Abstract
Context Oxidative stress and inflammation are implicated in the aging process and its related hepatic and renal function decline. Chlorogenic acid (CGA) is one of the most abundant polyphenol compounds in the human diet. Recently, CGA has shown in vivo and in vitro antioxidant properties.
Objective The current study investigates the effects of protective effects of chlorogenic acid (CGA) on d-galactose-induced liver and kidney injury.
Materials and methods Hepatic and renal injuries were induced in a mouse model by subcutaneously injection of d-galactose (d-gal; 100 mg/kg) once a day for 8 consecutive weeks and orally administered simultaneously with CGA included in the food (200 mg/kg of diet). The liver and renal functions were examined. Histological analyses of liver and kidney were done by haematoxylin and eosin staining. The oxidative stress markers and pro-inflammatory cytokines in the liver and the kidney were measured.
Results CGA significantly reduced the serum aminotransferase, serum creatinine (SCr) and blood urea nitrogen (BUN) levels in d-gal mice (p <0.05). CGA also restored superoxide dismutase, catalase, and malondialdehyde levels and decreased glutathione content in the liver and kidney in d-gal mice (p <0.05). Improvements in liver and kidney were also noted in histopathological studies. CGA reduced tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) protein levels in the liver and kidney in d-gal mice (p <0.05).
Discussion and conclusion These findings suggest that CGA attenuates d-gal-induced chronic liver and kidney injury and that this protection may be due to its antioxidative and anti-inflammatory activities.
Keywords: CGA, d-gal, oxidative stress
Introduction
Chronic hepatic and renal diseases are major health concerns worldwide with dramatically increasing prevalence and incidence. Despite tremendous advances in modern medicine, the prevention and the treatment of hepatic and renal diseases remain limited. The pathogenesis of hepatic and renal diseases, as well as the role of oxidative stress and inflammation, is well established (Malhi & Gores 2008). Therefore, inhibiting or blocking oxidative stress and inflammatory response is a promising therapeutic strategy for hepatic and renal injuries. Mounting evidence suggest that d-gal-induced liver and kidney injury is a well-established experimental model that closely similar to morphological and functional features of human hepatitis and nephritis (Dong et al. 2013; Li et al. 2015).
Research has shown that d-gal promotes oxidative damage in the liver and kidney of rodents (Li et al. 2005). Free radicals attack essential cell constituents and also induce lipid peroxidation, damage the membranes of cells and organelles in liver and kidney, cause the swelling and necrosis of hepatocytes and nephrocytes, and ultimately result in liver and kidney injury (Fan et al. 2009; Zhang et al. 2009b). d-Gal overload increases the production of free radicals, diminishes antioxidant enzyme activity, and attenuates immune responses.
Inflammation is one of the leading causes of many pathological processes associated with oxidative stress (Theiss et al. 2009). Accumulated data strongly suggest that chronic inflammation is also a major cause of liver and kidney injury (Tolosano et al. 2002; Kozlov et al. 2010). Emerging evidence shows a close link between oxidation and inflammation since excessive or uncontrolled free radicals production can induce an inflammatory response, while free radicals are inflammation effectors (Kulinsky 2007).
Chlorogenic acid (CGA) is one of the most abundant polyphenol compounds in coffee. It is one of the important ingredients of green coffee beans (Bekedam et al. 2008) and also in many kinds of fruits and vegetables such as apples, cherries, plums, berries, apricots, tomatoes, and potatoes (Heitman & Ingram 2014). Several recent studies have demonstrated that CGA exerts a variety of pharmacological effects, including antioxidative and anti-inflammatory effects (Xu et al. 2010; Sato et al. 2011). Previous research found that CGA protected mice against lipopolysaccharide-induced inflammatory responses in acute lung injury (Zhang et al. 2010). Furthermore, CGA protected against ischemia/reperfusion injury in rat liver through its antioxidative and anti-inflammatory properties (Yun et al. 2012). Little work has been done to clarify the protective effects of CGA on the d-gal-induced liver and kidney injury and its underlying mechanisms. Therefore, the aim of this study was to explore whether chronic CGA oral supplementation for 8 weeks could protect the mouse liver and kidney from d-gal-induced injury, and then to examine the underlying effects. The oxidative stress and inflammatory response were assessed in the liver and kidney for further clarification of the possible effects of CGA in the d-gal-induced liver and kidney injury process.
