Abstract
We report that the expression of the Bacillus megaterium bmlP1 gene is subject to negative regulation by the bmlP1 3′ flanking region. This repression occurred both in B. megaterium and in Escherichia coli. When the bmlP1 promoter was replaced with a heterologous promoter or when the orientation of the bmlP1 3′ flanking region was reversed, the inhibitory effect was still observed. However, the bmlP1 3′ flanking region was unable to exert repression on a heterologous gene when fused downstream in either orientation, and it was incapable of acting in trans. Dot blot and Northern blot analyses revealed that the repression occurred at the RNA level. Deletion analysis showed that the regulatory site responsible for the repression is located within a 116-bp region immediately following the bmlP1 gene. Possible mechanisms for this repression are discussed.
It is known that some structural elements in the 5′ or 3′ untranslated regions of bacterial genes can influence gene expression. For example, the ompA 5′ untranslated segment functions in Escherichia coli as an mRNA stabilizer, thus increasing OmpA production (2). The Bacillus subtilis senS gene contains a transcription attenuator located between the promoter and its coding region, which significantly reduces senS gene expression (19). There is also considerable evidence that stem-loop structures at the 3′ ends of bacterial transcripts play an important role in protecting mRNA from 3′ exonuclease digestion (1, 8, 9, 20). On the other hand, it has been shown that the cis-acting element sib, located about 260 bp downstream of the int gene, negatively regulates the int gene expression (4). This type of regulation is called retroregulation (3, 20).
The BmlP1 protein, which belongs to the AcrR/TetR family of transcriptional regulators and contains a typical helix-turn-helix DNA-binding motif (11) at its N-terminal region, is encoded by the bmlP1 gene, which is located upstream of the cytochrome P450BM-1 gene (5). BmlP1 was previously proposed to contain 98 amino acids and to function as a positive transcription factor for barbiturate-mediated induction of P450BM-1 (5). Recently, we provided evidence that BmlP1 is actually a protein of 217 amino acids and is not involved in barbiturate-mediated induction of P450BM-1 (15). We also found that a 53-bp inverted repeat located midway between the P450BM-1 gene and bmlP1 gene can act as a negative cis-acting element in controlling the expression of the divergently transcribed P450BM-1 and bmlP1 genes (16).
Our previously reported nucleotide sequence analysis revealed that an open reading frame encoding a putative ABC transporter (10) of 539 amino acids lies downstream of the bmlP1 gene (15). The contiguity of these two genes was confirmed by PCR with various pairs of synthetic oligonucleotides as primers and the genomic DNA of Bacillus megaterium as the template (15). Between these two genes is a 610-bp intergenic region where numerous translational stop codons are dispersed in all of the three reading frames. Located about 95 bp upstream of the translational start site of the ABC transporter gene is a 25-bp inverted repeat, which overlaps the −10 region of the promoter of the ABC transporter gene and may serve as an operator for a putative regulatory protein (Fig. 1). Except for the above-mentioned 25-bp putative operator sequence, no other inverted repeat which is followed by a stretch of thymidine residues and may potentially serve as a ρ-independent transcription terminator exists within the bmlP1-ABC transporter intergenic region. In this work, we demonstrate that a 116-bp region immediately following the bmlP1 gene can act as a negative cis-acting element in controlling the bmlP1 gene expression. We also discuss possible mechanisms for this control.
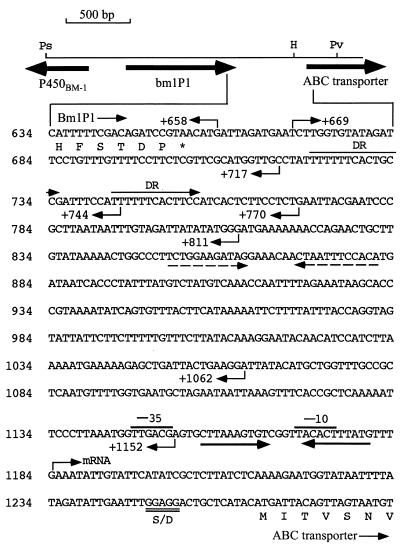
FIG. 1.
