Abstract
The present report concerns a case of sporotrichosis in a 3-year-old male crossbred cat. After 9 months of oral itraconazole (20 mg/kg) therapy, all skin lesions had resolved with the exception of a single nodular lesion located on the bridge of the nose. Therefore, a combined therapy that included intralesional (IL) amphotericin B (1 mg/kg) was started. The lesion resolved completely after three weekly administrations of IL amphotericin B, given in concert with oral itraconazole. The cat remains well 1 year after discontinuing therapy, with no signs of recurrence.
Sporotrichosis is caused by the dimorphic fungus Sporothrix schenckii. Feline sporotrichosis clinically ranges from a subclinical infection to a single or even multiple skin lesions and fatal systemic forms. 1
The first epidemic of sporotrichosis in humans as a result of zoonotic transmission was identified in Rio de Janeiro, Brazil. From 1998 to 2004, 759 humans, 64 dogs and 1503 cats were diagnosed with sporotrichosis in the Evandro Chagas Clinical Research Institute-Fiocruz. The evidence relies on the close relationship between humans and cats with severe feline sporotrichosis with cutaneous and mucosal lesions. Of them, 85% of dogs and 83.4% of patients were reported to have had contact with cats with sporotrichosis, and 55.8% of the latter reported cat bites or scratches. 2
Traditionally, sodium or potassium iodides have been used in the treatment of sporotrichosis. Nonetheless, serious adverse effects associated with the use of iodides in the cat have led to their replacement with safer and more effective antifungal drugs, such as the triazoles. 3
Ketoconazole and itraconazole have been used most recently to treat feline sporotrichosis. 1 Itraconazole is considered to be the best choice for cats with this disease,3,4 not only because of its effectiveness but also the absence of side effects at doses that are generally effective when compared to previously recommended antifungal agents. 5
Amphotericin B has been indicated for the treatment of rapidly progressive or severe systemic mycosis, imidazole-resistant cryptococcosis 6 and disseminated forms of sporotrichosis, 7 even though its use has been limited by nephrotoxicity. 8
Reports on the use of amphotericin B in feline sporotrichosis are few. Three cases have been described based on its intravenous (IV) use in cats.8,9 Reports on the intralesional (IL) use of the drug in cats with sporotrichosis have not been found.
Amphotericin B may be a worthwhile additional therapy for certain cases of feline sporotrichosis. In this study, we describe its IL use in concert with oral itraconazole in a cat with sporotrichosis, which presented with a persistent refractory skin lesion (Figs 1–3).
Fig 1.
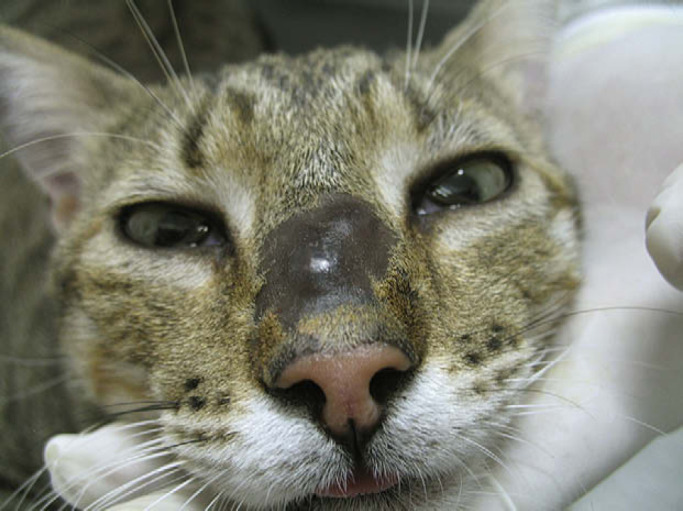
Nodular lesion on the bridge of the nose after 9 months of oral itraconazole therapy.
Fig 2.
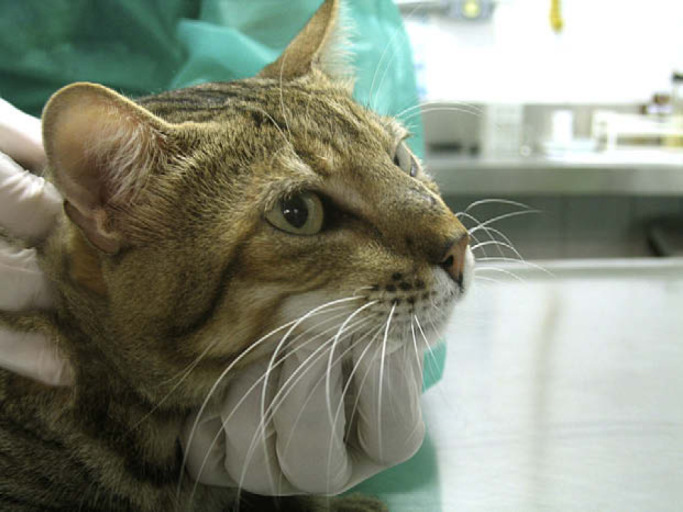
Scar tissue on the bridge of the nose after intralesional amphotericin B therapy and oral itraconazole.
