Abstract
Human Papilloma Virus (HPV) is considered one of the most common sexually transmitted infections and has been shown to play an important role in the pathogenesis of squamous cell carcinomas (SCC) of the cervix and head and neck. Manifestations of HPV infections can be manifold, ranging from asymptomatic infections to benign or potentially malignant lesions to intraepithelial neoplasms and invasive carcinomas. The heterogeneity of clinical manifestations from HPV infection depends on the interactions between the viral agent and the host, a direct consequence of the ability on the part of HPV is to remain silent and to evade and convey the action of the host immune system. The oral mucosa represents one of the tissues for which HPV has a distinct tropism and is frequently affected by infection. While much information is available on the role that HPV infection plays in the development of SCC in the oral cavity, there is less information on asymptomatic infections and benign HPV-induced oral lesions. Therefore, the purpose of this review is to analyze, in light of current knowledge, the early clinical and bio-humoral prognostic features related to the risk of HPV malignant transformation, focusing on subclinical conditions, benign lesions, and the correlation between oral infection and infection in other districts. The data show that the main risk associated with HPV infection is related to malignant transformation of lesions. Although HPV-driven OPSCC is associated with a better prognosis than non-HPV-driven OPSCC, primary prevention and early detection of the infection and affected genotype are essential to reduce the risk of malignant neoplastic complications and improve the prognosis.
Keywords: Human papilloma virus, Oral cell, Oral mucosa, Periodontitis, Prognostic markers
1. Introduction
Human Papilloma virus (HPV) is one of the most common sexually transmitted infections for both males and females [1,2]. It is often asymptomatic, underrated and underestimated. It can cause oropharyngeal and cervical cancers even after a long time of latent infection [3]. According to the American Center for Disease Control and Prevention (CDC), sexually active individuals go through HPV infection at least once during their lifetime, but it is not always symptomatic so they are not often aware about their condition [4]. Beside the asymptomatic variant, the manifestations of HPV infection are variable, in fact, there can be benign and potential malignant lesions or neoplastic lesions up to invasive carcinoma. The several manifestations of HPV's infections depend on the interaction between the host and the virus depends on the interaction between the host and the virus. HPV has been widely investigated in genital infections and it is considered the main etiological factor of uterine cervix cancer [[5], [6], [7], [8]]. Differently from uterine cervix cancer, HPV is involved in a small percentage of oral cancers [9,10]; instead, the main percentage of oral cancers are normally associated with smoking and alcohol abuse [11]; other suggested causes are related to the persistent traumas of lesions [12,13] and poor dental care [14]. The oral premalignant lesions caused by HPV infections include the following: verruca vulgaris (common wart) (VV), squamous papilloma (SP), condyloma acuminatum (CA), and multifocal epithelial hyperplasia (MEH) [15]. Other oral lesions have been associated with HPV infection too, including erythroplakia (HPV-16), proliferative verrucous leukoplakia (HPV-16), candidal leukoplakia, oral squamous cell carcinoma (HPV-16 and HPV-19) and lichen planus (HPV-6, HPV-11 and HPV-16). However, in a recent study it was observed that the prevalence of HR-HPV and that HR-HPV in OL does not appear to be a driver of oncogenesis [16]. Importantly, some types of HPV are associated with benignity and others with malignity lesions, in particular HPV types 2, 4, 6, 11, 13 and 32 have been associated with benign oral lesions, on the other hand, types 16 and 18 have been associated with malignant lesions [11].
In some cases, benign lesions may evolve into malignant lesions and such evolution is most related to the expression of two proteins of HPV (E6 and E7), defined oncogene proteins [17,18]. They are capable of inactivating two onco-suppressor proteins, called p53 and the retinoblastoma protein (pRb) [[19], [20], [21], [22]].
The aim of this review is to analyze the early prognostic clinical and bio humoral features linked with the epigenetic risk of malignant transformation of HPV, focusing on subclinical conditions, benign lesions, and the correlation between the HPV oral infection and the HPV infection affecting other anatomical districts.
2. Materials and methods
This review employed a comprehensive search strategy performed by two reviewers to identify relevant articles on HPV oral lesions and HPV infection. Major search engines such as PubMed and Google Scholar were utilized, using specific keywords related to the topic: “HPV oral lesions” and “HPV infection”. The search encompassed a wide timeframe from 1945 to 2024, with a focus on recent publications. The selection criteria included English papers designed as Randomized Clinical Trials (RCTs), cohort studies, case-control studies, case series, meta-analyses, and systematic reviews. Only articles that provided relevant and reliable evidence on HPV oral lesions and HPV infection have been considered for the discussion of the topic.
The inclusion of various study designs and involvement of multiple researchers enhanced the robustness and reliability of the review's findings.
3. HPV features
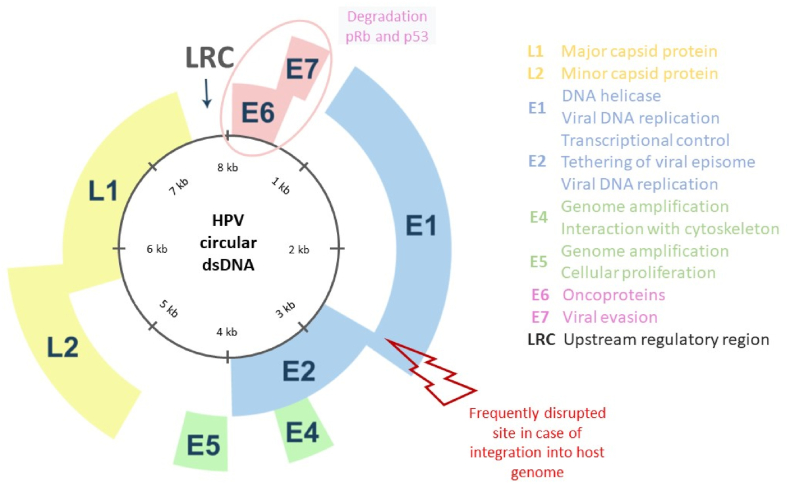
Papilloma virus belongs to the Papillomavirus family and together with Polyomaviruses form the species Papovaviridae. It is a DNA virus with a circular double DNA strand of almost 7.9 kb, covered by a small capsid. Such capsid is about 55 nm in diameter, and it is made of just two structural proteins. In the HPV genome all the putative open reading frames (ORFs) are contained in just one DNA strand; meanwhile the short ORFs are localized in the second-probably- non-coding strand. The single frames are divided into “early” (E) and late (L) genes according to the timing of action during the infection (Fig. 1). In fact, the early genes (E1-E8) are expressed immediately after the start of the infection and before the DNA replication into the host cells. The products of such genes control the DNA replication and the expression of the virus. When the infecting virus is oncogenic, its early genes and the related products cause the malignant transformation of the host cell. On the other hand, the late genes (L1-L2) code for the proteins that make the capsid structure, and they are activated during the late stages of the viral cycle [23].
Fig. 1.
The structure of human papillomavirus (HPV) and its function. HPV genome is composed of 8k base pairs and divided into early and late gene. The early genome encodes E1, E2, E4, E5, E6, and E7 and the late genome encodes L1 and L2. Oncoprotein E6 and E7 degrade tumor suppressor p53 and pRb, respectively. L1 and L2 build the structure of HPV capsid protein. LCR, long control region.
It has been discovered that each HPV type occurs in the form of “variants”. Nowadays, more than 200 types of HPV are known https://pave.niaid.nih.gov/search/search_database. Recently, the available data about the variants of HPV suggests that each variant of the same type of HPV may induce different pathogenic risks [22,[24], [25], [26]] and important related psychological risks [[27], [28], [29]].
HPV viruses are epitheliotropic and they normally give rise to benign epithelial proliferation, but in some cases, they may cause malignant transformation of benign lesions. The oncogenic potential is related to the type of the infecting HPV. They are divided into low oncogenic risk HPV (LR- HPV) and high oncogenic risk HPV (HR-HPV) [30]. Both classes of HPV can induce the development of unusual cells, but only the high oncogenic ones can cause cancer. In fact, the oncogenesis is influenced by the action of E7 protein, which is set by high-risk HPVs and can eternalize human epithelial cells. Among the HPVs which are classified as high risk and sexually transmitted there are: 16,18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68 and 73 [31]. The HPVs are also classified in α group, with tendency of infecting the mucosal epithelia and the β group, with the tendency of infecting the cutaneous epithelia [32].
3.1. Way of transmission
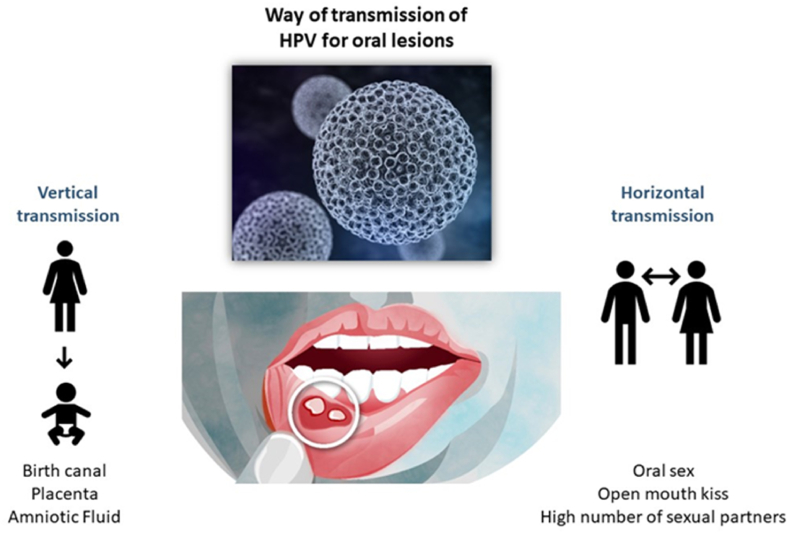
The transmission can be vertical, from mother to child or horizontal between individuals [33] (Fig. 2). In most cases the horizontal transmission is sexually, but the virus can spread non sexually through skin-to-skin, skin-to mucosa, or mucosa to mucosa paths [33,34]. The transmission from mother to child can occur not only by the birth canal but also through the placenta or amniotic fluid [[35], [36], [37], [38]]. Some scientists believe that oral infection, especially in children, may occur by autoinoculation from cutaneous lesions, fomites, breast feeding, or bathing [35,39]. In adults the infections are mostly related to oral sex and open-mouthed kissing, the risk augments proportionally with the increase of the number of sexual partners [[40], [41], [42]]. In addition, it has been demonstrated the association between the use of crack-cocaine and the HPV infection [43].
Fig. 2.
The way of transmission of HPV for oral lesions. Oral HPV infection may occur vertically, from mother to child and horizontally between sexual partners.
