Abstract
Glycaemic control and remission probabilities were compared in 24 newly diagnosed diabetic cats treated twice daily with either glargine, protamine zinc (PZI) or lente insulin and fed a low carbohydrate diet. After day 17, the probability of remission was substantially higher for cats with lower mean 12 h blood glucose concentrations on day 17, irrespective of insulin type. Glargine-treated cats had lower mean 12 h blood glucose concentrations on day 17 than PZI- or lente-treated cats, and all eight glargine-treated cats achieved remission compared to three PZI- and two lente-treated cats. The probability of remission was greater for cats treated with glargine than cats treated with PZI or lente insulin. In newly diagnosed diabetic cats, twice daily treatment with glargine provides better glycaemic control and higher probability of remission compared to twice daily treatment with PZI or lente insulin. Good glycaemic control soon after diagnosis is associated with increased probability of remission and should be the goal of insulin therapy.
Diabetes mellitus occurs with moderate frequency in cats 1–3 and requires effective treatment to avoid life-threatening complications. Dietary management 4 can improve outcomes in diabetic cats, but insulin therapy remains the most effective and reliable means of achieving glycaemic control.
The ideal insulin preparation for diabetic cats has not been identified. Many of the currently used insulins such as Neutral Protamine Hagedorn (NPH or isophane insulin) and lente fail to achieve good glycaemic control in cats for two reasons. Firstly, they have a relatively short duration of action, such that, when administered twice daily, marked hyperglycaemia (>18 mmol/l) occurs for several hours prior to the subsequent insulin injection. 5 Secondly, the potency of insulins such as lente and NPH may induce counter-regulatory mechanisms in response to rapidly falling blood glucose concentration. 6 Lente insulin is the most commonly used insulin that is registered for veterinary use in Australia, the United Kingdom and many other parts of Europe. Protamine zinc insulin (PZI) is the longest-acting insulin available for veterinary use.
Glargine has a very long duration of action in humans and healthy cats. 6 In healthy cats, glargine has a similar duration of action to PZI and longer duration of action than lente insulin. 6 A study in diabetic cats reported that glargine administered once daily provided effective control and achieved similar efficacy to lente administered twice daily. 7 Despite glargine's long duration of action, it is unclear whether glargine will achieve more effective glycaemic control and improved outcomes in diabetic cats compared with lente or PZI.
Many diabetic cats treated with insulin are able to maintain normoglycaemia without exogenous insulin within weeks to months of beginning therapy. This is commonly called diabetic remission and implies remission from requiring insulin therapy rather than true remission of their diabetes.
The aim of this study was to compare glycaemic control and remission probabilities in newly diagnosed diabetic cats treated with either glargine, PZI or lente insulin.
Materials and methods
A controlled trial was conducted where newly diagnosed diabetic cats were treated with either glargine, PZI or lente insulin. All cats were fed an ultra-low carbohydrate-high protein canned diet (Purina Diabetes Management, Ralston Purina, St Louis, MO, USA) for 16 weeks or until remission was achieved. The trial was approved by The University of Queensland Animal Ethics Committee (SVS/521/03) and conducted at a feline-only veterinary practice on an outpatient basis, except for assessment days that occurred in the practice hospital.
Animals
From March 2002 until September 2004, all cats presenting to a feline-only veterinary practice (The Cat Clinic, 189 Creek Road, Mt Gravatt, Qld 4122, Australia) with newly diagnosed diabetes but without serious concurrent disease, such as end-stage renal failure or neoplasia, were identified and the owners offered inclusion of their cat in the study. No owner declined participation in the study. Cats were started in the trial at the time they were first diagnosed with diabetes mellitus, and had no or minimal (less than 24 h) insulin therapy. Each cat was allocated to one of the three insulin treatment groups at the time of first diagnosis, with choice of treatment made to ensure equal numbers and similar proportions by breed (Burmese or non-Burmese) and previous corticosteroid administration in each group. Groups were not matched on gender, body weight or age. Inclusion in the trial was confirmed at day 10; cats were excluded up to day 10 if they did not eat the prescribed diet, or owners were unable to exclusively feed the diet or measure water intake.
Insulins
The lente, PZI and glargine insulins tested were, respectively, porcine lente insulin 40 U/ml (Caninsulin; Intervet, Netherlands Boxmeer, Netherlands), PZI 40 U/ml (PZI-Vet, Idexx Pharmaceuticals USA, Westbrook, Maine, USA) and glargine 100 U/ml (Lantus; Aventis Pharmaceuticals, Germany, Frankfurt, Germany).
Initial assessment
On beginning in the study, history, physical examination, body weight, body condition score (BCS; scale of 1–9), serum biochemistry profile, serum fructosamine concentration and urinalysis were obtained for each cat. Urine glucose and ketone concentration was assessed by urine dipstick analysis (Bayer Multistix 10 SG reagent urinalysis strips) using a scale 0 to 4+. Urine sediment was examined and culture and sensitivity testing performed if evidence of inflammation or infection were present.
