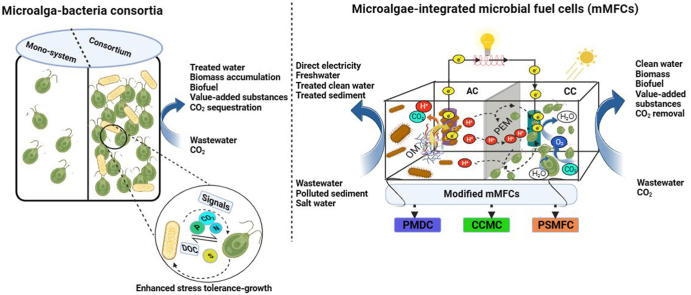
Abstract
Microalgae and bacteria, known for their resilience, rapid growth, and proximate ecological partnerships, play fundamental roles in environmental and biotechnological advancements. This comprehensive review explores the synergistic interactions between microalgae and bacteria as an innovative approach to address some of the most pressing environmental issues and the demands of clean and renewable freshwater and energy sources. Studies indicated that microalgae-bacteria consortia can considerably enhance the output of biotechnological applications; for instance, various reports showed during wastewater treatment the COD removal efficiency increased by 40%–90.5 % due to microalgae-bacteria consortia, suggesting its great potential amenability in biotechnology. This review critically synthesizes research works on the microalgae and bacteria nexus applied in the advancements of renewable energy generation, with a special focus on biohydrogen, reclamation of wastewater and desalination processes. The mechanisms of underlying interactions, the environmental factors influencing consortia performance, and the challenges and benefits of employing these bio-complexes over traditional methods are also discussed in detail. This paper also evaluates the biotechnological applications of these microorganism consortia for the augmentation of biomass production and the synthesis of valuable biochemicals. Furthermore, the review sheds light on the integration of microalgae-bacteria systems in microbial fuel cells for concurrent energy production, waste treatment, and resource recovery. This review postulates microalgae-bacteria consortia as a sustainable and efficient solution for clean water and energy, providing insights into future research directions and the potential for industrial-scale applications.
Keywords: Electron transfer mechanisms, Microalgae-bacteria consortia, Microalgae microbial fuel cell, Photosynthetic electrogens, Renewable energy resources, Wastewater treatment
Graphical abstract
Highlights
-
•
Microalgae-bacteria nexus enhance biohydrogen production and carbon sequestration.
-
•
Microalgae-bacteria consortia surpass the traditional application methods.
-
•
Microalgae-bacteria consortia welding in next-generation biotechnology applications.
-
•
Taxonomic exploration on potential microbes is pertinent for optimization.
-
•
Strain selection, genetic engineering and system design are pivot for optimization.
1. Introduction
Microalgae and heterotrophic bacteria are cosmopolitan microscopic organisms playing crucial roles in aquatic ecosystems as the basis of the food chain and recyclers of organic matter. While microalgae photosynthetically produce organic matter by reducing CO2 with protons and electrons that are derived from water photolysis, heterotrophic bacteria degrade the organic matter by oxidizing it and releasing CO2. In addition to some amenable versatile species, generally, microalgae and bacteria are endowed with key traits that are advantageous for technological applications. These traits include their rapid growth and biomass accumulation capability under optimum growth conditions, and their great diversity and robust adaptability. These key attributes make them suitable candidates for biotechnological application and exploitation.
Decades of research on microalgae-bacteria interactions have shed light on their interactive mechanisms and greatly contributed to the advancement of applied ecology, environmental microbiology and biotechnology. Consequently, synthetic consortia of these organisms have been leveraged in the past years to enhance biomass production, wastewater treatment, and biofuel generation, addressing essential societal needs for clean energy, environment and freshwater. As our energy and freshwater resources are currently dependent on finite and environmentally unsafe sources, that have been aggravated by rapid population growth and industrialization, it is imperative to explore alternative renewable sources before they adversely affect the quality of life on Earth.
For instance, reliance on fossil fuel is unsustainable and environmentally unsafe due to its finite nature, and it contributes to global warming and has health risks. Hence, renewable sources such as biofuel [1] and microbial fuel cells (MFC) technologies [2] have been promoted as promising alternatives to offer clean energy and environmentally safe solutions. Like energy, the demand for clean fresh water is among the crucial societal problems; while water is abundant globally, only a tiny fraction (0.5 %) is readily available for use and the majority (>97 %) of it is saline [3]. Although dissolved solids removal from salt waters has been done through membrane process and thermal desalination methods to maximize the accessibility of clean freshwater, the operational cost is very high [3]. Consequently, microbial desalination cells (MDCs) have emerged as an energy-efficient alternative technology for desalination.
Biotechnological applications of microalgae-bacteria interactions involve multiple disciplines, focusing on wastewater treatment via cocultivation, environmental remediation, improved biomass production, bioelectricity generation in MFCs, salinity reduction in photosynthetic desalination cells, biohydrogen production, biomass pretreatment for cell wall disruption, and biomass harvesting through advanced flocculation techniques. Review works of literature on microalgae-bacteria interactions have been growing since the 1990s, addressing distinct aspects such as metabolite and biomass enhancement [4,5], nutrient and micropollutant removal from wastewaters [[6], [7], [8], [9]], effects on wastewater treatment processes [10], remediation of hazardous wastes [11], treatment of acid mine drainage [12], removing CO2 while purifying wastewater and producing bioproducts [13], applications in photobioelectrochemical microbial fuel cells [14], heavy metal removal [15], downstream processing of microalgae biomass [16], and biomass pretreatment methods [17].
Despite the growth in review paper publications, there is a gap in comprehensive reviews that encapsulate the microalgae-bacteria interactions and their biotechnological applications from a broader perspective. This review paper addresses this gap, aiming to present a concise, yet thorough, overview of the environmental, economic, and biotechnological prospects of microalgae-bacteria interactions for a wide readership, including biologists, biotechnologists, environmental microbiologists, and engineers. This work is intended to cover topics such as biomass production, bioelectricity generation, saltwater desalination, and biohydrogen production to offer concise current knowledge and future perspectives in this dynamic area of study.
2. The interaction, application and optimization of microalgae-bacteria consortia
The exploitation of the synergetic interaction between microalgae and bacteria in consortia has been harnessed in the last few decades as an advanced technology to enhance the performance of aquaculture, biofuel production, and wastewater treatment, surpassing the outcome of either organism could achieve alone.
2.1. The interactions of microalgae and bacteria in consortia
Several studies indicated that microbes are interdependent and frequently dictate the life of each other through the exchange of materials and resources [18]. The interaction between bacteria and algae can be synergistic-positive (promoting) or antagonistic-negative (hindering) to the growth and physiology of the participant microorganisms. For instance, the algal surface can serve as a microbial habitat for bacteria, serving as a defense against predation, nutrient source and surface area to colonize, while the bacteria may favor the relationship by providing services such as antibiotic production, polysaccharide degradation, biosynthesis of allelochemical and growth stimulant biomolecules.
Recently, Takagi et al. [19] reported a symbiotic relationship between dinoflagellate and bacteria mutually benefitted each other, in which the dinoflagellate protected the symbiotic bacteria from antibiotics while the bacteria protected the algal cells from light stress through carotenoid production. Additionally, a study on the interaction of the microalga C. vulgaris and its phycospheric bacteria showed the two partners interact in various ways, harnessing efficient metabolization and removal of nutrients such as phosphorus, carbon and nitrogen from the wastewater [20]. Generally, the synergistic mutualism of microalgae and bacteria can be undertaken in two ways; by signal communication (materials are used for communication purposes but not as nutrients), and by exchanging materials and resources (Fig. S1).
2.1.1. Interaction via signaling in microalgae and bacteria consortia
The exchange of signal molecules can activate or inhibit the expression of genes or biological activities, resulting changes in metabolism and growth. A mutualistic interaction between bacteria and microalgae has been reported in which the bacteria influence the growth of microalgae by producing growth-promoting hormones and antibiotics [21]. Likewise, it has been reported that a Sulfitobacter species promotes the cell division of diatom by releasing indole-3-acetic acid (IAA).
Molecules like IAA, tryptophan, bacterial excreted-ammonium and diatom-excreted organosulfur molecules served as signaling molecules. During signaling and communication, the bacterium can synthesize the IAA using an endogenous source of tryptophan or tryptophan secreted by algae [22]. Metabolic and metatranscriptome analyses showed that there is a widespread IAA production by Sulfitobacter-related bacteria particularly in coastal environments, indicating this mode of signaling could also occur in the open ocean [22]. Such signaling is also reported in freshwater green algae, where organic molecules like tryptophan and thiamin that are exudated from Chlorella sorokiniana induce the IAA production by Azospirillum brasilense (plant growth-promoting bacterium), which in turn, promotes the growth of microalga [23].
2.1.2. Interaction via material exchange in microalgae and bacteria consortia
Interaction by exchanging materials and resources is another way that microalgae and bacterial communities influence each other. There are several materials that microalgae and bacteria can exchange during interactions. From these, the exchange of essential elements like iron is the cornerstone of mutualism. Iron is crucial for metabolic processes like catalyzing redox reactions and electron transfer. Other than being an essential element for photosynthesis and respiration, iron limits the primary productivity and growth of bacteria in much of the ocean. To counteract iron scarcity, several marine heterotrophic bacteria and some cyanobacteria produce siderophores, organic molecules that bind iron to enhance its availability [24]. Since eukaryotic algae are not known to produce or take up bacterial-originated siderophores, they obtain iron from siderophores or other chelates through ferrireductases and adjacent Fe (II) transport on their outer cell membranes. Amin et al. [25] proposed a mutual exchange of iron and fixed carbon between algae and bacteria. This was based on their study of Marinobacter′s production of a lower-affinity dicitrate siderophore (vibrioferrin) and its interaction with the dinoflagellate Scrippsiella trochoidea.
Vitamins are commonly used among prokaryotic and eukaryotic organisms for various metabolic functions, as a result of which a significant effect on the growth and composition of microbial communities is observed [26,27]. However, genomic data reveal many of these organisms cannot synthesize vitamins [28,29], with only about one-third of prokaryotes capable of producing vitamin B12 (cobalamin) [29]. Likewise, most of the harmful algal bloom-forming species are vitamin B12 and B1 auxotrophs [30]. In contrast to the limited distribution of these vitamins in most microbes, almost all marine prokaryotes, and more than half of marine eukaryotic microbes possess vitamin B12-dependent enzymes [28,31]. Thus, these organisms rely on an exogenous supply of vitamin B12. This discrepancy in dependency and supply of vitamin B12 results in a close microbial interaction. For this reason, microalgae might prefer to have a close association with bacteria and make a trade-off [32]. Therefore, during mutualism, the microalgae could acquire the vitamins and nutrients via active uptake from the soluble fraction or through episymbiosis [26].
A mathematical model showing the mutualistic interaction of microbes was also reported [33]. The green alga Chlamydomonas reinhardtii forms synthetic mutualism with Mesorhizobium loti and the genetically engineered gut bacterium Escherichia coli and can receive cobalamin [18]. The association of bacteria with other eukaryotic algae, such as picoeukaryotic alga [34] and diatoms [35] has also been documented. More information on sharing vitamins with microbes and their impact on microbial interactions has been provided by Sokolovskaya et al. [29].
In terms of material exchange, it is common that heterotrophic bacteria assimilate DOC for metabolic needs, this creates an opportunity for the algae to shape the diversity of the bacterial community in its surroundings by producing various types of DOC. In line with this, taxon- and substrate-specific responses of the bacterial community were observed during the degradation of diatom-derived extracellular carbohydrates [36].
Despite the large abundance of nitrogen in the atmosphere, the bioavailable form of nitrogen is limiting the growth of autotrophs. To alleviate this problem few prokaryotes developed a mechanism to convert atmospheric nitrogen into bioavailable forms. Other microorganisms and higher plants devise mechanisms to attract and develop associations with those prokaryotes that are capable of fixing atmospheric nitrogen. In this symbiosis, nitrogen-fixing prokaryotes and their algal hosts benefit mutually. This relationship can be further manipulated in microalgae and diazotrophs consortia under laboratory conditions to harness some biotechnological processes including reducing production costs and enhancing microalgal biomass and biochemical products [36]. Algae, besides getting fixed nitrogen from bacteria, also absorb nitrogen from the decomposed amino acids and peptides. For instance, the green alga C. reinhardtii is unable to utilize certain amino acids but thrives when co-cultured with Methylobacterium sp. This indicates there was a mutual carbon-nitrogen exchange; as Methylobacterium sp. degrades amino acids and releases ammonium for the alga, and the CO2 fixed by the alga provides glycerol for the bacterium [37].
The findings of synergistic interactions between microalgae and bacteria offer a clearer view into the complexities of microbial cooperation, revealing mechanisms that significantly enhance growth and productivity. The potential of these interactions in biotechnological applications, such as environmental remediation, wastewater treatment and biofuel production, is immense. However, to fully harness these benefits, a deeper understanding of the molecular and environmental factors governing these interactions is needed.
2.2. Applications of microalgae-bacteria consortia
2.2.1. Microalgae-bacteria consortia role in aquaculture production
As industrialization and the global population rapidly grow, the need for sustainable energy and food production also steadily increasing. In this prospect, aquaculture emerged as a viable sustainable solution with microalgae and bacteria nexus playing a pivotal role in improving the efficiency of energy and food production. The significance of microalgal-bacterial interactions for aquaculture has been reviewed by Natrah et al. [38]. The growth-promoting effect of bacteria on microalgae which have commercial values has been reported for several years [39]. For instance, the co-culture of microalga Isochrysis galbana with mutualistic heterotrophic bacteria such as Alteromonas sp. and Labrenzia sp. has led to notable increases in growth rate and biomass accumulation [40]. Co-culturing diatoms with specific bacteria can boost their production, providing improved feed yield for shellfish and finfish larvae [41].
The beneficial bacteria promote the growth of target microalgae while also inhibiting harmful pathogens and grazers. While outdoor pond cultivation of algae is cost-effective for mass production, it is challenged by pest organisms [42,43]. Several mitigation strategies such as creating non-permissive growth conditions with altered pH and salinity levels [44] or adding chemical treatments such as biocides have been developed to prevent the destruction of algal crops by pests [45]. Generally, mitigation strategies that are eco-friendly and do not hinder the growth of beneficial algae are highly preferred. Hence, as certain bacteria strains offer a promising approach to selectively inhibit unwanted bacteria without adversely affecting desired algae species, the microalgae-bacteria nexus provided another opportunity for pest control in the microalgae aquaculture system. For example, a Phaeobacter strain (BS52) showed antagonistic activity toward the bleaching pathogen and significantly increased the proportion of healthy individuals of the seaweed Delisea pulchra by preventing dysbiosis [46].
Algal biomass in the cultivation pond can be attacked by grazers such as rotifers, which requires an efficient mechanism to mitigate this problem. Recently, a study by Ward et al. [47] indicated that introducing a bacterium Janthinobacter lividum into Microchloropsis salina cultures effectively protected the algae from zooplankton Brachionus plicatilis predation without harming other microorganisms. This protection was confirmed through both laboratory and outdoor mesocosm experiments. Additionally, the nexus of algae with certain heterotrophic bacteria can lead to increased algal biomass as the bacteria recycle the organic matter and improve nutrient uptake in nutrient-deficient conditions [48].
2.2.2. Microalgae-bacteria consortia role in metabolite and biomass production
As the human population steadily increases, the demand for the production of high biomass in a short time and limited space and energy is imperative. Microalgae, providing formidable service as sources of biomass in biotechnological applications, have been exposed to various cultivation conditions to promote their growth and yield. Among these, altering the growth conditions through co-culturing with bacteria has been found to enhance the production of beneficial compounds in algae [49]. For instance, co-culturing Haematococcus lacustris with bacteria resulted in more than double biomass and a 60 % increase in the production of the antioxidant astaxanthin [50]. Similarly, the growth of the green algae Tetradesmus obliquus or Coelastrella sp. increased by 70 % when co-cultured with the bacterium Variovorax paradoxus [51]. The study by Cassan et al. [52] showed the lipid, carbohydrate and photosynthetic pigment productions of C. sorokiniana were promoted by the bacterium A. brasilense.
The synergetic association of the microalgae Chlorella sp., Scenedesmus sp. and the bacterium A. brasilense showed that there was production of signal molecules such as tryptophan and IAA by the microalgae and bacterium, respectively, under stress growth conditions of the co-culture, which permits the maintenance of their affinity and mutualistic association [53]. This promoted the CO2 fixation rate, biomass production, carbohydrate content and protein content of the microalgae [53]. Moreover, optimization of co-culture conditions with altered cell mobility, pH and CO2 concentration further augmented the production of carbohydrates and starch, particularly when C. sorokiniana and Chlorella vulgaris were co-cultured with A. brasilense within immobilizing alginate beads [54]. Higher CO2 fixation, algal growth and accumulation of carbohydrates were also reported in the cocultivation of the bacterium A. brasilense with microalgae Scenedesmus, Chlamydomonas and Chlorella under high CO2 provisions [55].
2.2.3. Microalgae-bacteria consortia role in biofuel
Microalgae constitute a considerable amount of their dry weight as lipids and carbohydrates. These have garnered the opportunity to apply algal biomass as feedstock for the production of biofuels including biodiesel, bioethanol, biohydrogen and biogas [1,56]. The utilization of microalgae as a biofuel source has been explored since the 1950s. Microalgae can serve as a sustainable energy source due to their vigorous growth characteristics and the ability to thrive in a wide range of media and growth conditions [57]. Moreover, microalgae are a potentially better candidate than edible crops, as their high specific growth rate can allow them to accumulate more biomass per acre [57]. In addition to that, microalgae cultivation requires less land area compared to the cultivation of land crops, offering ecological benefits by reducing pollutants released into the environment as well [58].
The biofuel production of microalgae can be promoted by enhancing the biomass production efficiency, which in turn, is improved by cultivating it in microalgae-bacteria consortia and altering the growth conditions. In line to this, Meng et al. [59] reported that nutrient removal and lipid accumulation for biofuel production significantly increased in microalgae-bacteria granules, coinciding with increased light intensity.
The cultivation of microalgae has been a field of potential interest due to their ability to produce high amounts of biooil [60]. Several studies have been reported on biooil production efficiencies of microalgae biomasses, including Spirulina [61] and Chlorella [62,63] species. Microalgae are suitable candidates for biooil due to their high lipid contents, and their lipid production is highly influenced by the growth conditions of the algae, which in turn, creates an opportunity to enhance production. For instance, a conspicuous increment of lipid production by C. sorokiniana under exogenous ethanol-stress [64] and increased biooil content by C. vulgaris under nitrogen-depleted media [65] have been reported. Moreover, microalgae can be integrated with other systems to enhance the output. For instance, an innovative integrated system for the production of biooil from C. sorokiniana that is grown in sewage has been reported recently [66]. Increased biomass production of C. vulgaris, C. reinhardtii, and Euglena gracilis due to co-culture with indigenous bacteria found in the effluent has been reported [67].
The biomass obtained from microalgae-bacteria consortia can also be valorized and applied in biodiesel production. Moreover, to increase the feasibility of the technology, cultivating microalgae-bacteria in wastewater has been indicated as a source of lipids for biodiesel. Toyama et al. [68] investigated the growth-promoting effect of some bacterial strains (Emticicia sp.) isolated from wastewater on a microalga (E. gracilis) suitable for biodiesel. They reported that biomass production and lipid production increased by 3.5 and 2.9-fold, respectively, compared to the monoculture. High biomass and lipid production, along with enhanced removal of nutrients, suitable for biodiesel production, has been reported under the microalga-bacteria consortium in municipal wastewater [69]. Co-cultivation of microalgae and bacteria provided a promising result in biocrude oil production through hydrothermal liquefaction of microbial biomass cultivated in a large-scale open raceway pond [70]. Additionally, they reported 90 % COD removal and 43 % biocrude oil energy recovery efficiencies. The enhanced accumulation of biomass for biodiesel production as well as the removal of contaminant nutrients and COD under microalgae-bacteria consortium have been extensively reported [71].
The lipids obtained from microalgal biomass can be refined into fatty acids, and processed to yield biodiesel through transesterification [72]. The cultivation of microalgae, drying of the biomass, extraction of the oil and then transesterification of the oil into fatty acid methyl esters are some of the crucial steps in the biodiesel production process. Biodiesel consisting of esters of methylated fatty acids is usually formed from oils through transesterification with alcohols. Mechanisms of lipid extraction and factors influencing transesterification process have been reviewed adequately by Anand et al. [73].
Microalgae, which contain a considerable amount of carbohydrates as their dry weight, can also be used as a feedstock for biofuels such as bioethanol production under anaerobic fermentation [74]. Although the utilization of microalgal biomass requires pretreatment during bioethanol production [75], the efficiency of the system can be enhanced by cultivating algal-bacterial aggregates [76]. Moreover, altering the metabolism of microorganisms in the consortium and altering the physicochemical growth conditions are also other mechanisms to enhance the production of biofuels. Given this, genetically engineered and metabolically altered E. coli was cultivated on a biomass constituted of a bacterium and four microalgal species to degrade proteins and produce ammonia, pyruvate and keto acids, which can be used as biofertilizers and sources of biofuels such as bioethanol and biobutanol [77]. In terms of altering growth conditions, Arcila and Buitrón [78] studied the effect of hydraulic retention time on the removal of pollutants, and the biochemical potential of methane production using a granular microalgae–bacteria system operated in a high-rate algal pond. They reported that high performance was found on the longer retention time with methane yield of 348 mL CH4 g−1.
2.2.4. Microalgae-bacteria consortia role in biohydrogen production
The use of hydrogen as a source of energy has rapidly increased in the past few decades, owing to its high energy content, which is clean and safe for the environment and health. Notably over 95 % (the other 4 % from water electrolysis and ∼1 % from biological production) of produced hydrogen comes from fossil fuel through thermochemical conversion and gasification [79], highlighting a reliance on non-renewable sources and environmentally unfriendly systems [80]. Therefore, the incorporation of advanced technologies that simultaneously produce hydrogen from renewable resources as well as reduce environmental pollution and feasible operational costs has received the most attention [81]. The abundant availability, high energy content, and non-toxic and less pollution effect make hydrogen a preferred choice of energy source [82,83].
The ambition of attaining renewable hydrogen production could be achieved in the long run, as several species of microorganisms from various phylogenetic groups, including bacteria (photosynthetic and fermentative), blue-green algae and some eukaryotic microalgae, have been reported to produce biological hydrogen [82,84]. Biological hydrogen production can be attained from three different processes; biophotolysis (hydrogen production under specific photosynthetic activities of eukaryotic algae and cyanobacteria, using light), photo-fermentation (hydrogen production from light and organic substrates without producing oxygen, this is performed by non-oxygenic photosynthetic bacteria) and dark fermentation (production of hydrogen and CO2 during degradation of organic matter) [79]. However, this paper only focuses on biophotolysis processes involving microalgae and bacteria.
