Abstract
Currently, only one selectable marker is available for genetic studies in the archaeal genus Methanosarcina. Here we report the generation of selectable markers that encode resistance to pseudomonic acid (PAr) in Methanosarcina species by mutagenesis of the isoleucyl-tRNA synthetase gene (ileS) from Methanosarcina barkeri Fusaro. The M. barkeri ileS gene was obtained by screening of a genomic library for hybridization to a PCR fragment. The complete 3,787-bp DNA sequence surrounding and including the ileS gene was determined. As expected, M. barkeri IleS is phylogenetically related to other archaeal IleS proteins. The ileS gene was cloned into a Methanosarcina-Escherichia coli shuttle vector and mutagenized with hydroxylamine. Nine independent PAr clones were isolated after transformation of Methanosarcina acetivorans C2A with the mutagenized plasmids. Seven of these clones carry multiple changes from the wild-type sequence. Most mutations that confer PAr were shown to alter amino acid residues near the KMSKS consensus sequence of class I aminoacyl-tRNA synthetases. One particular mutation (G594E) was present in all but one of the PAr clones. The MIC of pseudomonic acid for M. acetivorans transformed with a plasmid carrying this single mutation is 70 μg/ml of medium (for the wild type, the MIC is 12 μg/ml). The highest MICs (560 μg/ml) were observed with two triple mutants, A440V/A482T/G594E and A440V/G593D/G594E. Plasmid shuttle vectors and insertion cassettes that encode PAr based on the mutant ileS alleles are described. Finally, the implications of the specific mutations we isolated with respect to binding of pseudomonic acid by IleS are discussed.
In recent years, there has been significant progress in developing methods of genetic analysis applicable to methanoarchaea. Among the most important of these are the development of simple and reliable methods for growth and isolation of clonal populations (23, 42), the development of a functional selectable marker for puromycin resistance (16), the development of plasmid shuttle vectors (29, 45), and the development of highly efficient transformation protocols for Methanosarcina and Methanococcus species (29, 45).
Although the ability to select puromycin-resistant transformants has revolutionized genetic analysis among the methanoarchaea, few other selectable markers have been subsequently developed. This dearth of usable selectable markers is unfortunate because many genetic studies require multiple selectable markers, for example, the introduction of plasmids into strains that already carry a chromosomal insertion of one selectable marker. Selection for histidine prototrophy or neomycin resistance is possible in certain Methanococcus species (2, 35); however, neither of these have been shown to be useful in the Methanosarcina species under study in our laboratory. We have demonstrated that neither neomycin nor the related antibiotic G-418 is effective against Methanosarcina spp., even at a concentration of 1 mg/ml (P. Boccazzi and W. W. Metcalf, unpublished data). Indeed, few antibiotics that are effective against bacteria are effective against archaea (6). To complicate matters further, selectable markers that might confer resistance to the few effective antibiotics are lacking. This problem is exemplified by the antibiotic chloramphenicol, which is highly effective against many methanoarchaea. In bacteria, resistance to chloramphenicol can be achieved by acetylation of the antibiotic. Unfortunately, acetylchloramphenicol is an equally effective antibiotic among the methanoarchaea. Thus, the cat gene, encoding chloramphenicol acetyltransferase, is not expected to confer resistance on methanoarchaea (4). We have verified this in vivo by cloning the cat gene under the transcriptional control of a strong Methanosarcina promoter into a plasmid shuttle vector. Methanosarcina transformants carrying this plasmid were shown to express active Cat protein but were not resistant to either chloramphenicol or fusaric acid (M. A. Pritchett and W. W. Metcalf, unpublished data).
An attractive candidate for development of a usable selectable marker is the antibiotic pseudomonic acid A. Pseudomonic acid is an antibiotic produced by certain strains of Pseudomonas fluorescens (15). The antibiotic is an analog of the amino acid isoleucine that inhibits protein synthesis by blocking isoleucine charging to its cognate tRNA by isoleucyl-tRNA synthetase (19, 20, 47). Pseudomonic acid is an effective antibiotic against many methanoarchaea (21, 36). Importantly, mutants of Methanobacterium thermoautotrophicum that are resistant to pseudomonic acid (PAr) have been isolated (21). The mutations found in these resistant strains were shown to map to the ileS gene of this organism, which encodes the target of the antibiotic, isoleucyl-tRNA synthetase. These mutations could be transferred by phage transduction, indicating that, at least in this species, mutant ileS alleles can be used as selectable markers (26). However, it was unclear from these data whether the mutant ileS alleles would confer resistance on strains that also carry a wild-type allele of ileS, such as might occur if the genes were used as selectable markers on a plasmid construct. In Staphylococcus aureus, PAr is conferred by a plasmid-encoded IleS protein (13). The fact that this plasmid can be transferred to sensitive strains (that presumably carry an antibiotic-sensitive ileS gene), with concomitant transfer of PAr, suggests that resistant ileS alleles are dominant over sensitive alleles (33). Therefore, we reasoned that mutagenesis of a native ileS gene from Methanosarcina spp. might be used to develop an alternative selective marker for members of this genus. In this paper, we report the cloning and sequencing of the ileS gene from Methanosarcina barkeri Fusaro and its modification to allow its use as a selectable marker for PAr in Methanosarcina acetivorans C2A.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Standard conditions were used for growth of Escherichia coli strains (46). Strains DH5α and DH5α/λpir (32) were from S. Maloy (University of Illinois). Strain DH5α was used as the host for plasmid constructions using pTZ18R (Pharmacia, Piscataway, N.J.) or pBluescript KS(+) (Stratagene, La Jolla, Calif.) as a cloning vector. Strain DH5α/λpir or WM95 (28) was used as the host for construction of pir-dependent replicons. M. acetivorans C2A (= DSM 2834) and M. barkeri Fusaro (= DSM 804) were from laboratory stocks. Methanosarcina strains were grown in single-cell morphology (42) at 35°C in HS-methanol-acetate (HS-MA) broth medium under strictly anaerobic conditions (30). Growth of Methanosarcina strains on solid medium was done essentially as described in reference 42, with the exception that M. acetivorans could be plated without the use of top agar by spreading or streaking directly on the surface of agar-solidified medium (M. barkeri Fusaro was plated in top agar as described in reference 42). All plating manipulations were carried out under strictly anaerobic conditions in an anaerobic glove box. Plates with inoculated solid medium were incubated in an intrachamber anaerobic incubator as described in reference 31. Puromycin was used at 1 or 2 μg/ml for Methanosarcina species. Pseudomonic acid A, a gift from SmithKline Beecham Pharmaceuticals (Philadelphia, Pa.), was routinely used at 35 μg/ml, except as noted otherwise. Puromycin and pseudomonic acid were added to medium from sterile, anaerobic stock solutions. Stock solutions were sterilized through NALGENE 0.2-μm-pore-size filters (Nalge Company, Rochester, N.Y.) into sterile, sealed serum bottles and then made anaerobic by applying vacuum for three cycles of 15 min each. Inquiries regarding the availability of pseudomonic acid should be forwarded to P. G. Treagust, manager, Reference Materials Group, SmithKline Beecham Pharmaceuticals.
DNA methods.