Nucleotide sequence of the bmlP1 3′ flanking region. The nucleotide positions are numbered relative to the translational start site of bmlP1. The translational stop codon of bmlP1 is indicated by an asterisk. The −35 and −10 regions of the ςA-like promoter of the putative ABC transporter gene are overlined. The inverted repeat which overlaps the −10 region of the promoter of the ABC transporter gene and may serve as an operator is indicated by a pair of solid inverted arrows. The putative ribosome-binding site (S/D) of the ABC transporter gene is double underlined. A pair of dashed arrows denotes a pair of imperfect inverted repeats. DR represents a pair of imperfect direct repeats. The endpoint of each primer used in this work is indicated by an arrow as well as the number of the base position. H, HincII; Ps, PstI; Pv, PvuII.
Negative effect of the bmlP1 3′ flanking region on BmlP1 production in E. coli.
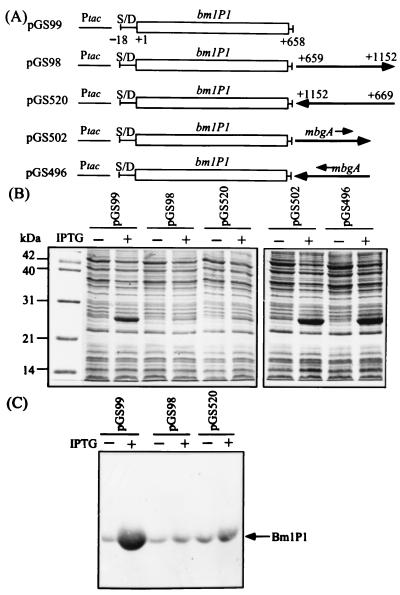
We first investigated whether the presence of such a long bmlP1-ABC transporter intergenic region would have any effect on bmlP1 expression. DNA fragments containing the bmlP1 coding region or bmlP1 coding region plus its 500-bp 3′ flanking region were amplified by PCR and cloned between the EcoRI and HindIII sites of the expression vector pKK223-3 (Amersham Pharmacia Biotech Ltd., Little Chalfont, United Kingdom) to generate plasmids pGS99 and pGS98 (Fig. 2A), respectively. Expression of the bmlP1 gene and its 3′ flanking sequence was thus under the control of the tac promoter. Whole-cell protein extracts prepared from E. coli cells carrying these plasmids were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (7). As shown in Fig. 2B, a substantial amount of the BmlP1 protein was produced in the extract from E. coli cells carrying plasmid pGS99 and grown in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG). In contrast, very little of the BmlP1 protein of the same size as that synthesized from pGS99 was produced from cells harboring plasmid pGS98 (Fig. 2B). Western blot analysis (18) using the polyclonal anti-BmlP1 antibodies (16) confirmed that the sizes of the BmlP1 proteins synthesized from plasmids pGS98 and pGS99 were identical (Fig. 2C).
FIG. 2.
Negative effect of the bmlP1 3′ flanking region on BmlP1 production in E. coli. (A) The expression vector pKK223-3-derived plasmids, pGS99 and pGS98, contain the bmlP1 coding region alone and the bmlP1 coding region plus the 500-bp 3′ flanking region, respectively. In the pKK223-3-derived plasmid pGS520, the 490-bp bmlP1 3′ flanking region is fused in the reverse orientation to the 3′ end of bmlP1. A 360-bp internal region (from positions +174 to +532 relative to the translational start site) of the B. megaterium mbgA gene is fused in both orientations to the 3′ end of bmlP1 in plasmid pGS99 to generate plasmids pGS496 and pGS502. Numbers of base positions are given relative to the translational start site of bmlP1. S/D, putative ribosome-binding site of bmlP1; Ptac, the tac promoter. (B) E. coli cells carrying the above plasmids were grown at 37°C to an absorbance at 600 nm of 0.5 and then treated with 0.3 mM IPTG for 2 h or left untreated. Whole-cell protein extracts from equal numbers of cells were subjected to SDS-PAGE. (C) Whole-cell protein extracts used for panel B were subjected to SDS-PAGE followed by immunoblotting with polyclonal anti-BmlP1 antibodies as the probe.
