Abstract
Signal transducer and activator of transcription 3 (STAT3) is frequently overexpressed in patients with acute myeloid leukemia (AML). STAT3 exists in two distinct alternatively spliced isoforms, the full-length isoform STAT3α and the C-terminally truncated isoform STAT3β. While STAT3α is predominantly described as an oncogenic driver, STAT3β has been suggested to act as a tumor suppressor. To elucidate the role of STAT3β in AML, we established a mouse model of STAT3β-deficient, MLL-AF9-driven AML. STAT3β deficiency significantly shortened survival of leukemic mice confirming its role as a tumor suppressor. Furthermore, RNA sequencing revealed enhanced STAT1 expression and interferon (IFN) signaling upon loss of STAT3β. Accordingly, STAT3β-deficient leukemia cells displayed enhanced sensitivity to blockade of IFN signaling through both an IFNAR1 blocking antibody and the JAK1/2 inhibitor Ruxolitinib. Analysis of human AML patient samples confirmed that elevated expression of IFN-inducible genes correlated with poor overall survival and low STAT3β expression. Together, our data corroborate the tumor suppressive role of STAT3β in a mouse model in vivo. Moreover, they provide evidence that its tumor suppressive function is linked to repression of the STAT1-mediated IFN response. These findings suggest that the STAT3β/α mRNA ratio is a significant prognostic marker in AML and holds crucial information for targeted treatment approaches. Patients displaying a low STAT3β/α mRNA ratio and unfavorable prognosis could benefit from therapeutic interventions directed at STAT1/IFN signaling.
Subject terms: Acute myeloid leukaemia, Tumour-suppressor proteins
Introduction
Acute myeloid leukemia (AML) is an aggressive form of leukemia occurring both in children and adults. The development of effective therapeutic strategies is complex due to the wide array of cytogenetic and molecular abnormalities observed in patients. Although targeted therapies with small molecule inhibitors of FLT3, BCL2, and IDH1/2 have improved disease outcomes for several AML subtypes, the 5-year survival rate remains only around 30% [1]. Thus, novel treatment combinations and prognostic markers are urgently needed.
Signal transducer and activator of transcription 3 (STAT3) is an important transcription factor regulating stem cell self-renewal, hematopoiesis, and inflammation [2]. Aberrant STAT3 signaling promotes proliferation and survival in various malignancies and therefore has been explored as a therapeutic target [3]. Moreover, enhanced STAT3 activity is associated with poor prognosis and increased resistance to chemotherapy [4–8]. In AML, constitutive STAT3 activation was reported in AML patient samples and cell lines and contributes to leukemia cell survival, uncontrolled proliferation, and evasion of apoptosis [4, 6–9]. Importantly, alternative splicing gives rise to two isoforms with distinct functions contributing to the diverse roles of STAT3 in cancer [10–12]. While STAT3α represents the full-length isoform, STAT3β is truncated due to an alternative acceptor site causing a C-terminal deletion of 55 amino acids. Consequently, STAT3β lacks the transactivation domain including the phosphorylation site serine 727 [13]. Therefore, STAT3β was initially considered as a negative regulator of STAT3α [14]. However, studies demonstrated that STAT3β plays a relevant role as a transcriptional regulator as it was capable of rescuing the embryonic lethality in STAT3α-deficient mice [11, 12]. Furthermore, STAT3β was reported to induce transcription of a distinct set of genes in a STAT3α-independent manner [11, 12, 15, 16]. In more recent studies, STAT3β gained attention as a tumor suppressive molecule in melanoma [17], lung cancer [18], and esophageal squamous cell carcinoma [16]. In our previous study, we demonstrated that a higher STAT3β/α mRNA ratio correlates with prolonged overall survival (OS) in AML patients [19]. Moreover, overexpression of STAT3β in murine leukemia cells delayed disease progression in mice [19]. However, the molecular mechanisms behind its protective role remained elusive.
Here, we provide insights into the cellular processes resulting in the tumor suppressive function of STAT3β in AML and assign a link between STAT3 isoform expression and interferon (IFN) signaling in leukemia cells. Absence of STAT3β led to an enhanced IFN response and unfavorable disease outcomes, both in an AML mouse model and in primary AML patient samples. Furthermore, STAT3β-deficient leukemia cells were specifically vulnerable to interference with IFN signaling through the JAK1/2 inhibitor Ruxolitinib. Patients with a lower STAT3β/α mRNA ratio and unfavorable prognosis might benefit from treatment combinations with Ruxolitinib.
Materials and methods
Additional methods can be found in Supplementary Materials and Methods.
AML patients
Diagnostic samples (peripheral blood and bone marrow aspirates) from 79 AML patients were collected after written informed consent at the Medical University of Graz (Austria) and processed as described [20]. Only patients receiving curative treatment were included [21]. Patients with FLT3 aberrations, associated with elevated STAT5 signaling, were excluded [22–26]. For patient characteristics see Supplementary Table 1. Publicly available gene expression data sets were analyzed with Kaplan-Meier Plotter [27] (n = 443) and BloodSpot [28] (n = 244).
Animal studies
Mice were kept under pathogen-free conditions at the Institute of Pharmacology, Medical University of Vienna (Austria). STAT3β-deficient mice were generated by Maritano et al. [11]. For the AML model, fetal liver cells were isolated and retrovirally transfected as described [19]. 2 × 105 Venus+ cells were transplanted into 6–8 weeks old male immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (The Jackson Laboratory) via tail vein injection.
Flow cytometry
Unspecific Fc-receptor binding was blocked using an anti-CD16/CD32 antibody (BD Bioscience, Franklin Lakes, NJ, USA). Antibodies (Supplementary Table 3) were added to cell suspension at 1:100–1:200 in PBS containing 2% FBS, incubated for 30 min on ice and protected from light, and afterwards washed twice using PBS. Samples were measured using a Cytoflex S (Beckman Coulter, Brea, California, USA), and data was analyzed with CytExpert (Beckman Coulter) or FlowJo software (TreeStar, Ashland, OR, USA). Living cells were determined according to FSC and SSC and viability was confirmed using 7-AAD staining. Gating was performed using negative controls (untransfected cells or unstained samples) and single-staining controls.
Colony formation assay
1×104 cells were seeded in 1 ml methylcellulose with recombinant cytokines (SCF, IL-3, IL-6, and EPO) for mouse cells (MethoCultTM GF M3434, Stemcell Technologies, Vancouver, Canada) in 35 mm cell culture dishes and incubated at 37 °C, 95% humidity and 5% CO2. After 7 days colonies were counted and harvested for immunophenotyping via flow cytometry. For treatments 2 µg/ml IFNAR1 blocking antibody (clone: MAR1-5A3, BioLegend, San Diego, CA, USA), 2 µg/ml IgG isotype control (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), 5 µM Ruxolitinib (LC Laboratories, MA, USA) or 0.05% DMSO (Carl Roth, Mannheim, Germany) was supplemented. All images were obtained after 7 days in methylcellulose using an Olympus CKX53 microscope (Olympus, Tokyo, Japan) and an Olympus EP50 camera.
Statistics
Patient data
Stratification in our cohort and in the Kaplan-Meier Plotter into high and low gene expression was performed according to Nagy et al. [29]. Survival analysis was performed using Kaplan-Meier estimates and log-rank (Mantel-Cox) test.
Animal and in vitro data
Survival was analyzed using Kaplan-Meier estimates and log-rank (Mantel-Cox) tests. Other data sets were analyzed with unpaired Student’s t-test using GraphPad Prism. The Shapiro-Wilk test was carried out to test for normal distribution. Outliers were excluded based on Grubbs’ test with significance level alpha = 0.05. Error bars represent means ± SD from at least three independent biological or technical replicates. p-values are indicated as p ≤ 0.05:*, ≤0.01:**, ≤0.001:***, and ≤0.0001:****. Lack of statistical significance was indicated with “ns”.