Standard methods were used throughout for isolation and manipulation of DNA (3). Plasmid DNA was isolated from Methanosarcina species as previously described (29). Alternatively, if the plasmids present in Methanosarcina strains were to be used solely for transfer to E. coli, the cells were lysed by resuspension in sterile H2O and the plasmid DNA was concentrated by precipitation and then used directly for transformation. Genomic DNA of high molecular weight was isolated from Methanosarcina species as follows. Cells from a stationary-phase culture grown in 200 ml of HS-MA medium were collected by centrifugation, resuspended in 10 ml of 50 mM Tris-HCl (pH 8.0) with 0.85 M sucrose, and lysed by addition of sodium dodecyl sulfate to a final concentration of 0.5%. The lysate was treated with proteinase K (150 μg/ml) at 37°C for 3 h, extracted three times with buffered phenol-CHCl3-isoamyl alcohol (25:24:1), and then extracted once with CHCl3-isoamyl alcohol (24:1). Finally, the DNA was precipitated from the treated, extracted lysate with NaCl and isopropanol; excess salt was removed by rinsing of the precipitate sequentially with 70% ethanol (three times) and 100% ethanol. The precipitated DNA was then dried and resuspended in 10 mM Tris-HCl (pH 8.0) with 1 mM EDTA. DNA hybridizations were performed as previously described (30). Colony hybridizations were performed as described in reference 3. Probes used for hybridization experiments were labeled with [α-32P]dATP using the Prime-a-Gene kit (Promega, Madison, Wis.) according to specifications. DNA sequences were determined from double-stranded templates by automated dye terminator sequencing. DNA sequencing was performed at the W. M. Keck Center for Comparative and Functional Genomics, University of Illinois.
Transformations.
E. coli was transformed by electroporation using an E. coli Gene Pulser (Bio-Rad, Richmond, Calif.) as recommended. Methanosarcina species were transformed by liposome-mediated transformation essentially as described in reference 29, except that the transformations were carried out in an anaerobic chamber with an atmosphere of N2-CO2-H2 (75:20:5) and that anaerobic HEPES buffer was replaced by sterile anaerobic sucrose (0.85 M) buffered with NaHCO3 (80 mM). The pH of the buffered sucrose solution was 7.4 under a 20% CO2 atmosphere.
Construction of the Methanosarcina-E. coli shuttle cosmids pWM348 and pWM349.
The shuttle cosmids pWM348 and pWM349 were constructed by cloning the dual cos sites and polylinker from Supercos (Stratagene) into the Methanosarcina-E. coli shuttle vector pWM307 (29). The cos sites and polylinker were PCR amplified from Supercos using Taq DNA polymerase and the primers 5′-GCCGGCGCGCCTATAAAAATAGGCGTATCACGAGG-3′ and 5′-GCCGGCGCGCCTTGAAGGCTCTCAAGGGCATCGGTCG-3′ (added AscI sites are in boldface). The PCR product was digested with AscI and cloned into the unique AscI site of pWM307 to generate pWM348 and pWM349. The two plasmids differ only with respect to the orientation of the insert.
Cloning of an internal ileS gene fragment from M. barkeri Fusaro and M. acetivorans C2A.
Internal ileS gene fragments were obtained from M. barkeri Fusaro and M. acetivorans C2A by PCR amplification from genomic DNA essentially as described in reference 7. The primers used for amplification were modified slightly to more closely match the archaeal consensus sequences and were 5′-GGNTGGGAYACNCAYGGNYTNCCNATHGA-3′ and 5′-GGNGTYTTRCANCKCCARCA-3′. Amplifications were performed using Taq DNA polymerase. The ca. 930-bp PCR products were made blunt by treatment with T4 DNA polymerase and deoxynucleoside triphosphates, phosphorylated by treatment with T4 polynucleotide kinase, and cloned into the SmaI site of pTZ18R to generate pWM351 and pWM352, which carry internal ileS fragments from M. barkeri Fusaro and M. acetivorans C2A, respectively. DNA sequence analysis of pWM351 and pWM352 strongly suggests that the two plasmids each carry fragments of the ileS gene of the respective Methanosarcina species.
Cloning and DNA sequence analysis of the complete ileS gene of M. barkeri Fusaro.
A cosmid-based genomic library of M. barkeri Fusaro was constructed by ligation of Sau3AI-digested genomic DNA with NheI- and BamHI-digested pWM348. After in vitro packaging, the library was transfected into DH5α/λpir and ca. 4,000 independent Ampr clones were saved for subsequent screening. A strain carrying the cosmid pJK63, which carries the intact ileS gene, was isolated from this library as a clone that hybridized to pWM351. Plasmid pJK64 was constructed by ligation of a 3,560-bp NotI-to-EcoRV fragment from pJK63 into NotI- and EcoRV-digested pBluescript KS(+). Plasmid pJK44 was constructed by ligation of a 3,812-bp NotI-to-SphI DNA fragment containing the M. barkeri Fusaro ileS gene from pJK63 into the same sites in the Methanosarcina-E. coli shuttle vector pWM321 (29). The majority of the complete DNA sequence of the M. barkeri Fusaro ileS gene was determined from pJK63 and pJK64 templates; the remainder was determined from pJK44. Standard primers were used to generate junction sequences from these plasmids. Internal sequences were obtained with primers designed based on the junction and subsequent sequences.
Phylogenetic analysis of IleS sequences was performed as follows. Sequences were automatically aligned using the CLUSTAL W program (version 1.74) (43). Segments that were deemed confidently aligned were manually masked and then extracted using the AE2 alignment editor (T. Macke, University of Illinois at Urbana-Champaign). PAUP* (version 4 beta 2, D. Swofford, published by Sinauer Associates, Inc.) was used to generate the 500 most parsimonious trees from the masked amino acid alignment. These trees were evaluated using maximum-likelihood criteria with the ProtML program (MOLPHY software package [reference 1]). A consensus of the trees, standardized and exponentially weighted using the protein maximum-likelihood scores and the Kishino-Hasegawa test for significance, was generated using the TreeCons program (version 1.0; L. S. Jermiin and O. Anpilogova) and the CONSENSE program (J. Felsenstein; PHYLIP, phylogeny inference package, version 3.5c; Department of Genetics, University of Washington, Seattle, 1993). The resultant consensus tree is topologically equivalent to the best tree evaluated by maximum-likelihood analysis (22). Branch lengths were calculated by supplying the consensus tree to the ProtML program as a user tree.
Mutagenesis of ileS.
Mutant forms of ileS that confer PAr were generated by in vitro mutagenesis of pJK44 with hydroxylamine (NH2OH · HCl) using a protocol adapted from reference 25. The reaction mixture contained 0.8 M NH2OH · HCl, 100 mM KHPO4, 1 mM EDTA, and 10 mM MgSO4. Mutagenesis reactions were carried out in a 100-μl total volume containing ca. 2.0 μg of plasmid DNA. Independent reactions were incubated for 0, 6, 12, 18, 24, 36, or 42 h at 37°C. Subsequently, each sample was dialyzed for 6 h at 4°C by incubation of the reaction mixture on the surface of a 0.025-μm-pore-size filter (type VS; Millipore, Bedford, Mass.) floating on 20 ml of 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffer. Each sample was then precipitated and used in its entirety to transform E. coli DH5α/λpir to Ampr. All of the colonies from each transformation were pooled independently and used for large-scale plasmid isolations to obtain the quantities of DNA needed for Methanosarcina transformation. The mutagenized pJK44 pools were then used to transform M. acetivorans with selection for PAr. A total of 12 mutagenized pools were screened by this method. From each of these pools, the first three colonies that appeared were purified by single-colony isolation on HS-MA agar with pseudomonic acid (35 μg/ml) and retained for subsequent analysis.