To rule out the possibility that the difference in the expression levels of BmlP1 is due to the difference in the copy numbers of plasmids pGS98 and pGS99, we isolated these two plasmids from equal numbers of E. coli cells and digested them with EcoRI and HindIII to cut out the insert. An aliquot was run on an agarose gel, stained with ethidium bromide, and photographed. Comparable band intensity was observed for the expression vector pKK223-3 (data not shown), indicating that the observed difference in the expression levels of BmlP1 is not caused by a difference in plasmid copy numbers. These results suggest that the presence of the 500-bp bmlP1 3′ flanking region inhibits BmlP1 production.
The bmlP1 3′ flanking region acts on BmlP1 production in an orientation-independent manner.
We next determined whether the bmlP1 3′ flanking region fused in the reverse orientation to the 3′ end of bmlP1 could still exert a negative effect on BmlP1 production. A DNA fragment containing the 490-bp bmlP1 3′ flanking region was fused in the reverse orientation to the 3′ end of bmlP1 in plasmid pGS99 to generate plasmid pGS520 (Fig. 2A). SDS-PAGE analysis of whole-cell protein extracts from E. coli cells carrying pGS520 showed that very little BmlP1 protein was produced, which was comparable to that observed in E. coli cells carrying pGS98 (Fig. 2B). Removal of the bmlP1 3′ flanking region from pGS520 restored the level of BmlP1 production to that observed in cells carrying pGS99 (data not shown). These results establish that the negative effect of the bmlP1 3′ flanking region on BmlP1 production is orientation independent.
Negative regulation of bmlP1 expression is a specific effect of the bmlP1 3′ flanking region.
To determine whether this negative effect on bmlP1 expression was a specific effect of the bmlP1 3′ flanking region or a nonspecific effect related to the presence of any other DNA fragment fused to the 3′ end of bmlP1, we amplified a 360-bp DNA fragment containing the internal region (from positions +174 to +532 relative to the translational start site) of the B. megaterium mbgA gene (17) by PCR and fused it to the 3′ end of bmlP1 in both orientations (Fig. 2A). Figure 2B shows that BmlP1 production was not significantly affected by the presence of the 360-bp mbgA internal region in either orientation, indicating that the negative effect on bmlP1 expression is a specific effect on the bmlP1 3′ flanking region.
The inhibitory effect of the bmlP1 3′ flanking region is specific to bmlP1 expression.
To examine whether the bmlP1 3′ flanking region could inhibit expression of a heterologous gene, we constructed a pKK223-3-based plasmid in which the expression of the B. megaterium ccpA gene (6) was under the control of the tac promoter. The ccpA gene encodes a DNA-binding protein involved in carbon catabolite repression. A PCR-amplified DNA fragment containing the bmlP1 3′ flanking region was then fused to the 3′ end of the B. megaterium ccpA gene in both orientations. SDS-PAGE analysis showed that CcpA production was not affected by the presence of the bmlP1 3′ flanking region in either orientation (data not shown). This result indicates that the inhibitory effect of the bmlP1 3′ flanking region is specific to bmlP1 expression.
The bmlP1 3′ flanking region acts only in cis.
To test whether the bmlP1 3′ flanking region was capable of acting in trans, we assayed BmlP1 production from E. coli cells. They were cotransformed with a pACYC184-based plasmid pGS280 containing the bmlP1 promoter region plus the bmlP1 coding region and with a compatible pBR322-based plasmid containing the 490-bp bmlP1 3′ flanking region, which was inserted into the unique EcoRV site. The transcription of the bmlP1 3′ flanking region was thus driven by the promoter of the tetracycline resistance gene of pBR322. Western blot analysis showed that the level of BmlP1 production from this E. coli transformant was comparable to that observed in E. coli cells cotransformed with plasmid pGS280 and with the control plasmid pBR322 (data not shown). This indicates that the bmlP1 3′ flanking region cannot act in trans but can act only in cis.
