Abstract
Clinical investigations are increasingly focusing on natural materials with medical benefits because, in contrast to medicines, they have extremely few adverse effects. Tinospora species of the Menispermaceae family has many bioactive principles for plant nutraceuticals. A thorough assessment of the existing literature revealed that Indian Tinospora species are an important group of medicinal herbs used for a variety of pharmacological activities. While, Tinospora cordifolia is widely recognized as a significant herb in the Indian System of Medicines (ISM) due to its bioactive components and has been used in the treatment of diabetes, cancer, urinary problems, fever, jaundice, helminthiasis, leprosy, dysentery, skin diseases, and many more.
Using the search phrases “phytochemistry,” “traditional uses,” and “pharmacological evaluation of Indian Tinospora species,” appropriate articles were carefully extracted from the MEDLINE/PubMed, Scopus, and WOS databases. Around 180 articles, related to the India Tinospora species, were selected from a pool of 200 papers published between 1991 and 2023. T. cordifolia has received a lot of scientific attention because of its diverse therapeutic characteristics in treating various diseases. Our present study in this review encompasses 1.) Phytochemistry, traditional uses and pharmacological potential of T. cordifolia as well as other Indian Tinospora species. 2.) Safety and toxicity study and available marketed formulation of T. cordifolia for the treatment of various diseases.
The chemical constitution and pharmacological characteristics of other Tinospora species must also be investigated, indicating a need for further scientific research.
Keywords: Tinospora cordifolia, Tinospora species (Menispermaceae), Phytochemistry, Pharmacological, Safety, Toxicity
Highlights
-
•
This paper provides a comprehensive analysis of Indian Tinospora cordifolia and several Tinospora species, highlighting their diverse bioactive components and their demonstrated potential in ethnopharmacology.
-
•
Phytochemistry and Pharmacological potential of Tinospora species.
-
•
Safety profile, Toxicity study and available Marketed formulation of T. cordifolia for the treatment of various diseases.
1. Introduction
Herbal formulations are pharmaceutical preparations of one or more plants present in precise quantities to offer cosmetic, diagnostic, and therapeutic benefits to humans or animals [1].
Phytomedicine is also known as plant medicine. Because herbal medicine has fewer adverse effects and is more compatible with the human body, 70–80 percent of people continue to use it for primary health care [2,3]. India's biodiversity and extensive comprehension of traditional herbal medicine systems such as Siddha, Ayurveda, and Unani provide a solid foundation for the general healthcare application of a wide variety of species and common ailments [4].
The Tinospora genus encompasses approximately 34 species that are distributed over the tropical and subtropical regions of Asia, Australia, and Africa [5]. There is a total of nine Tinospora species that have become naturalized in various states across India. Contemporary pharmacological investigations and clinical applications have revealed that Tinospora species exhibit a diverse range of actions. Tinospora cordifolia is widely recognized as a significant herb in the Indian System of Medicines (ISM) due to its bioactive components and numerous therapeutic properties, in comparison to other Indian species such as T. crispa (L.), T. sinensis (Lour.), T. baenzigeri. Whereas no ethnopharmacological activity has been reported for T. smilacina, T. maqsoodiana, T. formanii, T. glabra, and T. subcordata in ISM.
T. cordifolia (Wild) Hook. F & Thomson, Tinospora Gulancha Indian Tinospora, and Giloya are its Latin names T. cordifolia is also known as Tinospora sinensis (Lour.) Merr. and Guduchi/Amrita. It is a member of the Menispermaceae family and is native to China, Myanmar, and Sri Lanka [6]. The herb is commonly employed in conventional ayurvedic treatment. It can be used to treat jaundice, rheumatism, urinary diseases, skin conditions, diabetes, anemia, inflammation, allergic condition, anti-periodic qualities, radioprotective properties, and other conditions [7,8]. The root of the giloya plant (T. cordifolia) is used as an emetic and to relieve intestinal obstruction. This plant's starch is an effective at home treatment for chronic fever, relieving stinging sensations while increasing energy and appetite [9,10].
Giloya is useful in the treatment of helminthiasis, cardiovascular illness, leprosy, rheumatoid arthritis, and other disorders [11], and it also helps keep the immune system and the body's resistance to infections strong. It's also useful for treating gastrointestinal problems like hyperacidity, colitis, worm infestations, anorexia, stomach pain, excessive thirst, vomiting, and liver illnesses like hepatitis [12,13]. The root, stem, and entire plant all contain chemical components responsible for the plant's pharmacological activities, such as glycosides, diterpenoid lactones, sesquiterpenoids, steroids, aliphatic compounds, phenolics, essential oils, polysaccharides, a mixture of fatty acids [14].
It has garnered a great deal of scientific interest due to its diverse therapeutic properties in treating various diseases. In this review, our current work looks at 1.) Phytochemistry and pharmacological potential of T. cordifolia. 2.) Safety profile, toxicology study, and commercially available formulations for treating different illnesses.
2. Materials and methods
Using the search phrases “phytochemistry,” “chemical constituents,” “traditional uses,” and “pharmacological evaluation of Indian Tinospora cordifolia and Tinospora species,” appropriate articles were carefully extracted from the MEDLINE/PubMed, Scopus, and WOS databases. Around 170–175 articles, related to the India Tinospora species, were selected from a pool of 200 papers published between 1991 and 2023.
2.1. Growth Prerequisite
Several plant species can be used for medical purposes [15]. T. cordifolia is quickly disappearing from its natural habitat despite its widespread use in traditional and modern medicine. Biotechnological strategies for rapid distribution, scalability of secondary metabolites, and preservation of unusual, endangered, and scarce medicinal plants should be adopted [16] because the conventional approach falls short of easing depletion. Micropropagation in vitro is one of the best alternative strategies for rapid clonal mass replication of a disease-free, high-yielding plant. In vitro, metabolite creation and a few other technological methods for producing new species require cell culture [17]. Tissue culture allows for the rapid multiplication of this plant, which is mostly produced for its aesthetic value. Its optimal conditions for growth vary little from one soil type to the next or from one set of environmental conditions to another. If the plant growing on the neem tree is trained well, it will have a higher number of medicinal properties [18]. The plant is widespread across the tropics and subtropics, while it thrives best in dry, hot areas with plenty of moisture [19]. Discovery, reproduction, and survival of these fragile genotypes may rely heavily on biotechnological instruments.
3. Ethnomedicinal usage of Tinospora species
T. cordifolia is utilised to protect against a variety of human health issues. Particular attention has been paid to its efficacy in treating endocrine and metabolic diseases, as well as its capacity to boost immunity [20]. There have been reports of T. cordifolia as a key component of medications for treating metabolic, endocrine, and several other diseases receiving current protection.
In addition to reducing vascular endothelial growth factor, anti-inflammatory and analgesic properties prevent drug-resistant HIV and HIV from binding to TNF. The plant's roots, stem (branches), leaves, starch, flowers, and fruits, among other parts, all aid the human body in the treatment of a variety of maladies. T. cordifolia starch is an effective treatment for soothing searing sensations, persistent fever, enhancing appetite, and enhancing vitality. The roots of this plant is used as an emetic and to treat intestinal obstruction.
This herb can be used to treat a variety of ailments, such as stomach upset, diabetes, indigestion, high cholesterol, fever, gout, cancer, including lymphoma, liver disease, rheumatoid arthritis, stomach ulcer and gonorrhea, and syphilis.
Because it functions in conjunction with other ingredients, this plant is an essential component of polyherbal medicinal formulations used to treat a variety of diseases. It possesses preventative, immunomodulatory, anti-oxidant, and other properties that aid the body in repairing a variety of diseases. It can also enhance memory and function as a brain tonic in cases of senile and presenile dementia. Anti-spasmodic, anti-oxidant, anti-inflammatory, anti-diabetic, anti-allergic, anti-stress, anti-periodic, anti-arthritic, anti-leprotic, and anti-neoplastic properties of this plant have recently aroused interest. T. crispa has been used in the management of thirst, heat-clearing, and hunger resistance, as well as in the treatment of diabetes [12]. T. baenzigeri in traditional medicine is useful in curing antimalarial, diarrhoea, cold, headache, and fever [21], as indicated in Table 1.
Table 1.
Ethnomedicinal usage of Tinospora cordifolia and Tinospora species.
| Plant Species | Plant Part | Ethnomedicinal Uses | Reference |
|---|---|---|---|
| T. cordifolia | Whole plant, root | HIV drug resistance and HIV protease inhibitors. Tyramine is a neuro-modulator used to inhibit neurotransmitters and treat depression and anxiety. | [[22], [23], [24]] |
| Stem, root | Anticancer, antiviral. | [22,[25], [26], [27]] | |
| Whole plant | Antiviral, Anti-inflammatory, Anti-hypertensive, Anti-microbial. | [[28], [29], [30], [31], [32]] | |
| Stem | Treat neurological impairments, such as those brought on by ALS, Parkinson's disease, dementia, and aberrant movement. | [[33], [34], [35], [36], [37], [38], [39]] | |
| Stem, aerial part | Osteoporosis and IgA neuropathy are brought on by glucocorticoids in early inflammatory arthritis. | [[40], [41], [42], [43]] | |
| T. crispa | Whole plant | Anti-diabetic | [44] |
| Stem | Anti-inflammatory | [45] | |
| T. baenzigeri | stem | Antimalarial | [21] |
| T. sinensis | Root | Diuretic, immunomodulatory, acute rheumatoid arthritis, Antidepressant and aphrodisiac activity, | [46,47] |
| Whole plant | Antimicrobial, anti-inflammatory, Activity of hyperlipidemia |
The T. sinensis (Lour.) root powder was used to treat acute rheumatoid arthritis [48], and its main chemical component has been noted for its ability to suppress the development of malignant cells as well as metastasis and angiogenesis. The roots include Fe, K, Mg, and Ni, among other components, which are believed to have significant involvement in the diuretic and aphrodisiac effects. Its roots and leaves show notable action against Staphylococcus aureus [46,47]. Ethnomedicinal usage of Tinospora cordifolia and Tinospora species [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45]] are mentioned in Table 1. There is not enough scientific research documenting the traditional applications of T. maqsoodiana, T. formanii, T. glabra, T. smilacina, and T. subcordata species within the Indian traditional medicine system.