Methods and materials
Chemicals and reagents
d-Gal was purchased from Beijing Chemical-Regent Company (Beijing, China) and dissolved in 0.9% saline at concentrations of 20 mg/ml. Chlorogenic acid (the purity grade was 99.0%) was purchased from Shaanxi Sciphar Biotechnology Co., Ltd (Xi’an, China). SCr kit, BUN kit, MDA kit, GSH kit, SOD kit, CAT kit, ALT kit, and AST kit were obtained from Sigma Chemicals (St. Louis, MO). All other reagents used in the experiments were of analytical grade and purity.
Animals and treatments
Adult male Kunming mice (18–22 g) were provided by the Laboratory Animal Center, Xuzhou Medical College, Xuzhou, Jiangsu, China. The mice were housed with ad libitum access to food and water under controlled temperature (22 ± 2 °C) and humidity (50 ± 10%) conditions and maintained on a 12 h light/dark cycle for 1 week to acclimatise. The mice were randomly divided into four groups; (n = 10 each): Group 1 (control group), subcutaneously injected with an equal volume of saline instead of d-gal; Group 2 (d-gal group), subcutaneously injected with d-gal (100 mg/kg/day) (Wei et al. 2005); Group 3 (d-gal + CGA group), subcutaneously injected with d-gal (100 mg/kg/day) and received CGA (200 mg/kg of diet) (Cho et al. 2010; Tsuchiya et al. 1996); Groups 4 (CGA group), subcutaneously injected with an equal volume of normal saline instead of d-gal and received CGA (200 mg/kg of diet). The animals were sacrificed after treatment for 8 weeks. Blood samples were collected, from the orbital sinus, in 2 mL Eppendorf tubes and centrifugated for 10 min at 4000 rpm at 4 °C. The liver and kidney of the mice were quickly removed and stored at −80 °C for future biochemical and pathology analyses. Ten mice per group were used for the present study. The liver and the kidney of four mice in each group were used for histomorphological examinations. Six mice in each group were used to measure the alanine aminotransferase (ALT), aspartate aminotransferase (AST), SCr, BUN, oxidative stress markers, TNF-α and IL-6 levels.
All procedures were approved by the Animal Ethics Committee, Xuzhou Medical College, China, and complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Estimation of liver and kidney function
Liver function was assessed by the estimation of activities of ALT and AST in the serum using transaminases kit by the Reitman–Frankel method (Reitman & Frankel 1957). Briefly, ALT acts upon l-alanine and α-ketoglutaric acid to yield pyruvate that subsequently reacts with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenyl hydrazone while AST acts upon l-aspartate and α-ketoglutaric acid to yield oxaloacetate that subsequently reacts with 2,4-dinitrophenylhydrazine to form 2,4-dinitrophenylhydrazone. Colour development was measured photometrically at 505 nm.
The levels of SCr and BUN in plasma samples with kits were measured as indicators of renal function by microplate spectrophotometry, according to the protocol of the manufacturer.
Histological analyses of liver and kidney
For histomorphological examinations, four mice in each group were immediately anesthetised by an injection of sodium pentobarbital (50 mg/g, i.p.) and perfused with ice-cold normal saline followed by 4% paraformaldehyde via the left ventricle of the heart. The liver and kidney tissues were removed and postfixed in a fresh solution of 4% paraformaldehyde (pH 7.4) at 4 °C for 24 h. This was followed by embedment in paraffin and longitudinal slicing, with 5 μm thick sections obtained for hematoxylin–eosin (H&E) staining. The stained slides were examined by microscopy for histomorphological analyses.
Assessment of oxidative stress in liver and kidney
Tissue homogenates
For biochemical studies, animals were deeply anesthetised and sacrificed. Liver and kidney were homogenised in 1/5 (w/v) 50 mM (pH 7.4) ice-cold phosphate buffered saline solution (PBS) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) with 10 strokes at 1200 rev/min in a Potter homogeniser. Homogenates were directly centrifuged at 4000 rpm for 10 min to obtain the supernatant. Supernatant aliquots were used to determine MDA and GSH levels, SOD and CAT activities and protein contents. Protein contents were determined by using the BCA assay kit (Nanjing Jiancheng Biotechnology Co., Ltd).