Diagnosis
Diagnosis of diabetes mellitus was only made if blood glucose concentration was greater than 16 mmol/l at three consecutive times a least 4 h apart, serum fructosamine concentration was elevated (>406 μmol/l) and glycosuria was present. Presence of clinical signs consistent with diabetes mellitus (polyuria, polydipsia, polyphagia, weight loss) was used as supporting evidence. Each cat was classified as having either uncomplicated or complicated diabetes. Cats were classified as having uncomplicated diabetes if they were still eating. Cats were classified as having complicated diabetes mellitus if signs of systemic illness (depression and anorexia) and dehydration were detected.
Stabilisation
Cats entering the study with uncomplicated diabetes were treated with their allocated insulin type as soon as was practical (within 12 h).
Cats entering the study with complicated diabetes were treated with fluid therapy and regular insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark) administered intravenously until appetite returned or hydration status normalised, and were then started on subcutaneous insulin therapy (generally within 1–2 days of diagnosis).
Initial insulin dose
Cats received their allocated insulin twice daily subcutaneously (SC) at an initial dose of 0.5 U/kg ideal body weight if the blood glucose concentration on admission was greater than or equal to 20 mmol/l, and 0.25 U/kg if blood glucose concentration was less than 20 mmol/l. Blood glucose concentration was measured every 2 h for 12 h for each cat for the first 3 days of treatment with the allocated insulin, to ensure cats did not become hypoglycaemic. Insulin dose was adjusted using dosing rules set at the beginning of the study and based on blood glucose concentration (Table 1). Cats were then discharged from hospital and the owners requested to bring the cat back for re-examination in 7 days. No increase in insulin dose was made during the first 3 days, even if persistent hyperglycaemia was present.
Table 1.
Dosing rules for adjusting insulin dose on days 1, 2 and 3 in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with glargine, PZI or lente insulins
| Insulin type | Glucose variable and cutpoint | Dose change * |
|---|---|---|
| Lente | Nadir <3 mmol/l | Reduce by 50% |
| Nadir 3 to<5 mmol/l | Reduce by 1 U | |
| Nadir 5–7 mmol/l | Reduce by 0.5 U | |
| Nadir >7 mmol/l | No change | |
| Glargine and PZI | Nadir <3 mmol/l | Reduce by 50% |
| Nadir 3 to<5 mmol/l | Reduce by 1 U | |
| Nadir 5–7 mmol/l | Reduce by 0.5 U | |
| Pre-insulin <12 mmol/l | Reduce by 0.5 U | |
| Pre-insulin <12 mmol/l and dose was 1 Ubid | Reduce to 1 Usid | |
| Nadir >7 mmol/l | No change |
Note that no increase in insulin dose was allowed during the first 3 days.
For subsequent veterinary reassessments from day 10 onwards, insulin dose was adjusted using dosing rules based on blood glucose concentration (Table 2).
Table 2.
Dosing rules for determining insulin dose from day 10 onwards in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with glargine, PZI or lente insulins
| Insulin type | Glucose variable and cutpoint | Insulin dose change |
| Lente | Pre-insulin <12 mmol/l | Withhold and check for remission |
| Pre-insulin 13–16 mmol/l | Dose was no more than 1 U | |
| Pre-insulin >16 mmol/l | Same dose | |
| Nadir <3 mmol/l | Reduce by 50% | |
| Nadir 3–5 mmol/l | Reduce by 1 U | |
| Nadir 5–7 mmol/l | Reduce by 0.5 U | |
| Nadir 7–10 mmol/l | Same | |
| Nadir 10–12 mmol/l | Increase by 0.5 U | |
| Nadir >13 mmol/l | Increase by 1 U | |
| Glargine and PZI | Pre-insulin 12–14 mmol/l | Reduce by 0.5 U |
| Pre-insulin 15–20 mmol/l and/or nadir 7–9 mmol/l | No change | |
| Pre-insulin >20 mmol/l | Increase 0.5 U | |
| Pre-insulin <12 mmol/l | Withhold and check for remission | |
| Nadir 5–7 mmol/l | Reduce by 0.5 U | |
| Nadir <5 mmol/l | Reduce by 1 U | |
| Clinical hypoglycaemia | Reduce by 50% |
Withdrawing insulin and remission (non-insulin dependence)
After a minimum of 2 weeks of insulin therapy, if the pre-insulin blood glucose concentration was less than 12 mmol/l, insulin was withheld and serial blood glucose measurements were continued for 12 h to determine if the cat was no longer insulin dependent and thus in remission. Cats suspected of being in remission were discharged from hospital without insulin treatment and had their blood glucose concentration measured weekly for 3 months, to confirm remission. Remission date was defined as the first date at which normoglycaemia (blood glucose 4–7 mmol/l) was observed despite no exogenous insulin administration in the previous 2 weeks. Time to remission was defined as the time from initiation of insulin treatment to date of remission.