Results
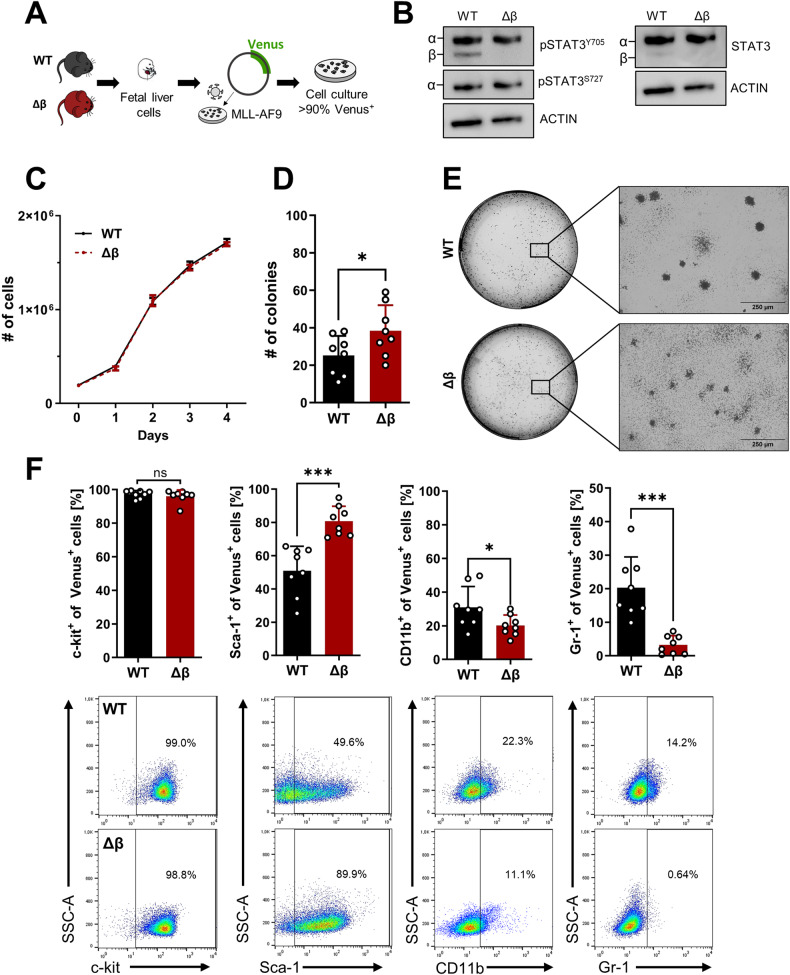
STAT3β-deficient leukemia cells display increased colony formation potential and are less committed to the myeloid lineage
To mimic the characteristics of AML blasts, we used an AML mouse model driven by the MLL-AF9 fusion oncogene that results from the t(9;11)(p22;q23) translocation, which is found in AML patients and associated with an intermediate prognosis [30–32]. Fetal liver cells (FLCs) were isolated from wildtype (WT) and STAT3β-deficient (Δβ) mice and transduced with a retroviral vector encoding MLL-AF9, coupled to a Venus fluorescence reporter (Fig. 1A) [11, 33]. The genotype was confirmed via PCR (Supplementary Fig. 1A). A similar expression of STAT3α on the mRNA and protein levels (Supplementary Fig. 1B, Fig. 1B) was observed in established homogenous Venus+ cells (Supplementary Fig. 1C) that were utilized for all in vitro experiments. Although the absence of STAT3β neither led to a proliferative advantage (Fig. 1C) nor affected cell cycle regulation (Supplementary Fig. 1D) or apoptosis in suspension culture (Supplementary Fig. 1E), STAT3β-deficiency resulted in increased colony formation capacity upon serial replating in methylcellulose (Fig. 1D). Both WT and STAT3β-deficient leukemia cells gave rise to myeloid-type colonies and exhibited similar viability after 7 days (Supplementary Fig. 1F) but displayed a different colony morphology (Fig. 1E, Supplementary Fig. 1G). Immunophenotyping revealed that STAT3β-deficient colonies were comprised of significantly fewer mature myeloid cells, as defined by the myeloid surface markers CD11b and Gr-1, but contained more Sca-1+ cells (Fig. 1F), while no discernible differences were observed in suspension culture (Supplementary Fig. 1H). In summary, these experiments demonstrate that loss of STAT3β does not accelerate proliferation but enhances colony forming ability of MLL-AF9-transformed FLCs. Furthermore, our findings suggest that STAT3β promotes myeloid differentiation of leukemia cells.
Fig. 1. STAT3β-deficient leukemia cells display increased colony formation potential and are less committed to the myeloid lineage.
A FLCs from WT and STAT3β–deficient (Δβ) mice were retrovirally transformed using a vector encoding MLL-AF9. B Western blot analysis of MLL-AF9 transformed FLCs with the indicated antibodies. C Growth curves of MLL-AF9 transformed FLCs (n = 6, 3 independent experiments, 2 cell lines). D Colony formation assay of WT and STAT3β-deficient leukemia cells (n = 8, 3 independent experiments with 3 serial replatings). E Representative pictures of colonies after 7 days in methylcellulose (40× magnification). F Immunophenotyping of leukemia cells after 7 days in methylcellulose via flow cytometry and representative dot plots (n = 8, 3 experiments, 3 serial replatings). Statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*, ≤0.01:**. Error bars represent means ± SD.
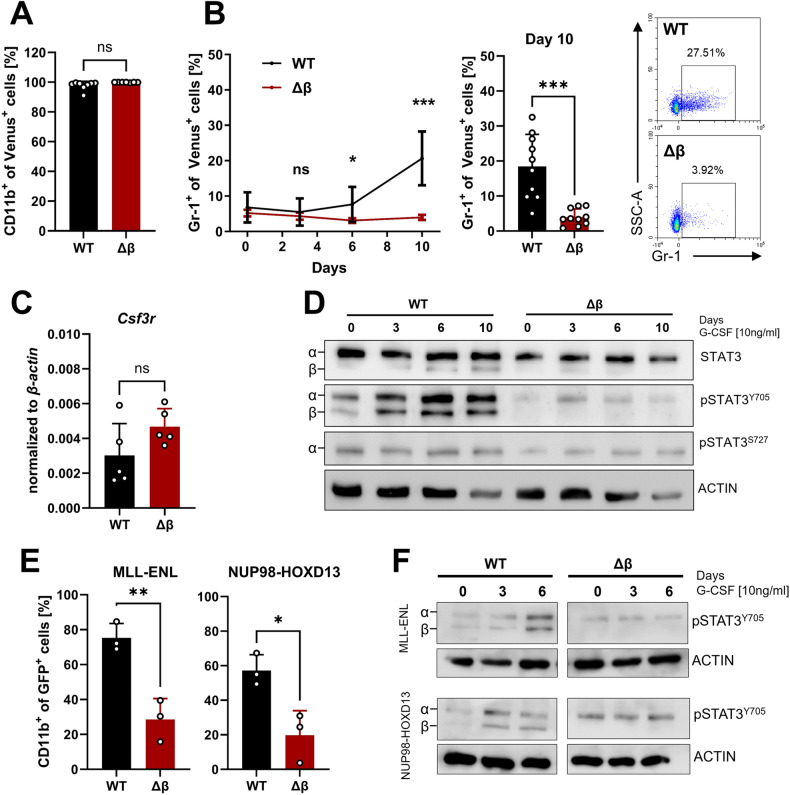
Leukemia cells lacking STAT3β exhibit reduced differentiation response to G-CSF
To explore the function of STAT3β during differentiation in AML cells, we used granulocyte colony-stimulating factor (G-CSF) as a stimulus. G-CSF regulates the differentiation and function of neutrophils and has been employed in multiple studies investigating the involvement of STAT3 in myeloid differentiation [34, 35]. Since G-CSF stimulation leads to a robust increase in STAT3β phosphorylation after long-term treatment [35, 36], we used G-CSF for up to 10 days to provoke differentiation and STAT3 activation in MLL-AF9 transformed FLCs. While all cells remained CD11b+ (Fig. 2A), solely leukemia cells expressing both STAT3 isoforms responded to G-CSF by notable upregulation of the granulocytic surface marker Gr-1 (Fig. 2B), while on the mRNA level expression of the G-CSF receptor (Csf3r) was comparable in suspension culture (Fig. 2C). Furthermore, G-CSF stimulation did not lead to alterations in the expression of the hematopoietic stem cell (HSC) markers c-kit and Sca-1 (Supplementary Fig. 2A). Three days of G-CSF stimulation resulted in a significant increase of STAT3 Y705 phosphorylation only in WT leukemia cells (Fig. 2D, Supplementary Fig. 2B). In contrast, serine 727 (S727) phosphorylation (Fig. 2D) and proliferation were not affected (Supplementary Fig. 2C). This phenomenon is not exclusive to MLL-AF9 transformed FLCs but was also observed in two additional leukemia models, driven by the fusion genes MLL-ENL or NUP98-HOXD13 [37, 38]. As on these cells Gr-1 was neither detected in suspension culture nor upon stimulation, we used the myeloid marker CD11b as a readout. Upon G-CSF stimulation we detected increased CD11b expression (Fig. 2E) and strong phosphorylation of STAT3 Y705 (Fig. 2F) only when both STAT3 isoforms were present. Similar to our observations in MLL-AF9-driven leukemia cells, we did not observe a proliferation advantage in the absence of STAT3β in MLL-ENL or NUP98-HOXD13-driven leukemia cells (Supplementary Fig. 2D). Taken together, this data demonstrates that STAT3β plays—independent of the driver oncogene—a significant role in orchestrating myeloid differentiation in AML cells as its absence led to impaired myeloid differentiation in response to G-CSF.
