normal limits. Palpation appeared to elicit head and neck pain.
Allison was presumptively diagnosed with a left forebrain lesion. She had two additional seizures on the day of admission and was treated with phenobarbital 2.5 mg/kg IV BID. Prior to anesthesia for advanced imaging, a CBC, chemistry profile and urinalysis were performed and all results were within normal limits. Three view thoracic radiographs were obtained and were also normal. General anesthesia was subsequently induced and MRI performed (see left).
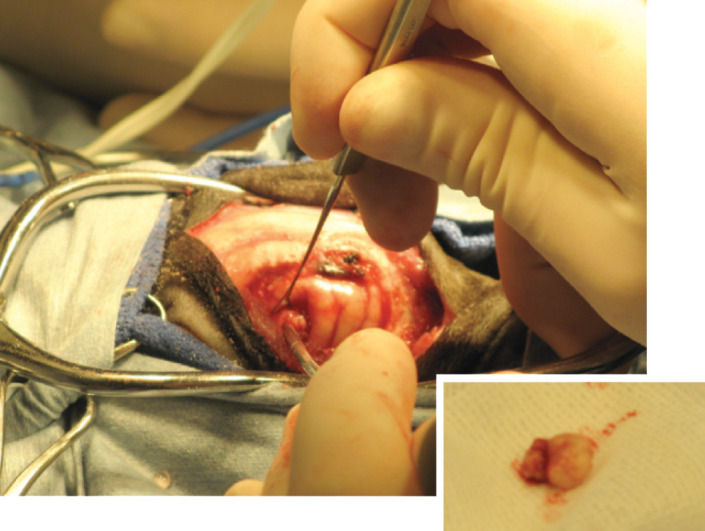
Diagnosis An extra-axial contrast-enhancing mass was identified in the left cerebrum, which was most consistent with a meningioma. Allison was taken to surgery the next day for mass removal via a craniotomy (see right). Histopathology of the mass was consistent with a meningioma. Prednisone 0.5 mg/kg BID was added to the treatment protocol.
Treatment and follow-up Allison initially responded favorably to surgery and phenobarbital treatment; her behavior returned to normal at home and she did not have any seizures. At her 3-week follow-up examination she continued to show a negative menace response OD, but the remainder of her examination was normal. A CBC and chemistry profile were performed and were normal. Her phenobarbital level was measured and the results were in the upper therapeutic range (28 μg/ml; ideal range 23–30 μg/ml).
Shortly after this examination, Allison re-presented following a cluster of five seizures in a 24 h period. Levetiracetam 20 mg/kg PO TID was added to her treatment protocol.
Six months later, Allison is doing well; she continues to have a negative menace response OD but is happy at home. She has had one generalized seizure, which occurred about 6 weeks after starting levetiracetam treatment. Her serum levetiracetam level is currently 41 μg/ml and serum phenobarbital level is 26 μg/ml. No changes to her anticonvulsant therapy have been made.
WHAT THIS CASE DEMONSTRATES
Despite therapy for the inciting cause of seizures (surgery, in Allison's case), anticonvulsants are a necessary, and often lifelong, component of care for the seizuring cat. In situations where phenobarbital is not adequately controlling seizures, and the serum drug concentration is adequate, addition of a second anticonvulsant can be successful. Levetiracetam may be considered the second-line anticonvulsant in cats.
Withdrawal or discontinuation of therapy
Therapy may be extremely successful and cats may become seizure-free. Although each cat requires an individual approach, a guideline is to consider reducing/eliminating drugs when the patient is seizure-free for at least 6 months. Drugs should never be discontinued abruptly as ‘withdrawal seizures’ may occur; this is most commonly seen with phenobarbital due to the development of physical dependence on the drug. 38 Rather, the cat should be slowly weaned off over several weeks.
Occasionally, cats will experience a negative reaction or severe side effects to a drug, necessitating rapid discontinuation of therapy. This may need to be done in a hospital setting, or with the client's understanding that seizures may occur with abrupt drug withdrawal.