Measurement of malondialdehyde (MDA) level
The levels of MDA in the liver and kidney tissue homogenates were determined using the method of Uchiyama and Mihara (1978). Half a milliliter of homogenate was mixed with 3 ml of H3PO4 solution (1%, v/v) followed by the addition of 1 ml of thiobarbituric acid solution (0.67%, w/v). Then the mixture was heated in a water bath at 95 °C for 45 min. The coloured complex was extracted into n-butanol, and the absorption at 532 nm was measured using tetramethoxypropane as standard. MDA levels were expressed as nmol per mg of protein.
Measurement of glutathione (GSH) level
The levels of GSH in the liver and kidney were determined as an index of antioxidant reserves in tissues. The homogenates were centrifuged at 10 000g for 20 min, and an aliquot of the clear supernatant (20 μl) was combined with 0.3 M Na2HPO4 (160 μl) and 0.04% 5,5-dithiobis-(2-nitrobenzoic acid) in 1% sodium citrate (20 μl). After 10 min of incubation at room temperature, A405 was read in a Spectramax microplate reader (ELX808, Bio Tek Instruments Inc, Winooski, VT). Concentrations of GSH were calculated from a standard curve constructed with known concentrations of GSH and were expressed in mg per g of protein.
Measurement of superoxide dismutase (SOD) activity
SOD activities in the liver and kidney were measured according to the method described by Lu et al. (2007). Solution A was prepared by mixing 100 ml of 50 mM PBS (pH 7.4) containing 0.1 mM EDTA and 2 μmol of cytochrome c with 10 ml of 0.001 N NaOH solution containing 5 μmol of xanthine. Solution B contained 0.2 U xanthine oxidase/ml and 0.1 mM EDTA. Fifty microlitre of a tissue supernatant was mixed with 2.9 ml of solution A and the reaction was started by adding 50 μl of solution B. Change in absorbance at 550 nm was monitored. A blank was run by substituting 50 μl of ultrapure water for the supernatant. SOD levels were expressed as U/mg protein with reference to the activity of a standard curve of bovine Cu/Zn SOD under the same conditions.
Measurement of catalase (CAT) activity
CAT activities in the liver and kidney were measured according to the method of Abei (1984). Briefly, to a quartz cuvette, 0.65 ml of the phosphate buffer (50 mmol/l; pH 7.0) and 50 μl sample were added, and the reaction was initiated by the addition of 0.3 ml fresh 30 mmol/L hydrogen peroxide (H2O2). The decomposition rate of H2O2 was spectrophotometrically measured by changes in absorbance at 240 nm at 25 °C. CAT activity was calculated as U/mg of tissue protein.
Measurement of TNF-α and IL-6 levels in the liver and kidney by ELISA
TNF-α and IL-6 levels in the liver and the kidney were determined with a commercial enzyme-linked immunosorbent assay (ELISA) kits (Adlitteram Diagnostic Laboratories, USA) according to the manufacturer’s instructions. At the end of all reactions, the absorbance of chromophore at 420 nm was measured using a microplate reader.
Statistical analysis
The results were expressed as mean ± SD. The data were analysed by one-way ANOVA, followed by Dunnett’s t-test. p <0.05 was considered to be statistically significant. Statistical analysis was conducted using SPSS 16.0 (SPSS Inc, Chicago, IL).