3.2. HPV infection
Epithelial stem cells are target of HPV infection [44]. It infects specific epithelia, that are predisposed to its infection, including tonsils, bulge region of the hair follicle and squamo-columnar junction of uterus cervix. It also gains access to non-predisposed epithelia thanks to trauma, wounds, or micro-abrasion, through a solution of continuity [44,45]. HPV enters in the intracellular compartment via endocytosis and viral episomal genome reaches the nucleus of the host cell, integrating into the host cell genome and initiating viral transcription [45]. Therefore, replication of the cell and the viral genome occur parallel; originating new genome, that receive the integration of the viral DNA to the mother cell DNA. The infection starts from the basilar cells, then, such cells differentiate into keratinocytes, which are also infected with the virus genome that is amplified during the replications. Since the keratinocytes exfoliates, several cell replications occur, which means that the cells are full of viruses and can infect other cells they come into contact with [46].
The establishment of HPV infection is facilitated by the ability of the virus to evade the host immune system control [47] and it is helped by the specific features of their target cells, keratinocytes. The first characteristic that represents an advantage for the action of the virus is that the infection occurs in the intraepithelial compartment with minimal interaction with immune cells [48,49]. A second advantageous factor is that it internalizes its genome into host cells and amplifies it using the host cell replication, which is not controlled by the immune system (Table 1).
Table 1.
The oral HPV related benign lesions. A summary of incidence most frequently interested zones of the oral cavity, clinical aspects and histological aspects of the benign lesions caused by the HPV in the mouth.
| Lesion | Incidence | Most frequent oral manifestations | Clinical aspect | Histological aspect | HPV type | Ref. |
|---|---|---|---|---|---|---|
| Squamous Papilloma |
Highest in adulthood, between 30 and 70 years. | Palate, tongue, inner surface of lips. | Sessile or pedunculated exophytic lesion, with a verrucous or papillary superficial aspect. | Multiple offshoots with a fibrovascular core and a keratinized squamous lining, acanthosis and basal cell hyperplasia. | HPV 6, 11 13, 16 and 36. | [[50], [51], [52]] |
| Condyloma acuminatum | Adulthood, mostly between 30 and 40 years. | Vestibular mucosa, anterior fornixes, labrum, palate. | Multiple lesions that coalesce to form big outgrows or isolated lesions with sessile or pedunculated aspect. | Papillary architecture with squat, anastomosed epithelial ridges converging to a broad base. Acanthosis. | HPV 2, 6, 11, 16, 18, 53 and 54. | [[53], [54], [55], [56]] |
| Verruca vulgaris | Children and adolescents, mostly between 12 and 16 years. | Labial mucosa and palate. | Exophytic, single or multiple lesions. | Hyperplasia of epithelial ridges with acanthosis and intense keratinization. | HPV 2, 4, 6 and 40. | [57] |
| Epithelial hyperplasia | / | Vestibular mucosa. | Multiple popular or nodular lesions that tend to converge. | Acanthosis of the epithelium with enlarged and anastomosed net ridges. Mitosoid bodies. | HPV13 and 32. | [15,58] |
Moreover, the virus actively works to evade the immune system detection; in fact, it interferes with the modulation of interferons, antigen processing and T cells and acts with other various strategies that differs among virus subtypes, since they are dependent on their proteins [44,49].
4. HPV related oral lesions
The HPV infection of oral mucosa may cause the following benign lesions: squamous papilloma, condyloma acuminatum, verruca vulgaris and multifocal epithelial hyperplasia [15]. These lesions have common histologic and clinical features with each other and with other diseases of inflammatory or malignant origin [53].
4.1. Squamous papilloma
Squamous Papilloma (SP) is the most frequent form of benign oral lesion induced by HPV infection in both children and adults [59,60] and has been found to be the second most commonly encountered disease entity overall [50]. The highest incidence is in adulthood; however, it can also occur in childhood. The age of most common occurrence is between 30 and 70 years of age without gender predilection [50]. The palate, tongue, and inner surface of the lips are the sites most prone to SP development, but other sites in the oral cavity may also be involved. The HPV types isolated, by molecular in situ hybridization, are HPV 6, 11 13. 16 and 36. The most frequent are types 6 and 11 [50,51]. Viral infections are also slightly related to periodontitis and gingival diseases [61,62].
Clinically, SP is a benign, sessile or pedunculated exophytic neoformation [50,51]. The superficial appearance may be verrucous or more commonly papillary. In the latter case, it takes on a "cauliflower-like" appearance, characterized by digitiform, white/reddish colored extroversions [50,51]. They are rarely larger than 5 mm in size and are usually solitary [63].
Histologically, there are multiple offshoots that possess a fibrovascular core and a keratinized squamous lining. The epithelium shows marked hyper- or orthokeratosis, acanthosis and basal cell hyperplasia. Coilocytes, with clear cytoplasm and small nuclei, are found visible in the spinous layer [50,52,64,65].
4.2. Condyloma acuminatum
Condyloma acuminatum (CA) is the most common HPV-induced lesion at the anogenital level and, therefore, is the most reported sexually transmitted infection in both the United Kingdom and the United States [54]. These lesions are uncommon in the oral cavity [53] and usually occur in adults with a higher incidence between the third and fourth decades of life. The HPV types involved are HPV 2, 6, 11, 16, 18, 53 and 54 [54].
Clinically, CA presents as multiple lesions that may coalesce to form voluminous outgrowths, more rarely, CA presents as a solitary lesion [66].
The lesions have a sessile, or more rarely pedunculated, appearance, with a smooth, pointed surface and white/pinkish color or a cauliflower-like surface appearance [54,[67], [68], [69]].
.The most affected sites are the vestibular mucosa of the anterior fornixes, labrum, palate, and [56,70].
Microscopically, the lesions show an exophytic, papillary architecture with squat, anastomosed epithelial ridges converging to a broad base [53,55]. The epithelium appears acanthotic with marked parakeratosis and invaginations filling the crypts between the papillae [53,55]. Coilcites are appreciated in the superior spinous layer [53,67].
4.3. Verruca vulgaris
Verruca vulgaris (VV) is the most common clinical form of HPV skin infection, constituting more than 70 % of warts [71,72]. It predominantly affects children and adolescents with a higher peak incidence between 12 and 16 years of age. The most encountered site is represented by periungual area of the hands and the associated HPV types are HPV 2, 4, 6 and 40 [57].
VV rarely affects the oral mucosa and autoinoculation is the main route of transmission [57,73]. Clinically, mucosal lesions appear like their cutaneous counterparts [57,73]. They appear pinkish/white in color, exophytic, single or, more rarely in groups [74]. The size is usually no more than 1 cm. The intraoral areas most prone to VV development are the labial mucosa and palate.
Histologically, the cutaneous and mucosal lesions also have no differences [57], hyperplasia of epithelial ridges with acanthosis and intense keratinization. Thick keratinization gives a chevron or "church spire" appearance [57]. At the margins of the lesion, the vertical axis of epithelial ridges takes on a cupped appearance that converges toward the center of the lesion. Coilcytes are cells exhibiting the characteristics of cytopathic alteration by HPV viral infection: shrunken and elongated nucleus, eccentrically positioned with a vacuolated cytoplasmic halo [57]. These cells are observed in the upper and proximal epithelial layers or within the granular cell layer [57].
4.4. Epithelial hyperplasia
Focal epithelial hyperplasia (FEH), defined as Heck's disease or multifocal epithelial hyperplasia (MEH) is a benign oral condition induced by infection with HPV13, HPV32, or both [75,76]. Clinically, the condition is characterized by multiple 0.2–3 cm, papular or nodular, which tend to converge, white-pink in color [58].The most affected sites are the anterior vestibular mucosa and the tongue. In the most serious cases, the lesions can spread over the entire vestibular mucosa, which takes on a cobblestone appearance [77]. The lesions tend to disappear when the mucosa is stretched [78]. Histological examination reveals exophytic areas with nodular or papillary surface features, characterized by an acanthotic epithelium with enlarged and anastomosed net ridges. Mitosoid bodies can be identified, present in enlarged epithelial cells, which present degenerated chromatin filaments that can mimic mitotic figures. These mitosoid bodies, in combination with koilocytosis, represent the outcomes of viral cytopathic effects [15].
5. Diagnosis of HPV-related oral benign lesions
The diagnosis of HPV-related oral benign lesions is based on the histopathologic exam, which represents the gold standard test.
Typical histological features of oral and periodontal [79] benign lesions caused by HPV infection include koilocytosis, acanthosis and papillomatosis which, coupled to the clinical appearance, suggest the existence of the infection [80,81].
There is also the possibility to perform cytologic exam of the lesion, that, on the contrary, allows studying just the cell morphology, intercepting any cellular atypia induced by HPV infection, which has the advantage of being less invasive [82].
To assess the presence of the HPV in such lesions, both histological and cytological samples of the lesions are effective to perform polymerase-chain reaction and detect HPV DNA. Detecting the genotype of the infecting HPV is important to identify HPV genotypes in LR-HPVs and HR-HPVs, allowing to foresee the possible evolution and prognosis of the existing lesion [70,[83], [84], [85], [86]].
5.1. Different methods of diagnosis and their effectiveness
HPV may be detected also in normal mucosa, but in general, according to the used detection method the viral load is variable [83,[87], [88], [89], [90], [91]]. It is important and challenging to clarify the importance of viral load in oral mucosa to possible interact variation into dysplastic and/or neoplastic tissues. In the study of Pierangeli et al. [82], the group investigated the difference of frequency of HPV infection in 62 patients with benign oral lesions (e.g. fibromas, papillomatosis, ulcers) or oral potentially premalignant disorders (OPMDs) (e.g. lichen, leukoplakia) compared with 54 controls performing cytobrushing and PCR. The results showed that the HPV detection rate was significantly higher in patients affected by benign oral lesion compared to patient with OPMDs and the percentage of high-risk HPVs (HR HPVs) was also higher in the first group than in the second group. HPV-DNA loads, particularly HPV16 and HPV18, were significantly higher in patients with benign HPV lesion compared to OPMDs' lesion. Unexpectedly the study observed high rates of HPV infection in cells of the oral mucosa, emphasizing the prevalence of HPV in oral tissues. The elevated HR HPV loads found in oral potentially malignant disorders (OPMD) suggest that quantitative polymerase chain reaction (PCR) is effective in testing and quantifying HPV presence in oral lesions. The study raises the possibility that elevated viral loads of high-risk HPVs could serve as a marker for the risk of malignant progression in oral lesions. However, prospective studies are needed to confirm whether these elevated viral loads can indeed predict the likelihood of the development of malignancy. To early detect precancerous lesions of tonsils, in the study by Franceschi et al. [92] deep brushing of the tonsils was tested. It was revealed the inapplicability of this method for early detection of HPV driven lesions of the tonsils in vivo since the results were unsatisfactory. However, cytobrushing has been assessed as valid option of detecting HPV infection in oral lesions, especially when it comes to malignant lesions and it's the most valid method of screening for uterine cervical cancer [34,[93], [94], [95], [96]].
