Abstract
Glycerol monolaurate (GML) inhibits the expression of virulence factors in Staphylococus aureus and the induction of vancomycin resistance in Enterococcus faecalis, presumably by blocking signal transduction. Although GML is rapidly hydrolyzed by bacteria, one of the products, lauric acid, has identical inhibitory activity and is metabolized much more slowly. At least four distinct GML-hydrolyzing activities are identified in S. aureus: the secreted Geh lipase, residual supernatant activity in a geh-null mutant strain, a novel membrane-bound esterase, and a cytoplasmic activity.
Glycerol monolaurate (GML) is a mild surfactant that is used in the food industry and in cosmetics as a preservative and emulsifier. At concentrations higher than 20 μg/ml, it inhibits growth of bacteria. However, at lower concentrations which do not significantly alter bacterial growth, GML blocks the production of various exoenzymes and virulence factors, including protein A, alpha-hemolysin, β-lactamase, and toxic shock syndrome toxin 1 (TSST-1) (12, 15) in Staphylococcus aureus. The action of GML is not restricted to S. aureus: it also blocks the induction of vancomycin resistance in another pathogen, Enterococcus faecalis (13).
The mechanism of GML inhibitory action is not known. It has been shown that GML affects neither secretion nor intracellular signaling and most likely acts through the inhibition of signal transduction (12, 13). As shown previously (12), GML does not inhibit synthesis of RNAIII, a key effector molecule, and therefore does not act through the agr system (4), which regulates RNAIII production. As proposed previously (12, 13), GML most likely affects exoprotein production through some uncharacterized signal transduction pathway(s). Studies of GML action are aimed toward identification and characterization of this pathway; however, they are complicated by the loss of activity in bacterial cultures (12), which has been assumed to be the result of hydrolysis to lauric acid and glycerol. In this paper, we describe a thin-layer chromatographic (TLC) method for monitoring GML and its hydrolysis and confirm that GML is very rapidly hydrolyzed to lauric acid and glycerol by staphylococci, with a half-life of ∼5 min in a typical culture. Nevertheless, in earlier studies, it was possible to maintain the inhibitory effect of GML by hourly additions to a growing culture (12). Thus, the kinetics of GML hydrolysis does not match the rate at which activity disappears. This result led us to demonstrate that lauric acid inhibits the same processes that are inhibited by GML and that its activity is equimolar with that of the ester.
These results raise the question of whether lauric acid is entirely responsible for the observed effects of GML. Because this question can be addressed only in the absence of GML hydrolysis, we have begun to identify the enzymes responsible for the hydrolysis. We find that there is GML esterase activity in culture supernatants, in association with the cell membrane, and in the cytoplasm. We find that the well-known Geh lipase is responsible for the majority (∼80%) of detectable GML-hydrolyzing activity in culture supernatants, and there is a previously undescribed membrane-bound esterase that is responsible for much, possibly all, of the cell-bound activity. Residual (∼20%) hydrolyzing activity in supernatants probably represents a previously described short-chain esterase, which has detectable activity with lauric acid esters (8, 16). The role of cytoplasmic esterases is uncertain and can be evaluated only after elimination of both extracellular and membrane-bound GML-hydrolyzing activities.
Development of a TLC method to monitor GML and lauric acid.
We tested solutions of GML and lauric acid in bacterial culture media by TLC. Samples were centrifuged, and 8 μl of supernatant was applied by micropipette to Whatman silica gel 60A TLC plates (20 by 20, with preadsorbent area). Emulsions of GML (40 μg/ml) and lauric acid (30 μg/ml) in CY/GP broth (9) were used as standards. Plates were air dried and developed with hexane-ethyl ether-methanol (70:20:10), air dried, baked at 100°C for 10 min, and sprayed with a 0.025% (wt/vol) solution of Coomassie R-250 in 20% (vol/vol) methanol until lipids were visible as white spots on a blue background. Picture taking and spot densitometry (not shown) were performed with the IS-1000 imaging system (Alpha Innotech Corp.). As shown in Fig. 1, we obtained satisfactory separation of GML (Rf = 0.24) and lauric acid (Rf = 0.35) from each other and from the lipid components of culture media (Rf = 0 to 0.07). The detection limit of negative staining was about 15 ng for GML (not shown). Several other lipid staining procedures (sulfuric acid and rhodamine B) were unsatisfactory.
FIG. 1.