Trial end-point
The trial endpoint for each cat was 112 days (16 weeks) after initiation of treatment or date of remission, whichever occurred first.
Hospital assessments
Cats were assessed by the same veterinarian on days 10, 17, 28 and then every 2 weeks (ie, days 42, 56, 70, 84, 98 and 112). Cats were admitted at each veterinary assessment and serial measurements of blood glucose concentration made every 2 h over 12 h. Based on the blood glucose concentrations, insulin dose was adjusted according to the dosing rules (Table 2) and the cat discharged the same day. Serum fructosamine concentrations were measured every 4 weeks until the trial end-point.
Diet
All cats were placed on a sole diet of ultra-low carbohydrate-high protein commercial canned diet (Purina Diabetes Management, Ralston Purina, St Louis, MO, USA) (distribution of metabolisable energy: 7% carbohydrate, 46% protein, 47% fat) for the duration of the trial. Food intake was not restricted for any cat during the first 2 weeks of insulin therapy. Dietary energy restriction was recommended after 2 weeks for cats with body condition scores of 7 or above (on 1–9 scale).
Measurement of blood glucose concentration
Serial whole blood glucose concentration was measured using a hand held glucometer (Bayer Esprit) which was regularly calibrated with an external laboratory.
Comparisons between insulin types
Effects of insulin type on 12 h mean blood glucose concentration and mean daily water intake at each veterinary assessment, and serum fructosamine concentration at days 28, 56, 84 and 112, were assessed for cats receiving insulin using analysis of variance. As each pair-wise comparison was of a priori interest, in addition to overall P-values, each pair-wise comparison was tested using the residual mean square of the full analysis of variance model. The effects of insulin type on cumulative probability of remission over time were compared using Kaplan–Meier estimates of survival functions for each insulin type. These survival functions described the probability that an individual's time from initiation of treatment to remission exceeded a specified time. Overall survival functions were compared between insulin types using log-rank tests. For the 22 cats not in remission by day 17, the association between mean 12 h blood glucose concentration on day 17 and occurrence of remission was also assessed by comparing overall survival functions using log-rank tests between cats with low and high mean blood glucose concentrations using two cutpoints (<16 versus ≥16 mmol/l and <10 versus ≥10 mmol/l) without and with stratification by insulin type. Overall survival functions were also compared between Burmese and non-Burmese cats using log-rank tests without and with stratification by insulin type. Statistical analyses were performed using Stata versions 9.2 and 10.1 (StataCorp, College Station, TX, US). Data are summarised as mean±standard error of the mean (SEM) unless indicated otherwise.
Results
Twenty-nine cats were enrolled in the study and five of these were subsequently excluded by day 10 because of failure to eat the diet (two cats in both the glargine and lente groups and one cat in the PZI group). Three groups, each consisting of eight cats, completed the study (n=24).
Mean age was 12 years and just over half the cats were purebred (12 were Burmese); four in each treatment group; (Table 3). At diagnosis all cats were markedly hyperglycaemic (mean 26 mmol/l), and had elevated serum fructosamine concentrations (mean 565 μmol/l) (Table 3). One cat in each of the lente and PZI groups had received corticosteroids in the 6 months prior to diagnosis (n=1, oral prednisone for 3 weeks just prior to diagnosis and intermittently for several years for inflammatory bowel disease; n=1, long-acting depo injection 5 weeks prior to diagnosis for flea allergy/military dermatitis). (Table 3). Four cats (lente=3 and PZI=1) had urine culture and sensitivity testing performed due to an active urine sediment, but only one cat (lente=1) had a positive culture (Enterococcus species). A minority of cats (12%) were diagnosed with complicated diabetes mellitus (lente=2 and PZI=1) and were treated with intensive fluid therapy and intravenous regular insulin administration (Table 3).
Table 3.