Results
Effects of CGA on liver and kidney functions in d-gal-induced mice
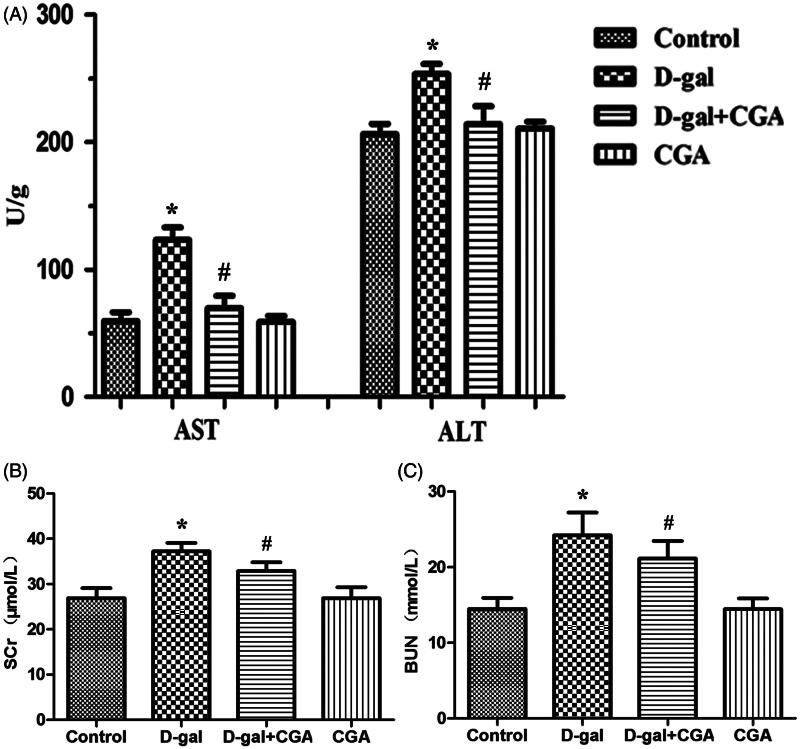
The effects of CGA on liver function in d-gal-induced mice are examined by measuring AST and ALT in the serum. The d-gal group mice exhibited a marked increase in the levels of AST and ALT (p <0.05). However, the d-gal + CGA group significantly decreased the levels of AST and ALT (P <0.05) in the serum when compared with d-gal group (Figure 1A).
Figure 1.
Effect of CGA on d-gal-induced changes in hepatic (A) and renal (B and C) functional markers. All data are represented as mean ± SD (n = 6). *p <0.05 as compared with the control group; #p <0.05 as compared with the d-gal group.
The effects of CGA on kidney function in d-gal-induced mice are examined by measurement of SCr and BUN in the serum. As shown in Figure 1, the concentrations of SCr and BUN (p <0.05) were significantly higher in the d-gal group when compared with the control group, while there were no significant differences between the CGA group and the control group. The d-gal + CGA group significantly decreased the levels of SCr and BUN (p <0.05) when compared with the d-gal group (Figure 1B and C).
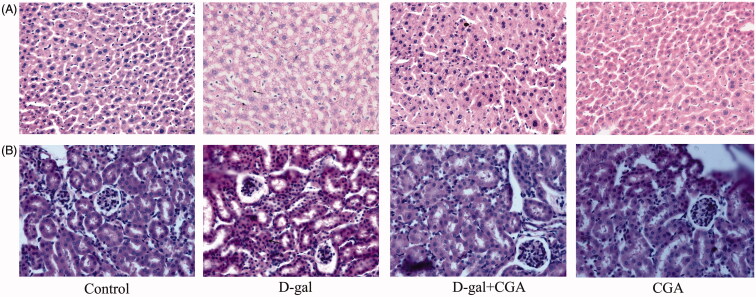
Effects of CGA on liver and kidney histomorphology in d-gal-induced mice
The histomorphological features of H&E stained liver and kidney sections showed that CGA exhibited protective effects against d-gal-induced liver and kidney damage (Figure 2A and B). As shown in Figure 2, compared with the control group, d-gal treatment caused visible histological changes including structure damage, degeneration, and necrosis of hepatocytes and nephrocytes. CGA significantly alleviated the liver and the kidney damage in d-gal-treated mice. No visible histological changes in the liver could be observed between the control group and the CGA group.
Figure 2.
Effect of CGA on histopathological changes of the d-gal-treated mouse liver (A) and kidney (B), (n = 4). Arrow indicated to the structure damage, degeneration, and necrosis of hepatocytes and nephrocytes. Original magnification, 10 × 40.