Negative control of BmlP1 production in B. megaterium by the bmlP1 3′ flanking region.
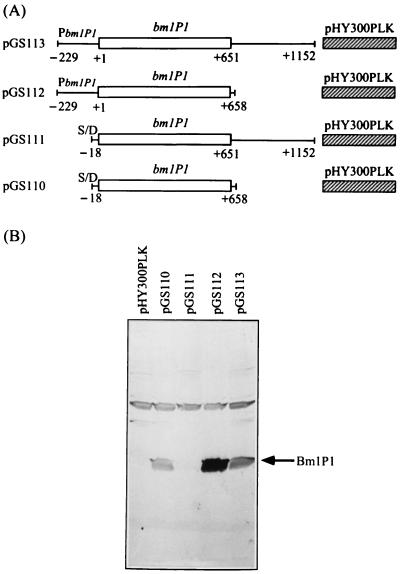
We further investigated whether the bmlP1 3′ flanking region also inhibited BmlP1 production in B. megaterium. A DNA fragment containing the bmlP1 promoter region, bmlP1 coding region, and 500-bp bmlP1 3′ flanking region was amplified by PCR and cloned between the EcoRI and HindIII sites of pHY300PLK (Takara Shuzo Co. Ltd., Kyoto, Japan) to generate pGS113 (Fig. 3A). Plasmid pGS112, which contains the bmlP1 promoter region and the bmlP1 coding region but no bmlP1 3′ flanking region, was constructed in a similar manner (Fig. 3A). Whole-cell protein extracts of B. megaterium cells harboring these plasmids were subjected to SDS-PAGE followed by Western blot analysis. As shown in Fig. 3B, the expression level of BmlP1 in B. megaterium cells carrying plasmid pGS113 was significantly lower than that observed in cells carrying pGS112. This result indicates that negative control of BmlP1 production by the bmlP1 3′ flanking region also occurs in B. megaterium.
FIG. 3.
Western blot analysis of the effect of the bmlP1 3′ flanking region on BmlP1 production in B. megaterium. (A) The expression of bmlP1 in the pHY300PLK-based plasmids, pGS110, pGS111, pGS112, and pGS113, is driven either by the bmlP1 promoter (as in pGS112 and pGS113) or by the promoter of the tetracycline resistance gene in pHY300PLK (as in pGS110 and pGS111). Numbers of base positions are given relative to the translational start site of bmlP1. S/D, the putative ribosome-binding site of bmlP1; PbmlP1, bmlP1 promoter. (B) B. megaterium cells carrying the above plasmids were grown at 37°C to the stationary phase. Equal numbers of cells were harvested, and whole-cell protein extracts were subjected to SDS-PAGE followed by Western blot analysis with the polyclonal anti-BmlP1 antibodies as the probe. A protein with lower mobility than that of BmlP1 on SDS-polyacrylamide gel that cross-reacted with the polyclonal anti-BmlP1 antibodies was used as the internal control.
Effect of the bmlP1 3′ flanking region on BmlP1 production is independent of the bmlP1 promoter region.
We previously demonstrated that the 53-bp inverted repeat located in the bmlP1 promoter region can negatively regulate bmlP1 expression (16). Figure 2 shows that in E. coli, the negative effect of the bmlP1 3′ flanking region on BmlP1 production occurred in the absence of the bmlP1 promoter region, suggesting that the presence of the 53-bp inverted repeat or another cis-acting element(s) in the bmlP1 promoter region was not required for the inhibitory effect of the bmlP1 3′ flanking region in E. coli. To examine if this is the case in B. megaterium, we replaced the bmlP1 promoter region with a heterologous promoter. The inserts removed from plasmids pGS98 and pGS99 (Fig. 2A) by double digestion with EcoRI and HindIII were recloned between the EcoRI and HindIII sites of pHY300PLK to generate plasmids pGS111 and pGS110, respectively (Fig. 3A). The bmlP1 gene expression was thus under the control of the promoter of the tetracycline resistance gene located in the vector pHY300PLK. Western blot analysis revealed that the expression level of BmlP1 in B. megaterium cells carrying plasmid pGS111 was substantially lower than that observed in cells carrying pGS110 (Fig. 3B). This result suggests that the bmlP1 3′ flanking region can act in the absence of cis-acting element(s) in the bmlP1 promoter region to modulate bmlP1 expression in B. megaterium.
