Abstract
An estimated 38 million people live with human immunodeficiency virus (HIV) worldwide and are at excess risk for multiple cancer types. Elevated cancer risks in people with HIV (PLWH) are driven primarily by increased exposure to carcinogens, most notably oncogenic viruses acquired through shared transmission routes, plus acceleration of viral carcinogenesis by HIV-related immunosuppression. In the era of widespread antiretroviral therapy (ART), life expectancy of PLWH has increased, with cancer now a leading cause of co-morbidity and death. Furthermore, the types of cancers occurring among PLWH are shifting over time and vary in their relative burden in different parts of the world. In this context, the International Agency for Research on Cancer (IARC) and the US National Cancer Institute (NCI) convened a meeting in September 2022 of multinational and multidisciplinary experts to focus on cancer in PLWH. This report summarizes the proceedings, including a review of the state of the science of cancer descriptive epidemiology, etiology, molecular tumor characterization, primary and secondary prevention, treatment disparities and survival in PLWH around the world. A consensus of key research priorities and recommendations in these domains is also presented.
Keywords: human immunodeficiency virus, people living with HIV, cancer, epidemiology, prevention
Graphical Abstract
Introduction
In 2021, there were an estimated 38 million people living with human immunodeficiency virus (HIV, PLWH) worldwide, of whom 26 million were living in sub-Saharan Africa (SSA) and 75% were accessing effective antiretroviral therapy (ART).1 Elevated rates of cancer have been observed among PLWH since the beginning of the global HIV epidemic. For some cancer types, risk is closely related to HIV-induced immunosuppression,2 along with an increased prevalence of co-infection with other oncogenic viruses as well as other major cancer risk factors, particularly, cigarette smoking. In 2009, the International Agency for Research on Cancer (IARC) identified six cancer types for which there is sufficient evidence that HIV is carcinogenic, and an additional five cancer types for which there is limited evidence (Table 1).3 With the introduction and widespread utilization of ART for HIV infection, the rates of some cancers have declined. However, PLWH are reaching older ages globally, when the incidence of many cancers increases.
Table 1.
Cancer sites for which HIV is considered by IARC to be a carcinogen with “sufficient” or “limited” evidence.
| Level of Evidence | Cancer site | Viral carcinogen |
|---|---|---|
| Sufficient Evidence | ||
|
| ||
| Kaposi sarcoma | Kaposi sarcoma herpesvirus (KSHV) | |
| Anus | Human papillomavirus (HPV) | |
| Non-Hodgkin lymphoma | Epstein-Barr virus (EBV), hepatitis C virus, HTLV-1, KSHV | |
| Hodgkin lymphoma | EBV | |
| Eye (conjunctiva) | Unknown | |
| Cervix | HPV | |
|
| ||
| Limited Evidence | ||
|
| ||
| Vulva | HPV | |
| Penis | HPV | |
| Vagina | HPV | |
| Liver | Hepatitis B and C viruses | |
| Skin | Unknown | |
In the context of the evolving HIV epidemic and the importance of cancer as a co-morbidity and leading cause of death among PLWH, a workshop was organized in September 2022 by the International Agency for Research on Cancer (IARC), in Lyon, France and the National Cancer Institute (NCI) in Rockville, Maryland, United States of America. This workshop focused on the state of the science of cancer among PLWH, highlighting the international cancer burden, etiology, tumor characteristics, treatment, and survival. Four cancers of special interest were Kaposi sarcoma (KS), cervical, lung, and anal, given their major contribution to the burden of cancer in PLWH and the potential for advancing their prevention and early detection. Here, we provide a summary of the meeting proceedings and highlight priority research questions. Clearly, primary prevention of HIV infection also remains a global priority, but this was not specifically addressed by this workshop.
International patterns in cancer incidence
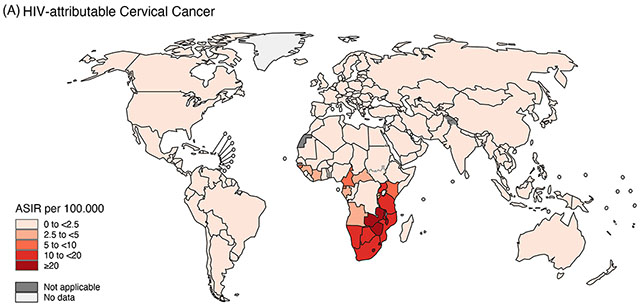
Cancer incidence and burden (i.e., the number of new cases) among PLWH is strongly influenced by the characteristics of the population living with HIV (e.g., demographics, co-infections, co-morbidities), availability of timely ART, and access to health care—all factors that differ across countries and regions of the world, and over time. The types of cancer that occur at higher rates among PLWH are largely consistent across countries and are typically caused by oncogenic viruses.4–8 However, rates of KS, cervical, and conjunctival cancer remain much higher in sub-Saharan Africa (SSA) than in Europe, Latin America, and North America (Figure 1).9, 10
Figure 1.
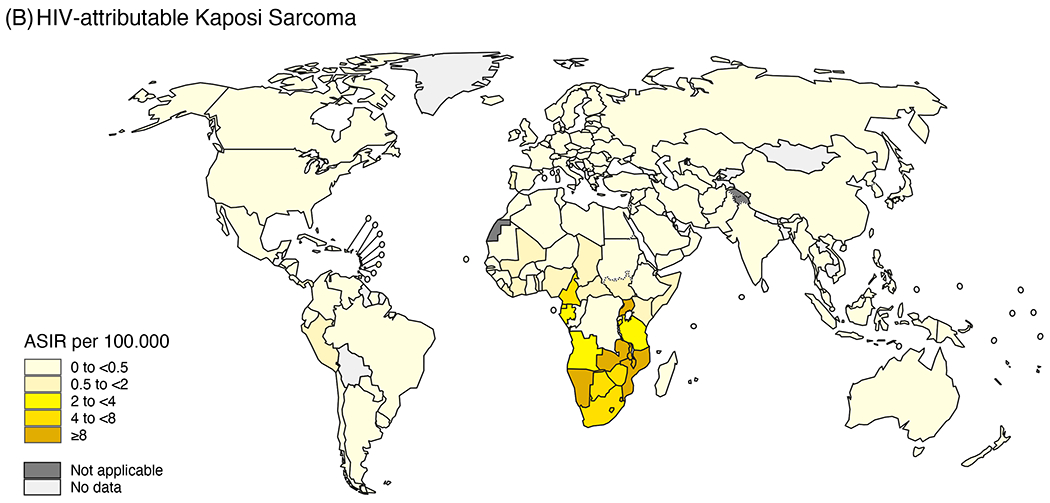
Age-standardized incidence rates (ASIR) in 2020 by country of (A) HIV-attributable cervical cancer (reproduced from Khalil et al. A specific burden of cervical cancer associated with HIV: a global analysis with a focus on sub-Saharan Africa, International Journal of Cancer, Volume 150, pages. 761-772. © 2021 World Health Organization. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.51) and (B) HIV-attributable Kaposi sarcoma (reproduced from Khalil et al. Burden of Kaposi sarcoma according to HIV status: A systematic review and global analysis, International Journal of Cancer, Volume 150, pages. 1948-1957. © 2022 World Health Organization. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.100), using the entire population (for KS) or the entire female population (for cervix) population as a denominator. The designations used and the presentation of the material in this article do not imply the expression of any opinion whatsoever on the part of WHO and the IARC about the legal status of any country, territory, city, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries.
