Two children presented with signs of raised intracranial pressure and sepsis. Computed tomography showed relatively large ventricles, suggesting obstructive hydrocephalus. Raised intracranial pressure was confirmed at ventriculostomy, but cerebrospinal fluid was sterile. Streptococcus pneumoniae infection was subsequently documented in both patients, and findings at postmortem examination in one confirmed that he had acute purulent basal meningitis.
Case reports
Case 1
A boy presented to a district general hospital on the eve of his first birthday. He had collapsed at home, and his parents reported that for three days he had had fever, anorexia, vomiting, and increasing drowsiness. On arrival at hospital he was poorly perfused, could barely be roused, and his anterior fontanelle was bulging. The boy’s blood pressure was 117/66 mm Hg and his occipitofrontal circumference was 50.6 cm (>90th centile). Initial investigations showed haemoglobin concentration 81 g/l, white cell count 23.9×109/l (neutrophils 18.9×109/l), platelets 226×109/l, serum urea and electrolytes normal, and C reactive protein 137 mg/l. He improved after resuscitation with plasma; then, because of recurrent episodes of bradycardia, he was intubated and ventilated. Intravenous ceftazidime and penicillin were given. The boy had been previously well, was fully immunised, and had been making appropriate neurodevelopmental progress. His large head had been noted previously but was attributed to a familial tendency and was not investigated.
The patient was transferred to the regional paediatric intensive care unit, where computed tomograms showed dilatation of the lateral and third ventricles with some reduction of the extra axial spaces (fig 1). There was no crowding of the foramen magnum, effacement of the basal cisterns, periventricular oedema, or loss of grey-white differentiation. Taking into account the history of a large head, it was concluded that this child had longstanding ventricular dilatation. Six hours later the boy’s condition deteriorated—he had fixed dilatation of the left pupil, loss of amplitude on the electroencephalogram, and increasing blood pressure. Intravenous mannitol (0.5 g/kg) was given before an intraventricular catheter was inserted. The initial cerebrospinal fluid pressure was 52 mm Hg, and analysis of the ventricular cerebrospinal fluid showed white cell count 3/mm3, red cell count 1500/mm3, protein 0.34 g/l, glucose 2.9 mmol/l, and latex tests for Neisseria meningitidis, Haemophilus influenzae, S pneumoniae, and group B streptococcus all negative. Direct culture of the cerebrospinal fluid had negative results; enriched culture produced only coagulase negative staphylococcal species.
Figure 1.
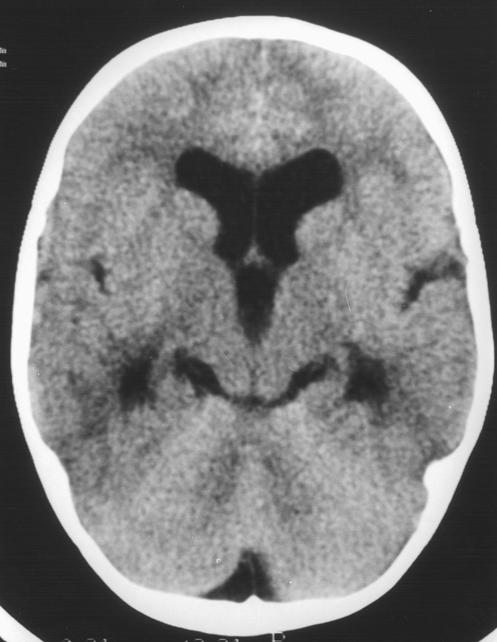
(left) Computed tomogram of patient in case 1 showing dilatation of the lateral and third ventricles with reduction of the extra axial spaces; grey-white differentiation is well preserved
The boy’s intracranial pressure continued to fluctuate at or above mean arterial pressure, and next morning repeat computed tomograms showed widespread cerebral necrosis (fig 2). Intensive care support was withdrawn. A lumbar puncture was performed after he had died. The results were white cell count 45/mm3, red cell count 3/mm3, protein 4.05 g/l, and glucose 2.2 mmol/l, in keeping with obstruction to flow of cerebrospinal fluid. Latex agglutination tests on the blood culture taken on admission to hospital and on lumbar cerebrospinal fluid were positive for S pneumoniae. Postmortem examination confirmed that he had acute purulent meningitis with exudate around the base of the brain; culture of cerebrospinal fluid grew S pneumoniae serotype 19. There was no congenital cerebral abnormality.