4. Chemical composition
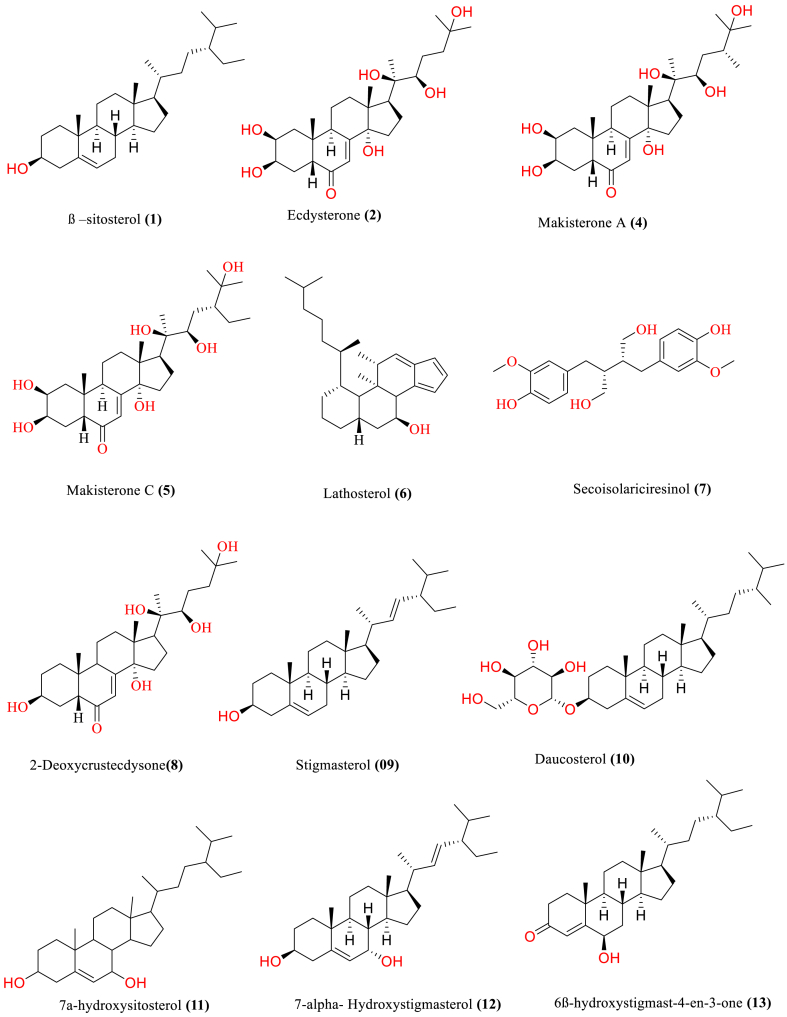
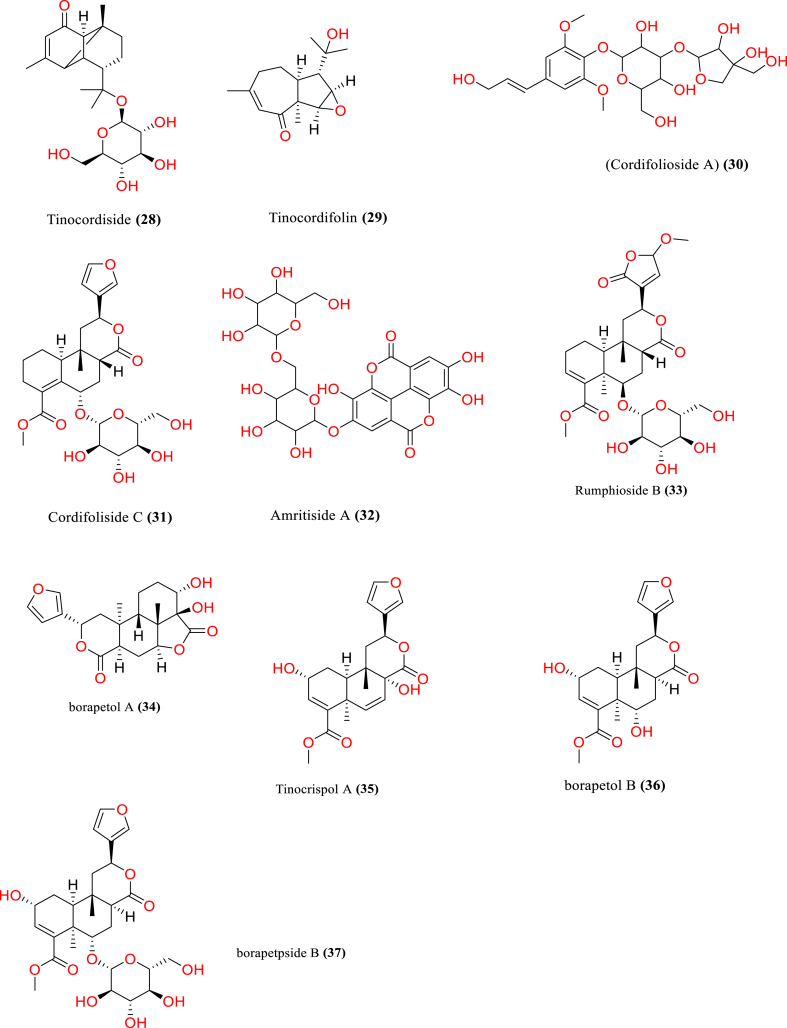
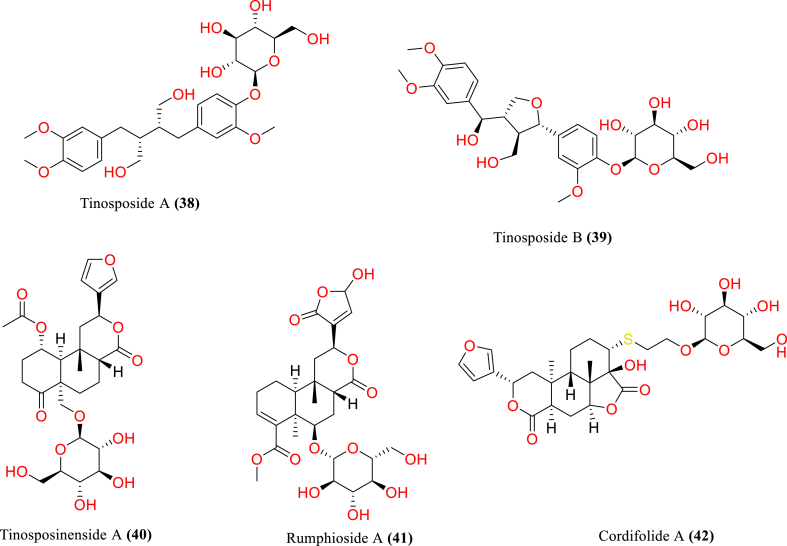
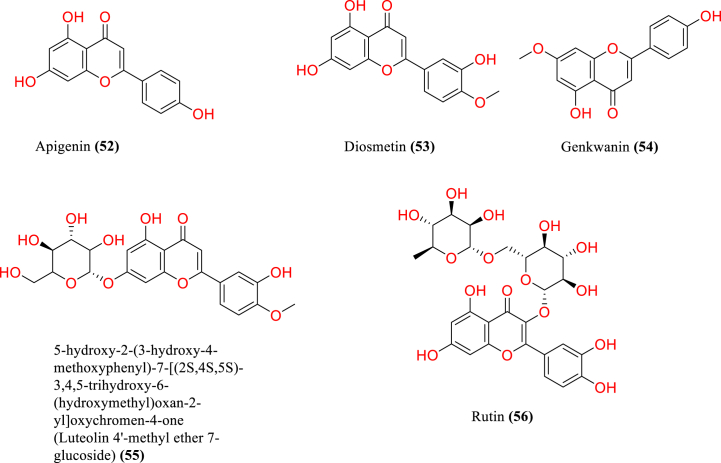
Table 2 and Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9 reports the chemical constituents of T. cordifolia and three other species viz (see Fig. 1). T. crispa (L.), T. sinensis (Lour.), T. baenzigeri belong to different classes such as glycosides, alkaloids, terpenoids, steroids, polysaccharides, sesquiterpenoids [49], etc. The main phytochemicals in T. cordifolia include tinosporide, tinosporine, tinosporaside, cordifol, cordifolide, diterpenoid furanolactone, clerodane furano diterpene, tinosporidine, columbine, and b-sitosterol [[49], [50], [51]]. The rest of five species have not been investigated for the presence of phytochemicals. No compound is reported from 5 Indian Tinospora species (T. glabra, T. formanii, T. smilacina, T. maqsoodiana, and T. subcordata).
Table 2.
Isolated Chemical constituents and mechanism of action of Indian Tinospora cordifolia and Tinospora species.
| Plant Species | Sr.no. | Isolated Compounds | Active Component | Plant parts | Mechanism of action | References |
|---|---|---|---|---|---|---|
| T. cordifolia | 1. | β-sitosterol | Steroids | Stem | Decrease inflammation | [41] |
| 2. | Ecdysterone | |||||
| 3. | Giloinsterol | |||||
| 4. | Makisterone A | |||||
| T. crispa | 5. | Makisterone C | Stems, aerial parts, vines stem | Remarkable α-glucosidase and α-amylase inhibition. | [52,53] | |
| 6. | Lathosterol | |||||
| 7. | Secoisolariciresinol | |||||
| 8. | 2-Deoxy crustecdysone | |||||
| 9. | Stigmasterol | Root | Reduce inflammation | |||
| T. sinensis | 10. | daucosterol | Stem | Anti-neuro-inflammation, regulation of immunity | [48,54,55] | |
| 11. | 7α-hydroxysitisterol | |||||
| 12. | 7α-hydroxy Stigmasterol | |||||
| 13. | 6β-hydroxystigmast-4-en-3-one | |||||
| T. cordifolia | 14. | Tembetarine | Alkaloids | Root, stem | Anti-histamine, CNS Stimulant. | [22,25,26,56] |
| 15. | Isocolumbin | |||||
| 16. | Palmetine | |||||
| 17. | Berberine | |||||
| 18. | Choline | |||||
| 19. | Magnoflorine | |||||
| T.crispa | 20. | N-Demethyl-N-formyldehydro-nuciferine | Stem | Important immune-modulatory effects include increased phagocytic activity, chemotaxis, ROS and NO generation, and TNF-α,IL-1β, IL6, PEG2, and MCP-1 production. | [52,57,58] | |
| 21. | N-cis-feruloyltyramine | |||||
| 22. | 4-(2-aminoethyl) phenol (Tyramine) | Substantial antioxidant activity and effects of positive inotropy on the isolated left atrium. | ||||
| 23. | (1S)-1-methyl-1,2,3,4-tetrahydro-isoquinoline-6,7-diol (Salsolinol) | |||||
| 24. | 3-methoxy-7-methyl-5,6,12,12a-tetrahydro indolo [2,1-a] iso-quinolin-7-ium-2, 9,10-triol (-litcubinine) | |||||
| T. sinensis | 25. | Tetrahydro palmatine | Stem | Antiviral action | [48,54] | |
| 26. | 4-[formyl-5-(hydroxyl-methyl)-1H-pyrrol-1-yl] butanoic acid | |||||
| 27. | 2,3,10- trimethoxy-5,6-dihydro-iso-quinolino [2,1-b] isoquinolin-7-ium-9-ol (Palmatrubine) | |||||
| T.cordifolia | 28. | Tinocordiside | Glycosides | Stem | Prevent cardiac Na+, K + ATPase activity | [33,34,36,37,39,59,60] |
| 29. | Tinocordifolin | |||||
| 30. | Cordifolioside A | |||||
| 31. | Cordifolioside C | |||||
| 32. | Amritiside A | |||||
| T. crispa | 33. | Rumphioside B | Stem, whole plant | Immunomodulatory action | [[61], [62], [63]] | |
| 34. | Borapetol A | |||||
| 35. | Tinocrispol A, | |||||
| 36. | Borapetol B | |||||
| 37. | Borapetpside B | |||||
| T. sinensis | 38. | Tinosposide A | Stem | Antioxidant activity | [64,65] | |
| 39. | Tinosposide B | |||||
| 40. | Tinosposinenside A | |||||
| 41. | Rumphioside A | |||||
| 42. | Cordifolide A | |||||
| 43. | Furanolactone | |||||
| T. cordifolia | 44. | Tinosporide | Diterpenoid Lactones and sesquiterpenoids | Whole plant | Cardio-protective | [28,[30], [31], [32],66] |
| 45. | Tinocordside | |||||
| 46. | Syringin | |||||
| 47. | Columbin | |||||
| 48. | Jateorine | |||||
| 49. | 14-dieno-17, 12S: 18, 1S dilactone], | |||||
| 50. | Tinosporon | |||||
| 51. | Clerodane derivatives [(5R,10R)-4R-8Rdi-hydroxy-2S-3R: 15, 16- diepoxy-cleroda-13 | |||||
| T. crispa | 52. | Apigenin | Flavonoids | Stem | Cardio-protective | [67] |
| 53. | Diosmetin | |||||
| 54. | Genkwanin | |||||
| 55. | Luteolin 4′-methyl ether 7-glucoside | |||||
| 56. | Rutin | |||||
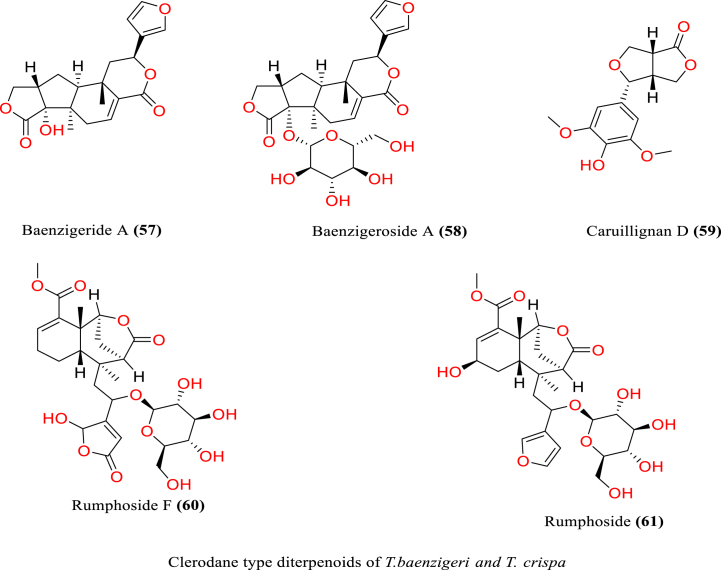
| T. baenzigeri | 57. | Baenzigeride A | Clerodane-type furano diterpenoids and diterpenoids | Stem | Diarrhoea, cold, fever and ulcers | [68,69] |
| 58. | Baenzigeroside A | |||||
| 59. | Caruillignan D | |||||
| T. crispa | 60. | Rumphioside F | Stem aerial parts | Substantial cytotoxicity against MDA-MB 231 breast cancer cells that are STAT3-dependent | [70] | |
| 61. | Rumphoside | |||||
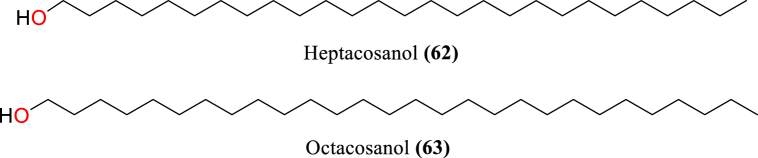
| T. cordifolia | 62. | Heptacosanol | Aliphatic compounds | Whole plant | – | [23] |
| 63. | Octacosanol | |||||
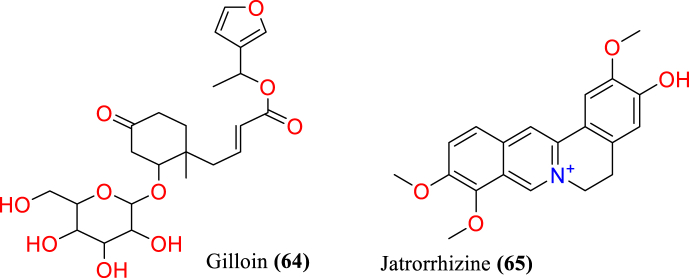
| 64. | Gilloin | Miscellaneous | ||||
| 65. | Jatrorrhizine |
Fig. 2.
Steroids of T. cordifolia, T. sinensis.
Fig. 3.
Alkaloids isolated from T. cordifolia, T. crispa and T. sinensis.
Fig. 4.
Glycosides of T. cordifolia and T. crispa and T. sinensis.
Fig. 5.
Terpenoids, Diterpenoid lactones and Sesquiterpenoids of T. cordifolia.
Fig. 6.
Flavonoids of T. crispa.
Fig. 7.
Clerodane type diterpenoids of T. baenzigeri and T. crisp.
Fig. 8.
Aliphatic compounds of T. crispa.
Fig. 9.
Miscellaneous compounds isolated from T. cordifolia.
Fig. 1.

T. cordifolia.
4.1. Phenolics
Four phenyl propanoids [[71], [72], [73]], two flavonoids [38,74], three lignans [72,73], and two benzenoid derivatives [75,76] have been discovered as phenolics.
4.2. Alkaloids
The alkaloids in a plant are a crucial secondary metabolite. The protoberberine alkaloids palmatine, berberine, magnoflorine, jatrorrhizine, and corydine [[77], [78], [79]] are among the thirteen alkaloids having aporphine and isoquinoline skeletons, as well as amide, and amine that have been discovered.
4.3. Terpenoids
T. cordifolia produced one monoterpene [72], five sesquiterpenoids [8,[79], [80], [81]], 32 diterpenoids and their glycosides with norclerodane and clerodane skeletons, as well as one triterpenoid called cycloeuphordenol [79]. The whole plant was used to get the bicyclic diterpenoid tinosporin.
4.4. Steroids
In addition to sitosterol, four steroids [[82], [83], [84], [85]] and 2, 3, 14, 20, 22, 25 -hexahydroxyl-5-cholest-7-en-6-one have also been discovered.
4.5. Aliphatic chemicals and essential oils
The essential oil extracted from fresh leaves using hydrodistillation contained various compounds, including phenols (16.6 %), alcohols (32.1 %), fatty acids (15.7 %), aldehydes (16.2 %), esters (3.2 %), alkanes (8.3 %), and terpenes (1.2 %) [86]. Additionally, the GC-MS analysis of a hexane extract of stems revealed the presence of hydroquinone (16.6 %), palmitic acid (14.1 %), 2-hexenal (14.2 %), phytol (11.4 %) ethyl-9,12-octadecadienoate, methyl 9-octadecenoate, methyl hexadecanoate, and methyl octadecanoate [87].
