Abstract
Tribolium castaneum and Rhyzopertha dominica are cosmopolitan, destructive postharvest pests. Although research has investigated how high densities of T. castaneum affect attraction to the aggregation pheromone by conspecifics, research into the behavioral response of both species to food cues after high density exposure has been lacking despite its importance to foraging ecology. Our goal was to manipulate and observe the effects of crowding on the behavioral response of both species to common food and pheromonal stimuli and to determine how the headspace emission patterns from grain differed under increasing densities. Densities of colonies for both species was altered (10–500 adults) on a fixed quantity of food (10 g of flour or whole wheat), then the behavioral response to common food and pheromonal cues was evaluated in a wind tunnel and release-recapture experiment, while volatiles were examined through gas chromatography coupled with mass spectrometry. Importantly, at least for T. castaneum, crowded conditions attenuate attraction to food-based stimuli, but not pheromonal stimuli. Crowding seemed to have no effect on R. dominica attraction to food and pheromonal stimuli in the wind tunnel, but exposure to high density cues did elicit 2.1–3.8-fold higher captures in traps. The relative composition and abundance of headspace volatiles emitted varied significantly with different densities of beetles and was also species-specific. Overall, our results have implications for expanding our understanding of the foraging ecology of two economically important pests.
Keywords: Lesser grain borer, Red flour beetle, Stored products, Semiochemicals, Taxis, Integrated pest management
Subject terms: Ecology, Behavioural ecology
Introduction
Much effort over the last century in ecology has been devoted to studying the effect of population density on populations. Density often negatively affects the growth rate of populations1, while it may modulate other key processes such as, behavior2, optimal foraging3, host-parasitoid interactions4, and insect-mold interactions5. Moreover, density along with trait-mediated indirect interactions, have both been found to contribute to structuring communities, leading to the coexistence of insect species6. As a species approaches its carrying capacity in an ecosystem, there may be several patterns that emerge. This may include an increase in avoidance behavior and decrease in joining behavior by individuals to a group7, differences in optimal foraging behavior8, changes in predation9, modulation of chemosensation10, as well as increasing intra- and interspecific competition11.
Prior to reaching high density situations (or after high density situations), a key challenge faced by individual insects is locating food patches at a distance typically through volatile organic compounds (VOCs). During this process, individuals must assess the quality of a patch, and determine when to leave or how long to stay. In some cases, early arrivals may recruit conspecifics that may arrive en masse, feed, and sometimes mate, as in the case of bark beetles12 and some stink bugs13. The density of conspecifics in the environment may affect volatile emissions by conspecifics14. At high densities, some species may emit anti-aggregation pheromones14 or alarm pheromones15 to disperse individuals. Some male broad-horned flour beetle, Gnatocerus cornutus (F.) (Coleoptera: Tenebrionidae) respond to male-perfumed females or increased presence of rival males by allocating more effort to sperm competition16. In another case, researchers found a density–dependent polyphenism in pathogen resistance to entomopathogenic fungus and immune function in the mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae)17. However, there have been few studies evaluating how an experience in a highly dense environment may then affect subsequent foraging decisions in an environment that is no longer densely populated.
Much work has been done on the foundational movement and dispersal ecology of red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) and lesser grain borer, Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae). This makes them particularly good models to evaluate density-mediated effects under controlled conditions that nonetheless reflect the real-world conditions they may encounter. In addition, T. castaneum has been used as a model species for at least the past five decades18,19 was the first beetle pest to have its genome sequenced20, and is easy to handle and manipulate for experiments21. Each species has significantly different life histories, with T. castaneum playing a role as a secondary pest feeding and reproducing on damaged or processed products22, and R. dominica attacking and ovipositing in intact whole kernels23. The male-produced aggregation pheromone for T. castaneum consists of four stereoisomers of (4S/R, 8S/R)-4,8-dimethyldecanal24 and is attractive to both sexes, while for R. dominica it is comprised of the male-produced stereoisomers, (S)-( +)-1-methylbutyl (E)-2-methyl-2-pentenoate and (S)-( +)-1-methylbutyl (E)-2,4-dimethyl-2-pentenoate, and is also attractive to both sexes25,26. Each species has different movement and dispersal patterns. For example, R. dominica are considered weak walkers but once they reach the adult stage, they are strong dispersers via flight, capable of immigrating between agricultural and non-agricultural landscapes27–30. By contrast, Tribolium castaneum adults are recognized to be strong walkers31, but have weaker dispersal by flight; typically, conspecifics are primarily found in food facilities and adjacent areas32–34. As a result, each species may respond differently to density-mediated pressures.
Prior work has already evaluated some density-mediated effects on the response and life history of T. castaneum. For example, it is known that increased density slowed down the development rate and decreased body weight of T. castaneum35, but did not appear to significantly increase flight initiation or change time to initial flight by conspecifics36. Generally, once a stored product insect finds a new suitable food patch, an aggregation pheromone will be released to attract conspecifics or a sex pheromone will be released by one sex to attract the opposite sex37. However, under high density situations some stored product insects will employ density-mediated stress cues that halt the release of aggregation pheromone and induce conspecifics nearby to disperse38. This may function to avoid competition and increase the survival rate. Density-mediated cues produced by T. castaneum include a variety of methyl- and benzoquinones, which function as anti-aggregation pheromones, causing beetles to rapidly disperse14,39. Prior work has found that density affects the release of these volatiles by T. castaneum, as well as the behavioral response of conspecifics to the presence of the aggregation pheromone14. Natal habitat experience was found to modulate density-dependent dispersal by T. castaneum40, suggesting an interaction between habitat cue perception and density.
There has also been prior work evaluating some density-mediated effects on the response and life history of R. dominica. For example, R. dominica of multiple strains (field and laboratory) reared under crowded conditions had significantly higher flight initiation than conspecifics reared in uncrowded conditions41. Indeed, while R. dominica reared in uncrowded cultures had a low flight response, the number of beetles initiating flight increased with exposure to increased concentrations of frass or uric acid from conspecifics42. In addition, with increasing density, aggregation behavior by R. dominica decreased in a grain bulk containing wheat or maize43. Prior work has shown that reducing the density of males per quantity of food, individual males release more pheromone than controls and are a stronger signal for responders44. Additionally, that same study found the quantity of pheromone emitted per male declined with increasing beetle density and that this effect is stronger in the presence of other males than of females. Thus, while quite a bit is known about how density affects the life history of both T. castaneum and R. dominica, it is unknown how extended experience in a high density environment affects later semiochemical-mediated foraging choices.
One of the key cues used by stored product insects in foraging are food cues from post-harvest commodities (reviewed in 45). For example, prior work has found that host cues from grain are broadly attractive to a range of stored product insects, including T. castaneum and R. dominica45–50. Out of seven plant species, R. dominica was most attracted to wheat, which was found to be most suitable for its development51. However, without presence of pheromone52, response to food cues may sometimes be limited by both T. castaneum and R. dominica. However, how prior experience with high density conditions affects the response of stored product insects to these and other food cues, and thus host-finding, is not well understood but can have significant impacts on populations and pest monitoring programs. Thus, the aim of this study was to elucidate the effects of density (e.g., crowding) on the behavioral response of R. dominica and T. castaneum to common food-based attractants and pheromonal stimuli. In order to accomplish this, the density of individuals for a species was varied between 10 and 500 adults for four weeks, and then their behavioral responses to a variety of food cues were observed. We assessed the behavioral responses of insects using wind tunnel and release-recapture assays. In addition, we also characterized how the volatile emissions patterns from grain with beetles of each species at various densities differed in order to identify potential cues that are used by these species to modulate dispersal and volatile response behaviors. The volatiles from beetles at different densities were characterized using a headspace collection system, and then examined through gas chromatography coupled with mass spectrometry (GC–MS).
Materials and methods
Colony maintenance and experimental insects
Beetles used in this study were obtained from stock colonies kept in the laboratory of the USDA situated in Manhattan, KS. Colonies of T. castaneum (strain from eastern KS, collected in 2012) were reared on 95% organic unbleached flour and 5% brewer’s yeast, while R. dominica (strain from outside a mill in central KS, collected in 2012) colonies were cultivated on organic whole kernel wheat. For both species, colonies were initially subcultured with 2- to 4- wk-old adults to create density treatments below, which were kept in 950-mL mason jars (8.5 D × 17 cm H) and stored in an environmental chamber (136VL, Percival Instruments, Perry, IA, USA) set at constant conditions (27.5 °C, 60% RH, and 14:10 L:D).