Figure 2.
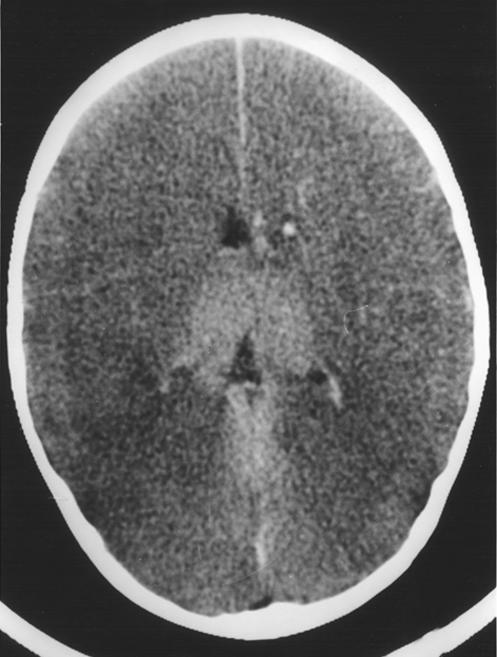
(right) Computed tomogram of patient in case 1 showing widespread cerebral necrosis
Case 2
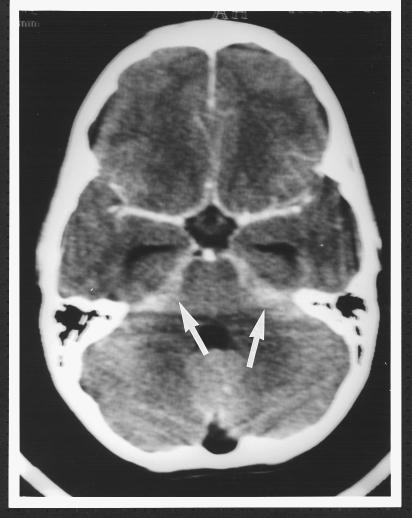
An 11½ year old boy presented to this hospital with a five day history of worsening fever, headache, anorexia, vomiting, and drowsiness. There was nothing noteworthy in his medical history and he had not been given any medication. On admission to hospital, he was poorly perfused, with fever and noticeable nuchal rigidity. The boy’s Glasgow coma score was 9 and deteriorating, his pupils were small and reactive, and his fundi were normal. The results of initial investigations included haemoglobin 140 g/l, white cell count 29.8×109/l (neutrophils 28.6×109/l), platelets 246×109/l, serum urea, electrolytes and ammonia normal, C reactive protein 63 mg/l. The boy was resuscitated with intravenous plasma and given intravenous cefotaxime, benzylpenicillin, and mannitol before emergency computed tomography. Scans showed prominent temporal horns with effacement of the basal cisterns and some diminution of grey-white differentiation, in keeping with early brain swelling and developing obstructive hydrocephalus. The contrast study showed no focal lesion but ill defined enhancement around the posterior cerebral arteries, suggesting basal meningitis (fig 3). An intraventricular catheter was inserted soon afterwards, and the initial cerebrospinal fluid pressure was >30 mm Hg. Cerebrospinal fluid analyses showed white cell count 180/mm3, red cell count >5000/mm3, protein 1.2 g/l, glucose 5.5 mmol/l, and negative results on latex tests for N meningitidis, H influenzae, S pneumoniae, and group B streptococcus. In addition, direct and enriched cultures had negative results. Blood culture taken on admission to hospital subsequently grew S pneumoniae serotype 14, although repeat latex agglutination of cerebrospinal fluid for this particular serotype was negative. Chest x ray showed left lobar pneumonia.
Figure 3.
Contrast study of patient in case 2 showing ill defined enhancement around the posterior cerebral arteries (arrows) suggestive of basal meningitis
Treatment with antibiotics and dexamethasone was continued, and by day 5 of his stay in hospital repeat computed tomograms were normal. The boy was successfully extubated next day, and discharged home on day 20 to make a full recovery.
