Abstract
The introduction of Human Papillomavirus (HPV) testing in cervical cancer screening enhanced the opportunity to introduce self-collection as an innovative approach to improve coverage rates. Validation and standardization of the pre-analytical and analytical procedures are crucial for the quality assurance of HPV tests on self-collected samples.
This study evaluated the analytical performance and the stability of self-collected vaginal samples resuspended in 5 mL of two non-alcohol-based media, eNat® and MSwab® compared to a professionally collected cervical sample, resuspended in 20 mL ThinPrep®, for the detection of high-risk HPV (hrHPV). The impact of the suspension volumes on analytical performance was also evaluated (2 and 5 ml).
A good analytical concordance in hrHPV detection in cervical and vaginal self-collected swabs suspended in 5 ml of both non-alcohol-based media was demonstrated (eNat®: 91.2 %, k = 0.821; MSwab®: 91.4 %; k = 0.798). A similar analytical performance was found for samples resuspended in 2 mL (eNat®: 92.9 %, k = 0.811; MSwab®: 92.9 %, k = 0.811) compared to cervical samples.
Good nucleic acid stability was demonstrated for vaginal samples stored at 20-25 °C and 37 °C for up to 4 weeks.
Results of this preliminary study support the introduction of these media for vaginal self-sampling-based prevention programs. Nevertheless, further research is necessary to evaluate clinical accuracy in larger settings.
Keywords: Human papillomavirus, HPV, Self-sampling, HPV testing, Cervical cancer, Suspension medium
Highlights
-
•
High-risk Human Papillomavirus (hrHPV) is the major cause of cervical cancer.
-
•
HPV test on self-samples as strategy to implement cervical cancer screening.
-
•
Importance of standardization of pre-analytical and analytical phases.
-
•
Evaluation of the best combination medium/volume for suspension of vaginal samples.
Abbreviations
- HPV
Human Papillomavirus
- hrHPV
high-risk Human Papillomavirus
- WHO
World Health Organization
- NILM
negative for intraepithelial lesion or malignancy
- ASCUS
Atypical squamous cells of undetermined significance
- LSIL
Low-grade squamous intraepithelial lesion
- ASCH
Atypical squamous cells suspicious for HSIL
- HSIL
High-grade squamous intraepithelial lesion
- AGC
Atypical glandular cells
- CIN1
cervical intraepithelial neoplasia grade 1
- CIN2
cervical intraepithelial neoplasia grade 2
- CIN3
cervical intraepithelial neoplasia grade 3
1. Introduction
Persistent infection of the cervical tract with high-risk Human Papillomavirus (hrHPV) has been recognized to be the major cause of cervical cancer. Cervical cancer is largely preventable through both HPV vaccination and screening for precursor lesions [1].
HPV nucleic acid testing of physician-collected cervical scrapes has demonstrated superior efficacy in reducing the incidence of cervical cancer compared to cervical cytology and its implementation in screening programs has been advocated by European guidelines [2]. Despite these advances in secondary prevention, there are still approximately 500,000 new cases of cervical cancer every year worldwide [3], occurring principally in developing countries, where women do not have access to screening programs, but also in industrialized countries in under- or never-screened women.
A promising strategy to implement cervical cancer screening programs in rural or low-resource settings as well as improving the participation rate of non-responders has been the implementation of self-collection-based screening [[4], [5], [6]]. The validation and standardization of both pre-analytical and analytical procedures of HPV testing on self-collected samples is however of fundamental importance in the quality assurance of HPV primary screening programs.
A protocol for the validation of human papillomavirus assays in combination with collections devices for HPV testing on self-samples has been published [7] allowing the evaluation of HPV test accuracy on self-samples as compared to physician-collected cervical scrapes. These studies allow to assess not only different sampling approaches using commercially available collection devices but also to extend the validation to different volumes and compositions of non-alcohol-based storage media for the resuspension of vaginal self-collected samples. Previously published validation studies of HPV tests on self-collected vaginal samples, using different vaginal collection devices, have shown great variability in the resuspension procedure used for vaginal samples, in terms of time from collection to sample processing, resuspension volume and type of liquid media being used [[8], [9], [10]].
Clinician-collected cervical samples are generally resuspended in 20 mL of ThinPrep® or PreservCyt® (Hologic, USA), an alcohol-based medium, which allows both HPV DNA molecular testing and liquid-based cytology for the triage of HPV-positive women from the same initial sample. ThinPrep® is however presently available only in 20 mL vials containing 60 % methanol, making it flammable and toxic (UN1993 Dangerous Good), with specialised handling and packaging requirements for air transportation. These aspects, together with the higher costs of alcohol-based media, represent important constraints, particularly in low-middle-income countries, making it unsuitable for vaginal sample resuspension, particularly as the latter is not an appropriate sample type for its use in cytology triage.
eNat® (Copan Italia Spa, Brescia, Italy) is a transport medium for the preservation and stabilization of nucleic acids compatible with numerous molecular assays and previously used for HPV molecular detection [11,12]. It also has the advantage of inactivating microbial agents and denaturing proteins, allowing safer sample handling in the laboratory. MSwab® medium (Copan Italia Spa, Brescia, Italy) is compatible with both bacterial and viral culture as well as nucleic acids detection. Moreover, it allows rapid nucleic acid heat extraction, thus reducing the time and costs of HPV molecular testing.
This pilot study aimed to evaluate the concordance of HPV testing of self-collected vaginal samples suspended in two alternative non-alcohol-based media, eNat® and MSwab®, using two different suspension volumes, 5 mL vs 2 mL, compared to the reference clinician-collected cervical samples suspended in 20 mL of ThinPrep®. Furthermore, viral nucleic acid stability of resuspended vaginal samples in both eNat® and MSwab® was evaluated following storage at both room temperature (RT) and 37 °C, over a timeframe of 4 weeks.