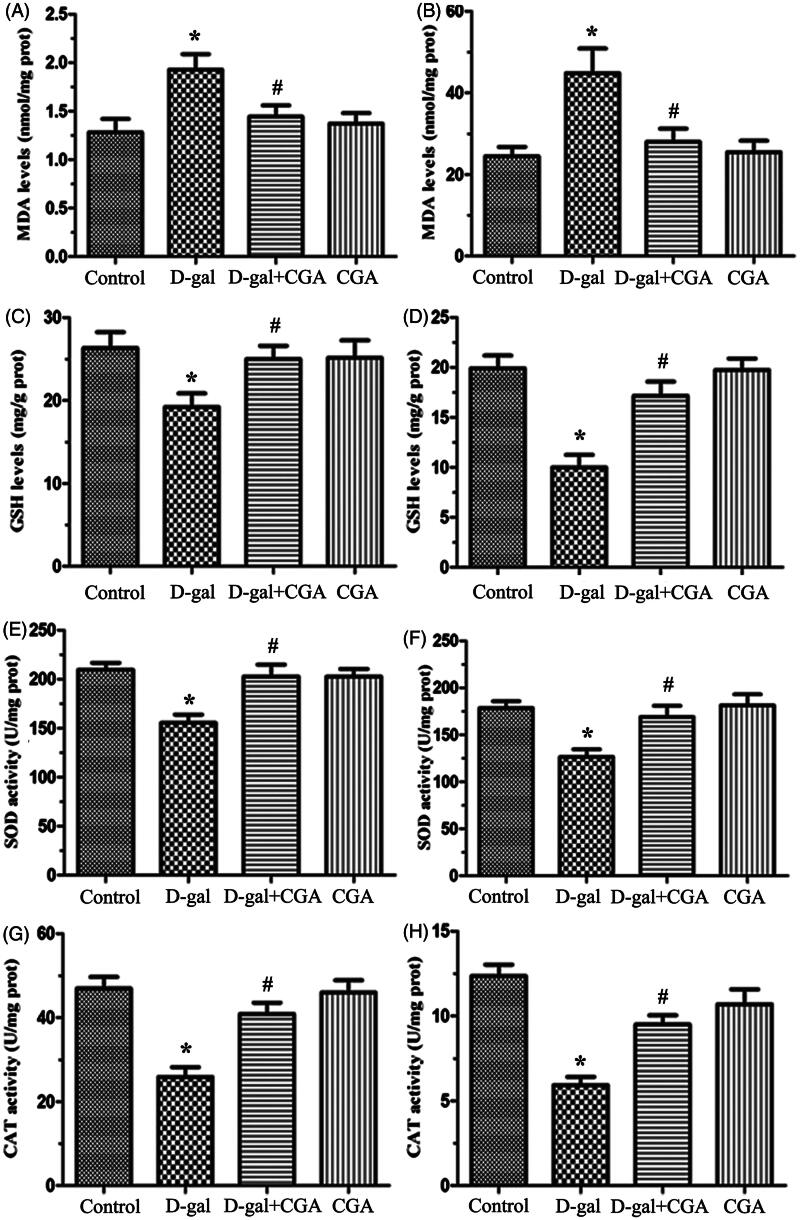
Effect of CGA on oxidative stress markers in liver and kidney of d-gal-induced mice
The levels of MDA in the liver and kidney of d-gal mice are significantly elevated when compared with the control group (p <0.05, Figure 3A and B), while CGA chronic treatment significantly decreased the MDA levels in the liver and the kidney. The d-gal group had lower levels of GSH (p <0.05, Figure 3C and D), SOD (p <0.05, Figure 3E and F), and CAT (p <0.05, Figure 3G and H) in the liver and kidney compared with the control group. Furthermore, the d-gal + CGA treatment group significantly increased GSH, SOD, and CAT (all p <0.05) levels in the liver and kidney of d-gal mice, respectively. CGA alone did not alter the parameters of oxidative stress compared to the control group.
Figure 3.
Effects of CGA on the levels of malondialdehyde (MDA) (A and B) and glutathione (GSH) (C and D) and the activities of superoxide dismutase (SOD) (E and F) and catalase (CAT) (G and H) in the liver and kidney of mice. All values are expressed as mean ± SD (n = 6).