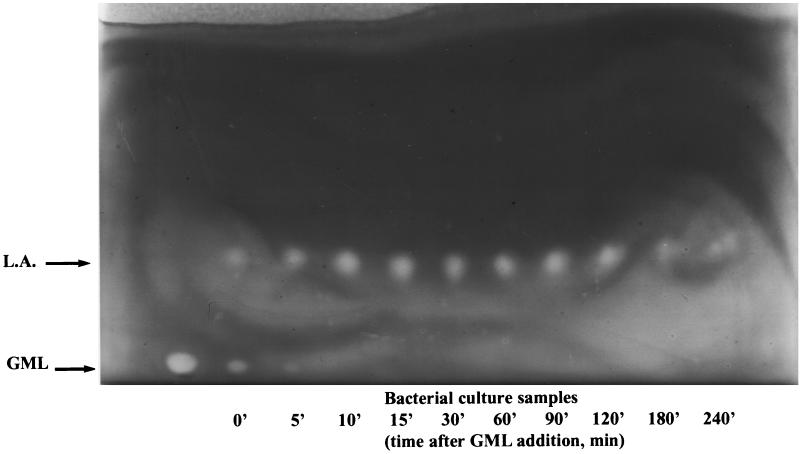
Degradation of GML monitored by TLC. GML was added to a growing culture of RN11, and samples (100 μl) were collected at the indicated time points and applied to a TLC plate. The plate was developed, stained with Coomassie blue, and photographed. GML and lauric acid (L.A.) appear as white spots on a dark background.
Fate of GML in growing bacterial cultures.
The bacterial strains used in this study are shown in Table 1. The culture medium was CY/GP broth (9). GML (Personal Products Co., New Brunswick, N.J.) and lauric acid (Sigma) were prepared at 1% (wt/vol) in 95% ethanol. Cultures were grown at 37°C with shaking at 240 rpm. To determine the fate of GML in staphylococcal cultures, we analyzed samples of RN11 culture growing in the presence of GML (20 μg/ml) by TLC. As shown in Fig. 1, GML disappears with a half-life of about 5 min and is replaced by lauric acid and, presumably, glycerol (which is not seen on TLC). Lauric acid persists in the culture for at least 2 h and then slowly decreases in quantity (Fig. 1).
TABLE 1.
Characteristics of the bacterial strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| MN8 | Naturally occurring TSST-1 producer | 14 |
| RN11 | NCTC8325(pI258) | 10 |
| RN6390B | NCTC8325 cured of 3 prophages; agr+ | 4 |
| RN6911 | Derivative of RN6390B with tetM replacing agr | 4 |
| FRI1169 | TSST-1+ clinical isolate | 3 |
| RN8083 | φL54a lysogen of FRI1169 | Kindly provided by S. Projan |
GML hydrolases.
Previous observations had shown that elimination of the major secreted esterase, Geh, by φL54a prophage-mediated insertional inactivation of its gene (7) had little if any effect on GML stability (S. Projan, personal communication), indicating that S. aureus produces one or more additional esterases capable of hydrolyzing GML.
As we found, both culture supernatant and cells had significant GML-hydrolyzing activity, (Table 2). Most of the supernatant activity could be accounted for by Geh esterase. A Geh-negative strain (RN8083), generated by lysogenization with phage φL54a, which has its attachment site within geh (7), showed less than 22% of normal supernatant activity, while cell-associated activity was undiminished (Table 2).
TABLE 2.
Lipolytic activities of individual fractionsa
| Strain and fraction | Lipolytic activity (U) |
|---|---|
| RN11 | |
| Culture supernatant | 814 |
| Cells (unwashed) | 1,301 |
| Cells (salt washed) | 1,261 |
| FRI1169 | |
| Culture supernatant | 2,480 |
| Cells (salt washed) | 4,140 |
| RN8083 | |
| Culture supernatant | 529 |
| Cells (salt washed) | 4,410 |
| Intracellular | 1,080 |
| Membrane (salt washed) | 3,120 |
Densitometry of GML spots (not shown) was performed with the IS-1000 imaging system. Lipolytic activities (obtained from densitometry data after correction for incubation time and sample dilution) are expressed in specific units, each of which corresponds to 1 ng of GML degraded per min ml−1.
One of the possibilities of cell-associated lipolytic activity—noncovalent binding of secreted lipases to cell wall—was excluded by washing cells in 1 M NaCl and testing for residual activity. As shown in Table 2, this treatment did not diminish the cell-associated activity.