Signalment and baseline data for treatment groups in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with either glargine, PZI or lente insulin
| Lente | Glargine | PZI | |
| Age (years) | 11.5±2.1 (7.9–15.5) | 11.4±3.2 (7.0–16.8) | 12.6±2.8 (7.3–16.5) |
| Body weight (kg) | 5.7±1.0 (4.6–7.2) | 6.0±1.2 (3.6–8.4) | 5.4±1.5 (4.5–7.6) |
| Male: female | 6:2 | 5:3 | 6:2 |
| Body condition score (on scale of 1–9) | 5.7±1.2 (4–7) | 6.6±1.1 (5–9) | 5.6±1.5 (5–8) |
| Blood glucose concentration (mmol/l) [ref=3.2–7.5 mmol/l] | 24.3±5.9 (17–31) | 27.3±5.3 (19–34) | 26.7±5.3 (19–33) |
| Fructosamine concentration(μmol/l) [ref=249–406 μmol/l] | 573±96 (495–793) | 554±70 (486–657) | 568±60 (449–660) |
| Serum bicarbonate (mmol/l) [ref=12–24 mmol/l] | 11.8±5.4 (1–20) | 15.3±3.8 (6–20) | 14.4±4.3 (8–19) |
| β-hydroxybutyrate (mmol/l) [ref=<0.6 mmol/l] | 5.7±2.7 (0.3–8.5) | 1.9±4.2 (0.1–8.6) | 3.5±2.9 (0.1–12.4) |
| Acidotic* | 4 | 1 | 2 |
| Ketotic† | 7 | 5 | 4 |
| Ketoacidotic‡ | 3 | 1 | 1 |
| Positive urine sediment | 3 | 0 | 1 |
| Previous diabetogenic drug administration§ | 1 | 0 | 1 |
| Burmese breed | 4 | 4 | 4 |
Data described using mean±SD. Values in parentheses indicate range; ref=reference range for healthy cats.
Serum bicarbonate concentration<12 mmol/l.
Serum β-hydroxybutyrate concentration>0.6 mmol/l and/or positive for urinary ketones.
Presence of both acidosis and ketosis.
Administered in the 3 months preceding diagnosis.
Glargine was associated with significantly lower mean 12 h blood glucose concentrations than lente and PZI, but daily water intake in glargine-treated cats was not different in the first 28 days of therapy (after which reduced group size made comparisons difficult; Table 4). Fructosamine concentration was significantly lower in glargine-treated cats from day 56 (Table 5). During the trial, two cats treated with lente insulin and one cat treated with PZI had severe clinical hypoglycaemia requiring intravenous glucose therapy. None of the glargine-treated cats exhibited signs of hypoglycaemia, although many had biochemical hypoglycaemia (blood glucose <3 mmol/l without clinical signs).
Table 4.
Mean 12 h blood glucose concentrations and water intakes in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with either glargine, PZI or lente insulins
| Day | Lente | Glargine | PZI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean daily glucose conc. (mmol/l) | Water intake (ml/kg/d) | n | Mean daily glucose conc. (mmol/l) | Water intake (ml/kg/d) | n | Mean daily glucose conc. (mmol/l) | Water intake (ml/kg/d) | n | P – mean glucose | P – water intake | |
| 1 | 16.4±2.4 | – | 8 | 16.9±1.2 | – | 8 | 17.2±2.6 | – | 8 | 0.967 | – |
| 2 | 19.4±1.9 | – | 8 | 10.8±2.9 | – | 8 | 18.9±3.2 | – | 8 | 0.065 | – |
| 3 | 20.4±2.0 | – | 8 | 18.4±2.6 | – | 8 | 20.3±2.5 | – | 8 | 0.808 | – |
| 10 | 17.7±2.7 | 41±9 | 8 | 13.1±2.7 | 32±14 | 8 | 16.4±3.3 | 47±10 | 8 | 0.531 | 0.648 |
| 17 | 15.6±6.9y | 39±32 | 7 | 11.4± 2.7x | 32±43 | 8 | 21.3±1.1y | 35±9 | 7 | 0.023 | 0.921 |
| 28 | 22.8±1.8 | 52±10 | 7 | 13.3±2.7 | 45±4 | 4 | 16.7±3.7 | 33±16 | 7 | 0.211 | 0.505 |
| 42 | 23.4±2.1 | 63±9 | 6 | 21.1±2.7 | 81±1 | 2 | 21.8±1.8 | 31±2 | 5 | * | * |
| 56 | 21.6±1.0 | 59±9 | 6 | 6.0±0.9 | 39±5 | 2 | 19.6±1.6 | 34±6 | 5 | * | * |
| 70 | 15.7±1.1 | 66±7 | 6 | 4.2 | 21 | 1 | 19.6±2.4 | 33±5 | 5 | * | * |
| 84 | 19.4±0.4 | 65±9 | 6 | 17.6 | 12 | 1 | 16.9±2.4 | 37±5 | 5 | * | * |
| 98 | 16.1±2.5 | 49±7 | 6 | 3.4 | 20 | 1 | 17.8±1.4 | 43±6 | 5 | * | * |
| 112a | 19.6±1.4 | 56±8 | 6 | – | – | 0 | 19.0±0.7 | 46±5 | 5 | 0.785† | 0.433† |
| 112b | 20.5±1.9 | 33±8 | 6 | – | – | 0 | 13.3± 0.9 | 43±7 | 5 | 0.023† | 0.450† |
| 112c | 21.2±2.1 | 34±9 | 6 | – | – | 0 | 15.5±1.7 | 47±4 | 5 | 0.124† | 0.321† |
n=number of cats receiving insulin and hence the number of individual water intake observations used in calculating group means. Data reported as mean±SEM. Water intake on days 1, 2 and 3 were not compared as some cats received parenteral fluids. Days 112a, 112b and 112c represent consecutive 12-h periods during the final 36 h blood glucose curve. Bolding highlights means that differ significantly (P<0.05) from other means in the same row, means with different superscripts are significantly different from each other.