Large studies of PLWH in many regions of the world have consistently found that rates of KS have declined over time with the introduction of ART, as KS is strongly associated with immunosuppression, and treatment with ART results in immune reconstitution.2, 4, 5, 7, 11, 12 Rates of non-Hodgkin lymphoma (NHL), a heterogeneous group of malignancies with varying associations with immunosuppression, have also generally declined in the ART era in Australia, Europe and the U.S., but not in SSA.4, 5, 7, 11, 13, 14 NHL trends among PLWH in SSA have been mixed, likely driven by a combination of delayed access to ART that varied by country and challenges in diagnosing NHL. Rates of human papillomavirus (HPV)-related cancers among PLWH continue to be high in SSA but are decreasing in Australia, Europe and the U.S.4–6, 8, 14, 15
Populations of PLWH accessing ART worldwide are aging, and there is an increasing burden of a range of cancers among PLWH, even in the setting of declining cancer incidence rates.16, 17 Shifts towards aging-associated cancers (e.g., prostate, breast and colorectal cancers) have already been observed in countries with early introduction of ART, and the impact of aging on cancer risk in countries with younger populations of PLWH will become apparent in the coming years.18
Large studies of PLWH followed over time are critical to quantify cancer incidence and mortality trends, and to understand what is driving cancer risks and outcomes to identify means of prevention and optimize treatment. Data linkages with records from data warehouses, disease registries, cohort studies and serial cross-sectional studies have been used thus far,19–23 but each approach has limitations. In some parts of the world, cancer registration is incomplete or lacking altogether, and health system limitations pose major barriers to accurate and timely cancer diagnosis. Even in settings where high-quality, population-based registries of cancer diagnoses are available, data linkage studies are often restricted to those elements collected for surveillance purposes. Large observational and clinical cohorts of PLWH have been established in many countries;24, 25 however, these cohorts often focus on collection of HIV-specific information and lack clinical information on most non-communicable co-morbidities.
Cancer etiology and tumor characteristics
Among PLWH, immunosuppression and loss of immune control of oncogenic viruses play prominent roles in the etiology of cancer. Immune abnormalities in PLWH do not always reverse with ART, and prior severe immunosuppression, as evidenced by low nadir CD4 count or opportunistic infections, is an important cancer risk factor.26, 27 During long-term HIV infection, T-cells exhibit chronic activation and can undergo terminal differentiation, which may contribute to impaired immune function.28 A low CD4:CD8 T-cell ratio indicates T-cell replicative senescence and is a risk factor for multiple cancer types, including KS, NHL, anal, lung, and colorectal.25
Many factors, including opportunistic infections and tobacco use, contribute to chronic inflammation in PLWH.28 For example, gastrointestinal microbial translocation, which occurs even in PLWH on virally-suppressive ART, is associated with elevated plasma levels of biomarkers indicating activation of the innate immune system (e.g., soluble CD14) and inflammation (e.g., C-reactive protein).29 Inflammation is a risk factor for certain cancers (e.g., lung cancer), and limited data support its role in carcinogenesis specifically among PLWH receiving ART.30 Treatment with lipid-lowering statins, which have anti-inflammatory properties, are also associated with reduced risk of liver cancer and NHL among PLWH.31
Even with ART, PLWH remain at elevated risk of developing certain diseases that are typically seen only at older ages, which has suggested that they experience “accelerated aging”.32 For instance, PLWH can develop end-organ damage and frailty at early ages, due to multiple processes associated with HIV including co-infections, chronic inflammation, oxidative stress, substance use (e.g., tobacco), and treatment toxicity.33 PLWH receiving ART also exhibit immune senescence and clonal hematopoiesis, which are potential cancer risk factors that are associated with aging in the general population.25, 34
Whether accelerated aging contributes to the development of cancer among PLWH is unknown. Although the average age at diagnosis for many cancer types appears to be younger among PLWH than among people without HIV, much of this is an artifact of the younger age distributions of the population of PLWH.35, 36 Moreover, the incidence of common age-related cancers (e.g., colorectal, prostate and breast) is not elevated among PLWH.36 Nonetheless, anal cancer and lung cancer occur at slightly younger ages compared to cancers diagnosed in people without HIV (an effect which could be driven by earlier exposure to carcinogens).36
Analysis of tumor specimens can help elucidate the etiology and clinical behavior of cancer among PLWH.37–40 Comprehensive molecular profiling can identify known or unknown oncogenic viruses, other biologic mechanisms, and potential therapeutic targets, especially for those cancers for which PLWH have markedly elevated risk or poor outcomes. For example, emerging data suggest that a virus (possibly EBV)41 is etiologically implicated in conjunctival squamous cell carcinoma.
Furthermore, as large-scale ‘omic’ technologies become more accessible for characterization of the tumor microenvironment in archival and small tumor samples, evaluation of tumors in PLWH could facilitate diagnostic and therapeutic advances.42 For example, expression of programmed death-ligand 1 (PD-L1), an immune checkpoint molecule that can be targeted by currently approved immunotherapy drugs, is frequently present in diffuse large B-cell lymphomas occurring in PLWH and is correlated with higher lymphoma-specific mortality.43 Among PLWH, the presence of CD8+ T-cell infiltrates in anal high-grade squamous intraepithelial lesions (HSIL) is associated with resistance to ablation therapy.44 Breast, lung, and anal cancers in PLWH exhibit expression of PD-L1 or a high tumor mutational burden,37, 39 both of which support the effectiveness of immunotherapy. It is also possible that within specific cancer types, biology may differ across PLWH depending on factors related to HIV treatment and control prior to cancer occurrence.
Studies of cancer etiology, including the role of oncogenic viruses, are greatly facilitated by the ability to obtain representative samples from blood and tissue repositories, such as the NCI-funded AIDS and Cancer Specimen Resource, which has sites in the U.S, South Africa and Brazil (https://acsr1.com/). Strengthening pathology core laboratories and expanding repositories in regions impacted by HIV will provide substantial benefit to the diagnosis and clinical management of cancer as well as enhance infrastructure to support research on HIV and cancer.
Cervical cancer
HIV-related immunodeficiency increases risk of cervical HPV persistence, progression to precancer, and (in unscreened populations) cervical cancer.45, 46 These risks can be partially reduced by ART.47 It is therefore predicted that the high age-standardized cervical cancer incidence observed in largely unscreened women with HIV in SSA48 should decline with wider and earlier access to ART.49, 50 Currently however, cervical cancer burden in women with HIV in SSA remains high,9, 51 perhaps partly due to sub-optimal ART use to date. Furthermore, because ART also increases life expectancy, even if age-standardized incidence rates decrease, the absolute numbers of women with HIV diagnosed with cervical cancer in SSA could still increase further, unless effective public health measures are implemented.