2. Materials and methods
2.1. Study design and samples collection
The study was conducted according to documentation approved by the Ethics Committee of the University of Milano-Bicocca (Protocol n. 0037320/2017 and subsequent update 0086409/2018).
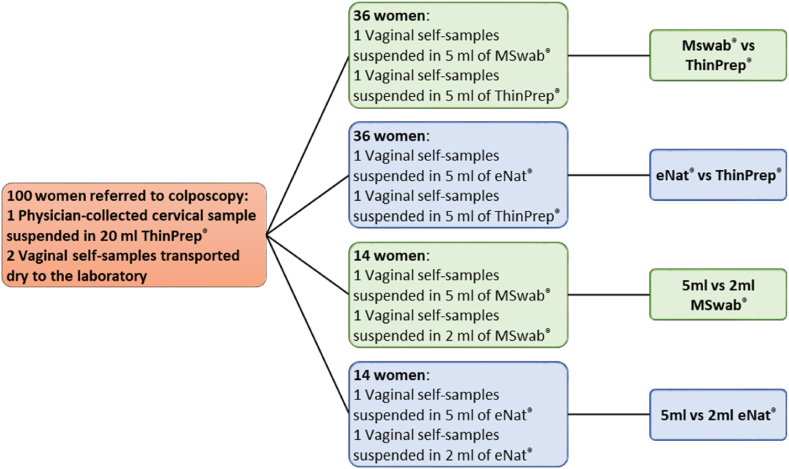
A clinician-taken cervical sample and 2 self-collected vaginal swabs were obtained from 100 women referred to colposcopy at Fondazione IRCCS San Gerardo dei Tintori (Monza, Italy) following a recent abnormal cervical cytology.
Women who agreed to participate in the study by the signature of an informed consent at the time of the colposcopy visit were instructed to collect 2 vaginal swabs using FLOQSwab® 552.80 (Copan Italia Spa, Brescia, Italy), prior to gynaecological examination, while cervical swabs were taken by the physician before performing colposcopy, using L-shaped Endo/Esocervical FLOQSwab® (Copan Italia Spa, Brescia, Italy) and resuspended immediately into 20 ml of ThinPrep® (Hologic, USA). All samples, including dry vaginal swabs, were transported within 24 h from collection to the Clinical Microbiology Laboratory of the University of Milano-Bicocca, Italy.
Women underwent biopsy or conization based on the outcome of the colposcopy and on the clinical judgment. Cytological lesions were classified according to the Bethesda system [13], whilst outcomes of histological analysis according to the World Health Organization (WHO) histological classification of tumours [14].
2.2. Pre-analytical samples processing and HPV testing
On arrival at the laboratory, cervical samples were vortexed for 30 s and aliquots of 1.5 ml were dispensed in sterile cryotubes and stored at −20 °C until testing.
Vaginal self-samples arrived dry at the laboratory where they were suspended in the different suspension media (ThinPrep®, eNat® and MSwab®) as described in Fig. 1. Arbitrarily, the first and second self-collected vaginal swabs were suspended respectively in the alternative non-alcohol based medium and ThinPrep® in the first two sets of 36 women. For the evaluation of the two different volumes of the alternative media, the first vaginal swab was suspended in 5 mL and the second self-sample in the 2 mL volume, respectively.
Fig. 1.
Study design.
200 μl of all sample types were used to perform nucleic acid extraction using STARMag 96 × 4 Universal Cartridge Kit (Seegene, Korea) on the Nimbus platform. The automated workstation also allowed the preparation of the real-time PCR assay Anyplex™II HR HPV (Seegene, Korea). The kit was able to individually detect 14 different genotypes of hrHPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) and a cellular gene target by melting curves analysis. Real-time assays were performed on the CFX96 (Bio-Rad, Hercules, USA) with 5 μl of template DNA in a total volume of 20 μl. As reported by the manufacturer, if sample positivity is detected before the first cyclic Catcher Melting Temperature Analysis (cyclic-CMTA) after the first 30 cycles of amplification, the result is indicated as “+++“; if positivity is detected between the first and the second cyclic-CMTA (30 < Amplification Cycles <40) as “++“; if between the second and the third cyclic-CMTA (40 < Amplification Cycles <50) as “+“. Samples with an invalid result (no detection of the cellular gene or cellular gene detected with just “+“) were retested and excluded from the analysis if the invalidity was confirmed.
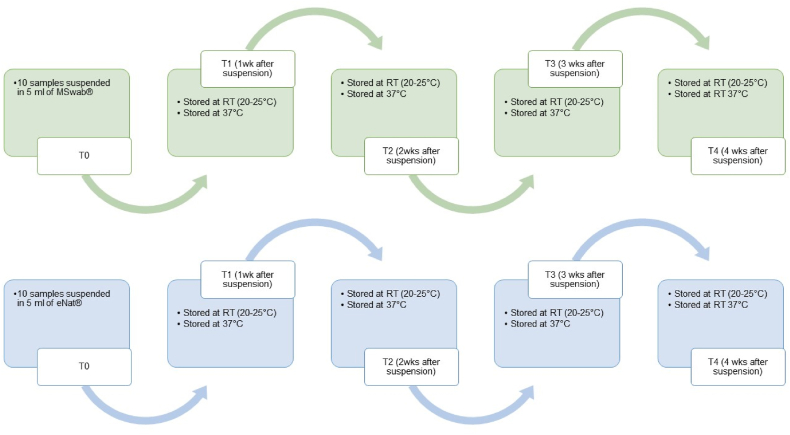
2.3. Evaluation of nucleic acid stability
In order to evaluate nucleic acid stability of 10 vaginal self-samples suspended in 5 ml of MSwab® and eNat®, aliquots of 0.4 mL of self-collected vaginal samples were dispensed in sterile cryotubes and stored at either RT (20–25 °C) and 37 °C until further testing. Nucleic acids were re-extracted starting from a new sample aliquot and hrHPV testing repeated at the time points and storage conditions described in Fig. 2.
