Abstract
Human diabetic patients routinely self-adjust their insulin dose using a protocol and home monitoring, and perform equally well or outperform physician directed adjustments. The objective of this study was to report the outcome of home monitoring of diabetic cats by owners using a protocol aimed at achieving euglycaemia, using ultra-low carbohydrate diets (≤10% metabolisable energy) and the insulin analogue glargine for >10 weeks and/or until remission was achieved. Fifty-five cats diagnosed with diabetes mellitus, whose owners joined the online German Diabetes-Katzen Forum, were included. An overall remission rate of 64% was achieved in the cohort. Significantly higher remission rates were observed if good glycaemic control was achieved soon after diagnosis: 84% for cats started on the protocol within 6 months of diagnosis went into remission, and only 35% for cats that began more than 6 months after diagnosis (P<0.001). Only one mild clinical hypoglycaemic episode occurred observed despite tight blood glucose control. In conclusion, intensive blood glucose control is safe and effective in diabetic cats using home monitoring and treatment with glargine.
Landmark clinical trials involving human type 1 and type 2 diabetic patients have shown that intensive control of blood glucose, in addition to blood pressure control, prevents and delays the progression of complications related to diabetes.1,2 While translating these research findings into real world practice is still ongoing, 3 these evidence-based practice standards show great promise in decreasing morbidity and mortality among human diabetics. Tight glycaemic control has not been studied in feline diabetics, but the possible benefits of increased remission rates and reduced levels of complications warrant investigation.
Achieving euglycaemia in feline patients is difficult, because the insulins available often have inadequate duration of action for twice daily administration, and because there is wide variability between and within cats in response to exogenous insulin.4,5 Glargine (Lantus; Sanofi-Aventis, Paris, France) is an insulin analogue which has a longer duration of action in healthy cats than intermediate-acting insulins 6 and initial studies in diabetic cats 7 suggest it is associated with high remission rates.
In Germany, porcine lente insulin (Caninsulin; Intervet BV, The Netherlands) is currently the only licensed insulin for feline use, and legislation requires that it is the first insulin to be used in treatment of diabetic cats. However, control of blood glucose concentration and remission rates were found to be inferior to glargine when both insulins were used twice daily in diabetic cats.7,8 Poor glycaemic control led some owners to seek alternative methods of treatment for cats responding inadequately to initial therapy with lente insulin. The web-based German Diabetes-Katzen Forum was established in its current form in April 6, 2006 as a forum for owners of diabetic cats using long-acting insulins such as glargine or detemir.
Recent studies in human diabetic patients have shown that patients that have been taught to use a self-adjusted dosing protocol, perform equally well as physician driven adjustments, 9 or outperform clinic-driven protocols. 10 It was also found that patients that were naive to insulin could effectively implement the dosing algorithm and achieved similar results to patients directed by a physician. 11 Similarly, diabetic cat owners participating in the Diabetes-Katzen Forum, adjusted insulin dose based on a dosing protocol, which encompassed daily home monitoring.
Home monitoring of blood glucose concentrations is standard practice for human diabetic patients, but relatively few pet owners are willing to measure blood glucose concentration multiple times daily to achieve tight glycaemic control for their pets. The German Diabetes-Katzen Forum attracted owners of diabetic cats who performed daily home monitoring and used glargine with a treatment protocol which aimed for euglycaemia. The dosing protocol was developed based on the veterinary literature with input from specialist veterinarians. 12
Diet is a cornerstone of successful management of diabetic cats. Recent studies demonstrated that diabetic cats fed low carbohydrate, high protein canned food had higher insulin sensitivity and remission rates than cats fed higher carbohydrate diets.13,14 Cats evolved as strict carnivores, but the diets which have become widespread in the last 20–30 years have moderate to high carbohydrate content (30 to >50% of energy).15,16 These diets promote post-prandial hyperglycemia and may contribute to the increasing incidence of diabetes in cats. 17 Therefore, low carbohydrate, high protein diets are recommended for management of diabetic cats. 13
The objectives of this study were to report the outcome of home monitoring of diabetic cats by owners using a protocol aimed at achieving euglycaemia, using an ultra-low carbohydrate diet (≤10% metabolisable energy) and glargine. Specifically the aims were to report (1) the remission rates of cats using such a protocol, (2) the safety and complication rate in cats using this protocol, and (3) the factors which might be prospectively used to differentiate diabetic cats that subsequently became non-insulin dependent from cats which remained insulin dependent.
Materials and methods
Data were provided by owners of diabetic cats who joined the German Diabetes-Katzen Forum (http://www.diabetes-katzen.net/forum/) or an earlier version of the website between October 3, 2004 and April 30, 2007. Data reported from the cats were collected until December 23, 2007 and 55 cats were included in the study. Owners were informed about the study and asked to provide information regarding their cats in spreadsheets and questionnaires. The cats were mostly located within Germany (n=49) and a small number in Switzerland (n=4) and other countries (n=2).
Inclusion criteria for the study were that the owner had to follow the forum's protocol (Table 1) using glargine for more than 10 weeks and/or until remission was achieved, and to supply blood glucose measurements for their cat. This included all daily insulin dosages and measured blood glucose concentrations from the time that they joined the forum, including for insulin used prior to changing to glargine. The owner also supplied as much additional clinical information as possible, collected in the form of questionnaires. The owner estimated the ideal weight of the cat, which was used to calculate the initial insulin dosage used. While on the protocol, cats were fed an ultra-low carbohydrate (generally <8–10% of energy) canned food, or in several cases, ultra-low carbohydrate veterinarian-developed home-made diets. 18
Table 1.
