Abstract
This report describes a nosocomial outbreak of feline calicivirus (FCV) associated virulent systemic disease (VSD) in a French veterinary teaching hospital in 2005. The outbreak started in March and resolved within 1 month. Signs, clinical course, clinicopathological findings and lesions were typical of FCV-induced VSD. FCV infection was confirmed by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). Among the eight infected cats, two had to be euthanased, three died, and three recovered after medical treatment. Virus could not be confined inside the animal hospital and on two occasions, students' own cats became infected. Subsequent genetic sequencing studies confirmed that the eight cats were infected with the same strain of virus, and that it was distinct from those involved in the US and UK outbreaks of VSD. Virulence and viral excretion patterns of the isolated strain were further characterised by experimental infection.
Feline calicivirus (FCV) is a common pathogen of cats worldwide. 1 FCV infection usually causes an acute, mild and self-limiting upper-respiratory tract disease characterised by oral vesicles/ulcers. 1–3
FCV is a small RNA virus characterised by a strong genetic and antigenic variability. 4 Region E, the immunodominant region of the capsid, contains two hypervariable areas. 1 The genetic and antigenic variabilities of FCV allow the virus to escape from the host immune response, and also explains the diversity in tissue tropism and virulence that accounts for a large spectrum of FCV-induced diseases. 3
FCV has been associated with diverse clinical manifestations including sudden death, 5 paw and mouth disease,6,7 ulcerative dermatitis,8,9 agitated state preceding death, 10 abortion, 11 jaundice, 12 feline lower urinary tract disease13,14 and feline chronic gingivo–stomatitis complex. 15
The importance of FCV-associated pneumonia may have been over emphasised by use of aerosol rather than natural oronasal route for experimental infection in the past. 1
By contrast, the role of FCV as a causative agent of spontaneous acute synovitis,16,17 acute faucitis 18 and more recently virulent systemic disease (VSD, formerly haemorrhagic-like fever 19 ) has been confirmed experimentally.
In the last decade, FCV-associated VSD (FCV-VSD) has emerged in the United States of America and the United Kingdom. Several outbreaks have been associated with a high (up to 50%) mortality rate and atypical severe clinical signs (high fever, cutaneous oedema, ulcerative dermatitis and jaundice). 19–22
This paper describes the first outbreak of FCV-VSD in France.
Materials and methods
Data collection
The outbreak occurred in March 2005 in the small animal clinics of the National Veterinary School of Toulouse (NVST). The clinical history of the cases was collected by one of the authors (BR), through personal intervention on some cases, examination of NVST's medical records of all affected cats and interviews with owners and clinicians involved in the outbreak.
Clinicopathological procedures
Haematological, plasma chemistry, cytological, clotting and histopathological examinations were performed by the clinical pathology laboratory of the small animal clinics and the pathology service of NVST, respectively, according to standard procedures.
FCV diagnosis by real-time RT-PCR
Samples from clinical cases were submitted to the Analysis Department of Scanelis Laboratory, France to detect FCV RNA by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) and quantify viral loads in the different samples. The limit of detection of the test as determined by probit analysis (European Pharmacopaeia (§ 2.6.1)) is 15 copies of viral RNA (detected in 95% of RT-PCR runs). The limit of quantification, defined as the lowest quantity of target nucleic acid that could be distinguished from a twofold quantity with a confidence level of 95%, is 200 copies of viral RNA.
Nucleic acid extraction, capsid gene amplification and sequencing
Total RNA was extracted from samples (200 μl of blood or plasma, cutaneous scrapings, oropharyngeal or conjunctival swabs) using an appropriate kit (NucleoSpin RNA Virus Kit, Macherey-Nagel, Hoerdt, France) as recommended by the supplier.
The FCV sequences available in the database were aligned (data not shown) in order to design primers needed in the study. The positions indicated for each primer refer to the F9 strain genome sequence M86379. Initially, two primers (FCVCapseq1F (5293–5312) tgatgtgttcgaagtttgag+FCVCapseq1R (7331–7355) agtrtcaatyaaaccyarwattgaa) were designed around the FCV capsid gene in order to amplify the complete gene. RT-PCR (FCVCapseq1F+FCVCapseq1R) was performed on an RNA sample using the Qiagen One-Step RT-PCR kit under the following conditions: 5 μl RNA sample, 10 μl 5× buffer, 2 μl dNTPs (10 mM each), 3 μl of each primer (10 μM), 2 μl RT/Taq mix in 50 μl total volume. Cycling conditions of the RT touchdown PCR were as follows: 50°C 30 min/95°C 15 min/5×[94°C 20 s/62°C 20 s/72°C 2 min]/5×[94°C 20 s/60°C 20 s/72°C 2 min]/40×[94°C 20 s/58°C 20 s/72°C 2 min]/72°C 5 min.
The RT-PCRs (FCVCapseq2F (6913–6932) ggttacacaggaattggaga+FCVCapseq2R: taagggtcaaccctgaatc (7549–7567); FCVCapseq3F (5867–5886) gcaaaatcctctttaagcaa+FCVCapseq2R) were designed to amplify the 3′ end of the capsid gene and were performed as described above, but with the following modifications in the annealing temperatures (62, 60, 58°C changed to 58, 56, 54°C, respectively). Polymerase chain reaction products were then cloned using a vector system (pGEM-T Easy phagemid Vector Systems, Promega, Charbonnières, France) as recommended by the supplier, and sequenced (Millegen, Labège, France).
Based on the sequence of the FCV capsid gene obtained, two specific primers were designed around the E region and their location on the sequence EU202915 was indicated (FCVCapseq4F (1127–1146) caatcgacattggactgaya+FCVCapseq4R (1883–1902) gatacacggcagadgartct). The RT-PCRs were performed as described, but with the following modifications in the cycling conditions 50°C 30 min/95°C 15 min/5×[94°C 20 s/56°C 20 s/72°C 2 min]/5×[94°C 20 s/54°C 20 s/72°C 2 min]/5×[94°C 20 s/52°C 20 s/72°C 2 min]/30×[94°C 20 s/50°C 20 s/72°C 2 min]/72°C. PCR products obtained with this specific PCR were directly sequenced without cloning.
Bioinformatics and phylogenetic analyses
Bioinformatics analyses (contigs assembly, sequence alignments) were performed using Vector NTI software (InVitrogen, Paisley, UK) and primers were designed using the on-line software Primer3. 23
Phylogenic nucleotide analysis was done with TREECON software 24 and nucleotide trees including 22 different sequences were generated by using the nearest-neighbour distance method. Bootstrapping was undertaken to generate 100 resamplings of the original data and rabbit haemorrhagic disease virus sequence was used as a root.