Fig. 2.
Time points and storage conditions of vaginal self-samples suspended in MSwab® and eNat® prior to testing.
2.4. Statistical analysis
Agreement between results of HPV testing of different types of samples was evaluated with Cohen's kappa (κ) statistics and defined as slight (0.00 < k < 0.20), fair (0.20 < k < 0.40), moderate (0.41 < k < 0.60), substantial (0.61 < k < 0.80) and almost perfect (0.81 < k < 1.00).
3. Results
3.1. Study population
100 women were enrolled between January 2020 and February 2022 as part of this pilot study. Three patients were excluded from the analysis: 2 of them had a reduced number of cells in the professionally collected cervical sample, resulting in an invalid HPV test result, while the other invalid result was obtained in a vaginal swab suspended in MSwab®. In total, the analysis of the results was performed on samples from 97 women (49 with one of the vaginal swabs suspended in 5 ml of MSwab® and 48 with one vaginal specimen suspended in 5 ml of eNat®).
Overall, forty-one women had a positive colposcopy examination, while in 56 no abnormalities were found. Table 1 reports the clinical data of the 97 women evaluated in this pilot study.
Table 1.
Clinical data of patients enrolled in the study.
| n (%) | |
|---|---|
| Cytology | Tot. 97 |
| HSIL | 14 (14.4) |
| ASCH | 3 (3.1) |
| LSIL | 36 (37.1) |
| ASCUS | 19 (19.6) |
| AGC | 2 (2.1) |
| NILM | 23 (23.7) |
| Biopsy/Conisation | Tot. 18 |
| Cervical cancer | 1 (5.6) |
| CIN3 | 9 (50.0) |
| CIN2 | 2 (11.1) |
| CIN1 | 5 (27.7) |
| Negative | 1 (5.6) |
3.2. hrHPV positivity and genotypes distribution: evaluation of alternative media
Among women whose self-collected vaginal samples were suspended in 5 ml of ThinPrep® and MSwab®, 23/35 (71.4 %) cervical samples and 26/35 (74.3 %) vaginal swabs suspended in each of the two media were found to be hrHPV-positive. Among cervical samples, 8/23 (34.8 %) women had single infections and 15/23 (65.2 %) had multiple infections. 8/26 (30.8 %) vaginal specimens suspended in ThinPrep® showed single infections and 18/26 (69.2 %) multiple infections, while those suspended in MSwab® showed 7 single infections and 19 multiple infections.
Among those with vaginal self-specimens suspended in 5 ml of ThinPrep® and eNat®, the hrHPV positivity rate in cervical samples was 52.9 % (18/34), while in vaginal self-samples suspended in each of the two media was 61.8 % (21/34). The distribution of co-infections was slightly different among the three sample types: 13 single and 5 multiple infections were detected in cervical specimens, 13 single and 8 multiple infections in vaginal specimens suspended in ThinPrep® and 11 single and 10 multiple infections in vaginal swabs suspended in eNat®.
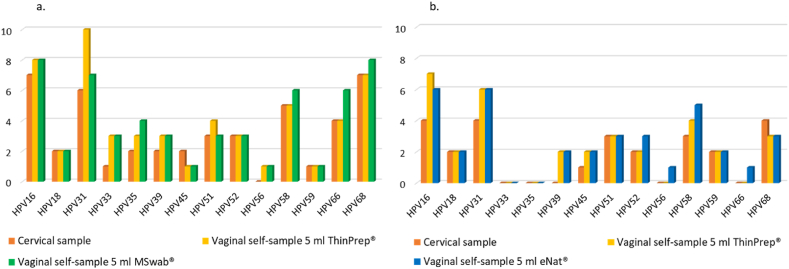
HPV16 and HPV31 were the genotypes most frequently detected. Fig. 3 and Supplementary Table 1 show the complete hrHPV genotypes distribution in cervical and vaginal self-samples in both groups of women. Among vaginal self-samples resuspended in MSwab®, 20/35 (57.1 %) samples showed the presence of the same hr-HPV types found in both the self-collected vaginal sample resuspended in ThinPrep® and in the cervical sample. A concordance for at least one hrHPV genotype among the three different types of samples was observed in 11/35 (31.4 %). On the contrary, among the 35 triplets of samples, 4 (11.4 %) have shown a discordant positive/negative result at least in one of the sample types. In 3 of these cases, the positivity found was related to just one ‘+‘, meaning that this positivity is associated with a very high Ct value ranging from 40 to 50 (Supplementary Table 1). Regarding the vaginal self-collected samples resuspended in eNat®, 24/34 (70.6 %) triplets of samples showed the presence of the same hrHPV types and 7/34 (20.6 %) demonstrated a concordance for at least one hrHPV genotype. Three triplets of samples (8.8 %) have shown a discordant result (positive/negative) and in one case the positivity found was associated with just one ‘+‘.
Fig. 3.
hrHPV genotypes distribution in cervical and vaginal self-samples suspended in 5 ml of ThinPrep® and 5 ml MSwab® (a) and 5 ml ThinPrep® and 5 ml of eNat® (b).
3.3. Analytical agreement between cervical and vaginal samples: evaluation of alternative media
As shown in Table 2, the concordance of hrHPV detection of cervical samples and vaginal self-collected swabs suspended in 5 ml of MSwab® and the same volume of ThinPrep® was demonstrated to be good (with k ranging from k = 0.798 to k = 0.821) irrespective of the medium used.
Table 2.