Fig 3.
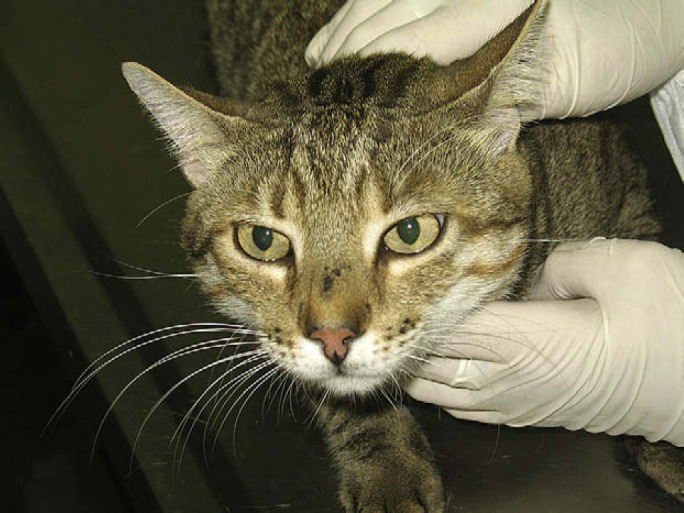
Scar tissue on bridge of the nose 1 year after the clinical cure.
A cat from the Rio de Janeiro metropolitan area was taken to the Laboratory of Clinical Research on Dermatozoonosis in Domestic Animals – LAPCLIN-DERMZOO – of the Evandro Chagas Clinical Research Institute (IPEC), at the Oswaldo Cruz Foundation (Fiocruz). The patient was a 3-year-old castrated male crossbred cat, weighing 5 kg. Its overall condition was good. Nevertheless, after a clinical examination, a nodule was found on the nasal bridge and ulcerated skin lesions were additionally detected on its face, left ear, left front leg and on its chest. It was reported that the lesions had been present for 60 days.
Material from the ulcerated lesion on its left ear was collected for cytological examination and mycological culture. Yeast-like cells were observed in Giemsa-stained semears. To isolate and identify S schenckii, the secretion from the lesion obtained with a swab was submitted to routine mycological examination as follows: seeding on to Saboraud-dextrose agar and mycobiotic agar (DIFCO), incubated at 25°C and observed during 4 weeks for fungal growth. Suspected isolates were subcultivated on potato-dextrose-agar medium (DIFCO) at 25°C for macroscopic and microscopic morphological studies and dimorphism was demonstrated by conversion to the yeast-like form on BHI agar medium (DIFCO) at 37°C. 10
Oral itraconazole (20 mg/kg) was prescribed to be taken once a day. The drug was well tolerated and no side effects were detected in the cat. After 9 months of antifungal therapy, all skin lesions had healed, with the exception of a single nodular lesion on the bridge of the nose. In order to collect biological samples, the cat was sedated with a combination of ketamine hydrochloride (10 mg/kg) and acepromazine (0.1 mg/kg) in the same syringe via intramuscular route. After antisepsis and local anesthesia with 2% lidocaine of the biopsy region, two fragments were collected from the rim of the persistent lesion: one for the mycological culture and the other for histopathological examination.
S schenckii was, again, isolated and identified in mycological culture. 10 In the histopathological examination, yeast-like cells, suggestive of this fungus, were identified on routine hematoxylin-eosin, periodic acid-Schiff (PAS) and Grocott's silver stains.
Due to the persistence of the lesion, we opted for the use of IL amphotericin B (Anforicin; Cristália) while continuing oral itraconazole.
Blood samples were collected for full blood examination and serum biochemical analyses before and during IL amphotericin B therapy. The drug was diluted in 10 ml of distilled water (5 mg/ml) and the necessary amount was aspirated and directly infiltrated in the lesion up to its intumesciment with a disposable needled 0.38×13 mm (27.5 G½) 1 ml insulin syringe. It was observed as the size of the lesion decreased, in each injection the volume of amphotericin B was 1 ml, 0.9 ml and 0.8 ml, in this order.
The cat was sedated as described before each injection and the procedure was undertaken weekly until the lesion regressed completely, which occurred after the third IL injection. Itraconazole was discontinued 1 week after the third application.