Comparisons between all three insulin types were not made after day 28 as only one or two cats remained in the glargine group. Means are presented for descriptive purposes only.
Comparisons are between PZI and lente only as there were no glargine-treated cats still receiving insulin.
Table 5.
Serum fructosamine concentrations from day 0 to day 112 in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with either glargine, PZI or lente insulin
| Day | Lente | Glargine | PZI | P value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | |||||||||
| 0 | 573 | 8 | 554 | 8 | 568 | 8 | * | ||||
| 28 | 465±49 | 8 | 343±38 | 8 | 444±42 | 8 | 0.125 | ||||
| 56 | 539±31a | 6 | 342±31b | 2 | 543±35a | 5 | 0.019 | ||||
| 84 | 517±24a | 6 | 182b | 1 | 540±32a | 5 | 0.002 | ||||
| 112 | 479±17 | 6 | 0 | 562±65 | 5 | 0.210 | |||||
n=number of cats receiving insulin within 7 days of test day. Data reported as mean±SEM. Bolding highlights means that differ significantly (P<0.05) from other means in the same row. Means with different superscripts are significantly different from each other.
Insulin therapy had not commenced so group comparison is of baseline data and not the effect of insulin type on fructosamine concentration.
There was no significant effect of insulin type (P=0.545) on change in body weight from initial hospital discharge to trial end. During the trial period, cats in the lente and PZI groups lost a small amount of weight (mean change±SD 68±34 g and 144±42 g, respectively) while cats in the glargine group gained a small amount of weight (mean change±SD 81±46 g).
From day 28 onwards, many cats achieved remission. By day 42, only 2/8 cats in the glargine group were receiving insulin while 6/8 cats in the lente group and 5/8 cats in the PZI group still required insulin therapy (Table 4). By day 112, all eight cats in the glargine group had achieved remission while 2/8 cats in the lente group and 3/8 cats in the PZI group had achieved remission (Table 4). There was a significant effect of insulin type (P=0.014) on probability of remission (Table 6, Fig 1 ). The probability of remission occurring with glargine was greater than for both PZI (P=0.010) and lente (P=0.013), but no significant difference was observed between lente and PZI (P=0.696). For the 22 cats not in remission by day 17, cats with lower mean 12 h blood glucose concentration on day 17 had a higher probability of subsequent remission than cats with higher mean glucose concentration, when mean glucose concentrations of <10 mmol/l versus ≥10 mmol/l and <16 mmol/l versus ≥16 mmol/l were compared. P-values for the non-stratified model and with stratification on insulin type were 0.007 and 0.058, respectively, for 16 mmol/l, and P<0.001 with and without stratification for 10 mmol/l.
Table 6.
Proportion of cats going into remission and time from initiation of treatment to remission in a controlled trial comparing glycaemic control and remission in 24 newly diagnosed diabetic cats treated with either glargine, PZI or lente insulin
| Lente | Glargine | PZI | P value | |
|---|---|---|---|---|
| Proportion of cats going into remission by day 42 (number of eight cats) | 0.25 (2) | 0.75 (6) | 0.38 (3) | 0.014 |
| Proportion of cats going into remission by day 112 (number of eight cats) | 0.25 (2) | 1.00 (8) | 0.38 (3) | |
| Median time (days) from initiation of treatment to remission for cats that achieved remission (range) | 19 (17–21) | 28 (28–100) | 35 (17–35) |
Time to remission reported as median and values in parentheses indicate range. P value is for testing the null hypothesis that the cumulative probability of remission over time does not differ between groups. Overall P value=0.014. Pair-wise comparisons for glargine versus PZI, P=0.016; lente versus PZI, P=0.696; glargine versus lente, P=0.012.
Fig 1.