Density treatments
To examine the effects of density on the behavioral response of T. castaneum and R. dominica to common food and pheromonal semiochemicals, adults 4- to 8-wk post-emergence of both species were removed from stock colonies described above and reared in subcolonies at different densities. Beetles of 1:1 sex ratio (T. castaneum) or mixed sex (R. dominica) were placed in glass containers (5 cm D × 6.6 cm H) with 20 g of 95% organic flour + 5% brewer’s yeast (T. castaneum) or organic whole kernel wheat (R. dominica). A total of 10, 50, 100, or 500 individuals per container were used for T. castaneum, while 10, 50 or 100 individuals per container were used for R. dominica. Because of limitations with colony populations, 500 individuals per container were not used for R. dominica in the experiments below. The control consisted of food only without beetles, and was the source of uncontaminated grain or flour described below. The beetles of both species were held under each density for 3–4 weeks before use in experiments described below.
Food & pheromonal semiochemicals
In the assays described below, we assessed the response of beetles to six semiochemical treatments that contained food stimuli alone or a combination of pheromonal and food stimuli (Table 1). Stored product beetle tab lures (SPB lures), wheat germ oil (WG), and Trécé Storgard Oil (TSO) was purchased in May 2018. Fresh samples of semiochemicals were used for each day of testing.
Table 1.
List of food and pheromone semiochemical treatments used.
| Abbreviations | Semiochemical treatment | Amount in wind tunnel | Amount in release-recapture | Function | Source | |
|---|---|---|---|---|---|---|
| Ctrl | Control | Empty | Empty | Unbaited control | ||
| WG | Wheat germ oil | 950 μl | 950 μl | Food stimuli | ||
| CF | Contaminated food | 20 g | 6 g | Food stimuli + potential repellents + attractants | Wheat flour or wheat kernels previously exposed to beetles: T. castaneum 500-beetle (flour + yeast) or R. dominica 100-beetle (whole wheat) densities for 3–4 weeks with beetles removed | |
| UCF | Uncontaminated food | 20 g | 6 g | Food stimuli | Analogous commodities to CF without exposure to beetles | |
| TSO | Trécé Storgard oil | 950 μl | 950 μl | Food stimuli | Trece, Inc., Adair, OK, USA | |
| SPB | Stored product beetle tab lure | 1 lure | 1 lure | Food stimuli + pheromone | Insect Limited,Westfield, IN, USA | |
Wind tunnel assay
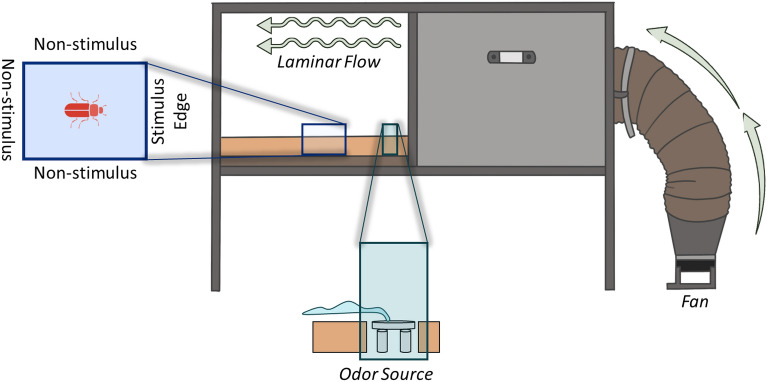
A laminar flow wind tunnel (94.5 × 73 × 80.5 cm L:H:W) was used to assess walking locomotory upwind orientation of T. castaneum and R. dominica that had been held at different densities, to food and pheromonal semiochemicals (Table 1). This is a common quick assay to assess horizontal locomotory response of stored product insects to stimuli53,54. The wind tunnel consisted of an electric fan, which pushed ambient air at 0.39 m/s, first through two metal sieves to straighten airflow, and then an activated carbon filter to purify the air (Fig. 1). The semiochemicals (as described above) were placed 5 cm downwind of the last sieve in a 100 × 15 mm plastic petri dish flush with the surface of the orienting beetle, and 13.5 cm upwind of the release arena. Each release arena consisted of a 21.6 × 27.9 cm piece of paper and was changed between trials to prevent cross-contamination by trace chemical stimuli deposited by beetles or the various treatments. A smoke test confirmed that the air flowing over the Petri dish reached the release arena. In each replicate, a single adult of either T. castaneum or R. dominica was placed in the center of the arena and given 2 min to leave the arena. The specific edge as well as the time required to make a decision was recorded for each individual. Beetles leaving the arena on the stimulus edge (e.g., upwind edge nearest to the attractant) were considered to have a positive response to the attractant, while beetles leaving on one of the other three boundaries (non-stimulus edges) of the arena were considered to have a negative response. If no choice was made within the timeframe, the individual was excluded from the final analysis. Beetles in this assay came from one of the density treatments described above. For both species there were 24 replicate individuals evaluated for each treatment combination (species × density × attractant type).
Figure 1.
Schematic of wind tunnel experimental setup in the laboratory under controlled conditions in a walk-in growth chamber.
Release-recapture assay
A release-recapture experiment was conducted for both T. castaneum and R. dominica using the stimuli listed in Table 1. Assays for T. castaneum were conducted in a 4.8 × 2.1 × 6 m L:H:W environmental chamber (Percival Instruments, Perry, IA, USA) set at constant conditions (27.5 °C, 60% RH, and 14:10 L:D) with a layer of craft paper on the floor to aid movement of beetles. Six commercial pitfall traps (Storgard Dome® Traps, Trécé, Inc., Adair, OK, USA) were each baited with one of the six different stimuli (Table 1). The pitfall traps were positioned around the perimeter of the environmental chamber. Trap position was re-randomized in each subsequent round of deployment to avoid positional effects. For each release, 100 mixed sex T. castaneum from the 100- and 500-beetle densities above as the high density treatments, or directly from stock colony individuals (effective density 18 beetles/jar) as a less crowded treatment, were settled in a piece of cardboard measuring 8 × 8 cm and released at the center of the environmental chamber at the start of the assay. Traps were collected after a period of 24 h and the number of T. castaneum in each trap was recorded.
Based on the lower walking capacity of Rhyzopertha dominica55, we conducted the release-recapture as above except for the following changes. In each replicate, a total of 20 mixed-sex adults reared at colony density (effective density 18 beetles/jar), 50 beetles/jar, or 100 beetles/jar (as above) were settled on corrugated cardboard (8 × 8 cm) and then placed in one corner of a plastic bin (86.3 × 30.5 × 39.4 cm L:H:W). In the opposite corner, diagonally across from the release point in the bin, a pitfall trap (Dome Trap™, Trécé, Inc., Adair, OK, USA) was deployed. The bins were located in a large (4.8 × 2.1 × 6 m, L:W:H) walk-in environmental chamber under constant conditions (as above). The adults were given 24 h to respond to the lures. In a given block by day, two replicates of each treatment listed above were performed. There was a total of n = 8 replicates for each combination of semiochemical treatment, beetle density, and species.
Volatile collection and characterization
Headspace samples were collected to characterize potential density-mediated volatiles produced by T. castaneum and R. dominica infested food (10 g of either whole wheat or flour taken from 0, 50, 100 and 500 densities for T. castaneum or 0, 10, 50 and 100 densities for R. dominica). Negative controls consisted of empty headspace chambers, and were used to identify and eliminate from analysis any background volatiles in the sampling room. Central air was pushed through an activated charcoal filter to purify the air, and then the flow rate was calibrated with a flow meter (Volatile Collection Systems, Gainsville, FL, USA) at 1 L per min. Four headspace volatile chambers (10.2 × 12.7 cm D:H) were attached to the headspace collection apparatus using Polytetrafluoroethylene (PTFE), chemically inert tubes (5 mm ID). Prior to headspace collection, all T. castaneum life stages were sieved out of the flour with first a No. 25 (710 μm) then a No. 70 (212 μm) mesh sieve. For R. dominica, all adults were removed from the wheat kernels using a No. 10 (2 mm) mesh sieve. The headspace volatiles were collected over a 3 h period using volatile collection traps (VCT; Volatile Collections Systems, Gainsville, FL, USA). Each VCT consisted of a borosilicate glass tube tapered at one end with a stainless-steel screen, followed by 20 mg of Porapak Q™ adsorbent material to trap volatiles, and held in place with borosilicate glass wool and a PTFE compression seal. The headspace volatiles were extracted from the VCTs using 300 or 150 µl of dichloromethane, with the solvent pushed through by inert N2 gas. After extraction, 1 μl of tetradecane (190.5 ng at 99% purity) was added as an internal standard for quantification in each of the labeled vials using a microsyringe (7000 Series Modified Microliter™ Syringe, Hamilton, Reno, NV, USA). Between uses all VCTs were washed with 700 μl of dichloromethane in triplicate. All volatile collection vials were sealed with Teflon tape and stored at − 4 °C until GC–MS analysis. There was a total of n = 4–8 replicates per density and species of beetle.