Prophylactic HPV vaccination programs have the potential to substantially reduce future cervical cancer risk for cohorts vaccinated prior to sexual debut. HPV vaccination is a key focus of the World Health Organization (WHO) initiative for cervical cancer elimination, and is crucial to reduce cervical cancer in settings with high HIV prevalence.49, 50 High efficacy of a single vaccine dose in young women without HIV52, 53 has prompted policy shifts towards single dose regimens.54, 55 However, there is no evidence regarding the level of protection maintained by HPV vaccine recipients who later acquire HIV. Whilst there are no strong precedents for breakthrough of vaccine-preventable diseases in PLWH, studies of immunogenicity and effectiveness should nonetheless be designed to address this issue. HPV vaccine coverage gaps (often inversely correlated with school attendance) may be higher among girls who later acquire HIV. Efficacy of reduced dose regimens for young females vaccinated when already living with HIV is also uncertain, but may become less relevant with the declining incidence of perinatally acquired HIV, at least for HPV vaccination at ages prior to sexual debut.
IARC recently convened an expert working group to update evidence-based evaluations of cervical screening methods,56 which informed a consensus update of WHO Guidelines for cervical screening, including special considerations for women with HIV, 57 albeit with recognition of scant evidence for this population. “Risk benchmarking,” which ensures similar management for women with similar risks, should be increasingly integrated into cervical screening research for women with HIV.58, 59 HPV infection and abnormal screening results are more common in women with HIV, but it is not known whether cervical cancer risk, conditional on test results, differs by HIV status. In two recent studies, risks of high-grade cervical lesions (conditional on screening test results) were similar between women with and without HIV for all evaluated algorithms, prompting the question of whether the two populations could potentially be screened more similarly,58, 60 which could simplify implementation of screening in settings with high HIV prevalence.
Kaposi sarcoma
The majority of KS is diagnosed in SSA, driven by high population prevalence of both HIV and KS-associated herpesvirus (KSHV) (Figure 1). In SSA, KS is most often diagnosed late, if at all. There are many reasons for this, including lack of community awareness, and lack of diagnostic pathology services. Thus, new methods to diagnose KS relevant to resource-limited settings are needed, perhaps using liquid biopsies or artificial intelligence-based digital imaging. Also, other severe KSHV-related diseases (e.g., multicentric Castleman disease, primary effusion lymphoma, and KSHV inflammatory cytokine syndrome) can be diagnosed in combination with KS, and it will be important to develop strategies to diagnose and manage these conditions.61
Studies describing the distribution of KS diagnoses in relation to ART (i.e., prior to or after initiation), as well as the effect of HIV viral suppression, have been reported in high-resource settings, but are particularly needed in SSA. KS onset and recurrence in virally-suppressed PLWH on ART are poorly understood and underscore the importance of developing novel therapeutics.62 Loss of anti-KSHV cellular immunity, (e.g., due to KSHV chronic antigen exposure and/or immune exhaustion) are mechanisms to investigate. Another concern is whether a resurgence of KS will occur with advancing age among PLWH chronically co-infected with KSHV.
Primary prevention of KS has been hampered by poor understanding of immunological control of KSHV (including in the oral cavity, where KSHV can be shed in saliva) and routes of transmission. This is particularly important in groups where KSHV prevalence is high, such as children in SSA and in men who have sex with men. An effective KSHV vaccine is the ultimate solution to KS prevention,63 but vaccine development faces several obstacles, particularly if prevention of KS must be the primary endpoint in clinical trials. Use of KSHV infection as the validated primary endpoint would greatly facilitate regulatory approval. An effective KSHV vaccine would also prevent morbidity and mortality from other diseases, including primary effusion lymphoma, multicentric Castleman disease, and KSHV inflammatory cytokine syndrome.
Lung cancer
Lung cancer incidence is elevated in PLWH,64, 65 in large part because of an elevated prevalence of smoking among PLWH.66, 67 Smoking cessation is key to prevent lung cancer, but clinical interventions are often inadequate, especially for individuals with comorbid substance use disorders. Screening for lung cancer using low-dose computed tomography (LDCT) is proven to reduce lung cancer-specific mortality among long-term smokers in the general population.68, 69 The U.S. Preventive Services Task Force recommends lung cancer screening for all individuals aged 50-80 years who have smoked at least 20 pack-years and are current smokers or former smokers who have quit for less than 15 years.70
There is interest in implementation of LDCT screening among PLWH. The feasibility of screening in PLWH has been demonstrated, including initiating screening at lower age thresholds than generally recommended.71, 72 Development and validation of a lung cancer risk score that integrates demographic information, smoking history, and HIV disease markers may be useful for selecting PLWH who would benefit most from enhanced LDCT screening.73–75 Ensuring follow-up of PLWH with positive LDCT screening results is also important.
Anal cancer
The prevalence of anal precancers (i.e., HSIL) is as high as 55% among men and 47% among women living with HIV in the U.S.76 The Anal Cancer HSIL Outcomes Research trial recently demonstrated that treatment of these lesions among PLWH aged ≥35 years decreased the incidence of subsequent invasive anal cancer.76 Unfortunately, there are insufficient providers skilled in high-resolution anoscopy (HRA) to perform this diagnostic test for all PLWH, and thus more effective screening strategies are needed.
Currently, anal cytology is the most common screening test used to triage PLWH for HRA, but there is a need for tests with improved sensitivity, specificity, and predictive values.77 Testing for carcinogenic HPV types is more sensitive than anal cytology and provides better reassurance that anal HSIL are not present, but the specificity is low. Novel screening biomarkers that could improve specificity are under consideration, including HPV genotyping (most notably for HPV16, which accounts for a large majority of anal cancers, even in PLWH), p16/Ki-67 dual staining of cytology specimens, and host and HPV DNA methylation markers.78, 79 Longitudinal studies evaluating these screening approaches are needed to understand how long negative tests provide reassurance to determine safe screening intervals.
Because current treatments for HSIL often require multiple procedures, and because of limited HRA and treatment capacity, accurate biomarkers that can predict which individuals with HSIL are at highest risk of progression are needed. It is also important to develop therapeutic regimens for HSIL that are less dependent on the ability of the HRA clinician to identify and target all lesions.
Cancer treatment and survival
PLWH are less likely to receive optimal, guideline-concordant cancer treatment compared with patients without HIV, particularly for individuals living in regions with low HIV prevalence.80–83 The largest treatment disparities are seen for those with early-stage cancers where treatment could be curative.80 There are also differences in the type of treatment provided.84 Drivers of cancer treatment disparities include social and structural determinants of health that impede access to care, including HIV stigma leading to decreased disclosure, limited training for oncologists and lack of interdisciplinary management with HIV specialists, and exclusion of PLWH from clinical trial eligibility.85, 86
Cancer survival is poorer among PLWH for many common cancers, including breast, lung, prostate, cervix, and colorectal.87–89 One U.S. study found that lung cancer-specific mortality was 30% higher among PLWH compared to individuals without HIV, and breast cancer-specific mortality was 2.6-times higher among women with HIV compared to women without HIV.89 Poor cancer survival among PLWH likely stems from a nexus of disparities, including social or structural determinants of health limited access to cancer treatment and HIV-related immunologic changes leading to accelerated tumor development and progression. Although delayed diagnosis and poorer access to standard oncologic treatments contribute, survival differences persist even after accounting for these factors.90–94 It should be noted, however, that PLWH can have similar outcomes to those without HIV in high- and low-resource settings. For example, survival among PLWH who are provided guideline-concordant care appears comparable to that in people without HIV for Hodgkin lymphoma, aggressive subtypes of NHL, and anal cancer.95–98 These results indicate that some disparities can be mitigated by ensuring high-quality treatment with appropriate social and medical support.