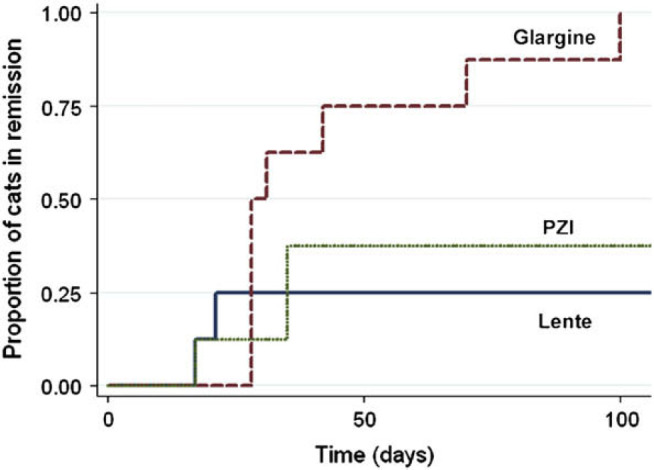
Cumulative proportion of cats going into remission by time after initiation of treatment in a controlled trial comparing glycaemic control and remission probabilities in 24 newly diagnosed diabetic cats treated with glargine (– –), PZI – –) or lente (– –) insulin.
Probabilities of remission were similar and did not differ significantly between Burmese and non-Burmese cats (P-values for non-stratified model and with stratification on insulin type were 0.903 and 0.688, respectively). Of the 12 Burmese and 12 non-Burmese cats, six and seven, respectively, achieved remission by 112 days. All four Burmese cats treated with glargine achieved remission as did the four non-Burmese cats in that group. In the PZI group, 3/4 Burmese cats achieved remission compared with 0/4 non-Burmese cats in that group. In the lente group, 0/4 Burmese cats achieved remission compared with 2/4 non-Burmese cats in that group.
Two cats (lente=1 and PZI=1) which had corticosteroids administered prior to diagnosis had their steroids withdrawn, and both cats achieved remission (lente=17 days, PZI=35 days). Of the cats that did not have steroids administered prior to diagnosis, all eight treated with glargine achieved remission, while only 2/7 PZI treated cats and 1/7 lente-treated cats achieved remission.
Discussion
The most important finding from this study in newly diagnosed diabetic cats was that treatment with glargine resulted in a higher probability of remission compared with PZI or lente insulin. Glargine-treated cats also had better glycaemic control based on lower mean blood glucose concentrations by 17 days after initiating therapy. Achieving non-insulin dependence (remission) is likely to enhance the quality of life of the owner and the cat, because daily insulin injections are no longer required and veterinary visits are usually fewer. The cost to the owner is also reduced.
The small group size limited the power of the study to detect small to modest differences between groups in the probability of remission and other parameters including mean glucose concentration. However, significant differences were detected for some variables. Assuming no bias, this is likely because in the populations represented by the study population, the true differences between insulin types were large. Prior steroid use is the only factor documented in the literature to pose a potential bias for remission, therefore, the glargine group was disadvantaged with respect to probability of remission compared to the other groups (one cat in both the lente and PZI groups had prior steroid administration and both achieved remission).8,9,21 There are no controlled studies reporting that cats with ketoacidosis have a poorer response to insulin or outcome than cats with uncomplicated diabetes and a recent study found 58% of ketoacidotic cats achieved remission. 9 Although obesity is associated with increased risk of diabetes, it was not found to be positively or negatively associated with remission. 8 If there was any bias associated with obesity, the glargine group was disadvantaged compared to the other groups, because glargine-treated cats tended to gain weight during the study and lente and PZI treated groups lost weight (although differences between groups were not significant). Therefore, of the known biases for remission and glycaemic control, the glargine group was disadvantaged, reinforcing the positive findings of the study.
Burmese cats in Australia, New Zealand and the United Kingdom are reported to be predisposed to diabetes.2,10,11 There is no evidence that diabetic Burmese cats are more or less likely to achieve remission than non-Burmese diabetic cats, but to avoid any potential bias, treatment groups were matched with four Burmese per group. The observed effects of insulin type were not confounded by breed, and Burmese cats had a similar probability of remission as non-Burmese cats.
Diets low in carbohydrate result in lower post-prandial blood glucose concentrations, 12 and most diabetic cats have reduced exogenous insulin requirements when fed a low carbohydrate diet. 4 To remove confounding effects of diet on blood glucose concentration, all cats were exclusively fed the same ultra-low carbohydrate, high protein canned diet (Purina Diabetes Management, Ralston Purina). The low carbohydrate diet was expected to lead to reduced insulin requirements, improved glycaemic control and increased probability of remission, but any effect was equally distributed between groups.
A second important finding was that probability of subsequent remission was substantially higher amongst cats with lower mean 12 h blood glucose concentrations on day 17 (<10 mmol/l versus ≥10 mmol/l and <16 mmol/l versus ≥16 mmol/l). Two interrelated factors probably explain this finding. Firstly, cats with lower mean 12 h blood glucose concentrations on day 17 probably had more β-cell function, a critical factor in cats becoming non-insulin dependent. Human diabetic patients with some endogenous insulin secretion have lower mean blood glucose concentrations and better glycaemic control than those with minimal β-cell function. 13 The second factor relates to glucose toxicity, a phenomenon of initially reversible and later irreversible loss of β-cell function secondary to high blood glucose concentrations.14,15 Good glycaemic control enhances β-cell recovery from glucose toxicity and facilitates non-insulin dependence in humans. 14 Thus, it is likely that the sooner a newly diagnosed diabetic cat has sufficiently low blood glucose concentrations for the effects of glucose toxicity to resolve, the greater the probability that β-cells will regain function, resulting in remission. This finding also suggests that low mean daily glucose concentration soon after diagnosis may be a good prognostic indicator for predicting remission, regardless of insulin type.