Subcloning and DNA sequence analysis.
The 3,812-bp SphI-NotI fragments containing the ileS gene from nine independent PAr-encoding plasmids were subcloned into the same sites in pWM321 (29), generating pPB3, pPB4, pPB5, pPB6, pPB7, pPB22, pPB23, pPB24, and pPB29. This ensured that the PAr phenotype associated with each mutant was the result of changes within the ileS region and not due to mutations within the vector backbone. The complete ileS DNA sequence present in each of these plasmids was then determined as described before.
Several additional plasmids were constructed that carried various combinations of the multiple mutations found in certain ileS mutants. Plasmid pPB8 was obtained by replacing the NotI-AlwNI DNA fragment of pPB4 with the same DNA fragment from pPB5. Plasmids pPB12 and pPB13 were obtained by replacing the Eco47III DNA fragment of pPB7 and pJK44, respectively, with the Eco47III DNA fragment of pPB5. Finally, the integration vector pPB18 was constructed by ligating the 3,812-bp SphI-NotI DNA fragment of pPB12 into the same sites in pJK41 (27).
Phenotypic characterization.
The MIC of pseudomonic acid and the generation times of M. acetivorans C2A strains carrying each mutant plasmid were determined by measuring the extent of growth in various media in terms of the optical density at 660 nm (OD660). For the MIC experiment, duplicate sets of HS-MA medium containing pseudomonic acid at concentrations of 0, 35, 70, 140, 280, 560, and 1,120 μg/ml were inoculated with 100 μl (1%) of a late-log-phase (OD660, ca. 0.5) culture grown in HS-MA broth plus puromycin. At this stage of growth, the inoculum was ca. 3 × 107 cells, which is well below the level expected to give rise to spontaneous PAr mutants (spontaneous PAr mutations occur in M. acetivorans with a frequency of 5.5 ± 1.8 × 10−9 [Boccazzi and Metcalf, unpublished]). A clone was considered PAr if the measured OD was greater than 0.05 after 15 days of incubation at 37°C. Our data indicate that cultures which reached this OD ultimately grew to levels similar to that obtained by wild-type cells grown in the absence of antibiotic, although this often required substantially longer incubations. In all cases, the results obtained from replicate cultures were identical. Growth rates were determined in HS-MA medium containing pseudomonic acid (35 μg/ml) at 37°C. The inoculum was 100 μl (1%) of a late-log-phase culture grown in HS-MA plus puromycin (2 μg/ml). Generation times were calculated during the exponential growth phase (OD660 between 0.05 and 0.45). The reported data are averages of six trials.
Construction of PAr-encoding Methanosarcina-E. coli shuttle vectors.
A variety of Methanosarcina-E. coli shuttle vectors encoding PAr were constructed in four steps as follows. First, pPB17 was constructed by self-ligation of a 1,482-bp EcoRI-digested PCR fragment amplified from pWM303 (29) using Taq DNA polymerase and the primers 5′-GAATTCGCGGCCGCGCTCTAGAGGCGCGCCAGGTGG-3′ and 5′-GAATTCGCATGCAATTCTGTCAGCCGTTAAGTG-3′ (added EcoRI, SphI, and NotI restriction sites are in boldface). Plasmid pPB17 carries the origin of replication from plasmid R6K and the bla gene for replication and selection for ampicillin resistance in E. coli, respectively. Second, to allow replication of the constructs in Methanosarcina, the pC2A plasmid replicon was isolated as a 5,467-bp SpeI fragment from pWM241 (29) and cloned into the compatible XbaI site of pPB17 to generate pPB20. Third, to allow selection for PAr in Methanosarcina, the 3,812-bp SphI-NotI DNA fragment from pPB12 was cloned into the same sites of pPB20 to generate pPB26. Finally, to allow blue-white screening of recombinant clones, PCR fragments carrying the lacZα gene and polylinker region amplified from pBluescript KS(+), pBluescript SK(+), and pMTL22 (12), respectively, using the primers 5′-GCCGGCGCGCCTTAACCATTCGCCATTCAGGCTGC-3′ and 5′-GCCGGCGCGCCAATACGCAAACCGCCTCTCC-3′ (added AscI restriction sites are in boldface) were cut with AscI and cloned into AscI-digested pPB26 to generate pPB32, pPB33, and pPB35, respectively. Plasmids pPB31 and pPB34 are similar to plasmids pPB32 and pPB33, respectively, but carry the PCR fragment in the opposite orientation.
Nucleotide sequence accession number.
The GenBank accession number for the M. barkeri Fusaro ileS gene and the surrounding sequence is AF208389.
RESULTS
Cloning and sequence analysis of the ileS gene from M. barkeri Fusaro.
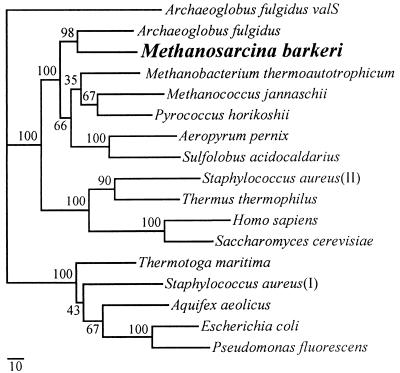
A cosmid clone, pJK63, carrying the ileS gene of M. barkeri Fusaro was identified by hybridization to an internal ileS gene fragment obtained by PCR as described in Materials and Methods. A 3,787-bp DNA sequence including the putative ileS gene, as well as 437 bp upstream and 174 bp downstream, was subsequently determined. The putative ileS gene is predicted to encode a protein of 1,058 amino acids. This putative IleS protein is highly homologous to the known IleS proteins from other organisms, including archaea, eucarya, and bacteria (29.7 to 52.2% identical to the 25 closest homologs in the Swiss-Prot database). It also has several conserved IleS motifs, including ones that correspond to the HIGH and KMSKS “signature” sequences of class I aminoacyl-tRNA synthetases (10). The consensus sequence KMSKS is perfectly conserved in archaea, eucarya, and bacteria and is located at positions 596 to 600 of the M. barkeri Fusaro IleS. In the case of the HIGH motif, the observed sequence HLGT, located at positions 55 to 58, is the same as that found in the IleS sequences of the Archaea Archaeoglobus fulgidus (24), M. thermoautotrophicum (41), and Methanococcus jannaschii (8). Consistent with this observation, phylogenetic analysis of the M. barkeri IleS protein indicates that it is specifically related to the other archaeal IleS proteins (Fig. 1).
FIG. 1.