No relevant alterations were identified in routine haematology or serum biochemistry panels (urea, creatinine, alanine aminotransferase, aspartate aminotransferase and alkaline phosphatase) before and during IL amphotericin B. One year after its clinical cure, the animal had no further active lesions, only a scar on the nasal bridge.
All of the procedures undertaken during this study were approved by the Commission of Ethics in the Use of Animals, of the Oswaldo Cruz Foundation (CEUA – Fiocruz).
Even though the treatment with itraconazole is effective with many feline patients and is convenient for owners, the expected clinical response is not observed in all feline patients. 1 The patient, in this study, presented with a persistent lesion in its nasal region despite 9 months of conventional treatment with oral itraconazole, given at a high dose rate. According to Malik et al , 11 the nasal region of felines does not have a rich supply of blood, nor does it possess easily accessible mobile skin nearby, which would facilitate reconstructive surgical procedures. Thus, the cure of infections in this specific area may be problematic.
Systemic administration of amphotericin B relies on either the IV route or subcutaneous infusions twice or three times a week in a large volume of 2.5% dextrose and 0.45% sodium chloride for many weeks. The duration of IV amphotericin B therapy is limited due to the tendency for thrombosis of cephalic and jugular veins in cats. 12
In the literature, the three reports on treatments with IV amphotericin B in cats with sporotrichosis document: (1) first case, although some initial improvement was noted, skin lesions increased after 3 weeks of potassium iodide therapy. This therapy was discontinued and the cat was given amphotericin B for 1 week. The cat's condition continued to deteriorate and it was euthanased. (2) In another case, nephrotoxicity limited the use of amphotericin B, although a moderate response was noted. (3) One more patient was euthanased due to neurological deterioration during amphotericin B and 5-fluorocytosine therapy.8,9 For those reasons, we opted for IL rather systemic administration of the drug.
Although there is a precedence for using IL amphotericin B to treat localised fungical infections in specific regions, 13–16 this treatment has not been described for feline sporotrichosis. This application produces high tissue concentrations, theoretically providing improved efficiency and fewer systemic effects.13,17
Vieira et al 14 were successful in using IL amphotericin B in association with surgical excision of non-responding lesion to treat cutaneous alternariosis in a human patient. Fifty milligrams of amphotericin B dissolved in 10 ml of distilled water was diluted with four volumes of 0.5% procaine hydrochloride to give a final amphotericin B concentration of 1 mg/ml and 1 ml was injected directly into the lesions twice a week for 5 weeks. The injections were well tolerated in spite of moderate pain where they had been applied.
Zamos et al 16 used 100 mg of IL amphotericin B (100 mg/ml) combined with 3 ml of dimethylsulfoxide solution via endoscopic guidance and systemic treatment with IV sodium iodide solution and oral potassium iodide in a case of nasopharyngeal conidiobolomycosis in a horse. Two months after the complete disappearance of the nasal masses, clinical signs recurred and the owner opted for euthanasia. Malik et al 12 successfully treated a case of feline cryptococcosis with the injection of IL amphotericin B in the nasal region, together with a treatment with subcutaneous amphotericin B and oral antifungal agents.
Studies have demonstrated that the combination of amphotericin B and oral antifungal agents was more effective than when the drug was used alone in the treatment of fungal infections.17,18 There was a reduction in both the duration of therapy and the necessary cumulative dosage of amphotericin B required for a cure.12,19 For those reasons, we opted for the IL administration of the drug, together with the treatment with oral antifungal agents. Consistent with the findings of Takahashi and Maie, Iwatsu and Vieira et al, 13–15 the present study has also showed efficacy of IL amphotericin B.
As in the Vieira et al 14 study, which did not observe the systemic effects in a human patient with cutaneous alternariosis after the use of this drug intralesionally. Blood and biochemical profiles in our cat failed to demonstrate any adverse changes, not even hepatotoxicity due to itraconazole, as reported by Medleau et al and Rosser and Dunstan.4,20
According to Malik et al, 12 it is possible to observe adverse effects related to the local administration of large volumes of amphotericin B, such as local irritation or a sterile abscess. In spite of that, the same authors have not observed such effects in a cat with cryptococosis. In this study, local adverse effects were also not identified after IL administration of the drug, either.
In the 10 years the LAPCLIN-DERMZOO-IPEC/Fiocruz has been monitoring the sporotrichosis epidemic, 1 several cases of cats with refractory focal lesions have been seen despite conventional oral antifungal treatment. Hence, we suggest that IL amphotericin B may represent a useful and cost effective therapeutic option for feline cutaneous sporotrichosis. Finally, it is worth mentioning that its costs greatly favour its use.