There is also the possibility to use other diagnostic methods, based on the use of light, that help in clinical evaluation of oral lesions. The used methods, including autoflourescence and chemiluminescence help in differentiating benign lesions from malignant lesions already at the clinical exam. Of these, autoflourescence device has shown to be superior to chemiluminescence and to the simple clinical examination of the lesion in detecting premalignant lesions and early malignant transformation, even though it remains undoubtable the superiority of biopsy and histological exam in diagnosis [[97], [98], [99], [100]].
6. Relationship between HPV oral lesions and uterine cervix lesions
Being HPV a sexual transmitted agent it involves primarily the lesions of genitals and, as said before, it is the main cause of uterine cervix cancer [5]. However considering the fact that it an induce benign and/or malignant lesions in both anatomical districts, an analyses of their similarities is required.
The histological similarities between oropharyngeal mucosa and cervix mucosa indicate an augmented susceptibility of the tissues for the HPV infection [101]. Both mucosae comprise a transition zone between the squamous epithelium and the cylindric epithelium, such zone allows the access of the viral particles up to the basal membrane [22,37,102]. This selective trophism may find an explication also in the reticular aspect of the epithelium of the crypts of the tonsils that imitate the microlesion of the cervix epithelium and allow the access of the virus to the basal membrane [103]. The HPV infection of uterine cervix and the oral mucosa may be associated to sexual behaviors, high number of sexual partners and compromission of immune system [[104], [105], [106], [107]], but it should be clarified if and in which way infections of both anatomical sites are related to each other. In a study [108] it was investigated the relationship between the prevalence of HPV in oral cavity of women with oral sex practices and cervical lesions. 46 patients with a previous CIN diagnosis were enrolled. They answered to a questionnaire about their sexual practices, then it was taken a swab of the check's mucosa and another one from palate/gums. The samples underwent though PR to detect the presence of HPV and its genotype. The results showed that the 72 % of the patients that affirmed to have oral sex practices were positive to HPV in oral mucosa, palate/gum. 35 % of them had HPV16 and among them the 26 % had regular oral sex practices, meanwhile the 9 % didn't. This data demonstrates that there is a high association between the detection of HPV in oral mucosa with oral sex behavior, but the 9 % of the population suggests that there may have other factors that favor the infection, maybe the autoinoculation, considering that such patients had HPV driven lesions in the uterine cervix. It was found an association between oral HPV16 positivity and progression of cervical CIN advanced lesions, suggesting that the dentist and the oral pathologist should be aware of CIN diagnosis of their patients. In fact, as already mentioned, the HPV 16 is associated with the high-risk group of the HPV family. The possibility to interact its presence in oral mucosa, when still asymptomatic, allows to monitor it. In this way, possible HPV 16 related oral lesions can be intercepted at a very early stage [109].
7. From the infection to cancer
The HPV infection is often cleared within 2 years, so it can also be silent and don't evolve into the aforementioned lesions; but sometimes it persists and may lead to the evolution of precancerous lesions, and if such lesions don't regress, they can transform into invasive oropharyngeal squamous cell carcinoma (OPSCC); moreover, persistent infections may evolve into cancer within 10 years [110,111]. The cancer onset is mostly related to two types of HPV: 16 and 18 [112,113]. The HPV-16 has been demonstrated to be present in almost 15 % of oral cavity tumors (OSCC) and in about 41 % of oropharyngeal tumors (OPSCC); HPV-18 type has been detected in about 6 % of OSCCs and 0.2 % of OPSCCs [114].
In a recent study, it was observed that the persistence of HPV-16 infection in male subjects would be influenced by advanced age, however, further studies are still needed [115].
The oncogenic risk of HPV is related to its proteins, in particular to E6 and E7, in fact, according to their features two groups can be distinguished: the LR-HPVs and the HR-HPV. The first one is associated with benign lesions of skin and mucosa, meanwhile the second one is associated with malignant lesions [[116], [117], [118]]. The oncogenic role of HR-HPV E6 has an impact in a lot of cellular functions, in fact it binds and degrades tumor suppressor protein p53, blocks the apoptosis, the differentiation of keratinocytes and the interferon responses [10,116,119]. On the other hand, HR-HPV E7 favors cell-cycle entry and progression, binds and degrades tumor suppressor pRb, promotes the release of the E2F transcription factor and causes genome instability. This leads to the de-regulation of the G1/S cell cycle check point and the activation of S-phase re-entry and viral replication. Because of E7 activation, p16INK4a is overexpressed. Normally, p16INK4a acts as a tumor suppressor by inhibiting the activity of cyclin-dependent kinases (CDKs) involved in cell cycle progression. However, in the context of HPV-associated tumors, p16INK4a overexpression is linked to cell survival and escaping oncogene-induced senescence [[120], [121], [122]]. About the other viral early proteins, E1, E2, E4 are not involved in the oncogenesis but are important to guarantee the viral cycle; E5 is not always expressed; when it is expressed, it impacts a lot of signaling pathways, such as the epidermal growth factor receptor (EGFR), the immune recognition and the regulation of apoptosis [123].
8. Differences between HPV- related OPSCC and HPV non-related OPSCC
Several studies have demonstrated that the HPV status of OPSCC patients is associated with improved survival outcomes [70,[124], [125], [126]]. One of the early studies conducted by Gillison and colleagues [127] showed a remarkable finding. Patients with HPV-related OPSCC had a 74 % reduction in the risk of death from cancer compared to those with non-HPV-related OPSCC. The hazard ratio (HR) was 0.26, and the 95 % confidence interval (CI) was 0.07–0. A subsequent meta-analysis [128] further confirmed the association between HPV-related OPSCC and improved survival outcomes. It showed that patients with HPV-related OPSCC had a 28 % reduced risk of death (meta HR: 0.72) compared to those with non-HPV-related OPSCC. The 95 % CI was 0.5–1. The studies establish HPV status as an independent predictor of better overall and disease-free survival in OPSCC patients. Patients with HPV-related OPSCC usually to have more favorable outcomes compared to those with non-HPV-related OPSCC.
The findings from these studies have had a significant impact in the management of OPSCC. HPV status has become a crucial factor in determining treatment strategies and prognostic assessments for patients with OPSCC. Patients with HPV-related OPSCC are generally considered to have a more favorable prognosis, and their treatment approaches may differ from those with non-HPV-related OPSCC. It's important to note that while HPV status is a powerful predictor of survival outcomes, other factors such as stage, grade, and patient characteristics also play a role in determining treatment plans and overall prognosis.
The combination of HPV infection with other cancer etiological factors, such as tobacco smoking, has bad influence in the prognosis of the malignant lesion [124].
From the clinical perspective the main difference between HPV-related and non-related OPSCC is that HPV-related OPSCC is often diagnosed in advanced stages, characterized by high nodal dissemination (lymph node involvement), but with relatively smaller tumor size (T). This implies that these cancers tend to spread to lymph nodes early in the disease course, even when the primary tumor size is small [129,130].
HPV-related OPSCC tumors have distinct histopathological features, often appearing as non-keratinizing, undifferentiated, or basaloid squamous cell carcinomas. These characteristics can be helpful in differentiating them from non-HPV-related OPSCC. Moreover, HPV-related OPSCC cases show a lower incidence of second primary neoplasms compared to non-HPV-related cases. This might be attributed to reduced exposure to other risk factors such as tobacco use or alcohol consumption, which are more commonly associated with non-HPV-related OPSCC. HPV-related OPSCC tumors typically lack transcriptionally active HPV in the surrounding mucosa. This suggests the absence of a cancerization field effect, where areas adjacent to the primary tumor may also have precancerous changes. As a result, there are fewer second primary neoplasms and locoregional recurrences associated with HPV-related OPSCC [38,127,129,[131], [132], [133], [134], [135]].
From a radiological standpoint, HPV-related OPSCC often presents with well-defined borders. Cystic nodal involvement is frequently observed, and in some cases, the primary tumor can be small or even occult, making it challenging to detect using conventional imaging techniques [136].
Understanding these distinct characteristics of HPV-related OPSCC is essential for accurate diagnosis, treatment planning, and prognosis assessment. The unique features of these tumors also highlight the importance of personalized and tailored approaches in managing patients with HPV-related OPSCC.
9. Prognostic factors of HPV induced cancer
In Literature it is largely assessed the positive prognostic value of HPV infection in OPSCC [70,[125], [126], [127]] and it is also known that OPSCC is mainly associated with HPV infection, meanwhile oral cancer is primarily associated with tobacco and alcohol [137]. For this reason, the role of HPV in oral cancer is unclear. Similar features have been noticed between OPSCC and oral cancer both induced by HPV. For example, the non-keratinizing morphology was supposed to be related to the typical tissue structure of tonsils, but it has been related to the HPV infection. In fact, it can be also found in HPV-induced non oropharyngeal cancers, even when such cancers emerge in tissues that are keratinized in normal conditions [138,139]. Even if classical features of HPV induced cancers are shared by OPSCC and non-OPSCCs, it has been demonstrated that there is a variant with different histological features. This variant has warty morphology defined by exophytic growth, prominent surface keratosis and parakeratosis, koilocytosis, multinucleation and marked nuclear pleomorphism and it is rare in head and neck area [140]. There are some data about the warty variant that suggest a better clinical outcome. In particular, it has been shown that HPV- induced non- OPSCCs, including oral ones, are associated with better clinical outcomes, short follow-up and sample size. Moreover, warty cancer lesions were associated with a lower stage disease, no metastases and no tumor recurrences [138]. This data should be further investigated and assessed by other studies, in fact the limitations are related to the short timelapse considered as follow-up (16 months) and the small sample size.
Another interesting information still coming from the study of Rooper et al. [138] is about the identification of a premalignant aspect of oral cavity and larynx mucosae infected by HPV. It can be identified by the assessment of the presence of p16 and by some typical histological characteristics. Premalignant lesions of oral cavity infected by HPV that may lead to HPV- induced cancer have the following histological features: full-thickness proliferation of basaloid cells with prominent apoptosis and karyorrhexis and corrugated surface parakeratosis. They have been associated with the developing of squamous cell carcinoma between the 15 % and the 70 % of the cases [[141], [142], [143], [144]]. So, intercepting this squamous dysplasia could be a good way to avoid future cancer developing. However, it should be considered that histological aspect is essential to assess this squamous dysplasia. In fact, the interception of p16 positivity is not sure. In fact, the presence of p16 suggests the presence of HPV infection, but the virus could be inactivated [145].
As already mentioned, the presence of HPV is related to a better prognosis of OPSCCs for sure. But there are some controversies about the risk associated with HPV positive non- OPSCCs. In fact, there are studies that suggest the positive impact of HPV infection in the prognosis [[146], [147], [148]], and others that affirm the opposite [139,149,150]. This incongruence may be related to the different histological variants, since warty variants have not been considered separately. In sum, while the prognostic value of HPV in OPSCCs is well known, it can be said the same for the oral cancer and other non- OPSCCs induced by HPV infection. There are limited data about them and other studies with longer follow-ups, larger samples sizes about selected lesions should be performed.