Agreement in hrHPV detection in vaginal self-samples suspended in 5 ml of ThinPrep® and 5 ml of MSwab® (a) and 5 ml of ThinPrep® and 5 ml of eNat® (b) as compared to the reference professionally collected cervical sample.
| a. |
Vaginal self-sample 5 ml ThinPrep® |
Vaginal self-sample 5 ml MSwab® |
||
| Overall concordance n (%) |
k (95 % c.i.) |
Overall concordance n (%) |
k (95 % c.i.) |
|
| Cervical sample |
31 (91.2) |
0.821 (0.631–1.000) |
31 (91.2) |
0.821 (0.631–1.000) |
| b. |
Vaginal self-sample 5 ml ThinPrep® |
Vaginal self-sample 5 ml eNat® |
||
| Overall concordance n (%) |
k (95 % c.i.) |
Overall concordance n (%) |
k (95 % c.i.) |
|
| Cervical sample | 32 (91.4) | 0.798 (0.583–1.000) | 32 (91.4) | 0.798 (0.583–1.000) |
When comparing the self-collected swabs, almost no differences were obtained between samples suspended in ThinPrep® and in the alternative media (5 mL MSwab® vs 5 mL ThinPrep®: 94.3 % [33/35]; k [95 % c.i.]: 0.850 [0.650–1.000]; 5 mL eNat® vs 5 mL ThinPrep®: 100.0 % [34/34]; k [95 % c.i.]: 1.000 [1.000–1.000]).
3.4. hrHPV positivity and genotypes distribution: evaluation of alternative volumes
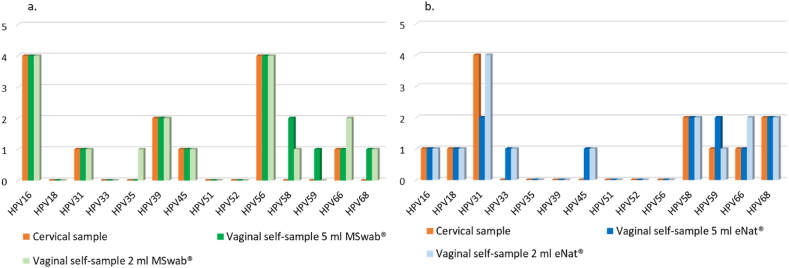
When considering the group of women whose vaginal specimens were suspended in both 2 and 5 ml of MSwab®, 10/14 (71.4 %) cervical samples and 11/14 (78.6 %) vaginal swabs suspended in the volumes of MSwab® were hrHPV-positive. Cervical specimens showed 8 single infections and 2 co-infections, while vaginal specimens suspended in 2 and 5 ml of MSwab® showed 7 single and 4 multiple infections.
In women with vaginal self-samples placed in 2 and 5 ml of eNat®, 10/14 cervical samples, 9/14 vaginal specimens suspended in 5 ml of eNat® and 11/14 vaginal swabs suspended in 2 ml of eNat® were hrHPV-positive. In the case of the two additional women whose vaginal specimen was hrHPV positive if suspended in 2 ml, but not in 5 ml of eNat®, the cervical samples were positive for HPV31 with “+” or “++” and the relative vaginal swabs suspended in 2 ml were HPV31 positive with “+“. 8 single infections and 2 multiple hrHPV infections were detected in cervical samples; 7 single infections and 4 co-infections on vaginal swabs suspended in 2 ml of eNat® and 4 single and 4 multiple infections on vaginal swabs suspended in 5 ml of eNat®.
hrHPV genotypes distribution in the two groups of enrolled patients is shown in Fig. 4 and Supplementary Table 2.
Fig. 4.
hrHPV genotypes distribution in cervical and vaginal self-samples suspended in 5 ml and 2 ml of MSwab® and 5 ml 2 ml of eNat®.
3.5. Analytical agreement between cervical and vaginal samples: evaluation of alternative volumes
In women with vaginal specimens suspended in 2 and 5 ml of MSwab®, an almost perfect concordance was demonstrated (k = 0.811) between the cervical sample and the vaginal swabs suspended in the two different volumes.
In the group of patients with double self-collection performed in the two volumes of eNat®, the agreement was higher when 2 ml instead of 5 ml of medium was used (92.9 %, k = 8.111 vs 78.6 %, k = 0.512), probably related to low hrHPV copies number as previously described.
Data are reported in Table 3.
Table 3.
Agreement in hrHPV detection in vaginal self-samples suspended in 5 ml and 2 ml of MSwab® (a) and 5 ml 2 ml of eNat® (b).
| a. |
Vaginal self-sample 2 ml MSwab® |
Vaginal self-sample 5 ml MSwab® |
||
| Overall concordance n (%) |
k (95 % c.i.) |
Overall concordance n (%) |
k (95 % c.i.) |
|
| Cervical sample |
13 (92.9) |
0.811 (0.460–1.000) |
13 (92.9) |
0.811 (0.460–1.000) |
| b. |
Vaginal self-sample 2 ml eNat® |
Vaginal self-sample 5 ml eNat® |
||
| Overall concordance n (%) |
k (95 % c.i.) |
Overall concordance n (%) |
k (95 % c.i.) |
|
| Cervical sample | 13 (92.9) | 0.811 (0.460–1.000) | 11 (78.6) | 0.512 (0.037–0.987) |
3.6. Stability
Good stability for up to 4 weeks was demonstrated for vaginal samples suspended in MSwab® and eNat® at room temperature (20–25 °C) and 37 °C. The results of HPV testing at the different time points and storage conditions are shown in Table 4. As reported in Table 4, the discrepancies are mainly related to a low number of viral copies with hrHPV detection with “+” using Anyplex™II HR HPV.