Discussion
Acute bacterial meningitis remains a relatively common and potentially fatal condition in childhood, and S pneumoniae is found to be the infecting organism in 10-20% of cases.1 As the box shows, presentation is generally with fever and signs of cerebral dysfunction. Classic nuchal rigidity may be absent in younger children, but most will have a peripheral leucocytosis.2
Obstructive hydrocephalus in bacterial meningitis
Presentation
Decreased level of consciousness
Low grade or fluctuating fever
Age appropriate signs of meningism
Management
Avoid lumbar puncture
Consider measures to reduce intracranial pressure (intravenous mannitol 0.25 g/kg, repeated up to a total dose of 1.5 g/kg if necessary)
Urgent brain imaging
Ventriculostomy and drainage
Cerebrospinal fluid is formed by the choroid plexus in the lateral ventricles, from where it flows via the third and fourth ventricles to the subarachnoid space. It is reabsorbed subsequently by the arachnoid villi in the intracranial venous sinuses.3 The flow of cerebrospinal fluid may be blocked at the third or fourth ventricles (obstructive hydrocephalus) or at the arachnoid villi (communicating hydrocephalus). In bacterial meningitis, neutrophil migration into the subarachnoid space follows bacterial invasion. The resultant purulent exudate tends to collect in the Rolandic and Sylvanian sulci over the cerebral hemispheres and in the basal cisterns, where the subarachnoid space is deepest4 and where, presumably, cerebrospinal fluid flow is most sluggish. The exudate interferes with absorption of cerebrospinal fluid by the arachnoid villi and may also cause obstructive hydrocephalus by obstructing the foramina of Luschka and Magendie. Typically, the obstruction occurs towards the end of the second week of the illness, when neutrophils begin to degenerate and fibroblasts proliferate in the exudate. In case 1, however, findings at postmortem examination were of an acute inflammatory exudate blocking the exit foramina, entirely consistent with the three day history of illness.
It was surprising that in both patients, culture of ventricular cerebrospinal fluid had negative results. Bacterial invasion into the cerebrospinal fluid from the nasopharynx in pneumococcal meningitis occurs via the choroid plexus and cerebral microvasculature,5 and in the primate model the highest concentration of organisms early in the course of H influenzae meningitis is in the lateral ventricles.6 Previous exposure to antibiotics does not explain failure to culture the infecting organism from ventricular cerebrospinal fluid in these two patients. Indeed, in case 1, S pneumoniae was grown from cerebrospinal fluid sampled after death and despite more than 48 hours of appropriate intravenous antibiotic treatment. A latex agglutination test performed with prior knowledge of the phage type of the pneumococcus (as in case 2) detects fragments of the infecting organism at a concentration of 5 ng/l (500 bacteria/ml) (LE Smart, personal communication). The absence of white blood cells in both ventricular and lumbar cerebrospinal fluid from the patient in case 1 is consistent with infection localised to the posterior fossa and obstructing the flow of cerebrospinal fluid. Presumably the route of infection was not via the choroid plexus. The acute inflammatory exudate in pneumococcal infection is particularly tenacious. Only one major textbook of paediatrics acknowledges that ventricular cerebrospinal fluid can be sterile at the same time as lumbar cerebrospinal fluid is purulent.7 We believe that this fact is not generally recognised.
Cerebral herniation is well recognised as a complication of pneumococcal meningitis.8,9 However, diagnosing critically high intracranial pressure is difficult—particularly after seizures and in sedated ventilated patients—and a high index of suspicion is necessary.9 Early use of osmotic agents, with or without ventricular drainage, may reduce the risks of long term morbidity or death, but mortality and morbidity are still appreciable.8–10 Computed tomography is not an accurate measure of intracranial pressure11 but is appropriate to exclude mechanical causes of raised intracranial pressure before lumbar puncture in children with suspected meningitis and a reduced level of consciousness.12 Ventriculomegaly is relatively common in acute bacterial meningitis in childhood and may be progressive in the absence of raised intraventricular pressure, presumably as a result of cerebral atrophy.13 Thus, computed tomograms must be interpreted in conjunction with the clinical status of the patient, and computed tomography may need to be repeated, particularly if there is no response to treatment. With hindsight, the patient in case 1 had simple familial macrocephaly—appreciation of this fact should have prompted earlier ventriculostomy.
Ventriculostomy is a safe and relatively simple procedure.12,14 It enables cerebral perfusion pressure (mean arterial pressure minus intracranial pressure) to be calculated and intracranial pressure reduced by removal of cerebrospinal fluid. Unlike cerebral oedema, which is common in meningitis,15 hydrocephalus can be treated by appropriate drainage of the cerebrospinal fluid, and therefore needs to be identified.