Cross-cutting themes
The international experts at this meeting identified a number of priority questions related to cancer among PLWH (see Table 2). In addition, attendees identified the following cross-cutting themes to guide future research.
Table 2.
Priority questions for research on cancer in people living with HIV (PLWH).
| Topic | Priority Questions |
|---|---|
| International cancer trends and burden | • How will the regional spectrum of cancers among PLWH change in the coming decades? • Who is at highest risk of cancer among PLWH and what are the determinants of elevated cancer risk among PLWH? • In the setting of widespread access to effective ART, what are the differences, if any, in cancer incidence between resource-rich and resource-limited regions? |
| Cancer etiology and tumor characteristics | • What role do disordered immunity, chronic inflammation, and accelerated aging play in explaining increased cancer risk among PLWH on ART? • Among PLWH, what is the contribution of oncogenic viruses to specific cancer types (beyond the generally accepted list of cancers and their associated viruses), and how do these associations vary across geographic settings and populations? • For specific cancer types, are there important biologic differences between tumors occurring among people living with and without HIV, and are there unique features of tumors among PLWH that can help guide treatment? |
| Cervical cancer | • How will wider and earlier access to ART impact cervical cancer burden in unscreened women with HIV, particularly in high HIV prevalence settings in sub-Saharan Africa? • What is the impact of HPV vaccination among immunocompetent vaccinees who later acquire HIV? • Is the risk of cervical cancer, conditional on a screening test result, different between women living with and without HIV? |
| Kaposi sarcoma | • How can we increase the fraction of KS in sub-Saharan Africa that is diagnosed in an early prognostic phase and better recognize concurrent severe diseases caused by KSHV? • What are the determinants of KS occurring in PLWH on ART with fully suppressed HIV? • What factors sustain high levels of KSHV transmission among children in sub-Saharan Africa and among MSM? • Is it possible to develop, approve and implement a vaccine against KSHV infection? |
| Lung and anal cancers | • Among PLWH, which novel biomarkers or risk scores can identify PLWH who will benefit most from screening for lung and anal cancers? • How can screening for these cancers be implemented effectively and at scale for the HIV population? |
| Cancer treatment and survival | • What are the mechanisms for the adverse effect of HIV infection on cancer survival by cancer site and subtype? • How much of the cancer survival differences in PLWH can be mitigated by reducing disparities in access to care, and how can we implement and disseminate evidence-based, community-engaged strategies to increase access to cancer therapy? |
ART: antiretroviral therapy, KSHV: Kaposi sarcoma herpesvirus HIV: human immunodeficiency virus, MSM: men who have sex with men; PLWH: people living with HIV
Retiring the term “AIDS-defining” cancer
There was strong consensus that the term “AIDS-defining” cancer (ADC) has become obsolete and should be phased out (along with the default opposing “non-ADC”). This is partly because the list of ADCs: a) does not capture all tumors associated with severe immunodeficiency (e.g., conjunctival cancer), b) refers to an outdated nomenclature for NHL subtypes, and c) includes cervical cancer, for which the association with HIV immunodeficiency is no stronger than for many other virus-associated tumors that occur at higher rates among PLWH, (e.g. anal cancer and Hodgkin lymphoma). Most importantly, any grouping of cancers, each of which have their individual specificities, has a likelihood of obscuring relevant biological and clinical differences. Hence, researchers are encouraged to consider separately each cancer type arising in PLWH. Cancers with sufficient evidence for HIV causality (after exclusion of confounding and bias) were judged by an expert consensus convened by IARC in 2009.3 Based on the data accumulated in the last 13 years, this consensus judgement on causality should be updated.
Enhancing and expanding surveillance
Ongoing surveillance of cancer incidence and mortality among PLWH at the regional and national level is critical to quantify and monitor cancer risk and burden over time. In many countries, there is a lack of vital statistic or disease-specific surveillance systems in place, and sustained infrastructure and funding are needed to build these resources. Until widespread surveillance infrastructure is in place in resource-limited settings, it will be the role of researchers to provide and maintain basic cancer monitoring in strategic samples of relevant populations. Within existing HIV-focused clinical cohorts across all global regions, broader capture of important data elements is needed, including information on behavioral risk factors, social determinants of health, environmental exposures, co-morbidities, medications, and details on cancer screening and occurrence, tumor characteristics, cancer treatment and survivorship. The next generation of studies of HIV and cancer should capture a more comprehensive set of data elements to address questions of etiology, and prevention, as well as data on cancer treatment access and quality of care. Approaches for rapid ascertainment of laboratory and clinical data during the COVID-19 pandemic may be transferrable and leveraged to improve completeness of cancer data in PLWH in some countries.
Understanding the impact of aging
Cancer prevention, screening, and treatment guidelines will need to be tailored to an increasingly older HIV population worldwide. With ART, PLWH are living longer, and consequently, the age distribution has changed. Rigorous epidemiologic approaches are required to account for this dramatic demographic shift to avoid biases.36 There is also a need to determine whether HIV accelerates biological aging and, if so, which mechanisms (e.g., immune senescence, inflammation), are particularly relevant to cancer development, prevention, and progression. An alternative possibility that should be assessed is whether elevated cancer rates at younger ages among PLWH can instead be explained by earlier, or more frequent, exposure to carcinogenic infections or tobacco.
Expanding molecular epidemiology and genetic studies
In the setting of partially restored immunity, pathogenic processes other than profound T-cell-mediated immunosuppression could play a key role in determining cancer incidence and outcomes among PLWH. Development and utilization of biomarkers of these processes (e.g., chronic inflammation, oxidative stress, and DNA damage) will provide insight into these pathways. In addition, detailed pathological and genetic studies of tumors from PLWH (e.g., tumor sequencing, assessment of the tumor microenvironment) will yield etiologic information and inform new and better therapeutic strategies. It is important to invest in epidemiologic and clinical studies that collect biological samples, and in pathology-based repositories of tumor tissues, which will enable such molecular epidemiology studies.
Expanding implementation and health services research
For many of the cancers discussed, the question of how to implement effective tools and approaches for prevention and screening is critical for control of cancer in PLWH.99 These tools/approaches could be for primary prevention (e.g., HPV vaccination), screening (e.g., cervical, anal, or lung cancer), or early detection by improved diagnosis (e.g., KS and NHL). The over-arching common need is to ensure that such research is performed in the specific context of PLWH and, as much as possible, in a manner allowing direct comparison with people without HIV. There is a need to determine whether approaches must be modified for PLWH or tailored to different geographic and community settings. These concepts can also be applied equally to cancer treatment research for PLWH. Community-based intervention design, implementation, and dissemination, in partnership with PLWH, is needed.