5. Pharmacological action
T. cordifolia has long been recognized as the predominant plant utilised in traditional medicine throughout history. The plant possesses numerous beneficial attributes and exerts a substantial impact on the defence system. The stem is employed as a bitter tonic for the stomach and as a laxative, while the root is utilised for stress relief and as an antimalarial agent [88], suggests that it enhances bile synthesis, improves blood quality, and alleviates jaundice. A lot of research has been done on the drug guduchi to find out how well it might work as a medicine. It improves several body systems. Ayurveda considers it a Rasayana with global effects [89,90]. Several research has reported various pharmacological effects of T. cordifolia and other Indian Tinospora species, which are covered in this article.
5.1. Anti-diabetic property
The stem of T. cordifolia is frequently used in traditional Indian medicine to treat diabetes by regulating blood glucose levels. According to reports, it possesses anti-diabetic properties that regulate blood sugar by increasing insulin secretion, reducing oxidative stress (OS), reducing gluconeogenesis, and reducing glycogenolysis. T. cordifolia contains anti-diabetic tannins, alkaloids, flavonoids, cardiac glycosides, saponins, and steroids as its primary phytoconstituents [[91], [92], [93]]. Isoquinoline alkaloid-rich stem fraction, including jatrorrhizine, palmatine, and magnoflorine, has been demonstrated to mimic and release insulin during in-vitro and in-vivo investigations. It has been shown that ingesting root extracts can reduce blood sugar levels, increase insulin production, and prevent OS symptoms. In-vitro studies demonstrated the activation and restoration of glutathione peroxidase (GPx), superoxide dismutase (SOD), and glutathione (GSH), as well as the inhibition of glucose 6-phosphatase and fructose 1, 6-diphosphatase, and the liver's glycogen content. The crude stem extracts of T. cordifolia in chloroforms, ethyl acetate, dichloromethane (DCM), and hexane inhibited salivary and pancreatic amylase and glycosidase, which increased postprandial glucose levels and may be useful in the treatment of diabetes mellitus [94].
The administration of T. cordifolia root extract to diabetic rats resulted in a reduction in their blood levels of glycosylated haemoglobin, hydroperoxides, ceruloplasmin, and vitamin E. Oral use of T. cordifolia extract has been shown to decrease blood levels of GSH and vitamin C [95]. Additionally, the Ayurvedic herbal composition “Ilogen Excel” consists of eight separate medicinal herbs. These include Curcuma longa, Salacia oblonga, Strychnos potatorum, Tinospora cordifolia, Coscinium fenestratum, Vetivelia zizanioides, and Andrographis paniculate. GSH, GPx, and SOD levels and catalytic activity are reduced in the hearts and brains of diabetic rodents [96]. TCE(Trichloroethylene) increases body weight, total hemoglobin, and hepatic hexokinase in diabetic rats while decreasing hepatic glucose-6-phosphatase, serum acid phosphates (ACP), alkaline phosphates (ALP), and lactate dehydrogenase (LDH) [97]. TCE was discovered to be protective in the presence of higher antioxidant molecule and enzyme concentrations. TCE has been shown to substantially reduce diabetes-related oxidative stress in the maternal liver by decreasing malondialdehyde and reactive oxygen species (ROS) and increasing GSH and total thiols [98].
In patients with low to severe hyperglycemia, the aqueous extract of T. cordifolia significantly decreased blood sugar levels. The group receiving 400 mg kg per day for moderate diabetes had the greatest decrease in glucose concentration by percentage. In experiments lasting 21–120 days, Alloxan rats served as test subjects. Significant glycemic control and an effect on key metabolic enzymes implicated in glucose metabolism in diabetic patients with mild to severe hyperglycemia. Body mass, total hemoglobin, and hepatic hexokinase concentrations all increase [99]. Streptozotocin rats exposed to aqueous T. cordifolia extract for six weeks exhibited significant anti-hyperglycemic activity a substantial decrease in blood and urine glucose levels [100].
T. cordifolia extracts have demonstrated anti-diabetic effects in vivo, according to pharmacological investigations. When the aqueous extract of the stem of another Tinospora species, “Tinospora crispa," was examined, anti-hyperglycemic properties were found. This is likely due to the fact that it stimulates insulin secretion by regulating cell and Ca2+ concentration [92]. Borapetoside C from Tinospora crispa (5 mg/kg, i. p.) decreased elevated plasma glucose levels, increased glucose intake, delayed the onset of insulin resistance, and ultimately improved insulin sensitivity in diabetic rats. Borapetaside C may have had a hypoglycemic effect due to the stimulation of insulin-induced IR-Akt-GLUT2 expression in the liver and its improvement of insulin sensitivity [101].
5.2. Antioxidant activity
The plant's capacity to enhance cell strength is derived from a polysaccharide known as arabinogalactan and a phenolic compound called epicatechin [74,102]. The leaf extract powder of the plant has higher antioxidant capacities compared to its stem extract powder [103]. The alkaloid components of this plant's root extract include antioxidant capabilities, which provide protection against nephrotoxicity caused by aflatoxin [56].
T. cordifolia root extracts can help replenish cell-reinforcement pointers like GPx, SOD, and GSH [22]. In the maternal livers of diabetic rats, T. cordifolia extracts have been demonstrated to decrease reactive oxygen species (ROS) and malondialdehyde while raising GSH levels [20,104].
5.3. Antimicrobial activity
Salmonella paratyphi, Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Salmonella flexneri, Salmonella typhimurium, Enterobacter aerogene, Pseudomonas aeruginosa, and Serratia marcesenses (Gram-positive bacteria) were tested for antibacterial activity against T. cordifolia extracts [105]. Extractions from T. cordifolia water, ethanol, and acetone are used to hook leaves and roots. In clinical isolates of urinary pathogens, F. Thoms demonstrated the strongest inhibitory efficacy against Klebsiella pneumoniae and Pseudomonas aeruginosa [106] strains obtained from burn victims show very great antibacterial activity when treated with silver nanoparticles made from the T. cordifolia stem [107]. It was shown that the active ingredient in T. cordifolia stem ethanol extracts [(5R, 10R)-4R, 8R-Dihydroxy-2S, 3R:15, 16-diepoxyclerod-13(16), 17, 12S, 18, 1S-dilactone] is effective against both bacteria and fungi. The lowest MIC values were found for Bacillus subtilis (200 g/ml) and Enterococcus faecalis (125 g/ml). The substance was also effective against fungi, with the lowest MIC values for Trichophyton rubrum 57 (62.5 g/ml), Trichophyton simii (31.25 g/ml), and Trichophyton rubrum 296 (62.5 g/ml) [108]. The components in T. cordifolia exhibited a greater inhibitory effect against reference microbiological strains and clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) and Klebsiella pneumoniae-developing carbapenemase, according to the findings of the Francesca Bonvicinia et al. investigation [109].
5.4. Antiulcer activity
Researchers tested the antiulcer effect of the roots using ethanolic extracts. It has been shown that T. cordifolia has a potent, diazepam-like preventive effect against ulceration caused by 8-h restriction tension [87,110].
5.5. Anticancer action
In mice, berberine exhibits anti-cancer properties. Ehrlich inhibits topoisomerase II at a dose of 10 mg/kg body weight in ascites carcinoma [[111], [112], [113]] [[111], [112], [113]] [[111], [112], [113]]. Columbin, a furanolactone diterpenoid, on the other hand, has demonstrated chemopreventive effectiveness against human colon cancer [28]. Octacosanol, long-chain aliphatic alcohol, inhibits MMP activity, NF kappa B translocation to the nucleus, and vascular endothelial growth factor synthesis by cancer cells into ascites fluid (in-vivo) [114]. By raising GSH, SOD, and catalase levels and reducing DNA damage, the plant alkaloid palmatine can reduce tumour size [115]. G1-4A can stimulate cytotoxic T lymphocytes that can destroy cancer cells by activating dendritic cells generated from bone marrow [116,117] By decreasing the population of drug-resistant cancer cells (rich in ATP-binding cassette transporters), its ethanolic extract might assist chemotherapy in overcoming such difficulties in cancer therapy [118]. Humans with MCF-7 breast cancer and chemically produced liver cell carcinoma can both be protected by ECD discovered in T. cordifolia [30,119]. ECD controls the expression of p53, Cdkn2A, and the mdm2 gene in cancer cells, leading to their demise [120]. This plant's octacosanol is an antiangiogenic drug that inhibits tumour spread and growth. It can increase pro-apoptosis and senescence while inhibiting mechanisms that reverse apoptosis, which has a therapeutic effect on neuroblastoma [121]. The capacity of this plant's extract to lessen CYP3A4, a crucial enzyme in the metabolism of chemotherapy drugs, makes it suitable for usage as an adjuvant with chemotherapy drugs. This might diminish the harmful effects that could otherwise have an influence on cells under normal circumstances and lower the number of hazardous drugs, such as those used to treat cancer [122]. By regulating pro-inflammatory cytokines and GSH like TNF, it also lessens the toxicity of anti-cancer drugs. A trademark has been filed for an eleven-part herbal remedy for the treatment of cancer that comprises 17 to 23 percent T. cordifolia. The hemoptysis and chest discomfort in a patient [123]with pulmonary epidermoid carcinomas (who refused previous therapies) completely stopped after one month of treatment with 450–480 mg of gelatinous capsule form TDS. Additionally, the patient's appetite had improved. When used as a tumoristatic drug, the same formulation was successful in treating a patient with third-stage pulmonary epidermoid carcinomas who had not responded to prior treatments [20].
5.6. Hepatoprotective activity
T. cordifolia has traditionally been recommended for reducing hepatosplenomegaly, stimulating bile flow, and treating Kamala (jaundice) in cases of obstructive lesions. Over 33 % of the hepatoprotective formulas available in the Indian market contain this substance, and it can also be consumed independently as a standalone agent [124]. Instead of inducing hepatotoxicity, this plant is crucial for its hepatoprotective properties [125]. In cases of carbon tetrachloride-induced liver injury, T. cordifolia has been seen to preserve normal metabolism and lower levels of particular enzymes such aspartate, alanine aminotransferase (ALT), alkaline phosphatase (ALP), aminotransferase (AST) and total bilirubin. The powerful hepatoprotective activities of T. cordifolia extract can be related to its ability to stimulate liver regeneration, as well as its antioxidant and free radical-scavenging properties [124,126] [[124], [126], [127], [128]] [[124], [126], [127], [128]]. A standardised aqueous extract was used to study patients with hepatic disorders who showed signs of immunosuppression and/or fibrosis. The most thorough clinical research was done on patients with obstructive jaundice, where it was discovered that adding T. cordifolia (16 mg/kg/day) to conventional therapy prior to surgical correction significantly decreased mortality, dropping it from 61.54 % to 25 % in patients with PTBD (percutaneous transhepatic biliary drainage) and from 39 % to 6.25 % in patients without PTBD. This was related to fewer individuals in the T. cordifolia treated group experiencing septicemia [129,130]. At a dosage of 200 mg/kg, T. sinensis significantly decreased paracetamol-induced elevated levels of serum ALT, AST, ALP, and bilirubin, displaying normal liver architecture and indicating a hepatoprotective potential [131].
5.7. Immunomodulator activities
Isolated chemical substances including cordifolioside A and guduchi syringin were referred to be immunomodulators in the clinical examination [8]. The stem of T. cordifolia influences the quantity of enzymes like catalase and stimulates lymphocyte cells, emphasizing the immune protective action of this shrub [132]. T. cordifolia extract causes macrophage cells to create more myeloperoxidase and other enzymes, which enhances antibacterial activity and increases immunity [133]. On the other hand, it increases the phagocytic activity of macrophages. Additionally, it stimulates macrophages and splenocytes, because there is a higher level of nitric oxide produced, which suggests anti-tumor and immune protective effect [134]. One research investigation found that T. cordifolia lotion lowers interleukin-1 and IL-6 levels in an animal model of scabies. Its anti-scabies effectiveness is demonstrated by the suppression of hyperkeratosis and inflammatory cell infiltration into scabietic wounds [135]. Aqueous extract induces cellular mitosis and increases the generation and activation of immune effector cells and cytokine.
T. cordifolia is a potent preventative for diseases with an immune system component since it may also enhance neutrophil and immune cell activity [136]. Alkaloids, steroids, aliphatic chemicals, and other guduchi substances showed a significant immuno protective effect when tested in a preclinical rat model. The polysaccharide G1-4A, which is generated from the T. cordifolia plant, stimulates the growth and development of T and B immune cells as well as the production of the anti-apoptotic gene [137]. It has been shown that the chemical D-glucan generated from TC maintains body physiology by stimulating lymphocyte cells [138]. T. cordifolia extracts caused PMN cells to undergo phagocytosis. Increases in bone marrow cells, white blood cell (WBC) count, and foot pad thickness are all brought on by T. cordifolia alcoholic extract (100 mg/kg), showing a stimulatory effect on the haemopoetic system with a strong immunomodulatory effect [139]. A traditional Ayurvedic preparation of T. cordifolia's aqueous extract known as “Ghana” reduced the edematogenic agents when tested on the edoema rat model, and as a result, exhibited potent immunostimulatory properties [97].
5.8. Antitoxic properties
As stated in literature [140], T. cordifolia possesses the ability to eliminate harmful free radicals and provides a protective effect on the body by modulating hormone and mineral levels. Evidence shows that T. cordifolia effectively reduces reactive oxygen species (ROS) and enhances hormone levels (such as glutathione) and enzyme activity (such as catalase and glutathione reductase) in the kidneys of Swiss albino mice. The presence of alkaloids in this plant is responsible for its inherent anti-toxin capabilities. T. cordifolia has shown efficacy in preventing damage and exhibiting its antitoxin activity in the urinary bladder and hepatic cells by significantly elevating the level of reduced glutathione content and cytokines, while gradually decreasing inflammatory cytokines (Tumour necrosis factor) [123]. T. cordifolia safeguards the kidneys by enhancing the activity of antioxidant enzymes, ascorbic acid, protein, and glutathione (GSH) [141].
5.9. Anti-HIV activity
The usefulness of T. cordifolia in treating HIV-positive patients by reducing resistance to the retroviral therapy has been investigated [142]. The anti-HIV action of T. cordifolia in HIV-positive patients increases CD4 T-cell count and decreases eosinophil count, demonstrating its usefulness in disease treatment. The T. cordifolia extract significantly improved the phagocytic and intracellular bactericidal activities. Activation of peritoneal macrophages was another effect of T. cordifolia. It also boosts intracellular killing and phagocytosis. B-lymphocytes, polymorph nuclear leucocytes, and macrophages are all significantly activated by T. cordifolia [[143], [144], [145]].