Table 4.
hrHPV positivity in cervical samples and vaginal self-specimens at the different time points and storage conditions suspended in MSwab® (a) and eNat® (b).
| (a) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSwab® | Clinical Data | Results at T0 | Results after 1 week of storage | Results after 2 weeks of storage | Results after 3 weeks of storage | Results after 4 weeks of storage | ||||||
| Sample ID | Colposcopy | Cytology | Histology | Vaginal self-sample | 20–25 °C | 37 °C | 20–25 °C | 37 °C | 20–25 °C | 37 °C | 20–25 °C | 37 °C |
| MO211 | NEG | LSIL | – | 33+ 59++ |
33+ 59++ |
33++ 59++ |
16+ 33+ 59++ |
33++ 59++ |
16+ 33+ 59++ |
33++ 59++ |
16+ 33 + 59++ |
33++ 59++ |
| MO213 | POS | HSIL | CIN 3 | 16++ | 16+++ | 16+++ | 16++ | 16++ | 16++ | 16++ | 16++ | 16++ |
| MO214 | NEG | LSIL | – | 16+ 68+ |
68+ | 68+ | 68+ | NEG | NEG | NEG | 68+ | 68+ |
| MO216 | NEG | LSIL | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO14 T24 | NEG | NILM | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO218 | NEG | NILM | – | 16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ |
16+++ 51+++ | 16+++ 51+++ |
| MO219 | NEG | ASCUS | – | 18++ 33++ |
18++ 33++ |
18++ 33++ |
18++ 33++ |
18++ 33++ |
18++ 33++ |
18++ 33++ |
18++ 33++ | 18++ 33++ |
| MO220 | POS | ASCUS | – | 31++ 66+ 68+ |
31++ 68+ |
31++ 68+ |
31++ 66+ 68+ |
31++ 66+ 68+ |
31++ 68+ |
31++ 68+ |
31+++ 68+ |
31+++ 68+ |
| MO221 | NEG | ASCUS | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO222 | NEG | NILM | – | 31+ 68+++ |
31+ 68++ |
68++ | 31+ 68++ |
31+ 68++ |
31+ 68++ |
31+ 68++ |
31++ 68++ | 31++ 68+++ |
| (b) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eNat® | Clinical Data | Results at T0 | Results after 1 week of storage | Results after 2 weeks of storage | Results after 3 weeks of storage | Results after 4 weeks of storage | ||||||
| Sample ID | Colposcopy | Cytology | Histology | Vaginal self-sample | 20–25 °C | 37 °C | 20–25 °C | 37 °C | 20–25 °C | 37 °C | 20–25 °C | 37 °C |
| MO235 T6 | NEG | LSIL | – | 16+++ | 16+++ 18+ |
16+++ | 16++ | 16+++ 18+ |
16+++ | 16+++ | 16++ | 16+++ 18+ |
| MO269 | POS | ASCUS | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO270 | NEG | ASCUS | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO230 T12 | NEG | NILM | – | 31+ 52++ |
31+ 52++ |
31+ 52++ |
52++ | 31+ 52++ |
31+ 52++ |
31+ 52++ |
52++ | 52++ |
| MO222 T12 | NEG | NILM | – | 52+ 68+++ |
68+++ | 52+ 68+++ |
68++ | 68++ | 68+++ | 68+++ | 68+ | 68+++ |
| MO271 | POS | ASCUS | – | 59+ | 59+ | 59+ | NEG | 59+ | 59+ | 59+ | NEG | 59+ |
| MO272 | POS | LSIL | – | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| MO239 T6 | NEG | NILM | – | NEG | NEG | 52+ | NEG | NEG | NEG | NEG | NEG | NEG |
| MO273 | POS | HSIL | CIN 3 | 16++ | 16+++ | 16+++ | 16++ | 16+++ | 16+++ | 16+++ | 16++ | 16+++ 68+ |
4. Discussion
In 2021, 17 countries recommended the use of HPV tests in combination with self-sampling devices in organised screening programs, while 10 countries were conducting pilot studies on self-collected samples [15]. The introduction of self-sampling-based HPV screening programs is an opportunity to increase coverage rate by reaching non-attendant women in countries with ongoing organised screening programs, but most of all to set up new procedures in low-middle income countries where resources are limited.
In order to evaluate the impact of different suspension media and volumes on HPV test conducted on self-collected samples, we investigated the performance of two non-alcohol-based media, eNat® and MSwab®, and two suspension volumes, 5 mL vs 2 mL of each alternative medium by assessing the analytical agreement with the clinician-collected cervical sample suspended in 20 mL of ThinPrep® from the same woman.
In this study, vaginal self-collected samples were transported dry to the laboratory where they were resuspended in the evaluated media. The use of dry swabs is a suitable solution to reduce costs and avoid the risk of leakage during transport. Costs related to vaginal specimens’ management are further reduced on using non-alcohol-based media for the resuspension of the vaginal swabs and by reducing the resuspension volume compared to that used for cervical samples.
Specific protocols to evaluate the performance of HPV tests in combination with self-collection devices have been introduced [7]. Optimal sample management is a crucial step to assure sample adequacy, particularly in the case of self-collection. Technical analysis to evaluate the best workflow procedure is required, as recently underlined by Arbyn and colleagues [16]. Up to now, self-collection procedures, volumes and types of suspension media have varied across different settings and just a few studies have been conducted to evaluate the impact of pre-analytical workflow on the HPV test result on clinical samples.
The different chemical compositions of the suspension media may influence the results of the test. Some previous studies have evaluated the impact of different suspension media and volumes on HPV test results [17,18] using artificially HPV-spiked samples. Badman and colleagues tested serial dilutions of HPV-positive cell lines with 5 different suspension media and suggested that MSwab® could be considered a valid alternative to ThinPrep® [17]. The results of our preliminary analysis confirm the good analytical performance of MSwab® in HPV detection also on clinical samples and further suggest that eNat® could also be considered as a good alternative to ThinPrep®. Furthermore, in a previous published study from our group conducted on clinical specimens, the analytical performance for HPV detection on vaginal samples collected with FLOQSwab® resuspended in 5 ml eNat® was demonstrated to be comparable to that of FLOQSwab® in 5 ml ThinPrep® [19].