Conclusions
The continuing evolution of the HIV epidemic has generated new and persistently unanswered questions regarding cancer among PLWH. These questions provide avenues for greater understanding of the etiology of cancer, and opportunities to apply translational science to improve cancer prevention, screening, and treatment among PLWH. It is recognized that large disparities remain with respect to cancer in PLWH, including differences in disease burden, access to prevention and treatment, and outcomes, both across the world and within single countries. Realizing scientific opportunities relevant to PLWH worldwide, and in lower- and middle-income countries in particular, will require focused, sustained and strategic investments to support the transdisciplinary science needed to advance the field. Because of the global nature of these issues, success in this next generation of research studies on HIV and cancer will depend on a robust system of international collaborations among laboratory scientists, clinicians, and epidemiologists as well as engagement of the many people who are affected by HIV and cancer worldwide.
Acknowledgements:
We are indebted to Philippine Gason (IARC), Nadia Nimley (NCI), and Kelly Yu (NCI) for providing logistic support to the workshop, and Nadia Akel (IARC) for editorial support.
Funding:
The work was funded by the Intramural Research Program of the National Cancer Institute (NCI), and by the International Agency for Research on Cancer.
Conflict of Interest:
RVB reports support from the US National Institutes of Health, the Bill and Melinda Gates Foundation, and the World Health Organization. Regeneron Pharmaceuticals covered the cost of abstract and manuscript writing outside the submitted work. RVB serves on a Gilead Sciences DMC for which she receives an honorarium. KL reports research support from Bristol Myers Squibb and CTI BioPharma through CRADAs with the NCI and receiving drugs for clinical trials from Merck, EMD-Serono, and Eli Lilly. JMP reports financial support as Vir Biotechnologies- consultant; Virion Therapeutics- consultant; Antiva Biosciences- research grant support; Abbott- consultant; Roche Diagnostics-consultant; Vaccitech- research grant support. RR reports funding for clinical trials via Cooperative Research and Development Agreements (CRADA) between the institution and the following companies - BMS/Celgene, EMD-Serano, PDS Biotech, CTI Biopharma, Merck, and Lilly. GS reports grant funding from NIH/NCI. RY reports receiving research support from Celgene (now Bristol Myers Squibb) and CTI BioPharma through CRADAs with the NCI. RY also reports receiving drugs for clinical trials from Merck, EMD-Serano, and Eli Lill through CRADAs with the NCI, and he has received drug supply for laboratory research from Janssen Pharmaceuticals. RY is a co-inventor on US Patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers.” An immediate family member of RY is a co-inventor on patents or patent applications related to internalization of target receptors, epigenetic analysis, and ephrin tyrosine kinase inhibitors. All rights, title, and interest to these patents have been assigned to the U.S. Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L.99-502).
Abbreviations:
- ART
antiretroviral therapy
- HIV
human immunodeficiency virus
- HPV
human papillomavirus
- HSIL
high-grade squamous intraepithelial lesions
- IARC
International Agency for Research on Cancer
- KS
Kaposi sarcoma
- KSHV
Kaposi sarcoma herpesvirus
- LDCT
low-dose computed tomography
- NCI
National Cancer Institute
- NHL
non-Hodgkin lymphoma
- PD-L1
programmed death-ligand 1
- PLWH
people living with HIV
- SSA
sub-Saharan Africa
- U.S.
United States of America
- WHO
World Health Organization
Footnotes
Disclaimer: The authors alone are responsible for the views expressed in this paper and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
References
- 1.UNAIDS. Global HIV & AIDS statistics — Fact sheet, vol. 2023. [Google Scholar]
- 2.Ruffieux Y, Muchengeti M, Egger M, Efthimiou O, Bartels L, Olago V, Davidovic M, Dhokotera T, Bohlius J, Singh E, Rohner E. Immunodeficiency and Cancer in 3.5 Million People Living With Human Immunodeficiency Virus (HIV): The South African HIV Cancer Match Study. Clin Infect Dis 2021;73: e735–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, A review of human carcinogens. Part B: Biological agents, 2009. [Google Scholar]
- 4.Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017;4: e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong IKJ, Grulich AE, Poynten IM, Polizzotto MN, van Leeuwen MT, Amin J, McGregor S, Law M, Templeton DJ, Vajdic CM, Jin F. Time trends in cancer incidence in Australian people living with HIV between 1982 and 2012. HIV Med 2022;23: 134–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hleyhel M, Hleyhel M, Bouvier AM, Belot A, Tattevin P, Pacanowski J, Genet P, De Castro N, Berger JL, Dupont C, Lavole A, Pradier C, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS 2014;28: 2109–18 [DOI] [PubMed] [Google Scholar]
- 7.Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, Genet P, De Castro N, Berger JL, Dupont C, Lavole A, Pradier C, Salmon D, et al. Risk of AIDS-defining cancers among HIV-1-infected patients in France between 1992 and 2009: results from the FHDH-ANRS CO4 cohort. Clin Infect Dis 2013;57: 1638–47 [DOI] [PubMed] [Google Scholar]
- 8.Sengayi-Muchengeti M, Singh E, Chen WC, Bradshaw D, de Villiers CB, Newton R, Waterboer T, Mathew CG, Sitas F. Thirteen cancers associated with HIV infection in a Black South African cancer patient population (1995-2016). Int J Cancer 2022 [DOI] [PubMed] [Google Scholar]
- 9.Rohner E, Butikofer L, Schmidlin K, Sengayi M, Maskew M, Giddy J, Taghavi K, Moore RD, Goedert JJ, Gill MJ, Silverberg MJ, D’Souza G, et al. Cervical cancer risk in women living with HIV across four continents: A multicohort study. Int J Cancer 2020;146: 601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IeDEA AI-dCPWGf, EuroCoord Ci. Comparison of Kaposi Sarcoma Risk in Human Immunodeficiency Virus-Positive Adults Across 5 Continents: A Multiregional Multicohort Study. Clin Infect Dis 2017;65: 1316–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhokotera T, Bohlius J, Spoerri A, Egger M, Ncayiyana J, Olago V, Singh E, Sengayi M. The burden of cancers associated with HIV in the South African public health sector, 2004-2014: a record linkage study. Infect Agent Cancer 2019;14: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pediatric Aids-Defining Cancer Project Working Group for IeDea Southern Africa T, EuroCoord Ci, Rohner E, Schmidlin K, Zwahlen M, Chakraborty R, Clifford G, Obel N, Grabar S, Verbon A, Noguera-Julian A, Collins IJ, et al. Kaposi Sarcoma Risk in HIV-Infected Children and Adolescents on Combination Antiretroviral Therapy From Sub-Saharan Africa, Europe, and Asia. Clin Infect Dis 2016;63: 1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aids-defining Cancer Project Working Group of IeDea CiE. Non-Hodgkin lymphoma risk in adults living with HIV across five continents. AIDS 2018;32: 2777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, Seage GR 3rd, Suneja G, Kayembe MK, Mmalane M, Rebbeck T, Rider JR, Essex M, Lockman S. Cancer Incidence following Expansion of HIV Treatment in Botswana. PLoS One 2015;10: e0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bukirwa P, Wabinga H, Nambooze S, Amulen PM, Joko WY, Liu B, Parkin DM. Trends in the incidence of cancer in Kampala, Uganda, 1991 to 2015. Int J Cancer 2021;148: 2129–38 [DOI] [PubMed] [Google Scholar]