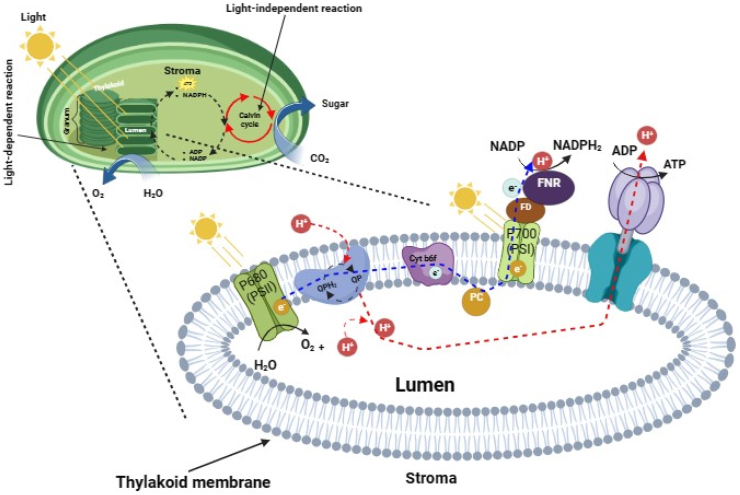
Photosynthetic microorganisms (both prokaryotic and eukaryotic microalgae) are endowed with hydrogenase enzyme, which gives them the capability to evolve hydrogen under specific conditions and to reduce CO2 in the next dark photosynthetic reaction (Fig. 1). Biohydrogen production by cyanobacteria and eukaryotic microalgae are preferred over non-oxygenic (photo-fermentation) or heterotrophic bacteria (dark fermentation), as they don't need external carbon sources. However, the requirement of restricted and specific growth conditions limited its wider applications [85]. For instance, biohydrogen production through biophotolysis has a shortfall as it requires an anoxic condition. Many microalgae showed activation of hydrogenases and evolved hydrogen when acclimatized in the anaerobic-dark condition, but the hydrogen production ceases when the cells are exposed to light and commence stable photosynthesis [85].
Fig. 1.
A diagram illustrating the light-dependent and light-independent reaction steps in the photosynthesis process. During the light-dependent reaction, the excited electron replacement and generation of hydrogen occur through the photolysis of water molecules, then ATP and NADPH are produced to be supplied to the Calvin cycle of light-independent reaction to fix carbon dioxide into sugar. The sketch was drawn based on the information provided by Marchand et al. [101].
The requirement of anoxic conditions in biohydrogen production systems poses technical difficulties and limitations for production efficiency [82]. Hydrogenase enzyme is sensitive to oxygen (a byproduct of photosynthesis); thus, continuous production of hydrogen can only occur in anoxic conditions that can be achieved by reversible inactivation of photosystem II and oxygen evolution. For example, the evolution of hydrogen by co-immobilizing spinach (higher plant) chloroplast and Clostridium butyricum (bacterium) with the help of electron carrier molecules such as benzyl viologen and ferredoxin was reported in early studies by Karube et al. [86]. They reported that there was an evolution of hydrogen through the oxidation of carrier molecules, although the PSII also evolved oxygen, which poses a major problem in chloroplast-hydrogenase coupled systems. Further research suggested that adding some substrates such as glucose, glucosidase and catalase, and immobilizing hydrogenases reduced interference of oxygen in biohydrogen production [86]. Subsequent studies showed that starvation with nutrients such as acetate, sulfide, phosphorus, nitrogen, magnesium and potassium [[87], [88], [89], [90]] inhibited photosystem II activity and consequently declined oxygen evolution.
Even though starving cells through the deprivation of nutrients temporarily suppress oxygen evolution and allows hydrogen production, hydrogen production does not sustain itself for a long time as the growth of the algae declines after some time due to the limited nutrients. Therefore, various strategies have been designed to increase the efficiency and durability of hydrogen production. These include changing culture conditions such as light/dark frequency, cell immobilization, the addition of fresh cells grown in sulfur-deprived medium, and using mutant strains with limited antenna features, suppressed photosystem II, altered PSII to PSI ratio and inhibited cycle electron flow or controlled CO2 fixation [ [91] and references therein]. Moreover, studies on C. reinhardtii [92] demonstrated that the decoupling of photosynthetically produced carbohydrates (during stage one) from cellular metabolism (stage two) leads to hydrogen and CO2 generation under anoxic conditions.
Even though most species require anoxic conditions for hydrogen production, there are nitrogen-fixing cyanobacterial species (Anabaena sp.) that can perform aerobic photolytic hydrogen evolution due to the hydrogenase's resistance to nitrogen and hydrogenase protection from oxygen [93]. Furthermore, hydrogen production by eukaryotic microalgae C. reinhardtii under aerobic conditions was also reported by Khetkorn et al. [94]. To address the limitation of hydrogenase inhibition by oxygen or nitrogen in cyanobacterial biophotolysis, an argon-based atmosphere has been used in hydrogen production [93].
Although the specific requirement of growth conditions for biohydrogen production hampered the wide application of microalgae for the production of biohydrogen, several reports indicated biohydrogen (photolysis) production can be enhanced by microalga-bacteria consortia [83 and references therein]. The advantage of co-culturing heterotrophic bacteria with microalgae for biohydrogen production includes the removal of oxygen by bacteria, which is a bottleneck for biohydrogen production, and the consumption of CO2 by microalgae, which is produced by the degradation of substrate by bacteria. The co-cultivation of bacteria with microalgae could enhance hydrogen production by increasing the light tolerance of the microalgae [83] and slowing chlorophyll reduction, enhancing starch accumulation, and maintaining protein content [95]. Additionally, several metabolites including acetic acid exchanged between the two partners, which could further promote hydrogen production [96]. For instance, co-cultivation of Chlamydomonas and E. coli under glucose provision showed a 60 % increase in hydrogen production compared to monocultures, where alga benefited from the consumption of the acetic acid excreted by bacteria and anoxic condition created [96].
Several research results indicated that the microalgae type, bacterial partner, substrate concentration, and media type have a significant impact on the hydrogen production efficiency of microalgae-bacteria consortia [83,[97], [98], [99]]. Furthermore, hydrogen production by the microalga-bacteria consortium can also be affected by the initial cell density and size of the microalgae [97]. He et al. [100] also found enhanced production of hydrogen under sulfide provision (Na2S2O3) by co-culturing of Chlamydomonas sp. and Thuomonas intermedia (sulfur-oxidizing facultative autotroph bacterium). Despite certain complexity, studies have demonstrated that the microalga-bacteria consortium presents a promising path forward in biohydrogen production, offering a potentially sustainable and efficient method for harnessing hydrogen as a clean energy source.
2.2.5. Microalgae-bacteria consortia role in promoting wastewater treatment
The increase in sewage generation, in parallel with the expansion of industrialization and human population growth, necessitates the development of efficient and economical wastewater management and treatment technologies for environmental remediation. Recently, the cultivation of microalgae-bacteria in effluent or sewage has gained attention for its dual benefits of nutrient removal and biomass production [7]. The co-cultivation of microalgae and bacteria offers mutual benefits to both organisms. Table 1 presents key studies highlighting the role of microalgae-bacteria consortia in enhancing wastewater treatment; indicating the removal efficiency of COD and other pollutants. For instance, the coculture of the microalgae C. sorokiniana and Chlorella sp. with the bacteria Klebsiella pneumoniae and Acinetobacter calcoaceticus enhanced microalgae growth, biomass, and nutrient/COD removal in wastewater, outperforming the monoculture systems [102]. Moreover, a consortium composed of Chlorella sp. and four bacterial strains was able to degrade ketoprofen with 40 % reduction in COD and 82 % reduction in toxicity in pharmaceutical wastes [103].
Table 1.
Studies of microalgae-bacteria consortia in promoting wastewater treatment.
| Microalgae-Bacteria Used | Medium/Condition | Biomass Yield (%) | Removal efficiency (%) | Findings | Reference |
|---|---|---|---|---|---|
| C. sorokiniana and Chlorella sp. with Klebsiella pneumoniae and Acinetobacter calcoaceticus | Artificial wastewater (AWW) and raw dairy wastewater (RDWW) | RDWW: 2.87 g/L | COD of RDWW: 90.49 % | Enhanced growth, biomass, nutrient/COD removal over monocultures. | [102] |
| AWW: 2.84 g/L | COD of AWW: 82.27 % | ||||
| C. sorokiniana with Streptomyces thermocarboxydus | Wastewater treatment | Bioflocculation efficiency: 93 %; Biomass productivity: increased 33 % Lipid productivity: 80 % increase due to the co-cultivation of Streptomyces and microalgal cells. | NA | Higher algal biomass and lipid content, suitable for biodiesel. | [112] |
| Chlorella sp. with four bacterial strains | Pharmaceutical waste degradation | NA | In dark condition: biodegradation was faster with a lag phase of 10 h; COD: 41 %; Toxicity reduction: 82 % | Effective ketoprofen degradation, reduced COD (40 %) and toxicity (82 %) | [103] |
| T. obliquus and Coelastrella sp. with V. paradoxus | Modified Bold's basal medium | NA | Nitrate: 88–99 %; Phosphate: 92–95 % | Improved microalgae growth, nutrient uptake nitrate and phosphate and biochemical composition. | [51] |
| C. vulgaris with various bacterial strains | Wastewater treatment | Biomass growth rate: 0.196 ± 0.06 d−1 (highest); mean daily biomass productivity 0.098 ± 0.009 g L−1 d−1 (for 10:1 ratio of S395-2 to C. vulgaris) | COD: 88.29 ± 5.03 %; TN: 88.31 ± 4.29 %; TP: 88.21 ± 4.51 %; CO2 68.13 ± 1.69 %. | Enhanced algal biomass accumulation, nutrient removal, and energy generation. | [113] |
| Consortia with microalgae | Photoreactor for wastewater treatment | TSS: ∼59 % increased; Chlorophyll: ∼64 % increased | NH4+–N: 65 ± 6 % −93 ± 2 % (with the change of light density) | Reduced aeration requirements due to inhibition of nitrite oxidizing bacteria. | [109] |
| Microalga-bacteria photoreactor system | Ammonium removal | TSS: 1006 ± 100 mg/L - 1930 mg/L |
NH4+ oxidized to NO2− at the rate of 8.09 mg NH4+-N L−1h−1. | Innovative method for nitrogen removal without external aeration. | [111] |
| VSS: 639 mg/L, - 1240 mg/L, | |||||
| Chlorophyll a: 217 % | |||||
| Chlorophyll b: 36 % | |||||
| Chlorophyll a and b indicate high algal biomass growth |
The co-cultivation of the bacterium V. paradoxus and microalgae T. obliquus and Coelastrella sp. in modified Bold's basal medium resulted in higher specific growth rate of the microalgae, uptake of nitrate (88–99 %) and phosphate (92–95 %), and accumulation of carbohydrate and proteins than the unialgal cultures. Additionally, numerous phytohormones, vitamins, polysaccharides and aminoamides are likely used during their interactions [51]. There are some important steps in the development and realization of microalgae-bacteria consortia; these include selection of the most appropriate cooperative partner species, wastewater characterization and acclimatization of the species, pretreatment of the cells including allowing biofilm formation or immobilization, adjusting the optimum growth condition such as temperature, pH, CO2 flow, nutrient concentrations, and finally inoculate the microalgae and bacteria in the reactor [104].
Recently, mathematical modeling and degradation efficiency of fluoroquinolone by considering microalgae and bacteria have been studied [105]. Compared to the utilization of microalgae or bacteria alone as bioremediation means, microalgae-bacteria consortia has provided more advantages by enhancing biomass accumulation, nutrient removal efficiency, energy generation and lower processing cost. For instance, increased efficiency of algal biomass accumulation, and removal of COD, nitrogen and phosphorous have been reported in the consortium of the microalga C. vulgaris and some bacterial strains [106]. Recently, Cai et al. [107] also showed high performance in volatile organic carbon degradation and accumulation of algal biomass by microalgae-bacteria consortia.
In addition to the enhanced performance, there is a job partition in the consortia. For example, there was primary (81–85 %) removal of ammonium through nitrification by nitrifying bacteria rather than uptake by microalgae [108] and removal of (up to 80 %) total nitrogen via bacterial nitrification/denitrification process in an anaerobically digested photobioreactor [109]. Effective carbon removal by bacteria and effective nitrogen removal by microalgae were reported in the microalga-bacteria consortium operated to treat dairy manure wastewater [110]. The microalgae in the consortium can supply oxygen that can be used by the heterotrophic bacteria to degrade the organic matter. This gives an additional advantage by minimizing the cost of aeration and alleviating the side effects that arise from the treatment of hazardous pollutants that need mechanical aeration but can volatilize during aeration [11]. Hence, in photobioreactors filled with consortia, microalgae can supply oxygen for nitrifying bacteria to oxidize the ammonium in the wastewater, potentially reducing the need for mechanical stirring or oxygenation.
Arun et al. [111] recently innovated an ammonium removal technique in a microalga-bacteria photoreactor system that operates without external aeration. By alternating between light and dark periods and adding methanol as the sole carbon source, they achieved ammonia oxidation to nitrite by bacteria during light periods and nitrite reduction in dark periods. This microalga-bacteria approach effectively removed nitrogen from the system, suggesting its valuable input for practical applications.
2.3. Factors influencing the performance of microalgae-bacteria consortia and optimization mechanisms
The performance of microalgae-bacteria consortia in wastewater treatment and biomass accumulation is significantly influenced by environmental factors, including physicochemical and biological conditions. Factors such as the availability of nutrients, granular size and presence of other pollutants could have negative or positive impacts on the degradation processes. Recently, the effect of carbon-to-nitrogen ratio, light, pH, salinity and temperature on the efficiency of microalga-bacteria consortia has been adequately reviewed [114]. Additionally, environmental factors such as temperature, light, pH, dissolved oxygen concentration and predators have been reported to affect the performance of microalga-bacteria consortia and have been reviewed by Muñoz and Guieysse [11]. Here we briefly discuss some important points that are not covered in those papers.
2.3.1. The type of growth media and hydraulic retention time
The type of growth media greatly influences the efficiency of microalgae-bacteria interactions, impacting biomass production and nutrient removal efficiency. Pérez-Nava et al. [115] demonstrated that C. vulgaris and Pseudomonas sp. have enhanced growth in wastewater compared to Bold's basal media, with microalga growth diminishing as the ratio of Bold's basal media increases. Wirth et al. [20] studied the growth of C. vulgaris and its phycosphere partner bacteria in various media; municipal, industrial, and agricultural liquid wastewaters, and compared with tris-acetate-phosphate medium. Their study revealed notable variations in nutrient removal and bioremediation efficiency depending on the media type, highlighting the alteration of microalga-bacteria cooperation. Tait et al. [116] found that bacterial strains from textile factory wastewater enhanced Chlorella sp. growth in Chu media, which has lower phosphorus and nitrogen, but not in BG11 media, demonstrating how growth-media type influences bacteria-stimulated microalgae growth.
Moreover, the effect of different ratios of tap water and lake water to wastewater was studied in municipal wastewater treatment photobioreactors operated on a lab scale [117]. The report showed that inoculation of lake water in the photobioreactor with the ratio of 70/30 v/v (wastewater/lake water) resulted in better algal biomass accumulation and nutrient removal efficiency than the wastewater alone.
The pretreatment of the growth media can also significantly influence the performance of the consortia. López-Patiño et al. [118] compared the performance of native microalgae-bacteria consortium processing sterilized and non-sterilized domestic wastewater. The result indicated that there was a higher nutrient removal rate and growth of microorganisms in non-sterilized wastewater, suggesting a potential synergy could be developed in the autochthonous consortia.
The hydraulic retention time also affects the performance of the consortia; for example, the effect of hydraulic retention time was studied in high-rate algal pond systems, which are primarily constituted by diatoms, filamentous microalgae and bacteria. The system's performance was initially low on day 2, but significantly improved by days 6 and 10, showing high removal rates of ammonium (>85 %), COD (>92 %), phosphorus (up to 30 %), and increased biomass and methane yields, particularly notable on day 10 [78]. Other researchers also reported the effect of hydraulic retention time on the performance of wastewater treatment [119].
2.3.2. Source of exogenous organic carbon and light intensity
The type of growth condition is one of the important factors that affect the performance of microalgae-bacteria consortia. Ferro et al. [120] investigated the co-cultivation of C. vulgaris and Rhizobium sp. in synthetic wastewater under photoautotrophic, heterotrophic, and mixotrophic conditions. They reported a twofold increase in biomass for axenic algal cultures compared to cocultures under heterotrophic conditions, suggesting potential resource competition. However, under the mixotrophic condition, the biomass increased (compared to axenic algal culture) threefold, along with a 13-fold increase in fatty acid content and higher wastewater treatment performance, indicating there is an exchange of nutrients between the alga and bacterium. Additionally, higher nutrient removal efficiency and biomass production of the microalga C. sorokiniana was reported under anaerobic conditions and in the presence of partner prokaryotic microbiomes [121].
Enhanced biomass production, inorganic nitrogen removal and biohydrogen production were also observed in the coculture of the bacterium M. oryzae and the microalga C. reinhardtii under the provision of ethanol and methanol [122]. Wang et al. [123] explored the impact of glucose and acetate on antibiotic removal (sulfadiazine and sulfamethoxazole) in a microalga-bacterium consortium. They found that glucose addition doubled biomass and promoted Proteobacteria, while acetate favored Bacteroidetes. Both substrates enhanced sulfadiazine degradation but did not affect sulfamethoxazole. The addition of these substrates had a positive effect on the physiology of microalga [123].
The source of exogenous organic carbon could also exert a negative impact on the performance of microalga-bacteria consortia. For example, Zhong et al. [124] showed a consortium that had high biomass accumulation and nutrient removal efficiency, but the system collapsed following the addition of glycerol in the late stage of co-cultivation.
The growth of microalgae and bacteria in the consortia can also be affected by the light condition of the system, as nitrifying bacteria have less light tolerance than microalgae. The light intensity above 450 μmol photons m−2s−1 leads to inhibition of both microalga and nitrifying bacteria, reflected in a reduction of nitrification [125,126]. Owing to this, Nishi et al. [127] proposed a new method by entrapping the bacteria in carbon black-added alginate hydrogel beads named “light-shielding hydrogel”, to enhance the growth performance of bacteria under high light conditions and advance its applications in microalgae-bacteria consortia. They showed the growth of the nitrifying bacteria together with C. sorokiniana in light-shielding hydrogel had better result of nitrification even at high light (1600 μmol photons m−2s−1) intensity.
2.3.3. The ratio of microalgae to bacteria and ratio of macronutrients
The inoculation ratio of microalgae and bacteria is another factor that can influence the performance of the consortia by affecting biomass accumulation and wastewater treatment efficiency. A study on the inoculation ratio of C. vulgaris and Shinella sp. in biogas slurry (50:1, 20:1, 10:1, 5:1 and 2:1) showed that a higher microalga ratio initially increased synergy, but the most effective nutrient removal, algal viability, and biomass were achieved at a 20:1 ratio [128]. The effect of different compositions (1:0, 9:1, 3:1, 1:1, 0:1 wt/wt) of microalgae on the activated sludge ratio was also investigated on nutrient removal and biomass production performance of a photobioreactor [129]. The authors indicated the 3:1 ratio was the optimum for yielding high total biomass accumulation (1.12 g/L) and nutrient removal efficiency. This study revealed that microalgae predominantly removed nitrogen, while bacteria were more effective in COD removal.
Some wastewaters contain disproportionally high concentrations of one of the macronutrients, which alter the general Redfield's ratio and result in stunted growth of microalgae or bacteria in the wastewater treatment system, which again results in low efficiency of the treatment systems. To curb this problem, the addition of scarce nutrients to balance the ratio has been introduced. For example, the effect of the nitrogen to phosphorus (N/P) ratio in swine wastewater was studied by adjusting the concentration of phosphorus in the consortium of Chlorella sp. and indigenous bacteria. The result showed variation in N/P ratio had a clear impact on the performance of the system, where the optimum growth of microalga, extracellular polymeric substance secretion of bacteria and removal efficiency of nutrients were observed at a ratio of 20 [130]. In addition to making various ratios, a better result has been reported by starvation of cells by avoiding some inorganic nutrients such as phosphorus for a limited time [131].
2.3.4. Effect of cations
Recently, besides the role of macro- and micronutrients, the effect of cations on microalga-bacteria cultivation has been reported. For example, owing to the low sedimentation and harvesting performance of microalgae biomass due to their low cell density and negative cell-surface charge [132], Tang et al. [133] studied the efficiency of wastewater treatment and the performance of microalgae harvest in microalga-bacteria cultivation by providing calcium ions (Ca2+). The result indicated that a low concentration of Ca2+ enhanced the removal of COD and nutrients, and the growth rate of microalga. Conversely, higher Ca2+ (10 mM) resulted in decreased removal efficiency of nutrients and COD but promoted microalgae aggregations. The study by Tang et al. [133] suggested that increased Ca2+ levels reduce microalga cell dispersibility by adsorbing onto cell aggregates, neutralizing charges, and compressing the electric double layer. This process, coupled with the promotion of extracellular secretions and flocculation by Ca2+, aids in efficient biomass harvesting.
Furthermore, the role of cations such as Mg2+, K+, and Li+ along with microalga-bacterial cocultivation was studied on wastewater treatment efficiency. The result showed improved pollutant removal efficiency at less than 1 mM of Mg2+ and K+, whereas the provision of Li+ inhibited the performance. Additionally, Tang et al. [134] observed that high concentrations (10 mM) of Mg2+, K+ and Li+ led to lower biomass buildup due to reduced absorption efficiency and an increase in extracellular polymeric substances. The study found that the high charge density of these ions facilitated algal aggregation by drawing water molecules closer, effectively shrinking the gap between algal cells. Nevertheless, recently Collao et al. [135] reported that high levels of Zn (100 mg/L), Cu (100 mg/L) and As (500 μg/L) disrupted Chlorella sp. growth and bacterial populations within a microalga-bacteria photobioreactor.
2.3.5. Effect of cell immobilization
The removal of biomass after wastewater treatment is one of the main challenges, which increases the cost of operation. This problem could be simplified by immobilization and granular formation techniques [114]. The co-immobilization of microalgae and bacteria could enhance the biomass and aggregate formation of the microorganisms. It reduces the distance between cells to ∼1 μm, and consequently induces an effective exchange of nutrients as well as signal molecules [23,136]. Under this condition, a relatively smaller quantity of signal molecules and nutrient production is required than without co-immobilization [23], which sustains the interaction between the microorganisms [137].
The co-immobilized (using alginate beads) consortium of C. vulgaris and bacterium A. brasilense in synthetic wastewater increased (compared to immobilization of microalga alone) the removal of ammonium and phosphorus [138]. Co-immobilization of C. vulgaris and C. sorokiniana with the bacterium A. brasilense showed the removal efficiency of ammonium, nitrate and phosphorus increased from 75 %, 6 % and 19 % (microalgae alone) to 100 %, 15 % and 36 % (microalgae co-immobilized with bacterium), respectively [139]. This indicates that co-immobilization can enhance nutrient removal efficiency. Furthermore, starvation and co-immobilization of C. sorokiniana and A. brasilense consortium showed enhanced removal of phosphorus [131]. The cultivation of the co-immobilized microalgae C. vulgaris and C. sorokiniana with the bacterium A. brasilense increased the accumulation of total carbohydrate and starch production in synthetic media [54].