Indirect immunofluorescence assay (IFA)
To further characterise the isolate, IFA was undertaken using a panel of fully characterised monoclonal antibodies (Mabs). 25 These Mabs were specific for FCV431 major neutralising sites. An IFA was carried out on monolayers of FCV-infected Crandell-Reese feline kidney (CRFK; Merial) cells fixed in microplates with acetone. Mock infected cells were used as negative controls. Following the addition of 100 μl of ascitis fluid or hybridoma supernatant, cultures were incubated for 30 min at 37°C. After washing with phosphate buffered saline (PBS; Sigma, Saint Louis, MO) without calcium or magnesium pH 7.2, cultures were incubated for 30 min at 37°C with an anti-mouse IgG goat antibody conjugated with fluorescein isothiocyanate (BioSys, Ann Arbor, MI) 1:1500 in PBS. The reaction was read microscopically using an ultraviolet light source.
Experimental infection
Ten specific pathogen-free kittens (Charles River, Larbresle, France), approximately 20–21 weeks of age, were confirmed to be seronegative for FCV and healthy at the beginning of the study. This animal experiment and the associated procedures were reviewed and approved by Merial Ethics Committee.
The cats were inoculated via the oronasal route with 107.2 CCID50 of the VS-NVST strain derived from the blood of an infected cat and passaged twice on CRFK cells for amplification (isolate 4b) (Table 5). Clinical examination was performed daily for 14 days post-challenge, and a clinical scoring adapted from the European Pharmacopeia monograph was implemented (2 for depression, 1 for hyperthermia above 39.5°C, 1 for few and small oral ulcers, 3 for large or numerous ulcers, 1 for slight nasal discharge, 2 for copious nasal discharge, 1 for ocular discharge, 1 for cutaneous oedema, 1 for cutaneous ulcers, 2 for weight loss).
Table 5.
RT-PCR results in different samples from cats 1–8 and accession numbers of capsid sequences in Genbank
| Cat | Sample | Date of sampling * | Sample name | RT-PCR results | Accession number | |
|---|---|---|---|---|---|---|
| Qualitative result | Quantitative result | |||||
| 1 | Plasma | J6 | Isolate 1 | + | <40 RNA copies/μl | EU202911 |
| 2 | Blood | J2 | Isolate 2 | + | 104.5 RNA copies/μl | EU202912 |
| 3 | Plasma | J2 | Isolate 3 | + | <40 RNA copies/μl | EU202913 |
| 4 | Blood | J4 | Isolate 4a | + | 102.6 RNA copies/μl | EU202914 |
| Strain after 2 cell culture passages (P2) (108 DICC50/ml) | Isolate 4b | + | 106.7 RNA copies/μl | EU202915 | ||
| 5 | Oropharyngeal swab | J14 | Isolate 5a | + | 104.7 RNA copies/swab | EU202916 |
| Cutaneous scraping | J14 | Isolate 5b | + | 106.8 RNA copies/swab | EU2029117 | |
| Blood | J14 | Isolate 5c | + | <40 RNA copies/μl | NS | |
| Conjunctival swab | J14 | Isolate 5d | + | 104 RNA copies/swab | NS | |
| Urine | J14 | Isolate 5e | + | <40 RNA copies/μl | NS | |
| Rectal swab | J14 | Isolate 5f | + | 105.2 RNA copies/swab | NS | |
| 6 | Blood | J14 | Isolate 6a | + | 102.5 RNA copies/μl | EU202918 |
| Oropharyngeal swab | J14 | Isolate 6b | + | 108 RNA copies/swab | EU202919 | |
| Conjunctival swab | J14 | Isolate 6c | + | 105.2 RNA copies/swab | EU202920 | |
| Cutaneous scraping | J14 | Isolate 6d | + | 107.3 RNA copies/swab | EU202921 | |
| Urine | J14 | Isolate 6e | + | <40 RNA copies/μl | NS | |
| Rectal swab | J14 | Isolate 6f | + | 105.9 RNA copies/swab | NS | |
| 7 | Blood | J-4 | Isolate 7 | + | 102.2 RNA copies/μl | EU202922 |
| 8 | Blood | J3 | Isolate 8 | + | 103.6 RNA copies/μl | EU202923 |
NS=not sequenced.
Days after or before onset of clinical disease.
Pharyngeal swabs were collected on the day of challenge and 2, 4, 6, 8, 11 and 14 days after challenge, in 1 ml of Dulbecco modified Eagle's minimum essential medium (DMEM; GibcoBRL, MD, USA) enriched with antibiotics. Swabs and supernatants were stored at −70°C until virus isolation and titration.
Blood was collected in dry tubes (SST Vacutainer, Becton Dickinson, NJ) before vaccination, on the day of challenge and 14 days later. Sera were stored at −20°C until they were assayed for enzyme-linked immunosorbent assay (ELISA) antibodies.
ELISA for antibodies against capsid protein
Humoral immune responses were assayed by a blocking ELISA. Flat-bottomed 96-well microplates (Maxisorp, Nunc, Naperville, IL) were coated with a capture polyclonal antibody diluted in 0.01 M sodium carbonate buffer (pH 9.6) and incubated overnight at +4°C. In 96-well culture plates (Microtest, Becton Dickinson, NJ), 100 μl of dilutions of sera on test were mixed with 100 μl of purified FCV whole virus particles and incubated overnight at +4°C. After washing with PBS containing 0.05% Tween 20 (PBS-T), the coated plates were incubated at 37°C for 3 h with 100 μl of the ‘antigen-serum on test’ mixture. The plates were then washed three times. One hundred microlitres of prediluted detection anti-FCV capsid protein monoclonal antibody, labelled with horseradish peroxidase (HRP), were added to all wells of the ELISA microplates. After 1 h at 37°C, plates were washed and incubated with 100 μl HRP substrate (5,5′ tetra methyl benzidine solution, Synbiotics, France) in obscurity for 30 min at 20°C±2. The reaction was then stopped by the addition of 50 μl of a 4 N sulphuric acid solution. Optical densities (OD) were read at 450/630 nm with an ELISA plate reader (EL311 BioTeck Instruments, Vinoosky, VT). The 50% ELISA serum titre was defined as the log10 of the reciprocal of the dilution of the serum on test, to obtain the OD50%. Validation of each ELISA plate was realised by a control card of the positive ELISA titre.
Virus isolation
Fourfold dilutions of swab samples were inoculated on to monolayers of CRFK cells grown in 96-well microtitre plates in DMEM supplemented with 5% of fetal calf serum and antibiotics. For each dilution, six wells were inoculated with 100 μl of viral suspension. Plates were observed daily for typical cytopathic effect. Virus titres were calculated using the Kärber statistical formula and expressed in log10 CCID50/ml.
Results
Description of the outbreak in small animal clinics – NVST (Table 1)
Table 1.