10. Risks of malignant transformation related to the HPV infection
The development of oropharyngeal malignities is associated with the persistent infection of HPV, moreover OPSCC is now becoming the most common cancer caused by HPV, surpassing the uterine cervix cancer [109,151,152]. The evidence coming from the Literature suggests that there are some circumstances, behaviors and conditions that enhance the probability to go through HPV- related OPSCC. First of all, as already mentioned, the viral types associated with carcinogenesis are HPV 16 and HPV 18 [51,53] and the involvement of gingival tissue is related to a higher risk of malignant transformation [51]. People with associated increased risk of developing HPV- related OPSCC are those with a lot of lifetime sexual partners and high-risk sexual behavior [[153], [154], [155]]. Moreover, it has also been demonstrated that poor oral care is associated with HPV16 infection and that chronic periodontitis [186] seems to be associated with the status of HPV infection of head and neck squamous cells carcinomas [156,157]. Another important fact to highlight is that immunocompromised subjects are exposed to higher risk of developing HPV-related cancers compared with general population [158]. Smoke of tobacco has a synergic carcinogenic role with HPV infection, since it has immunosuppressive impact and facilitates the integration of viral genome in HPV-infected cells [148]. After viral genome integration, HPV E6 inactivates p53 and HPV E7 eliminates pRB-E2F complexes in the host cells [17]. The result is augmented chromosomal instability and cell cycle deregulation that led to carcinogenesis.
11. A way to intercept malignant transformation
The specific role of HPV in malignant transformation of oral benign lesions is still controversial. Already in 2008 Acay et al. [159] suggested the possible impact of HPV infection the carcinogenesis of oral benign lesions; in fact, they demonstrated that, even with some limitations, HPV prevalence was higher in premalignant and malignant lesions and that the cases at highest risk were associated with HPV infection. For this reason, detecting the persistent infection of HPV, especially the 16 type may be a good source to intercept premalignant and malignant lesions. The challenge is to find the most accurate, safe and easy method to do that.
11.1. Salivary test
Saliva is arising as a promising tool that may give the possibility to early detect OPSCC driven by persistent HPV infection. Its clinical significance has been at the center of a scientific debate. Using saliva as a screening test may enable to detect early stage oropharynx cancer before development of clinical symptoms. Zhao et al. [160] investigated the feasibility and the efficacy of quantitative PCR-based saliva rinse screening of HPV 16 for head and neck cancer (HNSC); they tested the salivary rinses of 92 head and neck cancer-affected patients and 604 healthy controls. The results showed that HPV was detected in 50 % of the samples coming from patients with detectable HPV 16 in the tissue; HPV16 was detected in 18 % of saliva rinse samples from patients with HPV16-negative primary HNSC; HPV16 was detected in 2.8 % of saliva rinse samples from normal controls, the statistical significance of these findings is indicated by "p < 0.001." Using a cutoff of HPV16 > 0.001 copies/cell in saliva rinse samples yielded a sensitivity of 30.4 % (ability to correctly identify true positives) and a specificity of 98.3 % (ability to correctly identify true negatives). In addition, nonsmokers had significantly higher HPV16 levels in their saliva rinse samples compared to smokers among the cancer patients.
In conclusion, the presence of HPV16 DNA in saliva rinses was found to reflect the HPV16 status of primary HNSC. Quantitative analysis of HPV16 DNA in salivary rinses allowed for the detection of HPV-related HNSC. Other salivary tests have demonstrated the association between the detection of high expression of mucin 1 and oral potentially malignant lesions or oral cancer [161]. However, there are limitations that prevent the application of this technique as a screening tool for a broad population. While the sensitivity and specificity of the technique are provided, there are limitations to its broader application as a screening tool.
About the sensitivity and specificity of salivary rinses and oral swab samples the information coming from the review of Gibson et al. [162] states that oral HPV detection has good specificity (92 %, 95 % CI = 82–97 %) and moderate sensitivity (72 %, 95 % CI = 45–89 %) for HPV-positive HNSCC tumor. While oral HPV detection has a low rate of false positives (few false-positives), it may miss a significant proportion of HPV-related cases (false-negatives) in patients who already have head and neck squamous cell carcinoma (HNSCC). This indicates that the sensitivity of oral HPV detection as a screening tool is limited when it comes to identifying HPV-related HNSCC cases. A limitation of oral samples is the impossibility of reaching the non-keratinized strata of the epithelium, that, on the contrary, is reached by using cytobrush [163].
12. Oral microbiome
Scientists have investigated the role of oral microbiome and its characteristics in health conditions versus HPV induced head and neck cancers. In particular, there is evidence about the involvement of dysbiosis of oral microbiome in the pathogenesis OSCC and that Fusobacterium is associated with better OPSCC patient outcomes [164]. Another study [165] has studied the transition of oral and gut microbiome in patients affected by OPSCC up to the effect of the chemotherapy and discovered that chemotherapy induces changes that make oral microbiome like the gut one. Identifying the specific stage-features may help in evaluating the prognosis of OPSCC [166]. Saikia et al. [167] have underlined the importance of the microbiome in inducing cancer. According to their study specific oral microbiota, such as HPV, PG, and FN, may enhance the inflammatory microenvironment of OSCC and induce stemness in oral cancer cells. Additionally, these and other unidentified oral microbiota species may influence CSC niche defense by activating tumor stemness pathways and modulating epigenetic mechanisms. Hence, there is a need to develop novel in vitro and in vivo models to study the interaction between oral microbiota and oral cancer cells/CSCs, which leads to the enhancement of CSC stemness, including the induction of a TSD phenotype. Overall, new approaches are necessary to investigate the complex interaction between oral microbiota and cancer stemness in order to identify new targets against OSCC progression and relapse.
13. Prevention for HPV related lesions and cancer
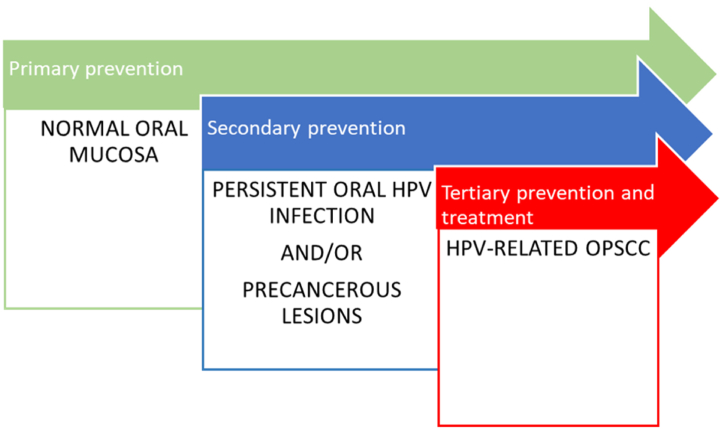
An important role is given by the prevention that can be act at different levels. It can be primary when the subject is still in health condition; secondary, when the subject has a persistent infection and/or a precancerous lesion and tertiary, when the subject has developed malignant lesion. The best option is to act by a primary prevention, but it is not always possible, so dentists have to know which tools we can use in every situation.
13.1. Primary prevention
Primary prevention (Fig. 3) can be done through information about the risks of HPV infection and its way of transmission and through vaccination.
Fig. 3.
Three types of prevention. The use of vaccines represents primary prevention, which represents intervention in healthy oral mucosa. Secondary prevention may be conducted during latent but persistent HPV infection and or in the presence of precancerous lesions. At the end, in the advanced cases, that is to say the oncological cases, in which the oral lesions have been transformed in cancer the only chance of intervention is represented by tertiary prevention and treatment.
It has been demonstrated that among adolescents there is a lack of knowledge about the HPV infection and its implications. In order to avoid such ignorance and the related low adherence to vaccination it should be highlighted the importance of spreading information through the framework of compulsory schooling, primary health care, and the development of informative interactive interventions [168].
The HPV vaccines target the L1 capsid proteins of the virus, which are crucial for viral entry into host cells. By inducing an immune response against these proteins, the vaccines aim to prevent HPV infection and the subsequent development of HPV-related cancers. Controlled clinical trials have shown that prophylactic HPV vaccines are highly effective in preventing vaccine-type HPV infections and associated anogenital precancerous lesions [169]. These vaccines have demonstrated 90 %–100 % efficacy in these trials. The use of HPV vaccines has the potential to significantly reduce the burden of anogenital cancers worldwide [170]. Anogenital cancers include cervical, anal, vaginal, vulvar, and penile cancers. Moreover, there is also evidence about the effectiveness of HPV vaccines against OP cancers [171]. While the vaccines have been highly effective in preventing anogenital and head and neck HPV infections, there is limited evidence regarding their impact on oral HPV infections specifically. Only one trial has shown a reduction in the prevalence of oral HPV infection four years after vaccination compared to a placebo [172]. A study by Pinto et al. [173] demonstrated that vaccination of males can induce HPV antibodies levels at the oral cavity, and these levels correlate with circulating levels of antibodies in the blood. Despite limited data, there is evidence suggesting that HPV vaccination can provide protection against vaccine-type oral HPV infection among both males and females in the general population [174].
It's important to note that while HPV vaccines have been successful in preventing certain types of HPV infections, they are most effective when administered before an individual becomes sexually active and exposed to the virus. Therefore, widespread vaccination efforts targeting adolescents and young adults have been recommended to achieve maximum benefits [174]. There are different formulations for the HPV vaccines: two-valent, four-valent, and nine-valent vaccines and there are also laws regulating their obligatory or recommendatory administration around the world, especially for the adolescents. For example, according to the US Food and Drug Administration (FDA) it recommended to vaccinate girls aged between 12 and 26 years and boys aged between 13 and 21 years old [175]. In addition, the FDA introduced the administration of nine-valent vaccine for women and men from 27 to 45 years old too [114]. In Italy, the access to this vaccination is free of charge and highly recommended for young people (both females and males) during the 12th year of age [114]. In Denmark, the HPV vaccine is free of charge for boys and girls from 12 to 18 years old; moreover, until 2021, men aged between 18 and 26 years, who had sexual relationships with other men could get the HPV vaccination for free too. The vaccination program of Netherlands provides HPV vaccines for free for both genders at the age of nine. Moreover, in Denmark, HPV vaccines are administered in the presence of precursor lesions in vaginal, vulvar, cervical and anal cancer, with the exception for the oropharyngeal cancer. Such exception is due to the fact that there is still not a clear demonstrated causal relationship between the administration of vaccine and the regression of premalignant and malignant oral lesions; even though the proven reduction of HPV oral infection after HPV vaccine administration suggests that such correlation may become real [176,177]. In recent decades, several preclinical and clinical studies have demonstrated the potential of genetically engineered lactic acid bacteria (LAB) to deliver recombinant antigens to induce mucosal, humoral, and cellular immunity in the host. This technique has also been applied to LAB-based oral mucosal HPV vaccines, noting the ability to express inducible surface-anchored antigens, showing increased potential to induce particularly specific systemic and mucosal cytotoxic cellular immune responses. However, they are still in the early stages of study and therefore further study is needed, but from the current data, it is not excluded that HPV vaccines obtained by LAB may be developed in the future [178].