Phylogeny of the M. barkeri IleS protein. The standardized, exponentially weighted consensus tree of the 500 most parsimonious trees, evaluated by maximum-likelihood analysis, is shown. A percentage at a node represents the relative-likelihood support for that branch, as calculated by the TreeCons program. The tree was rooted using the valS gene of A. fulgidus. The scale bar represents the estimated number of substitutions per 100 amino acid positions along each branch. The Swiss Protein database accession numbers of the sequences used are, from top to bottom, O28059 (A. fulgidus valS), O29622 (A. fulgidus), P26499 (M. thermoautotrophicum), Q58357 (M. jannaschii). P46215 (S. acidocaldarius), P41368 (S. aureus II, a plasmid-encoded copy that confers PAr). P56690 (T. thermophilus), P41252 (H. sapiens), P09436 (S. cerevisiae), P46213 (T. maritima), P41972 (S. aureus I, genomic copy), P46207 (A. aeolicus), P00956 (E. coli), and P18330 (P. fluorescens). The A. pernix and P. horikoshii sequences were from the respective genome databases.
Two other sequence features are worthy of note. First, four copies of a 28-bp direct repeat, TTTTTTTAAAAGGGTTGCGGTCAAGCAG, are found about 300 bp upstream of the putative ileS gene. Interestingly, four copies of the same direct repeat are found at a similar location upstream of the hdr operon in this organism (17). The function of these repeat sequences is unclear; however, direct repeat sequences appear to be quite common in Methanosarcina. Second, a portion of an open reading frame was identified beginning 72 bp downstream of the ileS translation stop. This putative gene encodes a highly conserved archaeal protein of unknown function (the accession numbers of homologs from M. jannaschii, M. thermoautotrophicum, Pyrococcus abyssi, A. fulgidus, and Pyrococcus horikoshii are MJ1634, MTH1600, PAB1706, AF0878, and PH1244, respectively).
ileS gene mutagenesis.
We mutagenized the M. barkeri Fusaro ileS gene in the hope of creating alleles that are insensitive to the isoleucyl-tRNA synthetase inhibitor pseudomonic acid. To do this, the ileS gene was cloned into a Methanosarcina-E. coli shuttle vector and treated in vitro with hydroxylamine. Pools of mutagenized plasmids were then used to transform the related species M. acetivorans C2A with selection for PAr (initially selected by using pseudomonic acid at 35 μg/ml). Preliminary experiments indicated that the MIC of pseudomonic acid for this species is approximately 12 μg/ml (W. W. Metcalf, unpublished data). Strains transformed with the ileS shuttle vector pJK44 (Fig. 2; Table 1) exhibit similar sensitivity to the antibiotic. Further, spontaneous resistance occurs at a relatively low frequency of 5.5 × 10−9 ± 1.8 × 10−9. However, when M. acetivorans was transformed with the mutagenized plasmid pools, numerous PAr colonies were obtained. Because we were interested in obtaining mutants that conferred the highest levels of antibiotic resistance, we routinely checked these plates and picked the first colonies to arise from each transformation for subsequent analysis.
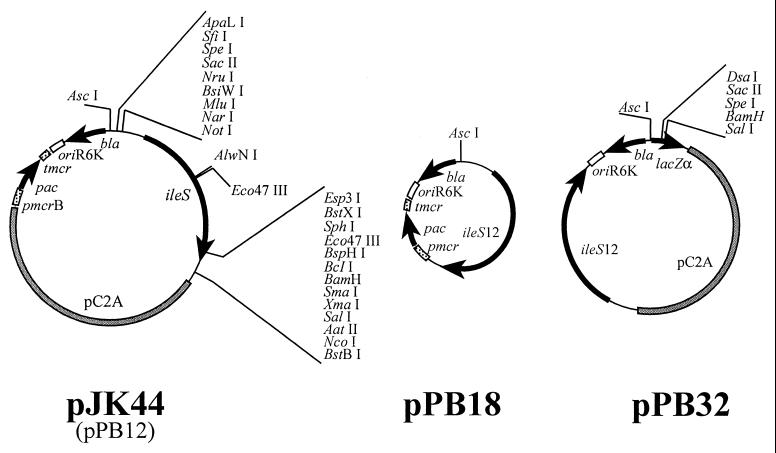
FIG. 2.
Plasmids used in this study. Plasmid pJK44 carries the M. barkeri strain Fusaro ileS gene in Methanosarcina-E. coli shuttle vector pWM321 (29). The origin of replication from plasmid R6K (oriR6K) allows plasmid replication in E. coli, and the pC2A replicon allows replication in the genus Methanosarcina. The pac cassette confers puromycin resistance upon methanoarchaea, and the β-lactamase gene bla encodes resistance to ampicillin on E. coli. Plasmid pPB12 is identical to pJK44, except that it carries the ileS12 allele in the place of wild-type ileS. Plasmid pPB18 differs from pPB12 by lacking the pC2A replicon and therefore is incapable of replication in Methanosarcina. Plasmid pPB32 is a Methanosarcina-E. coli shuttle vector that carries ileS12, lacZα for blue-white screening of recombinant clones, and a multiple cloning site with numerous unique sites. Plasmids pPB31, pPB33, pPB34, and pPB35 are similar to pPB32 and are described in the text. The promoter (pmcrB) and terminator (tmcr) of the Methanococcus voltae methyl reductase operon regulate expression of the puromycin acetyltransferase (pac) gene from Streptomyces alboniger in methanoarchaea.
TABLE 1.
Phenotypic characterization of M. acetivorans C2A transformed with various ileS-carrying plasmids
| Plasmida | Presence of mutation:
|
MICb (μg/ml) | Growth ratec (h) | ||||
|---|---|---|---|---|---|---|---|
| A440V | A482T | G587D | G593D | G594E | |||
| pJK44 | − | − | − | − | − | <35 | NG |
| pPB13 | + | − | − | − | − | 35 | 33.3 (1.1) |
| pPB3 | − | − | − | − | + | 70 | 37.1 (8.0) |
| pPB24 | − | − | − | − | + | 70 | 28.0 (0.8) |
| pPB29 | − | − | − | − | + | 70 | 28.3 (0.3) |
| pPB22 | − | − | + | − | − | 70 | 26.6 (1.0) |
| pPB4 | − | + | − | − | + | 70 | 32.9 (6.5) |
| pPB6 | − | − | − | + | + | 140 | 17.8 (0.9) |
| pPB7 | − | − | − | + | + | 140 | 15.8 (0.8) |
| pPB5 | + | − | − | − | + | 280 | 16.5 (1.1) |
| pPB8 | + | + | − | − | + | 560 | 15.1 (0.9) |
| pPB12 | + | − | − | + | + | 560 | 14.9 (1.8) |
| pPB23 | + | − | − | + | + | 560 | 20.5 (0.6) |
Plasmid pJK44 carries the wild-type ileS gene from M. barkeri Fusaro (Fig. 2). Other plasmids are identical to pJK44, except that each carries the mutations indicated.
MICs were determined as described in Materials and Methods in HS-MA medium plus pseudomonic acid added at concentrations of 0, 35, 70, 140, 280, 560, and 1,120 μg/ml. The MIC for M. acetivorans C2A was determined to be ca. 12 μg/ml in a different experiment (data not shown).