Signalment and history of cats involved in the outbreak
| Cat | Signalment | Date of last FCV vaccine according to accination file | Date when FCV-VSD signs were recorded | Hospitalisation at NVST | Outcome | Necropsy | Date/sample of first FCV-positive RT-PCR |
|---|---|---|---|---|---|---|---|
| Cat 1 | 2 yF DSH | N A | March 4 | Yes (March 4–6) | Dead | Yes | April 5/frozen plasma * |
| Cat 2 | 4 mM DSH | February 22, 2005 | March 8 | No | Dead | No | April 1/frozen blood * |
| Cat 3 | 12 yM DSH | June 12,2004 | March 14 | No | Dead | No | April 5/frozen plasma * |
| Cat 4 | 18 yF DSH | June 20, 2000 | March 17 | Yes (March 17–19) | Dead | Yes | March 21/blood |
| Cat 5 | 1 yF Siamese | N A | March 29 | Yes (February 26–June 15) | Recovered | N/A | March 30/blood |
| Cat 6 | 3 yM DSH | N A | March 29 | Yes (March 14–April 11) | Recovered | N/A | March 30/blood |
| Cat 7 | 4 yM DSH | N A | April 3 | Yes (March 26–April 1) | Dead | No | April 1/blood |
| Cat 8 | 1 yM DSH | N A | April 1 | No | Recovered | N/A | April 5/blood |
y=year(s), m=months, DSH=domestic shorthair, N A=not available, N/A=not applicable.
Retrospective testing.
Unexplained death of three cats (March 4–16)
On March 4, 2005 cat 1 was referred by a local veterinary practice with an 8-day history of disease. Clinical examination at admission revealed severe depression, hypothermia (37.2°C), facial and limb oedema and crusting, mucopurulent nasal discharge, oral ulceration and dyspnoea. Abdominal palpation was unremarkable but triggered vomiting. Some laboratory results were abnormal (Tables 2 and 3). Thoracic and abdominal effusions (transudates on cytological examination) were identified. The cat was hospitalised and treated with intravenous fluids, antibiotics and analgesics. It was euthanased 2 days later at the owner's request owing to its worsening condition. Necropsic examination was performed on March 7 and was initially inconclusive.
Table 2.
Results of haematological examination for the first four cats in the outbreak
| Variable (unit) | RI | Cat 1 | Cat 2 | Cat 3 | Cat 4 |
|---|---|---|---|---|---|
| WBC (109/μl) | 5.5–19.5 | 7.7 | 5.0 | 4.6 | 12.6 |
| N (109/μl) | 2.5–12.5 | 6.8 | 4.4 | 4.37 | 11.8 |
| E (109/μl) | 0–1.5 | 0.23 | 0 | 0 | 0 |
| B (109/μl) | Rare | 0 | 0 | 0 | 0 |
| L (109/μl) | 1.5–7.0 | 0.6 | 0.3 | 0.18 | 0.13 |
| M (109/μl) | 0–0.85 | 0.08 | 0.3 | 0.05 | 0.63 |
| RBC (1012/μl) | 5–10 | 9.97 | 6.84 | 9.06 | 7.23 |
| Hb (g/dl) | 8–15 | 13.1 | 9.5 | 12.9 | 10.2 |
| PCV (l/l) | 0.24–0.45 | 0.43 | 0.27 | 0.38 | 0.33 |
| Plt * (109/μl) | 300–800 | 112 | 140 | 185 | 239 |
| Fibrinogen (g/l) | 1–3 | 8 | 4.5 | 5 | NT |
| ACCT (s) | 10–14 | NT | 39.4 | NT | NT |
WBC=white blood cells, N=neutrophils, E=eosinophils, B=basophils, L=lymphocytes, M=monocytes, RBC=red blood cells, Hb=haemoglobin, PCV=packed cell volume, Plt=platelets, ACCT=activated cephalin clotting time, RI=reference interval of the laboratory, NT=not tested. Underlined values are outside the reference range of the laboratory.
No platelet aggregates were detected on blood smears and coulter counts were checked manually.
Table 3.
Results of plasma chemistry examination for the first two cats in the outbreak
| Variable (unit) | RI | Cat 1 | Cat 2 |
|---|---|---|---|
| Total proteins (g/l) | 55–71 | 45.2 | 62.8 |
| Albumin (g/l) | 27–39 | 16.3 | 26.3 |
| Bilirubin (μmol/l) | 1.7–8.4 | 51.5 | 13.4 |
| ALAT (U/l) | 20–107 | 159 | 105 |
| AP (U/l) | 23–107 | 37 | 62 |
| GGT (U/l) | <5 | 11 | 6 |
ALAT=alanine aminotransferase, AP=alkaline phosphatase, GGT=gammaglutamyl transferase, RI=reference interval of the laboratory. Underlined values are outside the reference interval of the laboratory.
On March 8, cat 2 owned by a veterinary student (student 1) was presented with a 1-day history of anorexia and fever. Clinical examination was unremarkable. It was placed on oral cefalexin and tolfenamic acid without improvement. Repeated examinations through the next 3 days revealed deterioration of the cat's general condition, oedema of the four limbs, diffuse pain and abnormal laboratory results (Tables 2 and 3). A septic condition with secondary vasculitis, hepatic impairment and disseminated intravascular coagulation was suspected, leading to euthanasia on March 11.
On March 14, cat 3 was presented with signs of acute upper-respiratory tract infection 3 days after a first visit at NVST for an unrelated problem (chronic respiratory stridor). It was sent home with a treatment including oral doxycyclin and steam inhalation. The attending veterinary surgeon called the day after to inform that he had to hospitalise the cat with oedema of the four limbs and icterus. Its condition declined and it died there 2 days later.
Positive testing for FCV on blood of a fourth cat dead after a similar clinical course (March 17–21)
On March 17, cat 4 was presented with acute depression and anorexia 3 days after a first visit at NVST for an unrelated problem (osteoarthritis). Clinical examination revealed sub-icteric mucous membranes, nasal discharge and abdominal pain. Abdominal radiographs, routine haematology and biochemistry were unremarkable. The cat was hospitalised in an isolation ward and treated with intravenous fluids, antibiotics and analgesics. Its condition progressively worsened with development of dyspnoea, ulcerative and necrotising stomatitis, ocular discharge and hypothermia. FCV-VSD was suspected. The cat died on March 19. Intracardiac blood was collected shortly after death and tested positive for FCV by RT-PCR on March 21 (isolate 4a). FCV was subsequently isolated on CRFK cells (isolate 4b). Necropsic examination was performed on March 21 and was considered unremarkable at that time.