The potential impact of the suspension volume on the clinical performance of vaginal specimens for the detection of high-grade cervical lesions has been discussed by Inturrisi and colleagues [20] and further confirmed by Arbyn and colleagues [21]. In a more recent study, FLOQSwab® and Evalyn®Brush were suspended in different volumes of ThinPrep® spiked with various concentrations of HPV16-positive cell lines. The resuspension in a volume of 5 ml or less was demonstrated to maximize HPV detection [18]. In other studies, conducted in a colposcopy setting, paired cervical and vaginal samples were collected and suspended in different types and volumes of media underlining the effect of these factors in terms of clinical accuracy [22,23]. Furthermore, a recent study conducted by our group demonstrated a similar accuracy in HPV detection of a vaginal sample collected using FLOQSwab® suspended in 5 ml of ThinPrep® as compared to clinician-collected scrape in a group of women referred to colposcopy [24]. In the current study, a concordance of over 91 % was found between vaginal swabs suspended in 5 ml of the three tested media (ThinPrep®, MSwab® and eNat®) and cervical specimens.
Looking at the comparison of the two suspension volumes of MSwab® and eNat®, no differences were detected in the analytical performance of MSwab®, while the use of 2 ml of eNat® seemed to improve the analytical sensitivity of the method as compared to the higher 5 mL volume; however, the small sample size of women evaluated in this pilot study does not allow to make definitive conclusions. A previously published paper already evaluated the performance of 2 ml of eNat® in a screening setting [25]. Furthermore, in the present study, the discordances were detected in two women in case of a low number of copies with HPV31 detected with “+” in vaginal swabs suspended in 2 ml of the medium and no HPV detected in those suspended in 5 ml. One of the two women in whom discordances were detected had an ASCUS cytology and a negative colposcopy outcome, the other a positive colposcopy outcome and an ASCH cytology. One of the two discordances could be considered as not clinically relevant. Since the comparison was performed in a small group of women, future studies on a larger number of participants will be necessary to further investigate the potential impact of suspension volume on the analytical and clinical performance of vaginal swabs. Moreover, the data need to be better correlated with the clinical outcomes of the patients to eventually establish different cut-off values to define HPV positivity in self-collected samples as compared to cervical swabs.
Some previous studies on clinician-collected cervical samples have investigated the stability of nucleic acids in SurePath® [26] and PreservCyt® [27]. Most of the studies conducted on vaginal samples, on the contrary, focused on demonstrating the stability of dry samples to highlight the possibility of transporting samples without any suspension media to reduce costs and improve safety [28,29]. Our preliminary results on real clinical samples, using clinician-collected cervical samples as reference, indicate that MSwab® and eNat® may offer two valid alternatives for the resuspension of self-collected samples because they allow for adequate preservation of nucleic acids for up to 4 weeks at room temperature (20–25 °C) and 37 °C. The small differences across the time points are mainly related to samples with low copy numbers (detected as “+” by the assay) which are likely to result from transient infections, not significant for the development of high-grade cervical lesions. Interestingly, no invalid results were obtained at the different time points underlining the good preservation of nucleic acids in MSwab® and eNat®. Future studies using quantitative real-time PCR assay may allow a more precise evaluation of nucleic acids’ stability at the different storage conditions.
5. Conclusions
These preliminary results demonstrated the good performance of MSwab® and eNat® as alternative media to ThinPrep® for the suspension of vaginal specimens for HPV detection.
The main limitation of this work is the reduced sample size. Since the preliminary results are promising, future analysis will be performed to evaluate the best combination of medium/volume for suspension of vaginal samples in order to achieve an accurate and cost-effective strategy for the implementation of self-sampling in cervical cancer screening programs.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics statement
The study was conducted according to documentation approved by the Ethics Committee of the University of Milano-Bicocca (Protocol n. 0037320/2017 and subsequent update 0086409/2018). Women agreed to participate to the study by the signature of a written informed consent.
Data availability statement
Final study datasets generated by the study are stored locally and securely at the University of Milano-Bicocca. Anonymized data will be available by request to the corresponding author on a case-by-case basis pending approval by the University of Milano-Bicocca.
CRediT authorship contribution statement
Chiara Giubbi: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Marianna Martinelli: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Data curation. Maria Letizia Di Meo: Writing – review & editing, Data curation. Ruth Chinyere Njoku: Writing – review & editing, Methodology, Data curation. Federica Perdoni: Writing – review & editing, Methodology. Robert Fruscio: Writing – review & editing. Fabio Landoni: Writing – review & editing. Clementina Elvezia Cocuzza: Writing – review & editing, Supervision, Project administration, Investigation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Clementina Elvezia Cocuzza received research funding or gratis consumables to support research from the following commercial entities in the last 3 years: BD, Seegene, Copan, Novosanis and Hiantis.
Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The study group received free vaginal self-sample collection devices and suspension media (MSwab® and eNat®) from Copan Italia Spa (Brescia, Italy), and a STARMag 96X4 Universal Cartridge Kit and Anyplex™II HR HPV kits from Seegene (Korea).
The authors want to thank Santina Castriciano for her support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31032.
Contributor Information
Chiara Giubbi, Email: chiara.giubbi@unimib.it.
Marianna Martinelli, Email: marianna.martinelli@unimib.it.
Maria Letizia Di Meo, Email: marialetizia.dimeo@gmail.com.
Ruth Chinyere Njoku, Email: njokuruthchinyere@gmail.com.
Federica Perdoni, Email: federica.perdoni@unimib.it.
Robert Fruscio, Email: robert.fruscio@unimib.it.
Fabio Landoni, Email: fabio.landoni@unimib.it.