Gas chromatography coupled with mass spectrometry
All headspace sample extracts were run on an Agilent 7890B gas chromatograph (GC) equipped with an Agilent Durabond HP-5 column (30 m length, 0.250 mm diameter and 0.25 μm film thickness) with He as the carrier gas at a constant 5 mL/min flow and 39 cm/s velocity, which was coupled with an Agilent 5997B mass spectrometer (MS) single-quadrupole detector. The compounds were injected with 1 μL of each sample under splitless mode into the machine. The program began at 35 °C for 1 min followed by 10 °C/min ramping to 300 °C over 26.5 min, and subsequently held for 4 min at 300 °C. After a solvent delay of 3 min, mass ranges between 50 and 550 atomic mass units were scanned. Preliminary assignment of compounds was obtained by comparing sample spectral data with the NIST 14 library through deconvolution. However, the primary goal was evaluating relative differences in emissions among treatments.
Statistical analysis
For statistical procedures, R Software was used56 and α = 0.05, unless otherwise noted. The glm function from the base R Software, Anova function from the car package, and glht function from the multcomp package were used for the univariate analyses.
To analyze the data from the wind tunnel assay, a generalized linear model based on a binomial distribution was used for each species. The response variable was the edge of the arena on which the beetles exited (stimulus or non-stimulus edge). The two fixed explanatory variables were the semiochemical treatments (TSO, WG, SPB, contaminated food [CF], uncontaminted food [UCF], and Ctrl) and beetle density (10, 50, 100, or 500 beetles), as well as their interaction. Overdispersion was evaluated and found not to be an issue with the model. Log-likelihood tests based on a χ2-distribution were used to calculate significance, and upon a significant result from the model, pairwise χ2-tests were used with a Bonferroni correction.
To evaluate whether the trap capture of beetles from different densities was affected by the semiochemical treatments, we analyzed the release-recapture assay for each beetle species using a 2-way ANOVA. Because T. castaneum exhibit an exponential decay in responding to semiochemicals by distance, with less than 40% making it to a food patch 16 cm away with airflow present, traps in the release-recapture assay were effectively independent from each other. The total number of adults recaptured by the trap was used as the response variable, while the beetle density (colony-reared, 100-, or 500-beetle density) and the semiochemical treatments (TSO, WG, SPB, CF, UCF, and Ctrl) were employed as fixed, explanatory variables, as well as their interaction. The data conformed to the assumptions of normality and homogeneity of variance, and thus no transformation was required. Upon a significant result from the model, Tukey HSD was used for multiple comparisons among the treatments.
To characterize the presence of headspace volatiles from the extracts, raw peak areas were extracted from the gas chromatograms using Unknowns Analysis (Quantitative Analysis Software, v7.1.524.1, Agilent Technologies, Inc., Santa Clara, CA, USA). Samples were analyzed based on deconvolution of spectra, and only those peaks were included that were at least 0.7% of the height of the tallest peak in order to exclude background noise. A single CSV file with the best hit for each compound and peak area was outputted to a CSV, then analyzed in R Software using the package uafR and a new automated protocol to streamline aligning peaks and removed contamination (Stratton et al. 2022). After alignment, background volatiles found in the negative control without grain were discarded from the other samples, since these represent transient background volatiles in the general vicinity of headspace collection but are not informative of differences among the treatments. Pairwise Bray–Curtis dissimilarities were calculated among all headspace samples, and non-metric multi-dimensional scaling (NMDS) was used to visualize the differences in volatile emissions among treatments. A total of n = 1000 permutations were used for the ordination procedure. Stress values for the NMDS procedure were < 0.13, indicating that good interpretation was possible. An analysis of similarity (ANOSIM) was used to determine significant differences for headspace volatiles among levels within density (0, 10, 50, 100, 500) and species (T. castaneum or R. dominica). A total of n = 1,000 permutations were performed for the test. For all multivariate statistics, the R Package vegan57 and ecodist58 was used. The mean volatile emissions for each compound in a treatment were analyzed with a generalized linear model based on a quasi-Poisson distribution to account for overdispersion in the dataset using a call to the function glm in R. Explanatory variables included density and species. A likelihood ration test based on a chi-square distribution was performed, with α = 0.05. Multiple comparisons employed Tukey HSD using the function glht from the multcomp package59.
Results
Wind tunnel assay
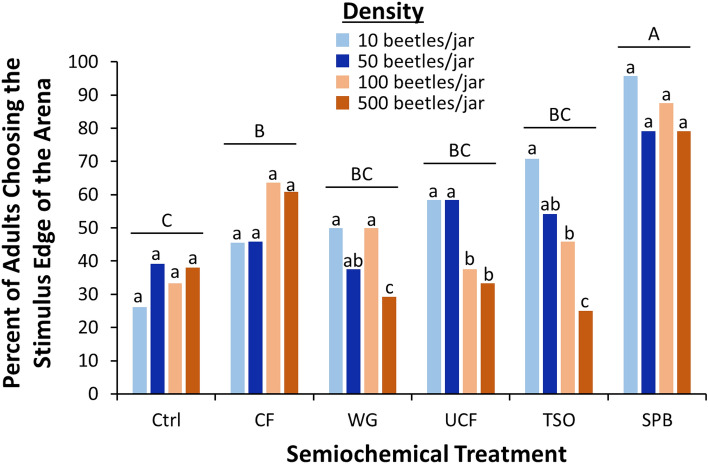
Overall, across treatments only 1.9% of T. castaneum did not respond. There were the most nonresponders for the Ctrl (5%) and the CF (5%). There were no nonresponders for any of the other treatments. For T. castaneum, density had a significant effect on the number of conspecifics responding positively to stimulus in the wind tunnel assay (χ2 = 5.70; df = 3; P < 0.05; Fig. 2). There was a 13% decreased positive response overall of adults reared at 500-beetle densities compared to those reared at 10-beetle densities. The semiochemical treatment also significantly affected the number of T. castaneum adults choosing the stimulus edge (χ2 = 65.5; df = 5; P < 0.0001; Fig. 2). There were 2.5 times more adults leaving on the stimulus edge for the SPB tab compared with the unbaited control, and CF also had a significantly greater positive response compared to the control but less strong than the response to the SPB. All remaining treatments were not significantly different from the controls. The interaction between density and semiochemical treatment was significant (χ2 = 10.9; df = 5; P < 0.05). Importantly, T. castaneum attraction to WG, UCF, and TSO (e.g., food semiochemicals) were suppressed by 2–threefold for beetles reared at the 500-beetle density compared to the 10-beetle density, while attraction to the contaminated food (CF) and food and pheromone lure (SPB) remained unchanged over the densities (Fig. 2, pairwise χ2-tests).
Figure 2.
Total percent of T. castaneum reared at different densities exiting the release arena in a wind tunnel assay on the upwind stimulus edge for various food and pheromone attractants. Upper case letters represent pairwise comparisons among semiochemical treatments across beetle densities, while lower case letters represent pairwise comparisons among beetle densities within a semiochemical treatment. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction). Ctrl unbaited control, CF contaminated food from 500 beetle density, WG wheat germ oil, UCF uncontaminated food with no beetles, TSO Trece Storgard Oil, and SPB Stored Product Beetle Pheromone Tab from Insects Limited.
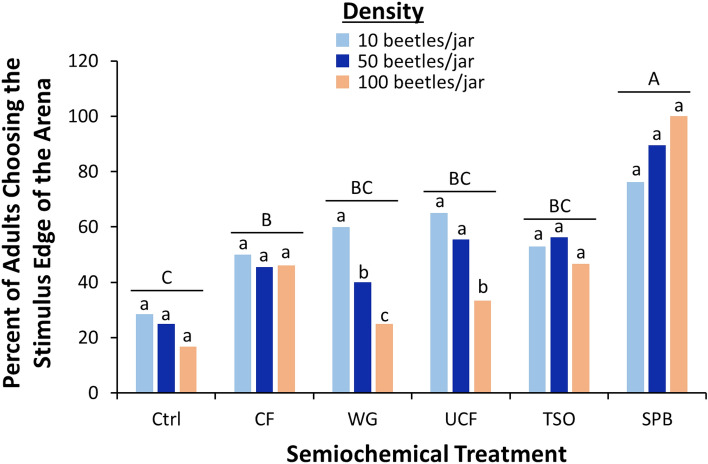
Overall, across treatments a total of 33% of R. dominica did not respond, which is typical for this species. There were the least number of nonresponders for the SPB lure (20%) and the most for the CF (47%). Generally, the density at which R. dominica were reared did not affect the number leaving on the stimulus edge of the arena (χ2 = 0.36; df = 3; P = 0.55). However, the semiochemical treatment significantly affected attraction of R. dominica (χ2 = 48.0; df = 5; P < 0.0001), with 3 times more adults exiting towards the SPB lure than the unbaited control (Fig. 3). The contaminated food was also significantly more attractive than the control, but significantly less than SPB, with all other treatments not being different from the control response. Finally, the interaction between the two variables was not significant (χ2 = 4.03; df = 15; P = 0.55).
Figure 3.