Generation times were determined as described in Materials and Methods in HS-MA medium plus pseudomonic acid (35 μg/ml). The values shown represent six independent determinations. Standard deviations are in parentheses. NG indicates that growth was not observed. Wild-type M. acetivorans C2A grew with a generation time of ca. 6 h in HS-MA medium but did not grow when PA was added to the medium.
Two steps were taken to demonstrate that the mutations conferring PAr in these clones were within the plasmid-encoded ileS gene. First, to ensure that the mutations did not map to the plasmid backbone (such as mutations that might alter the plasmid copy number), the mutagenized ileS fragments were subcloned into new shuttle vectors. Second, to ensure that the mutations were not spontaneous mutations in the genomic copy of ileS, each new subclone was retransformed into M. acetivorans with selection for the vector-encoded puromycin resistance and subsequently screened for PAr. Nine independent PAr clones passed this screening test and were characterized in detail. To ensure that all of the mutants analyzed were of independent origin, only a single clone from each mutagenized plasmid pool was examined. The nine plasmids isolated from these clones were designated pPB3, pPB4, pPB5, pPB6, pPB7, pPB22, pPB23, pPB24, and pPB29. The ileS alleles present on each plasmid correspond to the plasmid number, i.e., ileS3, ileS4, ileS5, ileS6, ileS7, ileS22, ileS23, ileS24, and ileS29, respectively.
Characterization of ileS mutants that confer PAr.
The mutational changes that confer PAr were determined by DNA sequence analysis of the entire ileS gene fragment from each mutant plasmid (Table 1). Surprisingly, six of the nine mutant plasmids carried multiple mutations in ileS. Most of the mutations observed were obtained multiple independent times, either alone or in combination with other mutations. Thus, eight of the nine plasmids carry the same G594E mutation, three of the nine carry the G593D mutation, and two of nine carry the A440V mutation. Mutations A482T and G587D were obtained only once. Mutation G594E was obtained as a single mutant three independent times (pPB3, pPB24, and pPB29), while the double mutant G593D/G594E was obtained independently twice (pPB6 and pPB7).
In the plasmids where multiple mutations were observed, it is possible that only one of the changes is responsible for the mutant phenotype. However, the fact that the same few mutations (and in some cases double mutations) were obtained multiple times suggests that most of these mutations contribute individually to the antibiotic resistance phenotype. To address this issue, the individual mutations found in the multiple mutants were separated or combined by subcloning in each case where available restriction endonuclease sites made this possible. Accordingly, plasmid pPB13 (ileS13) carries the single mutation A440V, originally found as one of two mutations in plasmid pPB5. Plasmid pPB8 (ileS8) carries this mutation in addition to the two mutations found in pPB4, while pPB12 (ileS12) combines the two mutations found in pPB6 with the A440V mutation found in pPB5. Interestingly, during the course of these subcloning experiments an ileS23 triple mutant which is identical to ileS12 was isolated directly from a mutagenized plasmid pool.
The MIC of pseudomonic acid and the growth rate in medium containing a low, but inhibitory, concentration of pseudomonic acid (35 μg/ml) were determined for M. acetivorans transformed with each of the mutant plasmids (Table 1). In general, the effects of the mutations with respect to these phenotypes were additive. Thus, the plasmids pPB8, pPB12, and pPB23, each containing three separate mutations, conferred PAr at levels at least twice that conferred by plasmids pPB4, pPB5, pPB6, and pPB7, containing two separate mutations each, and at least four times as high as that conferred by the plasmids pPB3, pPB13, pPB22, pPB24, and pPB29, containing only a single mutation each. One exception is that the A482T mutation does not significantly enhance the level of resistance conferred by the G594E mutation (compare pPB3 with pPB4) but doubles the level of resistance in combination with the mutations A440V and G594E (compare pPB5 with pPB8). Nonetheless, we feel that the A482T mutation in combination with the single mutation G594E must confer a minor advantage for it to have been isolated in our screening procedure.
The growth rate phenotypes were completely consistent with the MIC data. In each case, mutations that conferred higher MICs conferred faster growth rates in medium with pseudomonic acid at 35 μg/ml (Table 1). It should be noted that the growth rate data were somewhat less reproducible than the MIC data. In particular, we noted a difference in the growth rates of strains carrying identical plasmids pPB12 and pPB23. Such differences are not uncommon in the cultivation of these extremely oxygen-sensitive anaerobes, and it was for this reason that we utilized both MIC and growth rate data in the characterization of the ileS mutants.
The M. barkeri Fusaro ileS gene does not recombine with the M. acetivorans C2A chromosome.
A potential problem with the use of a native gene as a selectable marker is the possibility that recombination between the introduced gene and the resident genomic copy will give rise to antibiotic-resistant clones that are not the desired transformants. Sequence analysis of the internal ileS PCR fragments from M. barkeri Fusaro and M. acetivorans C2A indicates that the genes from the two species are approximately 90% identical at the DNA level (data not shown). Unfortunately, there are no data available regarding the degree of sequence similarity required for homologous recombination among the methanoarchaea. Because of this, we directly tested the ability of M. barkeri Fusaro ileS to recombine with the M. acetivorans gene.
To test for recombination between the two ileS genes, we transformed M. acetivorans C2A with the plasmids pPB12 and pPB18 (Fig. 2). Both clones carry the PAr ileS12 allele and the pac gene cassette, which encodes resistance to puromycin in Methanosarcina. The plasmids differ in that pPB12 carries the pC2A replicon allowing autonomous replication in M. acetivorans, while pPB18 does not. Therefore, transformation with pPB12 should result in antibiotic resistance regardless of the ability to recombine with the host gene; however, because pPB18 is incapable of replication in M. acetivorans C2A, antibiotic-resistant transformants are expected only if the plasmid can be integrated into the genome by recombination with the host chromosome. As shown in Table 2, transformation with the self-replicating vector pPB12 results in equal numbers of transformants, regardless of the antibiotic selection used. In contrast, puromycin-resistant transformants were not obtained after transformation with nonreplicating plasmid pPB18. A few PAr transformants were obtained after transformation with pPB18 (Table 2). These are likely to be spontaneous PAr mutants because they were not resistant to puromycin, indicating that the plasmid did not integrate into the chromosome. In separate experiments, the frequency of spontaneous PAr was shown to be 5.5 × 10−9 ± 1.8 × 10−9, consistent with this interpretation (Metcalf, unpublished).
TABLE 2.
Recombination of M. barkeri ileS12 with M. acetivoransa
| Plasmid | Transformation efficiencyb (CFU/μg of DNA)
|
||
|---|---|---|---|
| Pu | PA | Pu + PA | |
| pPB12 | 2.4 × 105 | 2.5 × 105 | 2.3 × 105 |
| pPB18 | 0 | 14c | 0 |
Recombination between the ileS alleles of M. barkeri and M. acetivorans was examined by transformation of M. acetivorans with plasmids carrying the M. barkeri ileS12 allele that confers PAr. Plasmid pPB12 is capable of autonomous replication and served as a positive control for transformation frequency. Plasmid pPB18 cannot replicate in Methanosarcina and therefore can only give antibiotic-resistant transformants if it recombines with the host chromosome.