5.10. Antipyretic properties
A 95 % ethanolic extract of T. cordifolia included a water-soluble component that was discovered to be antipyretic. Another study found that T. cordifolia stems exhibit antipyretic properties in the hexane and chloroform soluble parts. T. cordifolia has anti-infective and antipyretic effects, according to several research. Before being treated with T. cordifolia, rats didn't die from intra-abdominal sepsis after having their cecum tied, and it greatly reduced death rates in mice from E. coli caused peritonitis [146].
5.11. Nephroprotective properties
T. cordifolia's role in nephrotic syndrome (NS) shows that it contains antioxidant, immunomodulating, anti-inflammatory, and nephroprotective qualities that can treat nephritis. The effects of steroids and NS rebound are rare. T. cordifolia improves novel medication efficacy and safety. It can also be used with modern drugs to treat steroid-resistant and steroid-dependent Nephritic Syndrome [132,147]. Table 3 presents the main species, namely T. cordifolia, T. crispa (L.), and T. sinensis (Lour.), which have been demonstrated by researchers [[148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175]] to possess significant medicinal properties.
Table 3.
Pharmacological activities of Tinospora cordifolia and other Tinospora species.
| S. No | Activity | Part/Extract | Species | Animal Model/Cell Lines | Result | Reference |
|---|---|---|---|---|---|---|
| 1. | Analgesic activity | Whole plant/Alcohol extract C | T. cordifolia | Albino rats were subjected to abdominal writhing and a hot plate. | The anti-nociceptive activity was evaluated by the latency times. | [148] |
| 2. T. crispa | ||||||
| 3. T. sinensis |
Tests on adult male Wistar rats using the tail flick and acetic acid to cause writhing/p.o. | Significant (P < 0.001) increase in latency time was seen in the extract. | [47] | |||
| 4. | Antidyslipidemic activity | Stem extract | T. cordifolia | Charles Foster strain adult male rats who have developed diabetes due to alloxan. | As a natural remedy, T. cordifolia is more effective at reversing diabetic dyslipidemia and oxidative stress. Treatment had a positive impact on HDL synthesis, which may have helped to control lipid metabolism. | [149] |
| 5. T. crispa |
Male with alloxan diabetes model Wistar albino rats | The compound increased serum insulin levels while lowering fasting blood glucose levels. | [150] | |||
| 6. Flower and Leaf extract |
T. sinensis | Male Wistar rats were given hydrogenated groundnut oil to produce hypercholesterolemia using p.o. | Extract changed the inappropriate metabolic profile and significantly increased the risk of hypolipidemia (P 0.05). | [46] | ||
| 7. | Immunomodulatory activity | ethanol and aqueous extract from the whole plant | T. cordifolia | Swiss male albino mice. | T. cordifolia stem contains strong immunomodulatory properties, and these properties may be a result of the drug's individual chemical components or their combined effects. | [132] |
| 8. Aqueous |
T. sinensis | Anemia brought on by cyclophosphamide in male Wistar rats, p.o. | Extract shown a considerable effectiveness. | [125] | ||
| 9. Fraction and ethanolic |
T. crispa | MTT proliferation test and intracellular cytokine analysis in LPS-stimulated murine macrophage cell line RAW264.7. | RAW264.7 cell viability and the intracellular expression of the cytokines INF-c, IL-6, and IL-8 were increased by the extract and fractions. | [151] | ||
| 10. | Cardioprotective effect | Extract of whole plants |
T. cordifolia T. sinensis T. crispa |
Intravenous calcium chloride administration causes arrhythmia in rats | Strong evidence points to a potential function for T. cordifolia in cancer chemoprevention as a result of the therapy with Tinospora, which caused a significant induction in the particular activities of detoxifying enzymes in the kidney. | [56] |
| 11. | Aphrodisiac property | Hydroalcoholic and aqueous and extract | T. cordifolia | Adult albino rats of wistar strain. | In order to assess the potential mechanism of the medicine, it was recommended that in the future, the components in the hydro-alcoholic extract that are responsible for the aphrodisiac activity be isolated and their aphrodisiac potential be tested in both in-vitro and in-vivo models. | [152] |
| 12. | Antidiarrheal activity | Whole plant/Aqueous extract |
T. cordifolia T. sinensis T. crispa |
Magnesium sulphate and castor oil induced diarrhoea in albino rats | The outcomes established pharmacological support for the traditional uses of T. cordifolia as an antidiarrheal and antiulcer drug. | [110] |
| 13. | Antiulcer activity | Ethanol, aqueous, and whole plant extracts |
T. cordifolia, T. sinensis |
Pylorus ligation-induced ulcer in albino rats | ||
| 14. | Neuroprotective effect | Aerial parts/Ethanol extracts | T. cordifolia | Parkinson's disease rat models with 6-hydroxydopamine lesions. | The mitochondrial activity that TCEE was able to retain offered a potentially effective method for treating clinical PD. To put it to use in clinical researches, additional pharmacological and clinical studies are required. | [153] |
| 15. | Anti-inflammatory activity | Stem/Aqueous extract | T. cordifolia | Rat paw edema model caused by carrageenan. | Paw volume was significantly reduced by extract (P 0.05). | [97,154] |
| 16. Whole plant/Diosgenin |
T. sinensis | In comparison to indomethacin, diosgenin exhibited the most pronounced anti-inflammatory effect, with a percentage of 82.25 %. This effect was significant (P < 0.01), as it substantially reduced the average volume of paw edoema 3 h after the administration of carrageenan. | [155] | |||
| 17. Stem/aqueous and methanolic |
T. crispa | Inflammation was triggered by TNF-a in human umbilical vein endothelial cells. | Significant signaling molecule reduction was seen in both extracts for ICAM-1, VCAM-1, MCP-1, and M-CSF. | [156] | ||
| 18. | Gastroprotective activity | Whole plant | T. cordifolia | In rats, plant indomethacin caused stomach ulcers. | Based on our findings, we hypothesised that the ECD (Epoxy clerodane diterpene) substance found in T. cordifolia may be to blame. Due of ECD's strong biological activity, further research is appropriate to be done in order to turn it into a pharmaceutical. | [157] |
| 19. | Antioxidant activity | Whole plant/Ethanol extract | T. cordifolia | In male wistar albino rats, N-nitrosodiethylamine caused liver cancer. | T. cordifolia treatment of group III T. cordifolia treated mice considerably lowers the peroxidation reaction (P 0.01). | [158] |
| 20. T. sinensis |
In vitro tests for DPPH, lipid peroxidation, DMSO, ABTS, NO radical scavenging activity, and SOD scavenging. | In vitro experiments using the following extracts revealed considerable antioxidant activity: ABST (IC50 = 90.44 0.36 lg/mL); DPPH (IC50 = 94.66 0.049 lg/mL); DMSO (IC50 = 97.99 0.15 lg/mL); SOD (IC50 = 93.72 0; NO (IC50 = 87.25 2.72 lg/mL). | [159] | |||
| 21. T. crispa |
Free radical scavenging test using DPPH | Methanol extract considerably boosted vitamin E-like radical activity (100 %) and radical scavenging activity (IC50 12 mg/mL). | [158] | |||
| 22. | Radio protective and Cytoprotective activity | Stem/Ethanol extract | T. cordifolia | Genotoxicity was brought on by 4 Gy radiation and cyclophosphamide in albino mice. | Cordifolioside-A, which has radioprotective and cytoprotective activity, is present in the n-butanol fraction of T. cordifolia stem extract; however, the precise mechanism underlying these effects is yet unknown and requires further research. | [160] |
| 23. | Antifeedant activity | Whole plant/Chloroform Extract | T. cordifolia | Earias vitella, Plutella xylostella, and Spodoptera litura were the microorganisms used. | The most efficient diterpene tested was 8-hydroxy tinosporide 3. Only a small variation existed between the insects' susceptibilities to the diterpenoids, with S. litura being slightly more vulnerable than E. vitella. Compound 3's antifeedant efficacy at 5 and 10 mg doses was comparable to azadirachtin-A's antifeedant activity at 0.5 mg concentration. | [161] |
| 24. | Ameliorative effect | Root/Ethanol extract | T. cordifolia | Swiss male albino mice were exposed to aflatoxin B1. | Strong evidence points to a potential function for T. cordifolia in cancer chemoprevention as a result of the therapy with Tinospora, which caused a significant induction in the particular activities of detoxifying enzymes in the kidney. | [56] |
| 25. | Hepatoprotective activity | Whole plant/Aqueous Extract | T. cordifolia | Rats with bile duct ligation developed jaundice. | The outcomes allowed us to advise against using melatonin and T. cordifolia to lessen oxidative stress associated with cholestasis in humans. | [162] |
| 26. Root/Ethanol |
T. sinensis | Wistar albino rats with p.o. induced liver damage from CCl4. | Extract decreased sinusoidal dilation and minor inflammatory factors. Serum enzyme levels were found to have significantly decreased (P < 0.001) in treated mice. |
[163] | ||
| 27. | Nootropic effect | Whole plant/Ethanol extract | T. cordifolia | Amnesic rats accomplish tasks in the radial arm maze and the Barnes maze test. | It was determined that combining the ethanolic extracts of Evolvulus alsinoides, Bacopa monnieri, and T. cordifolia produced a stronger nootropic effect. | [164] |
| 28. | Hypoglycemic activity | Stem/Aqueous Extract | T. cordifolia | Rat pancreatic β-cell lines were used in vitro to assess the impact of insulin secreted. | The TC's alkaloid content contributed to its antihyperglycemic effects. The postprandial hyperglycemia is improved by isoquinoline alkaloid rich fraction (AFTC), which may have hypoglycemic effects through mechanisms of insulin releasing and insulin-mimicking activities. | [22] |
| 29. | Antipsychotic activity | Aqueous and Ethanol extract | T. cordifolia | Mouse model was hampered by amphetamine. | Based on the findings, it can be said that T. cordifolia aqueous ethanolic extract has no psychotropic potential at the dose levels tested. | [165] |
| 30. | Antidepressant activity | Stem, fresh leaves/Aqueous and Petroleum ether extract | T. cordifolia | The activity of Swiss albino mice was measured with the tail suspension test and the forced swim test. | The serotonin, monoamines noradrenaline, and dopamine were increased, while GABA was decreased, making T. cordifolia petroleum ether extract work in a manner similar to an antidepressant. | [166] |
| T. sinensis | After the first, second, and third weeks of treatment, the extract significantly raised anoxia stress tolerance (P 0.01); it also decreased high levels of serum biochemical parameters and blood cell count and avoided changes in the liver and adrenal gland weight. | [131] | ||||
| 31. | Anti osteoporotic activity | Stem/Ethanol extract | T. cordifolia | Sprague-Dawley female rats. | However, reproductive organs like the uterus and mammary gland did not exhibit estrogen-like actions from TC extract. As a result, our study shows that T. cordifolia extract has the potential to be employed as an antiosteoporotic drug. | [167] |
| 32. | Antineoplastic activity | Aerial parts/DCM extract | T. cordifolia | Transplants of Ehrlich ascites carcinoma in mice. | The TCE is an amalgam of several alkaloids, including berberine. The combination of other alkaloids and TCE's anti-cancer effects may have an impact. Optimistic outcomes from pre-clinical and clinical tests on this extract and its other components suggest that they could become a potent cancer therapy tool and a standard component of cancer treatment procedures. | [111] |
| 33. | Antifertility effect | Methanol extract | T. cordifolia | Male rats. | Accordingly, the findings imply that oral administration of a crude methanolic extract of T. cordifolia stem can cause male rats to become infertile as a result of interfering with the levels of androgen in the testes, which changes the process of spermatogenesis. | [168] |
| 34. | Antiasthamatic activity | Stem/Hydroalcoholic Extract | T. cordifolia | In an in vivo asthma model, mice were sensitized by intraperitoneal and intranasal administration of ovalbumin. | The T. cordifolia extract exhibits therapeutic potential for the treatment of inflammatory lung diseases, including asthma. | [169] |
| 35. | Antitumor activity | Aqueous alcoholic extract | T. cordifolia | Extract inhibited cell growth in a dose-dependent manner in C6 glioma cells. | Given the current research showing that TCE significantly reduced glioma cell migration and proliferation while also causing cell differentiation and programmed cell death, It's possible that this plant will show promise as a treatment for glioblastoma. |
[170] |
| 36. | Allergic rhinitis | Aqueous extract | T. cordifolia | Double blind placebo-controlled trial. | The age group of 20–30 and women were found to have a higher prevalence of allergic rhinitis. According to studies of the effects of TC and placebo after 8 weeks of treatment on allergic rhinitis symptoms (Table 1), patients who received TC experienced complete relief from sneezing, whereas patients in the placebo group saw no improvement at all. | [171] |
| 37. | Diabetic neuropathy | Stem/aqueous extract | T. cordifolia | Aldose reductase inhibition assay in vitro and Mann-Whitney test findings from streptozotocin-induced diabetic wistar albino rats were investigated. | The herb T. cordifolia inhibits hyperalgesia in diabetic experimental neuropathy. The positive benefits could be attributed to its in-vitro aldose reductase inhibitory action. | [172] |
| 38. | Hepatocellular carcinoma | Aerial parts/Ether extract | T. cordifolia | Male wistar rats developed hepatocellular cancer after exposure to diethyl nitrosamine. | We come to the conclusion that steryl glycosides, which are a significant component of bacteria and some plants, including cycads, can mediate nephrotoxicity through a convoluted cascade. Involves the CNS and immune system's interplay. | [30] |
| 39. | Antimalarial activity | Stem/Ethanolic extract | T. cordifolia | Microorganism Models using Plasmodium berghei on white Swiss mice. | The parasite was significantly inhibited by the extract (50 %; P 0.01) | [21,173] |
| 40. T. crispa |
Female ICR mice; Plasmodium berghei ANKA (PbANKA) strain that is chloroquine-sensitive; p.o. | |||||
| 41. T. banenzigeri | ||||||
| 42. | Anticancer activity | Aqueous and Ethanolic extract | T. cordifolia | As a model system, IMR 32 human neuroblastoma cell lines were used. | Our research suggests that this plant's active phytochemicals or crude extract could be a promising treatment option for malignant neuroblastoma cells that is based on differentiation. | [174] |
| 43. Ethanolic extract |
T. sinensis | Microculture tetrazolium assay/in vitro using human melanoma cancer cell line (A 375) and skin cancer cell line (A 431). | Against the cancer cells A375 and A431, the extract demonstrated considerable cytotoxicity (IC50 values of 49.87 lg/mL and 112.54 mg/mL, respectively). | [155] | ||
| 44. Stem/aqueous |
T. crispa | MTT assay; MCF-7 breast cancer cell lines | Significantly less cells were viable (IC50 = 42.75 mg/mL). | [158] | ||
| 45. | Antibacterial activity | Stem/Aqueous and Ethanolic Extract | T. cordifolia, T. crispa, T. sinensis | P. vulgaris, E. coli, S. typhi, S. aureus, E. faecalis, and S. marcesenses were the microorganisms employed. | The findings show that ethanolic plant stem extracts have more efficacy than other extracts, such as chloroform and aqueous, which only partially prevented bacterial growth. This might be because ethanol extracts make the chemical components that give antibacterial action more soluble. | [175] |
6. Bioactivity of major components
T. cordifolia and other Tinospora species possess a diverse range of bioactive chemicals. The many bioactive compounds, such as steroids, alkaloids, glycosides, diterpenoid lactones, sesquiterpenoids, flavonoids, furano diterpenoids, and aliphatic compounds, were investigated [48,72,82,52,54,55].