Parameters for changing insulin dosage when using insulin glargine together with home monitoring of blood glucose concentrations in a protocol aimed at achieving of intensive blood glucose control. Dose increases are per injection per cat. Blood glucose was measured at least three times daily with a glucometer designed for human diabetic patients that reported glucose concentrations for whole blood (not plasma-equivalent) and used ≤0.6 μl of blood per test. A low insulin dose generally is <3 IU, a high dose is ≥3 IU per injection per cat
| Parameter used for dosage adjustment | Change in dose |
|---|---|
| Phase 1: initial dose and first 3 days on glargine | |
| Begin with 0.25 IU/kg of ideal weight bid. If the cat received another insulin previously, increase or reduce the starting dose taking this information into account. Glargine has a lower potency than lente insulin or PZI in most cats | |
| Cats with a history of developing ketones that remain 16.6 mmol/l (>300 mg/dl) after 24–48 h | Increase by 0.5 IU |
| If blood glucose is <2.8 mmol/l (<50 mg/dl) | Reduce dose by 0.25–0.5 IU depending on if cat is on low or high dose of insulin |
| Phase 2: increasing the dose | |
| If nadir blood glucose concentration is >16.6 mmol/l (>300 mg/dl) | Increase every 3 days by 0.5 IU |
| If nadir blood glucose concentration is 11.1–16.6 mmol/l (200–300 mg/dl) | Increase every 3 days by 0.25–0.5 IU depending if on low or high dose of insulin |
| If nadir blood glucose concentration <11.1 mmol/l (<200 mg/dl) but peak is >11.1 mmol/l (>200 mg/dl) | Increase every 5–7 days by 0.25–0.5 IU depending if on low or high dose of insulin |
| If blood glucose is <2.8 mmol/l (<50 mg/dl) | Reduce dose by 0.25–0.5 IU depending if on low or high dose of insulin |
| If blood glucose at the time of the next insulin injection is 2.8–5.5 mmol/l (50–100 mg/dl) | Initially test which of the alternate methods is best suited to he individual cat: |
| a. Feed cat and reduce the dose by 0.25–0.5 IU depending if on low or high dose of insulin | |
| b. Feed the cat, wait 1–2 h and when the glucose concentration increases to >5.5 mmol/l (>100 mg/dl) give the normal dose. If the glucose concentration does not increase within 1–2 h, reduce the dose by 0.25 IU or 0.5 IU (as above) | |
| c. Split the dose: feed cat, and give most of dose immediately and then give the remainder 1–2 h later, when the glucose concentration has increased to >5.5 mmol/l (>100 mg/dl) | |
| If all these methods lead to increased blood glucose concentrations, give the full dose if pre-insulin blood glucose concentration is 2.8–5.5 mmol/l (50–100 mg/dl) and observe closely for signs of hypoglycaemia. In general for most cats, the best results in phase 2 occur when insulin is dosed as consistently as possible, giving the full normal dose at the regular injection time | |
| Phase 3: holding the dose. Aim to keep blood glucose concentration within 2.8–11.1 mmol/l (50–200 mg/dl) throughout the day | |
| If blood glucose is <2.8 mmol/l (<50 mg/dl) | Reduce dose by 0.25–0.5 IU depending if on low or high dose of insulin |
| If nadir or peak blood glucose concentration >11.1 mmol/l (>200 mg/dl) | Increase dose by 0.25–0.5 IU depending if on low or high dose of insulin and the degree of hyperglycaemia |
| Phase 4: reducing the dose. Phase out insulin slowly by 0.25–0.5 IU depending on dose | |
| When the cat regularly (every day for at least 1 week), has its lowest blood glucose concentration in the normal range of a healthy cat, and stays under 5.5 mmol/l (100 mg/dl) overall | Reduce dose by 0.25–0.5 IU depending if on low or high dose of insulin |
| If the nadir glucose concentration is 2.2–<2.8 mmol/l (40 to <50 mg/dl) at least three times on separate days | Reduce dose by 0.25–0.5 IU depending if on low or high dose of insulin |
| If glucose is <2.2 mmol/l (<40 mg/dl) once | Reduce dose immediately by 0.25–0.5 IU depending if on low or high dose of insulin |
| If peak blood glucose concentration >11.1 mmol/l (>200 mg/dl) | Immediately increase insulin dose to last effective dose |
| Phase 5: remission. Euglycaemia for a minimum of 14 days without insulin |
NB: For human-use meters which provide plasma-equivalent values, readings are approximately 11% higher than glucose measurements for whole blood in human patients. For veterinary-use meters calibrated to give plasma-equivalent values for feline blood, add approximately 1.7 mmol/l (30 mg/dl) to target glucose concentrations, for example, aim for 4.4–7.2 mmol/l (80–130 mg/dl), instead of 2.8–5.5 mmol/l (50–100 mg/dl).
Of the 209 diabetic cats whose owners joined the forum up until December 23, 2007, 78 cats were excluded from the study because they were not treated with glargine. Of the 131 remaining cats that were treated with glargine, further cats were excluded from the study for the following reasons: acromegaly (n=2), cats known to be on a high carbohydrate diet (n=1), cats whose owners joined the forum very close to the cutoff and did not have >10 weeks of tight regulation data or did not achieve remission by the cutoff date (n=11), cats whose owners dropped out of the forum (n=9), cats whose owners did very little blood glucose home monitoring or who chose not to aim for euglycaemia and used more traditional regulation protocols (n=21), and cats whose owners chose not to provide blood glucose concentration data for the study (n=32). The remaining 55 cats met the inclusion criteria and were included in the study.
Of the 55 cats reported in this study, blood glucose concentrations using the intensive regulation protocol were provided from the time the intensive regulation protocol with glargine was begun to the last insulin injection (median of 3.2 months of data provided (range=6 days to 25 months)). Two cats had less than 2 weeks of blood glucose data because they achieved remission very quickly (<2 weeks).
High and low blood glucose concentrations that were beyond the measurement ranges of glucometers were very rare occurrences within the cohort. For low values, a blood glucose value of 1.1 mmol/l (20 mg/dl) was used in the calculations and for high values, a blood glucose concentration of 33.3 mmol/l (600 mg/dl) was used. Owners were directed to measure blood glucose concentration a minimum of three times daily (including every pre-insulin concentration and then generally every 3–6 h), but most measured it more often and sometimes in shorter intervals.
Owners were asked to carefully read the protocol upon joining the forum, and were advised of the prerequisites for using the protocol (Table 1). Advice on using the protocol was provided on an ongoing basis within the forum by experienced owners. Owners were directed to general information in the forum or other websites with regard to potential issues such as hypoglycaemia and ketonuria, and were encouraged to maintain close contact with their veterinarian with regard to their cat's diabetic treatment and general health. Advice provided in the forum related only to non-ketotic diabetic cats with signs of uncomplicated diabetes. Owners of cats that developed ketonuria or signs indicating illness were directed to immediately seek veterinary attention.
Owners regularly tested their cat's urine for ketones, generally using urine dipsticks (Keto-Diastix or Ketostix; Bayer, Leverkusen, Germany). This was particularly recommended during the initial regulation phase on the protocol, before euglycaemia was achieved. Owners of cats with a history of ketonuria or diabetic ketoacidosis (DKA) generally tested twice a day in the first 3 days after switching to glargine.
Statistical analysis
Most data were supplied by owners in the form of spreadsheets and was subsequently imported into a database. Analysis was performed using the statistics software package R. 19
To minimise the effect on the weekly mean blood glucose concentration of more frequent testing during relatively short periods of low blood glucose concentration, means were calculated for each day and subsequently the seven daily means were used to generate a weekly mean.
Distributions were tested for normality using the Shapiro–Wilk normality test, 20 prior to further analysis. Differences in categorical data were analysed using Fisher's exact test for independence and the 95% confidence interval (CI) was computed. For comparing two distributions, the Wilcoxon rank sum test with continuity correction (Mann–Whitney) was used. For normal distributions, means and standard deviation were calculated, and for distributions that did not satisfy the test for normality, medians and ranges were calculated.
Results
Signalment and history of diabetic cats at diagnosis
Of the 55 cats included in the study of known signalment, all were neutered and most were male (74%), domestic cats (80%), and were more often overweight (40%) than underweight or normal weight based on the owners' assessment (Table 2). The median age when diagnosed was 10.3 years (range=3.1–16.7 years).
Table 2.