Transformation frequencies are averages of two trials with selection on HS-MA agar plus puromycin (Pu), pseudomonic acid (PA), or puromycin plus pseudomonic acid (Pu + PA).
None of the PAr transformants obtained with pPB18 were resistant to puromycin.
Construction of Methanosarcina-E. coli shuttle vectors that confer PAr.
The data indicate that mutant derivatives of M. barkeri ileS can be used as selectable markers for PAr in M. acetivorans. Because it confers the highest level of resistance and the fastest growth rate in medium containing pseudomonic acid, we chose to utilize the ileS12 allele for the construction of Methanosarcina-E. coli plasmid shuttle vectors. The plasmids pPB31, pPB32 (Fig. 2), pPB33, pPB34, and pPB35 were constructed as described in Materials and Methods and provide many useful features. Each plasmid is capable of replication in both E. coli and Methanosarcina utilizing the R6K and pC2A replicons, respectively. Transformants carrying these plasmids can be obtained by selection for ampicillin resistance encoded by the bla gene in E. coli and in Methanosarcina by selection for PAr encoded by the ileS12 gene. In addition, the various shuttle vectors possess a variety of restriction sites suitable for cloning and have a lacZα gene to allow blue-white screening for recombinant clones in E. coli.
DISCUSSION
The Methanosarcina-E. coli shuttle vectors encoding PAr reported here will be a valuable tool for genetic analysis in Methanosarcina. Prior to this report, only a single selectable marker, the pac cassette for resistance to puromycin, was available for use in Methanosarcina species (29). As a result, it was not previously possible to introduce a second genetic element (either a plasmid or a marker-tagged chromosomal mutation) into a strain that already carried the pac cassette. Therefore, standard genetic experiments involving two selectable markers, such as complementation of chromosomal mutations, identification of essential genes, and in vivo identification of genetic regulatory elements, were not possible. With the development of ileS12-encoded PAr selection, such experiments are now possible. Importantly, the finding that plasmid-encoded ileS alleles can confer PAr on otherwise wild-type (PA-sensitive) hosts indicates that the mutant alleles are dominant over the wild-type alleles. Therefore, these ileS alleles can be used as selectable markers in genetic experiments using wild-type hosts. Although we expected this to be the case, because in Staphylococcus a plasmid-carried ileS gene encodes PAr (13, 33), this finding was by no means certain. For example, the streptomycin sensitivity of the wild-type rpsL allele is dominant over streptomycin-resistant rpsL alleles in E. coli (38).
The utility of the ileS12 constructs reported here has been proven in two ongoing studies in our laboratory, the results of which will be reported elsewhere. We have used the PAr shuttle vectors reported here to study hydrogenase function and proline biosynthesis in Methanosarcina (W. W. Metcalf, J. K. Zhang, and H. C. Kuettner, unpublished data). Further, we have used the PAr marker to construct auxotrophic mutants by inserting the ileS12 gene into cloned Methanosarcina proline biosynthesis genes. The disrupted alleles were subsequently crossed onto the M. acetivorans chromosome by homologous recombination with selection for PAr provided by the ileS12 marker. Ninety percent (108 of 120) of the PAr recombinants tested were proline auxotrophs. In this regard, it is important to note that we clearly demonstrated that M. barkeri Fusaro ileS does not recombine with the gene of the closely related species M. acetivorans. It is, however, a functional gene in this heterologous host, as shown by its ability to confer PAr. As such, it is likely that this selectable marker will find use in other Methanosarcina species, although the possibility of recombination with the native ileS genes in these hosts must be kept in mind.
In addition to their utility in genetic experiments, the mutant ileS alleles reported here can further increase our understanding of pseudomonic acid action as an inhibitor of isoleucyl-tRNA synthetase. Pseudomonic acid is hypothesized to act as an analog of the active intermediate isoleucyl-adenylate and to contact residues within the enzyme involved in binding of both ATP and isoleucine (48). As such, the selection for PAr is quite stringent. Because IleS is required for growth, the mutant protein must remain capable of binding to its natural substrates isoleucine, ATP, and tRNAIle while no longer binding the antibiotic. At the same time, it must also remain capable of catalyzing tRNA charging. Thus, only subtle changes are expected to be allowed.
Considerable data have accumulated regarding the amino acid residues involved in substrate binding and catalysis by aminoacyl-tRNA synthetases. The known enzymes are divided into two classes of 10 enzymes each. Isoleucyl-tRNA synthetase is a class I aminoacyl-tRNA synthetase since it carries the two signature sequences, HIGH and KMSKS, that are not present in class II aminoacyl-tRNA synthetases (7, 10, 14). These consensus sequences are indicative of the presence of a nucleotide binding fold, designated the Rossman fold, that contains the active site for ATP binding (10, 14, 18, 37). Structural studies indicate that the amino acid binding site is immediately adjacent to these conserved residues within the active-site pocket of the enzyme (34). Two other consensus sequences have been implicated in IleS activity. The WCISR consensus sequence (amino acids 452 to 456 in M. barkeri IleS) has been implicated in the activation of isoleucine. In E. coli, ileS mutations in this region severely impair isoleucine activation (40). Mutational studies also led to the conclusion that the consensus sequence GWD (amino acids 84 to 86 in M. barkeri IleS) is important for isoleucine binding (12, 39). This idea was recently confirmed after determination of the crystal structure of the Thermus thermophilus IleS protein containing bound isoleucine indicated that the aspartate residue of the GWD sequence is hydrogen bonded to the NH3+ moiety of the amino acid (34).
Analysis of the PAr mutants isolated in our study, and PAr mutants of other organisms, supports the hypothesis that pseudomonic acid acts as an analog of the active intermediate isoleucyl-adenylate (48). Using the positions of the analogous amino acids in the crystal structure of the T. themophilus IleS protein, we modeled the approximate positions of the mutated amino acids found in our study (Fig. 3). Four of the five mutations we isolated (A440V, G587D, G593D, and G594E) and one mutation isolated in E. coli (48) result in alterations of amino acid residues predicted to lie within with the substrate binding pocket of the enzyme (Fig. 3). The single mutation G594E was present in all but one of the mutated clones we isolated. Further, an identical mutation was found to confer PAr on M. thermoautotrophicum Marburg (21). This mutation is only one amino acid residue upstream of the KMSKS consensus sequence thought to be involved in ATP binding. Similarly, a phenylalanine-to-leucine mutation seven amino acids upstream of the KMSKS consensus sequence was found to confer PAr on E. coli (48). The single mutations G593D and G594E were independently obtained together three times, indicating their importance. The A440V mutation, only 12 amino acids upstream of the WCISR sequence, was also obtained in two independent plasmids and is predicted to lie within the active-site pocket.
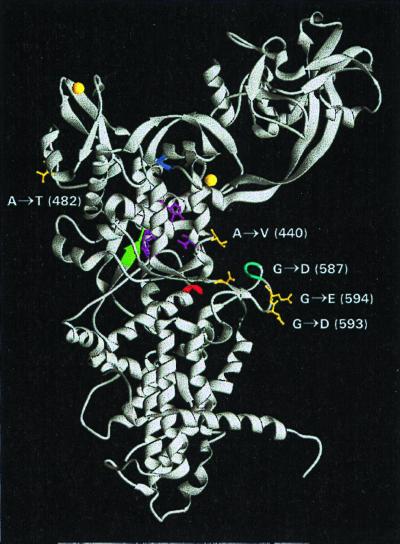
FIG. 3.