13.2. Secondary prevention
About secondary prevention (Fig. 3) transcervical ultrasonography is a technique that can be used to evaluate and visualize cancers at the base of the tongue [179]. It may play a role in identifying high-risk individuals who are at risk of developing OPSCC. The detection of HPV DNA in oral rinses is a potential screening tool for OPSCC too. However, there is currently very little data available on its effectiveness as a screening tool. Detecting antibodies against HPV proteins, such as HPV16 E6, in blood samples (seropositivity) has been studied as a potential screening marker for OPSCC [131,180]. HPV16 E6 seropositivity has been associated with a significantly increased risk of OPSCC [131,181]. While there may be strong associations between HPV seropositivity and OPSCC risk, this does not necessarily translate into a clinically useful screening tool. The prevalence of OPSCC is relatively low in the general population, so even with a highly specific test (over 99 % specificity), the positive predictive value (PPV) may still be low [182]. Currently, there are no well-established precancerous lesions of the oropharynx that can be reliably targeted for intervention. This further complicates the development of an effective preventive screening tool. As of now, there is no validated preventive screening tool for OPSCC in the general population. More research and investigation are needed to develop and validate effective screening approaches.
It should be highlighted that developing effective screening tools for OPSCC presents significant challenges due to the relatively low prevalence of the disease and the lack of clearly identifiable precancerous lesions. Until more data and research are available, primary prevention efforts through HPV vaccination remain crucial for reducing the incidence of HPV-related cancers, including OPSCC [183].
13.3. Tertiary prevention
After completing definitive therapy for OPSCC, some patients may still have persistent HPV infection. A preliminary study evaluated oral HPV-DNA detection using oral rinses at different time points (9, 12, 18, and 24 months) after treatment [184]. The study found that patients with persistent HPV16 infection had worse survival outcomes. Specifically, 5 out of 6 patients with persistent HPV-DNA detection showed disease progression, whereas only 9 out of 62 patients without persistent HPV-DNA detection progressed. The study suggests that persistent HPV infection may serve as a potential marker for disease progression and recurrence after treatment, making it a candidate for use in tertiary prevention strategies. Despite its potential utility, the test for oral HPV-DNA detection has some limitations in terms of its operating characteristics. The test showed a relatively low sensitivity (prevalence of a positive oral rinse at diagnosis was only 54 %), which means it may miss some cases of persistent infection. Additionally, the positive predictive value of the test may not be highly reliable, and the number needed to treat (to intervene based on the test results) might be high. Due to the limitations mentioned above, the test for oral HPV-DNA detection is not ready for immediate clinical adoption. Further research and validation are needed to improve its sensitivity, specificity, and predictive value before considering its routine clinical use [185]. In the treatment of OPSCC, important new findings show how the use of immunotherapy could change prognosis and long-term disease outcome in these patients, especially in relapsed forms. However, there is still little information in this context about the prognostic role played by HPV [81].
It's important to note that tertiary prevention strategies (Fig. 3) are still in the early stages of investigation, and more research is required to identify effective methods for detecting and managing persistent HPV infection post-treatment. As the field of HPV-related cancers continues to evolve, ongoing research may lead to the development of more reliable and clinically useful tools for tertiary prevention [185].
14. Conclusions
The main risk of HPV infection is its potential to cause malignant transformation, particularly in the context of oropharyngeal squamous cell carcinoma (OPSCC). HPV testing is crucial for diagnosis and prognosis, with early detection and genotyping essential for reducing the risk of cancer development. Primary prevention through education, screening, and vaccination is the key to minimize HPV transmission. However, current diagnostic tools have limitations, necessitating the development of more sensitive techniques like salivary tests. The suggestion for the ordinary practice is to appeal to multidisciplinary management, with dentists playing a role in monitoring oral health to intercept HPV-related lesions early, improving prognosis. Frequent visits to the dentist and accurate exam of oral tissues can help to reach this purpose. The present review tried to fill the existing gaps in the current research by giving a panoramic view of oral and oropharyngeal lesions HPV-induced, their malignant transformation, the possibility of prevention and, last but not least, the ways to diagnosticate them and to early intercept malignant transformation. Interesting is the role of oral microbiome, but the current data are still insufficient to clarify its role. In conclusion, the future research should focus on finding the most accurate ways to early diagnosticate HPV infection and the specific role of microbiome in oral cancer in order to reduce incidence of HPV driven oral cancers.
Funding
This research was funded by funds of the PRIN (Research Projects of National Interest) Program - Italian Ministry for Universities and Research (MIUR). Grant Number: 202254FLSB Head Prof. Gaetano Isola.
Institutional review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Mariacristina Amato: Data curation. Simona Santonocito: Writing – original draft, Resources. Maria Teresa Bruno: Methodology. Alessandro Polizzi: Investigation. Alessandro Mastroianni: Supervision. Akhilanand Chaurasia: Validation. Gaetano Isola: Writing – review & editing, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Alessandro Polizzi, Email: alessandro.polizzi@phd.unict.it.
Gaetano Isola, Email: gaetano.isola@unict.it.
References
- 1.Ciccarese G., et al. Prevalence of genital, oral, and anal HPV infection among STI patients in Italy. J. Med. Virol. 2017;89(6):1121–1124. doi: 10.1002/jmv.24746. [DOI] [PubMed] [Google Scholar]
- 2.Lu X.J.D., et al. Prognostic value and cost benefit of HPV testing for oropharyngeal cancer patients. Oral Dis. 2023;29(2):483–490. doi: 10.1111/odi.13938. [DOI] [PubMed] [Google Scholar]
- 3.Wierzbicka M., et al. Oral and laryngeal HPV infection: incidence, prevalence and risk factors, with special regard to concurrent infection in head, neck and genitals. Vaccine. 2021;39(17):2344–2350. doi: 10.1016/j.vaccine.2021.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Pazol K., et al. Receipt of selected preventive health services for women and men of reproductive age—United States, 2011–2013. MMWR Surveillance summaries. 2017;66(20):1. doi: 10.15585/mmwr.ss6620a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debrah O., et al. Prevalence of vaccine and non-vaccine human papillomavirus types among women in Accra and Kumasi, Ghana: a cross-sectional study. BMC Wom. Health. 2021;21(1):372. doi: 10.1186/s12905-021-01511-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno M.T., et al. Possible role of negative human papillomavirus E6/E7 mRNA as a predictor of regression of cervical intraepithelial neoplasia 2 lesions in hr-HPV positive women. Virol. J. 2022;19(1):95. doi: 10.1186/s12985-022-01822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno M.T., et al. Management of ASC-US/HPV positive post-menopausal woman. Virol. J. 2019;16(1):39. doi: 10.1186/s12985-019-1145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno M.T., et al. Conservative management of CIN2 p16 positive lesions in women with multiple HPV infection. BMC Infect. Dis. 2020;20(1):801. doi: 10.1186/s12879-020-05530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piperi E., et al. Management of oral herpes simplex virus infections: the problem of resistance. A narrative review. Oral Dis. 2023 doi: 10.1111/odi.14635. [DOI] [PubMed] [Google Scholar]
- 10.Drozdzik A. Covid-19 and SARS-CoV-2 infection in periodontology: a narrative review. J. Periodontal. Res. 2022;57(5):933–941. doi: 10.1111/jre.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testi D., et al. HPV and oral lesions: preventive possibilities, vaccines and early diagnosis of malignant lesions. Oral Implantol (Rome) 2015;8(2–3):45–51. doi: 10.11138/orl/2015.8.2.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piemonte E.D., et al. Chronic mechanical irritation enhances the effect of tobacco and alcohol on the risk of oral squamous cell carcinoma: a case-control study in Argentina. Clin Oral Investig. 2022;26(10):6317–6326. doi: 10.1007/s00784-022-04584-w. [DOI] [PubMed] [Google Scholar]
- 13.Pentenero M., et al. Chronic mechanical trauma/irritation and oral carcinoma: a systematic review showing low evidence to support an association. Oral Dis. 2022;28(8):2110–2118. doi: 10.1111/odi.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devoize L., et al. Poor dental condition is a factor of imbalance of the nutritional status at the outset of management of head and neck cancer. Clin Oral Investig. 2022;26(2):1251–1259. doi: 10.1007/s00784-021-04097-y. [DOI] [PubMed] [Google Scholar]
- 15.Betz S.J. HPV-related papillary lesions of the oral mucosa: a review. Head Neck Pathol. 2019;13(1):80–90. doi: 10.1007/s12105-019-01003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundberg J., et al. High-risk human papillomavirus in patients with oral leukoplakia and oral squamous cell carcinoma-A multi-centre study in Sweden, Brazil and Romania. Oral Dis. 2021;27(2):183–192. doi: 10.1111/odi.13510. [DOI] [PubMed] [Google Scholar]
- 17.Blatt S., et al. Clinical efficacy of an antibody-based detection system for human papilloma virus infection in oral squamous cell carcinoma. Clin Oral Investig. 2021;25(5):2837–2843. doi: 10.1007/s00784-020-03601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akagi M., et al. ACE2 expression and spike S1 protein-mediated immune responses in oral mucosal cells. Oral Dis. 2023 doi: 10.1111/odi.14670. [DOI] [PubMed] [Google Scholar]
- 19.Klaes R., et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59(24):6132–6136. [PubMed] [Google Scholar]
- 20.Kirschnick L.B., et al. Transcriptionally active HPV in OPMD and OSCC: a systematic review following the CAP/ASCO guidelines. Oral Dis. 2022;28(8):2309–2313. doi: 10.1111/odi.14154. [DOI] [PubMed] [Google Scholar]
- 21.Shewale J.G., et al. In vitro antiviral activity of stabilized chlorine dioxide containing oral care products. Oral Dis. 2023;29(3):1333–1340. doi: 10.1111/odi.14044. [DOI] [PubMed] [Google Scholar]
- 22.Qin R., et al. Effect of lentivirus-mediated BMP2 from autologous tooth on the proliferative and osteogenic capacity of human periodontal ligament cells. J. Periodontal. Res. 2022;57(4):869–879. doi: 10.1111/jre.13025. [DOI] [PubMed] [Google Scholar]
- 23.Della Fera A.N., et al. Persistent human papillomavirus infection. Viruses. 2021;13(2) doi: 10.3390/v13020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S.J., et al. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016;27(5):e51. doi: 10.3802/jgo.2016.27.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosseto J.H.F., et al. Epstein-Barr virus oral shedding and viremia and their association with oral hairy leukoplakia in HIV+ individuals. Oral Dis. 2023;29(2):796–802. doi: 10.1111/odi.14001. [DOI] [PubMed] [Google Scholar]