- 16.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med 2018;168: 866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ, Engels EA. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011;103: 753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruffieux Y, Muchengeti M, Olago V, Dhokotera T, Bohlius J, Egger M, Rohner E. Age and cancer incidence in 5.2 million people with HIV: the South African HIV Cancer Match study. Clin Infect Dis 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muchengeti M, Bartels L, Olago V, Dhokotera T, Chen WC, Spoerri A, Rohner E, Butikofer L, Ruffieux Y, Singh E, Egger M, Bohlius J. Cohort profile: the South African HIV Cancer Match (SAM) Study, a national population-based cohort. BMJ Open 2022;12: e053460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shamu T, Rohner E, Chokunonga E, Spoerri A, Mandiriri A, Chimbetete C, Egger M, Bohlius J, Borok M. Cancer incidence among people living with HIV in Zimbabwe: A record linkage study. Cancer Rep (Hoboken) 2022;5: e1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horner MJ, Chasimpha S, Spoerri A, Edwards J, Bohlius J, Tweya H, Tembo P, Nkhambule F, Phiri EM, Miller WC, Malisita K, Phiri S, et al. High Cancer Burden Among Antiretroviral Therapy Users in Malawi: A Record Linkage Study of Observational Human Immunodeficiency Virus Cohorts and Cancer Registry Data. Clin Infect Dis 2019;69: 829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sengayi M, Spoerri A, Egger M, Kielkowski D, Crankshaw T, Cloete C, Giddy J, Bohlius J. Record linkage to correct under-ascertainment of cancers in HIV cohorts: The Sinikithemba HIV clinic linkage project. Int J Cancer 2016;139: 1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohlius J, Maxwell N, Spoerri A, Wainwright R, Sawry S, Poole J, Eley B, Prozesky H, Rabie H, Garone D, Technau KG, Maskew M, et al. Incidence of AIDS-defining and Other Cancers in HIV-positive Children in South Africa: Record Linkage Study. Pediatr Infect Dis J 2016;35: e164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chammartin F, Lodi S, Logan R, Ryom L, Mocroft A, Kirk O, d’Arminio Monforte A, Reiss P, Phillips A, El-Sadr W, Hatleberg CI, Pradier C, et al. Risk for Non-AIDS-Defining and AIDS-Defining Cancer of Early Versus Delayed Initiation of Antiretroviral Therapy : A Multinational Prospective Cohort Study. Ann Intern Med 2021;174: 768–76 [DOI] [PubMed] [Google Scholar]
- 25.Castilho JL, Bian A, Jenkins CA, Shepherd BE, Sigel K, Gill MJ, Kitahata MM, Silverberg MJ, Mayor AM, Coburn SB, Wiley D, Achenbach CJ, et al. CD4/CD8 Ratio and Cancer Risk Among Adults With HIV. J Natl Cancer Inst 2022;114: 854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piketty C, Selinger-Leneman H, Bouvier AM, Belot A, Mary-Krause M, Duvivier C, Bonmarchand M, Abramowitz L, Costagliola D, Grabar S. Incidence of HIV-related anal cancer remains increased despite long-term combined antiretroviral treatment: results from the french hospital database on HIV. J Clin Oncol 2012;30: 4360–6 [DOI] [PubMed] [Google Scholar]
- 27.Hessol NA, Martínez-Maza O, Levine AM, Morris A, Margolick JB, Cohen MH, Jacobson LP, Seaberg EC. Lung cancer incidence and survival among HIV-infected and uninfected women and men. Aids 2015;29: 1183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis 2016;214 Suppl 2: S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, O’Connell RJ, Rupert A, Chomont N, Valcour V, Kim JH, Robb ML, et al. Persistent, Albeit Reduced, Chronic Inflammation in Persons Starting Antiretroviral Therapy in Acute HIV Infection. Clin Infect Dis 2017;64: 124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borges ÁH, Silverberg MJ, Wentworth D, Grulich AE, Fätkenheuer G, Mitsuyasu R, Tambussi G, Sabin CA, Neaton JD, Lundgren JD. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. Aids 2013;27: 1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedimo RJ, Park LS, Shebl FM, Sigel K, Rentsch CT, Crothers K, Rodriguez-Barradas MC, Goetz MB, Butt AA, Brown ST, Gibert C, Justice AC, et al. Statin exposure and risk of cancer in people with and without HIV infection. Aids 2021;35: 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breen EC, Sehl ME, Shih R, Langfelder P, Wang R, Horvath S, Bream JH, Duggal P, Martinson J, Wolinsky SM, Martinez-Maza O, Ramirez CM, et al. Accelerated aging with HIV begins at the time of initial HIV infection. iScience 2022;25: 104488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr 2012;60 Suppl 1: S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Pasca S, Post WS, Langan S, Pallavajjala A, Haley L, Gocke CD, Budoff M, Haberlen S, Brown TT, Ambinder RF, Margolick JB, et al. Clonal hematopoiesis in men living with HIV and association with subclinical atherosclerosis. Aids 2022;36: 1521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiels MS, Althoff KN, Pfeiffer RM, Achenbach CJ, Abraham AG, Castilho J, Cescon A, D’Souza G, Dubrow R, Eron JJ, Gebo K, John Gill M, et al. HIV Infection, Immunosuppression, and Age at Diagnosis of Non-AIDS-Defining Cancers. Clin Infect Dis 2017;64: 468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiels MS, Pfeiffer RM, Engels EA. Age at cancer diagnosis among persons with AIDS in the United States. Ann Intern Med 2010;153: 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caro-Vegas C, Ramirez C, Landis J, Adimora AA, Strickler H, French AL, Ofotokun I, Fischl M, Seaberg EC, Wang CJ, Spence AB, Dittmer DP. Molecular profiling of breast and lung cancer in women with HIV reveals high tumor mutational burden. AIDS 2022;36: 567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardi A, Porter VL, Zong Z, Bowlby R, Titmuss E, Namirembe C, Griner NB, Petrello H, Bowen J, Chan SK, Culibrk L, Darragh TM, et al. Analysis of Ugandan cervical carcinomas identifies human papillomavirus clade-specific epigenome and transcriptome landscapes. Nat Genet 2020;52: 800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanik EL, Kaunitz GJ, Cottrell TR, Succaria F, McMiller TL, Ascierto ML, Esandrio J, Xu H, Ogurtsova A, Cornish T, Lipson EJ, Topalian SL, et al. Association of HIV Status With Local Immune Response to Anal Squamous Cell Carcinoma: Implications for Immunotherapy. JAMA Oncol 2017;3: 974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crequit P, Ruppert AM, Rozensztajn N, Gounant V, Vieira T, Poulot V, Antoine M, Chouaid C, Wislez M, Cadranel J, Lavole A. EGFR and KRAS mutation status in non-small-cell lung cancer occurring in HIV-infected patients. Lung Cancer 2016;96: 74–7 [DOI] [PubMed] [Google Scholar]