Summary of signalment and historical data obtained from questionnaire information provided by owners of 55 diabetic cats
| Total known (n) | Total unknown (n) | Cats with characteristic (n) | Percentage of cats with characteristic (% of known) | |
|---|---|---|---|---|
| Signalment | ||||
| Breed | 50 | 5 | ||
| Domestic shorthair | 40 | 80 | ||
| Maine Coon | 3 | 6 | ||
| Persian | 1 | 2 | ||
| Siamese | 1 | 2 | ||
| Other or mix | 5 | 10 | ||
| Sex | 55 | 0 | ||
| Female | 14 | 26 | ||
| Male | 41 | 74 | ||
| Neutered | 41 | 14 | ||
| Entire | ||||
| Neutered | 41 | 100 | ||
| History/clinical signs | ||||
| Weight group | 53 | 2 | ||
| Overweight | 21 | 40 | ||
| Normal | 18 | 34 | ||
| Underweight | 14 | 26 | ||
| Neuropathy | 47 | 8 | ||
| Plantigrade stance/walking on hocks | 5 | 11 | ||
| Weakness jumping or climbing stairs/occasional weakness walking | 13 | 28 | ||
| Other signs of weakness/paralysis | 3 | 6 | ||
| No neuropathy | 26 | 55 | ||
| Corticosteroids previously | 45 | 10 | ||
| No | 31 | 69 | ||
| Yes | 14 | 31 | ||
| Tablets: <1 month before diagnosis | 1 | 7 | ||
| Tablets: <3 months before diagnosis | 0 | 0 | ||
| Tablets: <6 months before diagnosis | 1 | 7 | ||
| More than one depot injection: <1 month before diagnosis | 3 | 21 | ||
| More than one depot injection: <3 month before diagnosis | 7 | 50 | ||
| More than one depot injection:<6 month before diagnosis | 1 | 7 | ||
| Continuous therapy in 6 months before diagnosis | 1 | 7 | ||
| Diet before diagnosis | 46 | 9 | ||
| Dry food only | 11 | 24 | ||
| 50% dry, 50% canned | 26 | 57 | ||
| Mostly canned, some dry | 4 | 9 | ||
| Canned only | 5 | 11 |
Diabetic neuropathy appeared to be common with owners describing a plantigrade stance or walking on the hocks at diagnosis (11%) and other forms of weakness (34%) such as reduced jumping ability or an inability to climb stairs (Table 2). Corticosteroid treatment was frequent (31%) and was most often in the form of repeated injections of long-acting steroid in the 3 months prior to diagnosis. Most cats (81%) ate 50% or more of their diet as dry cat food prior to diagnosis, and only a minority ate exclusively canned food (11%). Following diagnosis of diabetes and institution of insulin therapy (but prior to treatment with glargine), 43 cats (78%) were changed to an ultra-low carbohydrate (≤10% of energy) wet food diet. All cats on the intensive blood glucose regulation protocol were fed an ultra-low carbohydrate diet.
Treatment history
Fifty cats (91%) in the cohort were initially treated with an insulin other than glargine for a median of 15 weeks, but failed to achieve remission prior to switching to glargine. Most cats (67%) were initially treated with porcine lente insulin and were then switched to glargine, and later began with the intensive blood glucose regulation protocol using glargine (Table 3). Some cats (18%) were switched from porcine lente to another insulin before beginning glargine, and only five cats received glargine as their first insulin.
Table 3.
Summary of previous treatment prior to glargine therapy, and types of blood glucose monitoring devices used by owners
| Total known (n) | Total unknown (n) | Cats with characteristic (n) | Percentage of cats with characteristic (% of known) | |
| Insulins | ||||
| Previous insulins | 55 | 0 | ||
| None, ie, glargine was first insulin | 5 | 9 | ||
| Porcine lente insulin only | 37 | 67 | ||
| Human lente insulin only | 3 | 6 | ||
| Porcine lente insulin and other insulins (eg, PZI) | 10 | 18 | ||
| Monitoring | ||||
| Glucometer | 52 | 3 | ||
| Ascensia Contour | 37 | 71 | ||
| Accu-Chek Aviva | 10 | 19 | ||
| Other brands | 5 | 10 |
The most commonly used glucometers were the Ascensia Contour (Bayer, Leverkusen, Germany; 37/52; 71%) and the Accu-Chek Aviva (Roche Diagnostics, Basel, Switzerland; 10/52; 19%). Both required 0.6 μl of blood for testing and reported glucose concentration in whole blood (Table 3). They are calibrated for human blood, and for feline blood in the normoglycaemic range they provide precise (r=0.97) measurements on average 1.9 mmol/l; 35 mg/dl (Bayer) and 1.4 mmol/l; 25 mg/dl (Roche) lower than an automated serum analyser (Abbott Laboratories, unpublished data).
Intensive protocol
Owners performed an average of 5±2 blood glucose measurements per day. No difference was observed in frequency of testing for cats that became non-insulin dependent (achieved remission) and cats that did not (P>0.05, Wilcoxon rank sum test with continuity correction). For cats on the protocol, there was no significant difference in the mean blood glucose concentration at week 4 for cats that subsequently went into remission (8.9 mmol/l; 160 mg/dl) and cats that did not (9.6 mmol/l; 173 mg/dl) (Table 4).
Table 4.