3. Microalgae-integrated-microbial fuel cells
Fossil fuel-derived energy production faces significant challenges, including its non-renewable nature, contribution to global warming as well as impacts on human health and the ecosystem. Consequently, biofuel energies from biomass [1] and direct electricity generation from microbial fuel cells (MFCs) [2] have emerged as viable and clean alternatives. The application of algal biomass (as a feedstock) has garnered significant attention for producing biodiesel, bioethanol, biohydrogen, and biogas [1], and the role of microalgae-bacteria interaction in biofuel technologies was discussed in the previous section. This section focuses on MFC technology integrated with microalgae. This section critically reviews the integration of microalgae with microbial fuel cells (MFCs) technology.
3.1. Microbial fuel cells
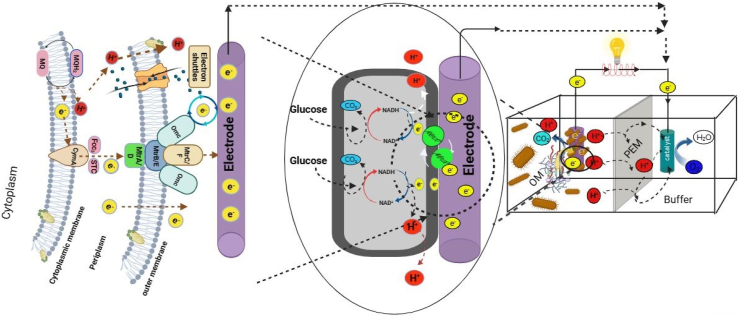
The MFC technology has already tracked a centurial history [[140], [141], [142], [143]]. It uses bioelectrochemical systems to convert organic matter into clean bioelectric energy by employing electroactive microorganisms like bacteria and yeast (Fig. 2, Fig. 3a). This technology leverages the redox reduction reaction that occurs during microbial interaction, with degradation of organic matter and transportation of electrons extracellularly to generate energy and value-added substances. The process of extracellular electron transport (EET) involves the transfer of electrons from the quinone pool within the cell membrane to the cell exterior (through periplasm and outer membrane). These electrons traverse a series of cytochrome complexes embedded in the membrane before reaching the surface.
Fig. 2.
Overview of MFC processes and extracellular electron transport (EET) mechanisms. Indirect transport of electrons with shuttle transfer and direct transfer with surface contact is depicted in the left and middle sections of the figure. The electron shuttle mediates the indirect transfer of electrons back and forth between the electrode and bacterial cells. Where, Mtr (metal-reducing) conduit system comprising several multi-heme c-type cytochormes (c-Cyts) including Fcc3-flavocytochrome c3 (tetraheme), MtrA (periplasmic, decamehe), MtrB (outer membrane-bounded), CymA- (cytoplasmic inner membrane-bounded, tetraheme), STC-small tetra heme cytochrome, MtrC (periplasmic protein, decaheme) and OmcA-outer membrane c-Cyt (decaheme). MQ and OM represent menaquinol and organic matter, respectively. The sketch was drawn based on the information provided by Zou et al. [144].
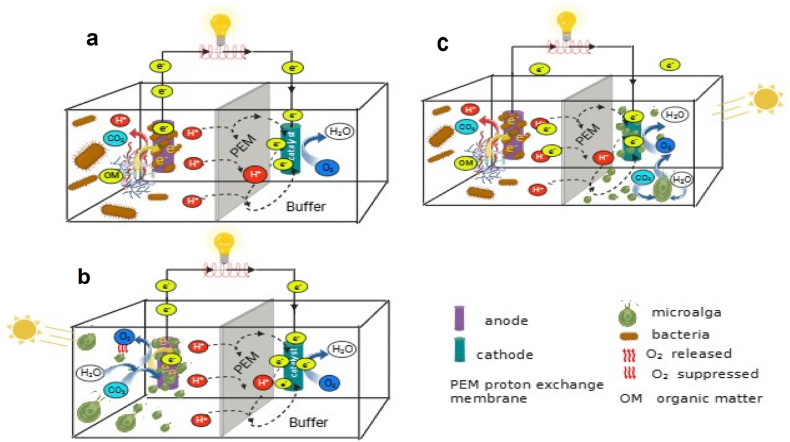
Fig. 3.
Illustration of microbial fuel cell (MFC). a) conventional MFC, where bacteria are electricigens to release electrons from organic matter (OM), b) photosynthetic-MFC (PMFC), where microalgae are electricigens to release electrons from photolysis, and c) microalgae-MFC (mMFC), where microalgae are biocathode by producing oxygen to reduce electron and release water. The diagrams show electrons are transported via external circuits and protons flow through a proton exchange membrane (PEM) to the cathode chambers to react with oxygen and electrons to produce water. The sketch was drawn based on the information provided by Saratale et al. [193].
Once the electrons are at the outer surface, they are transferred to electrodes either directly through physical contact, such as through nanowires in Geobacter sulfurreducens, or indirectly transferred through mediators like flavins, as seen in Shewanella oneidensis [144] (Fig. 2). In the intracellular metabolic process, NADH and FADH2 undergo reduction by accepting electrons and protons. This reduction allows them to participate in the electron transfer chain, thereby generating the cell's energy currency [145]. Electrochemically active microbes employ various EET mechanisms, including nanowires (pili-like structures), c-type cytochromes (heme-containing proteins in the periplasm and outer membrane), and electron shuttles (organic molecules like flavins and pyocyanin, capable of redox reactions) [145] (Fig. 2).
MFCs have shown potential in treating wastewater and generating bioelectricity, biohydrogen, and biomass for biofuel production such as bioethanol and biohydrogen [146,147]. Despite its environmental benefits, MFC technology faces high operational costs, particularly for membranes and mechanical aeration, which hinder widespread adaptation. To address this, microalgae-assisted microbial fuel cells (mMFCs) have been introduced, utilizing microalgae in the cathodic chamber as oxygen generators for wastewater treatment and bioelectricity production [148]. The mMFC technology can be applied in domestic wastewater to remove nutrients and generate electricity [[149], [150], [151]], acid drainage system [152] to generate electricity and remove ferrous iron by oxidizing it to insoluble iron (III) (a precipitate to settle down on the anode side [12], and to generate electricity and remove nutrients from agricultural wastewater [147], landfill leachate [153], industrial wastes [146,154,155], and ammonium-rich wastewaters [111].
It was reported that using the microalga Scenedesmus obliquus in the cathodic aeration process nearly doubled the efficiency of the mechanically aerated cathode [156]. Additionally, mMFCs offer benefits over conventional MFCs by eliminating the need for costly and environmentally detrimental catalysts and buffers [157]. Besides, Commault et al. [158] revealed increased electricity generation, nutrient removal and COD removal efficiencies in wastewater treatment of cathodic C. vulgaris, compared to catholytes of phosphorus buffer and anode effluent. Notably, higher COD removal efficiency was observed when microalgae were used as a substrate in a closed-circuit MFC, compared to an open-circuit system, indicating that bioelectricity generation enhanced the degradation of organic matter [159].
3.2. The roles of microalgae in the MFCs
Microalgae offer diverse functions within MFCs (Fig. 3b and c). In the anode chamber, it can act as a substrate for exoelectrogenic bacteria or a donor of photolytic hydrogen and electrons (as previously discussed in Section 2.2.3. and Section 2.2.4). Conversely, in the cathode chamber, microalgae primarily function as electron acceptors. However, the application of microalgae as bioanodes (electron and hydrogen donors) is challenged by their limited exoelectrogenic capacity and the inhibitory effect of oxygen on hydrogen production. Therefore, microalgae are predominantly utilized as substrates in the anode or as electron acceptors (biocathodes) in the cathode chamber. Unlike in the anode, microalgae are particularly effective as electron acceptors in the cathode. Their photosynthetic by-product, oxygen, efficiently facilitates the reduction of electrons transferred from the anode. For clarity, the term 'mMFC’ is used when microalgae are applied as feedstock in the anode or serve as biocatalysts and oxygen providers in the cathode. The term 'PMFC’ is used when microalgae function as electron providers in the anode chamber.
3.2.1. Microalgae biomass as sources of electrons in the anode
The living or dry biomass of microalgae can serve as substrates for bacterial degradation in MFCs. Strik et al. [160] demonstrated successful electricity generation using living algal biomass in an mMFC connected to a photobioreactor, though the electricity output was rather low. This approach also offers the opportunity to utilize microalgae from natural occurrences, particularly during algal blooms, thus aiding in environmental remediation and electricity generation. Algal biomass has been shown to produce electricity [161], along with simultaneous reduction of microcystin [162], disinfection by-products [163] and chromium [164]. As the energy recovery efficiency varies with different microalgal species, Velasquez-Orta et al. [165] reported different energy recovery efficiencies from C. vulgaris and Ulva lactuca. Furthermore, the complete degradation of microalgae biomass is hindered by several factors, such as cell wall resistance and enzyme deficiency, impeding complete degradation and electricity generation potential. For example, Kondaveeti et al. [159] found that the variation in concertation of degradation by-products in the anolyte was coupled with variation in electricity production levels.
Besides variation in cell content, the resistance of microalgae cell walls to hydrolysis presents a significant challenge. To enhance biomass degradability and electricity production, various pretreatment methods have been explored, such as autoclaving, ultrasonication, microwave irradiation, and exposure to acidic or alkaline solutions, which have proven to be effective in improving biomass suitability for mMFCs [166,167]. Notably, innovative approaches have been employed to utilize cyanobacteria biomass from blooming lakes [168]. They reported successful electricity production using cyanobacterial biomass from Taihu Lake, China, pretreated with alkaline solutions and acidic fermentation. Further enhancing the degradation process, an anaerobic digester connected to an MFC has been utilized for pretreating algal biomass, facilitating its breakdown [169]. Nishio et al. [170] demonstrated that Lactobacillus amylovorus, an algal-digesting bacterium, is essential for G. sulfurreducens to efficiently degrade C. reinhardtii biomass and generate electricity. The L. amylovorus ferments the algal biomass, producing by-products like lactate, which G. sulfurreducens then utilize for electricity production.
3.2.2. Photosynthetic electricigens in the anode-bioanode
Photosynthetic microbial fuel cells (PMFCs) utilize microalgae as electricigens in the anode, a concept distinct from conventional microalgae-integrated MFCs (mMFCs). PMFCs generate electrons through water photolysis, whereas mMFCs typically use microalgae as organic substrates for electron donation or oxygen production (Fig. 3b). The potential of microalgae in PMFCs was recognized following early experiments [171] on cyanobacteria and Rhodospirillum rubrum, demonstrating the conversion of light energy to electrical energy. In the subsequent studies, Ochiai et al. [172] demonstrated the potential application of cyanobacterium (Mastigocladus laminosus) as anodic photoelectrode (both immobilized and non-immobilized cells deposited on the electrode), indicating increased photocurrent output as well as the current potential of the algal film deposited on SnO2-electrode upon illumination. Moreover, the anodic photocurrent production has been forwarded as an electron-donating system to hydrogenases in biohydrogen production systems through the photolysis of water, particularly in two-stage operation systems [172]. Building on this concept, Yagishita et al. [173] observed a significant increment of current output with the addition of illumination and CO2 in a PMFC using Synechococcus sp. cultures, reinforcing the potential of this approach.
The ability of a photosynthetic organism to evolve oxygen through an efficient water oxidizing system (oxygen-evolving complex), has led scientists to explore a means to utilize or manipulate natural photosynthetic apparatus for energy conversion. It has been explored to redirect electrons from the photosynthetic electron transport chain towards bioelectricity or biofuel production [174]. In this exploration, promising results have been reported for the development of PMFC as a whole cell of photosynthetic microorganisms [175,176] or discrete photosynthetic machineries such as thylakoid [174], PSI [177] and PSII [178] have been implemented as biocatalysts to generate photocurrent in the anode side of photoelectrochemical cells. PMFCs, uniquely reliant on water and light, offer an advantage over traditional MFCs, which require organic sources.
Regarding electron generation in PMFCs, microalgae can be involved in two different processes: 1) transferring electrons from the photosynthetic electron transport chain directly to the anode. In support of this, Pisciotta et al. [179] indicated the electrons in PMFCs could originate from the photosynthetic electron transport chain of PSII. 2) transferring electrons from the respiratory electron transfer chain by oxidizing the organic matter, which is fixed through photosynthesis. For example, the photosynthetic activity of cyanobacterium (M. laminosus) was not completely halted upon the addition of PSI inhibitor (dichlorophenydimethylurea), suggesting that, apart from water molecules, other substances such as NADH and reduced metabolites were serving as electron donors to PSI [172]. Additionally, Tanaka et al. [180] also reported that the cyanobacterium Anabaena variabilis generates electricity from endogenous glycogens during the dark period, while electrons are generated from both glycogen and photosynthetic oxidation of water in the light period for electricity generation. Nonetheless, the transfer of electrons from microalgae to the anode is often hindered, requiring redox mediators like 2-hydrozy-1,4-naphthoquinone for efficient bioelectrochemical processes [173].
Effective electron production and transport to anodes typically occur under anoxic conditions. Thus, maintaining an anoxic environment in the anolyte media through nitrogen gas bubbling is essential [173]. Factors such as oxygen exposure, pH, temperature, electrode spacing, and light intensity also significantly influence PMFC performance [[181], [182], [183], [184]]. In general, microalgae are not suitable electricigens as they also produce oxygen, which is a perfect electron scavenger. Therefore, a consortium of photosynthetic microalgae and electrogenic bacteria could be cultivated in the anode to make them establish syntropy. For instance, the generation of electricity by non-phototrophic electricigens bacterium G. sulfurreducens using organic substrate supplied by photosynthetic green microalga C. reinhardtii has been reported [184].
3.2.3. Electron acceptor in the cathode-biocathode
The integration of microalgae into the cathodic chamber of MFCs has garnered increasing interest due to its multifaceted benefits [185]. These include the production of oxygen, i.e. an efficient electron acceptor, along with valuable biomass accumulation and environmental remediation through wastewater treatment and CO2 scavenging [166] (Fig. 3c). There is a preference for utilization of oxygen from the photosynthetic process of microalgae over mechanical aeration due to the high energy consumption in mechanical aeration [186]. The co-culture of C. vulgaris in the cathode chamber increased the power density by 41.7 % (from 175 mW m−2 to 248 mW m−2) [187]. Moreover, the presence of microalgae correlates with enhanced current production, as the generation of oxygen facilitates electron reduction in the cathode, especially under increased light conditions [188,189].
Beyond oxygen provision, microalgae function as biocatalysts in the cathode, introducing more potent electron acceptors like reactive oxygen species (ROS)-hydrogen superoxide (H2O2) and peroxide anion radicals (O2ˉ), which can further enhance the electron reduction process [190]. The role of ROS in electron reduction, which boosts electricity generation, has been evidenced by the decline in electricity generation when ROS production is inhibited by mannitol, as shown by Cai et al. [190] using the biocathode of cyanobacterium Microcystis aeruginosa. Additionally, microalgae scavenge CO2 during photosynthesis. The accumulation of biomass which serves as bioenergy [191] or value-added compounds such as pigments [192] promoted due to photosynthetic CO2 fixation of microalgae in biocathode.
As microalgae are capable of assimilating nutrients, wastewater treatment is another advantage that could be attained using microalgae in a biocathode [154]. If wastewater is supplied to microalgae in the cathode, then wastewater treatment and biomass accumulation can be achieved simultaneously [104,188]. However, the growth of heterotrophic bacteria that degrade the organic matter can consume oxygen and interfere with the cathodic reaction by diminishing electron reduction, which ultimately reduces electricity generation [166]. An alternative means to overcome this problem is to feed the wastewater first to the anode as pretreatment and then transfer effluent to the cathode for algal growth [166].
3.3. Variations in microalgae-integrated microbial fuel cells
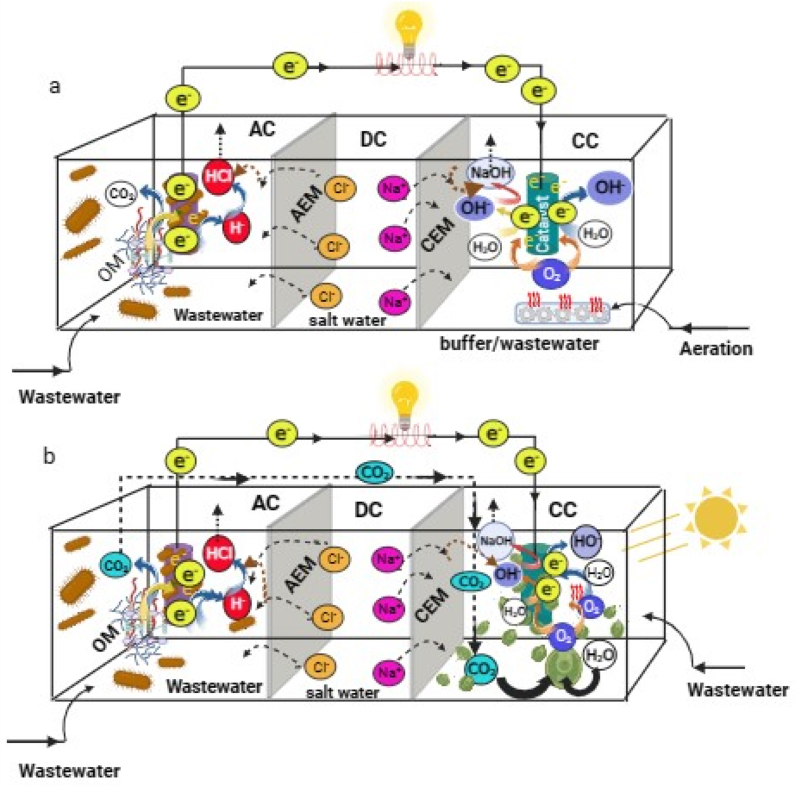
In recent decades, MFCs have evolved into various forms, including microbial carbon capture cells (MCCs), microbial desalination cells (MDCs) and sediment microbial fuel cells (SMFCs) (Table 2). These systems have shown promising results in bioenergy production, biofuel generation, wastewater treatment and environmental remediation. In the anode chamber of MFC, the exoelectrogenic bacteria transfer electrons to the anode by oxidizing organic matter, while in the cathode chamber, it can be oxygen reduction or hydrogen evolution. Microalgae are introduced in the cathode to supply oxygen for oxygenic reduction (Fig. 4a). These oxidation-reduction reactions create an electric field across the electrodes, facilitating ionic transport. By incorporating ion-exchange membranes (IEM) between the electrodes, this ionic movement can be harnessed in MDCs to segregate salt ions in the reactor chamber. Consequently, in MDCs, the cathode and anode chambers receive cations and anions, respectively from wastewater, leading to partial or complete desalination of the saltwater in the middle chamber [194].
Table 2.
Comparative evaluation of various microalgae integrated with microbial fuel cell technologies.
| Features | Microbial Carbon Capture Cells (MCCs) | Sediment Microbial Fuel Cells (SMFCs) | Microbial Desalination Cell (MDC) |
|---|---|---|---|
| Principle | CO2 is produced at the anode used by microalgae at the cathode. | The anode is buried in sediment, cathode is in the overlying water. | Electrical potential from microbes degrades organics and drives ion migration. |
| CO2 utilization | Efficient sequestration through microalgal photosynthesis. | Synergistic bioelectrochemical reactions under light exposure. | CO2 can be scavenged by biocathodes. |
| Integration with photosynthesis | Enabled CO2 consumption coincides with increased biomass. | Supported by photosynthetic microorganisms at the cathode. | Photosynthetic MDCs (PMDCs) use microalgae to generate oxygen and remove pollutants. |
| Electricity production | Enhanced algal biomass leads to increased power density. | Typically, low due to environmental conditions, but can be enhanced. | Bioelectricity generation is a primary function. |
| Biomass accumulation | Reported increased power density due to microalgae activity. | Stable power densities and nutrient removal under illumination. | Possible when using microalgae in the cathode or anode. |
| Wastewater treatment | Can be integrated for treatment and value-added compound production. | Potential in sediment bioremediation and biomass production. | Simultaneous wastewater treatment with desalination. |
| Challenges |
|
|
|
| References | [173,[195], [196], [197], [198], [199], [200]] | [168,[201], [202], [203], [204], [205], [206], [207], [208], [209], [210], [211]] | [[212], [213], [214], [215], [216], [217], [218], [219], [220]] |
Fig. 4.
Illustration of microalgae-MFC where microalgae inoculated as biocathode (a) and MCC where microalgae inoculated as biocathode and CO2 from the anodic chamber (AC) is channeled to cathodic chamber (CC) (b). Where OM is organic matter and BM is biomass accumulated. The sketch was drawn based on the information provided by Das et al. [196].
3.3.1. Microbial carbon capture cells
The MCCs represent an innovative modification of MFCs, where CO2 generated at the anode through organic matter degradation will be utilized by microalgae in the cathode. This process enables effective CO2 sequestration through photosynthesis, as shown in Fig. 4b. MCCs are integrated to attain wastewater treatment and value-added compounds [196,197]. Wang et al. [195] demonstrated that MCCs effectively reduce inorganic carbon concentration in the cathode (compared to the control) and contribute to enhanced algal biomass and power density. This capability was further evidenced by Pandit et al. [198], who reported increased power density when Anabaena sp. in the cathode was sparged with a CO2-air mixture.
The performance of MCCs varies with the type of microalgae used in the cathode. Jadhav et al. [197], found superior performances of MCCs in power production, Coulombic efficiency, COD removal, and biomass accumulation by Anabaena ambigua compared to Chlorella pyrenoidosa. This was attributed to C. pyrenoidosa's higher oxygen production and lower cathodic charge transfer resistance. Besides, the performance of MCCs is also affected by the concentration of CO2 received from the anode chamber, which again depends on the substrate given for the bacteria to degrade. Cui et al. [167] investigated the performance of MCC by providing either lyzed powder of microalga (Scenedesmus) or acetate as an electron source (substrate) at the anode, transferring the released CO2 by silicone tube, and inoculating C. vulgaris in the cathode. They found that, compared to MCC provided with acetate, the MCC provided with algal powder as the substrate showed higher CO2 to the cathode, which coincided with higher biomass and power output, however, the power generation duration was shorter may be related to incomplete degradation of the provided algal biomass-substrate. They also reported that the concentration of substrate is another factor that affects the performance of MCCs, whereby the performance of MCCs increased with the increase of COD until some maximum limit, beyond which the growth of microbes declined.