References
- 1.Barnes HL, Chrisman CL, Mariani CL, Sims M, Alleman A. Clinical signs, underlying cause and outcome in cats with seizures: 17 cases (1997–2002). J Am Vet Med Assoc 2004; 225: 1723–26. [DOI] [PubMed] [Google Scholar]
- 2.Platt SR. Feline seizure control. J Am Anim Hosp Assoc 2001; 37: 515–17. [DOI] [PubMed] [Google Scholar]
- 3.Quesnel AD, Parent JM, McDonell W. Clinical management and outcome of cats with seizure disorders: 30 cases (1991–1993). J Am Vet Med Assoc 1997; 10: 72–77. [PubMed] [Google Scholar]
- 4.Thomas WB, Dewey CW. Seizures and narcolepsy. In: Dewey CW, ed. A practical guide to canine and feline neurology. 2nd edn. Ames: Wiley Blackwell, 2008: 193. [Google Scholar]
- 5.Shell LG. Seizures in cats. Vet Med 1998; 93: 541–52. [Google Scholar]
- 6.Cizinauskas S, Fatzer R, Schenkel M, Gandini G, Jaggy A. Can idiopathic epilepsy be confirmed in cats? [abstract]. J Vet Intern Med 2003; 17: 246. [Google Scholar]
- 7.Schriefl S, Steinberg TA, Matiasek K, Ossig A, Fenske N, Fisher A. Etiologic classification of seizures, signalment, clinical signs, and outcome in cats with seizure disorders: 91 cases (2000–2004). J Am Vet Med Assoc 2008; 233: 1591–97. [DOI] [PubMed] [Google Scholar]
- 8.Quesnel AD, Parent JM, McDonell W, Percy D, Lumsden JH. Diagnostic evaluation of cats with seizure disorders: 30 cases (1991–1993). J Am Vet Med Assoc 1997; 210: 65–71. [PubMed] [Google Scholar]
- 9.Poncelet L. Electrophysiology. In: Platt S, Olby NJ, eds. BSAVA manual of canine and feline neurology. 3rd edn. Gloucester: BSAVA, 2004: 54. [Google Scholar]
- 10.Dewey CW. Encephalopathies: Disorders of the brain. In: Dewey CW, ed. A practical guide to canine and feline neurology. 2nd edn. Ames: Wiley Blackwell, 2008: 115–220. [Google Scholar]
- 11.Foster SF, Charles JA, Parker G, Krockenberger M, Churcher RM, Malik R. Cerebral cryptococcal granuloma in a cat. J Feline Med Surg 2001; 3: 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmann D, Cizinauskas S, Tomek A, Doherr M, Vandeveld M, Jaggy A. Retrospective analysis of seizures associated with feline infectious peritonitis in cats. J Feline Med Surg 2008; 10: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfohl JC, Dewey CW. Intracranial Toxoplasma gondii granuloma in a cat. J Feline Med Surg 2005; 7: 369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewey CW. Anticonvulsant therapy in dogs and cats. Vet Clin North Am Small Anim Pract 2006; 36: 1107–27. [DOI] [PubMed] [Google Scholar]
- 15.Dayrell-Hart B, Steinberg SA, VanWinkle RJ, Farnbach GC. Hepatotoxicity of phenobarbital in dogs: 18 cases (1985–1989). J Am Vet Med Assoc 1991; 199: 1060–66. [PubMed] [Google Scholar]
- 16.Gaskill CL, Miller LM, Mattoon JS, et al. Liver histopathology and liver and serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving phenobarbital. Vet Pathol 2005; 42: 147–60. [DOI] [PubMed] [Google Scholar]
- 17.Ducote JM, Coates JR, Dewey CW, Kennis RA. Suspected hypersensitivity to phenobarbital in a cat. J Feline Med Surg 1999; 1: 123–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boothe DM. Anticonvulsant therapy in small animals. Vet Clin North Am Small Anim Pract 1998; 28: 411–47. [DOI] [PubMed] [Google Scholar]
- 19.Center SA, Elston TH, Rowland PH, et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc 1996; 209: 618–25. [PubMed] [Google Scholar]
- 20.Hughes D, Moreau RE, Overall KL, VanWinkle TJ. Acute hepatic necrosis and liver failure associated with benzodiazepine therapy in six cats. J Vet Emerg Crit Care 1996; 6: 13–20. [Google Scholar]
- 21.Boothe DM, George KL, Couch P. Disposition and clinical use of bromide in cats. J Am Vet Med Assoc 2002; 221: 1131–35. [DOI] [PubMed] [Google Scholar]