Negative regulation of bmlP1 expression by the bmlP1 3′ flanking region occurs at the RNA level.
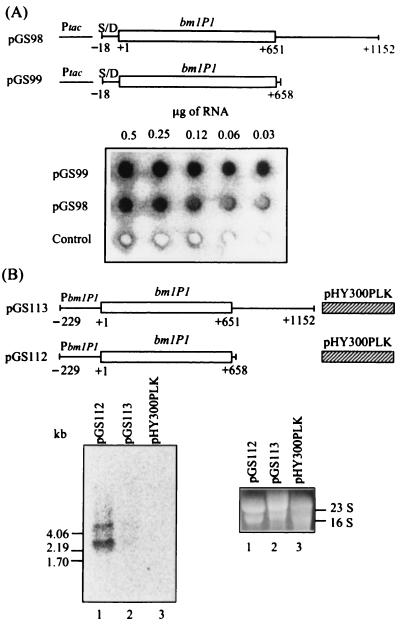
To investigate whether the observed negative effect of the bmlP1 3′ flanking region on BmlP1 production occurred at the RNA level, we carried out RNA dot blot analysis (13) using a 0.65-kb 32P-labeled DNA fragment corresponding to the bmlP1 coding region as the probe. RNA was isolated from E. coli cells carrying plasmid pGS98 or pGS99 or the control vector pKK223-3 and grown in the presence of IPTG as previously described (13). As shown in Fig. 4A, the level of the bmlP1-specific RNA transcript detected in cells carrying plasmid pGS98 was substantially lower than that detected in cells carrying plasmid pGS99. This finding indicates that negative regulation of bmlP1 expression by the bmlP1 3′ flanking region occurs at the RNA level.
FIG. 4.
Dot blot and Northern blot analyses of relative levels of the bmlP1 transcripts. (A) Dot blot analysis. Total cellular RNA was isolated from E. coli cells carrying plasmid pGS98 or pGS99 or the control vector pKK223-3 and grown in the presence of IPTG. After serial twofold dilutions, RNA was loaded onto a nylon membrane and probed with a 0.65-kb 32P-labeled DNA fragment corresponding to the bmlP1 coding region. Similar results were obtained from two independent experiments. Ptac, tac promoter; S/D, putative ribosome-binding site of bmlP1. (B) Northern blot analysis. Total cellular RNA extracted from B. megaterium cells carrying plasmid pGS112 or pGS113 or the control vector pHY300PLK during mid-exponential-phase growth was denatured, subjected to electrophoresis on a formaldehyde–1% agarose gel, and transferred to a nylon membrane. The membrane was then hybridized with a 0.65-kb 32P-labeled single-stranded DNA complementary to the upper strand of the bmlP1 coding region. Similar results were obtained from two independent experiments. PbmlP1, bmlP1 promoter. The migration positions of the single-stranded RNA markers (New England BioLabs, Inc.) are indicated. The lower right panel shows the ethidium bromide-stained rRNA on the gel used for Northern blotting.