Fig. 2. Leukemia cells lacking STAT3β exhibit reduced differentiation response to G-CSF.
A Flow cytometry quantification of CD11b+ cells after 10 days cultured with 10 ng/ml G-CSF. (n = 8, 3 independent experiments, 2–3 cell lines). B Quantification of Gr-1+ cells after 10 days of G-CSF stimulation via flow cytometry and representative dot plots from day 10 (n = 10, 4 independent experiments, 2–3 cell lines). C RT-qPCR analysis of G-CSFR (Csf3r) expression in suspension culture (n = 5, 3 independent experiments, 2 cell lines). D Western blot of G-CSF stimulated samples with the indicated antibodies and time points. E Immunophenotyping of MLL-ENL and NUP98-HODX13 transformed FLCs after 10 days G-CSF via flow cytometry (n = 3, independent experiments). F Western blot of G-CSF stimulated samples (MLL-ENL and NUP98-HOXD13 transformed FLCs) with the indicated antibodies and time points. Statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*, ≤0.01:**, ≤0.001:***. Error bars represent means ± SD.
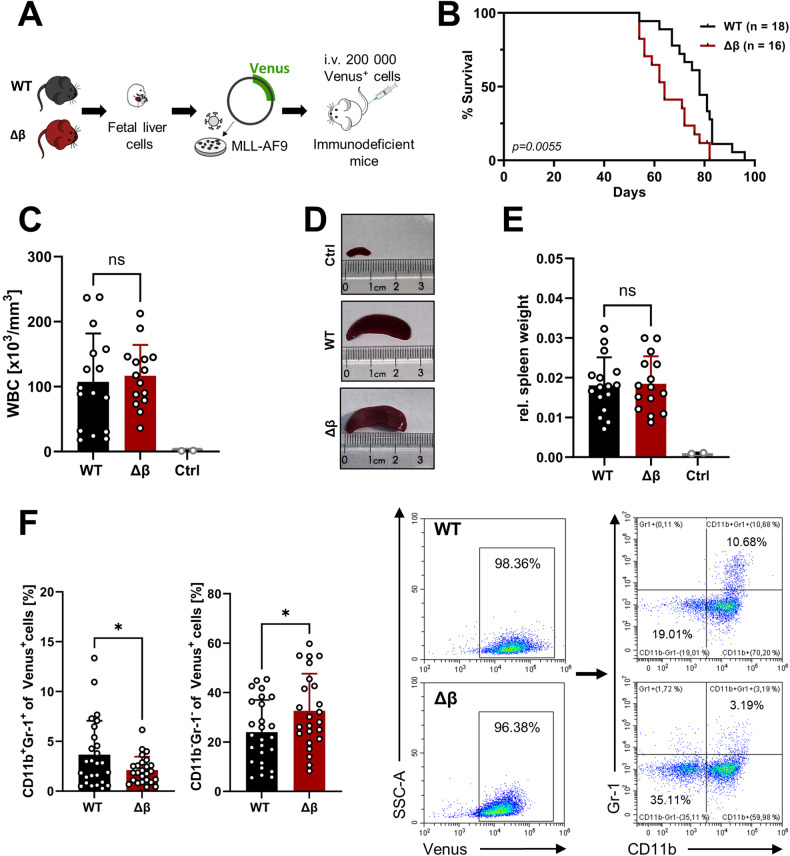
Absence of STAT3β accelerates disease progression in an MLL-AF9-dependent mouse model
We next intravenously transplanted MLL-AF9 transformed WT or STAT3β-deficient FLCs into immunocompromised NSG mice and monitored disease progression via white blood cell counts (Fig. 3A, Supplementary Fig. 3A). Animals transplanted with STAT3β-deficient leukemia cells exhibited significantly shorter OS (Fig. 3B) compared to mice receiving WT leukemia cells demonstrating its pivotal role as a tumor suppressor. At the endpoint no significant differences were observed between recipients of MLL-AF9 transformed WT or STAT3β-deficient cells in leukemia infiltration of the bone marrow (BM) and spleen (SP) (Supplementary Fig. 3B), blasts detected in the peripheral blood (Fig. 3C, Supplementary Fig. 3C), spleen size (Fig. 3D), or ex vivo proliferation (Supplementary Fig. 3D). In contrast, and consistent with our in vitro data, blasts lacking STAT3β were less committed to the myeloid lineage (CD11b+ Gr-1+) and comprise more CD11b-Gr-1- cells suggesting a more immature state in blasts lacking STAT3β (Fig. 3F). Together, these data indicate that the absence of STAT3β in blasts accelerates AML development and restricts myeloid differentiation in vivo, underscoring its role as a tumor suppressor.
Fig. 3. Absence of STAT3β accelerates disease progression in an MLL-AF9-dependent mouse model.
A 200 000 Venus+ cells were intravenously (i.v.) transplanted into immunocompromised NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice. B Kaplan-Meier plot of mice receiving STAT3β-deficient or WT leukemia cells (3 independent experiments, median survival: WT = 78 days; Δβ = 64 days). Log-rank (Mantel-Cox) test was performed to analyze the survival difference between the two groups (P = 0.0055). C WBC count of diseased animals at the time point of euthanasia (n = 15). D Representative pictures of spleens from diseased animals. E Spleen-to-body weight of diseased animals (n = 15, ctrl: n = 2). F Flow cytometry quantification of myeloid markers on leukemia cells isolated from the BM and SP of each animal (n = 24, BM and SP pooled from 12 animals) and representative dot plots. Further statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*. Error bars represent means ± SD. (WBC white blood cell).