Acknowledgements
This study was partially supported by the Health Strategic Research Support Program (PAPES V) – FIOCRUZ. TMP Schubach is a fellowship from the Technological and Scientific Development National Council (CNPq).
References
- 1.Schubach T.M., Schubach A., Okamoto T., et al. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998–2001), J Am Vet Med Assoc 224, 2004, 1623–1629. [DOI] [PubMed] [Google Scholar]
- 2.Schubach A., Barros M.B., Wanke B. Epidemic sporotrichosis, Curr Opin Infect Dis 21, 2008, 129–133. [DOI] [PubMed] [Google Scholar]
- 3.Welsh R.D. Sporotrichosis, J Am Vet Med Assoc 223, 2003, 1123–1126. [DOI] [PubMed] [Google Scholar]
- 4.Rosser E.J., Dunstan R.W. Sporotrichosis. Greene C.E. Infectious Diseases of the Dog and Cat, 3rd edn, 2006, Saunders Elsevier: Philadelphia, 608–612. [Google Scholar]
- 5.Sykes J.E., Torres S.M., Armstrong P.J., Lindeman C.J. Itraconazole for treatment of sporotrichosis in a dog residing on a Christmas tree farm, J Am Vet Med Assoc 218, 2001, 1440–1443. [DOI] [PubMed] [Google Scholar]
- 6.Greene C.E. Antifungal chemotherapy. Greene C.E. Infectious Diseases of the Dog and Cat, 3rd edn, 2006, Saunders Elsevier: Philadelphia, 542–550. [Google Scholar]
- 7.Peaston A. Sporotrichosis, J Vet Intern Med 7, 1993, 44–45. [DOI] [PubMed] [Google Scholar]
- 8.Werner A.H., Werner B.E. Sporotrichosis in man and animal, Int J Dermatol 33, 1994, 692–700. [DOI] [PubMed] [Google Scholar]
- 9.Dunstan R.W., Langham R.F., Reimann K.A., Wakenell P.S. Feline sporotrichosis: a report of five cases with transmission to humans, J Am Acad Dermatol 15, 1986, 37–45. [DOI] [PubMed] [Google Scholar]
- 10.Schubach T.M., Schubach A., Reis R.S., Cuzzy-Maia T., Blanco T.C., Monteiro D.F. Sporothrix schenckii isolated from domestic cats with and without sporotrichosis in Rio de Janeiro, Brazil, Mycopathol 153, 2002, 83–86. [DOI] [PubMed] [Google Scholar]
- 11.Malik R., Vogelnest L., O Brian C.R., et al. Infections and some other conditions affecting the skin and subcutis of the naso-ocular region of cats – clinical experience 1987–2003, J Feline Med Surg 6, 2004, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik R., Craig A.J., Wigney D.I., Martin P., Love D.N. Combination chemotherapy of canine and feline cryptococcosis using subcutaneously administered amphotericin B, Aust Vet J 73, 1996, 124–128. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S., Maie O. Cutaneous chromomycosis: therapy with intra-lesional amphotericin B injections, Hautarzt 32, 1981, 567–570. [PubMed] [Google Scholar]
- 14.Vieira M.R., Martins M.L., Afonso A., Rego F., Cardoso J. Cutaneous alternariosis, Rev Iberoam Micol 15, 1998, 97–99. [PubMed] [Google Scholar]
- 15.Iwatsu T. Cutaneous alternariosis, Arch Dermatol 124, 1988, 822–825. [PubMed] [Google Scholar]
- 16.Zamos D.T., Schumacher J., Loy J.K. Nasopharyngeal conidiobolomycosis in a horse, J Am Vet Med Assoc 208, 1996, 100–101. [PubMed] [Google Scholar]
- 17.Silva C.M.P., Marques S.G., Silva R.R., Souza S.S., Junior, Menezes D.R.T., Junior, Costa J.M.L. Cromoblastomicose tratada com itraconazol sistêmico associado a anfotericina B intralesional, An Bras Dermatol 74, 1999, 41–44. [Google Scholar]
- 18.Barchiesi F., Schimizzi A.N., Caselli F., et al. Interactions between triazoles and amphotericin B against Cryptococcus neoformans, Antimicrob Agents Chemother 44, 2000, 2435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennet J.E., Dismukes W.E., Duma R.J., Medoff G., Sande M.A., et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptoccal meningitis, N Engl J Med 301, 1979, 126–131. [DOI] [PubMed] [Google Scholar]
- 20.Medleau L., Jacobs G.J., Marks M.A. Itraconazole for the treatment of cryptococcosis in cats, J Vet Intern Med 9, 1995, 39–42. [DOI] [PubMed] [Google Scholar]