The total percent of R. dominica exiting the release arena in a wind tunnel assay on the upwind stimulus edge for various food and pheromone attractants. Upper case letters represent pairwise comparisons among semiochemical treatments across beetle densities, while lower case letters represent pairwise comparisons among beetle densities within a semiochemical treatment. Bars with shared letters are not significantly different from each other (χ2-test, Bonferroni correction). Bars with letters omitted are where within semiochemical treatments responses did not differ among beetle densities. Ctrl unbaited control, CF contaminated food from 500 beetle density, WG wheat germ oil, UCF uncontaminated food with no beetles, TSO Trece Storgard Oil, and SPB Stored Product Beetle Pheromone Tab from Insects Limited.
Release-recapture assay
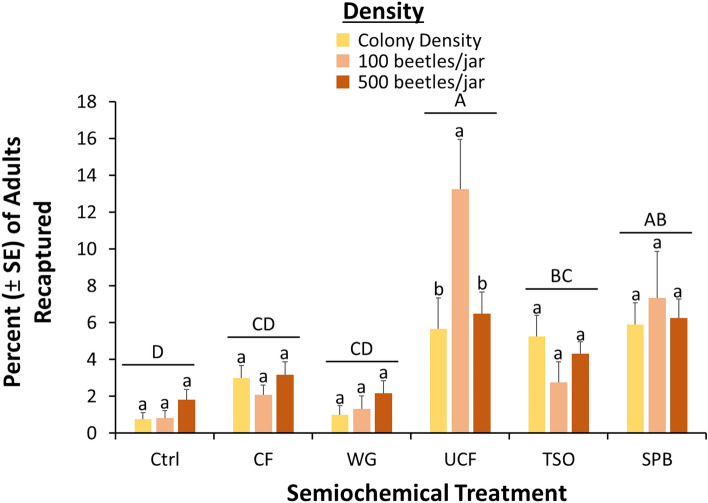
We recaptured 46% of the T. castaneum beetles that were released, suggesting that valid interpretation is possible. Density did not significantly affect the capture of T. castaneum adults in the overall model (χ2 = 1.50; df = 2; P = 0.47; Fig. 4), but for UCF the intermediate density had significantly greater captures than the colony density or the 500 beetles/jar density. Average captures ranged from 3.5 and 4.2 beetles per trap when reared at stock colony levels and the 100 beetles/jar density, respectively. However, the semiochemical treatments in traps significantly altered the capture of adults (χ2 = 118; df = 6; P < 0.0001). In particular, traps with the SPB tab and the uncontaminated food (UCF) captured 5.7 and 7.4 times more adults, respectively, compared to unbaited control traps. In addition, the interaction between density and the semiochemical treatment was significant (χ2 = 21.5; df = 12; P < 0.05), with greater recapture of adults reared at 100 adults/jar in traps baited with uncontaminated food compared to the other densities, but no significant differences among densities for traps baited with other stimuli.
Figure 4.
Percent (± SE) of T. castaneum adults reared at different densities and recaptured in pitfall traps baited with different semiochemical treatments under constant 27.5ºC and 60% RH after 24 h. A total of N = 8 replicates for each treatment combination. Upper case letters represent comparisons among semiochemical treatments across densities, while lower case letters represent comparisons within a semiochemical treatment among densities. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05). Ctrl unbaited control, CF contaminated food from 500 beetle density, UCF uncontaminated food with no beetles, TSO Trece Storgard Oil, and SPB Insects Limited SPB lure.
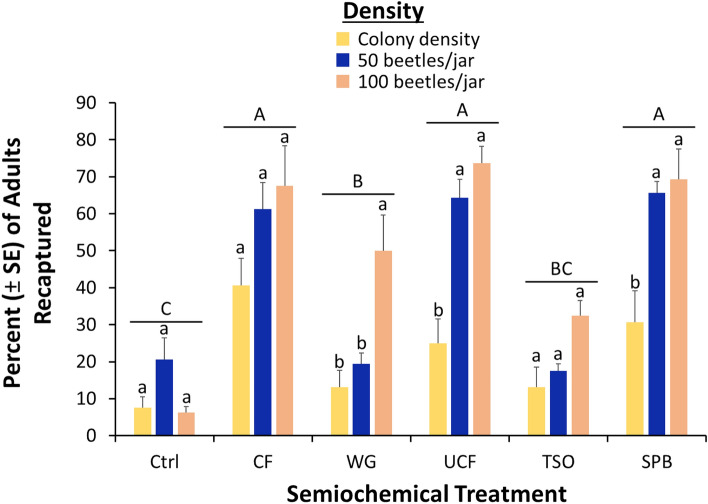
Similar to T. castaneum, we recaptured 38% of the R. dominica that were released, suggesting valid interpretation of the data was possible. Overall, density significantly affected the recapture of R. dominica in traps (χ2 = 64.1; df = 2; P < 0.0001; Fig. 5), with only about half as many adults captured in traps with colony densities compared to 50 and 100 adults/jar. The semiochemical treatment significantly affected recapture of R. dominica as well (χ2 = 157 df = 6; P < 0.0001), with traps baited with CF, SPB, or UCF capturing 4.7–4.9-fold more adults than unbaited controls. There was a significant interaction between density and semiochemical treatment interaction on trap captures of R. dominica (χ2 = 24.9, df = 12; P < 0.01), with 50 and 100 densities showing 2.1–3.8-fold higher increased response to the SPB lure, UCF, and WG relative to the lower colony density. Density did not affect response to the other semiochemical treatments.
Figure 5.
Percent (± SE) of R. dominica adults reared at different densities and recaptured in pitfall traps baited with different semiochemical treatments under constant 27.5ºC and 60% RH after 24 h. A total of N = 8 replicates for each treatment combination. Upper case letters represent comparisons among semiochemical treatments across densities, while lower case letters represent comparisons within a semiochemical treatment among densities. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05). Colony density was effectively 18 beetles/jar. Ctrl unbaited control, CF contaminated food from 100 beetle density, UCF uncontaminated food with no beetles, TSO Tréce Storgard oil, and SPB Insects Limited SPB lure.
Volatile characterization
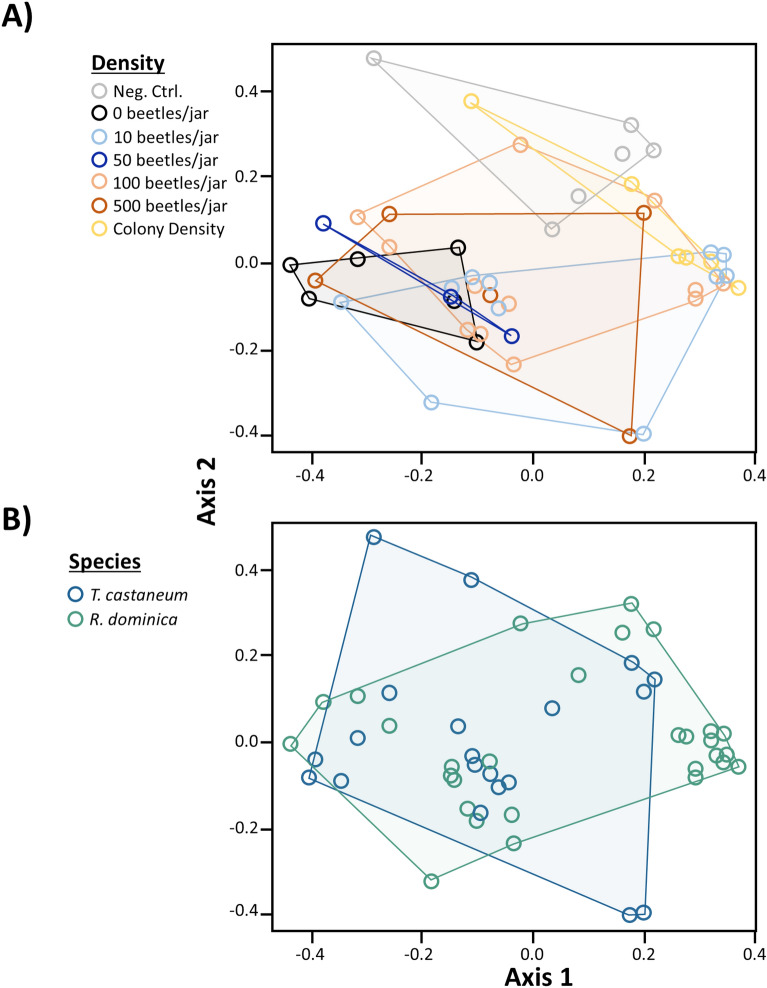
In total, we recorded the presence of 55 chemical compounds from whole grain or flour samples taken from containers containing different densities of T. castanuem and R. dominica (Table 2). Volatile emissions from the different densities of R. dominica contained 5–24 fewer compounds on average, compared to the treatment with wheat only (Table 2). Additionally, we found that as the density increased, so too did the abundance of most of the compounds. Importantly, there were significant density-dependent differences in the volatile composition of headspace emitted from the grain by R. dominica and T. castaneum (ANOSIM: R = 0.131, P < 0.01; Fig. 6A). The compounds most associated with the high density treatments of R. dominica (e.g., 50 and 100) were dodecanal, 2-pentyl ester cyclopentanecarboxylic acid, and 4-methylpentyl ester-2-thiophenecarboxylic acid (Table 2). By contrast, toluene, propyne, and heptadecane, were present in all densities of T. castaneum, but highest in the 100 beetle density. In addition, the 100 beetle density of T. castaneum was enriched in more compounds such as octane, dodecane, and nonanal, compared to the other densities. Moreover, there were significant species-specific differences in the emissions of volatiles (ANOSIM: R = 0.085, P < 0.02; Fig. 6B). Toluene, propyne, heptadecane, and (Z,Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene were most characteristic of T. castaneum, while dodecanal, 2-pentyl ester cyclopentanecarboxylic acid, and 4-methylpentyl ester-2-thiophenecarboxylic acid, and nonadecane were most dominant in R. dominica. Total volatile emissions were significantly affected by density (χ2 = 21.0; df = 5; P < 0.001; Fig. 7). There were 3-, 12-, 11-, and 11-fold higher volatile emissions in colonies with 10, 50, 100 beetles, and colony density compared to no beetles (Fig. 7). The species also significantly affected the total emissions from grain (χ2 = 18.8; df = 1; P < 0.0001; Fig. 7), but not its interaction with density (χ2 = 0.801; df = 3; P = 0.85). In particular, Rhyzopertha dominica colonies had about eightfold higher emissions than T. castaneum colonies.