We further carried out Northern blot analyses (13) to examine the RNA levels of bmlP1 driven by the native bmlP1 promoter in B. megaterium. Total cellular RNA was isolated from B. megaterium cells carrying plasmid pGS112 or pGS113 or the control vector pHY300PLK. After electrophoresis on a formaldehyde-containing denaturing gel, RNA was transferred to a nylon membrane and hybridized with 0.65-kb 32P-labeled single-stranded DNA complementary to the upper strand of the bmlP1 coding region. As shown in Fig. 4B, two RNA species were detected in B. megaterium cells carrying plasmid pGS112. The shorter bmlP1 transcript might be initiated from the native bmlP1 promoter. The longer bmlP1 transcript might be initiated from the promoter of the tetracycline resistance gene of pHY300PLK, span the tetracycline resistance gene, and extend into the bmlP1 coding region as well as its downstream region. The observation that the levels of the two bmlP1 transcripts detected in cells carrying plasmids pGS112 were substantially higher than those detected in cells carrying plasmid pGS113 further supports the idea that down-regulation of expression of bmlP1 by the bmlP1 3′ flanking region occurs at the RNA level, no matter whether the bmlP1 expression is driven by the tac promoter in E. coli or by the native bmlP1 promoter in B. megaterium.
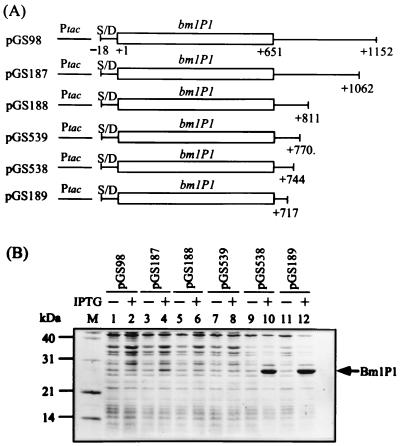
Effect of deletion from the 3′ end of the bmlP1 3′ flanking region on bmlP1 expression.
To further localize the sequence of the bmlP1 3′ flanking region responsible for the inhibitory effect, we constructed a series of deletion derivatives (Fig. 5A) by PCR and cloned these DNA fragments between the EcoRI and HindIII sites of pKK223-3. Whole-cell protein extracts of E. coli cells carrying these plasmids and grown in the absence or presence of IPTG were subjected to SDS-PAGE. As shown in Fig. 5B, very low levels of BmlP1 production were still observed when the sequences from positions +770 to +1152 relative to the translational start site of bmlP1 were deleted. Further deletion of the sequence between positions +744 and +770 resulted in a marked increase in BmlP1 production, suggesting that an important negative regulatory site was either partially or completely eliminated.
FIG. 5.
Effects of various deletions from the 3′ end of the bmlP1 3′ flanking region on bmlP1 expression. (A) The expression vector pKK223-3-derived plasmids, pGS98, pGS187, pGS188, pGS189, pGS538, and pGS539, contain the bmlP1 gene followed by various lengths of the bmlP1 3′ flanking region. Base positions are given relative to the translational start site of bmlP1. S/D, putative ribosome-binding site of bmlP1; Ptac, tac promoter. (B) E. coli cells carrying the above plasmids were grown at 37°C to an absorbance at 600 nm of 0.5 and then treated with 0.3 mM IPTG for 2 h or left untreated. Whole-cell protein extracts from equal numbers of cells were subjected to SDS-PAGE. Lane M, protein markers.
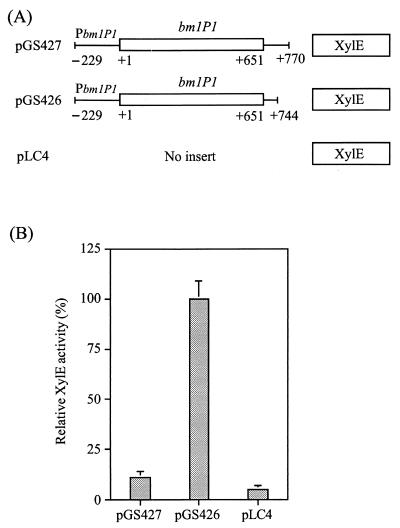
To further confirm the effect of this negative regulatory site on bmlP1 expression, PCR was carried out to amplify DNA fragments containing the bmlP1 promoter region, bmlP1 coding region, and deletion derivatives of the bmlP1 3′ flanking region as shown in Fig. 6A. These two DNA fragments were then individually cloned in front of a promoterless xylE reporter gene in pLC4 (12) to generate plasmids pGS426 and pGS427. Catechol 2,3-dioxygenase (XylE) activities were measured spectrophotometrically as previously described (12). Figure 6B shows that deletion of this negative regulatory site led to a marked increase in XylE activity, suggesting that the inhibitory effect of this site on bmlP1 expression occurs at the RNA level. This is consistent with the result obtained by the above-mentioned RNA dot blot analysis.