To localize cell-associated GML-hydrolyzing activity, we fractionated the cells. Cultures were grown with shaking in 2× Penassay broth (Difco), washed in SMM buffer (1 M sucrose, 35 mM sodium maleate, 85 mM MgCl2) plus 1 M NaCl, and treated with lysostaphin (10-μg/ml final concentration). The resulting protoplasts were lysed by osmotic shock and ultrasonication and centrifuged at 15,000 rpm for 10 min. The supernatant was then centrifuged at 70,000 rpm in a Beckman TLA100.4 rotor. Pellet (membrane fraction) was washed with 1 M NaCl in 0.1× SMM, recentrifuged, rinsed with 0.1× SMM, and resuspended in 0.1× SMM. We found activity in both intracellular and membrane fractions (Table 2). One or more uncharacterized cytoplasmic esterases are responsible for GML hydrolysis by the intracellular fraction; we suggest that one or more membrane hydrolases are responsible for the membrane-associated activity. Based on these results, we suggest that S. aureus possesses one or more novel membrane-bound lipases, which actively participate in degradation of GML.
As a first step toward purification of the membrane-bound enzyme(s), the membrane fraction was solubilized either with CHAPS {-3-[(3-cholamidopropyl)-dimethylammonia]-1-propanesulfonate} (5 mg/ml) or sodium deoxycholate (DOC, 2mg/ml) and subjected to ultracentrifugation, followed by dialysis of the supernatant against phosphate-buffered saline–0.1% β-mercaptoethanol. We found that material solubilized by CHAPS retained GML hydrolase activity, whereas that solubilized by DOC did not (not shown). Further purification of staphylococcal membrane-bound lipase(s) is currently in progress.
Inhibitory effects of lauric acid.
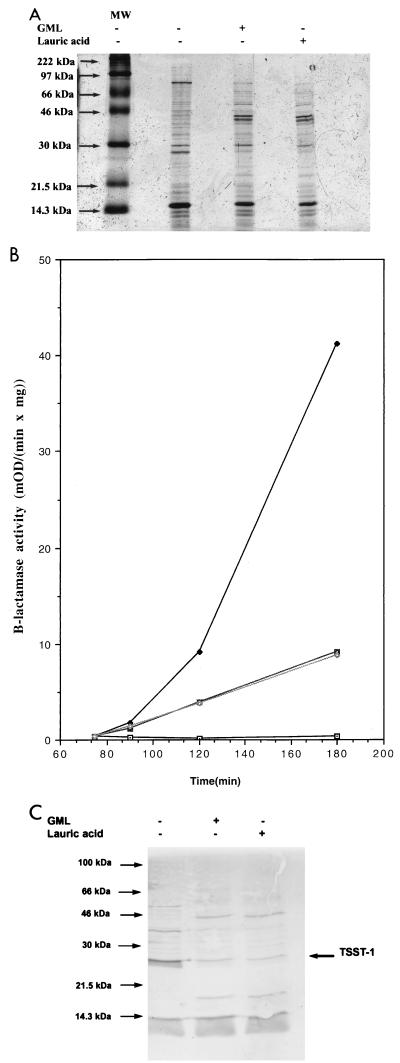
Given the observed rapid degradation of GML and persistence of lauric acid, we tested for the possibility that lauric acid might be at least partially responsible for the inhibitory effects of GML. Accordingly, we compared the effects of lauric acid and GML on the overall production of the exoproteins and on the induction of two different exoproteins, TSST-1 and β-lactamase, shown previously to be blocked by GML (12, 15). β-Lactamase activity was determined in a microplate reader with nitrocefin essentially as described previously (11). For analysis of bulk exoprotein production, MN8 cells were grown in broth, centrifuged and supernatants were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis SDS-PAGE (15% polyacrylamide) by the method of Laemmli (6). For analysis of TSST-1 production, separated proteins were transferred electrophoretically to nitrocellulose filters and immunoblotted with anti-TSST-1 rabbit serum (kindly provided by P. Schlievert) according to the method of Blake et al. (1). As shown in Fig. 2A, lauric acid and GML have identical effects on the production of staphylococcal exoproteins: some proteins are inhibited, while others are overproduced compared to those of untreated cells. In Fig. 2B and C are shown the results for β-lactamase induction and TSST-1 production, respectively. As can be seen, lauric acid and GML, at equimolar concentrations, have identical inhibitory effects.
FIG. 2.