The plant T. cordifolia and other Tinospora species possess a wide range of bioactive compounds that contribute to its analgesic, antidyslipidemic, immunomodulatory, cardioprotective, antidiarrheal, antiulcer, neuroprotective, antidiabetic, anti-inflammatory, antioxidant, antimalarial, anticancer, and antibacterial properties.
Studies have indicated that Magnoflorine, Tembetarine, Isocolumbin, and Isoquinoline alkaloids obtained from plants had strong anticancer, antidiabetic, anti-inflammatory, and antibacterial activities [22,58,115]. T. cordifolia has been found to contain various forms of diterpenoids that exhibit cardioprotective properties. Secoisolariciresinol steroid and lignans exhibit anti-inflammatory and antioxidative characteristics [25].
The flavonoids apigenin and diosmetin, which have been extracted from T. crispa, exhibit cardioprotective, anti-inflammatory, and antioxidant properties [44,52,99]. Diosgenin, which has been found in T. cordifolia, T. sinensis, and T. crispa, exhibits anti-inflammatory activities [[154], [155], [156]].
7. Safety and toxicity profile of T. cordifolia
Acute poisoning tests on T. cordifolia did not show any harmful effects or deaths, even when the plant was given at a very high dose of 9 g/kg body weight [101]. According to the acute toxicity tests, giving mice a 3 g/kg dose of T. cordifolia aqueous extract had no negative effects [171]. Studies on humans have shown that T. cordifolia does not have any harmful effects, even when given at very high amounts (900 mg/day) to HIV patients and people with allergic rhinitis. Clinical studies on T. cordifolia showed that a dose of 500 mg/day for 21 days was safe for people and did not seem to have any negative effects on their kidneys, heart, intestines, or nervous system [25]. In a study, scientists administered a dosage of 3 g/kg of T. cordifolia to rats and saw no adverse effects and did not result in any fatalities among the subjects [158]. Administering a dosage of 0.1 g/kg of T. cordifolia to rats for 12 weeks does not adversely affect their liver or renal function. Researchers gave the drug to rats, and it increased the number of white blood cells and neutrophils. However, this did not happen in healthy people [24,159]. During long-term monitoring studies, finding and displaying signs and symptoms can give researchers important information about the right way for drugs to work and how much to give during the experiment [176]. Adding Curcuma longa and T. cordifolia to normal anti-tuberculosis treatment for all TB patients was said to greatly reduce the number and severity of liver damage events [177]. In the first part of the study, giving T. cordifolia to healthy volunteers was found to be safe and well-accepted [[178], [179], [180]]. Several studies have been done on the phytochemical makeup and biological qualities of different medicinal plants. According to what they found, T. cordifolia does not have any significant side effects, acute or chronic toxins, or LD50 values. For therapeutic reasons, it is suggested that a half-dose of this drug, or 500 mg per kg, be given. Even so, the Tinospora species has not been the subject of many thorough toxicological tests and safety studies. Most of the time, low to moderate doses are appropriate to use.
8. Marketed formulation of T. cordifolia
There are numerous market formulations based on Tinospora cordifolia that each play a unique role in sustaining health and encouraging a disease-free lifestyle. Giloy products come in a variety of formulations, including powder, syrup, juice, pills, and many others. These products appear to treat a variety of ailments and to increase immunity [101,139]. Several of these items are listed in Table 4.
Table 4.
Marketed Formulation Tinospora cordifolia along with their application.
| S.No | Product Name | Brand Name | Application of Product |
|---|---|---|---|
| 1. | Giloy juice | Kapiva | Effective for gout, jaundice, anaemia, and fever patients. By emptying contaminants, it functions as a detoxifier, while also enhancing skin health and lowering respiratory diseases. |
| 2. | Giloy capsules | Zandu | Improves the digestive system, regulates blood sugar levels, and keeps the liver healthy. |
| 3. | Giloy ghanvati | Dabur | Promotes digestion, contributes to the formation of immunity, and prevents numerous illnesses |
| 4. | Guduchi ghrita | Guduchi | Teats skin conditions and gout |
| 5. | Giloy ghan vati | Patanjali | Aids in the treatment of gastroenteritis and offers protection from infectious disorders, persistent fever, cough, and cold |
| 6. | Brave heart capsule | Brave Heart | Reduces blood pressure, lipid levels, particularly cholesterol and LDL cholesterol, and regulates cardiac function. |
| 7. | Immuniveda Chyawanprash | Saffola | Cholesterol improves bioavailability, boosts immunity and respiratory health, and gives people more strength, energy, and endurance. |
| 8. | Cirrholiv-ds syrup | Paul Medicos | Used as an immune modulator and hepatoprotectant. Treats illnesses associated to the liver |
| 9. | Guduchi sattva | DAV Pharmacy | Effective in liver conditions, Diabetes, cough, and fever |
| 10. | Guduchi churna | Baidyanath | Treats malaria, swine fever, and dengue, a hypoglycemic agent that aids in the treatment of diabetes, possesses anti-aging qualities that aid in enhancing skin health. |
| 11. | Madhumehari | Baidyanath | Decreases blood and urine sugar levels, aids in energy recovery |
| 12. | Panchanim badi churna | Prakruti Remedies | Ascites, arthritis, diabetes, eczema, dermatitis, and other skin conditions. |
9. Conclusion and future prospective
Tinospora cordifolia and other species of the Tinospora genus are recognized as medicinal plants with recognized ethnopharmacological and therapeutic properties. It is widely observed in many clinical contexts within the Indian Systems of Medicine. T. cordifolia is a versatile medicinal plant with various types of bioactive compounds, including glycosides, alkaloids, sesquiterpenoids, aliphatic compounds, steroids, etc. The plant's anticancer, antidiabetic, analgesic, immunomodulatory, antioxidant, antiulcer, antibacterial, antipyretic, and nephroprotective effects are attributed to the presence of several bioactive compounds.
This review demonstrates that the primary areas of interest in phytochemistry, pharmacological research, and traditional clinical application on Tinospora cordifolia and other Tinospora species, including T. crispa (L.), T. sinensis (Lour.), and T. baenzigeri. The chemical and biological activities of other Tinospora species, namely T. glabra, T. formanii, T. smilacina, T. maqsoodiana, and T. subcordata remain unexplored or insufficiently documented. Additional research is required to elucidate the chemical diversity and fully exploit the biological importance of the chemicals and extracts derived from Tinospora species. However, in this regard, it is necessary to undertake additional scientific research to investigate the chemical composition and pharmacological properties of other Tinospora species in preventing and treating disease. This review will facilitate the future development of novel therapeutic products and help the advancement of knowledge in this particular field.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
All data required to support this study is already mentioned in the manuscript.
CRediT authorship contribution statement
Anu Chaudhary: Writing – review & editing. Rina Das: Data curation. Kiran Mehta: Formal analysis. Dinesh Kumar Mehta: Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
I am highly indebted to M M College of Pharmacy, Maharishi Markandeshwar (Deemed to be University) management for providing the facility to complete this review.
References
- 1.Olabiyi A.S., Nkemehule F.E., Odukoya O.A., Samuel T.A., Ogbonnia S.O. Inhibition of glycosylation as an index of activity in plants with antidiabetic potentials. Biochem. Pharmacol. 2013;2:181. [Google Scholar]
- 2.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:27–49. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singletary K. Turmeric: an overview of potential health benefits. Nutr. Today. 2010;45:216–225. [Google Scholar]
- 4.Niraj S., Varsha S. A review on scope of immuno-modulatory drugs in Ayurveda for prevention and treatment of Covid-19. Plant Science Today. 2020;7:417–423. [Google Scholar]
- 5.Udayan P.S., Pradeep A.K., Balachandran I. A new species of tinospora (Menispermaceae) from South India. Edinb. J. Bot. 2009;66:77–80. [Google Scholar]
- 6.Saha S., Ghosh S. Tinospora cordifolia: one plant, many roles. Ancient Sci. Life. 2012;31:151. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meena A.K., Singh A., Panda P., Mishra S., Rao M.M. Tinospora cordifolia: its bioactivities & evaluation of physicochemical properties. IJPPR. 2010;2:50–55. [Google Scholar]
- 8.Sharma U., Bala M., Kumar N., Singh B., Munshi R.K., Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Goel H.C., Prasad J., Singh S., Sagar R.K., Agrawala P.K., Bala M., Sinha A.K., Dogra R. Radioprotective potential of an herbal extract of Tinospora cordifolia. J. Radiat. Res. 2004;45:61–68. doi: 10.1269/jrr.45.61. [DOI] [PubMed] [Google Scholar]
- 10.V Sonkamble V., Kamble L.H. Antidiabetic potential and identification of phytochemicals from Tinospora cordifolia. Am. J. Phytomed. Clin. Ther. 2015;3:97–110. [Google Scholar]
- 11.Sinha K., Mishra N.P., Singh J., Khanuja S.P.S. 2004. Tinospora cordifolia (Guduchi), a Reservoir Plant for Therapeutic Applications: A Review. [Google Scholar]
- 12.Salkar K., Chotalia C., Salvi R. Tinospora cordifolia: an antimicrobial and immunity enhancer plant. Int. J. Sci. Res. 2017;6:1603–1607. [Google Scholar]
- 13.Upreti P., Chauhan R.S. Effect of leaf powder of giloy (Tinospora cordifolia) in fish feed on survival and growth of post larvae of Catla catla. Journal of Applied and Natural Science. 2018;10:144–148. [Google Scholar]
- 14.Khan M.M., Haque M.S., Chowdhury M.S.I. Medicinal use of the unique plant Tinospora cordifolia: evidence from the traditional medicine and recent research. Asian J. Med. Biol. Res. 2016;2:508–512. [Google Scholar]
- 15.Shrestha S., Shrestha J., Shah K.K. Non-timber forest products and their role in the Livelihoods of people of Nepal: a Critical review. Grassroots Journal of Natural Resources. 2020;3:42–56. doi: 10.33002/nr2581.6853.03024. [DOI] [Google Scholar]
- 16.Gururaj H.B., Giridhar P., Ravishankar G.A. Micropropagation of Tinospora cordifolia (willd.) Miers ex hook. F & Thoms - a multipurpose medicinal plant. Curr. Sci. 2007;92:23–26. [Google Scholar]
- 17.Mangal M., Sheoryan A., Mangal A.K., Kajla S., Choudhury A., Dhawan A. Biotechnological advances in Tinospora cordifolia (Willd.) Miers ex Hook. F. & Thoms: overview of present status and future prospects. Vegetos. 2012;25:182–191. [Google Scholar]
- 18.Ahsan R., Mishra A., Badar B., Owais M., Mishra V. Therapeutic application, Phytoactives and pharmacology of Tinospora cordifolia: an Evocative review. Chin. J. Integr. Med. 2023;29:549–555. doi: 10.1007/s11655-023-3733-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kattupalli S., Vesta V., Vangara S., Spandana U. The multi-activity herbaceous vine–Tinospora cordifolia. Asian J. Pharmaceut. Clin. Res. 2019;12:1–4. [Google Scholar]
- 20.Yoggeta D.Bhatia, Rani S. Tinospora cordifolia: a literature review on therapeutic uses and pharmacological actions. J Pharm Res Int. 2021;33:330–343. doi: 10.9734/jpri/2021/v33i57A34004. [DOI] [Google Scholar]
- 21.Ounjaijean S., Kotepui M., Somsak V. Antimalarial activity of Tinospora baenzigeri against Plasmodium berghei- infected mice. J. Trop. Med. 2019;2019 doi: 10.1155/2019/5464519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel M.B., Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phytomedicine. 2011;18:1045–1052. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee R., De U.K., Ram G.C. Evaluation of mammary gland immunity and therapeutic potential of Tinospora cordifolia against bovine subclinical mastitis. Trop. Anim. Health Prod. 2010;42:645–651. doi: 10.1007/s11250-009-9471-z. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh A.K., Martyr C.D., Steffey M., Wang Y.-F., Agniswamy J., Amano M., Weber I.T., Mitsuya H. Design of substituted bis-Tetrahydrofuran (bis-THF)-derived potent HIV-1 Protease Inhibitors, protein-ligand X-ray Structure, and Convenient Syntheses of bis-THF and substituted bis-THF ligands. ACS Med. Chem. Lett. 2011;2:298–302. doi: 10.1021/ml100289m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay A., Kumar K., Kumar A., Mishra H. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010;1:112. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rout G.R. Identification of Tinospora cordifolia (willd.) miers ex hook F. & thomas using RAPD markers. Zeitschrift Fur Naturforschung - Section C Journal of Biosciences. 2006;61:118–122. doi: 10.1515/znc-2006-1-221. [DOI] [PubMed] [Google Scholar]