Modeling of IleS mutations that confer PAr. The crystal structure of the isoleucyl-tRNA synthetase from T. thermophilus (34) was used to identify the homologous positions and spatial locations of amino acid changes in the IleS protein of M. barkeri Fusaro that confer PAr. Side chains of mutated amino acids are in yellow. Bound Zn2+ molecules are represented by yellow spheres. The GWD, HIGH, WCISR, and KMSKS consensus sequences of class I aminoacyl-tRNA synthetases are in green, red, blue, and aqua, respectively. The isoleucines binding residues Pro46, Asp85, Trp518, Gln554, and Trp558 in T. thermophilus IleS are in magenta. The IleS structure was drawn using the program Ribbons 2.0 (9). Coordinates were retrieved from the Protein Data Bank (accession no. 1ILE [5]). See the text for details.
The only change that does not appear to modify residues directly associated with those involved in substrate binding is the A482T mutation. As can be seen in Fig. 3, the altered amino acid residue is predicted to lie far from the enzyme active site. As such, it probably exerts its effect by a change in protein conformation; however, it should be noted that these conclusions are based on modeling with an enzyme from a distantly related organism. Therefore, it remains possible that this mutation will actually have a more direct effect in the Methanosarcina IleS protein.
Finally, this is the first report of an ileS gene sequenced in the genus Methanosarcina. Analysis of the sequence of IleS indicates that this protein is closely related to that of other archaea, most closely to that of A. fulgidus (Fig. 1). In other studies, IleS has been a valuable phylogenetic marker and has served to support 16S rRNA-based phylogenies, as well as to root the universal tree of life (7). Our finding that Methanosarcina IleS fits closely into this previously determined phylogeny is not unexpected, but it may be useful for future phylogenetic studies.
ACKNOWLEDGMENTS
We thank SmithKline Beecham for the kind gift of pseudomonic acid, Kjell Håkansson for assistance with drawing and analysis of the IleS structure, David E. Graham for assistance with the phylogenetic analysis of the IleS protein, and Stanley Maloy for providing strains.
This work was supported by grant MCB-987459 from the National Science Foundation and by a Searle Scholars Award to W.W.M. P.B. was supported in part by National Institutes of Health grant GM 51334.
REFERENCES
- 1.Adachi J, Hasegawa M. Computer science monographs n 28, MOLPHY version 2.3: programs for molecular phylogenetics based on maximum likelihood. Tokyo, Japan: Institute for Statistical Mathematics; 1996. [Google Scholar]
- 2.Argyle J L, Tumbula D L, Leigh J A. Neomycin resistance as a selectable marker in Methanococcus maripaludis. Appl Environ Microbiol. 1996;62:4233–4237. doi: 10.1128/aem.62.11.4233-4237.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. 1 and 2. New York, N.Y: John Wiley & Sons, Inc.; 1992. [Google Scholar]
- 4.Beckler G S, Hook L A, Reeve J N. Chloramphenicol acetyltransferase should not provide methanogens with resistance to chloramphenicol. Appl Environ Microbiol. 1984;47:868–869. doi: 10.1128/aem.47.4.868-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein F C, Koetzle T F, Williams G J B, Meyer E F, Brice M D, Rodgers J R, Kennard O, Shimanouchi T, Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 6.Bock A, Kandler O. Antibiotic sensitivity of archaebacteria. In: Woese C R, Wolfe R S, editors. Archaebacteria. Vol. 8. New York, N.Y: Academic Press, Inc.; 1985. pp. 525–544. [Google Scholar]
- 7.Brown J R, Doolittle W F. Root of the universal tree of life based on ancient aminoacyl-tRNA synthetase gene duplications. Proc Natl Acad Sci USA. 1995;92:2441–2445. doi: 10.1073/pnas.92.7.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, FitzGerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 9.Carson M. Ribbons 2.0. J Appl Crystallogr. 1991;24:958–961. [Google Scholar]
- 10.Carter C W. Cognition, mechanism, and evolutionary relationships in aminoacyl-tRNA synthetases. Annu Rev Biochem. 1993;62:715–748. doi: 10.1146/annurev.bi.62.070193.003435. [DOI] [PubMed] [Google Scholar]
- 11.Chambers S P, Prior S E, Barstow D A, Minton N P. The pMTL nic-cloning vectors. I. Improved pUC polylinker regions to facilitate the use of sonicated DNA for nucleotide sequencing. Gene. 1988;68:139–149. doi: 10.1016/0378-1119(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 12.Clarke N D, Lien D C, Schimmel P. Evidence from cassette mutagenesis for a structure-function motif in a protein of unknown structure. Science. 1988;240:521–523. doi: 10.1126/science.3282306. [DOI] [PubMed] [Google Scholar]
- 13.Dyke K G, Curnock S P, Golding M, Noble W C. Cloning of the gene conferring resistance to mupirocin in Staphylococcus aureus. FEMS Microbiol Lett. 1991;61:195–198. doi: 10.1016/0378-1097(91)90550-t. [DOI] [PubMed] [Google Scholar]
- 14.Eriani G, Delaure M, Poch O, Gangloff J, Moras D. Partition of tRNA synthetases into two classes based on mutually exclusive sets of sequence motifs. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- 15.Fuller A T, Mellows G, Woolford M, Banks G T, Barrow K D, Chain E B. Pseudomonic acid: an antibiotic produced by Pseudomonas fluorescens. Nature. 1971;234:416–417. doi: 10.1038/234416a0. [DOI] [PubMed] [Google Scholar]
- 16.Gernhardt P, Possot O, Foglino M, Sibold L, Klein A. Construction of an integration vector for use in the archaebacterium Methanococcus voltae and expression of a eubacterial resistance gene. Mol Gen Genet. 1990;221:273–279. doi: 10.1007/BF00261731. [DOI] [PubMed] [Google Scholar]
- 17.Hedderich R, Koch J, Linder D, Thauer R K. The heterodisulfide reductase from Methanobacterium thermoautotrophicum contains sequence motifs characteristic of pyridine-nucleotide-dependent thioredoxin reductases. Eur J Biochem. 1994;225:253–261. doi: 10.1111/j.1432-1033.1994.00253.x. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y M, Shiba K, Mottes C, Schimmel P. Sequence determining and modeling of structural motifs for the smallest monomeric aminoacyl transfer RNA synthetase. Proc Natl Acad Sci USA. 1991;88:976–980. doi: 10.1073/pnas.88.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes J, Mellows G. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J. 1978;176:305–318. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes J, Mellows G, Soughton S. How does Pseudomonas fluorescens, the producing organism of the antibiotic pseudomonic acid A, avoid suicide? FEBS Lett. 1980;122:322–324. doi: 10.1016/0014-5793(80)80465-0. [DOI] [PubMed] [Google Scholar]