Fructosamine can be useful in assessing longer term glycaemic control in diabetic cats. However, the long half-life of fructosamine resulted in serum fructosamine concentrations at day 28 not reflecting the significantly lower mean 12 h blood glucose concentrations seen in glargine-treated cats at day 17. This emphasises the value of blood glucose concentration rather than fructosamine concentration for adjusting insulin dose. For cats where serial blood glucose measurements in the hospital are problematic, home monitoring of blood glucose concentrations likely provides superior information to measurement of fructosamine concentration.
Severe clinical hypoglycaemia (blood glucose <3 mmol/l with clinical signs of ataxia, seizuring, etc) due to excessive insulin dose can be life-threatening and requires prompt treatment, usually including intravenous glucose supplementation. None of the glargine-treated cats exhibited signs of hypoglycaemia while three cats in the other treatment groups (lente=2, PZI=1) developed severe clinical hypoglycaemia requiring intravenous glucose therapy. Cats treated with glargine may be less likely to develop clinical hypoglycaemia and glargine may allow for tighter glycaemic control without increased risk of clinical hypoglycaemia. While humans with type 1 diabetes treated with glargine achieve superior glycaemic control compared with patients treated with shorter-acting insulins such as NPH, the major benefit is that fewer hypoglycaemic episodes occur. 16 Humans with type 2 diabetes treated with glargine also have a reduced incidence of hypoglycaemia compared with those treated with NPH insulin, but glycaemic control was similar. 17 The lower frequency of clinical hypoglycaemia is thought to be associated with glargine's consistent 24 h insulin profile compared to the sharp peak of insulin activity which occurs after administration of intermediate-acting insulin. Thus, glargine may result in reduced risk of severe clinical hypoglycaemia over other insulins, potentially allowing clinicians to safely aim for tight glycaemic control.
Glargine has a very long duration of action in healthy cats regardless of whether dosed once or twice daily. 18 A recent study reported that once daily glargine administration provided effective control and achieved similar efficacy to lente insulin administered twice daily. 7 The dosing protocol for lente and the proportion of lente-treated cats achieving remission was similar to our study and that of other studies using lente.19,20 However, the proportion of glargine-treated cats achieving remission in our study (8/8 cats) was much higher than in Weaver's (2006) study (1/6 cats). 7 This was probably due to the initial daily glargine dose being double (0.5 U/kg bid) that in the Weaver study (0.5 U/kgsid) and the inclusion of only newly diagnosed diabetic cats in our study. The substantially higher probability of diabetic remission seen in our study supports the use of twice daily administration of glargine in all newly diagnosed diabetic cats at an initial dose of 0.5 U/kg per injection.
Until recently, the goals of insulin therapy in diabetic cats have been to resolve clinical signs and prevent ketoacidosis. With the advent of longer-acting insulins and low carbohydrate diets, the goal of therapy has changed, and it is now expected that most newly diagnosed cats will achieve remission and not require insulin injections. To maximise the chance of remission, we suggest that all newly diagnosed diabetic cats initially be treated with twice daily glargine and a low carbohydrate diet. Because good glycaemic control soon after diagnosis is associated with increased probability of remission, and cats treated with glargine appear to have a reduced chance of clinical hypoglycaemia, we suggest that for the first 6–8 weeks of therapy, insulin dose be adjusted to achieve tight glycaemic control and cats monitored carefully. Slightly overdosing with insulin may increase the risk of biochemical hypoglycaemia, but the benefits of reversing glucose toxicity and rapidly achieving remission through early institution of good glycaemic control outweigh the low risk of clinical hypoglycemia. 8
In summary, this study in newly diagnosed diabetic cats showed that twice daily treatment with glargine insulin provides better glycaemic control and higher probability of remission compared to twice daily treatment with PZI or lente insulin. Good glycaemic control soon after diagnosis is associated with increased probability of remission and should be the goal of insulin therapy.
Acknowledgements
The authors gratefully acknowledge the Australian Companion Animal Health Foundation for funding of this study, Idexx Pharmaceuticals for supply of PZI insulin, Nestlé Purina Petcare for supply of cat food for the trial, and all the veterinary technicians from The University of Queensland Veterinary School.