FIG. 6.
XylE activities directed by the bmlP1 promoter region and its downstream regions. (A) The pLC4-based plasmids, pGS426 and pGS427, contain a xylE reporter gene preceded by the bmlP1 promoter region, bmlP1 coding region, and deletion derivatives of the bmlP1 3′ flanking region. Numbers of base positions are given relative to the translational start site of bmlP1. PbmlP1 denotes the bmlP1 promoter. (B) E. coli cells carrying the above plasmids were grown at 37°C to an absorbance at 600 nm of 1.0. The specific activity of XylE obtained from pGS426, 2,823 mU/mg, was assigned a relative value of 100%. Each value represents the mean of four determinations. Each error bar indicates the standard error of the mean.
In this study we present evidence that bmlP1 expression is subject to negative regulation at the RNA level by the bmlP1 3′ flanking region both in B. megaterium and in E. coli. This repression appears to be quite different from negative regulation of the int gene expression by its downstream sib site (4). The sib site can negatively modulate the expression of a heterologous galK gene when fused to the 3′ end of galK (14). RNase III is involved in processing the special RNA structure in the sib site (4, 14). In contrast, the bmlP1 3′ flanking region cannot affect the expression of a heterologous ccpA gene when fused to the 3′ end of ccpA. Based on the facts that the inhibitory effect of the bmlP1 3′ flanking region is specific to bmlP1 expression and that this negative effect on bmlP1 expression is specific to the bmlP1 3′ flanking region, it is possible that a certain RNA structure in the bmlP1 3′ flanking region folds back to interact with another RNA sequence within the bmlP1 coding region, thus making them targets for an as-yet-unidentified bacterial RNase existing both in E. coli and in B. megaterium. Another possibility is that the BmlP1 protein may interact with both the bmlP1 coding sequence and its flanking sequence in a loop model to exert an unusual type of negative autoregulation on bmlP1 expression at the transcriptional level.
When the nucleotide sequence of the 116-bp bmlP1 3′ flanking region was compared with that of the bmlP1 coding region, several pairs of direct repeats and inverted repeats were found to exist between these two regions, raising the possibility that a trans-acting factor may bind to them in a loop model. In addition, several RNA secondary structures may potentially be formed from the complementary sequences between the RNA transcribed from the bmlP1 coding region and the RNA transcribed from the 116-bp bmlP1 3′ flanking region, presumably making them sensitive to RNase cleavage. However, no matter what the proposed mechanism for this repression may be, one should take into account that the bmlP1 3′ flanking region can work in either orientation and can act only in cis. Further experiments will be necessary to pinpoint the structural features or elements, to identify the putative trans-acting factor(s) or RNase(s), and to elucidate precisely the mechanism responsible for this negative control.
Acknowledgments
This research was supported by grant NSC 88-2316-B-010-020 from the National Science Council of the Republic of China.