As CO2 utilization by microalgae is affected by the growth condition of the microalgae, it has been manipulated to enhance and optimize the performance of microalgae in MCCs. For example, Zhou et al. [199] showed the immobilized C. vulgaris cells in the cathode, had 84 % COD removal, 88 % increased maximum power density and 57.7 % increased Coulombic efficiency compared to suspended cells. This approach highlighted the feasibility of employing immobilized microalgae as electron acceptors in MCCs for steady voltage output.
Despite the promising aspects of MCC technology in CO2 sequestration, biomass accumulation, electricity production, and wastewater treatment, some challenges must be resolved to facilitate the commercialization of this technology. These include managing dissolved oxygen (DO) levels at the cathode, standardizing substrate and CO2 inputs, as well as enhancing overall MCC performance. Chiu et al. [200] and Yagishita et al. [173] noted that CO2 addition increases current output, but high CO2 concentrations can restrain microalgae growth. To address this, Chou et al. [221] developed mutant strains of C. vulgaris with enhanced tolerance to high CO2 and temperature, showing potential for improved MCC performance. The application of genetically engineered strains could thus play a key role in maximizing MCC efficiency and CO2 sequestration capabilities.
3.3.2. Sediment microbial fuel cells (SMFCs)
The SMFCs or benthic MFCs, represent an adaptation of conventional MFCs designed for natural environments with minimal engineering intervention (Fig. 5a). In SMFCs, an anode buried in sediment undergoes microbial oxidation, while a cathode placed in overlying water facilitates the electron-accepting process (Fig. 5b). In comparison to conventional MFCs, this configuration allows seamless electron transfer without a clear separator, which also exploits the natural stratification with low DO concentration in the sediments [201]. Although SMFCs typically exhibit low power output due to factors like limited electron donors or acceptors and environmental conditions (such as pH, temperature and large internal resistance), the easily-built structure and cost-effective construction of the SMFC makes it viable for remote area applications [[202], [203], [204]]. The power output in SMFCs can be enhanced through approaches such as increasing oxygen availability at the cathode [222], using macrophytes or rotating cathodes [223,224] and supplementing organic matter at the anode.
Fig. 5.
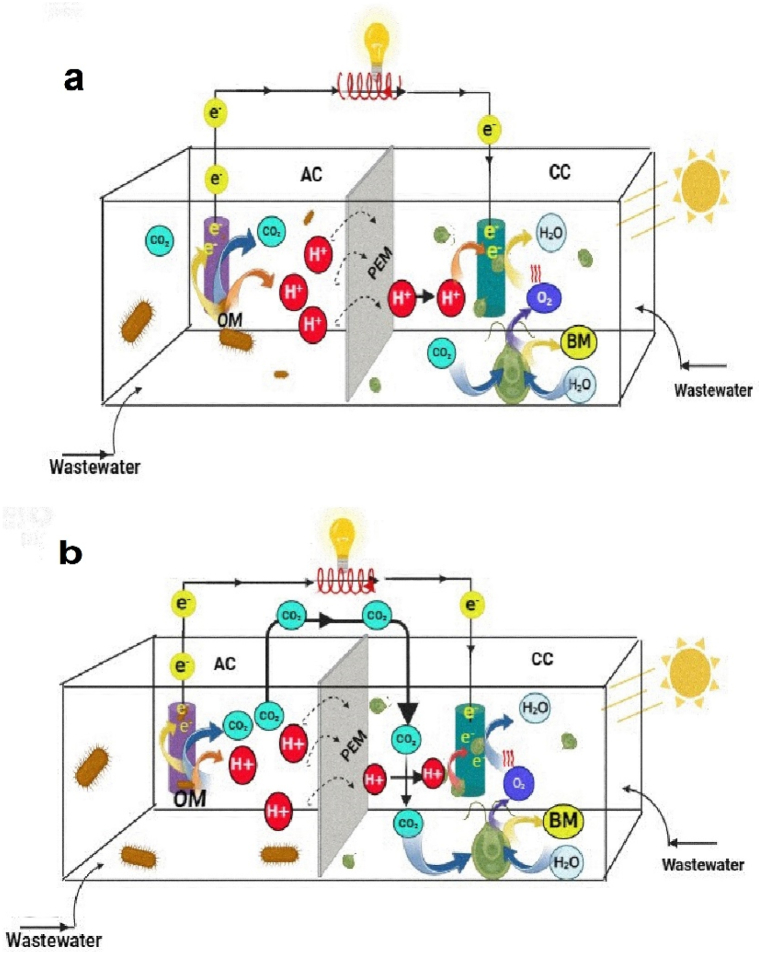
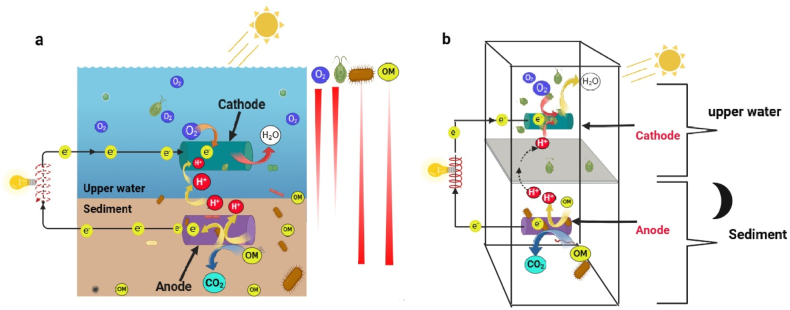
An illustration of SMFC (a) and PSMFC inoculated with microalgae (b), installed with an anode buried in sediment and a cathode hung in the surface water. Where OM is organic matter. The sketch was drawn based on the information provided by Yang and Chen [230].
The role of microalgae and heterotrophic bacteria in SMFCs has been extensively studied. He et al. [205] developed SMFC by inoculating lake sediment and water in a glass beaker and found that current production increased under light exposure, indicating the presence of a synergistic bioelectrochemical reaction between photosynthetic microorganisms and bacteria. Similarly, Malik et al. [206] constructed SMFC using marine sediment and water, whereby current production increased upon illumination. A recent investigation by Bardarov et al. [207] further confirmed the crucial role of cathodic biofilms, particularly photosynthetic microorganisms, in enhancing SMFC performance, with the observation of concurrent increment of pigmentation and current production during illumination.
Monocultures of microalgae, such as C. vulgaris have been utilized as biocathodes in SMFCs. Zhang et al. [208] reported stable power densities of 68±5 m W/m2 and improved biomass accumulation and nutrient removal under illuminated conditions. Additionally, Wang et al. [225] revealed that using microalgae as a biocathode resulted in increased electricity generation, corresponding to higher DO concentrations and electron reduction. Bardarov et al. [207] also noted that light irradiance (natural and artificial light) significantly influences biocathode biofilm performance, mainly correlated with photoperiod, duration and light quality. A recent study by Song et al. [209] demonstrated that cathode inoculated with C. vulgaris in SMFC enhanced COD removal efficiency and organic matter biodegradation, leading to a six-fold increase in maximum power density. It was explained that the conversion of chemical energy to electrical energy was enhanced due to lower internal resistance.
Furthermore, microalgae biomass has been employed as a substrate in the anode of SMFCs. For example, Zhao et al. [168] found that pretreated cyanobacterial biomass yielded better electricity generation and COD removal compared to other substrates. Other factors that influence the electricity generation of SMFC include electrode material [210], sediment pretreatment [226], number of anodes [227], distance of electrodes [228] and light [207]. Although SMFCs demonstrated potential input in sediment bioremediation [229] and algal biomass production [211], challenges such as the impact of photosynthetic microorganisms that reverse current production under illumination need to be addressed [205].
3.3.3. Microbial desalination cell
The MFC technology has evolved progressively over the past few decades, leading to the development of MDC (Fig. 6a) for simultaneous wastewater remediation, bioelectricity generation and saltwater desalination. MDCs incorporate a middle chamber that is partitioned by a cation exchange membrane (CEM) and an anion exchange membrane (AEM) for ion migration and water desalination (Fig. 6). Anion and cation migrate from the desalinating chamber to the anodic and cathodic chambers, respectively, as a result of the potential difference occurring between the electrodes [212]. Exoelectrogenic microorganisms in MDC degrade organic matter, and generate an electrical potential that drives ion transport through an ion-exchange membrane (IEM) for desalination [194,214]. MDC attracted great attention as it is capable of desalinating saline water, treating wastewater and generating electricity in a single device. In addition, MDC provides an energy-efficient and eco-friendly alternative to traditional desalination methods [214]. As the anaerobic condition creates a conducive environment in the anodic chamber to degrade various organic pollutants, the supply of clean and efficient electron donors at the anode and electron acceptor in cathodic chambers has been a focal point in the bioelectrochemical systems.
Fig. 6.
An illustration of MDC (a) and PMDC (b, inoculated with microalgae) installed into three chamber configurations mainly anodic chamber (AC), desalination chamber (DC) which is separated by cation exchange membrane (CEM) and anion exchange membrane (AEM), and cathodic chamber (CC). Showing the possibilities to produce HCl and NaOH, and connecting CO2 feeding the cathode (b). Where OM is organic matter. The sketch was drawn based on the information provided by Kim and Logan [213].
The technology of MDC has gone through several progressive steps. The first three-chambered MDCs used acetate as an electron donor and ferricyanide as an electron acceptor [212]. However, due to the environmental and economic concerns associated with ferricyanide, later designs employed air cathodes [215]. However, as the oxygen-reduction reaction was low/limited in the air cathode, an external aeration system or external aeration together with expensive catalysts such as platinum was introduced. Still, the performance of air cathode was limited and needed extra operational cost, hence, the technology was further advanced by using oxygen-producing microorganisms as biocathodes. This led to using oxygen-producing microorganisms as biocathodes to enhance performance and reduce costs [216,231]. The biocathode outperformed traditional air and ferricyanide cathodes by increasing oxygen concentration, thereby improving electron transfer efficiency [218]. Microalgae can either be passive oxygen providers or biocatalysts by producing enzymes like laccases in the biocathodic chamber [191]. The photosynthetic microbial desalination cells (PMDCs) further advanced this concept by using microalgae (as biofilm or suspension) as biocathodes (Fig. 6b) to produce oxygen, scavenge CO2, remove pollutants and generate biomass for biofuel [232].
There are several factors influencing the performance of PMDCs, such as biofilm formation, external resistance, internal resistance, pH, mode of operation and the type of microorganisms used in the MDC [214]. The efficiency of biofilms on electrodes influences the voltage of PDMC, which is directly correlated with the capacity of electricity generation. For example, the maximum voltage and duration of production of electricity increased over time, coinciding with the biofilm formation on the electrodes, which led to improved electron transport [217]. The performance of PMDC is significantly affected by the photosynthetic activity of microalgae and the bioelectrochemical activity of biofilm on the cathode, which can further be influenced by other factors such as pH, which could rise during inorganic carbon assimilations from the media [217].
The performance of MDCs can also be affected by the lifestyle of microorganisms in the device. For example, Zhang et al. [219] investigated the performance of MDC by operating for a long time (5500 h) and reported that the salt removal rate, power density and Coulombic efficiency decreased by 27 %, 71 % and 44 %, respectively. They explained that biofouling on the membrane was the primary reason by increasing internal resistance and reducing ionic transfer and energy conversion efficiency. They found changing the membrane was an efficient technique to recover the MDC performance [219]. Moreover, regular renewal of algae medium was also suggested due to the depletion of nutrients, which leads to lower MDC performance [233].
As the cations and anions diffuse from the desalination chamber to the cathode and anode chambers, the electrolyte conductivity increases and resulting in enhanced overall system performance. However, through time ions such as Cl− accumulate in the anode chamber and alter the activity of exoelectrogenic microorganisms [214]. For example, the performance of microbial communities was stable up to 41 g/L TSD but when the solute increased to 46 g/L TSD the exoelectrogenic activities were permanently lost [194]. Although the tolerance of microorganisms improved with a prolonged period of acclimation [214], exploring the possibility of hypersaline species or genetically modified salt-tolerant species could yield better results.
Treatment of low-strength wastewater by MDCs can give better output with enhanced electricity production as a result of improved conductivity in the anode chamber, which could occur due to the ions transported from the desalination chamber. The effect of bicarbonate and TDS on the PMDC has also been reported [234]. The effect of light and COD on the performance of PMDC was studied [233]. The result indicated that the supply of high concentrations of COD did not lead to an increase in the performance of MDC, while the light/dark frequency affected the performance, with the best result found in the natural light/dark cycle rather than continuing light condition.
The performance of the PMDC also varies depending on its configuration [232]. In this context, PMDCs can be configured into different modes; continuous-flow-PMDC (where the microalgae catholyte circulates with the help of a peristaltic pump) known for generating higher (compared to static PMDC) electricity, or static-PMDC (the conventional) known by generating higher (compared to continuous flow PMDC) biomass accumulation. This indicates that the selection of PMDC configuration could depend on whether the desired target is more biomass or more electricity production [217]. MDC can be applied to seawater desalination, brackish water desalination, water softening, hydrogen and chemical production and groundwater remediation [214]. Additionally, desalination plants can process various sources of water with high salt concentrations and simultaneously polish wastewater and accumulate biomass [220]. As the volume of wastewater required to desalinate saltwater is much higher than the water to be desalinated as well as the required volume of wastewater is also affected by the initial salt concentration, in a practical sense, MDCs have been recommended as a suitable technology for partial desalination of seawater [191].
As high voltage production in PMDC is associated with high electron release by exoelectrogenic microbes in anodic biofilm and high electron acceptors in the cathode, the exploration of microorganisms that are efficient electron donors or acceptors would be of great scientific importance. This far, most of the PMDC research has focused on using a few microalgae species (mostly C. vulgaris); however, given the diversity of microalgae in physiology, growth rate, tolerance, and adaptability, more research could be performed in the future to exploit the potential amenability of other microalgae in this technology.
3.4. Factors influencing the performance of microalgae-integrated-MFC
The key factors influencing mMFC performance include the distance between the anode and cathode [183], electrode material [14], the number of anodes [235], and the proton exchange membrane material [236]. These factors impact internal resistance, electron transfer efficiency, proton transfer rates and the passage of substrates and oxygen. Moreover, mMFC configurations, such as single-chamber and double-chamber setups, also affect performance [237]. In single-chamber systems, electricity is generated in one chamber, with variations like wet-cathodic or air-cathodic setups; while double-chamber systems involve separate cultivation of algal cells and fermentative microorganisms segregated by a proton exchange membrane [188].
The performance of mMFCs is also influenced by various environmental factors [238]. Specifically, light intensity [181,239,240] and duration of light/dark conditions [188,189,240] significantly influence photosynthesis and oxygen production, thereby affecting the electron flow and power output in mMFCs. For example, a three-fold increment of mMFC performance upon an increment of illumination in the Desmodesmus sp. culture in cathode has been reported [239]. A recent investigation on wastewater treatment using mixed algae cultures in mMFCs demonstrated that a sustainable voltage of 0.31V was achieved at 18/6 h of light/dark cycle [241]. Additionally, temperature is another critical factor that affects microbial growth and physiology, electrode potential, power density, Coulombic efficiency, and COD removal efficiency [14].
The pH affects the performance of mMFC [237] by influencing both optimal microbial growth and metabolic activity on the substrate [242]. In the context of this, Cheng et al. [152] tested the influence of conditions such as pH, solution chemistry, and iron concentration on mMFC performance and they found that pH was the most prominent environmental factor that affected the performance. Generally, the microbes in both anode and cathode best perform at pH of 6–8 [243]. However, the proton produced during organic matter degradation grades acidification of the anode side, which in turn, causes a loss of pH gradient and consequent reduction of current density and electrode potential [244]. Consequently, any process that causes an imbalance in proton, electron and oxygen levels marks a change in pH gradient and suppresses electricity production [14]. The application of some buffers, such as calcium carbonate, has been recommended to overcome the accumulation of pH [245,246].
The COD concentration also affects the mMFC performance [247]. For example, Yahampath Arachchige Don and Babel [248] studied the effect of trophic conditions on mMFC performance by inoculating C. vulgaris in autotropic (no organic carbon, as a control) or mixotrophic (sodium acetate as organic carbon) conditions at different COD concentrations (100, 300 and 500 mg/L) in cathodic side chamber, whereas bacterial sludge inoculant and constant concentration of COD (100 or 1500 mg/L) set in anode chamber. The result indicated that, the mixotrophic condition with 100 mg/L of COD had highest power density; conversely, the increasing of COD above 100 mg/L resulted increased algal biomass in cathode chamber but decreased power generation, likely due to promoted heterotrophic metabolism of the microalga.
Dissolved oxygen is another factor that impacts the power out of mMFC [228]. The higher dissolved oxygen in the anode can lower the performance of MFC. This problem can be alleviated by adding concentrated salt or nitrogen gas [248], however, the effect of these techniques on the growth and physiology of microalgae should be taken into consideration. For example, Venkata Subhash et al. [151] found that the dissolved oxygen generated during photosynthesis is a major limiting factor influencing the electrogenic activity of mixed microalgae inoculated with the anodic side of PMFC system. On the contrary, in the anode compartment, the high production of oxygen in the cathode compartment promotes the reduction reaction rate and consequent increase of power output. This compartment could have limitations as the oxygen production dropped at dark hours [14]. Corroborate, during the culturing of C. vulgaris in the cathode of mMFC, there was a cooccurrence of a high concentration of dissolved oxygen (evolved from photosynthesis) coinciding with high cell voltage in the light period, and low concentration of dissolved oxygen (due to consumption by the alga) coinciding with low voltage in the night [249].
As the algal density is directly related to the production of oxygen, it critically affects the mMFC performance both in the anode and cathode compartments. Concurrently, decreased electricity generation is reported by inoculating high algal biomass in the anode side of PMFC [250] and high power generation is obtained by high algal biomass in the cathode compartment [14,217,251]. In addition to this, the microalgae-bacterial species composition [252], pure culture or consortia [253], or the source of algal inoculant [254] considerably affects the performance of mMFC. The microbial assemblage in the sludge inoculant of the anode is another factor that could influence the mMFC performance [255].
The performance of mMFCs is significantly influenced by a multitude of factors, ranging from electrode configurations and microbial compositions to environmental conditions, highlighting the complex interaction between system design and operational environments essential for maximizing the potential in environmental remediation, wastewater treatment and sustainable energy production.
4. Techno-economic evaluation, environmental impacts and future perspective
4.1. Techno-economic evaluation
Although the application of microalgae-bacteria consortia and mMFCs technologies have gained great interest and consideration from researchers due to its several advantages in environmental remediation, clean energy, wastewater treatment, sediment treatment, desalination, biomass accumulation, and electricity generation, it also has some limitations. For example, while the microorganisms participating in microalgae-bacteria consortia should exchange materials with the environment and each other swiftly to maintain the system, several microorganisms are unculturable and pose technical challenges to understand their metabolic requirement and optimize the efficiency of the system. Moreover, light attenuation due to high algal biomass and rise in pH due to the elimination of CO2 by microalga also impair the normal growth and physiology of microorganisms in the chamber, which in turn diminishes the output efficiency of the system.
In terms of techno-economic evaluation, the current approach of converting microalgae biomass into biofuel is economically not feasible requiring more research in the future to make it suitable for commercialization [256]. Microalgae cultivation in the microalgae-bacteria consortium could significantly reduce this problem, and enhance the yield of biomass and biohydrogen [56]. While using microalgae as a bioethanol source offers carbo-neutral fuel alternatives, the requirement of pre-treatment of its biomass before the fermentation process has been one of the application challenges [75]. By overcoming this hurdle, a feasible bioethanol production combined with a high-performance wastewater treatment system at a pilot scale was reported using algal-bacterial aggregates [76].
Moreover, integrated systems have been proposed to increase the economic feasibility of microalga-bacteria consortia. For example, a naturally occurring microalgae-bacteria consortium employed to process urban wastewater operated in an 80 m2 raceway reactor, which resulted in the efficient removal of nutrients and incorporation into microbial biomass. Then, the treated effluent is used for watering edible crops, and the accumulated biomass is used as feedstock to stimulate plant growth [257]. As a requirement of high hydraulic retention time is one of the common limitations incurred in the application of microalgae-bacteria consortia, Arango et al. [258] studied the performance of microalgae-bacteria consortium operated in 50L raceway reactors to treat real municipal sewage with or without external microfiltration membrane at 4h and 7h retention times. The result showed that the deployment of an external microfiltration membrane significantly enhanced pollutant removal efficiency and produced effluent free of coliform and solids, suggesting its feasibility for application purposes.
The microalgae-bacteria consortia can gain other advantages, as the bacteria in consortia could play a crucial role in microalgae biomass processing, particularly in the harvesting and pretreatment stages. Harvesting is an important step that separates biomass from the culture media and can account for about 20–30 % of total production costs [259]. To alleviate this and enhance the flocculation of algal biomass, some bacterial strains can be applied. For example, Kim et al. [260] found that treating Scenedesmus sp. with the flocculant bacterium Paenibacillus polymyxa resulted in high (95 %) flocculating activity. In addition to this, processing microalgal biomass is challenged by its cell walls’ calcitrant properties. Several studies showed biological pretreatment of microalgal biomass yields enhanced outcomes compared to untreated biomass [261]. Treating C. vulgaris with Bacillus thuringiensis for lipid extraction increased biodiesel production by 44.3 % [262]. These suggest that the optimized utilization of microalgae and bacteria species not only enhances biomass accumulation and bioproducts but can facilitate downstream processes.
In mMFCs, the increase in internal resistance and high cost due to ion-exchange membranes bring additional limitations to practical applications [253]. Moreover, as power generation from an individual mMFC is not suitable in many practical applications [253], individual mMFCs are deployed into stacks, large-scale, and vertical cascade configurations [263,264]. For instance, microalgal productivity of 0.09 kg m−3d−1 and energy recovery of 11.53 kWm−3 with an operational cost of $11.225, reported for mMFC operated outdoor with 10L polyethylene bags and C. vulgaris at the cathode [265]. Moreover, a pilot system with 1500L capacity was operated outdoors in a municipal wastewater treatment plant and provided with 91 % COD removal, 64 % nitrogen removal and a power output of 406 Wm-3 [266]. The authors claimed that the low operational cost ($1135 m−3) and promoted real-world application pilot scale were attained by avoiding redundant structural materials and ion exchange membranes.
An mMFC with 1000L capacity operated in a municipal wastewater treatment plant also provided high (70–90 %) COD removal efficiency [267]. Moreover, a 90L capacity stackable pilot mMFC was operated on brewery wastewater, and the result showed the system ran in an energy-self-sufficient manner, indicating the potential of mMFC for the effective treatment of real wastewater with zero energy input [264]. Moreover, compared to the conventional wastewater treatment approach, mMFCs offer better technological and economic feasibility as they minimize the disposal of waste-activated sludge produced after wastewater treatment, which in turn, significantly minimizes the requirement of landfill sites and the potential risks from environmental contaminants.