References
- 1.Panciera D.L., Thomas C.B., Eicker S.W., Atkins C. Epizootiologic patterns of diabetes mellitus in cats: 333 cases (1980–1986), J Am Vet Med Assoc 197, 1990, 1504–1508. [PubMed] [Google Scholar]
- 2.Rand J.S., Bobbermein L.M., Hendrikz J.K. Over-representation of Burmese in cats with diabetes mellitus in Queensland, Austral Vet J 75, 1997, 402–405. [DOI] [PubMed] [Google Scholar]
- 3.Baral R.M., Rand J.S., Catt M.J., Farrow H.A. Prevalence of feline diabetes mellitus in a feline private practice, J Vet Int Med 17, 2003, 433–434. [Google Scholar]
- 4.Bennett N., Greco D.S., Peterson M.E., Kirk C., Mathes M., Fettman M.J. Comparison of a low carbohydrate–low fiber diet and a moderate carbohydrate–high fiber diet in the management of feline diabetes mellitus, J Feline Med Surg 8, 2006, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin G., Rand J.S. Pharmacology of a 40IU/ml porcine lente insulin preparation in diabetic cats. Findings during the first week and after five or nine weeks of therapy, J Feline Med Surg 3, 2001, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall R.D., Rand J.S., Morton J.M. Glargine and protamine zinc insulin have a longer duration of action and result in lower mean daily glucose concentrations than lente insulin in healthy cats, J Vet Pharmacol Therapeut 31, 2008, 205–212. [DOI] [PubMed] [Google Scholar]
- 7.Weaver K.E., Rozanski E.A., Mahony O.M., Chan D.L., Freeman L.M. Use of glargine and lente insulins in cats with diabetes mellitus, J Vet Int Med 20, 2006, 234–248. [DOI] [PubMed] [Google Scholar]
- 8.Roomp K., Rand J.S. Factors predictive of non-insulin dependence in diabetic cats initially treated with insulin [Abstract], J Vet Int Med 22, 2008, 696, (In review as: Intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine J Feline Med Surg) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieber-Ruckstuhl N.S., Kley S., Tschuor F., et al. Remission of diabetes mellitus in cats with diabetic ketoacidosis, J Vet Intern Med 22, 2008, 1326–1332. [DOI] [PubMed] [Google Scholar]
- 10.Wade C., Gething M., Rand J.S. Evidence of a genetic basis for diabetes mellitus in Burmese cats [Abstract], J Vet Int Med 13, 1999, 269. [Google Scholar]
- 11.McCann T.M., Simpson K.E., Shaw D.J., Butt J.A., Gunn-Moore D.A. Feline diabetes mellitus in the UK: the prevalence within an insured cat population and a questionnaire-based putative risk factor analysis, J Feline Med Surg 9, 2007, 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrow H.A., Rand J.S., Sunvold G.D. The effect of high protein, high fat or high carbohydrate diets on postprandial glucose and insulin concentrations in normal cats [Abstract], J Vet Int Med 16, 2002, 360. [Google Scholar]
- 13.Service F.J., Nelson R.L. Characteristics of glycemic stability, Diabetes Care 3, 1980, 58–62. [DOI] [PubMed] [Google Scholar]
- 14.Rossetti L., Giaccari A., DeFronzo R.A. Glucose toxicity, Diabetes Care 13, 1990, 610–630. [DOI] [PubMed] [Google Scholar]
- 15.Unger R.H., Grundy S. Hyperglycemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes, Diabetologia 28, 1985, 119–121. [DOI] [PubMed] [Google Scholar]
- 16.Fulcher G.R., Gilbert R.E., Yue D.K. Glargine is superior to NPH for improving glycated hemoglobin and fasting blood glucose levels during intensive insulin therapy, Int Med J 35, 2005, 536–542. [DOI] [PubMed] [Google Scholar]
- 17.Fonseca V., Bell D.S., Berger S., Thomson S., Mecca T.E. A comparison of bedtime insulin glargine with bedtime Neutral protamine Hagedorn in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study, Am J Med Sci 328, 2004, 274–280. [DOI] [PubMed] [Google Scholar]
- 18.Marshall R.D., Rand J.S., Morton J.M. Insulin glargine has a long duration of effect following administration either once daily or twice daily in divided doses in healthy cats, J Feline Med Surg 10, 2008, 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin G., Rand J. Control of diabetes mellitus in cats with porcine insulin zinc suspension, Vet Rec 161, 2007, 88–93. [DOI] [PubMed] [Google Scholar]
- 20.Michiels L., Reusch C.E., Boari A., Petrie G., Mandigers P., Thollot I.G., et al. Treatment of 46 cats with porcine lente insulin – a prospective, multicentre study, J Feline Med Surg 10, 2008, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rand J.S. Current understanding of feline diabetes: part 1, pathogenesis, J Feline Med Surg 1, 1999, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]