- 27.Patel S.S., Shah R.S., Goyal R.K. Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2009;47:564–570. [PubMed] [Google Scholar]
- 28.Kohno H., Maeda M., Tanino M., Tsukio Y., Ueda N., Wada K., Sugie S., Mori H., Tanaka T. A bitter diterpenoid furanolactone columbin from Calumbae Radix inhibits azoxymethane-induced rat colon carcinogenesis. Cancer Lett. 2002;183:131–139. doi: 10.1016/S0304-3835(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhao F., He E.Q., Wang L., Liu K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J. Asian Nat. Prod. Res. 2008;10:467–473. doi: 10.1080/10286020801948334. [DOI] [PubMed] [Google Scholar]
- 30.Dhanasekaran M., Baskar A.A., Ignacimuthu S., Agastian P., Duraipandiyan V. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs. 2009;27:347–355. doi: 10.1007/s10637-008-9181-9. [DOI] [PubMed] [Google Scholar]
- 31.Sriramaneni R.N., Omar A.Z., Ibrahim S.M., Amirin S., Mohd Zaini A. Vasorelaxant effect of diterpenoid lactones from Andrographis paniculata chloroform extract on rat aortic rings. Pharmacognosy Res. 2010;2:242–246. doi: 10.4103/0974-8490.69125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S., Evens A.M., Prachand S., Singh A.T.K., Bhalla S., David K., Gordon L.I. Mitochondrial-mediated apoptosis in lymphoma cells by the diterpenoid lactone andrographolide, the active component of Andrographis paniculata. Clin. Cancer Res. 2010;16:4755–4768. doi: 10.1158/1078-0432.CCR-10-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perona R., Sánchez-Pérez I. Control of oncogenesis and cancer therapy resistance. Br. J. Cancer. 2004;90:573–577. doi: 10.1038/sj.bjc.6601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S., Wu K., Knox R. Structure-function studies of DT-diaphorase (NQO1) and NRH:quinone oxidoreductase (NQO2) Free Radic. Biol. Med. 2000;29:276–284. doi: 10.1016/S0891-5849(00)00308-7. [DOI] [PubMed] [Google Scholar]
- 35.Jahfar M., Azadi P. Glycosyl composition of polysaccharide from Tinospora cordifolia. II. Glycosyl linkages. Acta Pharm. 2004;54:73–78. [PubMed] [Google Scholar]
- 36.Kim S.K., Kim H.J., Choi S.E., Park K.H., Choi H.K., Lee M.W. Anti-oxidative and inhibitory activities on nitric oxide (NO) and prostaglandin E2 (COX-2) production of flavonoids from seeds of Prunus tomentosa Thunberg. Arch Pharm. Res. (Seoul) 2008;31:424–428. doi: 10.1007/s12272-001-1174-9. [DOI] [PubMed] [Google Scholar]
- 37.Ly P.T.T., Singh S., Shaw C.A. Novel environmental toxins: steryl glycosides as a potential etiological factor for age-related neurodegenerative diseases. J. Neurosci. Res. 2007;85:231–237. doi: 10.1002/jnr.21147. [DOI] [PubMed] [Google Scholar]
- 38.Sengupta S., Mukherjee A., Goswami R., Basu S. Hypoglycemic activity of the antioxidant saponarin, characterized as α-glucosidase inhibitor present in Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009;24:684–690. doi: 10.1080/14756360802333075. [DOI] [PubMed] [Google Scholar]
- 39.Yang J.H., Kondratyuk T.P., Marler L.E., Qiu X., Choi Y., Cao H., Yu R., Sturdy M., Pegan S., Liu Y., Wang L.Q., Mesecar A.D., Breemen R.B.V., Pezzuto J.M., Fong H.H.S., Chen Y.G., Zhang H.J. Isolation and evaluation of kaempferol glycosides from the fern Neocheiropteris palmatopedata. Phytochemistry. 2010;71:641–647. doi: 10.1016/j.phytochem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv J., Xu D., Perkovic V., Ma X., Johnson D.W., Woodward M., Levin A., Zhang H., Wang H. Corticosteroid therapy in IgA nephropathy. J. Am. Soc. Nephrol. 2012;23:1108–1116. doi: 10.1681/ASN.2011111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mckeown E., Bykerk V.P., De Leon F., Bonner A., Thorne C., Hitchon C.A., Boire G., Haraoui B., Ferland D.S., Keystone E.C., Pope J.E. Quality assurance study of the use of preventative therapies in glucocorticoid-induced osteoporosis in early inflammatory arthritis: results from the CATCH cohort. Rheumatology. 2012;51:1662–1669. doi: 10.1093/rheumatology/kes079. [DOI] [PubMed] [Google Scholar]
- 42.Sundarraj S., Thangam R., Sreevani V., Kaveri K., Gunasekaran P., Achiraman S., Kannan S. γ-Sitosterol from Acacia nilotica L. induces G2/M cell cycle arrest and apoptosis through c-Myc suppression in MCF-7 and A549 cells. J. Ethnopharmacol. 2012;141:803–809. doi: 10.1016/j.jep.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 43.Lee I.A., Kim E.J., Kim D.H. Inhibitory effect of β-sitosterol on TNBS-induced colitis in mice. Planta Med. 2012;78:896–898. doi: 10.1055/s-0031-1298486. [DOI] [PubMed] [Google Scholar]
- 44.Thomas A., Rajesh E.K., Kumar D.S. The significance of Tinospora crispa in treatment of diabetes mellitus. Phytother Res. 2016;30:357–366. doi: 10.1002/ptr.555. [DOI] [PubMed] [Google Scholar]
- 45.Umapathy E., Ndebia E.J., Meeme A., Adam B., Menziwa P., Nkeh-Chungag B.N., Iputo J.E. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J. Med. Plants Res. 2010;4:789–795. [Google Scholar]
- 46.Sandhyarani G., Kumar K.P. Anti-hyperlipidaemic effect of Tinospora sinensis flowers on hydrogenated groundnut oil induced hypercholesterolemia in albino rats. International Journal of Experimental Pharmacology. 2014;4:101–104. doi: 10.1080/22311866.2016.1185968. [DOI] [Google Scholar]
- 47.Sandhyarani G., Kumar K.P. Evaluation of analgesic activity of ethanolic extract of Tinospora sinensis leaves in rats. Int. J. Preclin. Pharm. Res. 2014;5:34–37. [Google Scholar]
- 48.Lam S.H., Chen P.H., Hung H.Y., Hwang T.L., Chiang C.C., Thang T.D., Kuo P.C., Wu T.S. Chemical constituents from the stems of Tinospora sinensis and their bioactivity. Molecules. 2018;23:2541. doi: 10.3390/molecules23102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh D., Chaudhuri P.K. Nat Prod Commun. SAGE Publications Sage CA; Los Angeles, CA: 2017. Chemistry and pharmacology of Tinospora cordifolia; pp. 299–308. [DOI] [PubMed] [Google Scholar]
- 50.Singh S.S., Pandey S.C., Srivastava S., Gupta V.S., Patro B., Ghosh A.C. Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J. Pharmacol. 2003;35:83–91. [Google Scholar]
- 51.Spandana U., Ali S.L., Nirmala T., Santhi M., Sipai Babu S.D. A review on Tinospora cordifolia. International Journal of Current Pharmaceutical Review and Research. 2013;4:61–68. [Google Scholar]
- 52.Praman S., Mulvany M.J., Williams D.E., Andersen R.J., Jansakul C. Hypotensive and cardio-chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J. Ethnopharmacol. 2012;140:166–178. doi: 10.1016/j.jep.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 53.Rakib A., Paul A., Nazim Uddin Chy M., Sami S.A., Baral S.K., Majumder M., Tareq A.M., Amin M.N., Shahriar A., Zia Uddin M., Dutta M., Tallei T.E., Bin Emran T., Simal-Gandara J. Biochemical and computational approach of selected phytocompounds from Tinospora crispa in the management of COVID-19. Molecules. 2020;25:3936. doi: 10.3390/molecules25173936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurya R., Gupta P., Chand K., Kumar M., Dixit P., Singh N., Dube A. Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res. 2009;23:1134–1143. doi: 10.1080/14786410802682239. [DOI] [PubMed] [Google Scholar]
- 55.Dong L.P., Chen C.X., Ni W., Xie B.B., Li J.Z., Liu H.Y. A new dinorclerone diterpenoid glycoside from Tinospora sinensis. Nat. Prod. Res. 2010;24:13–17. doi: 10.1080/14786410802253197. [DOI] [PubMed] [Google Scholar]
- 56.Gupta R., Sharma V. Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by Aflatoxin-B 1 in mice kidney. Toxicol. Int. 2011;18:94–98. doi: 10.4103/0971-6580.84259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yusoff M., Hamid H., Houghton P. Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules. 2014;19:1201–1211. doi: 10.3390/molecules19011201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choudhary M.I., Ismail M., Ali Z. Alkaloidal constituents of Tinospora crispa. Nat. Prod. Commun. 2010;5:1747–1750. [PubMed] [Google Scholar]
- 59.Karpova E.A., Voznyi Y.V., Dudukina T.V., Tsvetkova I.V. 4-Trifluoromethylumbelliferyl glycosides as new substrates for revealing diseases connected with hereditary deficiency of lysosome glycosidases. Biochem. Int. 1991;24:1135–1144. [PubMed] [Google Scholar]
- 60.Kapil A., Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J. Ethnopharmacol. 1997;58:89–95. doi: 10.1016/S0378-8741(97)00086-X. [DOI] [PubMed] [Google Scholar]
- 61.Choudhary M.I., Ismail M., Shaari K., Abbaskhan A., Sattar S.A., Lajis N.H. Atta-Ur-Rahman, Cis- clerodane-type furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 2010;73:541–547. doi: 10.1021/np900551u. [DOI] [PubMed] [Google Scholar]
- 62.Lam S.H., Ruan C.T., Hsieh P.H., Su M.J., Lee S.S. Hypoglycemic diterpenoids from Tinospora crispa. J. Nat. Prod. 2012;75:153–159. doi: 10.1021/np200692v. [DOI] [PubMed] [Google Scholar]
- 63.Hossen F., Ahasan R., Haque M.R., Begum B., Hasan C.M., Crispene A. B, C and D, four new clerodane type furanoid diterpenes from Tinospora crispa (L.) Phcog. Mag. 2016;12:S37. doi: 10.4103/0973-1296.176116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu R.R., Zhang X.P., Wang F., Shang Z.P., Wang F., Liu Y., Lu J.Q., Zhang J.Y. Rapid screening and identification of sesquiterpene lactones in Kudiezi injection based on high-performance liquid chromatography coupled with linear ion trap-orbitrap mass spectrometry. Chin. J. Nat. Med. 2018;16:150–160. doi: 10.1016/S1875-5364(18)30042-6. [DOI] [PubMed] [Google Scholar]
- 65.Jiang H., Zhang G.J., Liao H.B., Liang D. New terpenoid and phenylpropanoid glycosides from Tinospora sinensis. Fitoterapia. 2018;131:127–133. doi: 10.1016/j.fitote.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Zhao F., He E.Q., Wang L., Liu K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J. Asian Nat. Prod. Res. 2008;10:467–473. doi: 10.1080/10286020801948334. [DOI] [PubMed] [Google Scholar]
- 67.Harwoko H., Warsinah W. Phytochemical analysis and evaluation of purified extract of Tinospora crispa stem for in vivo antihyperuricemic effect. Journal of Reports in Pharmaceutical Sciences. 2020;9:46–51. doi: 10.4103/jrptps.JRPTPS_45_19. [DOI] [Google Scholar]
- 68.Hanthanong S., Choodej S., Teerawatananond T., Pudhom K. Rearranged clerodane diterpenoids from the stems of Tinospora baenzigeri. J. Nat. Prod. 2019;82:1405–1411. doi: 10.1021/acs.jnatprod.8b00483. [DOI] [PubMed] [Google Scholar]
- 69.Hanthanong S., Choodej S., Aree T., Pudhom K. Two new rearranged clerodane diterpenes from Thai Tinospora baenzigeri. J. Nat. Med. 2021;75:201–206. doi: 10.1007/s11418-020-01450-5. [DOI] [PubMed] [Google Scholar]
- 70.Choudhary M.I., Ismail M., Shaari K., Abbaskhan A., Sattar S.A., Lajis N.H. Atta-Ur-Rahman, Cis- clerodane-type furanoditerpenoids from Tinospora crispa. J. Nat. Prod. 2010;73:541–547. doi: 10.1021/np900551u. [DOI] [PubMed] [Google Scholar]
- 71.Maurya R., Wazir V., Kapil A., Kapil R.S. Cordifoliosides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat. Prod. Lett. 1996;8:7–10. [Google Scholar]
- 72.Van Kiem P., Van Minh C., Dat N.T., Van Kinh L., Hang D.T., Nam N.H., Cuong N.X., Huong H.T., Van Lau T. Aporphine alkaloids, clerodane diterpenes, and other constituents from Tinospora cordifolia. Fitoterapia. 2010;81:485–489. doi: 10.1016/j.fitote.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Sipahimalani A., Nörr H., Wagner H. Phenylpropanoid glycosides and tetrahydrofurofuranlignan glycosides from the adaptogenic plant drugs Tinospora cordifola and Drypetes roxburghii. Planta Med. 1994;60:596–597. doi: 10.1055/s-2006-959587. [DOI] [PubMed] [Google Scholar]
- 74.Pushp P., Sharma N., Joseph G.S., Singh R.P. Antioxidant activity and detection of (-)epicatechin in the methanolic extract of stem of Tinospora cordifolia. J. Food Sci. Technol. 2013;50:567–572. doi: 10.1007/s13197-011-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaur H., Hindu D., Kumar S. Chemical investigation of epoxy-hydroxyoxyacetyl bicyclo-ketone from tinospora species. Orient. J. Chem. 2010;26:273–274. [Google Scholar]
- 76.Sarma D.N.K., Khosa R.L., Sahai M. Isolation of jatrorrhizine from Tinospora cordifolia roots. Planta Med. 1995;61:98–99. doi: 10.1055/s-2006-958022. [DOI] [PubMed] [Google Scholar]
- 77.Ghosal S., Vishwakarma R.A. Tinocordiside, a new rearranged cadinane sesquiterpene glycoside from Tinospora cordifolia. J. Nat. Prod. 1997;60:839–841. doi: 10.1021/np970169z. [DOI] [Google Scholar]
- 78.Maurya R., Handa S.S. Tinocordifolin, a sesquiterpene from Tinospora cordifolia1R.R.L. communication number 22501. Phytochemistry. 1998;49:1343–1345. doi: 10.1016/S0031-9422(98)00093-4. [DOI] [Google Scholar]