Assessment of in-hospital spread of the virus (March 29)
The possibility of a nosocomial transmission of the virus during hospitalisation of cat 4 was assessed on March 29. Six cats were staying in the small animal hospital setting of NVST. All were examined and tested for FCV by RT-PCR on EDTA blood or oropharyngeal swabs. Of these, two showed clinical signs of FCV-VSD (mostly fever, oedema, skin sores, pododermatitis and oral ulceration) ( Fig 1 A–D): cat 5 had been hospitalised on February 26 for surgical care of a traumatic wound, and cat 6 on March 14 for surgical repair of a tibio-tarsal dislocation. Both tested positive for FCV. The four remaining cats did not show any sign of FCV-VSD. Three were sent home on March 31. FCV-positive RT-PCR results were, however, obtained the day after release for one of these three (cat 7 hospitalised on March 26 for obstructive lower urinary tract disease).
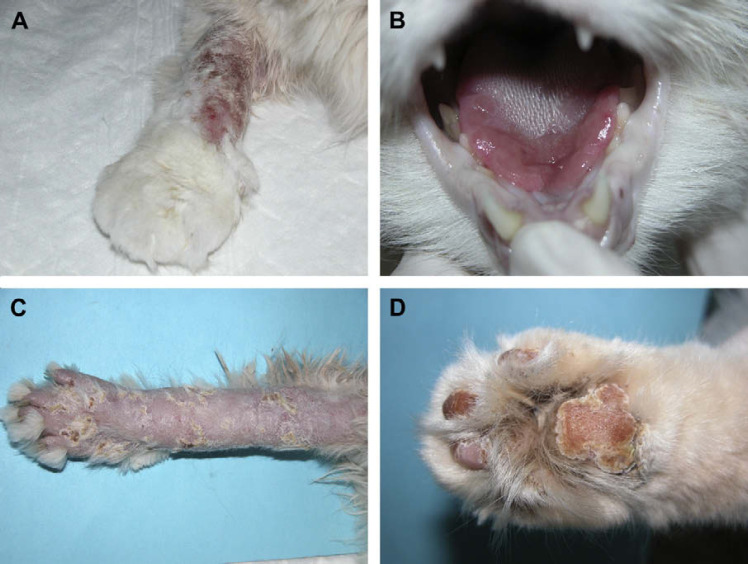
Fig 1.
Clinically apparent lesions observed in cat 6 during natural infection. (A) Oedema and skin lesion on the right forelimb. (B) Erosions on the tongue. (C) Alopecia and crusting on the right forelimb during healing process. (D) Aspect of the paw pads during healing process.
Implementation of sanitary control procedures (March 31–April 19)
The small animal clinics of NVST were closed for cats on March 31. Of the three cats still in the NVST hospital after March 31, one with a complex elbow fracture was euthanased at the owner's request for financial reasons on April 2. Cat 6, the unique indoor cat of its household, recovered from FCV infection and was sent home on April 11. Cat 5, living in a multi-cat household, was removed in strict isolation to other NVST facilities from April 14. The small animal hospital was re-opened on April 19 to feline patients after disinfection (9.6% hypochlorite solution diluted 1:30 in water after complete detergent and steam cleaning). No new case of FCV-VSD occurred at NVST. Cat 5 recovered and was released on June 15 (RT-PCR on oropharyngeal swab was negative for FCV on June 10).
Retrospective confirmation of FCV infection of cats 1–3 (April 1–5)
Infection by a virulent systemic FCV (VS-FCV) strain was considered retrospectively as a possible cause of the unexplained death of cats 1–3, because all had shown suggestive signs (oedema of the limbs, hepatic failure, upper-respiratory tract disease with oral ulcers, lymphopenia, thrombocytopenia). Frozen samples (heparinised plasma from cats 1 and 3 collected on March 4 and March 14, respectively, EDTA blood from cat 2 collected on March 9) tested positive for FCV by RT-PCR.
Assessment of confirmed or possible subsequent cases outside NVST (April 1–15)
Cat 8 owned by a second veterinary student (student 2) on duty at the NVST small animal hospital from March 28 had to be hospitalised in a private veterinary surgery on April 1, shortly after closure of the small animal clinics of NVST to cat admission. It showed fever, diffuse pain and oral ulceration. RT-PCR on EDTA blood proved to be positive for FCV on April 5. Cat 8 fully recovered from infection.
Cat 7 became ill soon after leaving NVST and was hospitalised on April 3 in a private practice suffering from oedema of the four limbs and signs of acute upper-respiratory tract infection. It died there on April 6. The two housemates of cat 7 developed clinical signs soon after him. Further laboratory diagnosis could not be performed. Both cats recovered.
History of exposure was documented for all the cats except for cat 1, considered as the index case of the outbreak (Table 4).
Table 4.
History of suspected exposure to FCV for the confirmed cases of the outbreak
| Cat | Day(s) of presumed exposure to FCV | Circumstances | Source | Suspected mode of transmission/vector |
|---|---|---|---|---|
| Cat 1 | Prior to February 28 | Unknown | Unknown | Unknown |
| Cat 2 | March 4–6 | At home | Cat 1 | Indirect at distance/owner (student 1 on duty in NVST small animal hospital and in charge of cat 1) |
| Cat 3 | March 11 | During outpatient consultation for unrelated problem | Cat 2 | Indirect/clinical team dealing also with cat 2 on March 11 |
| Cat 4 | March 14 | During outpatient consultation for unrelated problem | Cat 3 | Indirect/clinical team dealing also with cat 3 on March 14 |
| Cat 5 | March 4–29 | During hospitalisation for unrelated problem | Cat 1, 4 or 6 | Indirect/fomites in NVST Hospital |
| Cat 6 | March 14–29 | During hospitalisation for unrelated problem | Cat 1, 4 or 5 | Indirect/fomites in NVST Hospital |
| Cat 7 | March 26–31 | During hospitalisation for unrelated problem | Cat 1, 4, 5 or 6 | Indirect/fomites in NVST Hospital |
| Cat 8 | March 28–31 | At home | Cat 1, 4, 5, 6 or 7 | Indirect at distance/owner (student 2 on duty in NVST small animal hospital from March 28) |
No other possible cases were identified in any practice or household involved. Inquiry revealed that cat 1 could have contracted FCV infection from its housemate. Indeed, this latter had been presented to the same veterinarian 4 days before cat 1 with fever, facial dermatitis and oral ulcerations. It recovered uneventfully.
Histopathology
The most significant findings were observed in the liver of cat 1, and consisted of mild hepatocyte necrosis and severe hepatocellular dissociation ( Fig 2 ). Immunochemistry studies or attempts to detect/isolate FCV from this tissue sample were not performed.
Fig 2.