- 22.Wagner SO. Lower airway disease in cats on bromide therapy for seizures [abstract]. J Vet Intern Med 2001; 15: 562. [Google Scholar]
- 23.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol 1998; 353: 191–206. [DOI] [PubMed] [Google Scholar]
- 24.Hovinga CA. Levetiracetam: A novel antiepileptic drug. Pharmacotherapy 2001; 21: 1375–88. [DOI] [PubMed] [Google Scholar]
- 25.Leppik IE. The place of levetiracetam in the treatment of epilepsy. Epilepsia 2001; 42 (Suppl 4): 44–45. [PubMed] [Google Scholar]
- 26.Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci 2004; 101: 9861–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. Eur J Pharmacol 2006; 536: 102–8. [DOI] [PubMed] [Google Scholar]
- 28.Patsalos PN. Pharmacokinetic profile of levetiracetam: Toward ideal characteristics. Pharmacol Ther 2000; 85: 77–85. [DOI] [PubMed] [Google Scholar]
- 29.Carnes MB, Boothe DM, Axlund TW. Disposition of levetiracetam in cats [abstract]. J Vet Intern Med 2008; 22: 765–66. [Google Scholar]
- 30.Bailey KS, Dewey CW, Boothe DM, Barone G, Kortz GD. Levetiracetam as an adjunct to phenobarbital treatment in cats with suspected idiopathic epilepsy. J Am Vet Med Assoc 2008; 232: 867–72. [DOI] [PubMed] [Google Scholar]
- 31.Shorvon SD, van Rijckevorsel K. A new antiepileptic [editorial]. J Neurol Neurosurg Psychiatry 2002; 2: 426–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewey CW, Guiliano R, Boothe DM, et al. Zonisamide therapy for refractory idiopathic epilepsy in dogs. J Am Anim Hosp Assoc 2004; 40: 285–91. [DOI] [PubMed] [Google Scholar]
- 33.von Klopmann T, Rambeck B, Tipold A. Prospective study of zonisamide therapy for refractory idiopathic epilepsy in dogs. J Small Anim Pract 2007; 48: 134–38. [DOI] [PubMed] [Google Scholar]
- 34.Orito K, Saito M, Fukunaga K, et al. Pharmacokinetics of zonisamide and drug interaction with phenobarbital in dogs. J Vet Pharmacol Ther 2008; 31: 259–64. [DOI] [PubMed] [Google Scholar]
- 35.Hasegawa D, Kobayashi M, Kuwabara T, Ohmura T, Fujita M, Orima H. Pharmacokinetics and toxicity of zonisamide in cats. J Feline Med Surg 2008; 10: 418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar V, Dewey CW, Schwark WS, et al. Pharmacokinetics of single-dose oral pregabalin administration in dogs [abstract]. J Vet Intern Med 2008; 22: 765. [Google Scholar]
- 37.Dewey CW, Cerda-Gonzalez S, Levine JM, et al. Pregabalin therapy for refractory idiopathic epilepsy in dogs [abstract]. J Vet Intern Med 2008; 22: 765. [Google Scholar]
- 38.Podell M. Seizures. In: Platt SR, Olby NJ, eds. BSAVA manual of canine and feline neurology. Gloucester: BSAVA, 2004: 97. [Google Scholar]
- 39.Saito M, Munana KR, Sharp NJ, Olby NJ. Risk factors for development of status epilepticus in dogs with idiopathic epilepsy and effects of status epilepticus on outcome and survival time: 32 cases (1990–1996). J Am Vet Med Assoc 2001; 219: 618–23. [DOI] [PubMed] [Google Scholar]
- 40.Platt SR, McDonnell JJ. Status epilepticus: Patient management and pharmacologic therapy. Compend Contin Educ Pract Vet 2000; 22: 722–29. [Google Scholar]
- 41.Steffen F, Grasmueck S. Propofol for treatment of refractory seizures in dogs and a cat with intracranial disorders. J Small Anim Pract 2000; 41: 496–99. [DOI] [PubMed] [Google Scholar]
- 42.Dewey CW, Bailey KS, Boothe DM, Badgley BL, Cruz-Espindola C. Pharmacokinetics of single-dose intravenous levetiracetam administration in normal dogs. J Vet Emerg Crit Care 2008; 18: 153–57. [Google Scholar]
- 43.Ramael S, Daoust A, Otoul C, et al. Levetiracetam intravenous infusion: A randomized, placebo-controlled safety and pharmacokinetic study. Epilepsia 2006; 47: 1128–35. [DOI] [PubMed] [Google Scholar]