4.2. Environment impacts
As the cultivation of microalga-bacteria consortia provides enhanced outputs, the application of this technology on a mass scale is imminent, potentially creating a negative impact on the environment [268]. Whether the consortium is composed of genetically engineered or wild-type species, the risk of environmental contamination from the consortium pertains as long as mass-scale cultivation is undertaken. As the spill from the system finds its way into the natural environment, it might cause massive ecological effects by introducing non-native species or genetically engineered species with modified abilities of competition and survival strategies [269]. This might have unpredictable effects on the ecosystem and environmental health.
Genetic engineering techniques have been applied to microbes to increase their applicability in biotechnology. For instance, increased Cd accumulation and toxicity tolerance by genetically engineered C. reinhardtii have been reported [270]. Compared to the normal strain, a 4-fold increased power density was obtained in mMFC inoculated with genetically engineered Pseudomonas aeruginosa [271]. As genetic engineering techniques include the alteration of key genes involved in the cell's metabolism and growth performance, the impact of these genetically modified organisms on the natural environment should be addressed carefully. In engineered microalgae-bacteria consortia, bacterial genes have been incorporated into the microalgal genome to confer its stress adaptive advantages. Although it is considered advantageous to increase gene transfer between microalgae and bacteria in a consortium [272], the strains that receive new genes might have an unprecedented impact on the natural environment [268].
Evidence shows that several genes that have adaptive and survival advantages for the microbe have been transferred horizontally. For example, ice-binding proteins (IBPs) found in microalgae are largely independent of the phylogenetic distribution of microalgae, implying their acquisition through horizontal transfer from bacteria [273]. Additionally, the acquisition of genes related to metal detoxification, ferritin uptake and ornithine-urea cycle metabolism by microalgae living in iron-limited, nitrogen-limited and toxic environments has been suggested as horizontal gene transfer in microalga-bacteria ecosystems [274]. Evolved adaptative ability to environmental stress through the horizontal acquisition of bacterial genes by the green algae Zygnematophyceae has also been reported [275]. Although there is no concrete evidence to show horizontal gene transfer in short-term coexistence, it has been suggested that prolonged co-cultivation can produce gene transfer between microalgae and bacteria [8]. This indicates that prolonged cultivation of microbes in a synthetic microalgae-bacteria consortia might generate strains that acquire traits that are distinct from the natural environment, which in turn, suggests future research in this regard can be an interesting input for comprehensive understanding, full-scale optimization and alleviation of potential risks related to applications of microalgae-bacteria consortia.
Additionally, while the application of antibiotics and disinfectants is steadily increasing, their ever-increasing accumulation in wastewater is becoming worrying. To address this issue, microalgae-bacteria consortia have been applied as a means of environmental remediation [276]. Microalgae-bacteria consortia applied to treat wastewater that contains a considerable amount of antibiotics can develop either a short-term or long-term stress response upon their exposure to antibiotics. The short-term response promotes protoplasmic responses, whereas the long-term exposure could lead to changes in microbial community structures and the retention of bacteria with dominant antibiotic resistance and an increased abundance of antibiotic resistance genes. This was supported by the report of Li et al. [277], as the horizontal gene transfer rate was increased in the activated sludge bacterial community under the bacteriostatic drug triclosan and the antibiotic trimethoprim provided at concentrations commonly found in wastewater.
Studies showed that wastewater treatment plants can be a source of antibiotic-resistant genes in the environment, causing negative consequences for non-target organisms [276]. Moreover, it has been suggested that antibiotics can alter the composition of microbial communities and activities by promoting the development of antibiotic-resistant genes, and the spread of antibiotic-resistant bacteria [278]. The spill or leak from the consortium may harm the environment when some of these microorganisms join the natural aquatic systems [276], indicating that a necessary preparation and environmental impact analysis data should be developed.
4.3. Future perspectives
As microalgae-bacteria consortia and mMFC are relatively newly established technologies, there have been limited studies focused on large-scale applications till now. However, several reports are indicating its impedance and lucrativeness when discussing its economic feasibility for practical application. Despite extensive research on the benefits of microalga-bacteria consortia in wastewater treatment, Petrini et al. [279] found that microalgae's presence in such consortia did not significantly alter the performance. The study reported a minimal impact from the inclusion of microalgae in a 10-day study using two photobioreactors. Additionally, the study observed initial improvements in phosphorus removal with the inclusion of C. vulgaris in photobioreactors, but over time, there was no significant difference in COD and ammonium removal between reactors with and without the microalgae. Therefore, the study deduced that microalgae inoculation is not essential for treating municipal wastewater. In contrast to previous findings, studies by Raza et al. [280] and Verma et al. [281] demonstrated that microalgae in consortia enhanced nutrient removal in real textile wastewater and sewage-contaminated lake water, respectively. Moreover, the development of a microalgae-bacteria consortium and the efficiency of pollutant removal from urban wastewater in a pilot-scale high-rate pond were studied by Robles et al. [282]. The result showed microalga-bacteria consortium stabilized and fully functional through time, able to discharge environmentally acceptable effluent after one month. These results highlight the need for further research to fully understand the role of microalgae-bacteria consortia in real-world wastewater treatment scenarios.
Recent studies indicated that the application of the extract of nitrogen-fixing cyanobacteria and green algae consortium resulted in high production of phytohormones, exopolymers, macronutrients and micronutrients [283]. In addition to that, the consortium showed biostimulant properties that promoted seed germination of Capsicum annuum, as there was a significant increase in leaf number and seedling length. This suggests future studies on developing consortia consisting of nitrogen-fixing bacteria and microalgae will contribute to the advancement of biostimulant and biofertilizer technology in agricultural applications.
The mMFCs represent a rapidly evolving field with significant potential for breakthroughs. Recent developments in mMFCs have included diverse electrode configurations, varied substrate supplies, and complex microbial compositions. Notably, the application of immobilized microalgae cells has emerged as a promising strategy for enhancing MFC performance [199]. Future research is expected to concentrate on fast-growing microalgae species like Chlorella sorokiniana, whose incorporation into the cathodic side of mMFCs could substantially improve efficiency in bioelectricity and biofuel production. Key areas of focus, including the growth and use of algal biomass for bioelectricity and biofuel [238], and the resolution of challenges specific to mMFCs [284] have been reviewed previously.
Applications of mMFC technology in acid drainage systems [12], wastewater treatment and energy recovery [285], and biofuel, wastewater, biohydrogen, bioethanol, biodiesel and biogas [238] have also demonstrated its vast applicability. Furthermore, there is an increasing emphasis on scalability, cost-effectiveness, and the incorporation of genetic engineering and omics in mMFC research, according to Sharma et al. [253]. The potential of mMFCs in areas such as biofuel, wastewater treatment and various bioenergy forms [238] underlines its wide-ranging utility. Moreover, research focusing on the scale-up of MFCs and their operational conditions, including temperature, pH, organic loading rate, feed rate and shear stress has provided valuable guidance for optimizing mMFC performance [246]. The future trajectory of mMFCs is promising, offering sustainable wastewater treatment and bioenergy recovery through the synergistic integration of microalgae and microbial technologies.
5. Conclusion
This review offered a detailed examination of the interaction between microalgae and bacteria, highlighting their potential roles in addressing crucial environmental challenges and advancing biotechnological solutions for sustainable energy, water purification and environmental remediation. Although microalgae-bacteria consortia offer alternative technologies that are environmentally safe and enhance output, their practical implementation is challenged by several problems that require further research. This includes the selection of suitable microbial partners, scaling up for industrial applications with manageable costs, integrating advanced technologies to improve system efficiency and longevity, and a detailed investigation of the impacts of the participant microorganisms on the natural environment. Addressing these challenges will pave the way for these consortia to play a pivotal role in achieving a sustainable and environmentally friendly future.
Funding statement
This work was funded by the National Natural Science Foundation of China (No: 31971477) and the Hubei Provisional Science Fund (SFC2109150002).
Data availability
Has data associated with your study been deposited into a publicly available repository? NO.
Has data associated with your study been deposited into a publicly available repository? No data was used for the research described in the article.
Data will be made available on request.
CRediT authorship contribution statement
Rediat Abate: Writing – review & editing, Writing – original draft, Conceptualization. Yoong-Sin Oon: Writing – review & editing, Writing – original draft. Yoong-Ling Oon: Writing – review & editing, Writing – original draft. Yonghong Bi: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No: 31971477) and the Hubei Provisional Science Fund (SFC2109150002). We have used the free service of Biorender to draw illustrations.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31170.
Contributor Information
Rediat Abate, Email: rediat.abate@yahoo.com.
Yoong-Sin Oon, Email: oonyoongsin@ihb.ac.cn.
Yonghong Bi, Email: biyh@ihb.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sharma A., Arya S.K. Hydrogen from algal biomass: a review of production process. Biotechnol Rep (Amst) 2017;15:63–69. doi: 10.1016/j.btre.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabaey K., Verstraete W. Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol. 2005;23(6):291–298. doi: 10.1016/j.tibtech.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Gude V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Biotechnol. 2017;16(4):591–609. doi: 10.1007/s11157-017-9449-7. [DOI] [Google Scholar]
- 4.González-González L.M., de-Bashan L.E. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology. 2021;10 doi: 10.3390/biology10040282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuentes J.L., Garbayo I., Cuaresma M., Montero Z., González-del-Valle M., Vílchez C. Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs. 2016;14 doi: 10.3390/md14050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oruganti R.K., Katam K., Show P.L., Gadhamshetty V., Upadhyayula V.K.K., Bhattacharyya D. A comprehensive review on the use of algal-bacterial systems for wastewater treatment with emphasis on nutrient and micropollutant removal. Bioengineered. 2022;13:10412–10453. doi: 10.1080/21655979.2022.2056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saravanan A., Kumar P.S., Varjani S.J., Jeevanantham S., Yaashikaa P.R., Thamarai P., Abirami B.M., George C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2021.129540. [DOI] [PubMed] [Google Scholar]
- 8.Dai C., Wang F. Potential applications of microalgae–bacteria consortia in wastewater treatment and biorefinery. Bioresour. Technol. 2024;393 doi: 10.1016/j.biortech.2023.130019. [DOI] [PubMed] [Google Scholar]
- 9.Abdelfattah A., Ali S.S., Ramadan H., El-Aswar E.I., Eltawab R., Ho S.H., Elsamahy T., Li S., El-Sheekh M.M., Schagerl M., Kornaros M., Sun J. Microalgae-based wastewater treatment: mechanisms, challenges, recent advances, and future prospects. Environmental science and ecotechnology. 2023;13 doi: 10.1016/j.ese.2022.100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sial A., Zhang B., Zhang A., Liu K., Imtiaz S.A., Yashir N. Microalgal–bacterial synergistic interactions and their potential influence in wastewater treatment: a review. Bioenergy Research. 2020:1–16. [Google Scholar]
- 11.Muñoz R., Guieysse B. Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res. 2006;40(15):2799–2815. doi: 10.1016/j.watres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Abinandan S., Subashchandrabose S.R., Venkateswarlu K., Megharaj M. Microalgae–bacteria biofilms: a sustainable synergistic approach in remediation of acid mine drainage. Appl. Microbiol. Biotechnol. 2017;102:1131–1144. doi: 10.1007/s00253-017-8693-7. [DOI] [PubMed] [Google Scholar]
- 13.Kong W., Kong J., Feng S., Yang T., Xu L., Shen B., Bi Y., Lyu H. Cultivation of microalgae–bacteria consortium by waste gas–waste water to achieve CO2 fixation, wastewater purification and bioproducts production. Biotechnology for Biofuels and Bioproducts. 2024;17(1):26. doi: 10.1186/s13068-023-02409-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elshobary M.E., Zabed H.M., Yun J., Zhang G., Qi X. Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrogen Energy. 2021;46(4):3135–3159. doi: 10.1016/j.ijhydene.2020.06.251. [DOI] [Google Scholar]
- 15.Fang X., Wei C., Zhao-Ling C., Fan O. Effects of organic carbon sources on cell growth and eicosapentaenoic acid content of Nannochloropsis sp. J. Appl. Phycol. 2004;16(6):499–503. doi: 10.1007/s10811-004-5520-1. [DOI] [Google Scholar]
- 16.Nitsos C.K., Filali R., Taidi B., Lemaire J. Current and novel approaches to downstream processing of microalgae: a review. Biotechnol. Adv. 2020 doi: 10.1016/j.biotechadv.2020.107650. [DOI] [PubMed] [Google Scholar]
- 17.Sankaran R., Parra Cruz R.A., Pakalapati H., Show P.L., Ling T.C., Chen W.-h., Tao Y. Recent advances in the pretreatment of microalgal and lignocellulosic biomass: a comprehensive review. Bioresour. Technol. 2019 doi: 10.1016/j.biortech.2019.122476. [DOI] [PubMed] [Google Scholar]
- 18.Bunbury F., Deery E., Sayer A., Bhardwaj V., Harrison E., Warren M.J., Smith A.G. Exploring the onset of B12-based mutualisms using a recently evolved Chlamydomonas auxotroph and B12-producing bacteria. Environ. Microbiol. 2022;24(7):3134–3147. doi: 10.1111/1462-2920.16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi T., Aoyama K., Motone K., Aburaya S., Yamashiro H., Miura N., Inoue K. Mutualistic interactions between dinoflagellates and pigmented bacteria mitigate environmental stress. Microbiol. Spectr. 2023;11(1) doi: 10.1128/spectrum.02464-22. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirth R., Pap B., Böjti T., Shetty P., Lakatos G., Bagi Z., Kovács K.L., Maróti G. Chlorella vulgaris and its phycosphere in wastewater: microalgae-bacteria interactions during nutrient removal. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.557572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dao G., Wu G., Wang X.-X., Zhang T.-Y., Zhan X., Hu H.-Y. Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res. 2018;33:345–351. doi: 10.1016/j.algal.2018.06.006. [DOI] [Google Scholar]
- 22.Amin S.A., Hmelo L.R., Tol H.M.v., Durham B.P., Carlson L.T., Heal K.R., Morales R.L., Berthiaume C.T., Parker M.S., Djunaedi B., Ingalls A.E., Parsek M.R., Moran M.A., Armbrust E.V. Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature. 2015;522:98–101. doi: 10.1038/nature14488. [DOI] [PubMed] [Google Scholar]
- 23.Palacios O.A., Gómez-Anduro G.A., Bashan Y., de-Bashan L.E. Tryptophan, thiamine and indole-3-acetic acid exchange between Chlorella sorokiniana and the plant growth-promoting bacterium Azospirillum brasilense. FEMS Microbiol. Ecol. 2016;92(6) doi: 10.1093/femsec/fiw077. [DOI] [PubMed] [Google Scholar]
- 24.Vraspir J.M., Butler A. Chemistry of marine ligands and siderophores. Ann. Rev. Mar. Sci. 2009;1:43–63. doi: 10.1146/annurev.marine.010908.163712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin S.A., Green D.H., Hart M., Küpper F.C., Sunda W.G., Carrano C.J. Photolysis of iron–siderophore chelates promotes bacterial–algal mutualism. Proc. Natl. Acad. Sci. USA. 2009;106:17071–17076. doi: 10.1073/pnas.0905512106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-López R., Maske H. The vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sañudo‐Wilhelmy S., Gobler C., Okbamichael M., Taylor G. Regulation of phytoplankton dynamics by vitamin B12. Geophys. Res. Lett. 2006;33(4) [Google Scholar]
- 28.Wienhausen G., Dlugosch L., Jarling R., Wilkes H., Giebel H.-A., Simon M. Availability of vitamin B12 and its lower ligand intermediate α-ribazole impact prokaryotic and protist communities in oceanic systems. ISME J. 2022;16(8):2002–2014. doi: 10.1038/s41396-022-01250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sokolovskaya O.M., Shelton A.N., Taga M.E. Sharing vitamins: cobamides unveil microbial interactions. Science. 2020;369 doi: 10.1126/science.aba0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y.Z., Koch F., Gobler C.J. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc. Natl. Acad. Sci. USA. 2010;107(48):20756–20761. doi: 10.1073/pnas.1009566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nef C., Dittami S.M., Kaas R., Briand E., Noël C., Mairet F., Garnier M. Sharing vitamin b12 between bacteria and microalgae does not systematically occur: case study of the haptophyte Tisochrysis lutea. Microorganisms. 2022;10 doi: 10.3390/microorganisms10071337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-López R., Maske H., Yarimizu K., Holland N.A. The B-vitamin mutualism between the dinoflagellate Lingulodinium polyedrum and the bacterium Dinoroseobacter shibae. Front. Mar. Sci. 2018;5 doi: 10.3389/fmars.2018.00274. [DOI] [Google Scholar]
- 33.Grant M.A.A., Kazamia E., Cicuta P., Smith A.G. Direct exchange of vitamin B12 is demonstrated by modelling the growth dynamics of algal–bacterial cocultures. ISME J. 2014;8:1418–1427. doi: 10.1038/ismej.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cooper M.B., Kazamia E., Helliwell K.E., Kudahl U.J., Sayer A., Wheeler G.L., Smith A.G. Cross-exchange of B-vitamins underpins a mutualistic interaction between Ostreococcus tauri and Dinoroseobacter shibae. ISME J. 2019;13(2):334–345. doi: 10.1038/s41396-018-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durham B.P., Sharma S., Luo H., Smith C.B., Amin S.A., Bender S.J., Dearth S.P., Van Mooy B.A.S., Campagna S.R., Kujawinski E.B., Armbrust E.V., Moran M.A. Cryptic carbon and sulfur cycling between surface ocean plankton. Proc. Natl. Acad. Sci. USA. 2015;112(2):453–457. doi: 10.1073/pnas.1413137112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llamas A., Leon-Miranda E., Tejada-Jimenez M. Microalgal and nitrogen-fixing bacterial consortia: from interaction to biotechnological potential. Plants. 2023;12(13) doi: 10.3390/plants12132476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calatrava V., Hom E.F.Y., Llamas Á., Fernández E., Galván A. OK, thanks! A new mutualism between Chlamydomonas and methylobacteria facilitates growth on amino acids and peptides. FEMS Microbiol. Lett. 2018;365:1–9. doi: 10.1093/femsle/fny021. [DOI] [PubMed] [Google Scholar]
- 38.Natrah F.M.I., Bossier P., Sorgeloos P., Yusoff F.M., Defoirdt T. Significance of microalgal–bacterial interactions for aquaculture. Rev. Aquacult. 2014;6:48–61. [Google Scholar]
- 39.Fukami K., Nishijima T., Ishida Y. Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia. 2004;358:185–191. [Google Scholar]
- 40.S S.V., Vijayan K.K. Symbiotic association among marine microalgae and bacterial flora: a study with special reference to commercially important Isochrysis galbana culture. J. Appl. Phycol. 2019;31 doi: 10.1007/s10811-019-01772-2. [DOI] [Google Scholar]
- 41.Fukami K., Nishijima T., Ishida Y. Stimulative and inhibitory effects of bacteria on the growth of microalgae. Hydrobiologia. 1997;358(1):185–191. doi: 10.1023/A:1003139402315. [DOI] [Google Scholar]