- 26.Cui X.F., et al. Identification of competing endogenous RNA network in laryngeal squamous cell carcinoma. Oral Dis. 2023;29(2):574–583. doi: 10.1111/odi.13983. [DOI] [PubMed] [Google Scholar]
- 27.Myles L., Merlo E. Elucidating the Cognitive mechanisms Underpinning Behavioural activation. Int. J. Psychol. Res. 2022;15(1):126–132. doi: 10.21500/20112084.5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merlo Em S.F., Frisone F., Alibrandi A., Settineri S. Burden and professional quality of life of caregivers: the clinical role of suppression and resilience. Life Span and Disabilityy XXIV. 2021;1(1):55–83. [Google Scholar]
- 29.Popoviciu M.S., et al. Correlations between diabetes Mellitus Self-care Activities and Glycaemic control in the adult population: a cross-sectional study. Healthcare (Basel) 2022;10(1) doi: 10.3390/healthcare10010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi Y.J., Park J.S. Clinical significance of human papillomavirus genotyping. J Gynecol Oncol. 2016;27(2):e21. doi: 10.3802/jgo.2016.27.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walboomers J.M., et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Graham S.V. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin. Sci. (Lond.) 2017;131(17):2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 33.Sabeena S., et al. Possible non-sexual modes of transmission of human papilloma virus. J. Obstet. Gynaecol. Res. 2017;43(3):429–435. doi: 10.1111/jog.13248. [DOI] [PubMed] [Google Scholar]
- 34.Castillo P., et al. Accuracy of liquid-based brush cytology and HPV detection for the diagnosis and management of patients with oropharyngeal and oral cancer. Clin Oral Investig. 2022;26(3):2587–2595. doi: 10.1007/s00784-021-04228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petca A., et al. Non-sexual HPV transmission and role of vaccination for a better future. Exp. Ther. Med. 2020;20(6):186. doi: 10.3892/etm.2020.9316. Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryndock E.J., Meyers C. A risk for non-sexual transmission of human papillomavirus? Expert Rev. Anti Infect. Ther. 2014;12(10):1165–1170. doi: 10.1586/14787210.2014.959497. [DOI] [PubMed] [Google Scholar]
- 37.Yu W., et al. Identification of potentially functional circRNAs and prediction of circRNA-miRNA-mRNA regulatory network in periodontitis: Bridging the gap between bioinformatics and clinical needs. J. Periodontal. Res. 2022;57(3):594–614. doi: 10.1111/jre.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arduino P.G., et al. Herpes simplex virus type 1 in subgingival plaque and periodontal diseases. Meta-analysis of observational studies. J. Periodontal. Res. 2022;57(2):256–268. doi: 10.1111/jre.12968. [DOI] [PubMed] [Google Scholar]
- 39.Summersgill K.F., et al. Human papillomavirus in the oral cavities of children and adolescents. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(1):62–69. doi: 10.1067/moe.2001.108797. [DOI] [PubMed] [Google Scholar]
- 40.D'Souza G., et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J. Infect. Dis. 2009;199(9):1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Betz S.J. HPV-related papillary lesions of the oral mucosa: a review. Head and Neck Pathology. 2019;13(1):80–90. doi: 10.1007/s12105-019-01003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bruno M.T., et al. Oral HPV infection in women with HPV-positive cervix is Closely related to oral sex. Diagnostics. 2023;13(12) doi: 10.3390/diagnostics13122096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodrigues M.S.A., et al. Oral HPV among people who use crack-cocaine: prevalence, genotypes, risk factors, and key interventions in a remote Northern Brazilian region. Clin Oral Investig. 2021;25(2):759–767. doi: 10.1007/s00784-020-03698-3. [DOI] [PubMed] [Google Scholar]
- 44.Egawa N., et al. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7(7):3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham S.V. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clinical science. 2017;131(17):2201–2221. doi: 10.1042/CS20160786. [DOI] [PubMed] [Google Scholar]
- 46.Stanley M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012;25(2):215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bordignon V., et al. How human papillomavirus replication and immune Evasion strategies take advantage of the host DNA damage Repair Machinery. Viruses. 2017;9(12) doi: 10.3390/v9120390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Westrich J.A., Warren C.J., Pyeon D. Evasion of host immune defenses by human papillomavirus. Virus Res. 2017;231:21–33. doi: 10.1016/j.virusres.2016.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grabowska A.K., Riemer A.B. The invisible enemy - how human papillomaviruses avoid recognition and clearance by the host immune system. Open Virol. J. 2012;6:249–256. doi: 10.2174/1874357901206010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knapp M.J. Oral disease in 181,338 consecutive oral examinations. The Journal of the American Dental Association. 1971;83(6):1288–1293. doi: 10.14219/jada.archive.1971.0483. [DOI] [PubMed] [Google Scholar]
- 51.Frigerio M., Martinelli-Kläy C.P., Lombardi T. Clinical, histopathological and immunohistochemical study of oral squamous papillomas. Acta Odontol. Scand. 2015;73(7):508–515. doi: 10.3109/00016357.2014.996186. [DOI] [PubMed] [Google Scholar]
- 52.Abbey L.M., Page D.G., Sawyer D.R. The clinical and histopathologic features of a series of 464 oral squamous cell papillomas. Oral Surg. Oral Med. Oral Pathol. 1980;49(5):419–428. doi: 10.1016/0030-4220(80)90286-8. [DOI] [PubMed] [Google Scholar]
- 53.Eversole L.R. Papillary lesions of the oral cavity: relationship to human papillomaviruses. J. Calif. Dent. Assoc. 2000;28(12):922–926. [PubMed] [Google Scholar]
- 54.Panici P.B., et al. Oral condyloma lesions in patients with extensive genital human papillomavirus infection. Am. J. Obstet. Gynecol. 1992;167(2):451–458. doi: 10.1016/s0002-9378(11)91428-8. [DOI] [PubMed] [Google Scholar]
- 55.Eversole L., et al. Demonstration of human papillomavirus DNA in oral condyloma acuminatum. J. Oral Pathol. Med. 1987;16(5):266–272. doi: 10.1111/j.1600-0714.1987.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 56.Squires J., et al. Oral condylomata in children. Arch. Pediatr. Adolesc. Med. 1999;153(6):651–654. doi: 10.1001/archpedi.153.6.651. [DOI] [PubMed] [Google Scholar]
- 57.Pringle G.A. The role of human papillomavirus in oral disease. Dental Clinics. 2014;58(2):385–399. doi: 10.1016/j.cden.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 58.Agnew C., Alexander S., Prabhu N. Multifocal epithelial hyperplasia. J. Dent. Child. 2017;84(1):47–49. [PubMed] [Google Scholar]
- 59.Syrjänen S. Human papillomavirus infections and oral tumors. Medical microbiology and immunology. 2003;192:123–128. doi: 10.1007/s00430-002-0173-7. [DOI] [PubMed] [Google Scholar]
- 60.Esmeili T., Lozada-Nur F., Epstein J. Common benign oral soft tissue masses. Dental Clinics. 2005;49(1):223–240. doi: 10.1016/j.cden.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 61.Ramos Pena D.E., et al. Non-surgical periodontal debridement affects subgingival bacterial diversity in patients with HIV-1 and periodontitis. J. Periodontol. 2022;93(10):1455–1467. doi: 10.1002/JPER.21-0466. [DOI] [PubMed] [Google Scholar]
- 62.Sereme Y., et al. Gingival tissue as a reservoir for human immunodeficiency virus type 1: preliminary results of a cross-sectional observational study. J. Periodontol. 2022;93(4):613–620. doi: 10.1002/JPER.21-0345. [DOI] [PubMed] [Google Scholar]
- 63.Antezack A., et al. New putative periodontopathogens and periodontal health-associated species: a systematic review and meta-analysis. J. Periodontal. Res. 2023;58(5):893–906. doi: 10.1111/jre.13173. [DOI] [PubMed] [Google Scholar]
- 64.Bao Z., et al. Clinicopathologic features of oral squamous papilloma and papillary squamous cell carcinoma: a study of 197 patients from eastern China. Ann. Diagn. Pathol. 2012;16(6):454–458. doi: 10.1016/j.anndiagpath.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Hoffmann D. The role of the oral cavity in SARS-CoV-2- and other viral infections. Clin Oral Investig. 2023;27(Suppl 1):15–22. doi: 10.1007/s00784-023-05078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syrjänen S. Current concepts on human papillomavirus infections in children. Apmis. 2010;118(6‐7):494–509. doi: 10.1111/j.1600-0463.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 67.Kui L.L., Xiu H.Z., Ning L.Y. Condyloma acuminatum and human papilloma virus infection in the oral mucosa of children. Pediatr. Dent. 2003;25(2):149–153. [PubMed] [Google Scholar]
- 68.Eickholz P., et al. Clinical benefits of systemic amoxicillin/metronidazole may depend on periodontitis severity and patients' age: an exploratory sub-analysis of the ABPARO trial. J. Clin. Periodontol. 2019;46(4):491–501. doi: 10.1111/jcpe.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fonseca T.C., et al. Global prevalence of human papillomavirus-related oral and oropharyngeal squamous cell carcinomas: a systematic review and meta-analysis. Clin Oral Investig. 2023;28(1):62. doi: 10.1007/s00784-023-05425-0. [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y., et al. Correlation of immune makers with HPV 16 infections and the prognosis in oropharyngeal squamous cell carcinoma. Clin Oral Investig. 2023;27(4):1423–1433. doi: 10.1007/s00784-023-04926-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mammas I.N., Sourvinos G., Spandidos D.A. Human papilloma virus (HPV) infection in children and adolescents. Eur. J. Pediatr. 2009;168:267–273. doi: 10.1007/s00431-008-0882-z. [DOI] [PubMed] [Google Scholar]
- 72.Plasencia J.M. Cutaneous warts: diagnosis and treatment. Prim Care. 2000;27(2):423–434. doi: 10.1016/s0095-4543(05)70204-9. [DOI] [PubMed] [Google Scholar]
- 73.Eversole L., Laipis P., Green T. Human papillomavirus type 2 DNA in oral and labial verruca vulgaris. J. Cutan. Pathol. 1987;14(6):319–325. doi: 10.1111/j.1600-0560.1987.tb01531.x. [DOI] [PubMed] [Google Scholar]
- 74.Fatahzadeh M. Oral manifestations of viral infections. Atlas Oral Maxillofac Surg Clin North Am. 2017;25(2):163–170. doi: 10.1016/j.cxom.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Sethi S., et al. An update on Heck's disease-a systematic review. J. Public Health. 2022;44(2):269–285. doi: 10.1093/pubmed/fdaa256. [DOI] [PubMed] [Google Scholar]
- 76.Odell E., et al. Oral epithelial dysplasia: recognition, grading and clinical significance. Oral Dis. 2021;27(8):1947–1976. doi: 10.1111/odi.13993. [DOI] [PubMed] [Google Scholar]
- 77.Bozca B.C., Ozbudak I.H., Alpsoy E. A case of Heck's disease with primary intestinal lymphangiectasia treated with imiquimod. Indian J. Dermatol., Venereol. Leprol. 2020;86:724. doi: 10.4103/ijdvl.IJDVL_898_19. [DOI] [PubMed] [Google Scholar]
- 78.Schwenger J.U., von Buchwald C., Lindeberg H. Oral focal epithelial hyperplasia. Any risk of confusion with oral condylomas? Ugeskr Laeger. 2002;164(37):4287–4290. [PubMed] [Google Scholar]