Effects of GML and lauric acid on production of exoproteins, induction of β-lactamase synthesis, and production of TSST-1 in S. aureus. (A) Production of exoproteins. MN8 cultures were grown in broth with shaking with either GML, lauric acid, or no additives until the late-exponential phase (1,000 Klett units/ml). GML was added to 20 μg/ml at 0 min and to 10 μg/ml at 90, 150, 210 min. Lauric acid was added to 15 μg/ml at 0 min and to 7.5 μg/ml at 90, 150, and 210 min. Supernatants of final cultures were concentrated 10 times by precipitation with 10% trichloracetic acid, and proteins were separated by SDS-PAGE (12% polyacrylamide) and visualized by Coomassie staining. MW, molecular mass markers. (B) Induction of β-lactamase synthesis. Bacterial cultures (RN11) were grown in broth; equimolar subinhibitory amounts of GML (10 μg/ml) or lauric acid (7.5 μg/ml) were added at 0, 60, and 120 min; and β-lactamase inducer CBAP (carboxyphenylbenzoyl penicillanic acid) (5 μg/ml) was added at 75 min. Samples (1 ml) were collected at 60, 90, 120, and 180 min and assayed for β-lactamase activity. Each data point represents the average of two experiments. ⊡, no GML or lauric acid and no CBAP; ⧫, no GML or lauric acid and with CBAP added;  , GML and CBAP added; ◊, lauric acid and CBAP added. (C) Inhibition of TSST-1 production. Supernatants of MN8 cultures obtained as described for panel A were separated by SDS-PAGE (15% polyacrylamide). Separated proteins were transferred to a nitrocellulose filter (BA85; Schleicher & Schuell) and immunoblotted with anti-TSST-1 rabbit serum (kindly provided by P. Schlievert) according to the method of Blake et al. (1).
, GML and CBAP added; ◊, lauric acid and CBAP added. (C) Inhibition of TSST-1 production. Supernatants of MN8 cultures obtained as described for panel A were separated by SDS-PAGE (15% polyacrylamide). Separated proteins were transferred to a nitrocellulose filter (BA85; Schleicher & Schuell) and immunoblotted with anti-TSST-1 rabbit serum (kindly provided by P. Schlievert) according to the method of Blake et al. (1).
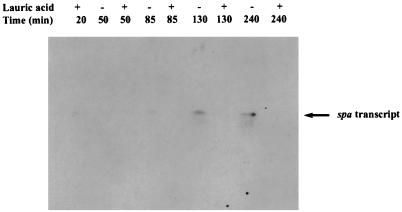
Additionally, GML inhibits the production of staphylococcal protein A (12), and as shown in Fig. 3, lauric acid blocks spa transcription. In this assay, whole-cell RNA was prepared from RN6911 and used for Northern blot hybridization according to the method of Kornblum et al. (5). Blots were hybridized with PCR-labeled (32P) spa-specific DNA probe. PCR was performed with the 5′ ATCTGGTGGCGTAACAC 3′ (forward) and 5′ CAGCTTTCGGTGCTTG 3′ (reverse) primers and RN6911 chromosomal DNA as a template.
FIG. 3.
Effect of lauric acid on spa transcription. RN6911 (agr-null mutant in which spa is overexpressed) was grown in broth either with or without lauric acid. Lauric acid was added at 0 min (15 μg/ml) and 65 min (7.5 μg/ml) and samples (10 ml) were collected at 0, 65, 110, and 220 min. Whole-cell lysates were prepared and used for RNA (Northern) blotting. The blot was hybridized overnight with spa-specific PCR-labelled probe, washed, and exposed to Kodak X-Omat AR film.
Conclusions.
In this report, we describe a new TLC method that has been used to monitor the GML concentration in growing cultures of S. aureus. We confirmed our earlier hypothesis that GML degradation is responsible for the transience of its inhibitory effects (12). Degradation of GML is very rapid (t1/2 of ∼5 min), while inhibitory effects of GML last for at least an hour. We noticed that lauric acid, a hydrolysis product of GML, persists in the culture for more than 2 h. We suggested that lauric acid might be responsible for prolonged inhibitory effects of GML. We found that indeed lauric acid at an equimolar concentration mimics the inhibitory effect of GML on the induction of staphylococcal β-lactamase activity and, like GML, blocks expression of protein A and TSST-1 in S. aureus. Thus, we currently hypothesize that lauric acid might be responsible for all of the inhibitory effects of GML described so far, although GML might be active as well.