Figure 6.
Ordination (non-metric multidimensional scaling plot) of density emissions from wheat based on Bray–Curtis indices calculated in a pairwise fashion among R. dominica and T. castaneum A) reared at different densities (0—black or grey, 10—dark blue, 50—light blue, 100—medium blue, 500—green, colony density—purple) after 3–4 weeks, and B) differences in volatiles by species-specific emissions with R. dominica in green and T. castaneum in blue. Stress was less than 0.07, suggesting valid interpretation is possible. There were a total of n = 5–12 replicates per density, and n = 20–29 replicates per species.
Figure 7.
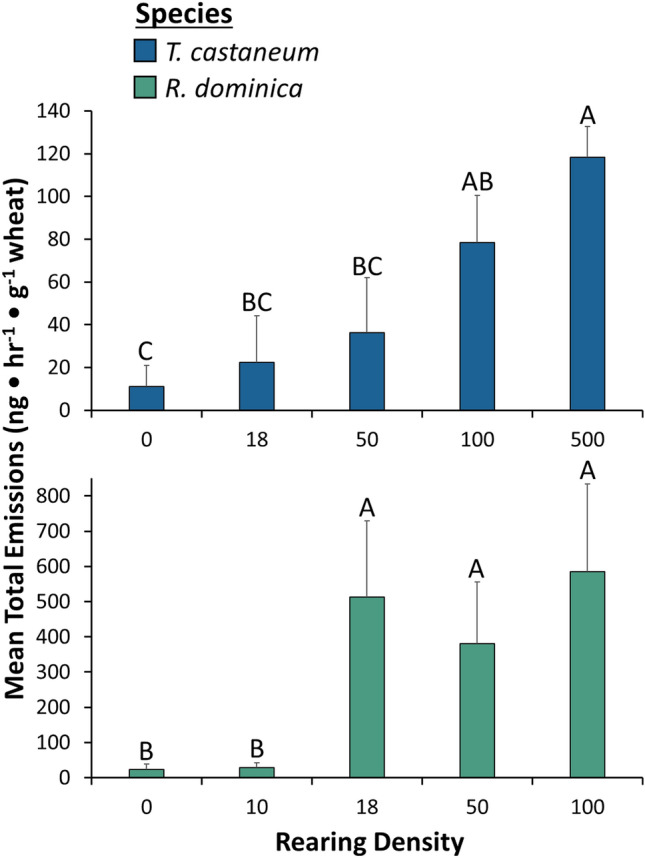
Mean total volatile density emissions by T. castaneum (top panel) and R. dominica (bottom panel) after 4 weeks based on density. Bars with shared letters are not significantly different from each other (Tukey HSD, α = 0.05). To be included, compounds were required to be at least 0.7% the height of the major peak in the sample.
Discussion
In this study, we examined the behavioral response of the stored product pests, T. castaneum and R. dominica, to food and pheromonal cues after exposure to varying densities. Tribolium castaneum held at certain higher population densities were less likely to respond positively to food cues in comparison to those reared at lower densities when pheromone was absent. For example, T. castaneum conspecific responses to contaminated flour or the lure that had food and pheromone was not changed. In fact, we found that the contaminated flour contained the pheromone from T. castaneum, supporting this point. This suggests that response to pheromones remained unchanged. By contrast, R. dominica reared at higher densities showed higher sensitivity to food and pheromone cues, exhibiting increased response compared to lower densities in the release-recapture study, though not in the wind tunnel. Interestingly, some unique compounds were emitted from high density treatments by each species. For example, nonadecane and pentanoic acid ester were unique to R. dominica, while 1-tridecene and (1E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol were unique to T. castaneum. Overall, there seemed to be species-specific responses to high density. Headspace from T. castaneum was dominated by toluene, propyne, heptadecane, and (Z,Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene, while dodecanal, 2-pentyl ester cyclopentanecarboxylic acid, and 4-methylpentyl ester-2-thiophenecarboxylic acid, and nonadecane comprised R. dominica headspace.
Here, we have considered how exposure to density-mediated cues affects stored product beetle behavior. These density-mediated cues may have included volatiles from oviposited eggs, excreted frass, deposited trace cuticular hydrocarbons and glandular secretions from tunneling by adults, and a food source imbued with a variety of volatile compounds, including aggregation pheromones and stress-related compounds such as methyl- and benzoquinones in the case of T. castaneum14,60. For the volatile collection treatments in our study, we used increasingly small sieves to remove beetles, larvae, and eggs. However, frass and residual volatiles in the grain (including density-related compounds) likely remained. For example, 1-tridecene has previously been collected from headspace of T. castaneum, and assumed to be a sex pheromone based on homology to the same compound from another tenebrionid61. There were also trace amounts of 1-pentadecene, which has been described as a larval frass volatile from Tribolium spp62. Thus, when we observed behavioral effects or volatile differences among different densities of beetles, we are primarily describing differential responses to these density-mediated cues. Prior to this research, the effect of density on response to conspecific aggregation pheromones for T. castaneum, but not food cues, was investigated14. In that study, researchers found that T. castaneum reared at 50 or 250 adults on 10 g of flour chose a clean grain volatile source 10- to 6-times more often than a grain volatile source from 1250 adult T. castaneum.
In the current study, we also found that T. castaneum was the most susceptible to modulation of its foraging behavior by density in the wind tunnel, but not in the release-recapture experiment. By contrast to T. castaneum, we observed that R. dominica was more affected in the release-recapture experiment than the wind tunnel assay to density-mediated cues at least under the levels evaluated here. It may be that such a pattern arises as a result of the difference in the life histories of our two species. Since R. dominica is a primary pest, it is less likely to be exposed to crowded conditions in environments that consist of many tonnes of commodities being stored for variable, sometimes short periods of time. In fact, we observed that R. dominica from high density rearings was captured more often in traps baited with cues that may also have pheromone. Over the range of densities tested, the total emissions by R. dominica plateaus by 50 individuals/jar, and remains steady at 100 individuals/jar and for the colony density (see Fig. 6). Interestingly, T. castaneum appeared somewhat more attracted to grain when contaminated by conspecifics compared to the negative control, though this effect was moderate, because their response to clean grain was not significantly different from the negative control or contaminated food. Indeed, we found trace amounts of pheromone in clean grain from headspace, which may explain why no differences were observed in our study. As an alternate hypothesis, it may be possible that the behavioral response of R. dominica to food semiochemicals was not affected, because they may only be responsive to host volatiles during foraging. For example, R. dominica are recognized as weak walkers and may locate food sources by chance when they are walking. Cordeiro et al.63 showed that R. dominica foraging in wheat tended to revisit the same areas where they had previously fed. In addition, those authors found there was evidence that R. dominica use cues associated with their feeding while in a grain mass. Finally, that study also showed incorporating fine materials from high infestation level grain by conspecifics at different ratios affected movement by R. dominica in wheat. By contrast, some studies found R. dominica may only respond to semiochemical cues when locating areas to land during flight from a distance, because of their strong dispersal flight capacity30. Future studies should employ a tethered flight mill64–67 to determine whether flight capacity in the presence of food cues by R. dominica is affected by density. Additionally, the conditions that trigger dispersal and the specific volatiles they may be using for orientation as they find routes to infest facilities are all open questions that should be addressed.