- 41.Julius P, Siyumbwa SN, Moonga P, Maate F, Kaile T, Haynatski G, Minhas V, Snow J, Peterson K, Gihozo P, Streeter S, Kaur S, et al. Epstein-Barr Virus, But Not Human Papillomavirus, Is Associated With Preinvasive and Invasive Ocular Surface Squamous Neoplasias in Zambian Patients. Front Oncol 2022;12: 864066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calkins KL, Chander G, Joshu CE, Visvanathan K, Fojo AT, Lesko CR, Moore RD, Lau B. Immune Status and Associated Mortality After Cancer Treatment Among Individuals With HIV in the Antiretroviral Therapy Era. JAMA Oncol 2020;6: 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao C, Young PA, Xu L, Silverberg MJ, Said JW, Timmerman JM. Analysis of Programmed Death Ligand 1 (PD-L1) Expression in Diffuse Large B Cell Lymphomas (DLBCL) in HIV-Negative Versus HIV-Positive Patients. Blood. 2015;126(23):146226265695 [Google Scholar]
- 44.Liu Y, Gaisa MM, Wang X, Swartz TH, Arens Y, Dresser KA, Sigel C, Sigel K. Differences in the Immune Microenvironment of Anal Cancer Precursors by HIV Status and Association With Ablation Outcomes. J Infect Dis 2018;217: 703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stelzle D, Tanaka LF, Lee KK, Ibrahim Khalil A, Baussano I, Shah ASV, McAllister DA, Gottlieb SL, Klug SJ, Winkler AS, Bray F, Baggaley R, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health 2021;9: e161–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018;32: 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly H, Weiss HA, Benavente Y, de Sanjose S, Mayaud P, Art, Group HPVR. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018;5: e45–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhokotera T, Asangbeh S, Bohlius J, Singh E, Egger M, Rohner E, Ncayiyana J, Clifford GM, Olago V, Sengayi-Muchengeti M. Cervical cancer in women living in South Africa: a record linkage study of the National Health Laboratory Service and the National Cancer Registry. Ecancermedicalscience 2022;16: 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall MT, Smith MA, Simms KT, Barnabas R, Murray JM, Canfell K. Elimination of cervical cancer in Tanzania: Modelled analysis of elimination in the context of endemic HIV infection and active HIV control. Int J Cancer 2021;149: 297–306 [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Mugo NR, Bayer C, Rao DW, Onono M, Mgodi NM, Chirenje ZM, Njoroge BW, Tan N, Bukusi EA, Barnabas RV. Impact of catch-up human papillomavirus vaccination on cervical cancer incidence in Kenya: A mathematical modeling evaluation of HPV vaccination strategies in the context of moderate HIV prevalence. EClinicalMedicine 2022;45: 101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim Khalil A, Mpunga T, Wei F, Baussano I, de Martel C, Bray F, Stelzle D, Dryden-Peterson S, Jaquet A, Horner MJ, Awolude OA, Trejo MJ, et al. Age-specific burden of cervical cancer associated with HIV: A global analysis with a focus on sub-Saharan Africa. Int J Cancer 2022;150: 761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnabas RV, Brown ER, Onono MA, Bukusi EA, Njoroge B, Winer RL, Galloway DA, Pinder LF, Donnell D, Wakhungu I, Congo O, Biwott C, et al. Efficacy of single-dose HPV vaccination among young African women. NEJM Evid 2022;1: EVIDoa2100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, Verma Y, Esmy PO, Poli URR, Shah A, Zomawia E, Pimple S, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol 2021;22: 1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joint Committee on Vaccination and Immunisation, JCVI statement on a one-dose schedule for the routine HPV immunisation programme, 2022. [Google Scholar]
- 55.World Health Organization, Meeting of the Strategic Advisory Group of Experts on Immunization, April 2022: conclusions and recommendations, 2022. [Google Scholar]
- 56.Bouvard V, Wentzensen N, Mackie A, Berkhof J, Brotherton J, Giorgi-Rossi P, Kupets R, Smith R, Arrossi S, Bendahhou K, Canfell K, Chirenje ZM, et al. The IARC Perspective on Cervical Cancer Screening. N Engl J Med 2021;385: 1908–18 [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization, WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, 2022. [PubMed] [Google Scholar]
- 58.Castle PE, Befano B, Schiffman M, Wentzensen N, Lorey T, Poitras N, Hyer M, Cheung LC. A comparison of high-grade cervical abnormality risks in women living with and without human immunodeficiency virus undergoing routine cervical-cancer screening. Prev Med 2022;162: 107157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins HA, Strickler HD, Massad LS, Pierce CB, Darragh TM, Minkoff H, Keller MJ, Fischl M, Palefsky J, Flowers L, Rahangdale L, Milam J, et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach. AIDS 2017;31: 1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kahesa C, Thomsen LT, Linde DS, McHome B, Katanga J, Swai P, Manongi R, Kjaerem M, Iftner T, Waldstrom M, Mwaiselage J, Rasch V, et al. Comparison of human papillomavirus-based cervical cancer screening strategies in Tanzania among women with and without HIV. Int J Cancer 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramaswami R, Lurain K, Polizzotto MN, Ekwede I, Waldon K, Steinberg SM, Mangusan R, Widell A, Rupert A, George J, Goncalves PH, Marshall VA, et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Adv 2021;5: 1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palich R, Makinson A, Veyri M, Guihot A, Valantin MA, Bregigeon-Ronot S, Poizot-Martin I, Solas C, Grabar S, Martin-Blondel G, Spano JP. Kaposi’s Sarcoma in Virally Suppressed People Living with HIV: An Emerging Condition. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casper C, Corey L, Cohen JI, Damania B, Gershon AA, Kaslow DC, Krug LT, Martin J, Mbulaiteye SM, Mocarski ES, Moore PS, Ogembo JG, et al. KSHV (HHV8) vaccine: promises and potential pitfalls for a new anti-cancer vaccine. NPJ Vaccines 2022;7: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haas CB, Engels EA, Horner MJ, Freedman ND, Luo Q, Gershman S, Qiao B, Pfeiffer RM, Shiels MS. Trends and risk of lung cancer among people living with HIV in the USA: a population-based registry linkage study. Lancet HIV 2022;9: e700–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health 2017;5: e578–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirk GD, Merlo CA, Lung HIVS. HIV infection in the etiology of lung cancer: confounding, causality, and consequences. Proc Am Thorac Soc 2011;8: 326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tron L, Lert F, Spire B, Dray-Spira R, group AN-Vs. Tobacco smoking in HIV-infected versus general population in france: heterogeneity across the various groups of people living with HIV. PLoS One 2014;9: e107451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, Sverzellati N, Sozzi G, Corrao G, Marchianò A. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol 2019;30: 1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Landefeld CS, Li L, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2021;325: 962–70 [DOI] [PubMed] [Google Scholar]
- 71.Makinson A, Eymard-Duvernay S, Raffi F, Abgrall S, Bommart S, Zucman D, Valour F, Cheret A, Poizot-Martin I, Duvivier C, Mauboussin JM, Bonnet F, et al. Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. Aids 2016;30: 573–82 [DOI] [PubMed] [Google Scholar]