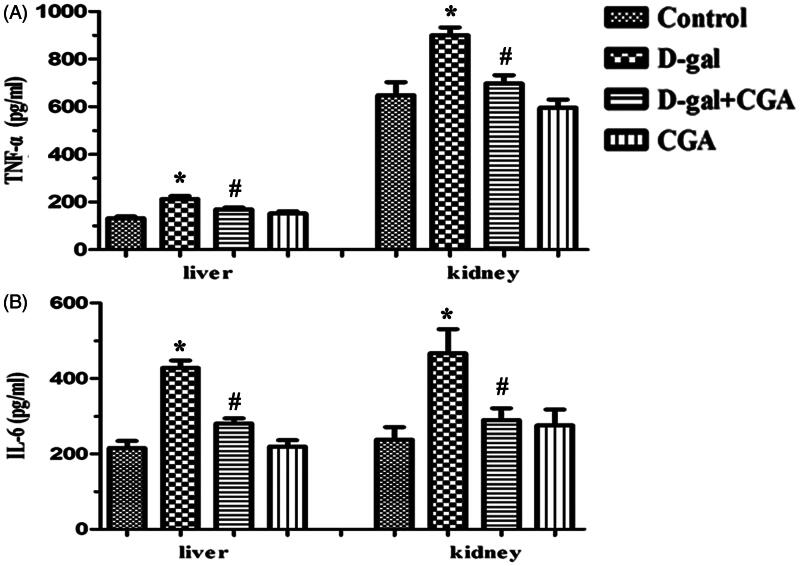
Effect of CGA on TNF-α and IL-6 levels in the liver and kidney of d-gal-induced mice
To investigate the protective effects of CGA on the d-gal-treated mice, we further examined the levels of pro-inflammatory cytokines, TNF-α, and IL-6 by ELISA. The d-gal group elevated TNF-α (p <0.05) and IL-6 (p <0.05) levels in the liver and the kidney when compared with the control group (Figure 4). The d-gal + CGA group produced a significant reduction in TNF-α and IL-6 in the liver (p <0.05) and the kidney (p <0.05) of d-gal mice.
Figure 4.
Effect of CGA on tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels in the liver (A) and kidney (B) of d-gal-induced mice. Values are expressed as mean ± SD (n = 6).
Discussion
The liver and the kidney are two main organs involved in d-gal metabolism and d-gal treatment increased MDA levels and caused oxidative stress in liver and kidney (Zhang et al. 2009a). The liver and the kidney injury model, induced by d-gal treatment (100–500 mg/kg body weight, s.c.) for 8 weeks in mice or rats, has been widely accepted (Fan et al. 2009; Ruan et al. 2013). The chronic subcutaneous administration of d-gal for 8 weeks induced oxidative stress, hepatopathy, and nephropathy in mice and significantly decreased the hepatic and renal SOD and CAT activities as well as GSH levels, increased the hepatic and renal MDA levels (Fan et al. 2009; Yu et al. 2015). However, there is no report about the protective effects of CGA and its underlying effects on d-gal-induced liver and kidney injury.
Previous studies have shown that treatment with d-gal causes liver and kidney injury and dysfunction, followed by elevated activities or levels of serum enzymes and histopathological damage (Fan et al. 2009; Yu et al. 2015). In the present study, our results showed that the activities or the levels of AST, ALT, SCr, and BUN in the serum of d-gal mice were markedly increased. Moreover, we observed hepatic and renal histological changes, such as structure damage, degeneration, necrosis, and infiltration of inflammatory cells of hepatocytes and nephrocytes, which were dramatically improved in mice treated with CGA. Our results showed that CGA remarkably alleviated the severity of liver and kidney injury of d-gal mice. Furthermore, CGA attenuated lipid peroxidation, activated antioxidant enzymes, and suppressed the inflammatory response in the d-gal-induced mice.
It is well known that the defence system of antioxidant enzymes containing SOD, CAT, and GSH-Px may reduce oxidative stress and potentially benefit oxidative-related diseases (Sun 1990). CGA restored the antioxidant defence system by increasing the activity of antioxidant enzymes (SOD and CAT) and suppressing lipid peroxidation (MDA) in the liver and the kidney of d-gal mice. CGA may improve the pro-oxidant–antioxidant disequilibrium, contributing to its prevention of liver and kidney injury.
It has been reported that TNF-α and IL-6 are the key pro-inflammatory cytokines to trigger an inflammatory cascade involving massive apoptosis of hepatocytes and nephrocytes, and consequent damages of hepatic and renal functions (Mei & Zheng 2009; Sarkar & Fisher 2006; Xu et al. 2010). TNF-α and IL-6 are critical players in liver and kidney damage (Nowak et al. 2000). Therefore, the liver and the kidney injury caused by various toxicants can be effectively attenuated by inhibiting synthesis or activity of TNF-α and IL-6. Our findings showed that CGA had an anti-inflammatory action by suppressing pro-inflammatory cytokines, TNF-α and IL-6, which may contribute to protecting against liver and kidney injury of d-gal mice.