- 79.Santonocito S., et al. A cross-Talk between Diet and the oral microbiome: Balance of Nutrition on inflammation and immune System's response during periodontitis. Nutrients. 2022;14(12) doi: 10.3390/nu14122426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhariwal S.K., Cubie H.A., Southam J.C. Detection of human papillomavirus in oral lesions using commercially developed typing kits. Oral Microbiol. Immunol. 1995;10(1):60–63. doi: 10.1111/j.1399-302x.1995.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 81.De Felice F., et al. Clinical outcomes in relapsed oropharyngeal cancer after definitive (chemo) radiotherapy. Oral Dis. 2023;29(2):595–603. doi: 10.1111/odi.13985. [DOI] [PubMed] [Google Scholar]
- 82.Pierangeli A., et al. Frequent detection of high human papillomavirus DNA loads in oral potentially malignant disorders. Clin. Microbiol. Infect. 2016;22(1) doi: 10.1016/j.cmi.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 83.Termine N., et al. Biopsy vs. brushing: comparison of two sampling methods for the detection of HPV-DNA in squamous cell carcinoma of the oral cavity. Oral Oncol. 2012;48(9):870–875. doi: 10.1016/j.oraloncology.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Hasegawa S., et al. Aromatase inhibitor anastrozole modifies cellular functions in gingival fibroblasts and endothelial cells: possible periodontal complications of aromatase inhibitor treatment. J. Periodontal. Res. 2021;56(4):828–836. doi: 10.1111/jre.12881. [DOI] [PubMed] [Google Scholar]
- 85.Oka S., et al. A deficiency of Dec2 triggers periodontal inflammation and pyroptosis. J. Periodontal. Res. 2021;56(3):492–500. doi: 10.1111/jre.12849. [DOI] [PubMed] [Google Scholar]
- 86.Jacome-Santos H., et al. Simultaneous occurrence of Epstein-Barr virus (EBV) in periodontal pockets and in oral squamous cell carcinoma: a cross-sectional study. Clin Oral Investig. 2022;26(3):2807–2815. doi: 10.1007/s00784-021-04258-z. [DOI] [PubMed] [Google Scholar]
- 87.Gillison M.L., et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouda M., et al. “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod. Pathol. 2000;13(6):644–653. doi: 10.1038/modpathol.3880113. [DOI] [PubMed] [Google Scholar]
- 89.Giovannelli L., et al. Human papillomavirus DNA in oral mucosal lesions. The Journal of infectious diseases. 2002;185(6):833–836. doi: 10.1086/339193. [DOI] [PubMed] [Google Scholar]
- 90.Edelstein Z.R., Schwartz S.M., Koutsky L.A. Incidence of oral human papillomavirus infection. Lancet. 2013;382(9904):1554. doi: 10.1016/S0140-6736(13)62326-0. [DOI] [PubMed] [Google Scholar]
- 91.Blioumi E., et al. Detection and typing of human papillomaviruses (HPV) in malignant, dysplastic, nondysplastic and normal oral epithelium by nested polymerase chain reaction, immunohistochemistry and transitional electron microscopy in patients of northern Greece. Oral Oncol. 2014;50(9):840–847. doi: 10.1016/j.oraloncology.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 92.Franceschi S., et al. Deep brush-based cytology in tonsils resected for benign diseases. Int. J. Cancer. 2015;137(12):2994–2999. doi: 10.1002/ijc.29660. [DOI] [PubMed] [Google Scholar]
- 93.Donà M.G., et al. Cytology and human papillomavirus testing on cytobrushing samples from patients with head and neck squamous cell carcinoma. Cancer. 2014;120(22):3477–3484. doi: 10.1002/cncr.28909. [DOI] [PubMed] [Google Scholar]
- 94.Ayilavarapu S., et al. Altered human alveolar bone gene expression in type 2 diabetes-A cross-sectional study. J. Periodontal. Res. 2022;57(1):142–151. doi: 10.1111/jre.12947. [DOI] [PubMed] [Google Scholar]
- 95.Li Y., et al. Inhibition of acid sphingomyelinase by imipramine abolishes the synergy between metabolic syndrome and periodontitis on alveolar bone loss. J. Periodontal. Res. 2022;57(1):173–185. doi: 10.1111/jre.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maver P.J., Poljak M. Primary HPV-based cervical cancer screening in Europe: implementation status, challenges, and future plans. Clin. Microbiol. Infect. 2020;26(5):579–583. doi: 10.1016/j.cmi.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 97.Buenahora M.R., et al. Diagnostic accuracy of clinical visualization and light-based tests in precancerous and cancerous lesions of the oral cavity and oropharynx: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(6):4145–4159. doi: 10.1007/s00784-020-03746-y. [DOI] [PubMed] [Google Scholar]
- 98.Buenahora M.R., et al. Correction to: diagnostic accuracy of clinical visualization and light-based tests in precancerous and cancerous lesions of the oral cavity and oropharynx: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(6):4161. doi: 10.1007/s00784-021-03810-1. [DOI] [PubMed] [Google Scholar]
- 99.Tamí-Maury I., et al. Testing the accuracy of autofluorescence device in diagnosing oral potentially malignant disorders among people with HIV seeking dental care. Clin Oral Investig. 2023;27(5):1899–1906. doi: 10.1007/s00784-022-04818-x. [DOI] [PubMed] [Google Scholar]
- 100.Lajolo C., et al. Patient perception after oral biopsies: an observational outpatient study. Clin Oral Investig. 2021;25(10):5687–5697. doi: 10.1007/s00784-021-03870-3. [DOI] [PubMed] [Google Scholar]
- 101.Zil A.R., et al. Human papilloma virus--role in precancerous and cancerous oral lesions of tobacco chewers. J Pak Med Assoc. 2013;63(10):1295–1298. [PubMed] [Google Scholar]
- 102.Chi A.C., Day T.A., Neville B.W. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015;65(5):401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 103.Morbini P., Benazzo M. Human papillomavirus and head and neck carcinomas: focus on evidence in the babel of published data. Acta Otorhinolaryngol. Ital. 2016;36(4):249–258. doi: 10.14639/0392-100X-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giraldo P., et al. Human papillomavirus in the oral mucosa of women with genital human papillomavirus lesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006;126(1):104–106. doi: 10.1016/j.ejogrb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 105.Kurose K., et al. Low prevalence of HPV infection and its natural history in normal oral mucosa among volunteers on Miyako Island, Japan. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;98(1):91–96. doi: 10.1016/j.tripleo.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 106.Coutlée F., et al. Risk factors for oral human papillomavirus in adults infected and not infected with human immunodeficiency virus. Sex. Transm. Dis. 1997;24(1):23–31. doi: 10.1097/00007435-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 107.Kreimer A.R., et al. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. J. Infect. Dis. 2004;189(4):686–698. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- 108.Sánchez-Vargas L.O., Díaz-Hernández C., Martinez-Martinez A. Detection of Human Papilloma Virus (HPV) in oral mucosa of women with cervical lesions and their relation to oral sex practices. Infect Agent Cancer. 2010;5(1):25. doi: 10.1186/1750-9378-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tang K.D., et al. Oral HPV16 DNA as a screening tool to detect early oropharyngeal squamous cell carcinoma. Cancer Sci. 2020;111(10):3854–3861. doi: 10.1111/cas.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mork J., et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2001;344(15):1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 111.Giuliano A.R., et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377(9769):932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Guan P., et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int. J. Cancer. 2012;131(10):2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 113.Rapado-González Ó., et al. Association of salivary human papillomavirus infection and oral and oropharyngeal cancer: a meta-analysis. J. Clin. Med. 2020;9(5) doi: 10.3390/jcm9051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verro B., Gallina S., Saraniti C. Papillomavirus infection and prevention: how much does the Sicilian population know? An observational study. Int J Environ Res Public Health. 2022;19(17) doi: 10.3390/ijerph191711032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bettampadi D., et al. Factors associated with persistence and clearance of high-risk oral human papillomavirus (HPV) among Participants in the HPV infection in men (HIM) study. Clin. Infect. Dis. 2021;73(9):e3227–e3234. doi: 10.1093/cid/ciaa1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coleman H.N., et al. CD8 T-cell responses in incident and prevalent human papillomavirus types 16 and 18 infections. ISRN Obstet Gynecol. 2012;2012 doi: 10.5402/2012/854237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ziauddin S.M., et al. Cytotoxic effects of dental calculus particles and freeze-dried Aggregatibacter actinomycetemcomitans and Fusobacterium nucleatum on HSC-2 oral epithelial cells and THP-1 macrophages. J. Periodontol. 2022;93(6):e92–e103. doi: 10.1002/JPER.21-0196. [DOI] [PubMed] [Google Scholar]
- 118.Upadhyaya J.D., et al. Marginal linear gingival leukoplakia progressing to "ring around the collar"-An ominous sign of proliferative verrucous leukoplakia. J. Periodontol. 2021;92(2):273–285. doi: 10.1002/JPER.19-0621. [DOI] [PubMed] [Google Scholar]
- 119.Scott M.L., et al. Human papillomavirus 16 E5 Inhibits interferon signaling and Supports episomal viral Maintenance. J. Virol. 2020;94(2) doi: 10.1128/JVI.01582-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pannone G., et al. Evaluation of a combined triple method to detect causative HPV in oral and oropharyngeal squamous cell carcinomas: p16 Immunohistochemistry, Consensus PCR HPV-DNA, and in Situ Hybridization. Infect Agent Cancer. 2012;7:4. doi: 10.1186/1750-9378-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rietbergen M.M., et al. Molecular characterization of p16-immunopositive but HPV DNA-negative oropharyngeal carcinomas. Int. J. Cancer. 2014;134(10):2366–2372. doi: 10.1002/ijc.28580. [DOI] [PubMed] [Google Scholar]
- 122.Fidler A., et al. Virus transmission by ultrasonic scaler and its prevention by antiviral agent: an in vitro study. J. Periodontol. 2022;93(7):e116–e124. doi: 10.1002/JPER.21-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.DiMaio D., Petti L.M. The E5 proteins. Virology. 2013;445(1–2):99–114. doi: 10.1016/j.virol.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ang K.K., et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu D., et al. Risk of second primary cancer in patients with head and neck squamous cell carcinoma: a systemic review and meta-analysis. Clin Oral Investig. 2023 doi: 10.1007/s00784-023-05066-3. [DOI] [PubMed] [Google Scholar]
- 126.Varoni E.M., et al. Oral Human Papillomavirus (HPV) and sexual behaviors in a young cohort of oral cancer survivors. Oral Dis. 2021;27(4):919–923. doi: 10.1111/odi.13622. [DOI] [PubMed] [Google Scholar]
- 127.Gillison M.L., et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 128.Ragin C.C., Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int. J. Cancer. 2007;121(8):1813–1820. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 129.Huang S.H., et al. Atypical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012;82(1):276–283. doi: 10.1016/j.ijrobp.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 130.Goldenberg D., et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30(7):898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]
- 131.Gillison M.L., et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–F54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 132.Peck B.W., et al. Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck. 2013;35(6):794–799. doi: 10.1002/hed.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Martel M., et al. The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol. 2017;64:37–43. doi: 10.1016/j.oraloncology.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 134.Rietbergen M.M., et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J. Oral Pathol. Med. 2014;43(2):137–142. doi: 10.1111/jop.12123. [DOI] [PubMed] [Google Scholar]