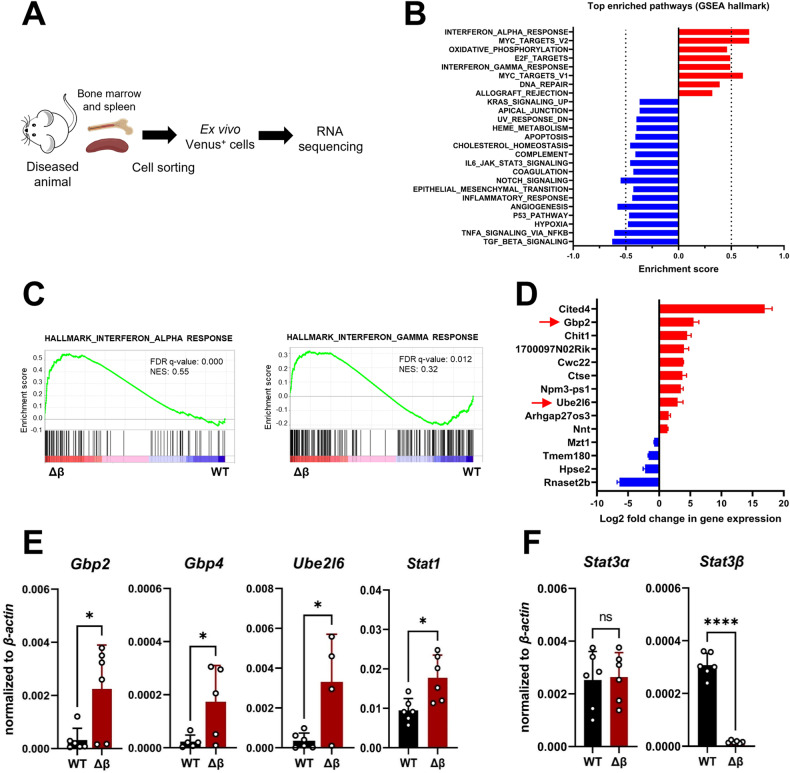
Gene expression analysis reveals enrichment of IFN-regulated genes in blasts lacking STAT3β
To identify STAT3β-regulated processes or target genes that affect disease outcome, blasts were isolated from the BM and SP of diseased animals, sorted based on the co-expressed fluorophore Venus, and subjected to RNA sequencing (Fig. 4A, Supplementary Fig. 4A, B). To assess which cellular processes are dysregulated due to STAT3β-deficiency, we performed gene set enrichment analysis (GSEA). An enrichment of genes involved in IFN response, Myc targets, and oxidative phosphorylation was observed in the absence of STAT3β (Fig. 4B). In line with previous reports [2, 10], pathways related to hypoxia, inflammatory response, and IL6-JAK-STAT3 signaling were reduced in STAT3β-deficient blasts (Fig. 4B, Supplementary Fig. 4C). KEGG pathway analysis revealed that genes that were differentially expressed in the absence of STAT3β were enriched in the processes “hematopoietic cell lineage” and “cytokine-receptor interaction” (Supplementary Fig. 4D). This is in accordance with the observation that loss of STAT3β reduces myeloid differentiation of leukemia cells both in vitro and in vivo (Figs. 1F, 3F). GSEA revealed that a strong enrichment in gene expression in STAT3β-deficient blasts was observed in genes involved in type I (IFNα/β) and type II (IFNγ) IFN signaling (Fig. 4C). By overlapping the significantly up- and downregulated genes between BM- and SP-derived blasts, we found Gbp2 and Ube2l6, which are directly involved in IFN signaling, upregulated in the absence of STAT3β (Fig. 4D). Additionally, Gbp4 and Stat1 were among the highest upregulated genes in GSEA data (Supplementary Fig. 4E). RT-qPCR confirmed a threefold increase in Ube2l6 and Gbp2 expression and a twofold increase in Stat1 expression, in STAT3β-deficient blasts (Fig. 4E). Moreover, Stat3α expression was comparable between blasts isolated from BM and SP confirming that the observed changes in gene expression are attributed to STAT3β rather than STAT3α (Fig. 4F). Taken together, this data suggests that STAT3β represses IFN response in AML cells.
Fig. 4. Gene expression analysis reveals enrichment of IFN-regulated genes in blasts lacking STAT3β isolated from diseased animals.
A Sample preparation for RNA sequencing (n = 3 per group and organ). For sorting strategy see Supplementary Fig. 4B. B Top enriched pathways (nominal p < 1%) in blasts lacking STAT3β. C GSEA plots of WT and STAT3β-deficient blasts isolated from SP of diseased animals (FDR False discovery rate, NES normalized enrichment score). D Significant hits shared between BM- and SP-derived blasts (n = 6). E RT-qPCR of ex vivo samples (n = 6, pooled BM and SP). F RT-qPCR on Stat3 isoform expression in ex vivo samples from BM and SP (n = 6, pooled). Statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*, ≤0.01:**, ≤0.001:*** and ≤0.0001:****. Error bars represent means ± SD.
STAT3β deficiency increases IFN responsiveness
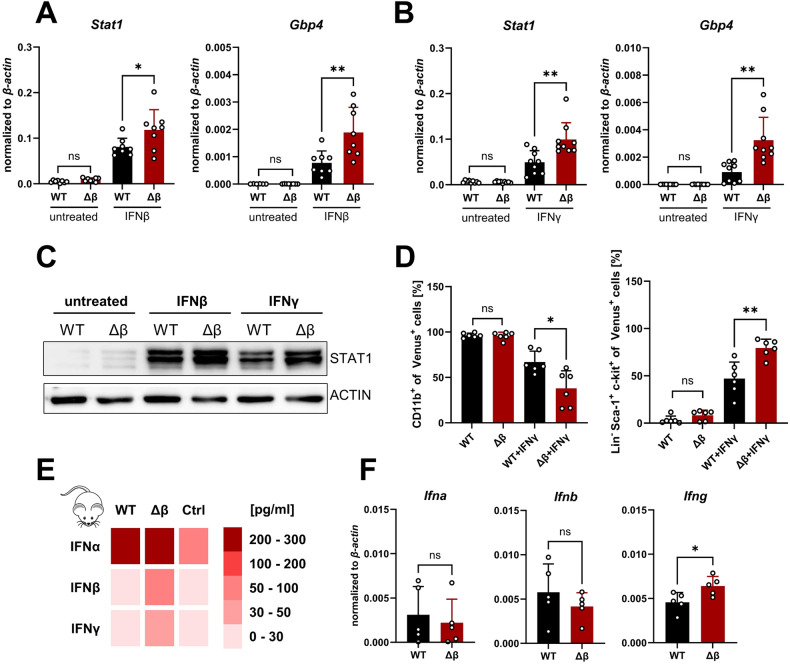
To investigate whether the IFN response depends on STAT3 isoform expression, we stimulated MLL-AF9 transformed FLCs for 3 h with either IFNβ or IFNγ. While no differences occurred in the naïve state, both agents caused a significantly stronger increase in the expression of IFN-inducible genes (Gbp4, Stat1) in STAT3β-deficient compared to WT leukemia cells (Fig. 5A, B). These differences were not caused by altered levels of interferon alpha/beta receptor 1 (IFNAR1) expression between genotypes (Supplementary Fig. 5A). After a 24 h stimulation with either IFNβ or IFNγ, we observed increased levels of STAT1 protein in both genotypes. Interestingly, a higher expression was observed when STAT3β was absent (Fig. 5C, Supplementary Fig. 5B). In healthy HSCs, IFNγ was shown to modulate lineage-specific myeloid differentiation [39] and to promote the expansion of Lin-Sca-1+c-kit+ (LSK) cells potentially through upregulation of Sca-1 via JAK/STAT signaling [40, 41]. To assess the potential impact of IFNγ on the differentiation state of AML cells, we treated the cells for 48 h with IFNγ followed by immunophenotyping. Strikingly and in line with STAT3β-deficient leukemia cells displaying attenuated commitment to the myeloid lineage, a significant decrease in CD11b expression and an increase of LSK blasts was detected upon IFNγ stimulation, which was stronger in leukemia cells lacking STAT3β (Fig. 5D, Supplementary Fig. 5C). These differences were independent of effects on cell viability (Supplementary Fig. 5D).
Fig. 5. STAT3β deficiency increases IFN responsiveness.