Accumulating evidence suggests that there is a close link between oxidative stress and inflammation (Kulinsky 2007). In this study, we found that the activation of d-gal-induced oxidative stress and inflammation. Importantly, our results showed that CGA dietary supplementation improved liver and kidney injury with both reduction of oxidative stress and the inflammatory response in hepatic and renal tissues. Oxidative stress is thought to aggravate inflammation and cause destruction of the liver and kidney tissues by the over-production of reactive oxygen species (ROS) (Chen et al. 2008; Shi et al. 2004). Furthermore, inflammatory cells are recruited to the site of damage, which leads to an increased uptake of oxygen (a “respiratory burst”), and thus, increases the accumulation of ROS at the site of damage and promotes oxidative stress (Reuter et al. 2010). Therefore, the antioxidative and anti-inflammatory effects of CGA supplementation might break the vicious cycle of oxidative stress/inflammation, and has the potential to protect against liver and kidney injury of d-gal mice.
CGA is a phenolic phytochemical which has been found to possess anti-inflammatory and antioxidative properties in previous in vitro and animal models (Sato et al. 2011; Yun et al. 2012). In the present study, the chronic subcutaneous administration with d-galactose for 8 weeks d-gal induced activation of oxidative stress and inflammation, and thereafter two systems aggravate each other to cause destruction of the liver and the kidney in mice. Importantly, CGA improved oxidative stress markers and decreased the levels of pro-inflammatory cytokines in the liver and kidney of d-gal mice, which means that the systems of oxidative stress and inflammation modulate each other to improve the function of liver and kidney in d-gal mice during chronic treatment of CGA. Therefore, CGA mitigates damage in liver and kidney from d-gal mice, and CGA might be used as a therapeutic agent. The exact mechanisms of CGA in suppressing inflammation and oxidative stress remain unclear. And thus, a further study of CGA on these inflammatory and oxidative stress-signalling pathways should be addressed in the liver and kidney injury.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (81341084), Superiority Academic Discipline Construction Project of Jiangsu Higher Education Institutions, the Post-doctoral Fund in Jiangsu Province (1201036B), the “Qing-Lan” Project of Jiangsu Province, “Six-Talents Summit” Project of Jiangsu Province (2011-YY-13), the Industrialization of Scientific Research Promotion Projects of Universities and Colleges in Jiangsu Province (2011-16), the Natural Science Fund for Universities and Colleges in Jiangsu Province (11KJB350005), Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy (2011YKJ004), the Foundation of Xuzhou Medical College Key Laboratory of Tumor Biology Therapy for Tumor (C0904, JSBL0803, and C0903), the Science and Technology Plan Projects of Xuzhou (XF11C037; XF11C062, XF11C062; XZZD1227; XZZD1219), the research project of Xuzhou (BRA201205).
References
- Abei H. 1984. Catalase in vitro. Methods Enzymol. 105:121–126. [DOI] [PubMed] [Google Scholar]
- Bekedam EK, Schols HA, Van Boekel MA, Smit G.. 2008. Incorporation of chlorogenic acids in coffee brew melanoidins. J Agric Food Chem. 56:2055–2063. [DOI] [PubMed] [Google Scholar]
- Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. 2008. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 74:1128–1138. [DOI] [PubMed] [Google Scholar]
- Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK. 2010. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol. 48:937–943. [DOI] [PubMed] [Google Scholar]
- Dong M, Hong T, Liu S, Zhao J, Meng Y, Mu J. 2013. Hepatoprotective effect of the flavonoid fraction isolated from the flower of Inula britannica against d-galactosamine-induced hepatic injury. Mol Med Rep. 7:1919–1923. [DOI] [PubMed] [Google Scholar]
- Fan SH, Zhang ZF, Zheng YL, Lu J, Wu DM, Shan Q, Hu B, Wang YY. 2009. Troxerutin protects the mouse kidney from d-galactose-caused injury through anti-inflammation and anti-oxidation. Int Immunopharmacol. 9:91–96. [DOI] [PubMed] [Google Scholar]
- Heitman E, Ingram DK.. 2014. Cognitive and neuroprotective effects of chlorogenic acid. Nutr Neurosci. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, van Griensven M, Haindl S, Kehrer I, Duvigneau JC, Hartl RT, Ebel T, Jafarmadar M, Calzia E, Gnaiger E, et al. 2010. Peritoneal inflammation in pigs is associated with early mitochondrial dysfunction in liver and kidney. Inflammation 33:295–305. [DOI] [PubMed] [Google Scholar]