Comparison of parameters between cats which subsequently became non-insulin dependent (n=35) after insulin treatment using the protocol described in Table 1 and cats which remained insulin dependent (n=20). Parameters shown in bold were significantly different between insulin-dependent and non-insulin-dependent cats
| Factor | Cats which achieved diabetic remission | Cats which remained insulin dependent | Statistical test | P-Value (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of cats (n) or years | Data available (n) | Data unavailable (n) | % of remission cases | Number (n) or years | Data available (n) | Data unavailable (n) | % of insulin-dependent cases | |||
| Average age at diagnosis | 10.6 years | 35 | 0 | NA | 9.2 years | 20 | 0 | NA | Wilcoxon | 0.06 |
| Proportion of female cats | 8 | 35 | 0 | 23 | 6 | 20 | 0 | 30 | Fisher's exact test | 0.75 |
| Overweight at diagnosis | 15 | 34 | 1 | 44 | 6 | 19 | 1 | 32 | Fisher's exact test | 0.40 |
| Underweight at diagnosis | 11 | 34 | 1 | 32 | 3 | 19 | 1 | 16 | Fisher's exact test | 0.33 |
| Cortisone treatment ≤6 months before diagnosis | 14 | 30 | 5 | 47 | 0 | 15 | 5 | 0 | Fisher's exact test | 0.0014 (2.45, inf) |
| Hyperthyroidism | 3 | 27 | 8 | 11 | 1 | 15 | 5 | 7 | Fisher's exact test | 1.0 |
| Neuropathic signs at diagnosis (plantigrade stance, weakness, etc) | 10 | 33 | 2 | 30 | 11 | 14 | 6 | 79 | Fisher's exact test | 0.0037 (0.018, 0.606) |
| Just plantigrade stance | 3 | 33 | 2 | 9 | 2 | 14 | 6 | 14 | Fisher's exact test | 0.63 |
| CKD | 8 | 34 | 1 | 24 | 5 | 15 | 5 | 33 | Fisher's exact test | 0.50 |
| Average age at CKD diagnosis | 11.4 years | 34 | 1 | NA | 10.6 years | 15 | 5 | NA | Wilcoxon | 0.72 |
| Mean insulin dose (IU) bid | 2.61 IU | 35 | 0 | NA | 3.86 IU | 20 | 0 | NA | Wilcoxon | 0.017 (NA) |
| Mean insulin dose (IU/kg) bid | 0.43 IU/kg | 32 | 3 | NA | 0.66 IU/kg | 18 | 2 | NA | Wilcoxon | 0.016 (NA) |
| Mean blood glucose concentration at week 4 under protocol | 8.9 mmol/l (160 mg/dl) | 35 | 0 | NA | 9.6 mmol/l (173 mg/dl) | 20 | 0 | NA | Wilcoxon | 1.0 |
| Biochemical hypoglycaemia ≥2.2 to <2.8 mmol/l (≥40 to <50 mg/dl) | 32 | 35 | 0 | 91 | 19 | 20 | 0 | 95 | Fisher's exact test | 1.0 |
| Biochemical hypoglycaemia ≥1.7 to <2.2 mmol/l (≥30 to <40 mg/dl) | 28 | 35 | 0 | 80 | 18 | 20 | 0 | 90 | Fisher's exact test | 0.46 |
| Biochemical hypoglycaemia ≥1.1 to <1.7 mmol/l (≥20 to <30 mg/dl) | 17 | 35 | 0 | 49 | 11 | 20 | 0 | 55 | Fisher's exact test | 0.78 |
| Biochemical hypoglycaemia <1.1 mmol/l (<20 mg/dl) | 1 | 35 | 0 | 3 | 0 | 20 | 0 | 0 | Fisher's exact test | 1.0 |
| Began protocol ≤6 months after diagnosis | 27 | 35 | 0 | 77 | 8 | 20 | 0 | 40 | Fisher's exact test | 0.0089 (1.32, 19.7) |
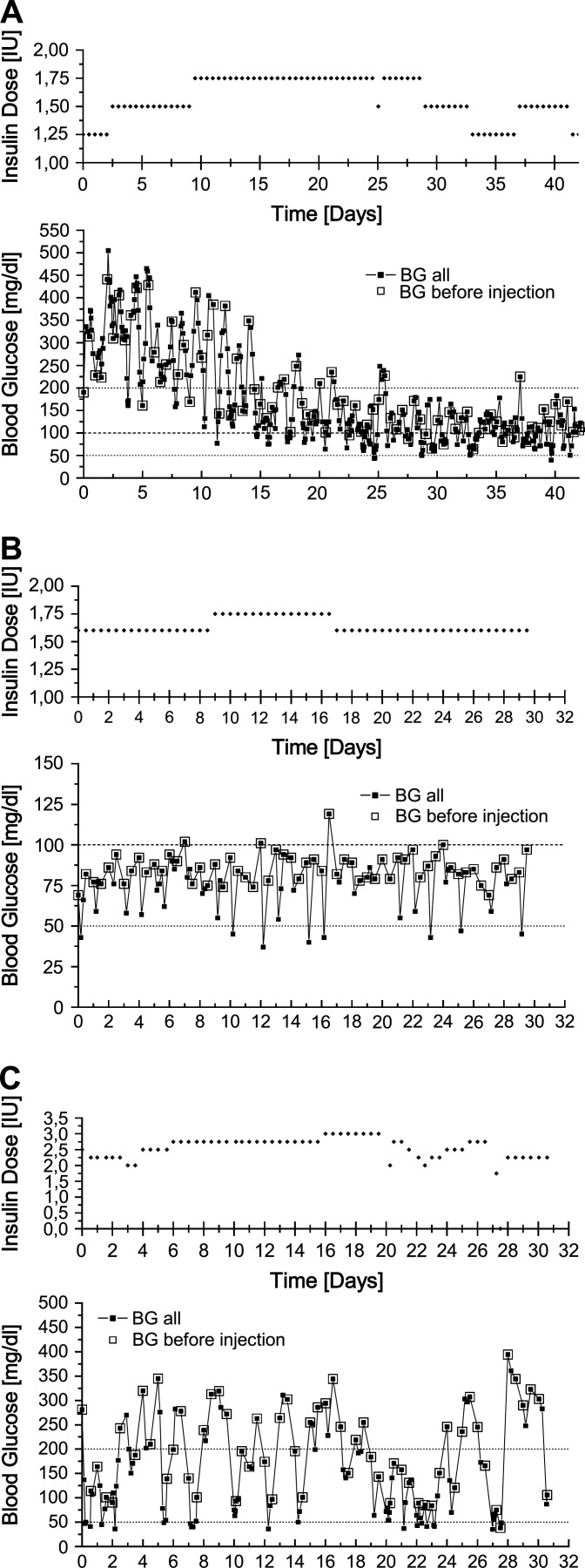
A typical blood glucose concentration profile of a cat on the intensive blood glucose regulation protocol and glargine treatment is shown in Fig 1 A. Fluctuations in the first weeks on glargine were common, with low blood glucose concentrations interspersed with high blood glucose concentrations. With time and consistent insulin doses, there was less variation within and between days.
Fig 1.
(A) Typical progress of a cat on the intensive blood glucose regulation protocol. The cat's first 42 days after switching on to glargine, and directly on to protocol, are shown. The top curve shows the glargine dose (bid) and the bottom curve shows all measured blood glucose concentrations. Blood glucose was measured on average five times daily with a glucometer designed for human diabetic patients that reported glucose concentrations for whole blood. This cat's ideal weight is 5.25 kg. (B) A well-regulated cat on glargine over the period of 1 month: the lowest blood glucose concentration is 2.1 mmol/l (37 mg/dl) and the highest is 6.6 mmol/l (119 mg/dl) measured with a whole blood glucose meter calibrated for human blood. This cat's ideal weight is 5.00 kg. (C) A difficult to regulate cat on glargine over the period of 1 month: the lowest glucose concentration is 2.0 mmol/l (36 mg/dl) and the highest is 21.9 mmol/l (394 mg/dl). This cat's ideal weight is 4.75 kg.
A phenomenon was observed in almost one quarter of all cats (13/55; 24%) that recently had their insulin dose increased. Within the first 2–3 days after a dose increase, the average and peak glucose concentration rose inexplicably without a preceding hypoglycaemia blood glucose concentration being measured.
Asymptotic or biochemical hypoglycaemia was common among cats using this protocol. Most cats (51/55; 93%) had blood glucose concentrations ≥2.2 to <2.8 mmol/l (≥40 to <50 mg/dl) measured at some point during the study, 46 cats (84%) had glucose concentrations ≥1.7 to <2.2 mmol/l (≥30 to <40 mg/dl), 28 cats (51%) had glucose concentrations ≥1.1 to <1.7 mmol/l (≥20 to <30 mg/dl) and one cat (2%) had glucose concentrations <1.1 mmol/l (<20 mg/dl). However, when calculated as a percentage of all blood glucose concentration curves for an individual cat, low blood glucose concentrations were typically not frequent. For example, of all curves measured for individual cats, the percentage of curves with nadir blood glucose concentrations in the hypoglycaemic range were 7.3% (0–25%) for glucose ≥2.2 to <2.8 mmol/l (≥40 to <50 mg/dl); 4.4% (0–25%) for glucose ≥1.7 to <2.2 mmol/l (≥30 to <40 mg/dl); and 0.19% (0–10.7%) for glucose ≥1.1 to <1.7 mmol/l (≥20 to <30 mg/dl). Measurement of blood glucose concentration <1.1 mmol/l (<20 mg/dl) consisted of a single event in one cat. There were no significant differences in the rates of asymptomatic hypoglycaemia between cats that went into remission and those cats that did not (Table 4).