A mRNA expression after 3 h 100 U/ml IFNβ stimulation (n = 8, 3 independent experiments, 2–3 cell lines). B mRNA expression after 3 h 100 ng/ml IFNγ stimulation (n = 9, 3 independent experiments, 3 cell lines). C Western blot after 24 h IFN treatment. D Flow cytometry quantification of CD11b+ and Sca-1+ cells after 48 h IFNγ treatment (n = 6, 3 independent experiments, 2 cell lines). E Heatmap represents the average concentration measured in the plasma of diseased animals via multiplex assay. (n = 5 per group, 3 controls). F mRNA expression of Ifna, Ifnb, and Ifng in leukemia cells after 7 days in methylcellulose analyzed via RT-qPCR (n = 5, 3 independent experiments, 2 cell lines). Statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*, ≤0.01:**. Error bars represent means ± SD.
To investigate whether increased expression of IFN-inducible genes is linked to elevated cytokine production, we analyzed IFN levels in the plasma of leukemic mice. Remarkably, elevated levels of IFNα, IFNβ, and IFNγ were detected in comparison to healthy controls (Fig. 5E). While high IFNα concentrations were found in both groups, we observed higher IFNβ and IFNγ levels in the absence of STAT3β. In line with previous work demonstrating AML blasts themselves are capable of releasing IFNγ, associated with poorer OS [42], we detected Ifna, Ifnb and Ifng expression after 7 days in methylcellulose, with a significantly higher Ifng expression in colonies formed by STAT3β-deficient leukemia cells (Fig. 5F). In summary, these data indicate that loss of STAT3β leads to enhanced IFN signaling, and potentially increased IFN production in leukemia cells.
Interference with IFN signaling is a vulnerability of STAT3β-deficient leukemia cells
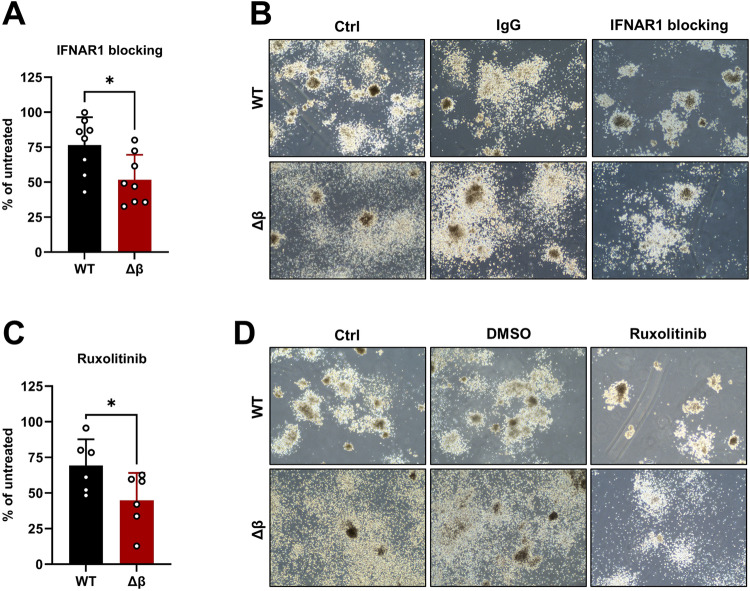
Thus far, our data indicated that STAT3β-deficient leukemia cells induced disease at a significantly accelerated rate compared to WT cells (Fig. 3B), accompanied by enhanced IFN signaling (Fig. 4C). This suggests that increased expression of IFN-inducible genes may contribute to an adverse disease outcome. As we detected significantly higher IFN levels in the plasma of leukemic mice (Fig. 5E) and IFN-inducible genes in colonies, particularly in the absence of STAT3β (Supplementary Fig. 6A), we hypothesized that IFN signaling might contribute to the leukemogenic potential of AML blasts. To assess whether interference with type I IFN signaling could ameliorate disease outcomes, we conducted colony formation assays (CFAs) in the presence and absence of an IFNAR1-blocking antibody. Indeed, we observed a substantial reduction of colony numbers after 7 days in methylcellulose for both WT and STAT3β-lacking leukemia cells with a stronger impact on the latter (Fig. 6A, B, Supplementary Fig. 6B, D). Accordingly, IFNAR1 blocking reduced IFN-inducible genes (Stat1, Gbp4, Ube2l6), in both groups (Supplementary Fig. 6C). To inhibit both type I and type II IFN signaling, we tested the JAK1/2 inhibitor Ruxolitinib. As expected, treatment with Ruxolitinib was sufficient to completely inhibit IFNγ-induced upregulation of Stat1 and Gbp2 in MLL-AF9 transformed FLCs (Supplementary Fig. 6E). Furthermore, Ruxolitinib treatment resulted in a significant reduction in colony numbers of STAT3β-deficient leukemia cells compared to WT cells (Fig. 6C, D, Supplementary Fig. 6F). To conclude, this data suggests that blockade of IFN signaling is a STAT3β-isoform specific vulnerability of leukemia cells, which could be of therapeutic relevance.
Fig. 6. Interference with IFN signaling is a vulnerability of STAT3β-deficient cells.
A Colonies formed in the presence of 2 µg/ml IFNAR1 blocking antibody normalized to untreated (n = 8 from 3 independent experiments, 3 cell lines). B Representative images of colonies formed after 7 days in methylcellulose ± IFNAR1 blocking antibody or IgG control. C Colonies formed in the presence of 5 µM Ruxolitinib normalized to untreated (n = 6, 3 independent experiments, 2 cell lines). D Representative images of colonies formed ± Ruxolitinib or DMSO control. Statistical analysis was performed using Student’s t test and indicated as p ≤ 0.05:*. Error bars represent means ± SD.
High expression of STAT1/IFN-inducible genes is associated with low STAT3β expression and with adverse outcomes in patients with AML
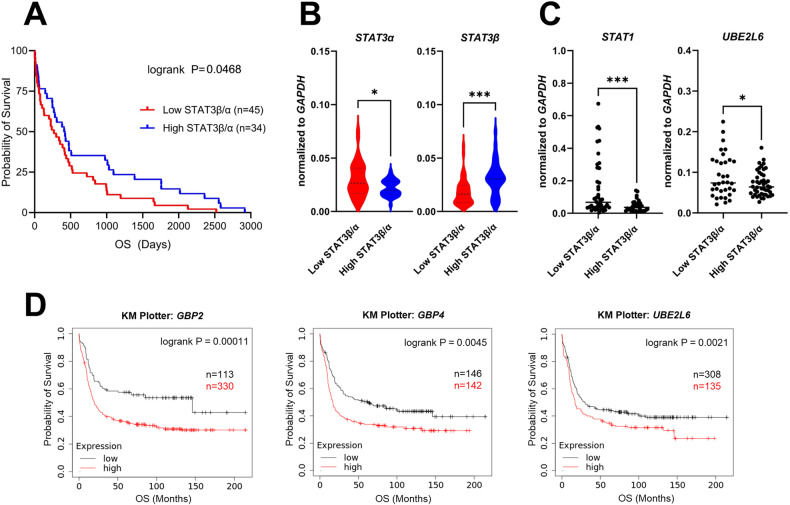
As our results indicate a link between IFN signaling and disease outcome, we explored the relevance of these findings in the clinical context. To validate the association between high expression of IFN-inducible genes and imbalanced STAT1/3 signaling, we accessed a patient cohort comprising 79 newly diagnosed AML patients (Supplementary Table 1). In line with our previous study, we confirmed in this new cohort via RT-qPCR that a low STAT3β/α mRNA ratio correlates with adverse disease outcomes (Fig. 7A) which is independent of age and gender of the patients (Supplementary Fig. 7A, B). Notably, STAT3β levels were primarily responsible for a lower STAT3β/α ratio, while STAT3α expression appears to be of less relevance (Fig. 7B). Intriguingly, patients with low STAT3β/α ratio displayed a significantly higher STAT1 and UBE2L6 gene expression (Fig. 7C). In this cohort we noted a trend suggesting higher expression of STAT1 and IFN-inducible genes is potentially associated with adverse disease outcomes (Supplementary Fig. 7C) We therefore tested this in a larger patient cohort using two different publicly available RNA sequencing data sets of AML patients (KM Plotter: Affymetrix, BloodSpot: TCGA), and correlated the expression of IFN-inducible genes to OS. Remarkably, high expression of GBP2 and UBE2L6 correlated with significantly poorer OS in both cohorts (Fig. 7D, Supplementary Fig. 7D). To conclude, we here describe a specific contribution of STAT3β in fine-tuning IFN response by repressing STAT1-mediated IFN signaling in AML blasts, which improves disease outcome.