Clementina Elvezia Cocuzza, Email: clementina.cocuzza@unimib.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bouvard V., Wentzensen N., Mackie A., Berkhof J., Brotherton J., Giorgi-Rossi P., Kupets R., Smith R., Arrossi S., Bendahhou K., Canfell K., Chirenje Z.M., Chung M.H., Del Pino M., de Sanjosé S., Elfström M., Franco E.L., Hamashima C., Hamers F.F., Herrington C.S., Murillo R., Sangrajrang S., Sankaranarayanan R., Saraiya M., Schiffman M., Zhao F., Arbyn M., Prendiville W., Indave Ruiz B.I., Mosquera-Metcalfe I., Lauby-Secretan B. The IARC perspective on cervical cancer screening. N. Engl. J. Med. 2021 Nov 11;385(20):1908–1918. doi: 10.1056/NEJMsr2030640. PMID: 34758259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyrgiou M., Arbyn M., Bergeron C., Bosch F.X., Dillner J., Jit M., Kim J., Poljak M., Nieminen P., Sasieni P., Kesic V., Cuzick J., Gultekin M. Cervical screening: ESGO-EFC position paper of the European society of gynaecologic oncology (ESGO) and the European federation of colposcopy (EFC) Br. J. Cancer. 2020 Aug;123(4):510–517. doi: 10.1038/s41416-020-0920-9. Epub 2020 Jun 8. PMID: 32507855; PMCID: PMC7434873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin S., Gao K., Gu S., You L., Qian S., Tang M., Wang J., Chen K., Jin M. Worldwide trends in cervical cancer incidence and mortality, with predictions for the next 15 years. Cancer. 2021 Nov 1;127(21):4030–4039. doi: 10.1002/cncr.33795. Epub 2021 Aug 9. PMID: 34368955. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M., Castle P.E., Schiffman M., Wentzensen N., Heckman-Stoddard B., Sahasrabuddhe V.V. Meta-analysis of agreement/concordance statistics in studies comparing self- versus clinician-collected samples for HPV testing in cervical cancer screening. Int. J. Cancer. 2022 Feb;18 doi: 10.1002/ijc.33967. Epub ahead of print. PMID: 35179777. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M., Smith S.B., Temin S., Sultana F., Castle P. Collaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. BMJ. 2018 Dec 5;363:k4823. doi: 10.1136/bmj.k4823. PMID: 30518635; PMCID: PMC6278587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M., Verdoodt F., Snijders P.J., Verhoef V.M., Suonio E., Dillner L., Minozzi S., Bellisario C., Banzi R., Zhao F.H., Hillemanns P., Anttila A. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014 Feb;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. Epub 2014 Jan 14. PMID: 24433684. [DOI] [PubMed] [Google Scholar]
- 7.Arbyn M., Peeters E., Benoy I., Vanden Broeck D., Bogers J., De Sutter P., Donders G., Tjalma W., Weyers S., Cuschieri K., Poljak M., Bonde J., Cocuzza C., Zhao F.H., Van Keer S., Vorsters A. VALHUDES: a protocol for validation of human papillomavirus assays and collections devices for HPV testing on self-samples and urine samples. J. Clin. Virol. 2018;107:52–56. doi: 10.1016/j.jcv.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Viviano M., Willame A., Cohen M., Benski A.C., Catarino R., Wuillemin C., Tran P.L., Petignat P., Vassilakos P. A comparison of cotton and flocked swabs for vaginal self-sample collection. Int J Womens Health. 2018 May 15;10:229–236. doi: 10.2147/IJWH.S157897. PMID: 29805267; PMCID: PMC5960235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haguenoer K., Sengchanh S., Gaudy-Graffin C., Boyard J., Fontenay R., Marret H., Goudeau A., Pigneaux de Laroche N., Rusch E., Giraudeau B. Vaginal self-sampling is a cost-effective way to increase participation in a cervical cancer screening programme: a randomised trial. Br. J. Cancer. 2014 Nov 25;111(11):2187–2196. doi: 10.1038/bjc.2014.510. Epub 2014 Sep 23. PMID: 25247320; PMCID: PMC4260034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sechi I., Cocuzza C.E., Martinelli M., Muresu N., Castriciano S., Sotgiu G., Piana A. Comparison of different self-sampling devices for molecular detection of human papillomavirus (HPV) and other sexually transmitted infections (STIs): a pilot study. Healthcare. 2022;10:459. doi: 10.3390/healthcare10030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juliana N.C.A., Juma M.H., Heijmans R., Ouburg S., Ali S.M., Chauhan A.S., Pemba A.B., Sazawal S., Morré S.A., Deb S., Ambrosino E. Detection of high-risk human papillomavirus (HPV) by the novel AmpFire isothermal HPV assay among pregnant women in Pemba Island, Tanzania. Pan Afr Med J. 2020 Oct 27;37:183. doi: 10.11604/pamj.2020.37.183.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seyoum A., Seyoum B., Gure T., Alemu A., Belachew A., Abeje D., Aseffa A., Howe R., Mulu A., Mihret A. Genotype heterogeneity of high-risk human papillomavirus infection in Ethiopia. Front. Microbiol. 2023 Feb 10;14 doi: 10.3389/fmicb.2023.1116685. Erratum in: Front Microbiol. 2023 Mar 23;14:1178530. PMID: 36846744; PMCID: PMC9951590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith J.H.F. Bethesda 2001: Bethesda 2001. Cytopathology. 2002;13(1):4–10. doi: 10.1046/j.1365-2303.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 14.WHO Classification of Tumours Editorial Board . World Health Organization Classification of Tumors. Female Genital Tumours. fifth ed. IARC Press; Lyon: 2020. [Google Scholar]
- 15.Serrano B., Ibáñez R., Robles C., Peremiquel-Trillas P., de Sanjosé S., Bruni L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022;154 doi: 10.1016/j.ypmed.2021.106900. ISSN 0091-7435. [DOI] [PubMed] [Google Scholar]