Both T. castaneum and R. dominica have evolved a complex system of glandular and volatile emissions, as well as odor-reception for dealing with crowded situations10,68. In support of this, we found the density volatile bouquets for both T. castaneum and R. dominica, were species-specific, and vary with the density of beetles. In particular, we found increasing total emissions of volatiles compared with the control with increasing density for both T. castaneum and R. dominica. Generally, we found that as beetle density increased, the diversity of compounds decreased for R. dominica but not T. castaneum. It is likely that increased densities of insects supported more microbial contamination, which may also be involved in emissions42. In fact, we observed octane, 2-methyl-1-butanol, and hexanal enriched at the high density treatments of the species, which are all considered to be microbial volatiles69.
In prior work, the amount of volatiles produced by T. castaneum adults increased with increasing insect density, including for methyl-1,4-benzoquinone, ethyl-1,4-benzoquinone, and 1-tridecene61,70. For example, Duehl et al.14 tested a range of T. castaneum densities from near zero to over 1200, and found pheromone production (4,8-dimethyldecanal) peaked at 500 conspecifics, which produced about 200 ng/h, and methyl and ethyl 1,4-benzoquinone,which can act as repellents, peaked above 1200 individuals, producing about 600 ng/h. In contrast to our study, T. castaneum from this previous was found not prefer cues from high densities cultures of 250–1250 conspecifics when reared at 50–1250 individuals per 10 g flour14. However, we reared beetles on double the amount of flour (20 g) as prior work (10 g), leading to absolute beetle densities that were effectively halved by comparison. This may mean that there was a lower concentration of density cues that would likely act as repellents (e.g., benzoquinones) in the current study than in the prior one, which may be why repellency was not observed in our study.
Both R. dominica and T. castaneum produce pheromone when feeding on a high quality food source. Rhyzopertha dominica is strongly attracted to infested wheat when its pheromone is present, regardless of other factors. or volatiles71. In our study, R. dominca were still attracted to wheat even when high levels of volatiles associated with feces were present. For example, fenchone was associated with R. dominica-infested wheat, while 1-tetradecanol and methyl-decanoate were associated with R. dominica feces. However, response to infested grain in high density situations seems to have a disproportionate effect on the foraging of T. castaneum. Generally, T. castaneum or most species with an aggregation pheromone may be expected to exhibit a positive response to the food resource and increase up to a certain density, then subsequently to show decreased response as density continues to increase. In addition, in prior work, researchers found that the production of 4,8-dimethyldecanal was weakly correlated with the production of methyl and ethyl benzoquinones16. In our study, we found that the behavioral response by T. castaneum to food cues decreased after exposure to high densities, but notable exceptions were where pheromone or both pheromone and food were present. It is possible that exposure to repellents (e.g., quinones) from the high-density treatment could be responsible for the lower response to food in beetles from the high density treatment, but perhaps the pheromone can override that behavioral response. This latter aspect merits further research to disentangle specific mechanisms, identify if there is an eliciting compound for this behavior, and whether it can be used for behaviorally-based management of T. castaneum. In addition to behavioral mechanisms, there may be physiological processes at play in exposure to different densities, which may cause cascades internally in metabolites and gene regulation. Future work should evaluate how density may alter the homeostasis of these internal mechanisms. Finally, future work should explore 1) whether there is a robust response to the compounds by beetles via gas chromatography coupled with electroantennographic detection (GC-EAD), and 2) for promising volatiles identified from GC-EAD, culture the beetles with these compounds to assess whether the compounds affect beetles’ behavioral responses to food cues.
While the current study was primarily focused on the effect of intraspecific density cues on conspecific response to food and pheromone cues, it may be worthwhile to determine whether density cues from R. dominica, for instance, affect the behavioral response of T. castaneum and vice versa, and whether they can in fact perceive them. Under typical grain storage conditions, multiple species will often be present in a single area, composing a unique ecological community adapted to utilizing anthropogenic granaries. In the vast majority of cases, there will be multiple primary and secondary pests attacking grain at a food facility72,73, and it may be worthwhile to understand how crowding cues from one species affects another species. For example, perhaps the density-mediated cues from T. castaneum have a similarly downregulating effect on other species, and thus may be of broader interest than if they were specific only to T. castaneum.
Currently, behaviorally-based management approaches are being developed for stored product insects74. For the first time, we have shown that crowding may affect the attractiveness of food cues to T. castaneum, and thus may affect the beetles’ ability to locate new host food patches. However, when common commercial lures were deployed in traps, they did not affect capture of T. castaneum or R. dominica. Thus, food facilities experiencing different densities of infestation may experience relatively stable trap performance in long-term monitoring programs. Nonetheless, there are some worthwhile follow-up studies to be performed at the intersection of behaviorally-based management and how density affects performance. For example, it would be useful to understand whether exposure to high concentrations of benzoquinones or other isolated density cues impact orientation to traps. The stimuli in this study may be most useful as a repellent, but in high concentrations it would be interesting to understand if it overrides orientation to pheromone with behaviorally-based tools. Follow-up studies could then assess whether briefer bouts of exposure result in the same behavioral changes to pheromones. Overall, this study presents an interesting step forward in understanding the basic ecological relationships between stored product insects, density, and the repercussions that this has on host-finding behavior. Future work will be well-positioned to further elucidate these relationships and determine whether any of the volatiles tentatively identified in this study may be useful from an integrated pest management perspective.
Supplementary Information
Acknowledgements
We would like to thank the excellent technical assistance of all members of the Morrison Lab. This work was funded in part by a United States Department of Agriculture, National Institute of Food and Agriculture, Crop Protection and Pest Management Grant (#2020-70006-33000). This project was also supported by the USDA Agricultural Research Service through Congress-appropriated funds. M.P. was supported through an NSF Graduate Research Fellowship #2019287083. The use of trade names is for the purposes of providing scientific information only and does not constitute endorsement by the United States Department of Agriculture. The USDA is an equal opportunity employer.
Author contributions
WRM and MAP conceived the experiment. MAP collected the data. AB helped with methodology. MAP conducted the analysis, and wrote up the initial draft. WRM produced the figures and provided feedback. All authors including SRM , TVW, and JFC reviewed and edited manuscript for final approval.
Data availability
Ponce, Marco A.; Ranabhat, Sabita; Bruce, Alexander; Van Winkle, Taylor; Campbell, James F.; Morrison, William R. (2024). Data from: Density-mediated emissions by Rhyzopertha dominica (Coleoptera: Bostrichidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) modulates foraging by conspecifics. Ag Data Commons. Dataset. 10.15482/USDA.ADC/24851604.v1. Accessed 2024-05-21.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-62277-8.