- 42.Lee P.A., Martinez K.J.L., Letcher P.M., Corcoran A.A., Ryan R.A. A novel predatory bacterium infecting the eukaryotic alga Nannochloropsis. Algal Res. 2018;32:314–320. doi: 10.1016/j.algal.2018.04.003. [DOI] [Google Scholar]
- 43.Carney L.T., Lane T.W. Parasites in algae mass culture. Front. Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganuza E., Sellers C.E., Bennett B.W., Lyons E.M., Carney L.T. A novel treatment protects Chlorella at commercial scale from the predatory bacterium Vampirovibrio chlorellavorus. Front. Microbiol. 2016;7:848. doi: 10.3389/fmicb.2016.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McBride R., Smith V., Carney L., Lane T. 2016. Crop Protection in Open Ponds; pp. 165–182. [DOI] [Google Scholar]
- 46.Li J., Majzoub M.E., Marzinelli E.M., Dai Z., Thomas T., Egan S. Bacterial controlled mitigation of dysbiosis in a seaweed disease. ISME J. 2021;16(2):378–387. doi: 10.1038/s41396-021-01070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward C.S., Rolison K., Li M., Rozen S., Fisher C.L., Lane T.W., Thelen M.P., Stuart R.K. Janthinobacter additions reduce rotifer grazing of microalga Microchloropsis salina in biotically complex communities. Algal Research-Biomass Biofuels and Bioproducts. 2021;58 [Google Scholar]
- 48.Le Chevanton M., Garnier M., Bougaran G., Schreiber N., Lukomska E., Bérard J.B., Fouilland E., Bernard O., Cadoret J.P. Screening and selection of growth-promoting bacteria for Dunaliella cultures. Algal Res. 2013;2(3):212–222. doi: 10.1016/j.algal.2013.05.003. [DOI] [Google Scholar]
- 49.Zhu S., Higa L., Barela A., Lee C., Chen Y., Du Z.-Y. Microalgal consortia for waste treatment and valuable bioproducts. Energies. 2023;16(2):884. [Google Scholar]
- 50.Lee S.-A., Kim M., Esterhuizen M., Le V.V., Kang M., Ko S.-R., Oh H.-M., Kim Y.J., Ahn C.-Y. An acceleration of carotenoid production and growth of Haematococcus lacustris induced by host-microbiota network interaction. Microbiol. Res. 2022;262 doi: 10.1016/j.micres.2022.127097. [DOI] [PubMed] [Google Scholar]
- 51.Perera I.A., Abinandan S., Subashchandrabose S.R., Venkateswarlu K., Naidu R., Megharaj M. Combined inorganic nitrogen sources influence the release of extracellular compounds that drive mutualistic interactions in microalgal‒bacterial co-cultures. J. Appl. Phycol. 2022;34(3):1311–1322. doi: 10.1007/s10811-022-02711-4. [DOI] [Google Scholar]
- 52.Cassan F.D., Coniglio A., Amavizca E., Maroniche G.A., Cascales E., Bashan Y., de-Bashan L.E. The Azospirillum brasilense Type VI secretion system promotes cell aggregation, biocontrol protection against phytopathogens and attachment to the microalgae Chlorella sorokiniana. Environ. Microbiol. 2021;23(10):6257–6274. doi: 10.1111/1462-2920.15749. [DOI] [PubMed] [Google Scholar]
- 53.Barbosa-Nuñez J.A., Palacios O.A., Mondragón-Cortéz P.M., Ocampo-Alvarez H., Becerril-Espinosa A., Nevárez-Moorillón G.V., Choix F.J. Chemical and physical affinity of microalga–Azospirillum consortium co-cultured in suspension during CO2 fixation from biogas. BioEnergy Research. 2022:1–14. [Google Scholar]
- 54.Choix F.J., de-Bashan L.E., Bashan Y. Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: I. Autotrophic conditions. Enzym. Microb. Technol. 2012;51(5):294–299. doi: 10.1016/j.enzmictec.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 55.Choix F.J., López-Cisneros C.G., Méndez-Acosta H.O. Azospirillum brasilense Increases CO2 fixation on microalgae Scenedesmus obliquus, Chlorella vulgaris, and Chlamydomonas reinhardtii cultured on high CO2 Concentrations. Microb. Ecol. 2017;76:430–442. doi: 10.1007/s00248-017-1139-z. [DOI] [PubMed] [Google Scholar]
- 56.Chia S.R., Ling J., Chia W.Y., Nomanbhay S., Kurniawan T.A., Chew K.W. Future bioenergy source by microalgae–bacteria consortia: a circular economy approach. Green Chem. 2023;25(22):8935–8949. doi: 10.1039/D3GC02228E. [DOI] [Google Scholar]
- 57.Kilbane J.J. Shining a light on wastewater treatment with microalgae. Arabian J. Sci. Eng. 2022;47(1):45–56. doi: 10.1007/s13369-021-06444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith V.H., Sturm B.S.M., deNoyelles F.J., Billings S.A. The ecology of algal biodiesel production. Trends Ecol. Evol. 2010;25(5):301–309. doi: 10.1016/j.tree.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Meng F., Xi L., Liu D., Huang W., Lei Z., Zhang Z., Huang W. Effects of light intensity on oxygen distribution, lipid production and biological community of algal-bacterial granules in photo-sequencing batch reactors. Bioresour. Technol. 2019;272:473–481. doi: 10.1016/j.biortech.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 60.Aravind S., Kumar P.S., Kumar N.S., Siddarth N. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ. Chem. Lett. 2020;18(3):829–849. doi: 10.1007/s10311-020-00990-2. [DOI] [Google Scholar]
- 61.Chaiwong K., Kiatsiriroat T., Vorayos N., Thararax C. Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy. 2013;56:600–606. doi: 10.1016/j.biombioe.2013.05.035. [DOI] [Google Scholar]
- 62.Mahfud M., Qadariyah L., Haqqyana H., Aswie V. Optimization bio-oil production from Chlorella sp. Through microwave-assisted pyrolysis using response surface methodology. Green Energy and Resources. 2024 doi: 10.1016/j.gerr.2024.100057. [DOI] [Google Scholar]
- 63.Singh S., Meena P., Bhoi R., Saharan V.K., George S. Optimization of bio-oil extraction from Chlorella biomass via a green approach to obtain algal-based Di-ethyl phthalate. Environ. Sci. Pollut. Control Ser. 2023 doi: 10.1007/s11356-023-30866-1. [DOI] [PubMed] [Google Scholar]
- 64.Abate R., Bi Y., Song G., Mi W., Cheng F., Zhu Y. Exogenous ethanol induces cell giantism accompanied by enhanced accumulation of lipid and carbohydrates in Chlorella sorokiniana. J. Appl. Phycol. 2024 doi: 10.1007/s10811-024-03199-w. [DOI] [Google Scholar]
- 65.Adamakis I.-D., Lazaridis P.A., Terzopoulou E., Torofias S., Valari M., Kalaitzi P., Rousonikolos V., Gkoutzikostas D., Zouboulis A., Zalidis G., Triantafyllidis K.S. Cultivation, characterization, and properties of Chlorella vulgaris microalgae with different lipid contents and effect on fast pyrolysis oil composition. Environ. Sci. Pollut. Control Ser. 2018;25(23):23018–23032. doi: 10.1007/s11356-018-2368-5. [DOI] [PubMed] [Google Scholar]
- 66.Lamba B.Y., Shah S.V., Sharma R., Tiwari A.K., Jain S., Kumar S. A pilot scale hydrothermal liquefaction of sewage water grown Chlorella sorokiniana for bio-oil production—a sustainable integrated approach. Biomass Conversion and Biorefinery. 2023 doi: 10.1007/s13399-023-04155-3. [DOI] [Google Scholar]
- 67.Toyama T., Kasuya M., Hanaoka T., Kobayashi N., Tanaka Y., Inoue D., Sei K., Morikawa M., Mori K. Growth promotion of three microalgae, Chlamydomonas reinhardtii, Chlorella vulgaris and Euglena gracilis, by in situ indigenous bacteria in wastewater effluent. Biotechnol. Biofuels. 2018;11(1):176. doi: 10.1186/s13068-018-1174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toyama T., Hanaoka T., Yamada K., Suzuki K., Tanaka Y., Morikawa M., Mori K. Enhanced production of biomass and lipids by Euglena gracilis via co-culturing with a microalga growth-promoting bacterium, Emticicia sp. EG3. Biotechnol. Biofuels. 2019;12:205. doi: 10.1186/s13068-019-1544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leong W.H., Kiatkittipong K., Kiatkittipong W., Cheng Y.W., Lam M.K., Shamsuddin R., Mohamad M., Lim J.W. Comparative performances of microalgal-bacterial Co-cultivation to bioremediate synthetic and municipal wastewaters whilst producing biodiesel sustainably. Processes. 2020;8(11):1427. [Google Scholar]
- 70.Makut B.B., Goswami G., Das D. Evaluation of bio-crude oil through hydrothermal liquefaction of microalgae-bacteria consortium grown in open pond using wastewater. Biomass Conversion and Biorefinery. 2022;12(7):2567–2581. doi: 10.1007/s13399-020-00795-x. [DOI] [Google Scholar]
- 71.Xie B., Gong W., Tian Y., Qu F., Luo Y., Du X., Tang X., Xu D., Lin D., Li G., Liang H. Biodiesel production with the simultaneous removal of nitrogen, phosphorus and COD in microalgal-bacterial communities for the treatment of anaerobic digestion effluent in photobioreactors. Chem. Eng. J. 2018;350:1092–1102. doi: 10.1016/j.cej.2018.06.032. [DOI] [Google Scholar]
- 72.Gonçalves A.L., Pires J.C.M., Simões M. Green fuel production: processes applied to microalgae. Environ. Chem. Lett. 2013;11(4):315–324. doi: 10.1007/s10311-013-0425-3. [DOI] [Google Scholar]
- 73.Anand U., Dey S., Parial D., Federici S., Ducoli S., Bolan N.S., Dey A., Bontempi E. Algae and bacteria consortia for wastewater decontamination and transformation into biodiesel, bioethanol, biohydrogen, biofertilizers and animal feed: a review. Environ. Chem. Lett. 2023;21(3):1585–1609. doi: 10.1007/s10311-023-01562-w. [DOI] [Google Scholar]
- 74.El-Dalatony M.M., Salama E.-S., Kurade M.B., Hassan S.H.A., Oh S.-E., Kim S., Jeon B.-H. Utilization of microalgal biofractions for bioethanol, higher alcohols, and biodiesel production: a review. Energies. 2017;10(12):2110. [Google Scholar]
- 75.Kumar S., Gupta R., Kumar G., Sahoo D., Kuhad R.C. Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013;135:150–156. doi: 10.1016/j.biortech.2012.10.120. [DOI] [PubMed] [Google Scholar]
- 76.Papadopoulos K.P., Economou C.N., Stefanidou N., Moustaka-Gouni M., Genitsaris S., Aggelis G., Tekerlekopoulou A.G., Vayenas D.V. A semi-continuous algal-bacterial wastewater treatment process coupled with bioethanol production. J. Environ. Manag. 2023;326 doi: 10.1016/j.jenvman.2022.116717. [DOI] [PubMed] [Google Scholar]
- 77.Huo Y.-X., Cho K.M., Rivera J.G.L., Monte E., Shen C.R., Yan Y., Liao J.C. Conversion of proteins into biofuels by engineering nitrogen flux. Nat. Biotechnol. 2011;29(4):346–351. doi: 10.1038/nbt.1789. [DOI] [PubMed] [Google Scholar]
- 78.Arcila J.S., Buitrón G. Microalgae–bacteria aggregates: effect of the hydraulic retention time on the municipal wastewater treatment, biomass settleability and methane potential. J. Chem. Technol. Biotechnol. 2016;91:2862–2870. [Google Scholar]
- 79.Mahidhara G., Burrow H., Sasikala C., Ramana C.V. Biological hydrogen production: molecular and electrolytic perspectives. World J. Microbiol. Biotechnol. 2019;35(8):116. doi: 10.1007/s11274-019-2692-z. [DOI] [PubMed] [Google Scholar]
- 80.Jain I.P. Hydrogen the fuel for 21st century. Int. J. Hydrogen Energy. 2009;34(17):7368–7378. doi: 10.1016/j.ijhydene.2009.05.093. [DOI] [Google Scholar]
- 81.Kadier A., Simayi Y., Abdeshahian P., Azman N.F., Chandrasekhar K., Kalil M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016;55(1):427–443. doi: 10.1016/j.aej.2015.10.008. [DOI] [Google Scholar]
- 82.Y. Yin, J. Wang, Chapter 8 - Production of biohydrogen, in: F.I. Gómez Castro, C. Gutiérrez-Antonio (Eds.), Biofuels and Biorefining, Elsevier2022, pp. 283-337. https://doi.org/ 10.1016/B978-0-12-824116-5.00002-7.. [DOI]
- 83.Fakhimi N., González-Ballester D., Fernández E., Galván A., Dubini A. Algae-bacteria consortia as a strategy to enhance H2 production. Cells. 2020;9 doi: 10.3390/cells9061353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruiz-Marin A., Canedo-López Y., Chávez-Fuentes P. Biohydrogen production by Chlorella vulgaris and Scenedesmus obliquus immobilized cultivated in artificial wastewater under different light quality. Amb. Express. 2020;10(1):191. doi: 10.1186/s13568-020-01129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Das D., Veziroǧlu T.N. Hydrogen production by biological processes: a survey of literature. Int. J. Hydrogen Energy. 2001;26(1):13–28. doi: 10.1016/S0360-3199(00)00058-6. [DOI] [Google Scholar]
- 86.Karube I., Matsunaga T., Otsuka T., Kayano H., Suzuki S. Hydrogen evolution by co-immobilized chloroplasts and Clostridium butyricum. Biochim. Biophys. Acta. 1981;637(3):490–495. doi: 10.1016/0005-2728(81)90055-4. [DOI] [Google Scholar]
- 87.Morsy F.M. Acetate versus sulfur deprivation role in creating anaerobiosis in light for hydrogen production by Chlamydomonas reinhardtii and Spirulina platensis: two different organisms and two different mechanisms. Photochem. Photobiol. 2011;87(1):137–142. doi: 10.1111/j.1751-1097.2010.00823.x. [DOI] [PubMed] [Google Scholar]
- 88.Volgusheva A., Kukarskikh G., Krendeleva T., Rubin A., Mamedov F. Hydrogen photoproduction in green algae Chlamydomonas reinhardtii under magnesium deprivation. RSC Adv. 2015;5(8):5633–5637. doi: 10.1039/C4RA12710B. [DOI] [Google Scholar]
- 89.Gonzalez-Ballester D., Jurado-Oller J.L., Fernandez E. Relevance of nutrient media composition for hydrogen production in Chlamydomonas. Photosynth. Res. 2015;125(3):395–406. doi: 10.1007/s11120-015-0152-7. [DOI] [PubMed] [Google Scholar]
- 90.Pewnual T., Jampapetch N., Saladtook S., Raksajit W., Klinsalee R., Maneeruttanarungroj C. Response of green alga Tetraspora sp. CU2551 under potassium deprivation: a new promising strategy for hydrogen production. J. Appl. Phycol. 2022;34(2):811–819. doi: 10.1007/s10811-021-02672-0. [DOI] [Google Scholar]
- 91.Krishna P.S., Styring S., Mamedov F. Photosystem ratio imbalance promotes direct sustainable H2 production in Chlamydomonas reinhardtii. Green Chem. 2019;21(17):4683–4690. doi: 10.1039/C9GC01416K. [DOI] [Google Scholar]
- 92.Melis A., Zhang L., Forestier M., Ghirardi M.L., Seibert M. Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2000;122(1):127–136. doi: 10.1104/pp.122.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Asada Y., Kawamura S. Aerobic hydrogen accumulation by a nitrogen-fixing cyanobacterium, Anabaena sp. Appl. Environ. Microbiol. 1986;51(5):1063–1066. doi: 10.1128/aem.51.5.1063-1066.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khetkorn W., Rastogi R.P., Incharoensakdi A., Lindblad P., Madamwar D., Pandey A., Larroche C. Microalgal hydrogen production – a review. Bioresour. Technol. 2017;243:1194–1206. doi: 10.1016/j.biortech.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 95.Ban S., Lin W., Wu F., Luo J. Algal-bacterial cooperation improves algal photolysis-mediated hydrogen production. Bioresour. Technol. 2018;251:350–357. doi: 10.1016/j.biortech.2017.12.072. [DOI] [PubMed] [Google Scholar]
- 96.Fakhimi N., Dubini A., Tavakoli O., González-Ballester D. Acetic acid is key for synergetic hydrogen production in Chlamydomonas-bacteria co-cultures. Bioresour. Technol. 2019;289 doi: 10.1016/j.biortech.2019.121648. [DOI] [PubMed] [Google Scholar]
- 97.Lakatos G.E., Balogh D., Farkas A.E., Ördög V., Nagy P.I., Bíró T., Maróti G. Factors influencing algal photobiohydrogen production in algal-bacterial co-cultures. Algal Research-Biomass Biofuels and Bioproducts. 2017;28:161–171. [Google Scholar]
- 98.Xu L., Li D., Wang Q., Wu S. Improved hydrogen production and biomass through the co-cultivation of Chlamydomonas reinhardtii and Bradyrhizobium japonicum. Int. J. Hydrogen Energy. 2016;41:9276–9283. [Google Scholar]
- 99.Miura Y., Saitoh C., Matsuoka S., Miyamoto K. Stably sustained hydrogen production with high molar yield through a combination of a marine green alga and a photosynthetic bacterium. Biosci., Biotechnol., Biochem. 1992;56(5):751–754. doi: 10.1271/bbb.56.751. [DOI] [PubMed] [Google Scholar]
- 100.He J., Xi L.-j., Sun X., Ge B., Liu D., Han Z., Pu X., Huang F. Enhanced hydrogen production through co-cultivation of Chlamydomonas reinhardtii CC-503 and a facultative autotrophic sulfide-oxidizing bacterium under sulfurated conditions. Int. J. Hydrogen Energy. 2018;43(32):15005–15013. doi: 10.1016/j.ijhydene.2018.06.081. [DOI] [Google Scholar]
- 101.Marchand J., Heydarizadeh P., Schoefs B., Spetea C. Ion and metabolite transport in the chloroplast of algae: lessons from land plants. Cell. Mol. Life Sci. 2018;75(12):2153–2176. doi: 10.1007/s00018-018-2793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Makut B.B., Das D., Goswami G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: a sustainable approach. Algal Res. 2019;37:228–239. doi: 10.1016/j.algal.2018.11.020. [DOI] [Google Scholar]
- 103.Ismail M.M., Essam T.M., Ragab Y.M., Mourad F.E. Biodegradation of ketoprofen using a microalgal–bacterial consortium. Biotechnol. Lett. 2016;38:1493–1502. doi: 10.1007/s10529-016-2145-9. [DOI] [PubMed] [Google Scholar]
- 104.Olguín E.J. Dual purpose microalgae–bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a Biorefinery. Biotechnol. Adv. 2012;30(5):1031–1046. doi: 10.1016/j.biotechadv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 105.Fu R., Li X., Zhao Y., Pu Q., Li Y., Gu W. Efficient and synergistic degradation of fluoroquinolones by bacteria and microalgae: design of environmentally friendly substitutes, risk regulation and mechanism analysis. J. Hazard Mater. 2022;437 doi: 10.1016/j.jhazmat.2022.129384. [DOI] [PubMed] [Google Scholar]
- 106.Xu M., Xue Z., Sun S., Zhao C., Liu J., Liu J., Zhao Y. Co-culturing microalgae with endophytic bacteria increases nutrient removal efficiency for biogas purification. Bioresour. Technol. 2020;314 doi: 10.1016/j.biortech.2020.123766. [DOI] [PubMed] [Google Scholar]
- 107.Cai Z., Karunakaran E., Pandhal J. Bottom-up construction and screening of algae-bacteria consortia for pollutant biodegradation. Front. Microbiol. 2024;15 doi: 10.3389/fmicb.2024.1349016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karya N., van der Steen N.P., Lens P.N.L. Photo-oxygenation to support nitrification in an algal-bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013;134:244–250. doi: 10.1016/j.biortech.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 109.Wang M., Yang H., Ergas S.J., van der Steen P. A novel shortcut nitrogen removal process using an algal-bacterial consortium in a photo-sequencing batch reactor (PSBR) Water Res. 2015;87:38–48. doi: 10.1016/j.watres.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 110.Chang Y.-L., Nagarajan D., Chen J.-H., Yen Chen C., Wu Y.-J., Whang L.-M., Lee D.-J., Chang J.-S. Microalgae-bacteria consortia for the treatment of raw dairy manure wastewater using a novel two-stage process: process optimization and bacterial community analysis. Chem. Eng. J. 2023;473 doi: 10.1016/j.cej.2023.145388. [DOI] [Google Scholar]
- 111.Arun S.K., Manikandan N.A., Pakshirajan K., Pugazhenthi G. Novel shortcut biological nitrogen removal method using an algae-bacterial consortium in a photo-sequencing batch reactor: process optimization and kinetic modelling. J. Environ. Manag. 2019;250 doi: 10.1016/j.jenvman.2019.109401. [DOI] [PubMed] [Google Scholar]
- 112.Lakshmikandan M., Wang S., Murugesan A.G., Saravanakumar M., Selvakumar G. Co-cultivation of Streptomyces and microalgal cells as an efficient system for biodiesel production and bioflocculation formation. Bioresour. Technol. 2021;332 doi: 10.1016/j.biortech.2021.125118. [DOI] [PubMed] [Google Scholar]
- 113.Xu M., Zeng Q., Li H., Zhong Y., Tong L., Ruan R., Liu H. Contribution of glycerol addition and algal–bacterial cooperation to nutrients recovery: a study on the mechanisms of microalgae‐based wastewater remediation. J. Chem. Technol. Biotechnol. 2020;95(6):1717–1728. doi: 10.1002/jctb.6369. [DOI] [Google Scholar]
- 114.Kant Bhatia S., Ahuja V., Chandel N.P., Mehariya S., Kumar P., Vanayak V., Saratale G.D., Raj T., Kim S.H., Yang Y.-H. An overview on microalgal-bacterial granular consortia for resource recovery and wastewater treatment. Bioresour. Technol. 2022 doi: 10.1016/j.biortech.2022.127028. [DOI] [PubMed] [Google Scholar]
- 115.Pérez-Nava J., Hernández-Aldana F., Martínez-Valenzuela C., Rivera A. Pseudomonas sp. isolated from wastewater and their interaction with microalgae. J. Biochem. Technol. 2021;12:1–5. doi: 10.51847/yaQC7KnNoY. [DOI] [Google Scholar]
- 116.Tait K., White D.A., Kimmance S.A., Tarran G.A., Rooks P.A., Jones M., Llewellyn C.A. Characterisation of bacteria from the cultures of a Chlorella strain isolated from textile wastewater and their growth enhancing effects on the axenic cultures of Chlorella vulgaris in low nutrient media. Algal Res. 2019;44:101666. doi: 10.1016/j.algal.2019.101666. [DOI] [Google Scholar]
- 117.Krustok I., Truu J., Odlare M., Truu M., Ligi T., Tiirik K., Nehrenheim E. Effect of lake water on algal biomass and microbial community structure in municipal wastewater-based lab-scale photobioreactors. Appl. Microbiol. Biotechnol. 2015;99:6537–6549. doi: 10.1007/s00253-015-6580-7. [DOI] [PubMed] [Google Scholar]
- 118.López-Patiño A.M., Cárdenas-Orrego A., Torres A.F., Navarrete D., Champagne P., Ochoa-Herrera V. Native microalgal-bacterial consortia from the Ecuadorian Amazon region: an alternative to domestic wastewater treatment. Front. Bioeng. Biotechnol. 2024;12 doi: 10.3389/fbioe.2024.1338547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Medina M., Neis U. Symbiotic algal bacterial wastewater treatment: effect of food to microorganism ratio and hydraulic retention time on the process performance. Water Sci. Technol. : a journal of the International Association on Water Pollution Research. 2007;55 11:165–171. doi: 10.2166/wst.2007.351. [DOI] [PubMed] [Google Scholar]
- 120.Ferro L., Colombo M., Posadas E., Funk C., Muñoz R. Elucidating the symbiotic interactions between a locally isolated microalga Chlorella vulgaris and its co-occurring bacterium Rhizobium sp. in synthetic municipal wastewater. J. Appl. Phycol. 2019:1–12. [Google Scholar]