- 72.Díaz-Álvarez J, Roiz P, Gorospe L, Ayala A, Pérez-Pinto S, Martínez-Sanz J, Sánchez-Conde M, Casado JL, Pérez-Elías MJ, Moreno A, Ron R, Vivancos MJ, et al. Implementation of a lung cancer screening initiative in HIV-infected subjects. PLoS One 2021;16: e0260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, Rodriguez-Barradas MC, Gibert C, Goetz MB, Bedimo R, Park LS, Dubrow R. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV 2017;4: e67–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makinson A, Tron L, Grabar S, Milleron B, Reynes J, Le Moing V, Morquin D, Lert F, Costagliola D, Guiguet M. Potential lung cancer screening outcomes using different age and smoking thresholds in the ANRS-CO4 French Hospital Database on HIV cohort. HIV Med 2020;21: 180–8 [DOI] [PubMed] [Google Scholar]
- 75.Kong CY, Sigel K, Criss SD, Sheehan DF, Triplette M, Silverberg MJ, Henschke CI, Justice A, Braithwaite RS, Wisnivesky J, Crothers K. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+ cell count at least 500 cells/μl. Aids 2018;32: 1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palefsky JM, Lee JY, Jay N, Goldstone SE, Darragh TM, Dunlevy HA, Rosa-Cunha I, Arons A, Pugliese JC, Vena D, Sparano JA, Wilkin TJ, et al. Treatment of Anal High-Grade Squamous Intraepithelial Lesions to Prevent Anal Cancer. N Engl J Med 2022;386: 2273–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clarke MA, Deshmukh AA, Suk R, Roberts J, Gilson R, Jay N, Stier EA, Wentzensen N. A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int J Cancer 2022;151: 1889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen CM, Wentzensen N, Lahrmann B, Tokugawa D, Poitras N, Bartels L, Krauthoff A, Keil A, Miranda F, Castle PE, Lorey T, Hare B, et al. Automated Evaluation of p16/Ki-67 Dual-Stain Cytology as a Biomarker for Detection of Anal Precancer in Men Who Have Sex With Men and Are Living With Human Immunodeficiency Virus. Clin Infect Dis 2022;75: 1565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van der Zee RP, Meijer C, Cuming T, Kreuter A, van de Sandt MM, Quint WGV, de Vries HJC, Prins JM, Steenbergen RDM. Characterisation of anal intraepithelial neoplasia and anal cancer in HIV-positive men by immunohistochemical markers p16, Ki-67, HPV-E4 and DNA methylation markers. Int J Cancer 2021;149: 1833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, Macomber KE, Melville SK, Engels EA. Cancer treatment disparities in HIV-infected individuals in the United States. J Clin Oncol 2014;32: 2344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suneja G, Shiels MS, Melville SK, Williams MA, Rengan R, Engels EA. Disparities in the treatment and outcomes of lung cancer among HIV-infected individuals. AIDS 2013;27: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suneja G, Lin CC, Simard EP, Han X, Engels EA, Jemal A. Disparities in cancer treatment among patients infected with the human immunodeficiency virus. Cancer 2016;122: 2399–407 [DOI] [PubMed] [Google Scholar]
- 83.Rositch AF, Jiang S, Coghill AE, Suneja G, Engels EA. Disparities and Determinants of Cancer Treatment in Elderly Americans Living With Human Immunodeficiency Virus/AIDS. Clin Infect Dis 2018;67: 1904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Islam JY, Nogueira L, Suneja G, Coghill A, Akinyemiju T. Palliative Care Use Among People Living With HIV and Cancer: An Analysis of the National Cancer Database (2004-2018). JCO Oncol Pract 2022;18: e1683–e93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knettel B, Corrigan K, Cherenack E, Ho N, Carr S, Cahill J, Chino J, Ubel P, Watt M, Suneja G. HIV, cancer, and coping: The cumulative burden of a cancer diagnosis among people living with HIV. J Psychosoc Oncol 2021;39: 734–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corrigan KL, Knettel BA, Ho N, Carr S, Shah B, Cahill J, Chino J, Watt MH, Suneja G. Improving Access to Cancer Care in the HIV Population: Qualitative Research to Identify Barriers to Care. Health Equity 2020;4: 468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coghill AE, Engels EA, Schymura MJ, Mahale P, Shiels MS. Risk of Breast, Prostate, and Colorectal Cancer Diagnoses Among HIV-Infected Individuals in the United States. J Natl Cancer Inst 2018;110: 959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer 2019;125: 2868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol 2015;33: 2376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ayeni OA, O’Neil DS, Pumpalova YS, Chen WC, Nietz S, Phakathi B, Buccimazza I, Cacala S, Stopforth LW, Farrow HA, Mapanga W, Joffe M, et al. Impact of HIV infection on survival among women with stage I-III breast cancer: Results from the South African breast cancer and HIV outcomes study. Int J Cancer 2022;151: 209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chasimpha S, McCormack V, Cubasch H, Joffe M, Zietsman A, Galukande M, Parham G, Pinder LF, Anele A, Adisa CA, Offiah AU, Anderson BO, et al. Disparities in breast cancer survival between women with and without HIV across sub-Saharan Africa (ABC-DO): a prospective, cohort study. Lancet HIV 2022;9: e160–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, Chiyapo S, Ramogola-Masire D, Kebabonye-Pusoentsi M, Clayman R, Mapes AC, Tapela N, Asmelash A, et al. HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol 2016;34: 3749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fuzzell LN, Vadaparampil ST, Giuliano AR, Liu Y, Coghill AE. Patterns of HIV Self-Disclosure in the Oncology Setting. JNCI Cancer Spectr 2021;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marcus JL, Chao C, Leyden WA, Xu L, Yu J, Horberg MA, Klein D, Towner WJ, Quesenberry CP Jr., Abrams DI, Silverberg MJ. Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev 2015;24: 1167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bryant AK, Huynh-Le MP, Simpson DR, Gupta S, Sharabi AB, Murphy JD. Association of HIV Status With Outcomes of Anal Squamous Cell Carcinoma in the Era of Highly Active Antiretroviral Therapy. JAMA Oncol 2018;4: 120–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gopal S, Fedoriw Y, Kaimila B, Montgomery ND, Kasonkanji E, Moses A, Nyasosela R, Mzumara S, Varela C, Chikasema M, Makwakwa V, Itimu S, et al. CHOP Chemotherapy for Aggressive Non-Hodgkin Lymphoma with and without HIV in the Antiretroviral Therapy Era in Malawi. PLoS One 2016;11: e0150445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milligan MG, Bigger E, Abramson JS, Sohani AR, Zola M, Kayembe MKA, Medhin H, Suneja G, Lockman S, Chabner BA, Dryden-Peterson SL. Impact of HIV Infection on the Clinical Presentation and Survival of Non-Hodgkin Lymphoma: A Prospective Observational Study From Botswana. J Glob Oncol 2018;4: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moahi K, Ralefala T, Nkele I, Triedman S, Sohani A, Musimar Z, Efstathiou J, Armand P, Lockman S, Dryden-Peterson S. HIV and Hodgkin Lymphoma Survival: A Prospective Study in Botswana. JCO Glob Oncol 2022;8: e2100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taghavi K, Mandiriri A, Shamu T, Rohner E, Butikofer L, Asangbeh S, Magure T, Chimbetete C, Egger M, Pascoe M, Bohlius J. Cervical Cancer Screening Cascade for women living with HIV: a cohort study from Zimbabwe. PLOS Glob Public Health 2022;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ibrahim Khalil A, Franceschi S, de Martel C, Bray F, Clifford GM. Burden of Kaposi sarcoma according to HIV status: A systematic review and global analysis. Int J Cancer 2022;150: 1948–57 [DOI] [PubMed] [Google Scholar]