Fig. 7. High expression of STAT1/IFN-inducible genes is associated with low STAT3β expression and adverse outcomes in AML patients.
A RT-qPCR analysis of STAT3 isoform expression in 79 AML patient samples. A low STAT3β/α mRNA ratio correlates with poor OS. Patients were stratified according to the best cut-off value. Statistical analysis was performed using log-rank (Mantel-Cox) test. B RT-qPCR of STAT3α and STAT3β (normalized to GAPDH) in patients with a low or high STAT3β/α ratio (n = 79). Data were compared using the Student’s t test. C STAT1 and UBE2L6 expression in patients with a low or high STAT3β/α ratio (n = 79). Data were compared using the Student’s t test. D KM Plotter analysis of RNA sequencing data of AML patients showing a correlation between high expression of GBP2, GPB4, and UBE2L6 with OS. Patients were stratified according to the best cut-off value. (kmplot.com), status: 15.01.2024. Statistical analysis was performed using log-rank (Mantel-Cox) test. p ≤ 0.05:*, ≤0.01:**, ≤0.001:***.
Discussion
We previously demonstrated that the STAT3β/α mRNA ratio serves as a prognostic marker, correlating with disease outcomes in AML patients [19]. Here, we provide strong evidence for STAT3β acting as a tumor suppressor in AML and uncovered the underlying mechanism behind its protective role. The absence of STAT3β in AML cells led to impaired G-CSF-induced myeloid differentiation and STAT3α phosphorylation in vitro. Using an MLL-AF9-driven AML mouse model we confirmed a reduced commitment to the myeloid lineage in leukemia cells in vivo. The imbalance of STAT3 isoforms in leukemia cells resulted in enhanced IFN signaling in the absence of STAT3β together with shorter survival of leukemic mice. Intriguingly, we confirmed that higher expression of IFN-inducible genes correlates with shorter OS in AML patients.
The impact of STAT3 on regulating the balance between self-renewal and differentiation of HSCs is highly dependent on the type of hematopoietic progenitor and the stimulus. Especially during myeloid differentiation in response to G-CSF, robust and sustained STAT3 activation is crucial for differentiation and survival of myeloid progenitors [43–45]. Several studies reported increased STAT3β activity upon G-CSF-induced myeloid differentiation in healthy but also transformed leukemia cells [34, 35]. Accordingly, phosphorylation of STAT3 in response to G-CSF in murine leukemia cells only occurred if both isoforms were present. Interestingly, phosphorylation of STAT3α in STAT3β-deficient blasts also remained absent, together with impaired myeloid differentiation. These findings indicate that STAT3β is pivotal for a G-CSF-induced myeloid differentiation program in AML blasts. As we did not observe alterations in G-CSF receptor (Csf3r) mRNA expression or in proliferation upon G-CSF stimulation, we assume that the absence of STAT3β does not affect receptor expression per se, but rather impairs endosomal recycling of the receptor causing reduced signaling, as previously suggested [46–48]. However, to obtain more detailed mechanistic insights further research is required. In vivo, we also identified a reduction in myeloid lineage commitment in blasts lacking STAT3β, together with more rapid disease progression. Consistent with this, pediatric AML patient samples with low STAT3 phosphorylation upon G-CSF stimulation showed poorer OS than patients with a stronger STAT3 response [46].
As anticipated, the absence of STAT3β led to accelerated disease progression, together with enhanced IFN signaling in vivo. Since IFN signaling plays a central role in cancer progression by promoting inflammation and reprogramming the tumor microenvironment, but also in multiple anti-tumor properties, its effects are highly context-dependent [49–51]. In AML patients, IFN signatures have been reported to correlate with favorable prognosis [52, 53], and negative outcomes as well [42]. One possible explanation for these contradictory results could be a concentration-dependent effect of IFN leading to either an acute or chronic response [51, 54]. While we observed high IFNα concentrations in the plasma of diseased animals in both groups, we detected IFNβ and IFNγ to a higher extent in the absence of STAT3β. Constant production of IFN at a low level, also known as tonic signaling [55], was reported for AML blasts leading to rearrangement of the tumor microenvironment driving leukemogenesis [42]. Accordingly, we observed that blocking type I IFN signaling leads to impaired colony formation ability, particularly in the absence of STAT3β. Furthermore, interference with both type I and type II IFN signaling with Ruxolitinib resulted in significantly decreased colony formation of STAT3β-deficient blasts. Ruxolitinib, approved for myeloproliferative neoplasms, was tested in clinical trials including patients with secondary AML or relapsed patients with moderate effectiveness [56].
Since STAT1 expression was elevated in STAT3β-deficient leukemia cells along with IFN-inducible genes, we postulate that the enhanced IFN signaling results from an imbalance in STAT1/3 signaling due to the absence of STAT3β. While STAT1 and STAT3 activate transcription of an overlapping set of genes, they do not bind to identical DNA elements, which is likely to account for their distinct biological effects [57]. In line, the balance between STAT1/STAT3 activation in response to IFN determines the inflammatory potency in healthy but also transformed cells [58, 59]. IFNβ and IFNγ stimulation in the absence of STAT3β in vitro revealed significantly higher expression of STAT1 as well as other IFN-inducible genes. In accordance, STAT3 has been described to attenuate STAT1-mediated IFN signaling [58, 59]. However, in our setting, loss of STAT3β alone was sufficient to enhance STAT1 expression in AML blasts. To ascertain the clinical relevance, we analyzed 79 AML patient samples. STAT1 and the IFN-inducible gene UBE2L6 were significantly higher expressed in patients with a low STAT3β/α mRNA ratio. Using two distinct publicly available RNA sequencing data sets of AML patients, we confirmed a correlation between higher expression of a set of IFN-inducible genes with poorer OS.
To conclude, in this study, we gained novel insights into the protective role of STAT3β in AML. STAT3β favors myeloid differentiation and fine-tunes tonic IFN signaling in experimental mice and human AML patients. We here describe for the first time STAT3β as a repressor of IFN signaling in AML blasts by maintaining the balance of STAT1-mediated IFN signaling. Thus, using the STAT3β/α mRNA ratio as a prognostic marker could hold crucial information for targeted treatment approaches. Specifically, patients exhibiting a low STAT3β/α ratio could benefit from therapeutic interventions targeting STAT1/IFN signaling.
Supplementary information
Acknowledgements
The authors thank Johannes Zuber for the pMSCV-MLL-AF9-IRES-Venus construct. We thank Christoph Bock and the Biomedical Sequencing Facility of the Medical University of Vienna for help with RNA sequencing. We are grateful to Birgit Strobl, Karoline Kollmann, and Sebastian Kollmann for helpful discussions. We thank Petra Aigner and Tatsuaki Mizutani who provided expertise that assisted the research. Lastly, we would like to thank Jaqueline Horvath and all members of the animal facility for their support. This research was funded in whole or in part by the Austrian Science Fund (FWF, 10.55776/P32693 to DS, 10.55776/P36728, 10.55776/P33430, 10.55776/P32900 and 10.55776/DOC59 to EC), City of Vienna Fund for Innovative, Interdisciplinary Cancer Research to EC, Italian Cancer Research Association (AIRC IG16930) to VP, and Gesellschaft für Forschungsförderung Niederösterreich m.b.H. (GFF, SC19-019 and LSC19-019) to DS. For open access purposes, the author has applied for a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission. We also acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria. BG was supported by the National Research, Development, and Innovation Office (PharmaLab, RRF-2.3.1-21-2022-00015). SE is a PhD candidate at the Medical University of Vienna. This work is submitted in partial fulfillment of the requirement for a PhD.