- 79.Sharma A., A G., S S., A B. Tinospora cordifolia (Willd.) Hook. F. and Thomson A plant with immense economic potential. J. Chem. Pharmaceut. Res. 2010;2:327–333. [Google Scholar]
- 80.Ghosal S., Vishwakarma R.A. Tinocordiside, a new rearranged cadinane sesquiterpene glycoside from Tinospora cordifolia. J. Nat. Prod. 1997;60:839–841. [Google Scholar]
- 81.Maurya R., Handa S.S. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry. 1998;49:1343–1345. [Google Scholar]
- 82.Ahmad F., Ali M., Alam P. New phytoconstituents from the stem bark of Tinospora cordifolia Miers. Nat. Prod. Res. 2010;24:926–934. doi: 10.1080/14786410802435679. [DOI] [PubMed] [Google Scholar]
- 83.Gupta S., Singh R., Ashwlayan V.D. Pharmacological activity of Tiospora cordifolia. Pharmacologyonline. 2011;1:644–652. [Google Scholar]
- 84.Pathak A.K., Agarwal P.K., Jain D.C. NMR studies of 20p-hydroxyecdysone, a steroid; isolated from Tinospora cord∼ roliatt. Indian J. Chem. 1995;34:674–676. [Google Scholar]
- 85.Gangan V.D., Pradhan P., Sipahimalani A.T. 1997. Phytoecdysones from Tinospora Cordifolia: Structural Elucidation of Ecdysterone and Makisterone A by 2D NMR Spectroscopy. [Google Scholar]
- 86.Naik D., Dandge C., Rupanar S. Determination of chemical composition and evaluation of antioxidant activity of essential oil from Tinospora cordifolia (willd.) leaf. Journal of Essential Oil-Bearing Plants. 2014;17:228–236. doi: 10.1080/0972060X.2013.831568. [DOI] [Google Scholar]
- 87.Sarma D.N.K., Khosa R.L., Chansauria J.P.N., Sahai M. Antiulcer activity of Tinospora cordifolia Miers and Centella asiatica Linn extracts. Phytother Res. 1995;9:589–590. [Google Scholar]
- 88.Tiwari P., Nayak P., Prusty S.K., Sahu P.K. Phytochemistry and pharmacology of Tinospora cordifolia: a review. Sys. Rev. Pharm. 2018;9:70–78. doi: 10.5530/srp.2018.1.14. [DOI] [Google Scholar]
- 89.Tiwari P., Nayak P., Prusty S.K., Sahu P.K. Phytochemistry and pharmacology of Tinospora cordifolia: a review. Sys. Rev. Pharm. 2018;9:70–78. [Google Scholar]
- 90.B K., N K., V R., G V. Tinospora cordifolia (willd.) miers.: nutritional, ethnomedical and therapeutic utility. Int. J. Res. Ayurveda Pharm. 2015;6:195–198. doi: 10.7897/2277-4343.06240. [DOI] [Google Scholar]
- 91.Chougale A.D., Ghadyale V.A., Panaskar S.N., Arvindekar A.U. Alpha glucosidase inhibition by stem extract of Tinospora cordifolia. J. Enzym. Inhib. Med. Chem. 2009;24:998–1001. doi: 10.1080/14756360802565346. [DOI] [PubMed] [Google Scholar]
- 92.Umamaheswari S., Mainzen Prince P.S. Antihyperglycaemic effect of “ilogen-excel”. an ayurvedic herbal formulation in streptozotocin-induced diabetes mellitus., Acta Pol Pharm. 2007;64:53–61. [PubMed] [Google Scholar]
- 93.Devi P., Chaudhary A., Sharma R., Arora D. Antidiabetic activity of magic herb giloe: a review. World journal of pharmaceutical and technology. 2021;10:320–331. [Google Scholar]
- 94.Stanely Mainzen Prince P., Menon V.P. Hypoglycaemic and hypolipidaemic action of alcohol extract of Tinospora cordifolia roots in chemical induced diabetes in rats. Phytother Res.: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2003;17:410–413. doi: 10.1002/ptr.1130. [DOI] [PubMed] [Google Scholar]
- 95.Stanely Mainzen Prince P., Padmanabhan M., Menon V.P. Restoration of antioxidant defence by ethanolic Tinospora cordifolia root extract in alloxan-induced diabetic liver and kidney. Phytother Res. 2004;18:785–787. doi: 10.1002/ptr.1567. [DOI] [PubMed] [Google Scholar]
- 96.Shivananjappa M.M. Muralidhara, Abrogation of maternal and fetal oxidative stress in the streptozotocin-induced diabetic rat by dietary supplements of Tinospora cordifolia. Nutrition. 2012;28:581–587. doi: 10.1016/j.nut.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Patgiri B., Umretia B., Vaishnav P., Prajapati P., Shukla V., Ravishankar B. Anti-inflammatory activity of Guduchi Ghana (aqueous extract of Tinospora cordifolia Miers. AYU (An International Quarterly Journal of Research in Ayurveda) 2014;35:108. doi: 10.4103/0974-8520.141958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smirin P., Taler D., Abitbol G., Brutman-Barazani T., Kerem Z., Sampson S.R., Rosenzweig T. Sarcopoterium spinosum extract as an antidiabetic agent: in vitro and in vivo study. J. Ethnopharmacol. 2010;129:10–17. doi: 10.1016/j.jep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Noor H., Ashcroft S.J.H. Pharmacological characterisation of the antihyperglycaemic properties of Tinospora crispa extract. J. Ethnopharmacol. 1998;62:7–13. doi: 10.1016/s0378-8741(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 100.Ruan C.T., Lam S.H., Chi T.C., Lee S.S., Su M.J. Borapetoside C from Tinospora crispa improves insulin sensitivity in diabetic mice. Phytomedicine. 2012;19:719–724. doi: 10.1016/j.phymed.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 101.Mittal J., Sharma M.M., Batra A. Tinospora cordifolia: a multipurpose medicinal plant-A, Review. Journal of Medicinal Plants Studies. 2014;2:32–47. [Google Scholar]
- 102.Subramanian M., Chintalwar G.J., Chattopadhyay S. Antioxidant properties of a Tinospora cordifolia polysaccharide against iron-mediated lipid damage and γ-ray induced protein damage. Redox Rep. 2002;7:137–143. doi: 10.1179/135100002125000370. [DOI] [PubMed] [Google Scholar]
- 103.Sarala M., Velu V., Anandharamakrishnan C., Singh R.P. Spray drying of Tinospora cordifolia leaf and stem extract and evaluation of antioxidant activity. J. Food Sci. Technol. 2012;49:119–122. doi: 10.1007/s13197-011-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shivananjappa M.M. Muralidhara, Abrogation of maternal and fetal oxidative stress in the streptozotocin-induced diabetic rat by dietary supplements of Tinospora cordifolia. Nutrition. 2012;28:581–587. doi: 10.1016/j.nut.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Narayanan A.S., Raja S.S.S., Ponmurugan K., Kandekar S.C., Natarajaseenivasan K., Maripandi A., Mandeel Q.A. Antibacterial activity of selected medicinal plants against multiple antibiotic resistant uropathogens: a study from Kolli Hills, Tamil Nadu, India. Benef. Microbes. 2011;2:235–243. doi: 10.3920/BM2010.0033. [DOI] [PubMed] [Google Scholar]
- 106.Shanthi V., Nelson R. Original research article anitbacterial activity of Tinospora cordifolia (willd) hook.F.thoms on urinary tract pathogens. Int.J.Curr.Microbiol.App.Sci. 2013;2:190–194. [Google Scholar]
- 107.Singh K., Panghal M., Kadyan S., Chaudhary U., Yadav J.P. Antibacterial activity of synthesized silver nanoparticles from Tinospora cordifolia against multi drug resistant strains of Pseudomonas aeruginosa isolated from burn patients. J. Nanomed. Nanotechnol. 2014;5:1. doi: 10.4172/2157-7439.1000192. [DOI] [Google Scholar]
- 108.Duraipandiyan V., Ignacimuthu S., Balakrishna K., Al-Harbi N.A. Antimicrobial activity of Tinospora cordifolia: an ethnomedicinal plant. Asian J of trad Med. 2012;7:59–65. [Google Scholar]
- 109.Bonvicini F., Mandrone M., Antognoni F., Poli F., Gentilomi G.A. Ethanolic extracts of Tinospora cordifolia and Alstonia scholaris show antimicrobial activity towards clinical isolates of methicillin-resistant and carbapenemase-producing bacteria. Nat. Prod. Res. 2014;28:1438–1445. doi: 10.1080/14786419.2014.909421. [DOI] [PubMed] [Google Scholar]
- 110.Kaur M., Singh A., Kumar B. Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J Adv Pharm Technol Res. 2014;5:122–128. doi: 10.4103/2231-4040.137417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jagetia G.C., Rao S.K. Evaluation of the antineoplastic activity of guduchi (Tinospora cordifolia) in Ehrlich ascites carcinoma bearing mice. Biol. Pharm. Bull. 2006;29:460–466. doi: 10.1248/bpb.29.460. [DOI] [PubMed] [Google Scholar]
- 112.Jagetia G.C., Baliga M.S. Effect of Alstonia scholaris in enhancing the anticancer activity of berberine in the Ehrlich ascites carcinoma-bearing mice. J. Med. Food. 2004;7:235–244. doi: 10.1089/1096620041224094. [DOI] [PubMed] [Google Scholar]
- 113.Makhey D., Gatto B., Yu C., Liu A., Liu L.F., LaVoie E.J. Coralyne and related compounds as mammalian topoisomerase I and topoisomerase II poisons. Bioorg. Med. Chem. 1996;4:781–791. doi: 10.1016/0968-0896(96)00054-5. [DOI] [PubMed] [Google Scholar]
- 114.Thippeswamy G., Salimath B.P. Induction of caspase-3 activated DNase mediated apoptosis by hexane fraction of Tinospora cordifolia in EAT cells. Environ. Toxicol. Pharmacol. 2007;23:212–220. doi: 10.1016/j.etap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 115.Ali H., Dixit S. Extraction optimization of Tinospora cordifolia and assessment of the anticancer activity of its alkaloid palmatine. Sci. World J. 2013;2013 doi: 10.1155/2013/376216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pandey V.K., Amin P.J., Shankar B.S. G1-4A, a polysaccharide from Tinospora cordifolia induces peroxynitrite dependent killer dendritic cell (KDC) activity against tumor cells. Int. Immunopharm. 2014;23:480–488. doi: 10.1016/j.intimp.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 117.Pandey V.K., Shankar B.S., Sainis K.B. G1-4 A, an arabinogalactan polysaccharide from Tinospora cordifolia increases dendritic cell immunogenicity in a murine lymphoma model. Int. Immunopharm. 2012;14:641–649. doi: 10.1016/j.intimp.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 118.Maliyakkal N., Appadath Beeran A., Balaji S.A., Udupa N., Ranganath Pai S., Rangarajan A. Effects of withania somnifera and Tinospora cordifolia extracts on the side population phenotype of human epithelial cancer cells: toward targeting multidrug resistance in cancer. Integr. Cancer Ther. 2015;14:156–171. doi: 10.1177/1534735414564423. [DOI] [PubMed] [Google Scholar]
- 119.Subash-Babu P., Alshammari G.M., Ignacimuthu S., Alshatwi A.A. Epoxy clerodane diterpene inhibits MCF-7 human breast cancer cell growth by regulating the expression of the functional apoptotic genes Cdkn2A, Rb1, mdm2 and p53. Biomed. Pharmacother. 2017;87:388–396. doi: 10.1016/j.biopha.2016.12.091. [DOI] [PubMed] [Google Scholar]
- 120.Thippeswamy G., Sheela M.L., Salimath B.P. Octacosanol isolated from Tinospora cordifolia downregulates VEGF gene expression by inhibiting nuclear translocation of NF-<kappa>B and its DNA binding activity. Eur. J. Pharmacol. 2008;588:141–150. doi: 10.1016/j.ejphar.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 121.Mishra R., Kaur G. Tinospora cordifolia induces differentiation and senescence pathways in neuroblastoma cells. Mol. Neurobiol. 2015;52:719–733. doi: 10.1007/s12035-014-8892-5. [DOI] [PubMed] [Google Scholar]
- 122.Patil D., Gautam M., Gairola S., Jadhav S., Patwardhan B. Effect of botanical immunomodulators on human CYP3A4 inhibition: implications for concurrent use as adjuvants in cancer therapy. Integr. Cancer Ther. 2014;13:167–175. doi: 10.1177/1534735413503551. [DOI] [PubMed] [Google Scholar]
- 123.Hamsa T.P., Kuttan G. Tinospora cordifolia ameliorates urotoxic effect of cyclophosphamide by modulating GSH and cytokine levels. Exp. Toxicol. Pathol. 2012;64:307–314. doi: 10.1016/j.etp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 124.Panneer selvam K., Payyappallimana U., Ravikumar K., Venkatasubramanian P. Can Guduchi (Tinospora cordifolia), a well-known ayurvedic hepato-protectant cause liver damage? J. Ayurveda Integr. Med. 2023;14 doi: 10.1016/j.jaim.2022.100658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Haque MdA., Jantan I., Abbas Bukhari S.N. Tinospora species: an overview of their modulating effects on the immune system. J. Ethnopharmacol. 2017;207:67–85. doi: 10.1016/j.jep.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 126.Dhama K., Sachan S., Khandia R., Munjal A., Iqbal H.M.N., Latheef S.K., Karthik K., Samad H.A., Tiwari R., Dadar M. Medicinal and beneficial health applications of Tinospora cordifolia (guduchi): a miraculous herb countering various diseases/disorders and its immunomodulatory effects. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017;10:96–111. doi: 10.2174/1872214811666170301105101. [DOI] [PubMed] [Google Scholar]
- 127.Onkar P., Bangar J., Karodi R. Evaluation of antioxidant activity of traditional formulation giloy satva and hydroalcoholic extract of the Curculigo orchioides gaertn. J. Appl. Pharmaceut. Sci. 2012;2:209–213. doi: 10.7324/JAPS.2012.2733. [DOI] [Google Scholar]
- 128.Stansbury J., Saunders P.R., Zampieron E.R., Winston D. The treatment of liver disease with botanical agents. J Restor Med. 2013;2:84–93. doi: 10.14200/jrm.2013.2.0108. [DOI] [Google Scholar]
- 129.Rege N., Bapat R.D., Koti R., Desai N.K., Dahanukar S. Immunotherapy with Tinospora cordifolia: a new lead in the management of obstructive jaundice. Indian J. Gastroenterol. 1993;12:5–8. [PubMed] [Google Scholar]