- 16.Arbyn M., Costa S., Latsuzbaia A., Kellen E., Girogi Rossi P., Cocuzza C.E., Basu P., Castle P.E. HPV-Based cervical cancer screening on self-samples in The Netherlands: challenges to reach women and test performance questions. Cancer Epidemiol. Biomarkers Prev. 2023;32(2):159–163. doi: 10.1158/1055-9965.EPI-22-1041. [DOI] [PubMed] [Google Scholar]
- 17.Badman S.G., Vallely A.J., Pardo C., Mhango L.P., Cornall A.M., Kaldor J.K., Whiley D. A comparison of ThinPrep against four non-volatile transport media for HPV testing at or near the point of care. Pathology. 2021 Feb;53(2):264–266. doi: 10.1016/j.pathol.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Connor L., Elasifer H., Sargent A., Bhatia R., Graham C., Cuschieri K. Influence of resuspension volume on dry sampling devices taken for human papillomavirus testing: implications for self-sampling. Biotechniques. 2023 doi: 10.2144/btn-2022-0084. [DOI] [PubMed] [Google Scholar]
- 19.Giubbi C., Martinelli M., Vallini I., Paganoni S., Dafa’alla T., Perdoni F., Musumeci R., Wu W., Castriciano S., Romano P., Cocuzza C.E. Human papillomavirus (HPV) detection in vaginal self-samples: evaluation of eNat® as an alternative suspension medium to ThinPrep®PreservCyt® for vaginal swabs [version 2; peer review: 2 approved] Open Res Europe. 2022;2:35. doi: 10.12688/openreseurope.14344.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inturrisi F., Aitken C.A., Melchers W.J.C., van den Brule A.J.C., Molijn A., Hinrichs J.W.J., Niesters H.G.M., Siebers A.G., Rob Schuurman R., Heideman D.A.M., de Kok I.M.C.M., Bekkers R.L.M., van Kemenade F.J., Berkhof J. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in The Netherlands: an observational study. The Lancet Regional Health - Europe. 2021;11 doi: 10.1016/j.lanepe.2021.100235. ISSN 2666-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbyn M., Latsuzbaia A., Castle P.E., Sahasrabuddhe V.V., Vanden Broeck D. HPV testing of self-samples: influence of collection and sample handling procedures on clinical accuracy to detect cervical precancer. The Lancet Regional Health - Europe. 2022;14 doi: 10.1016/j.lanepe.2022.100332. ISSN 2666-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinelli M., Latsuzbaia A., Bonde J., Pedersen H., Iacobone A.D., Bottari F., Piana A.F., Pietri R., Cocuzza C.E., Arbyn M. Extended Valhudes Study Group. Performance of BD Onclarity HPV assay on FLOQSwabs vaginal self-samples. Microbiol. Spectr. 2024;12(3):e0287223. doi: 10.1128/spectrum.02872-23. Epub 2024 Feb 7. PMID: 38323823; PMCID: PMC10913526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latsuzbaia A., Van Keer S., Vanden Broeck D., Weyers S., Donders G., De Sutter P., Tjalma W., Doyen J., Vorsters A., Arbyn M. Clinical accuracy of alinity m HR HPV assay on self- versus clinician-taken samples using the VALHUDES protocol. J. Mol. Diagn. 2023 Dec;25(12):957–966. doi: 10.1016/j.jmoldx.2023.09.008. Epub 2023 Oct 20. PMID: 37865293. [DOI] [PubMed] [Google Scholar]
- 24.Martinelli M., Giubbi C., Di Meo M.L., Perdoni F., Musumeci R., Leone B.E., Fruscio R., Landoni F., Cocuzza C.E. Accuracy of human papillomavirus (HPV) testing on urine and vaginal self-samples compared to clinician-collected cervical sample in women referred to colposcopy. Viruses. 2023;15:1889. doi: 10.3390/v15091889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sechi I., Muresu N., Puci M.V., Saderi L., Del Rio A., Cossu A., Muroni M.R., Castriciano S., Martinelli M., Cocuzza C.E., Sotgiu G., Piana A. Preliminary results of feasibility and acceptability of self-collection for cervical screening in Italian women. Pathogens. 2023 Sep 17;12(9):1169. doi: 10.3390/pathogens12091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tardif K.D., Pyne M.T., Malmberg E., Lunt T.C., Schlaberg R. Cervical cytology specimen stability in surepath preservative and analytical sensitivity for HPV testing with the cobas and hybrid capture 2 tests. PLoS One. 2016 Feb 23;11(2) doi: 10.1371/journal.pone.0149611. PMID: 26905067; PMCID: PMC4764333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuschieri K.S., Beattie G., Hassan S., Robertson K., Cubie H. Assessment of human papillomavirus mRNA detection over time in cervical specimens collected in liquid based cytology medium. J. Virol Methods. 2005 Mar;124(1–2):211–215. doi: 10.1016/j.jviromet.2004.11.005. Epub 2004 Dec 16. PMID: 15664071. [DOI] [PubMed] [Google Scholar]
- 28.Ejegod D.M., Pedersen H., Alzua G.P., Pedersen C., Bonde J. Time and temperature dependent analytical stability of dry-collected Evalyn HPV self-sampling brush for cervical cancer screening. Papillomavirus Res. 2018 Jun;5:192–200. doi: 10.1016/j.pvr.2018.04.005. Epub 2018 Apr 22. PMID: 29689311; PMCID: PMC6026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilardo C., Marguerettaz M., Breton A., Azzoug T., Schmitt N., Fournier R., Bayette J., Teissier G., Lelarge C., Ponseille B. Performance and pre-analytical stability of self-collected samples versus clinician cervical samples for the detection of HPV16, HPV18 and a pool of 12 other HPV types on the Roche Cobas 8800 System. New Microbiol. 2022 Apr;45(2):111–114. PMID: 35699559. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Final study datasets generated by the study are stored locally and securely at the University of Milano-Bicocca. Anonymized data will be available by request to the corresponding author on a case-by-case basis pending approval by the University of Milano-Bicocca.