- 121.Paddock M.B., Fernández-Bayo J.D., VanderGheynst J.S. The effect of the microalgae-bacteria microbiome on wastewater treatment and biomass production. Appl. Microbiol. Biotechnol. 2019;104:893–905. doi: 10.1007/s00253-019-10246-x. [DOI] [PubMed] [Google Scholar]
- 122.Jesus Torres M., González-Ballester D., Gomez-Osuna A., Galván A., Fernández E., Dubini A. Chlamydomonas-Methylobacterium oryzae cooperation leads to increased biomass, nitrogen removal and hydrogen production. Bioresour. Technol. 2022 doi: 10.1016/j.biortech.2022.127088. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., Li J., Lei Y., Cui R., Liang A., Li X., Kit Leong Y., Chang J.-S. Enhanced sulfonamides removal via microalgae-bacteria consortium via co-substrate supplementation. Bioresour. Technol. 2022;358 doi: 10.1016/j.biortech.2022.127431. [DOI] [PubMed] [Google Scholar]
- 124.Zhong Y., Liu H., Li H., Lu Q., Sun Y. Does exogenous carbon source always promote algal biomass and nutrients removal in algal-bacterial wastewater remediation? J. Clean. Prod. 2021;281 [Google Scholar]
- 125.Arun S.K., Ramasamy S., Pakshirajan K. Mechanistic insights into nitrification by microalgae-bacterial consortia in a photo-sequencing batch reactor under different light intensities. J. Clean. Prod. 2021;321 [Google Scholar]
- 126.Akizuki S., Kishi M., Cuevas-Rodríguez G., Toda T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2019;171 doi: 10.1016/j.watres.2019.115445. [DOI] [PubMed] [Google Scholar]
- 127.Nishi K.-i., Akizuki S., Toda T., Matsuyama T., Ida J. Advanced light-tolerant microalgae-nitrifying bacteria consortia for stable ammonia removal under strong light irradiation using light-shielding hydrogel. Chemosphere. 2022 doi: 10.1016/j.chemosphere.2022.134252. [DOI] [PubMed] [Google Scholar]
- 128.Huang Q., Yan H., Liu Y., Cui X., Wang Y., Yu Z., Ruan R., Zhang Q. Effects of microalgae-bacteria inoculation ratio on biogas slurry treatment and microorganism interactions in the symbiosis system. J. Clean. Prod. 2022;362:132271. doi: 10.1016/j.jclepro.2022.132271. [DOI] [Google Scholar]
- 129.Nguyen T.T.V., Nguyen T.-T., An Binh Q., Bui X.-T., Ngo H.h., Vo H.N.P., Andrew Lin K.-Y., Vo T.-D.-H., Guo W., Lin C., Breider F. Co-culture of microalgae-activated sludge for wastewater treatment and biomass production: exploring their role under different inoculation ratios. Bioresour. Technol. 2020;314 doi: 10.1016/j.biortech.2020.123754. [DOI] [PubMed] [Google Scholar]
- 130.Liu X.-y., Hong Y., Zhai Q.-Y., Zhao G., Zhang H., Wang Q. Performance and mechanism of Chlorella in swine wastewater treatment: roles of nitrogen-phosphorus ratio adjustment and indigenous bacteria. Bioresour. Technol. 2022 doi: 10.1016/j.biortech.2022.127402. [DOI] [PubMed] [Google Scholar]
- 131.Hernández J.-P., de-Bashan L.E., Bashan Y. Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. co-immobilized with Azospirillum brasilense. Enzym. Microb. Technol. 2006;38:190–198. [Google Scholar]
- 132.Xia L., Huang R., Li Y., Song S. The effect of growth phase on the surface properties of three oleaginous microalgae (Botryococcus sp. FACGB-762, Chlorella sp. XJ-445 and Desmodesmus bijugatus XJ-231) PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang C.-C., Zhang X.-Y., Wang R., Wang T., He Z., Wang X.C. Calcium ions-effect on performance, growth and extracellular nature of microalgal-bacterial symbiosis system treating wastewater. Environ. Res. 2021 doi: 10.1016/j.envres.2021.112228. [DOI] [PubMed] [Google Scholar]
- 134.Tang C.-C., Wang T., Zhang X.-Y., Wang R., He Z., Li Z., Wang X.C. Role of types and dosages of cations with low valance states on microalgal-bacterial symbiosis system treating wastewater. Bioresour. Technol. 2022 doi: 10.1016/j.biortech.2022.127755. [DOI] [PubMed] [Google Scholar]
- 135.Collao J., García-Encina P.A., Blanco S., Bolado-Rodríguez S., Fernandez-Gonzalez N. Current concentrations of Zn, Cu, and as in piggery wastewater compromise nutrient removals in microalgae–bacteria photobioreactors due to altered microbial communities. Biology. 2022;11 doi: 10.3390/biology11081176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de-Bashan L.E., Schmid M., Rothballer M., Hartmann A., Bashan Y. Cell-cell interaction in the eukaryote-prokaryote model of the microalgae Chlorella vulgaris and the bacterium Azospirillum brasilense immobilized in polymer beads. J. Phycol. 2011;47(6):1350–1359. doi: 10.1111/j.1529-8817.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 137.Palacios O.A., López B.R., Bashan Y., Bashan L.E.d. Early changes in nutritional conditions affect formation of synthetic mutualism between Chlorella sorokiniana and the bacterium Azospirillum brasilense. Microb. Ecol. 2018;77:980–992. doi: 10.1007/s00248-018-1282-1. [DOI] [PubMed] [Google Scholar]
- 138.de-Bashan L.E., Moreno M., Hernandez J.-P., Bashan Y. Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth-promoting bacterium Azospirillum brasilense. Water Res. 2002;36(12):2941–2948. doi: 10.1016/S0043-1354(01)00522-X. [DOI] [PubMed] [Google Scholar]
- 139.de-Bashan L.E., Hernández J.-P., Morey T., Bashan Y. Microalgae growth-promoting bacteria as "helpers" for microalgae: a novel approach for removing ammonium and phosphorus from municipal wastewater. Water Res. 2004;38(2):466–474. doi: 10.1016/j.watres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 140.Arends J.B., Verstraete W. 100 years of microbial electricity production: three concepts for the future. Microb. Biotechnol. 2012;5(3):333–346. doi: 10.1111/j.1751-7915.2011.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cohen B. The bacterial culture as an electrical half-cell. J. Bacteriol. 1931;21(1):18–19. [Google Scholar]
- 142.Aboelela D., Soliman M.A. Hydrogen production from microbial electrolysis cells powered with microbial fuel cells. Journal of King Saud University - Engineering Sciences. 2022 doi: 10.1016/j.jksues.2022.05.008. [DOI] [Google Scholar]
- 143.Jalili P., Ala A., Nazari P., Jalili B., Ganji D.D. A comprehensive review of microbial fuel cells considering materials, methods, structures, and microorganisms. Heliyon. 2024;10(3) doi: 10.1016/j.heliyon.2024.e25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zou L., Zhu F., Long Z.-e., Huang Y. Bacterial extracellular electron transfer: a powerful route to the green biosynthesis of inorganic nanomaterials for multifunctional applications. J. Nanobiotechnol. 2021;19(1):120. doi: 10.1186/s12951-021-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mekuto L., Olowolafe A.V.A., Pandit S., Dyantyi N., Nomngongo P., Huberts R. Microalgae as a biocathode and feedstock in anode chamber for a self-sustainable microbial fuel cell technology: a review. S. Afr. J. Chem. Eng. 2020;31:7–16. doi: 10.1016/j.sajce.2019.10.002. [DOI] [Google Scholar]
- 146.Rahman A., Borhan M.S., Rahman S. Evaluation of microbial fuel cell (MFC) for bioelectricity generation and pollutants removal from sugar beet processing wastewater (SBPW) Water Sci. Technol. 2018;77(1–2):387–397. doi: 10.2166/wst.2017.549. [DOI] [PubMed] [Google Scholar]
- 147.Min B., Kim J., Oh S., Regan J.M., Logan B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005;39(20):4961–4968. doi: 10.1016/j.watres.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 148.Hou Q., Pei H., Hu W., Jiang L., Yu Z. Mutual facilitations of food waste treatment, microbial fuel cell bioelectricity generation and Chlorella vulgaris lipid production. Bioresour. Technol. 2016;203:50–55. doi: 10.1016/j.biortech.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 149.JiangHai-ming Combination of microbial fuel cells with microalgae cultivation for bioelectricity generation and domestic wastewater treatment. Environ. Eng. Sci. 2017;34:489–495. [Google Scholar]
- 150.Ren L., Ahn Y., Logan B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014;48(7):4199–4206. doi: 10.1021/es500737m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Venkata Subhash G., Chandra R., Venkata Mohan S. Microalgae mediated bio-electrocatalytic fuel cell facilitates bioelectricity generation through oxygenic photomixotrophic mechanism. Bioresour. Technol. 2013;136:644–653. doi: 10.1016/j.biortech.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 152.Cheng S., Dempsey B.A., Logan B.E. Electricity generation from synthetic acid-mine drainage (AMD) water using fuel cell technologies. Environ. Sci. Technol. 2007;41(23):8149–8153. doi: 10.1021/es0712221. [DOI] [PubMed] [Google Scholar]
- 153.Nguyen H.T.H., Kakarla R., Min B. Algae cathode microbial fuel cells for electricity generation and nutrient removal from landfill leachate wastewater. Int. J. Hydrogen Energy. 2017;42(49):29433–29442. doi: 10.1016/j.ijhydene.2017.10.011. [DOI] [Google Scholar]
- 154.Firdous S., Jin W., Shahid N., Bhatti Z.A., Iqbal A., Abbasi U., Mahmood Q., Ali A. The performance of microbial fuel cells treating vegetable oil industrial wastewater. Environ. Technol. Innovat. 2018;10:143–151. doi: 10.1016/j.eti.2018.02.006. [DOI] [Google Scholar]
- 155.Wang X., Feng Y.J., Lee H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008;57(7):1117–1121. doi: 10.2166/wst.2008.064. [DOI] [PubMed] [Google Scholar]
- 156.Kakarla R., Min B. Photoautotrophic microalgae Scenedesmus obliquus attached on a cathode as oxygen producers for microbial fuel cell (MFC) operation. Int. J. Hydrogen Energy. 2014;39(19):10275–10283. doi: 10.1016/j.ijhydene.2014.04.158. [DOI] [Google Scholar]
- 157.Wang C.-T., Huang Y.-S., Sangeetha T., Chen Y.-M., Chong W.-T., Ong H.-C., Zhao F., Yan W.-M. Novel bufferless photosynthetic microbial fuel cell (PMFCs) for enhanced electrochemical performance. Bioresour. Technol. 2018;255:83–87. doi: 10.1016/j.biortech.2018.01.086. [DOI] [PubMed] [Google Scholar]
- 158.Commault A.S., Laczka O., Siboni N., Tamburic B., Crosswell J.R., Seymour J.R., Ralph P.J. Electricity and biomass production in a bacteria-Chlorella based microbial fuel cell treating wastewater. J. Power Sources. 2017;356:299–309. doi: 10.1016/j.jpowsour.2017.03.097. [DOI] [Google Scholar]
- 159.Kondaveeti S., Choi K.S., Kakarla R., Min B. Microalgae Scenedesmus obliquus as renewable biomass feedstock for electricity generation in microbial fuel cells (MFCs) Front. Environ. Sci. Eng. 2014;8(5):784–791. [Google Scholar]
- 160.Strik D.P.B.T.B., Terlouw H., Hamelers H.V.M., Buisman C.J.N. Renewable sustainable biocatalyzed electricity production in a photosynthetic algal microbial fuel cell (PAMFC) Appl. Microbiol. Biotechnol. 2008;81(4):659–668. doi: 10.1007/s00253-008-1679-8. [DOI] [PubMed] [Google Scholar]
- 161.Walter X.A., Greenman J., Taylor B., Ieropoulos I.A. Microbial fuel cells continuously fuelled by untreated fresh algal biomass. Algal Res. 2015;11:103–107. doi: 10.1016/j.algal.2015.06.003. [DOI] [Google Scholar]
- 162.Yuan Y., Chen Q., Zhou S., Zhuang L., Hu P. Bioelectricity generation and microcystins removal in a blue-green algae powered microbial fuel cell. J. Hazard Mater. 2011;187(1):591–595. doi: 10.1016/j.jhazmat.2011.01.042. [DOI] [PubMed] [Google Scholar]
- 163.Wang H., Liu D., Lu L., Zhao Z., Xu Y., Cui F. Degradation of algal organic matter using microbial fuel cells and its association with trihalomethane precursor removal. Bioresour. Technol. 2012;116:80–85. doi: 10.1016/j.biortech.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 164.Singhvi P., Chhabra M. Simultaneous chromium removal and power generation using algal biomass in a dual chambered salt bridge microbial fuel cell. J. Biorem. Biodegrad. 2013;4(5) [Google Scholar]
- 165.Velasquez-Orta S.B., Curtis T.P., Logan B.E. Energy from algae using microbial fuel cells. Biotechnol. Bioeng. 2009;103(6):1068–1076. doi: 10.1002/bit.22346. [DOI] [PubMed] [Google Scholar]
- 166.Xiao L., He Z. Applications and perspectives of phototrophic microorganisms for electricity generation from organic compounds in microbial fuel cells. Renew. Sustain. Energy Rev. 2014;37:550–559. doi: 10.1016/j.rser.2014.05.066. [DOI] [Google Scholar]
- 167.Cui Y., Rashid N., Hu N., Rehman M.S.U., Han J.-I. Electricity generation and microalgae cultivation in microbial fuel cell using microalgae-enriched anode and bio-cathode. Energy Convers. Manag. 2014;79:674–680. doi: 10.1016/j.enconman.2013.12.032. [DOI] [Google Scholar]
- 168.Zhao J., Li X.-F., Ren Y.-P., Wang X.-H., Jian C. Electricity generation from Taihu Lake cyanobacteria by sediment microbial fuel cells. J. Chem. Technol. Biotechnol. 2012;87(11):1567–1573. doi: 10.1002/jctb.3794. [DOI] [Google Scholar]
- 169.Inglesby A.E., Fisher A.C. Downstream application of a microbial fuel cell for energy recovery from an Arthrospira maxima fed anaerobic digester effluent. RSC Adv. 2013;3(38):17387–17394. doi: 10.1039/C3RA42277A. [DOI] [Google Scholar]
- 170.Nishio K., Hashimoto K., Watanabe K. Digestion of algal biomass for electricity generation in microbial fuel cells. Biosci., Biotechnol., Biochem. 2013;77(3):670–672. doi: 10.1271/bbb.120833. [DOI] [PubMed] [Google Scholar]
- 171.Berk R.S., Canfield J.H. Bioelectrochemical energy conversion. Appl. Microbiol. 1964;12(1):10–12. doi: 10.1128/am.12.1.10-12.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ochiai H., Shibata H., Sawa Y., Katoh T. "Living electrode" as a long-lived photoconverter for biophotolysis of water. Proc. Natl. Acad. Sci. U. S. A. 1980;77(5):2442–2444. doi: 10.1073/pnas.77.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yagishita T., Horigome T., Tanaka K. Effects of light, CO2 and inhibitors on the current output of biofuel cells containing the photosynthetic organism Synechococcus sp. J. Chem. Technol. Biotechnol. 1993;56(4):393–399. doi: 10.1002/jctb.280560411. [DOI] [Google Scholar]
- 174.Sekar N., Ramasamy R.P. Recent advances in photosynthetic energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2015;22:19–33. doi: 10.1016/j.jphotochemrev.2014.09.004. [DOI] [Google Scholar]
- 175.Zou Y., Pisciotta J., Billmyre R.B., Baskakov I.V. Photosynthetic microbial fuel cells with positive light response. Biotechnol. Bioeng. 2009;104(5):939–946. doi: 10.1002/bit.22466. [DOI] [PubMed] [Google Scholar]
- 176.Torimura M., Miki A., Wadano A., Kano* K., Ikeda* T. Electrochemical investigation of cyanobacteria Synechococcus sp. PCC7942-catalyzed photoreduction of exogenous quinones and photoelectrochemical oxidation of water. J. Electroanal. Chem. 2001;496(1):21–28. doi: 10.1016/S0022-0728(00)00253-9. [DOI] [Google Scholar]
- 177.Terasaki N., Yamamoto N., Hiraga T., Sato I., Inoue Y., Yamada S. Fabrication of novel photosystem I–gold nanoparticle hybrids and their photocurrent enhancement. Thin Solid Films. 2006;499(1):153–156. doi: 10.1016/j.tsf.2005.07.050. [DOI] [Google Scholar]
- 178.Guskov A., Kern J., Gabdulkhakov A., Broser M., Zouni A., Saenger W. Cyanobacterial photosystem II at 2.9-Å resolution and the role of quinones, lipids, channels and chloride. Nat. Struct. Mol. Biol. 2009;16(3):334–342. doi: 10.1038/nsmb.1559. [DOI] [PubMed] [Google Scholar]
- 179.Pisciotta J.M., Zou Y., Baskakov I.V. Role of the photosynthetic electron transfer chain in electrogenic activity of cyanobacteria. Appl. Microbiol. Biotechnol. 2011;91(2):377–385. doi: 10.1007/s00253-011-3239-x. [DOI] [PubMed] [Google Scholar]
- 180.Tanaka K., Tamamushi R., Ogawa T. Bioelectrochemical fuel-cells operated by the cyanobacterium, Anabaena variabilis. J. Chem. Technol. Biotechnol. Biotechnol. 1985;35(3):191–197. doi: 10.1002/jctb.280350304. [DOI] [Google Scholar]
- 181.Fu C.-C., Su C.-H., Hung T.-C., Hsieh C.-H., Suryani D., Wu W.-T. Effects of biomass weight and light intensity on the performance of photosynthetic microbial fuel cells with Spirulina platensis. Bioresour. Technol. 2009;100(18):4183–4186. doi: 10.1016/j.biortech.2009.03.059. [DOI] [PubMed] [Google Scholar]
- 182.Fu C.-C., Hung T.-C., Wu W.-T., Wen T.-C., Su C.-H. Current and voltage responses in instant photosynthetic microbial cells with Spirulina platensis. Biochem. Eng. J. 2010;52(2):175–180. doi: 10.1016/j.bej.2010.08.004. [DOI] [Google Scholar]
- 183.Raman K., Lan J.C.-W. Performance and kinetic study of photo microbial fuel cells (PMFCs) with different electrode distances. Appl. Energy. 2012;100:100–105. doi: 10.1016/j.apenergy.2012.03.011. [DOI] [Google Scholar]
- 184.Nishio K., Hashimoto K., Watanabe K. Light/electricity conversion by defined cocultures of Chlamydomonas and Geobacter. J. Biosci. Bioeng. 2013;115(4):412–417. doi: 10.1016/j.jbiosc.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 185.Yang Z., Pei H., Hou Q., Jiang L., Zhang L., Nie C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: nutrient, organics removal and bioenergy production. Chem. Eng. J. 2018;332:277–285. doi: 10.1016/j.cej.2017.09.096. [DOI] [Google Scholar]
- 186.Lobato J., González del Campo A., Fernández F.J., Cañizares P., Rodrigo M.A. Lagooning microbial fuel cells: a first approach by coupling electricity-producing microorganisms and algae. Appl. Energy. 2013;110:220–226. doi: 10.1016/j.apenergy.2013.04.010. [DOI] [Google Scholar]
- 187.Aiyer K.S. Synergistic effects in a microbial fuel cell between co-cultures and a photosynthetic alga Chlorella vulgaris improve performance. Heliyon. 2021;7(1) doi: 10.1016/j.heliyon.2021.e05935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Xiao L., Young E.B., Berges J.A., He Z. Integrated photo-bioelectrochemical system for contaminants removal and bioenergy production. Environ. Sci. Technol. 2012;46(20):11459–11466. doi: 10.1021/es303144n. [DOI] [PubMed] [Google Scholar]
- 189.Wu X.-y., Song T.-s., Zhu X.-j., Wei P., Zhou C.C. Construction and operation of microbial fuel cell with Chlorella vulgaris biocathode for electricity generation. Appl. Biochem. Biotechnol. 2013;171(8):2082–2092. doi: 10.1007/s12010-013-0476-8. [DOI] [PubMed] [Google Scholar]
- 190.Cai P.-J., Xiao X., He Y.-R., Li W.-W., Zang G.-L., Sheng G.-P., Hon-Wah Lam M., Yu L., Yu H.-Q. Reactive oxygen species (ROS) generated by cyanobacteria act as an electron acceptor in the biocathode of a bio-electrochemical system. Biosens. Bioelectron. 2013;39(1):306–310. doi: 10.1016/j.bios.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 191.Kokabian B., Gude V.G. Photosynthetic microbial desalination cells (PMDCs) for clean energy, water and biomass production. Environmental science. Processes & impacts. 2013;15(12):2178–2185. doi: 10.1039/c3em00415e. [DOI] [PubMed] [Google Scholar]
- 192.Gouveia L., Neves C., Sebastião D., Nobre B.P., Matos C.T. Effect of light on the production of bioelectricity and added-value microalgae biomass in a Photosynthetic Alga Microbial Fuel Cell. Bioresour. Technol. 2014;154:171–177. doi: 10.1016/j.biortech.2013.12.049. [DOI] [PubMed] [Google Scholar]
- 193.Saratale R.G., Kuppam C., Mudhoo A., Saratale G.D., Periyasamy S., Zhen G., Koók L., Bakonyi P., Nemestóthy N., Kumar G. Bioelectrochemical systems using microalgae – a concise research update. Chemosphere. 2017;177:35–43. doi: 10.1016/j.chemosphere.2017.02.132. [DOI] [PubMed] [Google Scholar]
- 194.Kim Y., Logan B.E. Simultaneous removal of organic matter and salt ions from saline wastewater in bioelectrochemical systems. Desalination. 2013;308:115–121. doi: 10.1016/j.desal.2012.07.031. [DOI] [Google Scholar]
- 195.Wang X., Feng Y., Liu J., Lee H., Li C., Li N., Ren N. Sequestration of CO2 discharged from anode by algal cathode in microbial carbon capture cells (MCCs) Biosens. Bioelectron. 2010;25(12):2639–2643. doi: 10.1016/j.bios.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 196.Das S., Das S., Das I., Ghangrekar M.M. Application of bioelectrochemical systems for carbon dioxide sequestration and concomitant valuable recovery. A review, Materials Science for Energy Technologies. 2019;2(3):687–696. doi: 10.1016/j.mset.2019.08.003. [DOI] [Google Scholar]
- 197.Jadhav D.A., Jain S.C., Ghangrekar M.M. Simultaneous wastewater treatment, algal biomass production and electricity generation in clayware microbial carbon capture cells. Appl. Biochem. Biotechnol. 2017;183(3):1076–1092. doi: 10.1007/s12010-017-2485-5. [DOI] [PubMed] [Google Scholar]
- 198.Pandit S., Nayak B.K., Das D. Microbial carbon capture cell using cyanobacteria for simultaneous power generation, carbon dioxide sequestration and wastewater treatment. Bioresour. Technol. 2012;107:97–102. doi: 10.1016/j.biortech.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 199.Zhou M., He H., Jin T., Wang H. Power generation enhancement in novel microbial carbon capture cells with immobilized Chlorella vulgaris. J. Power Sources. 2012;214:216–219. doi: 10.1016/j.jpowsour.2012.04.043. [DOI] [Google Scholar]
- 200.Chiu S.-Y., Kao C.-Y., Chen C.-H., Kuan T.-C., Ong S.-C., Lin C.-S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008;99(9):3389–3396. doi: 10.1016/j.biortech.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 201.De Schamphelaire L., Boeckx P., Verstraete W. Evaluation of biocathodes in freshwater and brackish sediment microbial fuel cells. Appl. Microbiol. Biotechnol. 2010;87(5):1675–1687. doi: 10.1007/s00253-010-2645-9. [DOI] [PubMed] [Google Scholar]
- 202.Xu B., Ge Z., He Z. Sediment microbial fuel cells for wastewater treatment: challenges and opportunities. Environ. Sci. J. Integr. Environ. Res.: Water Research & Technology. 2015;1(3):279–284. doi: 10.1039/C5EW00020C. [DOI] [Google Scholar]