REFERENCES
- 1.Chen C Y, Beatty J T, Cohen S N, Belasco J G. An intercistronic stem-loop structure functions as an mRNA decay terminator necessary but insufficient for puf mRNA stability. Cell. 1988;52:609–619. doi: 10.1016/0092-8674(88)90473-4. [DOI] [PubMed] [Google Scholar]
- 2.Emory S A, Bouvet P, Belasco J G. A 5′-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman M, Oppenheim A, Court D. Retroregulation: control of gene expression from sites distal to the gene. Cell. 1982;29:727–728. doi: 10.1016/0092-8674(82)90434-2. [DOI] [PubMed] [Google Scholar]
- 4.Guarneros G, Montanez C, Hernandez T, Court D. Posttranscriptional control of bacteriophage lambda gene expression from a site distal to the gene. Proc Natl Acad Sci USA. 1982;79:238–242. doi: 10.1073/pnas.79.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J S, Liang Q, Fulco A J. The molecular cloning and characterization of BmlP1 and BmlP2 proteins, putative positive transcription factors involved in barbiturate-mediated induction of the genes encoding cytochrome P450BM-1 of Bacillus megaterium. J Biol Chem. 1995;270:18615–18625. doi: 10.1074/jbc.270.31.18615. [DOI] [PubMed] [Google Scholar]
- 6.Hueck C J, Kraus A, Hillen W. Sequences of ccpA and two downstream Bacillus megaterium genes with homology to the motAB operon from Bacillus subtilis. Gene. 1994;143:147–148. doi: 10.1016/0378-1119(94)90621-1. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Mott J E, Galloway J L, Platt T. Maturation of Escherichia coli tryptophan operon mRNA: evidence for 3′ exonucleolytic processing after ρ-dependent termination. EMBO J. 1985;4:1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newbury S F, Smith N H, Robinson E C, Hiles I D, Higgins C F. Stabilization of translationally active mRNA by prokaryotic REP sequences. Cell. 1987;48:297–310. doi: 10.1016/0092-8674(87)90433-8. [DOI] [PubMed] [Google Scholar]
- 10.Ouellette M, Legare D, Papadopoulou B. Microbial multidrug-resistance ABC transporters. Trends Microbiol. 1994;2:407–411. doi: 10.1016/0966-842x(94)90620-3. [DOI] [PubMed] [Google Scholar]
- 11.Pabo C O, Sauer R T. Protein-DNA recognition. Annu Rev Biochem. 1984;53:293–321. doi: 10.1146/annurev.bi.53.070184.001453. [DOI] [PubMed] [Google Scholar]
- 12.Ray C, Hay R E, Carter H L, Moran C P., Jr Mutations that affect utilization of a promoter in stationary-phase Bacillus subtilis. J Bacteriol. 1985;163:610–614. doi: 10.1128/jb.163.2.610-614.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 14.Schmeissner U, McKenney K, Rosenberg M, Court D. Removal of a terminator structure by RNA processing regulates int gene expression. J Mol Biol. 1984;176:39–53. doi: 10.1016/0022-2836(84)90381-4. [DOI] [PubMed] [Google Scholar]
- 15.Shaw G C, Sung C C, Liu C H, Lin C H. Evidence against the BmlP1 protein as a positive transcription factor for barbiturate-mediated induction of cytochrome P450BM-1 in Bacillus megaterium. J Biol Chem. 1998;273:7996–8002. doi: 10.1074/jbc.273.14.7996. [DOI] [PubMed] [Google Scholar]
- 16.Shaw G-C, Sung C-C, Liu C-H, Kao H-S. A 53-base-pair inverted repeat negatively regulates expression of the adjacent and divergently oriented cytochrome P450BM-1 gene and its regulatory gene, bmlP1, in Bacillus megaterium. J Bacteriol. 1997;179:280–283. doi: 10.1128/jb.179.1.280-283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw G-C, Kao H-S, Chiou C-Y. Cloning, expression, and catabolite repression of a gene encoding β-galactosidase of Bacillus megaterium ATCC 14581. J Bacteriol. 1998;180:4734–4738. doi: 10.1128/jb.180.17.4734-4738.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L-F, Doi R H. Complex character of senS, a novel gene regulating expression of extracellular-protein genes of Bacillus subtilis. J Bacteriol. 1990;172:1939–1947. doi: 10.1128/jb.172.4.1939-1947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong H C, Chang S. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc Natl Acad Sci USA. 1986;83:3233–3237. doi: 10.1073/pnas.83.10.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]