Only a single clinical hypoglycaemic event associated with a blood glucose concentration <1.7 mmol/l (<30 mg/dl) was recorded in a cat on the intensive protocol, and involved mild signs which included restlessness. The cat was fed a high carbohydrate meal, and blood glucose concentration rapidly increased within 1 h and signs resolved. The insulin dose was subsequently reduced by 20%. This cat was difficult to regulate and did not go into remission.
No cats tested positive for urinary ketones either in the first days on the protocol or later, and there were no instances of DKA occurring in cats after beginning the protocol.
Non-insulin dependence (diabetic remission)
Thirty-five cats (64%) were able to maintain normoglycaemia on a low carbohydrate diet without insulin therapy within a median of 59 days (1.9 months) of beginning the protocol (range=6 days to 10.2 months). The majority of these remission cats (26/35; 74%) remained off insulin and the median duration of remission for non-relapsing cats was 10.8 months (range=2.8 months to 3 years). Nine (9/35; 26%) remission cats relapsed and required insulin again. Two of these nine relapsed cats achieved a second remission.
The mean maximum dose per injection during the stabilisation period for cats which eventually became non-insulin dependent was 2.6 IUbid (range=1.0–9.0 IU). The mean maximum dose/kg of ideal weight per injection was 0.43 IU/kgbid (range=0.20–0.90 IU/kg).
Of the cats which became non-insulin dependent during the study, 30% had signs of neuropathy at diagnosis. During the study, two cats in remission died, one of bladder cancer and the other of unknown causes.
Insulin-dependent diabetic cats
Twenty cats (36%) in the cohort required insulin throughout the study to control blood glucose concentrations and did not achieve remission (Table 4). The median length of time on protocol was 12.7 months (range=2.6 months to 2.1 years). The level of glycaemic control was assessed using the median blood glucose concentration in the last week of the study (12 cats) or for the last day for which data was available (eight cats). Most (70%) long-term diabetics were considered well-regulated with a mean daily blood glucose concentration of ≤8.3 mmol/l (≤150 mg/dl, Table 5). Most well-regulated cats had mean daily blood glucose concentrations between 2.8 and 5.5 mmol/l (50 and 100 mg/dl, Fig 1 B). Cats with mean blood glucose concentrations from >5.5 to ≤11.1 mmol/l (>100 to ≤200 mg/dl) or >11.1 mmol/l (>200 mg/dl) generally had nadir glucose concentrations in the appropriate range (<6.7 mmol/l; <100 mg/dl), but had rapid blood glucose concentration changes following a nadir and still had substantial periods of hyperglycaemia ( Fig 1 C).
Table 5.
Level of glycaemic control in insulin-dependent diabetic cats after treatment with glargine and a protocol for intensive blood glucose control for a median of 12 months (range=2.4 months to 2.1 years). Mean blood glucose concentrations were calculated for the last week from daily mean concentrations where data were available. Blood glucose was measured on average five times daily with a glucometer designed for human diabetic patients that reported glucose concentrations for whole blood
| Mean glucose concentration | Insulin-dependent diabetic cats (n) |
|---|---|
| <8.3 mmol/l (<150 mg/dl) | 14 |
| ≤11.1 mmol/l (≤200 mg/dl) | 3 |
| >11.1 mmol/l (>200 mg/dl) (difficult to regulate) | 3 |
For cats which remained insulin dependent, mean maximum dose/injection was 3.86 IUbid (range=1.00–9.00 IU). The mean maximum dose/injection/kg of ideal weight per injection was 0.66 IU/kgbid (range=0.22–1.64 IU/kg).
A high percentage (11/14; 79%) of cats that did not go into remission had signs of neuropathy at diagnosis or during the study. Five cats which remained insulin dependent died during the study: two of cancer, one of chronic kidney disease (CKD), one likely of CKD related problems and one of unknown causes.
Predictors for non-insulin dependence (remission)
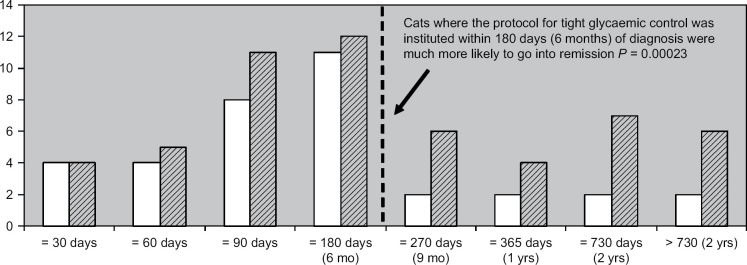
For cats that started with intensive blood glucose control within 180 days (6 months) of diagnosis of diabetes, the remission rate was 84%. The remission rate dropped to 35% for cats that were started on protocol later than 180 days after diagnosis ( Fig 2 ). This was highly significant (P<0.001, Fisher's exact test, 95% CI 2.42, 45.48). Of cats that went into remission, 77% began with the protocol within 180 days of diagnosis. In contrast, of cats that remained insulin dependent, 40% began with the protocol later than 180 days of diagnosis (Table 4). This was also highly significant (P=0.0089, Fisher's exact test, 95% CI 1.32, 19.7). The median time from diagnosis to starting the protocol was 3.2 months (range=5 days to 3.1 years) for cats achieving remission and 10.9 months (range=39 days to 3.5 years) for cats not achieving remission.
Fig 2.
The number of cats which went into remission (white bar), relative to the total number of cats (hatched bar), categorised by when they began on the protocol of intensive glucose concentration regulation relative to the day of their diabetes diagnosis. The median time to start the protocol for cats starting the protocol within 6 months was 2.4 months (range=5 days to 6 months) and for cats starting later than 6 months the median time to start the protocol was 13.7 months (range=6.2 months to 3.5 years).
Cats that were treated with corticosteroid in the 6 months prior to being diagnosed with diabetes were much more likely to go into remission than cats without prior corticosteroid treatment (P=0.0014, Fisher's exact test, 95% CI 2.45, inf); with 47% of cats that achieved remission having prior corticosteroid treatment (Table 4). All 14 cats that were treated with steroids in the 6 months prior to diagnosis achieved remission.
Cats which displayed a plantigrade stance at diagnosis or milder signs of peripheral neuropathy, such as a difficulty climbing stairs, were significantly less likely to go into remission (P=0.0036, Fisher's exact test, 95% CI 0.018, 0.606). However, when cats with only a plantigrade stance are examined, that is, cats with milder forms of peripheral neuropathy were excluded, the results were no longer significant (P=0.63, Fisher's exact test) (Table 4).