Acute obstructive hydrocephalus is thought to be an uncommon presenting feature of bacterial meningitis, usually occuring in younger children who have had previous treatment with antibiotics.8,13 We could find no record of the incidence of this complication in published reports, nor any other case report, but since preparing this manuscript we have seen two other children with obstructive hydrocephalus as a presenting feature of bacterial meningitis. All patients with suspected meningitis and decreased level of consciousness should have urgent brain imaging to exclude obstructive hydrocephalus before lumbar puncture.
Footnotes
Funding: None.
Conflict of interest: None.
References
- 1.Roos KL, Tunkel AR, Scheld WM. Acute bacterial meningitis in children and adults. In: Scheld WM, Whitley RJ, Durack DT, editors. Infections of the central nervous system. Philadelphia: Lippincott-Raven; 1997. pp. 335–401. [Google Scholar]
- 2.Geiseler PJ, Nelson KE. Bacterial meningitis without clinical signs of meningeal irritation. S Med J. 1982;75:448–450. doi: 10.1097/00007611-198204000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Walton JN, editor. Brain’s diseases of the nervous system. 8th ed. New York: Oxford University Press; 1977. pp. 121–122. [Google Scholar]
- 4.Adams RD, Kubik CS, Bonner FJ. The clinical and pathological aspects of B influenzal meningitis. Arch Pediatr. 1948;65:408–441. [PubMed] [Google Scholar]
- 5.Saez-Llorens X, Ramilo O, Mustafa MM, Mertsola J, McCracken GH. Molecular pathophysiology of bacterial meningitis: Current concepts and therapeutic implications. J Pediatr. 1990;116:671–684. doi: 10.1016/s0022-3476(05)82647-2. [DOI] [PubMed] [Google Scholar]
- 6.Smith AL, Daum RS, Scheifele D, Syriopolou V, Averill DR, Roberts MC, et al. Pathogenesis of Haemophilus influenzae meningitis. In: Sell SH, Wright PF, editors. Haemophilus influenzae: epidemiology, immunology, and prevention of disease. New York: Elsevier; 1982. pp. 89–109. [Google Scholar]
- 7.Campbell AGM. Infections. In: Campbell AGM, McIntosh N, editors. Forfar and Arneil’s textbook of paediatrics. Edinburgh: Churchill Livingstone; 1992. p. 1345. [Google Scholar]
- 8.Stephenson JBP. Timing of lumbar puncture in severe childhood meningitis. BMJ. 1985;291:1123. doi: 10.1136/bmj.291.6502.1123-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz SJ, Boxerbaum B, O’Bell J. Cerebral herniation in bacterial meningitis in childhood. Ann Neurol. 1980;7:524–528. doi: 10.1002/ana.410070605. [DOI] [PubMed] [Google Scholar]
- 10.Goitein KJ, Tamir I. Cerebral perfusion pressure in central nervous system infections of infancy and childhood. J Pediatr. 1983;103:40–43. doi: 10.1016/s0022-3476(83)80772-0. [DOI] [PubMed] [Google Scholar]
- 11.Hyderman RS, Robb SA, Kendall BE, Levin M. Does computerised tomography have a role in the evaluation of complicated bacterial meningitis in childhood? Dev Med Child Neurol. 1992;34:870–875. doi: 10.1111/j.1469-8749.1992.tb11384.x. [DOI] [PubMed] [Google Scholar]
- 12.McWilliam R, Stephenson JBP. Bedside intracranial pressure monitoring. Lancet. 1985;ii:341. doi: 10.1016/s0140-6736(85)90395-2. [DOI] [PubMed] [Google Scholar]
- 13.Snyder RD. Ventriculomegaly in childhood bacterial meningitis. Neuropediatrics. 1984;15:136–138. doi: 10.1055/s-2008-1052356. [DOI] [PubMed] [Google Scholar]
- 14.Polpe IK, Muhlbauer MS, Sanford RA, Kirk E. Results and complications of intracranial pressure monitoring in 303 children. Pediatr Neurosurg. 1995;23:64–67. doi: 10.1159/000120938. [DOI] [PubMed] [Google Scholar]
- 15.Odio CM, Fainezicht I, Paris M, Nassar M, Baltodano A, Rogers J, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med. 1991;324:1525–1531. doi: 10.1056/NEJM199105303242201. [DOI] [PubMed] [Google Scholar]