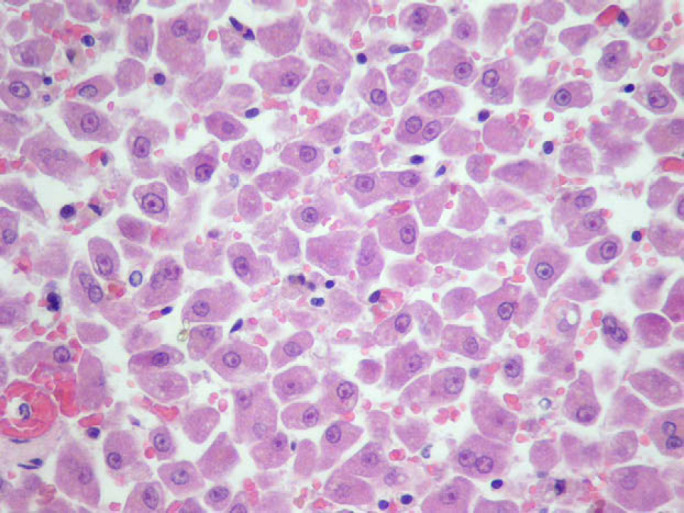
Histopathological view of the liver of cat 1 showing generalised hepatocellular dissociation.
FCV capsid gene amplification
The 18 samples collected from the eight clinical cases were positive for FCV RNA (Table 5). The large amount of total RNA purified from the cell culture supernatant of the FCV isolate from cat 4 (isolate 4b) was used to amplify the FCV capsid gene. Amplification with FCVCapseq1F+1R primers did not yield a full length capsid gene PCR product, but two partially overlapping fragments which were cloned and sequenced. A first contig of about 1000 nucleotides was then constructed. Amplification with FCVCapseq2F/3F+2R primers produced two other clones which partially overlapped the first contig from which it was possible to construct a full length contig of the FCV capsid gene from cat 4 (accession number EU202915).
Sequencing of the E region of the FCV capsid gene and phylogenetic analysis
In order to amplify and sequence the E region of the FCV capsid gene from different isolates, primers FCVCapseq4F and 4R were designed and RT-PCRs were performed on 13 out of 19 isolates (Table 5). Comparison between the 13 sequences (677 nucleotides) revealed less than 1.5% divergence among 677 nucleotides between isolates (Table 6). All the sequences from different samples of the same cat (cats 5 and 6) were 100% identical.
Table 6.
Nucleotide mismatches in the hypervariable E region of capsid gene of the different isolates of VS-NVST
| Nucleotide | N 1242 | N 1386 | N 1518 | N 1568 | N 1571 | N 1583 | N 1594 | N 1754 |
|---|---|---|---|---|---|---|---|---|
| Codon | 422 | 462 | 506 | 523 | 524 | 528 | 532 | 585 |
| F9 | CCT | AAC | ATA | GCG | CAA | GAT | CCT | GTC |
| Isolate 1 | CCC | AAT | ATA | GTG | CAA | GAC | ATT | GTG |
| Isolate 2 | CCC | AAY | ATA | GTG | CGA | GAC | ATT | GTG |
| Isolate 3 | CCC | AAC | ATA | GTG | CGA | GAC | ATT | GTG |
| Isolate 4a | CCC | AAC | ATA | GTG | CGA | GAC | ATT | GTG |
| Isolate 4b | CCT | AAC | ATG | GTG | CGA | GGC | ATT | GCG |
| Isolate 5a | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 5b | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 6a | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 6b | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 6c | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 6d | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 7 | CCC | AAT | ATA | GCG | CAA | GAC | ATT | GTG |
| Isolate 8 | CCC | AAT | ATA | GCG | CAA | GAC | RTT | GTG |
The position of nucleotides in bold is related to F9 strain sequence and the position of codons is the same as for the amino acids alignment in Fig 4 . Codons for which ambiguities were detected in two isolates' sequences appear in grey boxes.
Sequences obtained from cat 2 and cat 8 samples showed ambiguities (N 1242, codon 422 and N 1594, codon 532, respectively) (Table 6). Due to the fact that the nucleic acids from these samples were not cloned, it is probable that these two cats were infected by at least two variants of the VS-NVST. The sequences of isolates 3 and 4a were 100% identical, as well as those of isolates 5–7. The most divergent sequence was an FCV 4b sequence obtained after two passages of the virus in cell culture. As far as direct isolates (1; 2; 3; 4a; 5a, b; 6a, b, c, d; 7; 8) are concerned, there are five nucleic acid polymorphisms, two of which induce a change in the amino acid sequence between some isolates (N 1568, codon 523 V/A, N 1571, codon 524 Q/R), while another induces a change for the same isolate 8 (N 1594, codon 532, I/L) due to an ambiguity in the sequence. Altogether, these analyses show that the various FCV isolates are very close. Although compatible, analysis of mismatches in the E hypervariable sequences (Table 6) could not confirm the history of transmission described in Table 4. Indeed this would have needed sequencing of different locations of the genome.
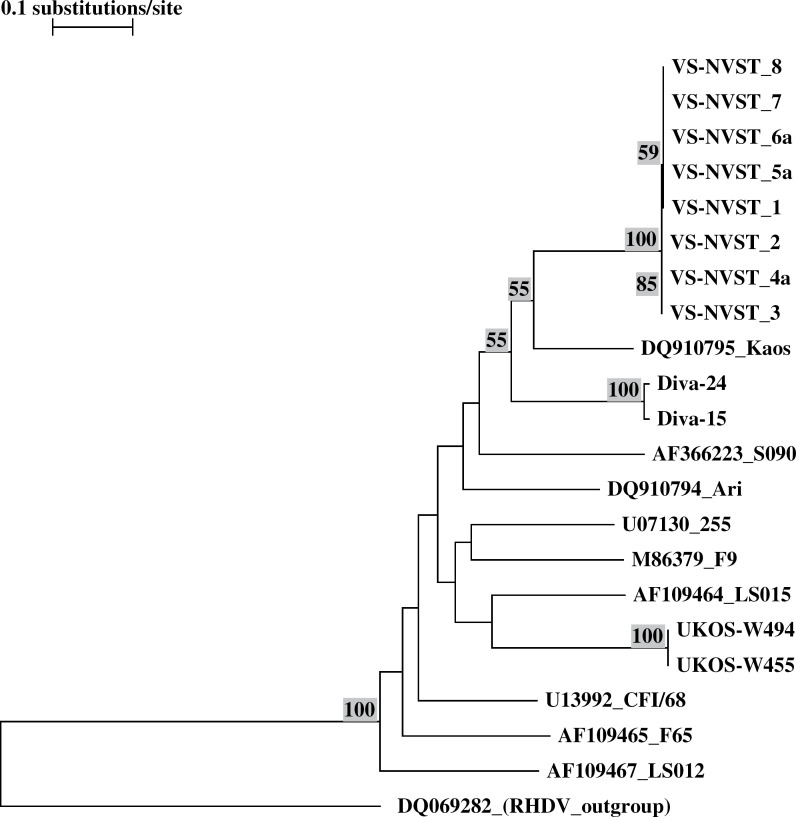
Comparison with published sequences from different hypervirulent strains Ari, 19 Diva, 20 Kaos 21 and Ukos-W 22 showed no relationship between VS-NVST and FCV strains isolated during other FCV outbreaks ( Fig 3 ). However, some amino acid substitutions already reported for other VS-FCV strains and located in the N-terminal hypervariable area of region E at positions 430 (isoleucine/valine to threonine), 438 (threonine to valine), 448 (alanine to lysine), and/or 452 (aspartic acid to glutamic acid)26,27 were identified in VS-NVST region E ( Fig 4 ). Nevertheless, some of these substitutions were not consistently observed in VS-FCV capsid amino acid sequences (Diva (aa 430, 438), Ukos-W (aa 438, 448, 452)).