There was a significant difference in mean maximum insulin dose between cats which became non-insulin dependent during the study (0.43 IU/kg ideal body weight bid) and cats which remained insulin dependent (0.66 IU/kg ideal body weight bid) (P=0.016, Wilcoxon rank sum test with continuity correction) (Table 4).
Other factors which were examined but were not predictors of remission were age at diagnosis, gender, weight at diagnosis, evidence of DKA at diagnosis, CKD, hyperthyroidism, number of daily blood glucose measurements and frequency of asymptotic hypoglycaemia (Table 4). Obesity at diagnosis was not negatively correlated with remission.
Concurrent disease
Persistent azotaemia (increased plasma creatinine concentration) while receiving insulin or while in remission was detected in 13 cats (13/49; 26%) in the cohort (Table 6). Azotaemic cats were generally diagnosed while receiving insulin (n=10), but a small number of cats (n=3) were diagnosed when in remission.
Table 6.
Summary of secondary disease in 55 diabetic cats
| Total known (n) | Total unknown (n) | Cats with characteristic (n) | Percentage of cats with characteristic (% of known) | |
|---|---|---|---|---|
| Secondary disease | ||||
| Hyperthyroidism: | 42 | 13 | ||
| Developed before diagnosis | 1 | 2 | ||
| Developed while on insulin | 0 | 0 | ||
| Developed in remission | 2 | 5 | ||
| Yes, but time point unknown | 1 | 2 | ||
| Tested negative at diagnosis | 38 | 91 | ||
| Dental problems developed: | 26 | 29 | ||
| Preceding diabetes diagnosis | 3 | 12 | ||
| First discovered after diabetes diagnosis, might have existed before | 11 | 42 | ||
| Definitely developed after diabetes diagnosis | 4 | 15 | ||
| No dental problems | 8 | 31 | ||
| Dental problems type: | 28 | 27 | ||
| Gingivitis only | 3 | 11 | ||
| Tartar only | 5 | 18 | ||
| FORL plus tartar and/or gingivitis | 4 | 14 | ||
| FORL only | 5 | 18 | ||
| Peritonitis±tartar | 2 | 7 | ||
| Stomatitis and gingivitis | 1 | 4 | ||
| No dental problems | 8 | 29 | ||
| CKD | 49 | 6 | ||
| Developed before diagnosis | 0 | 0 | ||
| Developed while on insulin | 10 | 20 | ||
| Developed in remission | 3 | 6 | ||
| No CKD | 36 | 74 |
FORL=feline oral resorptive lesions; CKD=chronic kidney disease.
The proportion of azotaemic cats increased with age, with the highest frequency (31%) in the 10 to <15 year age group, but a surprising 18% of cats had azotaemia in the 5 to <10 year age group (Table 7). There were only two deaths among azotaemic cats during the study, which was most likely associated with advanced CKD. No significant differences were detected in the rate of CKD between cats which subsequently achieved remission and those that did not (P=0.50, Fisher's exact test), nor was the mean time between diagnosis and beginning the protocol associated with the rate of azotaemia. Most owners whose cats had azotaemia continued to feed a high protein diet, but attempted to regulate phosphate intake by selecting relatively low phosphate foods or using phosphate binders.
Table 7.
Age of cats and the proportion of diabetic cats with CKD (n=13) in each age group
| Age today | CKD (n) | Total tested (n) | % |
|---|---|---|---|
| <5 years | 0 | 1 | 0 |
| 5 to <10 years | 2 | 11 | 18 |
| 10 to <15 years | 8 | 26 | 31 |
| ≥15 years | 3 | 11 | 20 |
For cats with peripheral neuropathies, treatment usually involved methylcobalamin in addition to regulating blood glucose concentration. In the most severe cases, it took several months for cats to completely recover, despite having achieved euglycaemia and receiving methylcobalamin. All cats recovered from their peripheral neuropathy.
Dental disease was evident in (18/26; 69%) of cats examined before or after the diagnosis of diabetes. There was no association between dental disease and insulin resistance based on insulin dose. However, almost half the cats (29/55; 49%) did not have a dental examination, making it difficult to accurately estimate the occurrence of dental disease in this cohort (Table 6).
Hyperthyroidism was relatively rare within the cohort with only four cats diagnosed either prior to or after diagnosis of diabetes (Table 6).
Discussion
There were several important findings in this study. Firstly, committed owners were able to follow a protocol with home monitoring of blood glucose concentrations and achieve remission rates of 84% for cats started on the protocol within 6 months of diagnosis of diabetes.
Secondly, cats switched within 6 months of diagnosis to intensive blood glucose monitoring and a protocol of insulin dosing designed to achieve tight glycaemic control, achieved significantly higher rates of non-insulin dependence than cats that were started on the protocol longer than 6 months after diagnosis. This finding is important because cats which maintain euglycaemia without insulin, often termed diabetic remission, have improved health and quality of life, and it decreases the cost and inconvenience to the owner. Prolonged hyperglycaemia initially causes reversible suppression of β-cell function, and later permanent loss of β-cells. 21–23 This is the most likely cause for the decreasing remission rate in cats changed later to the protocol, even when blood glucose was eventually well controlled. This was reflected in our cohort. All four cats that began with the tight regulation protocol within 30 days of diagnosis went into remission, and the longer a cat had unregulated diabetes, the less likely remission became. The longest term diabetic in our cohort that went into remission was diabetic for 3 years, but had been reasonably well-regulated with glargine during this period, and therefore arguably not subjected to prolonged periods of severe hyperglycaemia.
Glargine is well suited to the goal of achieving euglycaemia in feline diabetic patients because its duration of action is more than 12 h, producing overlap between the doses when using a twice daily regimen. 6 This prevents marked hyperglycaemia from occurring around the time of the next insulin injection, which is typical of an intermediate-acting insulin such as lente. 6 This is also the likely reason for the increased remission rates using glargine than are reported for lente, because with glargine, glucose toxic damage to the β-cells is minimised, insulin resistance associated with hyper- and hypoglycaemia is reduced, and insulin secretion by β-cells is facilitated. 15
A previously undescribed phenomenon was observed in approximately 1/4 of the cats that recently had their insulin dose increased, which consisted of inexplicably higher blood glucose concentrations for the first 2–3 days after a dose increase, without a preceding hypoglycaemic blood glucose concentration being detected. The cause of the effect was unknown, but a low-grade counter-regulatory response might have been involved.
A third important finding was the identification of factors which were statistically significant predictors of remission in diabetic cats. These were (1) early institution of a protocol for rapid and effective control of hyperglycaemia, (2) cortisone treatment prior to the diagnosis of diabetes (3) lack of signs of neuropathy at diagnosis (plantigrade stance and other milder peripheral neuropathic signs such as difficulty jumping) and (4) a lower maximum insulin dose required for blood glucose regulation. Obesity at diagnosis was not negatively correlated with remission. However, the effect of weight loss in obese cats on the probability of remission needs further investigation. It could be hypothesised that cats which developed peripheral neuropathies had longer duration of hyperglycaemia before effective treatment was instituted compared to cats without neuropathy. Conversely, cats with corticosteroid treatment might have had shorter duration of hyperglycaemia, because acute precipitation of signs of diabetes after corticosteroid administration might lead owners to promptly seek veterinary treatment. Insulin resistance associated with corticosteroid administration is temporary, so once β-cells have recovered from glucose toxicity, providing there is sufficient β-cell mass remaining to maintain euglycaemia, remission is more likely. Seventy percent of cats which developed diabetes within 3 months of steroid administration had two or more depot injections.