References
- 1.Getz WM. A hypothesis regarding the abruptness of density dependence and the growth rate of populations. Ecology. 1996;77:2014–2026. doi: 10.2307/2265697. [DOI] [Google Scholar]
- 2.Lauer MJ, James AS, Krupa J. Male density, female density and inter-sexual conflict in a stream-dwelling insect. Anim. Behav. 1996;52:929–939. doi: 10.1006/anbe.1996.0241. [DOI] [Google Scholar]
- 3.Gunton RM, Pöyry J. Scale-specific spatial density dependence in parasitoids: A multi-factor meta-analysis. Funct. Ecol. 2016;30:1501–1510. doi: 10.1111/1365-2435.12627. [DOI] [Google Scholar]
- 4.Thierry M, Hrček J, Lewis OT. Mechanisms structuring host–parasitoid networks in a global warming context: A review. Ecol. Entomol. 2019;44:581–592. doi: 10.1111/een.12750. [DOI] [Google Scholar]
- 5.Rohlfs M. Density-dependent insect-mold interactions: Effects on fungal growth and spore production. Mycologia. 2005;97:996–1001. doi: 10.1080/15572536.2006.11832749. [DOI] [PubMed] [Google Scholar]
- 6.Van Veen FJF, van Holland PD, Godfray HCJ. Stable coexistence in insect communities due to density- and trait-mediated indirect effects. Ecology. 2005;86:3182–3189. doi: 10.1890/04-1590. [DOI] [Google Scholar]
- 7.Prokopy RJ, Roitberg BD. Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol. 2001;46:631–665. doi: 10.1146/annurev.ento.46.1.631. [DOI] [PubMed] [Google Scholar]
- 8.Morris DW, Mukherjee S. Is density-dependent resource harvest a reliable habitat indicator for conservation and management? Israel J. Ecol. Evol. 2007;53:371–387. doi: 10.1560/IJEE.53.3.371. [DOI] [Google Scholar]
- 9.Krivan V, Sikder A. Optimal foraging and predator prey. Theor. Popul. Biol. 1999;126:111–126. doi: 10.1006/tpbi.1998.1399. [DOI] [PubMed] [Google Scholar]
- 10.Maille J, et al. Exploiting chemosensory genomics for improved monitoring and control of stored product pests. Insects. 2024;12:391. [Google Scholar]
- 11.Quellhorst H, Athanassiou CG, Bruce A, Scully ED, Morrison WR. Temperature-mediated competition between the invasive larger grain borer (Coleoptera: Bostrichidae) and the cosmopolitan maize weevil (Coleoptera: Curculionidae) Environ.Entomol. 2020;49:255–264. doi: 10.1093/ee/nvz151. [DOI] [PubMed] [Google Scholar]
- 12.Blomquist GJ, et al. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010;40:699–712. doi: 10.1016/j.ibmb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Morrison WR, Lee DH, Short BD, Khrimian A, Leskey TC. Establishing the behavioral basis for an attract-and-kill strategy to manage the invasive Halyomorpha halys in apple orchards. J. Pest Sci. 2016;89:81–96. doi: 10.1007/s10340-015-0679-6. [DOI] [Google Scholar]
- 14.Duehl AJ, Arbogast RT, Teal PEA. Density-related volatile emissions and responses in the red flour beetle, Tribolium castaneum. J. Chem. Ecol. 2011;37:525–532. doi: 10.1007/s10886-011-9942-3. [DOI] [PubMed] [Google Scholar]
- 15.Vandermoten S, Mescher MC, Francis F, Haubruge E, Verheggen FJ. Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions. Insect Biochem. Mol. Biol. 2012;42:155–163. doi: 10.1016/j.ibmb.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Lane SM, et al. Rival male chemical cues evoke changes in male pre- and post-copulatory investment in a flour beetle. Behav. Ecol. 2015;26:1021–1029. doi: 10.1093/beheco/arv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes AI, Siva-Jothy MT. Density–dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): Cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B. 2000;267:177–182. doi: 10.1098/rspb.2000.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell JF, Athanassiou CG, Hagstrum DW, Zhu KY. Tribolium castaneum: A model insect for fundamental and applied research. Annu. Rev. Entomol. 2022;67:347–365. doi: 10.1146/annurev-ento-080921-075157. [DOI] [PubMed] [Google Scholar]
- 19.Brown SJ, et al. The red flour beetle, Tribolium castaneum (Coleoptera): A model for studies of development and pest biology. Cold Spring Harb. Protoc. 2009;4:1–10. doi: 10.1101/pdb.emo126. [DOI] [PubMed] [Google Scholar]
- 20.T. G. S. Consortium The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–955. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- 21.Yamada, Y. Tribolium as a biological model in quantitative genetics. in 1st World Congress on Genetics Applied to Livestock (1974).
- 22.Hagstrum DW, Subramanyam B. Fundamentals of Stored-Product Entomology. AACC International; 2006. [Google Scholar]
- 23.Morrison WR, Lanba A, Hall B, Bruce A. Novel implementation of laser ablation tomography as an alternative technique to assess grain quality and internal insect development in stored products. J. Stor. Prod. Res. 2020;86:101552. doi: 10.1016/j.jspr.2019.101552. [DOI] [Google Scholar]
- 24.Suzuki T. 4, 8-dimethyldecanal: The aggregation pheromone of the flour beetles, Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae) Agric. Biol. Chem. 1980;44:2519–2520. [Google Scholar]
- 25.Khorramshahi A, Burkholder WE. Behavior of the lesser grain borer Rhyzopertha dominica (Coleoptera: Bostrichidae)—Male-produced aggregation pheromone attracts both sexes. J. Chem. Ecol. 1981;7:33–38. doi: 10.1007/BF00988633. [DOI] [PubMed] [Google Scholar]
- 26.Williams HJ, Silverstein RM, Burkholder WE, Khorramshahi A. Dominicalure 1 and 2: Components of aggregation pheromone from male lesser grain borer Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) J. Chem. Ecol. 1981;7:759–780. doi: 10.1007/BF00990308. [DOI] [PubMed] [Google Scholar]
- 27.Gates MW. Population Dynamics of Lesser Grain Borer, Rusty Grain Beetle, and Cephalonomia waterstoni in Commercial Elevators. Oklahoma State University; 1995. [Google Scholar]
- 28.Edde PA, Phillips TW, Toews MD. Responses of Rhyzopertha dominica (Coleoptera: Bostrichidae) to its aggregation pheromones as influenced by trap design, trap height, and habitat. Environ. Entomol. 2005;34:1549–1557. doi: 10.1603/0046-225X-34.6.1549. [DOI] [Google Scholar]
- 29.Mahroof RM, Phillips TW. Stable isotopes as markers to investigate host use by Rhyzopertha dominica. Entomologia Experimentalis et Applicata. 2007;125:205–213. doi: 10.1111/j.1570-7458.2007.00618.x. [DOI] [Google Scholar]
- 30.Mahroof RM, Edde PA, Robertson B, Puckette JA, Phillips TW. Dispersal of Rhyzopertha dominica (Coleoptera: Bostrichidae) in different habitats. Environ. Entomol. 2010;39:930–938. doi: 10.1603/EN09243. [DOI] [PubMed] [Google Scholar]
- 31.Morrison WR, Larson NL, Brabec D, Zhang A. Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored product insects. J. Econ. Entomol. 2019;112:2458–2468. doi: 10.1093/jee/toz179. [DOI] [PubMed] [Google Scholar]
- 32.Ridley AW, et al. The spatiotemporal dynamics of Tribolium castaneum (Herbst): Adult flight and gene flow. Mol. Ecol. 2011;20:1635–1646. doi: 10.1111/j.1365-294X.2011.05049.x. [DOI] [PubMed] [Google Scholar]
- 33.Daglish GJ, Ridley AW, Reid R, Walter GH. Testing the consistency of spatio-temporal patterns of flight activity in the stored grain beetles Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) J. Stor. Prod. Res. 2017;72:68–74. doi: 10.1016/j.jspr.2017.03.005. [DOI] [Google Scholar]
- 34.Campbell JF, Toews MD, Arthur FH, Arbogast RT. Long-term monitoring of Tribolium castaneum in two flour mills: Seasonal patterns and impact of fumigation. J. Econ. Entomol. 2010;103:991–1001. doi: 10.1603/EC09347. [DOI] [PubMed] [Google Scholar]
- 35.Dukić N, et al. The effects of population densities and diet on Tribolium castaneum (Herbst) life parameters. J. Stor. Prod. Res. 2016;69:7–13. doi: 10.1016/j.jspr.2016.05.007. [DOI] [Google Scholar]
- 36.Perez-Mendoza J, Campbell JF, Throne JE. Effects of rearing density, age, sex, and food deprivation on flight initiation of the red flour beetle (Coleoptera: Tenebrionidae) J. Econ. Entomol. 2011;104:443–451. doi: 10.1603/EC10430. [DOI] [PubMed] [Google Scholar]
- 37.Burkholder WE. Practical use of pheromones and other attractants for stored-product insects. In: Burkholder WE, editor. Behavior-Modifying Chemicals for Insect Management: Applications of Pheromones and Other Attractants. Marcel Dekker, Inc.; 1990. pp. 497–516. [Google Scholar]
- 38.Sonleitner FJ, Guthrie PJ. Factors affecting the oviposition rate in the flour beetle Tribolium castaneum and the origin of the population regulation mechanism. Res. Popul. Ecol. 1991;33:1–11. doi: 10.1007/BF02514569. [DOI] [Google Scholar]
- 39.Applebaum SW, Heifetz Y. Density-dependent physiological phase in insects. Annu. Rev. Entomol. 1999;44:317–341. doi: 10.1146/annurev.ento.44.1.317. [DOI] [PubMed] [Google Scholar]
- 40.Van Allen BG, Bhavsar P. Natal habitat effects drive density-dependent scaling of dispersal decisions. Oikos. 2014;123:699–704. doi: 10.1111/oik.01240. [DOI] [Google Scholar]