Fig 3.
Phylogenic tree based on 186 nucleotides of the E hypervariable region of the capsid from different VS-FCV strains, including VS-NVST. Only bootstrap values >50% are indicated.
Fig 4.
Amino acid alignments (aa 426–481) of E hypervariable region of capsid protein from different VS-FCV compared to F9.
Viral loads
All samples collected during the outbreak were tested for viral loads by real-time RT-PCR (Table 5). Blood, plasma, oropharyngeal swabs, cutaneous scrapings, conjunctival swabs, rectal swabs and urines were submitted to analysis. All the samples tested were positive and high viral loads were detected in blood, oropharyngeal and conjunctival swabs, skin scrapings and rectal swabs (tested for cats 5 and 6 on April 8, in the second week after onset of clinical disease).
Identity test by indirect IFA
An IFA with a panel of Mabs confirmed the identity of the virus. Two Mabs binding to a conserved neutralising region and two Mabs targeting linear neutralising epitopes in the 5′ hypervariable area bound to the VS-NVST strain.
Experimental infection
All inoculated cats presented clinical signs typical of upper-respiratory tract disease, such as apathy, weight loss, hyperthermia, oronasal ulcers, and nasal and/or ocular discharge. In addition to those signs, 8/10 cats had ulcerations of paw pads and ears. The mean clinical score was 46 (Table 7). All cats recovered from infection.
Table 7.
Clinical scores after experimental infection with VS-NVST FCV strain
| Viral strain | General signs | Ocular/nasal signs | Other signs | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| General body condition | Weight | Temperature | Oronasal ulceration | Nasal discharge | Ocular discharge | Oedema | Cutaneous ulceration or necrosis | ||
| Individual clinical scores | |||||||||
| VS-NVST | |||||||||
| SPF Kitten 1 | 4 | 4.0 | 4 | 6 | 1 | 5 | 0 | 0 | 24 |
| SPF Kitten 2 | 6 | 4.0 | 4 | 17 | 10 | 0 | 0 | 10 | 51 |
| SPF Kitten 3 | 4 | 4.0 | 8 | 23 | 5 | 8 | 0 | 3 | 55 |
| SPF Kitten 4 | 8 | 4.0 | 6 | 34 | 10 | 0 | 0 | 0 | 62 |
| SPF Kitten 5 | 0 | 4.0 | 4 | 31 | 3 | 2 | 0 | 8 | 52 |
| SPF Kitten 6 | 4 | 4.0 | 8 | 16 | 8 | 1 | 0 | 5 | 46 |
| SPF Kitten 7 | 8 | 4.0 | 5 | 13 | 8 | 2 | 0 | 8 | 48 |
| SPF Kitten 8 | 2 | 4.0 | 5 | 28 | 7 | 1 | 0 | 9 | 56 |
| SPF Kitten 9 | 4 | 4.0 | 6 | 4 | 3 | 1 | 0 | 8 | 30 |
| SPF Kitten 10 | 6 | 4.0 | 4 | 8 | 5 | 1 | 0 | 8 | 36 |
| Mean clinical scores | |||||||||
| VS-NVST | 4.6 | 4.0 | 5.4 | 18 | 6 | 2.1 | 0 | 5.9 | 46 |
| FCV 255 | 0.4 | 3.4 | 1.4 | 15.6 | 0.7 | 1.75 | 0 | 0 | 23.1 |
As a reference, the mean clinical scores induced by a reference classical strain (FCV 255) are indicated at the bottom of the table.
Virus shedding peaked at day 6 post-inoculation (mean titre: 104.3 CCID50/ml). Virus was not detectable in a fraction of the swabs collected on day 11 and in any of the swabs collected on day 14 ( Fig 5 ).
Fig 5.
FCV excretion after experimental infection with VS-NVST strain.
All cats were seronegative at the time of inoculation, consistent with their specific pathogen-free status. Two weeks after infection, all cats had seroconverted to high ELISA antibody titres (mean titre: 2.44 log10).
Discussion
Outbreaks of FCV-VSD have been reported in North America 19–21 and in the United Kingdom. 22 This paper describes the first outbreak identified in France. Since this first occurrence, other outbreaks have come to our knowledge by chance, suggesting that the incidence of FCV-VSD may be underestimated. The epizootic described here shares many characteristic features with those previously described.
Clinical signs (depression, fever, anorexia, oedema of the face and limbs, cutaneous ulceration, alopecia, icterus, oral ulceration and upper-respiratory signs) and the high mortality rate were comparable to previous descriptions. 19–22 Laboratory (hypoproteinaemia, hyperbilirubinaemia, lymphopenia, thrombocytopenia) and pathological (hepatocellular dissociation) findings have also been described in other outbreaks and were suggestive of FCV-VSD. Nevertheless, suspicion of VS-FCV infection and implementation of strict control procedures were not immediate which emphasises the need for veterinarians to be aware of this emerging syndrome.
Most of the outbreaks described to date were also nosocomial. 19–21 The severity of the condition is likely to lead to hospitalisation and its highly contagious nature accounts for rapid spread of the virus within and outside the hospital through people carrying the virus, 19–21 as occurred here for cats 2 and 8 owned by two veterinary students. Several peculiarities of VS-FCV strains can account for the easy indirect contamination observed inside and outside hospitals. High replication rates and abundant viral excretion, as recorded in naturally infected cats and also in experimentally infected cats in the present study, seem to be characteristic features of these strains. 1 The presence of large amounts of virus on the skin, at least when cutaneous lesions are present, as observed here, suggests that any infected cat is likely to disseminate the virus on material and people even during brief contacts. 4 Moreover, FCV is generally considered to remain infective for at least 2 weeks in the environment 1 and is not inactivated by some commonly used disinfectants. 28 Spread of the virus is, therefore, possible through brief indirect contact such as restraint or physical examination even if hands have been routinely washed between cats. 4
These characteristics justify previous recommendation of attempts to treat affected cats on an outpatient basis whenever possible1,20 and implementing strict sanitary measures when dealing with an outbreak.4,29 Temporary closure to cat admission was ultimately necessary here and in all nosocomial outbreaks described to date. 19–21 It may, therefore, be recommended as soon as a VS-FCV has been identified. Early cat closure is indeed likely to be the most efficient measure in preventing additional contaminations and controlling the outbreak.