- 130.Bapat R.D., Rege N.N., Koti R.S., Desai N.K., Dahanukar S.A. Can we do away with PTBD? HPB Surg. 1995;9:5–11. doi: 10.1155/1995/90362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hegde S., Jayaraj M. A review of the medicinal properties, phytochemical and biological active compounds of Tinospora sinensis (lour.) Merr. Journal of Biologically Active Products from Nature. 2016;6:84–94. doi: 10.1080/22311866.2016.1185968. [DOI] [Google Scholar]
- 132.Aher V., Wahi A.K. Biotechnological approach to evaluate the immunomodulatory activity of ethanolic extract of Tinospora cordifolia stem (mango plant climber) Iran. J. Pharm. Res. (IJPR) 2012;11:863–872. [PMC free article] [PubMed] [Google Scholar]
- 133.More P., Pai K. In vitro NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of macrophages after Tinospora cordifolia (guduchi) treatment. Immunopharmacol. Immunotoxicol. 2012;34:368–372. doi: 10.3109/08923973.2011.606324. [DOI] [PubMed] [Google Scholar]
- 134.Upadhya R., Pandey R., Sharma V., Verma A. Assessment of the multifaceted immunomodulatory potential of the aqueous extract of Tinospora cordifolia. Res. J. Chem. Sci. 2011;1:71–79. [Google Scholar]
- 135.Castillo A.L., Ramos J., De Francia J.L., Quilala P.F., Dujunco M.U. Immunomodulatory effects of Tinospora cordifolia lotion on interleukin-1, interleukin-6 and interleukin-8 levels in scabies-infected pediatric patients: a single blind, randomized trial. 2014. www.ijpsdr.com
- 136.Samuel Sudhakaran D., Srirekha P., Devasree L.D., Premsingh S., Dinakaran Michael R. Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J. Exp. Biol. 2006;44:726–732. [PubMed] [Google Scholar]
- 137.Raghu R., Sharma D., Ramakrishnan R., Khanam S., Chintalwar G.J., Sainis K.B. Molecular events in the activation of B cells and macrophages by a non-microbial TLR4 agonist, G1-4A from Tinospora cordifolia. Immunol. Lett. 2009;123:60–71. doi: 10.1016/j.imlet.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 138.Koppada R., Norozian F.M., Torbati D., Kalomiris S., Ramachandran C., Totapally B.R. Physiological effects of a novel immune stimulator drug, (1,4)-α-d-glucan, in rats. Basic Clin. Pharmacol. Toxicol. 2009;105:217–221. doi: 10.1111/j.1742-7843.2009.00383.x. [DOI] [PubMed] [Google Scholar]
- 139.Aher V., Wahi A. Pharmacological study of Tinospora cordifolia as an immunomodulator. Int J Curr Pharm Res. 2010;2:50–52. http://www.ijcpr.org/Issues/Vol2Issue4/245.pdf [Google Scholar]
- 140.Promila S., Singh P., Devi U. Pharmacological potential of tinospora cordifolia (willd.) miers ex hook. & thoms.(giloy): a review. J. Pharmacogn. Phytochem. 2017;6:1644–1647. https://www.phytojournal.com/archives?year=2017&vol=6&issue=6&ArticleId=2275 [Google Scholar]
- 141.Tiwari P., Nayak P., Prusty S.K., Sahu P.K. Phytochemistry and pharmacology of Tinospora cordifolia: a review. Sys. Rev. Pharm. 2018;9:70–78. doi: 10.5530/srp.2018.1.14. [DOI] [Google Scholar]
- 142.Gupta G.D., Sujatha N., Dhanik A., Rai N.P. Clinical evaluation of Shilajatu Rasayana in patients with HIV infection. Ayu. 2010;31:28. doi: 10.4103/0974-8520.68205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Patel A., Bigoniya P., Singh C.S., Patel N.S. Radioprotective and cytoprotective activity of Tinospora cordifolia stem enriched extract containing cordifolioside-A. Indian J. Pharmacol. 2013;45:237–243. doi: 10.4103/0253-7613.111919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kalikar M., Thawani V., Varadpande U., Sontakke S., Singh R., Khiyani R. Immunomodulatory effect of Tinospora cordifolia extract in human immuno-deficiency virus positive patients. Indian J. Pharmacol. 2008;40:107–110. doi: 10.4103/0253-7613.42302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Akhtar S. Use of Tinospora cordifolia in HIV infection. Indian J. Pharmacol. 2010;42:57. doi: 10.4103/0253-7613.62402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jeyachandran R., Xavier T.F., Anand S.P. Antibacterial activity of stem extracts of Tinospora cordifolia (Willd) Hook. f & Thomson. Ancient Sci. Life. 2003;23:40–43. [PMC free article] [PubMed] [Google Scholar]
- 147.Modi B., Shah K.K., Shrestha J., Shrestha P., Basnet A., Tiwari I., Aryal S.P. Morphology , biological activity , chemical composition , and medicinal value of Tinospora cordifolia (willd .) Miers. Advanced Journal of Chemistry-Section B. 2021;3:36–53. https://www.researchgate.net/profile/Jiban-Shrestha/publication/344819737_Morphology_Biological_Activity_Chemical_Composition_and_Medicinal_Value_of_Tinospora_Cordifolia_willd_Miers/links/5f91bb33299bf1b53e3d6f83/Morphology-Biological-Activity-Chemical-Co [Google Scholar]
- 148.Goel B., Pathak N., Nim D.K., Singh S.K., Dixit R.K., Chaurasia R. Clinical evaluation of analgesic activity of Guduchi (Tinospora cordifolia) using animal model. J. Clin. Diagn. Res. 2014;8 doi: 10.7860/JCDR/2014/9207.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tinospora cordifolia regulates lipid metabolism in allaxon induced diabetes in rats. Int. J. of Pharm. & Life Sci. (IJPLS) 2012;3:1858–1867. [Google Scholar]
- 150.Xu Y., Niu Y., Gao Y., Wang F., Qin W., Lu Y., Hu J., Peng L., Liu J., Xiong W., Borapetoside E. A clerodane diterpenoid extracted from Tinospora crispa, improves hyperglycemia and hyperlipidemia in high-fat-diet-induced type 2 diabetes mice. J. Nat. Prod. 2017;80:2319–2327. doi: 10.1021/acs.jnatprod.7b00365. [DOI] [PubMed] [Google Scholar]
- 151.Abood W.N., Fahmi I., Abdulla M.A., Ismail S. Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement Altern Med. 2014;14:205. doi: 10.1186/1472-6882-14-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wani J.A., Achur R.N., Nema R.K. Phytochemical screening and aphrodisiac property of Tinospora cordifolia. International Journal of Pharmaceutical and Clinical Research. 2011;3:21–26. [Google Scholar]
- 153.Kosaraju J., Chinni S., Roy P.D., Kannan E., Antony A.S., Kumar M.N.S. Neuroprotective effect of Tinospora cordifolia ethanol extract on 6-hydroxy dopamine induced Parkinsonism. Indian J. Pharmacol. 2014;46:176–180. doi: 10.4103/0253-7613.129312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rajalakshimi V., Gauthaman K., Adel Ali A., Nazima N. Analgesic and anti-inflammatory evaluation of ethanolic extract of Seenthil churanam. Int. J. Pharmaceut. Sci. Drug Res. 2014;7:63–67. [Google Scholar]
- 155.Punitha D., Udhayasankar M.R., Danya U., Arumugasamy K., Shalimol A. Anti-inflammatory activity of characterized compound diosgenin isolated from Tinospora malabarica Miers in Ann.(Menispermaceae) in animal model. Int. J. Herb. Med. 2013;1:76–78. [Google Scholar]
- 156.Kamarazaman I.S. Inhibitory properties of Tinospora crispa extracts on TNF-cz induced inflammation on human umbilical vein endothelial cells (HUVECS)* ihsan safwan kamarazaman,“Zulkhairi Hj. Amom," Rasadah Mat Ali," Abdah Md Akim,“Khairunnuur Fairuz Azman," Daryl Jesus Ar. Int. J. Trop. Med. 2012;7:24–29. [Google Scholar]
- 157.Antonisamy P., Dhanasekaran M., Ignacimuthu S., Duraipandiyan V., Balthazar J.D., Agastian P., Kim J.H. Gastroprotective effect of epoxy clerodane diterpene isolated from Tinospora cordifolia Miers (Guduchi) on indomethacin-induced gastric ulcer in rats. Phytomedicine. 2014;21:966–969. doi: 10.1016/j.phymed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 158.Jayaprakash R., Ramesh V., Sridhar M.P., Sasikala C. Antioxidant activity of ethanolic extract of Tinospora cordifolia on N-nitrosodiethylamine (diethylnitrosamine) induced liver cancer in male Wister albino rats. J. Pharm. BioAllied Sci. 2015;7:S40–S45. doi: 10.4103/0975-7406.155791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jain B.N., Jain V., Shete A. Antipsychotic activity of aqueous ethanolic extract of Tinospora cordifolia in amphetamine challenged mice model. J Adv Pharm Technol Res. 2010;1:30–33. [PMC free article] [PubMed] [Google Scholar]
- 160.Patel A., Bigoniya P., Singh C.S., Patel N.S. Radioprotective and cytoprotective activity of Tinospora cordifolia stem enriched extract containing cordifolioside-A. Indian J. Pharmacol. 2013;45:237–243. doi: 10.4103/0253-7613.111919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sivasubramanian A., Narasimha K.K.G., Rathnasamy R., Campos A.M.F.O. A new antifeedant clerodane diterpenoid from Tinospora cordifolia. Nat. Prod. Res. 2013;27:1431–1436. doi: 10.1080/14786419.2012.722088. [DOI] [PubMed] [Google Scholar]
- 162.Stanca M.H., Nagy A., Toæa M., Vlad L. Hepatoprotective effects of per os administrated melatonin and Tinospora cordifolia in experimental induced jaundice. Chirurgia (Romania) 2011;108:205–210. [PubMed] [Google Scholar]
- 163.Chinna Eshwaraiah M., Manasa N., Kavitha K., Bardalai D. Evaluation of Hepato protective activity of ethanolic root extract of Punica granatum. Int J Pharm Pharm Sci. 2013;5:220–223. [Google Scholar]
- 164.GuptaHemRaj A., Karchuli M.S., Upmanyu N. Comparative evaluation of ethanolic extracts of bacopa monnieri, evolvulus alsinoides, Tinospora cordifolia and their combinations on cognitive functions in rats. Curr. Aging Sci. 2014;6:239–243. doi: 10.2174/18746098112059990036. [DOI] [PubMed] [Google Scholar]
- 165.Jain B.N., Jain V., Shete A. Antipsychotic activity of aqueous ethanolic extract of Tinospora cordifolia in amphetamine challenged mice model. J Adv Pharm Technol Res. 2010;1:30–33. [PMC free article] [PubMed] [Google Scholar]
- 166.Dhingra D., Goyal P.K. Evidences for the involvement of monoaminergic and GABAergic systems in antidepressant-like activity of Tinospora cordifolia in mice. Indian J Pharm Sci. 2008;70:761–767. doi: 10.4103/0250-474X.49118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kapur P., Jarry H., Wuttke W., Pereira B.M.J., Seidlova-Wuttke D. Evaluation of the antiosteoporotic potential of Tinospora cordifolia in female rats. Maturitas. 2008;59:329–338. doi: 10.1016/j.maturitas.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 168.Gupta R.S., Sharma A. Antifertility effect of Tinospora cordifolia (Willd.) stem extract in male rats. Indian J. Exp. Biol. 2003;41:885–889. [PubMed] [Google Scholar]
- 169.Tiwari M., Dwivedi U.N., Kakkar P. Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1, pro-inflammatory cytokines and redox status in murine model of asthma. J. Ethnopharmacol. 2014;153:326–337. doi: 10.1016/j.jep.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 170.Mishra R., Kaur G. Aqueous ethanolic extract of Tinospora cordifolia as a potential candidate for differentiation based therapy of glioblastomas. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Badar V.A., Thawani V.R., Wakode P.T., Shrivastava M.P., Gharpure K.J., Hingorani L.L., Khiyani R.M. Efficacy of Tinospora cordifolia in allergic rhinitis. J. Ethnopharmacol. 2005;96:445–449. doi: 10.1016/j.jep.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 172.Nadig P., Revankar R., Dethe S., Narayanswamy S., Aliyar M. Effect of Tinospora cordifolia on experimental diabetic neuropathy. Indian J. Pharmacol. 2012;44:580–583. doi: 10.4103/0253-7613.100380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Niljan J., Jaihan U., Srichairatanakool S., Uthaipibull C., Somsak V. Antimalarial activity of stem extract of Tinospora crispa against Plasmodium berghei infection in mice. J Health Res. 2014;28:199–204. https://thaiscience.info/Journals/Article/JHRE/10935094.pdf [Google Scholar]
- 174.Mishra R., Kaur G. Tinospora cordifolia induces differentiation and senescence pathways in neuroblastoma cells. Mol. Neurobiol. 2015;52:719–733. doi: 10.1007/s12035-014-8892-5. [DOI] [PubMed] [Google Scholar]
- 175.Jeyachandran R., Xavier T.F., Anand S.P. Antibacterial activity of stem extracts of Tinospora cordifolia (Willd) Hook. f & Thomson. Ancient Sci. Life. 2003;23:40. [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma M., Pandey G., Khanna A. Studies on phytochemistry and toxicities of Tinospora cordifolia (Giloe) Anusandhan. 2011;5:64–68. doi: 10.13140/2.1.3938.7205. [DOI] [Google Scholar]
- 177.Balkrishna A., et al. Traditional uses, hepatoprotective potential, and phytopharmacology of Tinospora cordifolia: a narrative review. J. Pharm. Pharmacol. 2024;76:183–200. doi: 10.1093/jpp/rgae013. [DOI] [PubMed] [Google Scholar]
- 178.Rege N.N., Thatte U.M., Dahanukar S.A. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 179.Manjrekar P.N., Jolly C.I., Narayanan S. Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia. 2000;71:254–257. doi: 10.1016/S0367-326X(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 180.Nayampalli S.S., Ainapure S.S., Samant B.D., Kudtarkar R.G., Desai N.K., Gupta K.C. A comparative study of diuretic effects of Tinospora cordifolia and hydrochlorothiazide in rats and a preliminary phase I study in human volunteers. J. Postgrad. Med. 1998;34:233–236. PMID: 3254990. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data required to support this study is already mentioned in the manuscript.