- Kulinsky V. 2007. Biochemical aspects of inflammation. Biochem (Mosc). 72:595–607. [DOI] [PubMed] [Google Scholar]
- Li JJ, Zhu Q, Lu YP, Zhao P, Feng ZB, Qian ZM, Zhu L. 2015. Ligustilide prevents cognitive impairment and attenuates neurotoxicity in d-galactose induced aging mice brain. Brain Res. 1595:19–28. [DOI] [PubMed] [Google Scholar]
- Li L, Ng T, Gao W, Li W, Fu M, Niu S, Zhao L, Chen R, Liu F. 2005. Antioxidant activity of gallic acid from rose flowers in senescence accelerated mice. Life Sci. 77:230–240. [DOI] [PubMed] [Google Scholar]
- Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q. 2007. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem Pharmacol. 74:1078–1090. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores GJ.. 2008. Cellular and molecular mechanisms of liver injury. Gastroenterology 134:1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei C, Zheng F.. 2009. Chronic inflammation potentiates kidney aging. Semin Nephrol. 555–568. [DOI] [PubMed] [Google Scholar]
- Nowak M, Gaines GC, Rosenberg J, Minter R, Bahjat F, Rectenwald J, MacKay SL, Edwards CK, Moldawer LL. 2000. LPS-induced liver injury in d-galactosamine-sensitized mice requires secreted TNF-α and the TNF-p55 receptor. Am J Physiol Regul Integr Comp Physiol. 278:R1202–R1209. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S.. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 49:1603–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q, Liu F, Gao Z, Kong D, Hu X, Shi D, Bao Z, Yu Z. 2013. The anti-inflamm-aging and hepatoprotective effects of huperzine A in d-galactose-treated rats. Mech Ageing Dev. 134:89–97. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Fisher PB.. 2006. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 236:13–23. [DOI] [PubMed] [Google Scholar]
- Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. 2011. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 403:136–138. [DOI] [PubMed] [Google Scholar]
- Shi M, Xu B, Wang X, Aoyama K, Michie SA, Takeuchi T. 2004. Oxidative damages in chronic inflammation of a mouse autoimmune disease model. Immunol Lett. 95:233–236. [DOI] [PubMed] [Google Scholar]
- Sun Y. 1990. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 8:583–599. [DOI] [PubMed] [Google Scholar]
- Theiss AL, Vijay-Kumar M, Obertone TS, Jones DP, Hansen JM, Gewirtz AT, Merlin D, Sitaraman SV. 2009. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology 137:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, Altruda F. 2002. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood 100:4201–4208. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Suzuki O, Igarashi K.. 1996. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem. 60:765–768. [DOI] [PubMed] [Google Scholar]
- Uchiyama M, Mihara M.. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 86:271–278. [DOI] [PubMed] [Google Scholar]
- Wei H, Li L, Song Q, Ai H, Chu J, Li W. 2005. Behavioural study of the d-galactose induced aging model in C57BL/6J mice. Behav Brain Res. 157:245–251. [DOI] [PubMed] [Google Scholar]
- Xu Y, Chen J, Yu X, Tao W, Jiang F, Yin Z, Liu C. 2010. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm Res. 59:871–877. [DOI] [PubMed] [Google Scholar]
- Yu Y, Bai F, Liu Y, Yang Y, Yuan Q, Zou D, Qu S, Tian G, Song L, Zhang T. 2015, et al. Fibroblast growth factor (FGF21) protects mouse liver against d-galactose-induced oxidative stress and apoptosis via activating Nrf2 and PI3K/Akt pathways. Mol Cell Biochem. 403:287–299. [DOI] [PubMed] [Google Scholar]
- Yun N, Kang JW, Lee SM.. 2012. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: molecular evidence of its antioxidant and anti-inflammatory properties. J Nutr Biochem. 23:1249–1255. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang H, Yang T, Ye Y, Shan J, Yin Z, Luo L. 2010. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 41:746–752. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. 2009a. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem Toxicol. 47:496–501. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Fan SH, Zheng YL, Lu J, Wu DM, Shan Q, Hu B. 2009b. Troxerutin protects the mouse liver against oxidative stress-mediated injury induced by d-galactose. J Agric Food Chem. 57:7731–7736. [DOI] [PubMed] [Google Scholar]