A fourth finding of this study was that azotaemia was common (26%) in this cohort. Nephropathy has not yet been linked to prolonged periods of hyperglycaemia in diabetic cats. However, because of its importance as a devastating chronic complication in human diabetics, nephropathy was examined in the cohort. Because an age and breed matched control population was not available for comparison, no definitive statement can be made about the frequency of azotaemia in diabetic cats. In addition, because concurrent urine specific gravity measurements were often not available, pre-renal azotaemia cannot be excluded as a cause. However, persistent azotaemia was surprisingly frequent (18%) in 5- to 9-year-old diabetic cats. Further large epidemiological studies are required to investigate the association of renal disease and diabetes in cats.
Substantial evidence has accumulated for humans that there is a bidirectional relationship between periodontal disease and diabetes, and the presence of one condition tends to promote the other. 24 No correlation between insulin resistance and periodontal disease was found in this cohort of cats. However, as almost half the cats did not have a dental examination and the remainder had a high prevalence of dental disease, the study may have lacked sufficient power to find such a correlation.
The frequency of cats in remission returning to insulin dependency was lower than reported in a small study of cats treated with glargine, 7 where 3/8 cats later required insulin therapy. In contrast to the reported study, the cats in our study remained on an ultra-low carbohydrate diet after insulin therapy was terminated. High carbohydrate diets increase post-prandial glucose concentrations significantly 17 and are not recommended for cats that have become non-insulin dependent, because these cats likely have reduced β-cell mass. Reducing the glucose load using an ultra-low carbohydrate would be beneficial in helping to maintain euglycaemia for non-insulin-dependent cats.
In contrast to other studies with glargine 7 or intermediate-acting insulins25,26 which studied newly diagnosed diabetics, the vast majority of cats in this cohort were already treated, having failed to go into remission using, typically, lente insulin; the cats in this cohort were probably more difficult candidates for remission than newly diagnosed diabetics. Hence, these cats are particularly likely to benefit from being closely monitored at home (and, therefore, tightly regulated) rather than the less optimal dosage adjustments that could be achieved during hospital monitoring using glargine, 7 or alternatively home monitored with a lente insulin which is not suited for tight regulation in cats.25,26
Home monitoring provides a number of advantages including decreasing stress which can lead to spuriously high glucose concentrations if the cat struggles, or lower glucose concentrations if the cat does not eat in hospital.25,27 It provides increased information on which to make blood glucose adjustments compared to less frequent monitoring by the veterinarian; this facilitates early optimisation of blood glucose concentration and achieving remission. Blood glucose concentrations can be assessed immediately by the owner if abnormal behaviour is observed, which is helpful for identifying and managing clinical hypoglycaemia. Importantly, a protocol which aims for euglycaemia can only be used with home monitoring because blood glucose needs to be tested several times daily. Home monitoring also decreases the cost and inconvenience to the owner, and can be more profitable for the veterinarian than hospitalising patients for the day. Regular appointments, initially weekly and increasing to every 2–4 weeks, are recommended for review of the owner's log book of blood glucose concentrations, insulin dose and clinical signs, and for physical assessment of the cat, including body condition. Once the cat is stabilised and the owner becomes knowledgeable about insulin dose adjustments, appointments can become less frequent and the overall analysis of blood glucose concentration data can often be done via fax or e-mail.
In human studies into intensive blood glucose control, the typical adverse event measured is severe, symptomatic hypoglycaemic episodes, which occur not uncommonly.1,2 In contrast in our study, although biochemical hypoglycaemia was common, clinical hypoglycaemia was rare, with only a single mild event being recorded. In human patients, clinical hypoglycaemia is reported to be less common when using glargine compared to intermediate-acting insulin.28,29 This is thought be associated with glargine's consistent insulin action over 24 h compared to a sharp peak of insulin action which occurs with intermediate-acting insulin. Based on the very low frequency of clinical hypoglycaemia observed in this trial and another trial in cats, it appears that glargine use in cats might be similarly advantageous. 7 The frequency of biochemical hypoglycaemia reported in our study was likely over estimated because blood glucose concentrations were measured using whole blood glucose meters designed for human-use, which give readings that are 1–2 mmol/l (18–36 mg/dl) lower than measurements using automated serum chemistry analysers calibrated for feline plasma. 30
The protocol reported in this study advised owners of diabetic cats to eventually aim to maintain most blood glucose concentrations between 2.8 and 5.5 mmol/l (50 and 100 mg/dl). A blood glucose concentration of 2.8 mmol/l (50 mg/dl) is below the usual reference range measured in plasma for healthy cats. The recommendation only relates to blood glucose concentrations measured using human-use whole blood glucose meters, which are currently the most commonly available portable glucose meters. These meters report glucose concentration in whole blood and are calibrated for use in human beings. Newer meters calculate the plasma-equivalent value and report plasma glucose concentration. In human beings, plasma glucose concentration is on average 11% higher than that measured in whole blood.31,32 Veterinary-use glucose meters are now available in some countries (eg, Abbott AlphaTrak; Abbott Laboratories, IL, USA), and provide plasma-equivalent blood glucose measurements calibrated for feline blood. Values obtained using human-use whole blood glucose meters are up to 30–40% lower in the lower end of the glucose range (hypo- and normoglycaemia) than plasma-equivalent meters calibrated specifically for cats and measurements obtained from serum chemistry analysers. This is assumed to be because the distribution of glucose between red blood cells and plasma in cats is substantially different from human beings (7:93 for cats and 42:58 for human beings).33,34 Therefore, if this protocol is being used together with measurement of blood glucose concentration using a glucose meter internally calibrated for feline blood, or using a serum chemistry analyser, it is recommended that approximately 1.7 mmol/l (30 mg/dl) be added to target glucose concentrations used in this study that are in the lower range. For example, instead of aiming for a blood glucose concentration of 2.8–5.6 mmol/l (50–100 mg/dl), aim for 4.4–7.2 mmol/l (80–130 mg/dl) when using a meter calibrated for feline use or a serum chemistry analyser. Importantly, this protocol has not been tested and shown safe for diabetic cats only monitored once every 1–2 weeks at a veterinary practice. It has only been shown safe when combined with home monitoring of blood glucose concentration a minimum of three times daily.
In summary, we have shown for the first time that owners of diabetic cats are able to use a protocol aimed at achieving euglycaemia, using home monitoring, glargine and an ultra-low carbohydrate diet, and that it is a safe and effective method of insulin therapy. Importantly, this method allows for achieving high rates of non-insulin dependence in cats that have already failed to achieve remission with traditional approaches, provided it is instituted within 6 months of diagnosis.