- 135.Iwasaki M., et al. Interruption of regular dental visits during the COVID-19 pandemic due to concerns regarding dental visits was associated with periodontitis in Japanese office workers. J. Periodontal. Res. 2021;56(6):1091–1098. doi: 10.1111/jre.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cantrell S.C., et al. Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: a blinded matched-pair analysis. AJNR Am J Neuroradiol. 2013;34(10):2005–2009. doi: 10.3174/ajnr.A3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Speight P.M., Farthing P.M. The pathology of oral cancer. Br. Dent. J. 2018;225(9):841–847. doi: 10.1038/sj.bdj.2018.926. [DOI] [PubMed] [Google Scholar]
- 138.Rooper L.M., et al. HPV-Positive squamous cell carcinoma of the larynx, oral cavity, and Hypopharynx: Clinicopathologic characterization with recognition of a novel warty variant. Am. J. Surg. Pathol. 2020;44(5):691–702. doi: 10.1097/PAS.0000000000001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chakravarthy A., et al. Human papillomavirus Drives tumor development throughout the head and neck: improved prognosis is associated with an immune response largely Restricted to the oropharynx. J. Clin. Oncol. 2016;34(34):4132–4141. doi: 10.1200/JCO.2016.68.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Piattelli A., et al. Warty carcinoma of the oral mucosa in an HIV+ patient. Oral Oncol. 2001;37(8):665–667. doi: 10.1016/s1368-8375(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 141.Zhang L., et al. Nonkeratinizing squamous cell carcinoma in situ of the upper aerodigestive tract: an HPV-related entity. Head and Neck Pathology. 2017;11:152–161. doi: 10.1007/s12105-016-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lerman M.A., et al. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod. Pathol. 2017;30(12):1646–1654. doi: 10.1038/modpathol.2017.71. [DOI] [PubMed] [Google Scholar]
- 143.McCord C., et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral surgery, oral medicine, oral pathology and oral radiology. 2013;115(4):541–549. doi: 10.1016/j.oooo.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 144.Woo S.-B., Cashman E.C., Lerman M.A. Human papillomavirus-associated oral intraepithelial neoplasia. Mod. Pathol. 2013;26(10):1288–1297. doi: 10.1038/modpathol.2013.70. [DOI] [PubMed] [Google Scholar]
- 145.Lafaurie G.I., et al. Human papilloma virus: an etiological and prognostic factor for oral cancer? J Investig Clin Dent. 2018;9(2) doi: 10.1111/jicd.12313. [DOI] [PubMed] [Google Scholar]
- 146.Jiang H., Lin P.-F. Human papillomavirus infection a favorable prognostic factor in laryngeal squamous cell carcinoma is associated with the expression of proliferating cell nuclear antigen. Pakistan J. Med. Sci. 2013;29(5):1173. doi: 10.12669/pjms.295.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhao D., et al. Human papillomavirus as an independent predictor in oral squamous cell cancer. Int. J. Oral Sci. 2009;1(3):119–125. doi: 10.4248/IJOS.09015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wei L., et al. Tobacco exposure results in increased E6 and E7 oncogene expression, DNA damage and mutation rates in cells maintaining episomal human papillomavirus 16 genomes. Carcinogenesis. 2014;35(10):2373–2381. doi: 10.1093/carcin/bgu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fakhry C., et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer. Cancer. 2017;123(9):1566–1575. doi: 10.1002/cncr.30353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Young R.J., et al. Frequency and prognostic significance of p16(INK4A) protein overexpression and transcriptionally active human papillomavirus infection in laryngeal squamous cell carcinoma. Br. J. Cancer. 2015;112(6):1098–1104. doi: 10.1038/bjc.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Agalliu I., et al. Associations of oral α-, β-, and γ-human papillomavirus types with risk of incident head and neck cancer. JAMA Oncol. 2016;2(5):599–606. doi: 10.1001/jamaoncol.2015.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilson Westmark N.L., et al. Vitamin D status in patients with oropharyngeal cancer: association with HPV status and prognosis. Oral Dis. 2023;29(2):542–546. doi: 10.1111/odi.13965. [DOI] [PubMed] [Google Scholar]
- 153.Dahlstrom K.R., et al. Differences in history of sexual behavior between patients with oropharyngeal squamous cell carcinoma and patients with squamous cell carcinoma at other head and neck sites. Head Neck. 2011;33(6):847–855. doi: 10.1002/hed.21550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.D'Souza G., et al. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 155.Bahl A., et al. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36(4):505–510. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 156.Sun C.X., et al. A pilot study into the association between oral health status and human papillomavirus—16 infection. Diagnostics. 2017;7(1):11. doi: 10.3390/diagnostics7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tezal M., et al. Local inflammation and human papillomavirus status of head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 2012;138(7):669–675. doi: 10.1001/archoto.2012.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Frisch M., Biggar R.J., Goedert J.J. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 159.Acay R., et al. Human papillomavirus as a risk factor in oral carcinogenesis: a study using in situ hybridization with signal amplification. Oral Microbiol. Immunol. 2008;23(4):271–274. doi: 10.1111/j.1399-302X.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 160.Zhao M., et al. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. Int. J. Cancer. 2005;117(4):605–610. doi: 10.1002/ijc.21216. [DOI] [PubMed] [Google Scholar]
- 161.Abdelwhab A., Shaker O., Aggour R.L. Expression of Mucin1 in saliva in oral squamous cell carcinoma and oral potentially malignant disorders (case control study) Oral Dis. 2023;29(4):1487–1494. doi: 10.1111/odi.14138. [DOI] [PubMed] [Google Scholar]
- 162.Gipson B.J., et al. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol. 2018;77:52–56. doi: 10.1016/j.oraloncology.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Poate T., et al. An audit of the efficacy of the oral brush biopsy technique in a specialist Oral Medicine unit. Oral Oncol. 2004;40(8):829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 164.Chan J.Y.K., et al. Characterization of oral microbiota in HPV and non-HPV head and neck squamous cell carcinoma and its association with patient outcomes. Oral Oncol. 2022;135 doi: 10.1016/j.oraloncology.2022.106245. [DOI] [PubMed] [Google Scholar]
- 165.Oliva M., et al. Transitions in oral and gut microbiome of HPV+ oropharyngeal squamous cell carcinoma following definitive chemoradiotherapy (ROMA LA-OPSCC study) Br. J. Cancer. 2021;124(9):1543–1551. doi: 10.1038/s41416-020-01253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lim Y., et al. The performance of an oral microbiome Biomarker Panel in predicting oral cavity and oropharyngeal cancers. Front. Cell. Infect. Microbiol. 2018;8:267. doi: 10.3389/fcimb.2018.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Saikia P.J., et al. The emerging role of oral microbiota in oral cancer initiation, progression and stemness. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1198269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thanasas I., et al. Understanding of young adolescents about HPV infection: how health education can improve vaccination rate. J. Cancer Educ. 2020;35(5):850–859. doi: 10.1007/s13187-019-01681-5. [DOI] [PubMed] [Google Scholar]
- 169.Lehtinen M., Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat. Rev. Clin. Oncol. 2013;10(7):400–410. doi: 10.1038/nrclinonc.2013.84. [DOI] [PubMed] [Google Scholar]
- 170.Jit M., et al. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health. 2014;2(7):e406–e414. doi: 10.1016/S2214-109X(14)70237-2. [DOI] [PubMed] [Google Scholar]
- 171.Macilwraith P., Malsem E., Dushyanthen S. The effectiveness of HPV vaccination on the incidence of oropharyngeal cancers in men: a review. Infect Agent Cancer. 2023;18(1):24. doi: 10.1186/s13027-022-00479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herrero R., et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pinto L.A., et al. Quadrivalent human papillomavirus (HPV) vaccine induces HPV-specific antibodies in the oral cavity: results from the Mid-Adult male vaccine trial. J. Infect. Dis. 2016;214(8):1276–1283. doi: 10.1093/infdis/jiw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hirth J.M., Chang M., Resto V.A. Prevalence of oral human papillomavirus by vaccination status among young adults (18-30years old) Vaccine. 2017;35(27):3446–3451. doi: 10.1016/j.vaccine.2017.05.025. [DOI] [PubMed] [Google Scholar]
- 175.Petrusek J., Thorpe E., Britt C.J. HPV vaccination practices and attitudes among primary care physicians since FDA approval to age 45. Am. J. Otolaryngol. 2020;41(6) doi: 10.1016/j.amjoto.2020.102685. [DOI] [PubMed] [Google Scholar]
- 176.Nielsen K.J., et al. The effect of prophylactic HPV vaccines on oral and oropharyngeal HPV infection—a systematic review. Viruses. 2021;13(7):1339. doi: 10.3390/v13071339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kaczmarczyk K.H., Yusuf H. The impact of HPV vaccination on the prevention of oropharyngeal cancer: a scoping review. Community Dent. Health. 2022;39(1):14–21. doi: 10.1922/CDH_00072Kaczmarczyk08. [DOI] [PubMed] [Google Scholar]
- 178.Taghinezhad S.S., et al. Twenty years of research on HPV vaccines based on genetically modified lactic acid bacteria: an overview on the gut-vagina axis. Cell. Mol. Life Sci. 2021;78(4):1191–1206. doi: 10.1007/s00018-020-03652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Blanco R.G., et al. Transcervical ultrasonography is feasible to visualize and evaluate base of tongue cancers. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Anantharaman D., et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105(8):536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 181.Gillison M.L., et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015;33(29):3235–3242. doi: 10.1200/JCO.2015.61.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morice, P., et al., Reply to M.S. Rajagopalan et al and P.G. Rose. J. Clin. Oncol., 2014. 32(4): p. 358-359.. [DOI] [PubMed]
- 183.Taberna M., et al. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017;28(10):2386–2398. doi: 10.1093/annonc/mdx304. [DOI] [PubMed] [Google Scholar]
- 184.Rettig E.M., et al. Prognostic implication of persistent human papillomavirus type 16 DNA detection in oral rinses for human papillomavirus–related oropharyngeal carcinoma. JAMA Oncol. 2015;1(7):907–915. doi: 10.1001/jamaoncol.2015.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bussu F., et al. HPV infection in squamous cell carcinomas arising from different mucosal sites of the head and neck region. Is p16 immunohistochemistry a reliable surrogate marker? Br. J. Cancer. 2013;108(5):1157–1162. doi: 10.1038/bjc.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Isola G., et al. Impact of periodontitis on gingival crevicular fluid miRNAs profiles associated with cardiovascular disease risk. J. Periodontal Res. 2023;58(10):165–174. doi: 10.1111/jre.13078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.