- 203.Arias-Thode Y.M., Hsu L., Anderson G., Babauta J., Fransham R., Obraztsova A., Tukeman G., Chadwick D.B. Demonstration of the SeptiStrand benthic microbial fuel cell powering a magnetometer for ship detection. J. Power Sources. 2017;356:419–429. doi: 10.1016/j.jpowsour.2017.03.045. [DOI] [Google Scholar]
- 204.Song N., Yan Z., Xu H., Yao Z., Wang C., Chen M., Zhao Z., Peng Z., Wang C., Jiang H.-L. Development of a sediment microbial fuel cell-based biosensor for simultaneous online monitoring of dissolved oxygen concentrations along various depths in lake water. Sci. Total Environ. 2019;673:272–280. doi: 10.1016/j.scitotenv.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 205.He Z., Kan J., Mansfeld F., Angenent L.T., Nealson K.H. Self-Sustained phototrophic microbial fuel cells based on the synergistic cooperation between photosynthetic microorganisms and heterotrophic bacteria. Environ. Sci. Technol. 2009;43(5):1648–1654. doi: 10.1021/es803084a. [DOI] [PubMed] [Google Scholar]
- 206.Malik S., Drott E., Grisdela P., Lee J., Lee C., Lowy D.A., Gray S., Tender L.M. A self-assembling self-repairing microbial photoelectrochemical solar cell. Energy Environ. Sci. 2009;2(3):292–298. doi: 10.1039/B816417G. [DOI] [Google Scholar]
- 207.Bardarov I., Mitov M., Ivanova D., Hubenova Y. Light-dependent processes on the cathode enhance the electrical outputs of sediment microbial fuel cells. Bioelectrochemistry. 2018;122:1–10. doi: 10.1016/j.bioelechem.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Y., Noori J.S., Angelidaki I. Simultaneous organic carbon, nutrients removal and energy production in a photomicrobial fuel cell (PFC) Energy Environ. Sci. 2011;4(10):4340–4346. doi: 10.1039/C1EE02089G. [DOI] [Google Scholar]
- 209.Song X., Wang W., Cao X., Wang Y., Zou L., Ge X., Zhao Y., Si Z., Wang Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138011. [DOI] [PubMed] [Google Scholar]
- 210.Ren Y., Pan D., Li X., Fu F., Zhao Y., Wang X. Effect of polyaniline-graphene nanosheets modified cathode on the performance of sediment microbial fuel cell. J. Chem. Technol. Biotechnol. 2013;88(10):1946–1950. doi: 10.1002/jctb.4146. [DOI] [Google Scholar]
- 211.Jeon H.J., Seo K.-w., Lee S.H., Yang Y.-H., Kumaran R.S., Kim S., Hong S.W., Choi Y.S., Kim H.J. Production of algal biomass (Chlorella vulgaris) using sediment microbial fuel cells. Bioresour. Technol. 2012;109:308–311. doi: 10.1016/j.biortech.2011.06.039. [DOI] [PubMed] [Google Scholar]
- 212.Cao X., Huang X., Liang P., Xiao K., Zhou Y., Zhang X., Logan B.E. A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 2009;43(18):7148–7152. doi: 10.1021/es901950j. [DOI] [PubMed] [Google Scholar]
- 213.Kim Y., Logan B.E. Microbial desalination cells for energy production and desalination. Desalination. 2013;308:122–130. doi: 10.1016/j.desal.2012.07.022. [DOI] [Google Scholar]
- 214.Sevda S., Yuan H., He Z., Abu-Reesh I.M. Microbial desalination cells as a versatile technology: functions, optimization and prospective. Desalination. 2015;371:9–17. doi: 10.1016/j.desal.2015.05.021. [DOI] [Google Scholar]
- 215.Mehanna M., Saito T., Yan J., Hickner M., Cao X., Huang X., Logan B.E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy Environ. Sci. 2010;3(8):1114–1120. doi: 10.1039/C002307H. [DOI] [Google Scholar]
- 216.Tawalbeh M., Al-Othman A., Singh K., Douba I., Kabakebji D., Alkasrawi M. Microbial desalination cells for water purification and power generation: a critical review. Energy. 2020;209 doi: 10.1016/j.energy.2020.118493. [DOI] [Google Scholar]
- 217.Kokabian B., Ghimire U., Gude V.G. Water deionization with renewable energy production in microalgae - microbial desalination process. Renew. Energy. 2018;122:354–361. doi: 10.1016/j.renene.2018.01.061. [DOI] [Google Scholar]
- 218.Wen Q., Zhang H., Chen Z., Li Y., Nan J., Feng Y. Using bacterial catalyst in the cathode of microbial desalination cell to improve wastewater treatment and desalination. Bioresour. Technol. 2012;125:108–113. doi: 10.1016/j.biortech.2012.08.140. [DOI] [PubMed] [Google Scholar]
- 219.Zhang H., Wen Q., An Z., Chen Z., Nan J. Analysis of long-term performance and microbial community structure in bio-cathode microbial desalination cells. Environ. Sci. Pollut. Res. Int. 2016;23(6):5931–5940. doi: 10.1007/s11356-015-5794-7. [DOI] [PubMed] [Google Scholar]
- 220.Gude V.G. Desalination and sustainability - an appraisal and current perspective. Water Res. 2016;89:87–106. doi: 10.1016/j.watres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 221.Chou H.-H., Su H.-Y., Song X.-D., Chow T.-J., Chen C.-Y., Chang J.-S., Lee T.-M. Isolation and characterization of Chlorella sp. mutants with enhanced thermo- and CO2 tolerances for CO2 sequestration and utilization of flue gases. Biotechnol. Biofuels. 2019;12:251. doi: 10.1186/s13068-019-1590-9. 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wang A., Cheng H., Ren N., Cui D., Lin N., Wu W. Sediment microbial fuel cell with floating biocathode for organic removal and energy recovery. Front. Environ. Sci. Eng. 2012;6(4):569–574. doi: 10.1007/s11783-011-0335-1. [DOI] [Google Scholar]
- 223.Kabutey F.T., Ding J., Zhao Q., Antwi P., Quashie F.K., Tankapa V., Zhang W. Pollutant removal and bioelectricity generation from urban river sediment using a macrophyte cathode sediment microbial fuel cell (mSMFC) Bioelectrochemistry. 2019;128:241–251. doi: 10.1016/j.bioelechem.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 224.Guo F., Shi Z., Yang K., Wu Y., Liu H. Enhancing the power performance of sediment microbial fuel cells by novel strategies: overlying water flow and hydraulic-driven cathode rotating. Sci. Total Environ. 2019;678:533–542. doi: 10.1016/j.scitotenv.2019.04.439. [DOI] [PubMed] [Google Scholar]
- 225.Wang D.-B., Song T.-S., Guo T., Zeng Q., Xie J. Electricity generation from sediment microbial fuel cells with algae-assisted cathodes. Int. J. Hydrogen Energy. 2014;39(25):13224–13230. doi: 10.1016/j.ijhydene.2014.06.141. [DOI] [Google Scholar]
- 226.Song T.-S., Jiang H.-L. Effects of sediment pretreatment on the performance of sediment microbial fuel cells. Bioresour. Technol. 2011;102(22):10465–10470. doi: 10.1016/j.biortech.2011.08.129. [DOI] [PubMed] [Google Scholar]
- 227.Zhao Q., Ji M., Li R., Ren Z.J. Long-term performance of sediment microbial fuel cells with multiple anodes. Bioresour. Technol. 2017;237:178–185. doi: 10.1016/j.biortech.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 228.Hong S.W., Chang I.S., Choi Y.S., Chung T.H. Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresour. Technol. 2009;100(12):3029–3035. doi: 10.1016/j.biortech.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 229.Li W.-W., Yu H.-Q. Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol. Adv. 2015;33(1):1–12. doi: 10.1016/j.biotechadv.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 230.Yang X., Chen S. Microorganisms in sediment microbial fuel cells: ecological niche, microbial response, and environmental function. Sci. Total Environ. 2021;756 doi: 10.1016/j.scitotenv.2020.144145. [DOI] [PubMed] [Google Scholar]
- 231.Kokabian B., Gude V.G., Smith R., Brooks J.P. Evaluation of anammox biocathode in microbial desalination and wastewater treatment. Chem. Eng. J. 2018;342:410–419. doi: 10.1016/j.cej.2018.02.088. [DOI] [Google Scholar]
- 232.Kokabian B., Smith R., Brooks J.P., Gude V.G. Bioelectricity production in photosynthetic microbial desalination cells under different flow configurations. J. Ind. Eng. Chem. 2018;58:131–139. doi: 10.1016/j.jiec.2017.09.017. [DOI] [Google Scholar]
- 233.Kokabian B., Gude V.G. Sustainable photosynthetic biocathode in microbial desalination cells. Chem. Eng. J. 2015;262:958–965. doi: 10.1016/j.cej.2014.10.048. [DOI] [Google Scholar]
- 234.Arana T.J., Gude V.G. A microbial desalination process with microalgae biocathode using sodium bicarbonate as an inorganic carbon source. Int. Biodeterior. Biodegrad. 2018;130:91–97. doi: 10.1016/j.ibiod.2018.04.003. [DOI] [Google Scholar]
- 235.Yang Z., Zhang L., Nie C., Hou Q., Zhang S., Pei H. Multiple anodic chambers sharing an algal raceway pond to establish a photosynthetic microbial fuel cell stack: voltage boosting accompany wastewater treatment. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114955. [DOI] [PubMed] [Google Scholar]
- 236.Ghassemi Z., Slaughter G. Biological fuel cells and membranes. Membranes. 2017;7(1):3. doi: 10.3390/membranes7010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chiranjeevi P.V., Patil S.A. 2020. Microbial Fuel Cell Coupled with Microalgae Cultivation for Wastewater Treatment and Energy Recovery. [Google Scholar]
- 238.Shukla M., Kumar S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018;82:402–414. doi: 10.1016/j.rser.2017.09.067. [DOI] [Google Scholar]
- 239.Wu Y.-c., Wang Z.-j., Zheng Y., Xiao Y., Yang Z.-h., Zhao F. Light intensity affects the performance of photo microbial fuel cells with Desmodesmus sp. A8 as cathodic microorganism. Appl. Energy. 2014;116:86–90. doi: 10.1016/j.apenergy.2013.11.066. [DOI] [Google Scholar]
- 240.Bazdar E., Roshandel R., Yaghmaei S., Mardanpour M.M. The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresour. Technol. 2018;261:350–360. doi: 10.1016/j.biortech.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 241.Kakarla R., Min B. Sustainable electricity generation and ammonium removal by microbial fuel cell with a microalgae assisted cathode at various environmental conditions. Bioresour. Technol. 2019;284:161–167. doi: 10.1016/j.biortech.2019.03.111. [DOI] [PubMed] [Google Scholar]
- 242.Patil S.A., Harnisch F., Koch C., Hübschmann T., Fetzer I., Carmona-Martínez A.A., Müller S., Schröder U. Electroactive mixed culture derived biofilms in microbial bioelectrochemical systems: the role of pH on biofilm formation, performance and composition. Bioresour. Technol. 2011;102(20):9683–9690. doi: 10.1016/j.biortech.2011.07.087. [DOI] [PubMed] [Google Scholar]
- 243.Puig S., Serra M., Coma M., Cabré M., Balaguer M.D., Colprim J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010;101(24):9594–9599. doi: 10.1016/j.biortech.2010.07.082. [DOI] [PubMed] [Google Scholar]
- 244.Zhang E., Liu L., Cui Y. Effect of pH on the performance of the anode in microbial fuel cells. Adv. Mater. Res. 2013:884–888. [Google Scholar]
- 245.Fan Y., Hu H., Liu H. Sustainable power generation in microbial fuel cells using bicarbonate buffer and proton transfer mechanisms. Environ. Sci. Technol. 2007;41(23):8154–8158. doi: 10.1021/es071739c. [DOI] [PubMed] [Google Scholar]
- 246.Oliveira V.B., Simões M., Melo L.F., Pinto A.M.F.R. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013;73:53–64. doi: 10.1016/j.bej.2013.01.012. [DOI] [Google Scholar]
- 247.Gonzalez del Campo A., Lobato J., Cañizares P., Rodrigo M.A., Fernandez Morales F.J. Short-term effects of temperature and COD in a microbial fuel cell. Appl. Energy. 2013;101:213–217. doi: 10.1016/j.apenergy.2012.02.064. [DOI] [Google Scholar]
- 248.Yahampath Arachchige Don C.D.Y., Babel S. Effects of organic loading on bioelectricity and micro-algal biomass production in microbial fuel cells using synthetic wastewater. J. Water Proc. Eng. 2021;39 doi: 10.1016/j.jwpe.2020.101699. [DOI] [Google Scholar]
- 249.González del Campo A., Cañizares P., Rodrigo M.A., Fernández F.J., Lobato J. Microbial fuel cell with an algae-assisted cathode: a preliminary assessment. J. Power Sources. 2013;242:638–645. doi: 10.1016/j.jpowsour.2013.05.110. [DOI] [Google Scholar]
- 250.Xu C., Poon K., Choi M.M., Wang R. Using live algae at the anode of a microbial fuel cell to generate electricity. Environ. Sci. Pollut. Res. Int. 2015;22(20):15621–15635. doi: 10.1007/s11356-015-4744-8. [DOI] [PubMed] [Google Scholar]
- 251.He H., Zhou M., Yang J., Hu Y., Zhao Y. Simultaneous wastewater treatment, electricity generation and biomass production by an immobilized photosynthetic algal microbial fuel cell. Bioproc. Biosyst. Eng. 2014;37(5):873–880. doi: 10.1007/s00449-013-1058-4. [DOI] [PubMed] [Google Scholar]
- 252.Ribeiro V.R., Osorio H., Ulrich A.C., Rizzetti T.M., Barrios A.S., de Cassia de Souza Schneider R., Benitez L.B. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Proc. Eng. 2022;48:102882. doi: 10.1016/j.jwpe.2022.102882. [DOI] [Google Scholar]
- 253.Sharma M., Salama E.-S., Zhang P., Zhang L., Xing X., Yue J., Song Z., Nan L., Yujun S., Li X. Microalgae-assisted microbial fuel cells for electricity generation coupled with wastewater treatment: biotechnological perspective. J. Water Proc. Eng. 2022;49 doi: 10.1016/j.jwpe.2022.102966. [DOI] [Google Scholar]
- 254.Xiao L., Young E.B., Grothjan J.J., Lyon S., Zhang H., He Z. Wastewater treatment and microbial communities in an integrated photo-bioelectrochemical system affected by different wastewater algal inocula. Algal Res. 2015;12:446–454. doi: 10.1016/j.algal.2015.10.008. [DOI] [Google Scholar]
- 255.Rajesh P.P., Jadhav D.A., Ghangrekar M.M. Improving performance of microbial fuel cell while controlling methanogenesis by Chaetoceros pretreatment of anodic inoculum. Bioresour. Technol. 2015;180:66–71. doi: 10.1016/j.biortech.2014.12.095. [DOI] [PubMed] [Google Scholar]
- 256.Aditya L., Mahlia T.M.I., Nguyen L.N., Vu H.P., Nghiem L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022;838 doi: 10.1016/j.scitotenv.2022.155871. [DOI] [PubMed] [Google Scholar]
- 257.Morillas-España A., Pérez-Crespo R., Villaró-Cos S., Rodríguez-Chikri L., Lafarga T. Integrating microalgae-based wastewater treatment, biostimulant production, and hydroponic cultivation: a sustainable approach to water management and crop production. Front. Bioeng. Biotechnol. 2024;12 doi: 10.3389/fbioe.2024.1364490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Arango J., Contreras C., Crouchett-Catalán F., Gallardo Mejías J., Donoso-Bravo A., Muñoz R., Lesty Y., Olivares D., Jeison D. The potential of membrane-assisted microalgae-bacteria consortia for the treatment of real municipal sewage. J. Water Proc. Eng. 2023;56 doi: 10.1016/j.jwpe.2023.104527. [DOI] [Google Scholar]
- 259.Uduman N., Qi Y., Danquah M.K., Forde G.M., Hoadley A. Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J. Renew. Sustain. Energy. 2010;2(1) doi: 10.1063/1.3294480. [DOI] [Google Scholar]
- 260.Kim D.-G., La H.-J., Ahn C.-Y., Park Y.-H., Oh H.-M. Harvest of Scenedesmus sp. with bioflocculant and reuse of culture medium for subsequent high-density cultures. Bioresour. Technol. 2011;102(3):3163–3168. doi: 10.1016/j.biortech.2010.10.108. [DOI] [PubMed] [Google Scholar]
- 261.Carrillo-Reyes J., Barragán-Trinidad M., Buitrón G. Biological pretreatments of microalgal biomass for gaseous biofuel production and the potential use of rumen microorganisms: a review. Algal Res. 2016;18:341–351. doi: 10.1016/j.algal.2016.07.004. [DOI] [Google Scholar]
- 262.Bai M.-D., Chen C.-Y., Lu W.-C., Wan H.-P., Ho S.-H., Chang J.-S. Enhancing the oil extraction efficiency of Chlorella vulgaris with cell-disruptive pretreatment using active extracellular substances from Bacillus thuringiensis ITRI-G1. Biochem. Eng. J. 2015;101:185–190. doi: 10.1016/j.bej.2015.05.020. [DOI] [Google Scholar]
- 263.Nookwam K., Cheirsilp B., Maneechote W., Boonsawang P., Sukkasem C. Microbial fuel cells with Photosynthetic-Cathodic chamber in vertical cascade for integrated Bioelectricity, biodiesel feedstock production and wastewater treatment. Bioresour. Technol. 2022;346 doi: 10.1016/j.biortech.2021.126559. [DOI] [PubMed] [Google Scholar]
- 264.Dong Y., Qu Y., He W., Du Y., Liu J., Han X., Feng Y. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015;195:66–72. doi: 10.1016/j.biortech.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 265.Khandelwal A., Chhabra M., Yadav P. Performance evaluation of algae assisted microbial fuel cell under outdoor conditions. Bioresour. Technol. 2020;310 doi: 10.1016/j.biortech.2020.123418. [DOI] [PubMed] [Google Scholar]
- 266.He W., Dong Y., Li C., Han X., Liu G., Liu J., Feng Y. Field tests of cubic-meter scale microbial electrochemical system in a municipal wastewater treatment plant. Water Res. 2019;155:372–380. doi: 10.1016/j.watres.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 267.Liang P., Duan R., Jiang Y., Zhang X., Qiu Y., Huang X. One-year operation of 1000-L modularized microbial fuel cell for municipal wastewater treatment. Water Res. 2018;141:1–8. doi: 10.1016/j.watres.2018.04.066. [DOI] [PubMed] [Google Scholar]
- 268.Usher P.K., Ross A.B., Camargo-Valero M.A., Tomlin A.S., Gale W.F. An overview of the potential environmental impacts of large-scale microalgae cultivation. Biofuels. 2014;5(3):331–349. doi: 10.1080/17597269.2014.913925. [DOI] [Google Scholar]
- 269.Gressel J., van der Vlugt C.J.B., Bergmans H.E.N. Environmental risks of large scale cultivation of microalgae: mitigation of spills. Algal Res. 2013;2(3):286–298. doi: 10.1016/j.algal.2013.04.002. [DOI] [Google Scholar]
- 270.Ibuot A., Dean A.P., McIntosh O.A., Pittman J.K. Metal bioremediation by CrMTP4 over-expressing Chlamydomonas reinhardtii in comparison to natural wastewater-tolerant microalgae strains. Algal Res. 2017;24:89–96. doi: 10.1016/j.algal.2017.03.002. [DOI] [Google Scholar]
- 271.Yong X.-Y., Shi D.-Y., Chen Y.-L., Jiao F., Lin X., Zhou J., Wang S.-Y., Yong Y.-C., Sun Y.-M., OuYang P.-K., Zheng T. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014;152:220–224. doi: 10.1016/j.biortech.2013.10.086. [DOI] [PubMed] [Google Scholar]
- 272.Li S., Li X., Chang H., Zhong N., Ren N., Ho S.-H. Comprehensive insights into antibiotic resistance gene migration in microalgal-bacterial consortia: mechanisms, factors, and perspectives. Sci. Total Environ. 2023;901 doi: 10.1016/j.scitotenv.2023.166029. [DOI] [PubMed] [Google Scholar]
- 273.Raymond J.A., Remias D. Ice-binding proteins in a chrysophycean snow alga: acquisition of an essential gene by horizontal gene transfer. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fard M.B., Wu D. Potential interactive effect on biomass and bio-polymeric substances of microalgal-bacterial aerobic granular sludge as a valuable resource for sustainable development. Bioresour. Technol. 2023;376 doi: 10.1016/j.biortech.2023.128929. [DOI] [PubMed] [Google Scholar]
- 275.Cheng S., Xian W., Fu Y., Marin B., Keller J., Wu T., Sun W., Li X., Xu Y., Zhang Y., Wittek S., Reder T., Günther G., Gontcharov A., Wang S., Li L., Liu X., Wang J., Yang H., Xu X., Delaux P.-M., Melkonian B., Wong G.K.-S., Melkonian M. Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell. 2019;179(5):1057–1067.e14. doi: 10.1016/j.cell.2019.10.019. [DOI] [PubMed] [Google Scholar]
- 276.Eheneden I., Wang R., Zhao J. Antibiotic removal by microalgae-bacteria consortium: metabolic pathways and microbial responses. Sci. Total Environ. 2023;891 doi: 10.1016/j.scitotenv.2023.164489. [DOI] [PubMed] [Google Scholar]
- 277.Li B., Qiu Y., Song Y., Lin H., Yin H. Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics. Environ. Int. 2019;131 doi: 10.1016/j.envint.2019.105007. [DOI] [PubMed] [Google Scholar]
- 278.Amaro H.M., Salgado E.M., Nunes O.C., Pires J.C.M., Esteves A.F. Microalgae systems - environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023;337 doi: 10.1016/j.jenvman.2023.117678. [DOI] [PubMed] [Google Scholar]
- 279.Petrini S., Foladori P., Beghini F., Armanini F., Segata N., Andreottola G. How inoculation affects the development and the performances of microalgal-bacterial consortia treating real municipal wastewater. J. Environ. Manag. 2020;263 doi: 10.1016/j.jenvman.2020.110427. [DOI] [PubMed] [Google Scholar]
- 280.Raza N., Rizwan M., Mujtaba G. Bioremediation of real textile wastewater with a microalgal-bacterial consortium: an eco-friendly strategy. Biomass Conversion and Biorefinery. 2022 doi: 10.1007/s13399-022-03214-5. [DOI] [Google Scholar]
- 281.Verma K., Kumar P.K., Krishna S.V., Himabindu V. Phycoremediation of sewage-contaminated lake water using microalgae–bacteria Co-culture. Water Air Soil Pollut. 2020;231:1–16. [Google Scholar]
- 282.Robles Á., Capson-Tojo G., Gales A., Ruano V., Sialve B., Ferrer J., Steyer J.P. Microalgae-bacteria consortia in high-rate ponds for treating urban wastewater: elucidating the key state indicators under dynamic conditions. J. Environ. Manag. 2020;261 doi: 10.1016/j.jenvman.2020.110244. [DOI] [PubMed] [Google Scholar]
- 283.Jose S., Renuka N., Ratha S.K., Kumari S., Bux F. Bioprospecting of microalgae from agricultural fields and developing consortia for sustainable agriculture. Algal Res. 2024;78 [Google Scholar]
- 284.Jaiswal K.K., Kumar V., Vlaskin M.S., Sharma N., Rautela I., Nanda M., Arora N., Singh A., Chauhan P.K. Microalgae fuel cell for wastewater treatment: recent advances and challenges. J. Water Proc. Eng. 2020;38 doi: 10.1016/j.jwpe.2020.101549. [DOI] [Google Scholar]
- 285.Guo Y., Wang J., Shinde S., Wang X., Li Y., Dai Y., Ren J., Zhang P., Liu X. Simultaneous wastewater treatment and energy harvesting in microbial fuel cells: an update on the biocatalysts. RSC Adv. 2020;10:25874–25887. doi: 10.1039/d0ra05234e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Has data associated with your study been deposited into a publicly available repository? NO.
Has data associated with your study been deposited into a publicly available repository? No data was used for the research described in the article.
Data will be made available on request.