Author contributions
SE, AW-S, BZ, and DS designed the research; SE, AW-S, KH, BZ, and SW performed experiments, analyzed, and interpreted data; BZ and TE performed bioinformatics analysis of RNA sequencing data; SD and HS provided AML patient samples and clinical information; UG and SK helped with statistical analysis; OS, RW, BG, VP, EC, and FG provided essential material and discussion. SE and DS wrote the manuscript. AW-S, BZ and DS revised the manuscript. The manuscript was approved by all authors.
Data availability
RNA sequencing data is available via Gene Expression Omnibus (GEO) database under accession number GSE261198.
Competing interests
The authors declare no competing interests.
Ethics
Analysis of patient-derived samples was approved by the Scientific Integrity and Ethics Committee of the Karl Landsteiner University of Health Sciences (Austria) under license 1030/2023 and conducted according to the Declaration of Helsinki. All animal experiments were approved by the Animal Ethics Committee of the Medical University of Vienna and the Austrian Ministry of Education, Science and Research under license GZ BMBWF-66.009/0410-V/3b/2018 and performed according to the FELASA guidelines.
Footnotes
Edited by Marc Diederich
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-024-06749-9.
References
- 1.Cancer SEER Statistics Review (CSR) 1975-2017. 2024: https://seer.cancer.gov/archive/csr/1975_2017/.
- 2.Levy DE, Lee C. What does Stat3 do? J Clin Invest. 2002;109:1143–114. doi: 10.1172/JCI0215650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh J, Chand A, Gough D, Ernst M. Therapeutically exploiting STAT3 activity in cancer—using tissue repair as a road map. Nat Rev Cancer. 2018;19:82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 4.Benekli M, Xia Z, Donohue KA, Ford LA, Pixley LA, Baer MR, et al. Constitutive activity of signal transducer and activator of transcription 3 protein in acute myeloid leukemia blasts is associated with short disease-free survival. Blood. 2002;99:252–7. doi: 10.1182/blood.V99.1.252. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Bi C, Janakakumara JV, Liu SC, Chng WJ, Tay KG, et al. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 2009;113:4052–62. doi: 10.1182/blood-2008-05-156422. [DOI] [PubMed] [Google Scholar]
- 6.Steensma DP, McClure RF, Karp JE, Tefferi A, Lasho TL, Powell HL, et al. JAK2 V617F is a rare finding in de novo acute myeloid leukemia, but STAT3 activation is common and remains unexplained. Leukemia. 2006;20:971–8. doi: 10.1038/sj.leu.2404206. [DOI] [PubMed] [Google Scholar]
- 7.Gouilleux-Gruart V, Gouilleux F, Desaint C, Claisse J, Capiod J, Delobel J, et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood. 1996;87:1692–7. doi: 10.1182/blood.V87.5.1692.1692. [DOI] [PubMed] [Google Scholar]
- 8.Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117:5701–9. doi: 10.1182/blood-2010-04-280123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuringa JJ, Wierenga ATJ, Kruijer W, Vellenga E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood. 2000;95:3765–70. doi: 10.1182/blood.V95.12.3765.012k50_3765_3770. [DOI] [PubMed] [Google Scholar]
- 10.Aigner P, Just V, Stoiber D. STAT3 isoforms: alternative fates in cancer? Cytokine. 2018;118:27–34. doi: 10.1016/j.cyto.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Maritano D, Sugrue ML, Tininini S, Dewilde S, Strobl B, Fu X, et al. The STAT3 isoforms α and β have unique and specific functions. Nat Immunol. 2004;5:401–9. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 12.Dewilde S, Vercelli A, Chiarle R, Poli V. Of alphas and betas: distinct and overlapping functions of STAT3 isoforms. Front Biosci. 2008;13:6501–14. doi: 10.2741/3170. [DOI] [PubMed] [Google Scholar]
- 13.Shao H, Quintero AJ, Tweardy DJ. Identification and characterization of cis elements in the STAT3 gene regulating STAT3α and STAT3β messenger RNA splicing. Blood. 2011;98:3853–6. doi: 10.1182/blood.V98.13.3853. [DOI] [PubMed] [Google Scholar]
- 14.Caldenhoven E, Van Dijk TB, Solari R, Armstrong J, Raaijmakers JAM, Lammers JWJ, et al. STAT3β, a splice variant of transcription factor STAT3, is a dominant negative regulator of transcription. J Biol Chem. 1996;271:13221–7. doi: 10.1074/jbc.271.22.13221. [DOI] [PubMed] [Google Scholar]
- 15.Ng IHW, Ng DCH, Jans DA, Bogoyevitch MA. Selective STAT3-α or -β expression reveals spliceform-specific phosphorylation kinetics, nuclear retention and distinct gene expression outcomes. Biochem J. 2012;447:125–36. doi: 10.1042/BJ20120941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang HF, Chen Y, Wu C, Wu ZY, Tweardy DJ, Alshareef A, et al. The opposing function of STAT3 as an oncoprotein and tumor suppressor is dictated by the expression status of STAT3β in esophageal squamous cell carcinoma. Clin Cancer Res. 2016;22:691–703. doi: 10.1158/1078-0432.CCR-15-1253. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov VN, Zhou H, Partridge MA, Hei TK. Inhibition of ataxia telangiectasia mutated kinase activity enhances TRAIL-mediated apoptosis in human melanoma cells. Cancer Res. 2009;69:3510–9. doi: 10.1158/0008-5472.CAN-08-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu G, Zhang C, Zhang J. Dominant negative STAT3 suppresses the growth and invasion capability of human lung cancer cells. Mol Med Rep. 2009;2:819–24. doi: 10.3892/mmr_00000178. [DOI] [PubMed] [Google Scholar]
- 19.Aigner P, Mizutani T, Horvath J, Eder T, Heber S, Lind K, et al. STAT3β is a tumor suppressor in acute myeloid leukemia. Blood Adv. 2019;3:1989–2002. doi: 10.1182/bloodadvances.2018026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lal R, Lind K, Heitzer E, Ulz P, Aubell K, Kashofer K, et al. Somatic TP53 mutations characterize preleukemic stem cells in acute myeloid leukemia. Blood. 2017;129:2587–91. doi: 10.1182/blood-2016-11-751008. [DOI] [PubMed] [Google Scholar]
- 21.Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, Iino T, Rocnik JL, Kikushige Y, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009;114:5034–43. doi: 10.1182/blood-2008-12-196055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotecha N, Flores NJ, Irish JM, Simonds EF, Sakai DS, Archambeault S, et al. Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell. 2008;14:335–43. doi: 10.1016/j.ccr.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choudhary C, Müller-Tidow C, Berdel WE, Serve H. Signal transduction of oncogenic Flt3. Int J Hematol. 2005;82:93–99. doi: 10.1532/IJH97.05090. [DOI] [PubMed] [Google Scholar]
- 25.Mizuki M, Schwäble J, Steur C, Choudhary C, Agrawal S, Sargin B, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–73. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 26.Spiekermann K, Bagrintseva K, Schwab R, Schmieja K, Hiddemann W. Overexpression and constitutive activation of FLT3 induces STAT5 activation in primary acute myeloid leukemia blast cells. Clin Cancer Res. 2003;9:2140–50. [PubMed] [Google Scholar]