Vaccination files of most of the cats involved in that particular outbreak were not available so that their status remained uncertain. During previous outbreaks, most of the affected cats were vaccinated and some succumbed to the infection. 19–22 Although all induce some degree of cross protection, no vaccine is likely to be able to protect against all field isolates of FCV because of its high antigenic variability.1,15 It may, therefore, be that only the ‘vaccine resistant’ strains of hypervirulent FCV that consequently caused serious outbreaks have eventually been brought to the attention of the veterinary community. Known occurrence of vaccine breakdowns and lack of specific treatment for the condition further emphasises the importance of rapid identification and implementation of sanitary measures when dealing with VS-FCV.
The reason why this outbreak and all thus far reported have been self-limiting and fortunately did not spread to the general cat population remains an enigma, however. Even when drastic control procedures are implemented, these cannot account for the extinction of such obviously contagious strains of FCV. It may, therefore, be that passages during outbreaks progressively yield to less virulent strains or that non-viral cofactors are needed for the disease's expression and spread.20,21
More often in previous epizootics, the initial source of infection appeared to be a shelter kitten or less frequently adult cat. 4,19–21,29 Such large groups of cats are indeed associated with high levels of replication and biodiversity of FCV strains and may provide the conditions for the emergence of hypervirulent strains.1,4 The most severe forms of disease are then seen outside the originating colony on a feline population deprived of protective matched immune response. 1 Interestingly, in our report, a posteriori inquiry revealed that there was a large rescue colony of cats dependant on a foster home in the immediate neighbourhood of cat 1's household.
The identity of the virus was confirmed by indirect immunofluorescence and sequencing of its capsid gene. All the sequences obtained from the different isolates are very close, thus confirming that all the cases are related. These techniques cannot, however, be used to confirm the virulent nature of the virus, as no strict correlation has been found so far between the antigenic, genetic and clinical profiles. Indeed, several phylogenetic analyses of FCV protease-polymerase or whole capsid protein did not differentiate VS-FCV strains from classical isolates.22,30 Nevertheless, the VS-NVST strain shares some specific amino acid changes in the 5′ end of region E with some other VS-FCV strains.26,27 The N-terminal hypervariable area contains linear neutralising epitopes, 31 and its interaction with the JAM-1 receptor was confirmed in structural analyses.32,33 FCV was also shown to bind to α2,6-linked sialic acid 34 and a dual receptor usage cannot be ruled out. The tropism of VS-FCV strains for endothelial cells 35 might be the result of an altered receptor usage, associated with specific changes within the N-terminal hypervariable region.
After experimental infection, this strain induced severe clinical signs of the classical upper-respiratory tract disease, and cutaneous lesions usually associated with FCV-VSD. However, experimental infection did not reproduce the other signs commonly associated with FCV-VSD, like facial and limb oedema, dyspnoea, icterus, depression and a high mortality rate. Nevertheless, the overall clinical score was about twice as high as that of a classical strain, using the scoring of the European Pharmacopeia monograph. Consistently with the severity of the clinical signs, cats excreted large amounts of virus, higher than those usually measured in this model. 36 This observation is consistent with the higher yields attained by VS-FCV strains in vitro in multiple-cycle kinetics of growth on CRFK cells. 30
In vitro amplification of the strain from the blood of an infected cat required two passages on CRFK cells because of the poor quality of the initial sample (frozen whole blood sample). The virus may have lost some of its virulence during in vitro culture, explaining the difference of clinical signs between the natural outbreak and the experimental infection. Several mismatches have been identified in the sequence of the hypervariable region E of this cell culture strain (isolate 4b) compared with the original isolate 4a, thus confirming that the virus evolved during the cell culture passages. In addition, the specific pathogen-free kittens included in the experimental study may have been less sensitive than the infected cats in the field. Others have reported that adult cats might be more sensitive to VS-FCV strains, 21 but this observation has never been confirmed. The results of the experimental infection suggest that some less severe forms of FCV-VSD might also occur in the field. Cutaneous lesions associated with FCV infection have already been reported in the absence of other FCV-VSD clinical signs. 7
The reason for the emergence of the outbreaks is not known. Phylogenic analyses have highlighted the strong genetic diversity as confirmed in this study but, so far, no evolution of the FCV population towards new genotypes. In addition, the outbreaks are independent from one another, and no relation can be found between VS-FCV strains. Cross-neutralisation studies show that the FCV population is subjected to an antigenic drift probably driven by the vaccine-induced immune pressure.25,37 It may be speculated that this evolution in the FCV field isolates has increased the risk of emergence of hypervirulent strains. Indeed, most VS-FCV strains are poorly neutralised by antisera specific of the classical vaccine strains, FCV F9 and FCV 255.19,20,36
Acknowledgements
We are very grateful to Alan Radford for providing the sequence of W strain described in Coyne et al. 22
References
- 1.Radford A.D., Coyne K.P., Dawson S., Porter C.J., Gaskell R.M. Feline calicivirus, Vet Res 38, 2007, 319–335. [DOI] [PubMed] [Google Scholar]
- 2.Gaskell R.M., Dawson S., Radford A. Feline respiratory disease. Greene G.E. Infectious diseases of the dog and cat, 2006, Saunders Elsevier: St Louis, 145–154. [Google Scholar]
- 3.Foley J.E. Calicivirus: spectrum of disease. August J.R. Consultations in feline internal medicine Vol 5, 2006, Elsevier Saunders: St Louis, 3–9. [Google Scholar]
- 4.Hurley K.F., Sykes J.E. Update on feline calicivirus: new trends, Vet Clin North Am Small Anim Pract 33, 2003, 759–772. [DOI] [PubMed] [Google Scholar]
- 5.Love D.N., Baker K.D. Sudden death in kittens associated with a feline picornavirus, Austral Vet J 48, 1972, 643. [DOI] [PubMed] [Google Scholar]
- 6.Cooper L.M., Sabine M. Paw and mouth disease in a cat, Austral Vet J 48, 1972, 644. [DOI] [PubMed] [Google Scholar]
- 7.Hubert B., Cadilhac I., Magnol J.P. Manifestations cutanéo-muqueuses de la calicivirose féline chez un chat, Prat Méd Chirurgic Anim Compagnie 41, 2006, 141–144. [Google Scholar]
- 8.Love D.N., Zuber R.M. Feline calicivirus associated with pyrexia, profound anorexia and oral and perianal ulceration in a cat, Aust Vet Pract 17, 1987, 136–137. [Google Scholar]
- 9.Declercq J. Pustular calicivirus dermatitis on the abdomen of two cats following routine ovariectomy, Vet Dermatol 16, 2005, 395–400. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y., Ohe K., Fukuyama M., et al. Properties of a calicivirus isolated from cats dying in an agitated state, Vet Rec 155, 2004, 800–805. [PubMed] [Google Scholar]
- 11.Van Vuuren M., Geissler K., Gerber D., Nöthling J.O., Truyen U. Characterization of a potentially abortigenic strain of feline calicivirus isolated from a domestic cat, Vet Rec 144, 1999, 636–638. [DOI] [PubMed] [Google Scholar]
- 12.Ellis T.M. Jaundice in a Siamese cat with in utero feline calicivirus infection, Aust Vet J 57, 1981, 383–385. [DOI] [PubMed] [Google Scholar]
- 13.Osborne A.C., Kruger J.M., Lulich J.P., Polzin D.J., Lekcharoensuk C. Feline lower urinary tract diseases. Ettinger S.J., Feldman E.C. Textbook of veterinary internal medicine, diseases of the dog and cat, 2000, WB Saunders: Philadelphia, 1710–1747. [Google Scholar]
- 14.Rice C.C., Kruger J.M., Venta P.J., et al. Genetic characterization of 2 novel feline caliciviruses isolated from cats with idiopathic lower urinary tract disease, J Vet Intern Med 16, 2002, 293–302. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen N.C. Feline calicivirus infection, Proceedings of the 13th ECVIM-CA Congress, Uppsala, 2003, 108–111.