Acknowledgements
We would like to thank Sandra Palm for her help with the figures of the blood glucose curves.
References
- 1.DCCT Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group, N Engl J Med 329, 1993, 977–986. [DOI] [PubMed] [Google Scholar]
- 2.UKPDS Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group, Lancet 352, 1998, 837–853. [PubMed] [Google Scholar]
- 3.Garfield S.A., Malozowski S., Chin M.H., et al. Considerations for diabetes translational research in real-world settings, Diabetes Care 26, 2003, 2670–2674. [DOI] [PubMed] [Google Scholar]
- 4.Martin G., Rand J. Current understanding of feline diabetes: part 2, treatment, J Feline Med Surg 2, 2000, 3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin G.J., Rand J.S. Control of diabetes mellitus in cats with porcine insulin zinc suspension, Vet Rec 161, 2007, 88–94. [DOI] [PubMed] [Google Scholar]
- 6.Marshall R.D., Rand J.S., Morton J.M. Glargine and protamine zinc insulin have a longer duration of action and result in lower mean daily glucose concentrations than lente insulin in healthy cats, J Vet Pharmacol Ther 31, 2008, 205–212. [DOI] [PubMed] [Google Scholar]
- 7.Marshall R.D., Rand J. Treatment with glargine results in higher remission rates than lente or protamine zinc insulins in newly diagnosed diabetic cats, 2005, ACVIM: Baltimore, Maryland, USA, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin G.J., Rand J.S. Pharmacology of a 40IU/ml porcine lente insulin preparation in diabetic cats: findings during the first week and after 5 or 9 weeks of therapy, J Feline Med Surg 3, 2001, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meneghini L., Koenen C., Weng W., et al. The usage of a simplified self-titration dosing guideline (303 algorithm) for insulin detemir in patients with type 2 diabetes – results of the randomized, controlled predictive 303 study, Diabetes Obes Metab 9, 2007, 902–913. [DOI] [PubMed] [Google Scholar]
- 10.Davies M., Lavalle-Gonzalez F., Storms F., et al. Initiation of insulin glargine therapy in type 2 diabetes subjects suboptimally controlled on oral antidiabetic agents: results from the AT.LANTUS trial, Diabetes Obes Metab 10, 2008, 387–399. [DOI] [PubMed] [Google Scholar]
- 11.Selam J.L., Koenen C., Weng W., et al. Improving glycemic control with insulin detemir using the 303 algorithm in insulin naive patients with type 2 diabetes: a subgroup analysis of the US Predictive 303 study, Curr Med Res Opin 24, 2008, 11–20. [DOI] [PubMed] [Google Scholar]
- 12.Rand J.S., Marshall R.D. Diabetes mellitus in cats, Vet Clin North Am Small Anim Pract 35, 2005, 211–224. [DOI] [PubMed] [Google Scholar]
- 13.Bennett N., Greco D.S., Peterson M.E., et al. Comparison of a low carbohydrate-low fiber diet and a moderate carbohydrate-high fiber diet in the management of feline diabetes mellitus, J Feline Med Surg 8, 2006, 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoenig M., Thomaseth K., Waldron M., et al. Insulin sensitivity, fat distribution, and adipocytokine response to different diets in lean and obese cats before and after weight loss, Am J Physiol Regul Integr Comp Physiol 292, 2007, R227–R234. [DOI] [PubMed] [Google Scholar]
- 15.Rand J.S., Fleeman L.M., Farrow H.A., et al. Canine and feline diabetes mellitus: nature or nurture?, J Nutr 134, 2004, 2072S–2080S. [DOI] [PubMed] [Google Scholar]
- 16.Zoran D.L. The carnivore connection to nutrition in cats, J Am Vet Med Assoc 221, 2002, 1559–1567. [DOI] [PubMed] [Google Scholar]
- 17.Rand J., Farrow H., Fleeman L., et al. Diet in the prevention of diabetes and obesity in companion animals, Asia Pac J Clin Nutr 12 (suppl), 2003, S6. [Google Scholar]
- 18.Pierson L. Making cat food. Available from: <http://www.catinfo.org/makingcatfood.htm>2007.
- 19.Ihaka R., Gentleman R. R: a language for data analysis and graphics, J Comput Graph Stat 5, 1996, 299–314. [Google Scholar]
- 20.Shapiro S.S., Wilk M.B. An analysis of variance test for normality (complete samples), Biometrika, 1965, 591–611.
- 21.Dohan F.C., Lukens F.D. Experimental diabetes produced by the administration of glucose, Endocrinology 42, 1948, 244–262. [DOI] [PubMed] [Google Scholar]
- 22.Kaneto H., Katakami N., Kawamori D., et al. Involvement of oxidative stress in the pathogenesis of diabetes, Antioxid Redox Signal 9, 2007, 355–366. [DOI] [PubMed] [Google Scholar]
- 23.Zini E., Osto M., Franchini M., et al. Hyperglycaemia but not hyperlipidaemia causes beta cell dysfunction and beta cell loss in the domestic cat, Diabetologia 52, 2009, 336–346. [DOI] [PubMed] [Google Scholar]
- 24.Mealey B.L., Oates T.W. Diabetes mellitus and periodontal diseases, J Periodontol 77, 2006, 1289–1303. [DOI] [PubMed] [Google Scholar]
- 25.Casella M., Hassig M., Reusch C.E. Home-monitoring of blood glucose in cats with diabetes mellitus: evaluation over a 4-month period, J Feline Med Surg 7, 2005, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reusch C.E., Kley S., Casella M. Home monitoring of the diabetic cat, J Feline Med Surg 8, 2006, 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rand J.S., Kinnaird E., Baglioni A., et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine, J Vet Intern Med 16, 2002, 123–132. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca V., Bell D.S., Berger S., et al. A comparison of bedtime insulin glargine with bedtime neutral protamine hagedorn insulin in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study, Am J Med Sci 328, 2004, 274–280. [DOI] [PubMed] [Google Scholar]
- 29.Fulcher G.R., Gilbert R.E., Yue D.K. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy, Intern Med J 35, 2005, 536–542. [DOI] [PubMed] [Google Scholar]
- 30.Cohen T., Nelson R., Kass P., et al. Evaluation of six portable blood glucose meters in dogs, 2008, ACVIM: San Antonio, Texas, USA, p 43. [Google Scholar]
- 31.D'Orazio P., Burnett R.W., Fogh-Andersen N., et al. Approved IFCC recommendation on reporting results for blood glucose (abbreviated), Clin Chem 51, 2005, 1573–1576. [DOI] [PubMed] [Google Scholar]
- 32.Steffes M.W., Sacks D.B. Measurement of circulating glucose concentrations: the time is now for consistency among methods and types of samples, Clin Chem 51, 2005, 1569–1570. [DOI] [PubMed] [Google Scholar]
- 33.Coldman M.F., Good W. The distribution of sodium, potassium and glucose in the blood of some mammals, Comp Biochem Physiol 21, 1967, 201–206. [DOI] [PubMed] [Google Scholar]
- 34.Mackay E. The distribution of glucose in human blood, J Biol Chem, 1932, 685–688.