To test this hypothesis, we first needed to identify the enzyme or enzymes that are responsible for GML hydrolysis and later to eliminate them by mutation. Both supernatant and salt-washed cells had activity. That in the supernatant is mainly attributed to the well-known Geh lipase. The residual GML-hydrolyzing activity in supernatant of RN8083 could correspond to an additional secreted esterase that has recently been described for S. aureus NCTC8530 (8, 16). Although the preferred substrates for this enzyme are short-chain (propionyl- and butyryl-) acyl esters, it has detectable activity with lauric acid esters. Cell-associated lipolytic activity might be explained by the action of either intracellular or membrane-bound lipases. However, intracellular lipases, previously described in S. aureus (2), are not expected to play any significant role in GML degradation, since, as mentioned above, GML is a surfactant and is considered unlikely to reach the cytoplasm. Membrane-bound lipases have been identified in other bacterial species, e.g., Escherichia coli, Bacillus megaterium, Mycobacterium phlei, and members of the genus Vibrio (17). These enzymes belong to class of acyl hydrolases, functions of which are still poorly understood. Although they are presumed to play role in bacterial membrane metabolism, mutants defective in the production of such enzymes are viable and have a normal lipid composition, possibly due to redundancy. Perhaps lipases and esterases have a role in nutrition in that their products can be metabolized by the bacteria. Membrane-bound lipases have not been previously described for S. aureus, and our results represent their initial identification in this species. The membrane-bound GML hydrolase or hydrolases may or may not be analogs of the acyl hydrolases of other species. Our next steps toward identification of such enzymes in S. aureus will include protein purification, isolation of their genes by reverse genetics, and subsequent inactivation. Our goal in this project is to create a mutant unable to degrade GML in order to determine whether GML has any inhibitory activity of its own.
Acknowledgments
This work was supported by NIH grants AI R01-22159 and AI R01-30138.
REFERENCES
- 1.Blake M S, Johnson K H, Russell-Jones G J, Gotschlich E C. A rapid, sensitive method for detection of alkaline phosphatase conjugated anti-antibody on Western blots. Anal Biochem. 1984;136:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- 2.Branger C, Goullet P. Esterase electrophoretic polymorphism of methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1987;24:275–281. doi: 10.1099/00222615-24-3-275. [DOI] [PubMed] [Google Scholar]
- 3.de Azavedo J C S, Arbuthnott J P. Toxicity of staphylococcal toxic shock syndrome toxin 1 in rabbits. Infect Immun. 1984;46:314–317. doi: 10.1128/iai.46.2.314-317.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornblum J, Kreiswirth B, Projan S J, Ross H, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 5.Kornblum J, Projan S J, Moghazeh S L, Novick R P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63:75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- 6.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 7.Lee C Y, Iandolo J J. Lysogenic conversion of staphylococcal lipase caused by insertion of the bacteriophage phage L54a genome into the lipase structural gene. J Bacteriol. 1986;166:385–391. doi: 10.1128/jb.166.2.385-391.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikoleit K, Rosenstein R, Verheij H M, Gotz F. Comparative biochemical and molecular analysis of the Staphylococcus hyicus, Staphylococcus aureus and a hybrid lipase. Indication for a C-terminal phospholipase domain. Eur J Biochem. 1995;228:732–738. [PubMed] [Google Scholar]
- 9.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 10.Novick R P, Murphy E, Gryczan T J, Baron E, Edelman I. Penicillinase plasmids of Staphylococcus aureus: restriction-deletion maps. Plasmid. 1979;2:109–129. doi: 10.1016/0147-619x(79)90010-6. [DOI] [PubMed] [Google Scholar]
- 11.O'Callaghan C H, Morris A, Kirby S M, Shingler A H. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Projan S J, Brown-Skrobot S, Schlievert P M, Vandenesch F, Novick R P. Glycerol monolaurate inhibits the production of β-lactamase, toxic shock syndrome toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J Bacteriol. 1994;176:4204–4209. doi: 10.1128/jb.176.14.4204-4209.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruzin A, Novick R P. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J Bacteriol. 1998;180:182–185. doi: 10.1128/jb.180.1.182-185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlievert P, Blomster D. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 15.Schlievert P M, Deringer J R, Kim M H, Projan S J, Novick R P. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob Agents Chemother. 1992;36:626–631. doi: 10.1128/aac.36.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons J W, Adams H, Cox R C, Dekker N, Gotz F, Slotboom A J, Verheij H M. The lipase from Staphylococcus aureus. Expression in Escherichia coli, large-scale purification and comparison of substrate specificity to Staphylococcus hyicus lipase. Eur J Biochem. 1996;242:760–769. doi: 10.1111/j.1432-1033.1996.0760r.x. [DOI] [PubMed] [Google Scholar]
- 17.Waite M. Bacterial acyl hydrolyses (phospholipases A, B, and lysophospholipases) In: Hanahan D J, editor. The phospholipases. Vol. 5. New York: Plenum Press; 1987. pp. 17–28. [Google Scholar]