- 16.Pedersen N.C., Laliberte L., Ekman S. A transient febrile limping syndrome of kittens caused by two different strains of feline calicivirus, Feline Pract 13, 1983, 26–35. [Google Scholar]
- 17.Dawson S., Bennett D., Carter S.D., et al. Acute arthritis of cats associated with feline calicivirus infection, Res Vet Sci 56, 1994, 133–143. [DOI] [PubMed] [Google Scholar]
- 18.Reubel G.H., Hoffmann D.E., Pedersen N.C. Acute and chronic faucitis of domestic cats: a feline calicivirus induced disease, Vet Clin North Am Small Anim Pract 22, 1992, 1347–1360. [DOI] [PubMed] [Google Scholar]
- 19.Pedersen N.C., Elliott J.B., Glasgow A., Poland A., Keel K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus, Vet Microbiol 73, 2000, 281–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schorr-Evans E.M., Poland A., Johnson W.E., Pedersen N.C. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England, J Feline Med Surg 5, 2003, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurley K.E., Pesavento P.A., Pedersen N.C., et al. An outbreak of virulent systemic feline calicivirus disease, J Am Vet Med Assoc 224, 2004, 241–249. [DOI] [PubMed] [Google Scholar]
- 22.Coyne K.P., Jones B.R., Kipar A., et al. Lethal outbreak of disease associated with feline calicivirus infection in cats, Vet Rec 158, 2006, 544–550. [DOI] [PubMed] [Google Scholar]
- 23.Rozen S., Skaletsky H.J. Primer3 on the WWW for general users and for biologist programmers. Krawetz S., Misener S. Bioinformatics methods and protocols in the series methods in molecular biology, 2000, Humana Press: Totowa, NJ, 365–386. [DOI] [PubMed] [Google Scholar]
- 24.Van de Peer Y., De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment, Comput Appl Biosci 10, 1994, 569–570. [DOI] [PubMed] [Google Scholar]
- 25.Poulet H., Brunet S., Soulier M., et al. Comparison between acute oral/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: pathogenicity, antigenic profile and cross-neutralisation studies, Arch Virol 145, 2000, 243–261. [DOI] [PubMed] [Google Scholar]
- 26.Abd-Eldaim M., Potgieter L., Kennedy M. Genetic analysis of feline caliciviruses associated with a hemorrhagic-like disease, J Vet Diag Invest 17, 2005, 420–429. [DOI] [PubMed] [Google Scholar]
- 27.Foley J., Hurley K., Pesavento P.A., Poland A., Pedersen N.C. Virulent systemic feline calicivirus infection: local cytokine modulation and contribution of viral mutants, J Feline Med Surg 8, 2006, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eleraky N.Z., Potgieter L.N., Kennedy M.A. Virucidal activity of four new disinfectants, J Am Anim Hosp Assoc 38, 2002, 231–234. [DOI] [PubMed] [Google Scholar]
- 29.Hurley K.F. Virulent systemic feline calicivirus: recognition and control, Proceedings of the North American Veterinary Conference, 2005, 1096–1097.
- 30.Ossiboff R.J., Sheh A., Shotton J., Pesavento P.A., Parker J.S. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates, J Gen Virol 88, 2007, 506–517. [DOI] [PubMed] [Google Scholar]
- 31.Radford A.D., Willoughby K., Dawson S., McCracken C., Gaskell R.M. The capsid gene of feline calicivirus contains linear B-cell epitopes in both variable and conserved regions, J Virol 73, 1999, 8496–8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makino A., Shimojima M., Miyazawa T., et al. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus, J Virol 80, 2006, 4482–4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhella D., Gatherer D., Chaudhry Y., Pink R., Goodfellow I.G. Structural insights into calicivirus attachment and uncoating, J Virol 82, 2008, 8051–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart A.D., Brown T.D. Alpha2,6-linked sialic acid acts as a receptor for feline calicivirus, J Gen Virol 88, 2007, 177–186. [DOI] [PubMed] [Google Scholar]
- 35.Pesavento P.A., MacLachlan N.J., Dillard-Telm L., Grant C.K., Hurley K.F. Pathologic, immunohistochemical, and electron microscopic findings in naturally occurring virulent systemic feline calicivirus infection in cats, Vet Pathol 41, 2004, 257–263. [DOI] [PubMed] [Google Scholar]
- 36.Poulet H., Jas D., Lemeter C., Coupier C., Brunet S. Efficacy of a bivalent inactivated non-adjuvanted feline calicivirus vaccine: relation between in vitro cross-neutralization and heterologous protection in vivo, Vaccine 26, 2008, 3647–3654. [DOI] [PubMed] [Google Scholar]
- 37.Lauritzen A., Jarrett O., Sabara M. Serological analysis of feline calicivirus isolates from the United States and United Kingdom, Vet Microbiol 56, 1997, 55–63. [DOI] [PubMed] [Google Scholar]