- 21.Jenal U, Rechsteiner T, Tan P Y, Buhlmann E, Meile L, Leisinger T. Isoleucyl-tRNA synthetase of Methanobacterium thermoautotrophicum Marburg. Cloning of the gene, nucleotide sequence, and localization of a base change conferring resistance to pseudomonic acid. J Biol Chem. 1991;266:10570–10577. [PubMed] [Google Scholar]
- 22.Jermiin L S, Olsen G J, Mengersen K L, Easteal Majority-rule consensus of phylogenetic trees obtained by maximum-likelihood analysis. Mol Biol Evol. 1997;14:1296–1302. [Google Scholar]
- 23.Jones W J, Whitman W B, Fields R D, Wolfe R S. Growth and plating efficiency of methanococci on agar media. Appl Environ Microbiol. 1983;46:220–226. doi: 10.1128/aem.46.1.220-226.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenk H P, Clayton R A, Tomb J F, White O, Nelson K E, Ketchum K A, Dodson R J, Gwinn M, Hickey E K, Peterson J D, Richardson D L, Kerlavage A R, Graham D E, Kyrpides N C, Fleischmann R D, Quackenbush J, Lee N H, Sutton G G, Gill S, Kirkness E F, Dougherty B A, McKenney K, Adams M D, Loftus B, Venter J C, et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature. 1997;390:364–370. doi: 10.1038/37052. [DOI] [PubMed] [Google Scholar]
- 25.Maloy S R. Hydroxylamine mutagenesis. In: Maloy S R, editor. Experimental techniques in bacterial genetics. Boston, Mass: Jones and Bartlett; 1990. pp. 50–54. [Google Scholar]
- 26.Meile L, Abendschein P, Leisinger T. Transduction in the archaebacterium Methanobacterium thermoautotrophicum Marburg. J Bacteriol. 1990;172:3507–3508. doi: 10.1128/jb.172.6.3507-3508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalf W W. Genetic analysis in the domain Archaea. Methods Microbiol. 1999;29:277–326. [Google Scholar]
- 28.Metcalf W W, Jiang W, Daniels L L, Kim S K, Haldimann A, Wanner B L. Conditionally replicative and conjugative plasmids carrying lacZα for cloning, mutagenesis, and allele replacement in bacteria. Plasmid. 1996;35:1–13. doi: 10.1006/plas.1996.0001. [DOI] [PubMed] [Google Scholar]
- 29.Metcalf W W, Zhang J K, Apolinario E, Sowers K R, Wolfe R S. A genetic system for Archaea of the genus Methanosarcina: liposome-mediated transformation and construction of shuttle vectors. Proc Natl Acad Sci USA. 1997;94:2626–2631. doi: 10.1073/pnas.94.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metcalf W W, Zhang J K, Shi X, Wolfe R S. Molecular, genetic, and biochemical characterization of the serC gene of Methanosarcina barkeri Fusaro. J Bacteriol. 1996;178:5797–5802. doi: 10.1128/jb.178.19.5797-5802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalf W W, Zhang J K, Wolfe R S. An anaerobic, intrachamber incubator for growth of Methanosarcina spp. on methanol-containing solid media. Appl Environ Microbiol. 1998;64:768–770. doi: 10.1128/aem.64.2.768-770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morton T M, Johnston J L, Patterson J, Archer G L. Characterization of a conjugative staphylococcal mupirocin resistance plasmid. Antimicrob Agents Chemother. 1995;39:1272–1280. doi: 10.1128/aac.39.6.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nureki O, Vassylyev Dmitry G, Tateno M, Shimada A, Nakama T, Fukai S, Konno M, Hendrickson Tamara L, Schimmel P, Yokoyama S. Enzyme structure with two catalytic sites for double-sieve selection of substrate. Science. 1998;280:578–582. doi: 10.1126/science.280.5363.578. [DOI] [PubMed] [Google Scholar]
- 35.Pfeiffer M, Bestgen H, Burger A, Klein A. The vhuU gene encoding a small subunit of a selenium-containing [NiFe]− hydrogenase in Methanococcus voltae appears to be essential for the cell. Arch Microbiol. 1998;170:418–426. doi: 10.1007/s002030050662. [DOI] [PubMed] [Google Scholar]
- 36.Possot O, Gernhardt P, Klein A, Sibold L. Analysis of drug resistance in the archaebacterium Methanococcus voltae with respect to potential use in genetic engineering. Appl Environ Microbiol. 1988;54:734–740. doi: 10.1128/aem.54.3.734-740.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossman M G, Liljas A, Branden C I, Banaszak L J. Evolutionary and structural relationships among dehydrogenases. 3rd ed. Vol. 11. Orlando, Fla: Academic Press; 1975. [Google Scholar]
- 38.Russell C B, Dahlquist F W. Exchange of chromosomal and plasmid alleles in Escherichia coli by selection for loss of a dominant antibiotic sensitivity marker. J Bacteriol. 1989;171:2614–2618. doi: 10.1128/jb.171.5.2614-2618.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt E, Schimmel P. Dominant lethality by expression of a catalytically inactive class I tRNA synthetase. Proc Natl Acad Sci USA. 1993;90:6919–6923. doi: 10.1073/pnas.90.15.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt E, Schimmel P. Residues in a class I tRNA synthetase which determine selectivity of amino acid recognition in the context of tRNA. Biochemistry. 1995;34:11204–11210. doi: 10.1021/bi00035a028. [DOI] [PubMed] [Google Scholar]
- 41.Smith D R, Doucette-Stamm L A, Deloughery C, Lee H, Dubois J, Aldredge T, Bashirzadeh R, Blakely D, Cook R, Gilbert K, Harrison D, Hoang L, Keagle P, Lumm W, Pothier B, Qiu D, Spadafora R, Vicaire R, Wang Y, Wierzbowski J, Gibson R, Jiwani N, Caruso A, Bush D, et al. Complete genome sequence of Methanobacterium thermoautotrophicum ΔH: functional analysis and comparative genomics. J Bacteriol. 1997;179:7135–7155. doi: 10.1128/jb.179.22.7135-7155.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowers K R, Boone J, Gunsalus R P. Disaggregation of Methanosarcina spp. and growth as single cells at elevated osmolarity. Appl Environ Microbiol. 1993;59:3832–3839. doi: 10.1128/aem.59.11.3832-3839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tumbula D L, Bowen T L, Whitman W B. Characterization of pURB500 from the archaeon Methanococcus maripaludis and construction of a shuttle vector. J Bacteriol. 1997;179:2976–2986. doi: 10.1128/jb.179.9.2976-2986.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tumbula D L, Makula R A, Whitman W B. Transformation of Methanococcus maripaludis and identification of a PstI-like restriction system. FEMS Microbiol Lett. 1994;121:309–314. [Google Scholar]
- 46.Wanner B L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986;191:39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- 47.Ward A, Campoli-Richards D M. Mupirocin: a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1986;32:425–444. doi: 10.2165/00003495-198632050-00002. [DOI] [PubMed] [Google Scholar]
- 48.Yanagisawa T, Lee J T, Wu H C, Kawakami M. Relationship of protein structure of isoleucyl-tRNA synthetase with pseudomonic acid resistance of Escherichia coli: a proposed mode of action of pseudomonic acid as an inhibitor of isoleucyl-tRNA synthetase. J Biol Chem. 1994;269:24304–24309. [PubMed] [Google Scholar]