- 27.Lánczky A, Győrffy B. Web-based survival analysis tool tailored for medical research (KMplot): development and implementation. J Med Internet Res. 2021;23:e27633. doi: 10.2196/27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagger FO, Sasivarevic D, Sohi SH, Laursen LG, Pundhir S, Sønderby CK, et al. BloodSpot: a database of gene expression profiles and transcriptional programs for healthy and malignant haematopoiesis. Nucleic Acids Res. 2016;44:917–24. doi: 10.1093/nar/gkv1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy Á, Munkácsy G, Győrffy B. Pancancer survival analysis of cancer hallmark genes. Sci Rep. 2021;11:6047. doi: 10.1038/s41598-021-84787-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuo Y, MacLeod RAF, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23) Leukemia. 1997;11:1469–77. doi: 10.1038/sj.leu.2400768. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs MC, Krivtsov AV. Murine retrovirally-transduced bone marrow engraftment models of MLL-fusion-driven acute myelogenous leukemias (AML) Curr Protoc Pharmacol. 2017;78:14.42.1–14.42.19. doi: 10.1002/cpph.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winters AC, Bernt KM. MLL-rearranged leukemias—an update on science and clinical approaches. Front Pediatr. 2017;5:240371. doi: 10.3389/fped.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skucha A, Ebner J, Schmöllerl J, Roth M, Eder T, César-Razquin A, et al. MLL-fusion-driven leukemia requires SETD2 to safeguard genomic integrity. Nat Commun. 2018;9:1983. doi: 10.1038/s41467-018-04329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty A, White SM, Schaefer TS, Ball ED, Dyer KF, Tweardy DJ. Granulocyte colony-stimulating factor activation of Stat3α and Stat3β in immature normal and leukemic human myeloid cells. Blood. 1996;88:2442–9. doi: 10.1182/blood.V88.7.2442.bloodjournal8872442. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty A, Tweardy DJ. Stat3 and G-CSF-induced myeloid differentiation. Leuk Lymphoma. 1998;30:433–42. doi: 10.3109/10428199809057555. [DOI] [PubMed] [Google Scholar]
- 36.Hevehan DL, Miller WM, Papoutsakis ET. Differential expression and phosphorylation of distinct STAT3 proteins during granulocytic differentiation. Blood. 2002;99:1627–37. doi: 10.1182/blood.V99.5.1627. [DOI] [PubMed] [Google Scholar]
- 37.Bach C, Mueller D, Buhl S, Garcia-Cuellar MP, Slany RK. Alterations of the CxxC domain preclude oncogenic activation of mixed-lineage leukemia 2. Oncogene. 2009;28:815–23. doi: 10.1038/onc.2008.443. [DOI] [PubMed] [Google Scholar]
- 38.Lin YW, Slape C, Zhang Z, Aplan PD. NUP98-HOXD13 transgenic mice develop a highly penetrant, severe myelodysplastic syndrome that progresses to acute leukemia. Blood. 2009;106:287. doi: 10.1182/blood-2004-12-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11:685–92. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao X, Ren G, Liang L, Ai PZ, Zheng B, Tischfield JA, et al. Brief report: interferon-gamma induces expansion of Lin(-)Sca-1(+)C-Kit(+) Cells. Stem Cells. 2010;28:122–6. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 41.Snapper CM, Yamaguchi H, Urban JF, Finkelman FD. Induction of Ly-6A/E expression by murine lymphocytes after in vivo immunization is strictly dependent upon the action of IFN-alpha/beta and/or IFN-gamma. Int Immunol. 1991;3:845–52. doi: 10.1093/intimm/3.9.845. [DOI] [PubMed] [Google Scholar]
- 42.Corradi G, Bassani B, Simonetti G, Sangaletti S, Vadakekolathu J, Fontana MC, et al. Release of IFNγ by acute myeloid leukemia cells remodels bone marrow immune microenvironment by inducing regulatory T cells. Clin Cancer Res. 2022;28:3141–55. doi: 10.1158/1078-0432.CCR-21-3594. [DOI] [PubMed] [Google Scholar]
- 43.Shimozaki K, Nakajima K, Hirano T, Nagata S. Involvement of STAT3 in the granulocyte colony-stimulating factor-induced differentiation of myeloid cells. J Biol Chem. 1997;40:25184–9. doi: 10.1074/jbc.272.40.25184. [DOI] [PubMed] [Google Scholar]
- 44.Ward AC, Smith L, De Koning JP, Van Aesch Y, Touw IP. Multiple signals mediate proliferation, differentiation, and survival from the granulocyte colony-stimulating factor receptor in myeloid 32D cells. J Biol Chem. 1999;93:113–24. doi: 10.1074/jbc.274.21.14956. [DOI] [PubMed] [Google Scholar]
- 45.De Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, Van Leeuwen D, et al. STAT3-mediated differentiation and survival of myeloid cells in response to granulocyte colony-stimulating factor: role for the cyclin-dependent kinase inhibitor p27 Kip1. Oncogene. 2000;19:3290–8. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- 46.Narayanan P, Man TK, Gerbing RB, Ries R, Stevens AM, Wang YC, et al. Aberrantly low STAT3 and STAT5 responses are associated with poor outcome and an inflammatory gene expression signature in pediatric acute myeloid leukemia. Clin Transl Oncol. 2021;23:2141. doi: 10.1007/s12094-021-02621-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbone CJ, Fuchs SY. Eliminative signaling by Janus kinases: role in the downregulation of associated receptors. J Cell Biochem. 2014;115:8–16. doi: 10.1002/jcb.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmid SL. Reciprocal regulation of signaling and endocytosis: Implications for the evolving cancer cell. J Cell Biol. 2017;216:2623–32. doi: 10.1083/jcb.201705017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kollmann S, Grundschober E, Maurer B, Warsch W, Grausenburger R, Edlinger L, et al. Twins with different personalities: STAT5B-but not STAT5A-has a key role in BCR/ABL-induced leukemia. Leukemia. 2019;33:1583–97. doi: 10.1038/s41375-018-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciciarello M, Corradi G, Sangaletti S, Bassani B, Simonetti G, Vadakekolathu J, et al. Interferon-γ-dependent inflammatory signature in acute myeloid leukemia cells is able to shape stromal and immune bone marrow microenvironment. Blood. 2019;134:1212. doi: 10.1182/blood-2019-126512. [DOI] [Google Scholar]
- 51.Parker BS, Rautela J, Hertzog PJ. Antitumour actions of interferons: implications for cancer therapy. Nat Rev Cancer. 2016;16:131–44. doi: 10.1038/nrc.2016.14. [DOI] [PubMed] [Google Scholar]
- 52.Holicek P, Truxova I, Rakova J, Salek C, Hensler M, Kovar M, et al. Type I interferon signaling in malignant blasts contributes to treatment efficacy in AML patients. Cell Death Dis. 2023;14:209. doi: 10.1038/s41419-023-05728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Curran E, Bell J, Venkataraman G, Fidai S, Godley L, Kline J. Type I interferon signaling predicts inferior survival in patients with AML. Clin Lymphoma Myeloma Leuk. 2019;19:S241. doi: 10.1016/j.clml.2019.07.136. [DOI] [Google Scholar]
- 54.Boukhaled GM, Harding S, Brooks DG. Opposing roles of type I interferons in cancer immunity. Annu Rev Pathol. 2021;16:167–98. doi: 10.1146/annurev-pathol-031920-093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gough DJ, Messina NL, Clarke CJP, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–74. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eghtedar A, Verstovsek S, Estrov Z, Burger J, Cortes J, Bivins C, et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood. 2012;119:4614–8. doi: 10.1182/blood-2011-12-400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horvath CM, Wen Z, Darnell JE. A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–94. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 58.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses: negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2008;281:14111–8. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 59.Qing Y, Stark GR. Alternative activation of STAT1 and STAT3 in response to interferon-γ. J Biol Chem. 2004;279:41679–85. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data is available via Gene Expression Omnibus (GEO) database under accession number GSE261198.