- 41.Perez-Mendoza J, Dover BA, Hagstrum DW, Hopkins TL. Effect of crowding, food deprivation, and diet on flight initiation and lipid reserves of the lesser grain borer, Rhyzopertha dominica. Entomologia Exp. Applicata. 1999;91:317–326. doi: 10.1046/j.1570-7458.1999.00498.x. [DOI] [Google Scholar]
- 42.Perez-Mendoza J, Dover BA, Hagstrum DW, Baker JE. Flight activity of Rhyzopertha dominica (Coleoptera: Bostrichidae) in response to feeding damage and accumulation of waste. J. Econ. Entomol. 1998;91:1445–1448. doi: 10.1093/jee/91.6.1445. [DOI] [Google Scholar]
- 43.Jian F, Larson R, Jayas DS, White NDG. Three-dimensional temporal and spatial distribution of adult Rhyzopertha dominica in stored wheat and corn under different temperatures, moisture contents, and adult densities. J. Econ. Entomol. 2012;105:1194–1204. doi: 10.1603/EC12072. [DOI] [PubMed] [Google Scholar]
- 44.Edde PA, Phillips TW. Pheromone emission rate by Rhyzopertha dominica (Coleoptera: Bostrichidae) in response to adult starvation and presence of conspecifics. Ann. Entomol. Soc. Am. 2010;103:796–801. doi: 10.1603/AN10014. [DOI] [Google Scholar]
- 45.Phillips TW, Jiang X-L, Burkholder WE, Phillips JK, Tran HQ. Behavioral responses to food volatiles by two species of stored-product coleoptera, Sitophilus oryzae (Curculionidae) and Tribolium castaneum (Tenebrionidae) J. Chem. Ecol. 1993;19:723–734. doi: 10.1007/BF00985004. [DOI] [PubMed] [Google Scholar]
- 46.Morrison WR, Grosdidier RF, Arthur FH, Myers SW, Domingue MJ. Attraction, arrestment, and preference by immature Trogoderma variabile and Trogoderma granarium to food and pheromonal stimuli. J. Pest Sci. 2020;93:135–147. doi: 10.1007/s10340-019-01171-z. [DOI] [Google Scholar]
- 47.Van Winkle T, et al. Microbial volatile organic compounds mediate attraction by a primary but not a secondary stored product insect pest in wheat. J. Chem. Ecol. 2021;48:27–40. doi: 10.1007/s10886-021-01312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ponce MA, Lizarraga S, Bruce A, Kim TN, Morrison WR. Grain inoculated with different growth stages of the fungus, Aspergillus flavus, affect the close-range foraging behavior by a primary stored product pest, Sitophilus oryzae (Coleoptera: Curculionidae) Environ. Entomol. 2022;51:927–939. doi: 10.1093/ee/nvac061. [DOI] [PubMed] [Google Scholar]
- 49.Romero SA, Campbell JF, Nechols JR, With KA. Movement behavior of red flour beetle: Response to habitat cues and patch boundaries. Environ. Entomol. 2010;39:919–929. doi: 10.1603/EN09324. [DOI] [PubMed] [Google Scholar]
- 50.Gerken AR, Dryer D, Campbell JF. Distance-based decision-making in oviposition by Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) on low- and no-gluten flours. J. Econ. Entomol. 2023;116:605–614. doi: 10.1093/jee/toad003. [DOI] [PubMed] [Google Scholar]
- 51.Edde PA, Phillips TW. Potential host affinities for the lesser grain borer, Rhyzopertha dominica: Behavioral responses to host odors and pheromones and reproductive ability on non-grain hosts. Entomologia Experimentalis et Applicata. 2006;119:255–263. doi: 10.1111/j.1570-7458.2006.00417.x. [DOI] [Google Scholar]
- 52.Campbell JF. Attraction of walking Tribolium castaneum adults to traps. J. Stor. Prod. Res. 2012;51:11–22. doi: 10.1016/j.jspr.2012.06.002. [DOI] [Google Scholar]
- 53.Harman RR, Morrison WR, III, Bruce A, Ranabhat S, Quellhorst HE, Wilkins RV, Campbell JF, Gerken AR. The behavioral response to the putative necromones from dead Tribolium castaneum (Coleoptera: Tenebrionidae) in traps by conspecifics as a function of density and time since capture. Environ. Entomol. 2023;52:1020–1032. doi: 10.1093/ee/nvad098. [DOI] [PubMed] [Google Scholar]
- 54.Van Winkle T, Ponce M, Quellhorst H, Bruce A, Albin CE, Kim TN, Zhu KY, Morrison WR. Microbial volatile organic compounds from tempered and incubated grain mediate attraction by a primary but not secondary stored product insect pest in wheat. J. Chem. Ecol. 2022;48:27–40. doi: 10.1007/s10886-021-01312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrison WR, Arthur FH, Bruce A. Characterizing and predicting sublethal shifts in mobility by multiple stored product insects over time to an old and novel contact insecticide in three key stored commodities. Pest Manag. Sci. 2021;77:1990–2006. doi: 10.1002/ps.6228. [DOI] [PubMed] [Google Scholar]
- 56.Team, R. C. R: A language and environment for statistical computing. (2022).
- 57.Oksanen, J. et al. vegan: Community Ecology Package. R package version 2.5–6https://CRAN.R-project.org/package=vegan. 10.1007/978-94-024-1179-9_301576 (2019).
- 58.Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. J. Stat. Softw. 2007;22:1–19. doi: 10.18637/jss.v022.i07. [DOI] [Google Scholar]
- 59.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometr. J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 60.Hodges RJ, Robinson R, Hall DR. Quinone contamination of dehusked rice by Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) J. Stor. Prod. Res. 1996;32:31–37. doi: 10.1016/0022-474X(95)00036-7. [DOI] [Google Scholar]
- 61.Flinn PW, Hagstrum DW, Muir WE. Effects of time of aeration, bin size, and latitude on insect populations in stored wheat: A simulation study. J. Econ. Entomol. 1997;90:646–651. doi: 10.1093/jee/90.2.646. [DOI] [Google Scholar]
- 62.Awater, S., & Furstenau, B. The potential of host-specific volatiles from Tribolium confusum larval faeces for luring the ectoparasitoid Holepyris sylvanidis. 12th International Working Conference on Stored Product Protection (IWCSPP) in Berlin, Germany, October 7–11, (2018).
- 63.Cordeiro EMG, Campbell JF, Phillips TW. Movement and orientation decision modeling of Rhyzopertha dominica (Coleoptera: Bostrichidae) in the grain mass. Environ. Entomol. 2016;45:410–419. doi: 10.1093/ee/nvv232. [DOI] [PubMed] [Google Scholar]
- 64.Morrison WR, III, Poling B, Leskey TC. The consequences of sublethal exposure to insecticide on the survivorship and mobility of Halyomorpha halys (Hemiptera: Pentatomidae) Pest Manag. Sci. 2017;73:389–396. doi: 10.1002/ps.4322. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz KP, et al. Field trapping and flight capacity of Eucosma giganteana (Riley) (Lepidoptera: Tortricidae) in response to behaviorally active congeneric semiochemicals in novel silflower agroecosystems. Insects. 2022;13:350. doi: 10.3390/insects13040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cui J, et al. Flight capacity of Sitophilus zeamais Motschulsky in relation to gender and temperature. Southwest. Entomol. 2016;41:667–674. doi: 10.3958/059.041.0309. [DOI] [Google Scholar]
- 67.Maille J, Reed J, Brabec D, Morrison WR, III, Scully E. Diurnal Flight Behavior of Indianmeal Moth. Environmental Entomology in press; 2024. [Google Scholar]
- 68.Engsontia P, et al. The red flour beetle’s large nose: An expanded odorant receptor gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2008;38:387–397. doi: 10.1016/j.ibmb.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Ming Q-L, Lewis SM. Pheromone production by male Tribolium castaneum (Coleoptera: Tenebrionidae) is influenced by diet quality. J. Econ. Entomol. 2010;103:1915–1919. doi: 10.1603/EC10110. [DOI] [PubMed] [Google Scholar]
- 70.Han S, et al. Detection of specific volatile organic compounds in Tribolium castaneum (Herbst) by solid-phase microextraction and gas chromatography-mass spectrometry. Foods. 2023;12:2484. doi: 10.3390/foods12132484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dowdy AK, Howard RW, Seitz LM, McGaughey WH. Response of Rhyzopertha dominica (Coleoptera, Bostrichidae) to its aggregation pheromone and wheat volatiles. Environ. Entomol. 1993;22:965–970. doi: 10.1093/ee/22.5.965. [DOI] [Google Scholar]
- 72.Gerken AR, Morrison WR. Viewing Farm2Fork agriculture through the lens of community ecology: The who, why, and what of IPM in the postharvest agricultural supply chain. Front. Agron. 2023;7:1137683. [Google Scholar]
- 73.Nansen C, Phillips TW, Parajulee MN, Franqui RA. Comparison of direct and indirect sampling procedures for Plodia interpunctella in a maize storage facility. J. Stor. Prod. Res. 2004;40:151–168. doi: 10.1016/S0022-474X(02)00084-X. [DOI] [Google Scholar]
- 74.Morrison WR, Scully ED, Campbell JF. Towards developing areawide semiochemical-mediated, behaviorally-based integrated pest management programs for stored product insects. Pest Manag. Sci. 2021;77:2667–2682. doi: 10.1002/ps.6289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Ponce, Marco A.; Ranabhat, Sabita; Bruce, Alexander; Van Winkle, Taylor; Campbell, James F.; Morrison, William R. (2024). Data from: Density-mediated emissions by Rhyzopertha dominica (Coleoptera: Bostrichidae) and Tribolium castaneum (Coleoptera: Tenebrionidae) modulates foraging by conspecifics. Ag Data Commons. Dataset. 10.15482/USDA.ADC/24851604.v1. Accessed 2024-05-21.